Abstract
Background: Meta-analyses on the prevalence and clinical significance of breast incidental uptake (BIU) at PET/CT are available only for [18F]FDG, showing that BIU is rare but malignant in a substantial proportion of cases. This study aimed to update the pooled prevalence and malignancy risk of BIU using different PET radiotracers, expanding [18F]FDG-based evidence. Methods: A comprehensive literature search of studies on BIU was carried out in two bibliographic databases, and the literature was screened up to 25 May 2025. Only original articles reporting BIU were selected. A proportion meta-analysis was conducted on a patient-based analysis using a random-effects model to estimate pooled prevalence, malignancy rate, and histological distribution. Results: In total, 29 studies were included in the systematic review and meta-analysis. PET/CT was performed using [18F]FDG (n = 25), radiolabeled somatostatin analogues (SSAs) (n = 3), or [18F]fluorocholine (n = 1). The pooled prevalence of BIU was 0.5% for [18F]FDG PET/CT, 3.4% for SSA PET/CT, and 2.6% for [18F]fluorocholine. The pooled malignancy rate among BIUs (female patients) was 33.5% for [18F]FDG, 86.4% for SSA, and 70% for [18F]fluorocholine PET/CT. Histological data were mainly available for [18F]FDG PET/CT, showing ductal carcinoma as the most frequent malignant histotype (pooled value 42.2%) and fibroadenoma (pooled value 14.8%) as the most frequent benign histotype. Conclusions: Similar to the case for [18F]FDG, BIU using other PET radiopharmaceuticals is uncommon but often malignant. Therefore, BIU should prompt dedicated breast imaging and, when indicated, histopathological confirmation. Further well-designed studies are needed to clarify the clinical impact of BIU detection and the prevalence and clinical significance of BIU using tracers other than [18F]FDG.
1. Introduction
Incidental imaging findings, often called incidentalomas, are lesions detected on examinations performed for indications unrelated to the organ imaged. Their observed frequency has risen in parallel with the wider use of advanced imaging and with improvements in spatial and contrast resolution [1,2]. Although many incidental findings prove clinically relevant, their prevalence and subsequent implications depend on both anatomical site and imaging technique [1,2].
Within molecular imaging, positron emission tomography (PET) has become integral to oncologic and non-oncologic care. PET is commonly combined with computed tomography (CT) or magnetic resonance imaging (MRI) as hybrid techniques, and several radiopharmaceuticals are available to evaluate distinct metabolic pathways or receptor profiles [3]. The most widely used tracer is [18F]fluorodeoxyglucose ([18F]FDG), a glucose analogue; however, other tracers, such as radiolabeled somatostatin analogues (SSAs) and [18F]fluorocholine, are increasingly used for specific clinical purposes [4]. In particular, the use of SSA and [18F]fluorocholine PET/CT has expanded beyond traditional indications, such as neuroendocrine tumors and prostate cancer, to include additional areas like parathyroid disorders for [18F]fluorocholine and meningiomas for SSAs, thus broadening the patient populations undergoing these scans [2,3].
Incidental focal uptake in the breast on PET/CT (breast incidental uptake, BIU) is a well-known occurrence in everyday practice. Despite organized breast-cancer screening with mammography, unsuspected lesions may be encountered on PET/CT studies acquired for non-breast indications [5,6]. Because [18F]FDG uptake is not tumor specific and may also occur in benign inflammatory or hyperplastic conditions, the mere presence of focal activity does not equate to malignancy. Conversely, a considerable proportion of BIUs represent previously unrecognized breast cancer, making accurate estimates of prevalence and malignancy risk essential to guide subsequent diagnostic work-up and to avoid both over- and under-management [5,6].
Previous reviews have mainly focused on BIU detected by [18F]FDG-PET/CT in women, reporting variable prevalence and malignancy rates across studies [5,6]. However, the clinical landscape has evolved: PET technology and usage have expanded, and incidental uptakes are now reported also with tracers beyond [18F]FDG, including SSAs (in the setting of neuroendocrine tumors) and [18F]fluorocholine (in parathyroid adenoma localization, prostate-cancer imaging, and other indications) [2,4,7]. The prevalence and clinical significance of BIU may differ by tracer because of distinct biological targets and background biodistribution [4].
We conducted an updated systematic review and meta-analysis to estimate the pooled prevalence of BIU on PET/CT across tracers, overall and in women; to quantify the pooled malignancy rate among BIUs (overall and among histologically verified lesions); and to describe the histopathologic spectrum of malignant and benign BIUs. We hypothesized that both prevalence and risk of malignancy vary according to tracer type.
2. Materials and Methods
2.1. Review Protocol, Working Group, and Review Question
This review followed a predefined protocol [8] and is reported in accordance with PRISMA 2020 (PRISMA checklist reported in Table S1) [9]. Registration on PROSPERO was not performed, consistent with PRISMA guidance that registration is recommended but not mandatory [9].
Three reviewers (C.M.I., A.M., and G.T.) independently performed screening and selection; disagreements were resolved by discussion with senior authors via videoconference. The review question was defined a priori as follows: “What are the prevalence and clinical significance of breast incidental uptake (BIU) at PET/CT across radiopharmaceuticals?”.
2.2. Search Strategy
A comprehensive search was conducted in two bibliographic databases (PubMed/MEDLINE and Cochrane Library) until May 2025. The search string combined free-text terms reflecting the organ, the incidental nature of findings, and the imaging modality:
(A) “breast” OR “mammary” AND (B) “incidental” OR “incidentaloma*” OR “incidental*” OR “unexpected” OR “unusual” AND (C) “PET” OR “positron”.
No language or date limits were applied. To enhance sensitivity, the reference lists of eligible studies and relevant reviews were hand-searched for additional records.
2.3. Study Selection
Eligibility criteria were prespecified. The inclusion criteria were as follows: original articles reporting prevalence and/or clinical significance of BIU detected on PET/CT using different radiopharmaceuticals performed for indications unrelated to primary breast disease. The exclusion criteria were as follows: articles outside the scope, articles lacking extractable data on BIU prevalence or malignancy, reviews, editorials, comments, letters, and case reports.
Titles/abstracts obtained with the predefined strategy were screened independently by three reviewers (C.M.I.; A.M.; G.T.). Full texts of potentially relevant records were then assessed. Final inclusion was established by consensus. Studies were entered into the quantitative analysis (meta-analysis) only when sufficient data were available to calculate at least one prespecified proportion (e.g., number of BIUs over PET/CT examinations, or number of malignant BIUs over histologically verified BIUs).
2.4. Data Extraction and Quality Assessment
Three reviewers (C.M.I.; A.M.; G.T.) independently extracted data using standardized forms: basic study characteristics; PET tracer; study population and sex distribution; number of PET/CT examinations; number of BIUs overall and in women; number of BIUs that underwent histopathology (BIU-H); number of malignant BIUs; and histopathologic diagnoses (malignant and benign subtypes). Discrepancies were resolved by consensus. Risk of bias and study quality were evaluated with the NIH Quality Assessment Tools online appropriate to each design.
2.5. Statistical Analysis
We performed proportion meta-analyses using random-effects models to account for between-study variability. Results are reported as pooled proportions with 95% confidence intervals. Heterogeneity was quantified with I2. Prespecified subgroup analyses included different radiopharmaceuticals. Sensitivity analyses were planned to exclude studies with evident selection bias from pooling, while retaining them in the qualitative synthesis. Publication bias/small-study effects were explored visually with funnel plots and formally with Egger’s regression test (α = 0.05). Analyses were executed with the web platform MetaAnalysisOnline (https://metaanalysisonline.com/, last accessed on 14 November 2025, module “Proportion”; forest and funnel plots generated with default settings).
3. Results
3.1. Literature Search
The selection process is summarized in Figure 1 and Figure S1. The search of two databases (PubMed/MEDLINE and the Cochrane Library) yielded 411 records screened by three reviewers. After title/abstract screening and full-text assessment, 29 studies met the inclusion criteria [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]: 25 used [18F]FDG [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34], 3 used somatostatin receptor tracers (SSAs) [35,36,37], and 1 used [18F]fluorocholine [38]. Case reports and non-original articles were excluded. One [18F]FDG study with selection bias (Benveniste et al. [23]) was retained for the narrative synthesis but excluded from meta-analysis. Most of the included studies were retrospective.
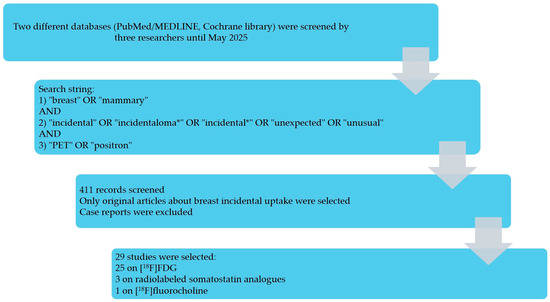
Figure 1.
Results of the literature search.
3.2. Qualitative Synthesis
Table 1 summarizes the main characteristics and outcomes of the 29 included studies on breast incidental uptake (BIU) detected with PET tracers. In this review, BIU was defined as incidentally detected focal breast uptake on PET/CT performed for non-breast indications. Across studies, clinical indications, sex distribution, and verification pathways (biopsy vs. imaging follow-up) differed. When tissue diagnosis was obtained, a substantial proportion of BIUs proved malignant, with higher rates reported in SSA and fluorocholine series, and the most frequent malignancy was ductal carcinoma, whereas benign outcomes most often included fibroadenoma and other non-specific benign lesions.

Table 1.
Prevalence and malignancy rate of breast incidental uptake detected by PET/CT with different tracers.
3.2.1. [18F]FDG PET/CT
Across studies, BIU on all scans was uncommon (pooled prevalence 0.5%) with higher values observed in female-only cohorts (pooled prevalence 1%). The outlier series by Benveniste et al. [23] (22.5% BIU) reflected referral/selection bias and was not included in meta-analysis. Histology was available for a large subset of [18F]FDG-BIUs, allowing pooled estimates of the malignant and benign spectrum. Among malignant BIUs, ductal carcinoma (DC) was the most frequent diagnosis, followed by other/unspecified primary malignancies, metastases, ductal carcinoma in situ (DCIS), and lobular carcinoma (LC); among benign entities, other/unspecified benign lesions and fibroadenoma predominated (Table 2).
3.2.2. Somatostatin Receptor PET/CT (SSA)
Three eligible studies using DOTA-peptides were identified. SSA exams were mostly performed for neuroendocrine tumor work-up. Reported BIU prevalence ranged from 0.8% to 12.1% of scans overall, with higher values in smaller female-only cohorts (pooled prevalence 5.2%). Histology was available in a minority of BIUs; when obtained, malignancy among biopsied BIUs was frequent; however, the corresponding estimates showed wide confidence intervals due to the small number of contributing studies and events.
3.2.3. [18F]Fluorocholine PET/CT
One study (Broos et al. [38]) provided extractable data. BIU prevalence was 2.6% overall and 3.4% among women; all 7/7 BIUs with histopathologic confirmation were malignant. Because only a single study was available, no pooling was performed for fluorocholine.
3.2.4. Overall Study Quality
Using the NIH tools, the overall quality was moderate, mainly limited by retrospective design, variable verification with histology, and incomplete sex-specific denominators in some cohorts.
3.3. Quantitative Synthesis
3.3.1. Prevalence of BIU
With [18F]FDG PET/CT the pooled prevalence of BIU across 19 analyzable series (n = 261,563 scans; Figure 2) was 0.5% (95% CI 0.3–0.6). Statistical heterogeneity was high (I2 = 96.8%), reflecting differences in populations and referral indications. Restricting to women (n = 119,557 scans; Figure 3), pooled BIU prevalence was 1.0% (95% CI 0.6–1.6), again with substantial heterogeneity (I2 = 97.8%).
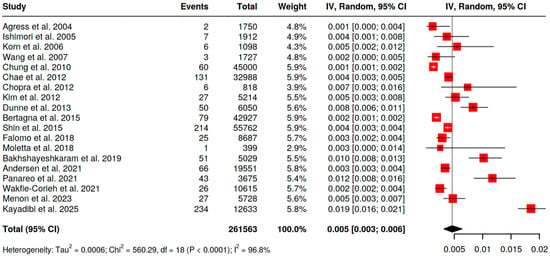
Figure 2.
Pooled prevalence of BIU in both genders [10,11,12,13,16,18,19,20,21,24,26,27,28,29,30,31,32,33,34].
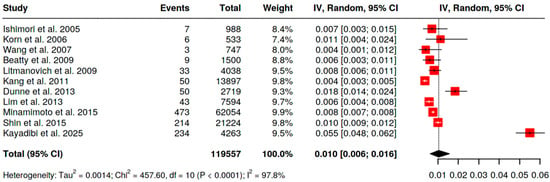
Figure 3.
Pooled prevalence of BIU in female patients [11,12,13,14,15,17,21,22,25,26,34].
With SSA PET/CT, based on three small studies, pooled prevalence was 3.4% (95% CI 0.0–11.5) with considerable heterogeneity (I2 = 72.4%). Female-only prevalence was 5.2% (95% CI 0.1–14.7; I2 = 55.9%). The wide CIs reflect sparse data and between-study variation.
With [18F]fluorocholine PET/CT the single eligible study reported 2.6% overall and 3.4% BIU in women (no heterogeneity measure applicable).

Table 2.
Histological findings of breast incidentalomas at PET/CT.
Table 2.
Histological findings of breast incidentalomas at PET/CT.
| PET Tracer | First Author [Ref.] | BIU-H | DC | % of DC Among BIU-H | DCIS | % of DCIS Among BIU-H | LC | % of LC Among BIU-H | OUM | % of OUM Among BIU-H | MET | % of MET Among BIU-H | FA | % of FA Among BIU-H | OBL | % of OBL Among BIU-H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [18F]FDG | Agress et al. [10] | 2 | 1 | 50 | - | - | 1 | 50 | - | - | - | |||||
| Ishimori et al. [11] | 2 | 2 | 100 | - | - | - | - | - | - | |||||||
| Korn et al. [12] | 6 | 5 | 83.3 | - | - | - | - | 1 | 16.7 | - | ||||||
| Wang et al. [13] | 3 | 2 | 66.7 | - | - | - | - | 1 | 33.3 | - | ||||||
| Beatty et al. [14] | 8 | 5 | 62.5 | - | - | - | - | 2 | 25 | 1 | 12.5 | |||||
| Litmanovich et al. [15] | 21 | 7 | 33.3 | - | 2 | 9.5 | - | 3 | 14.3 | 2 | 9.5 | 7 | 33.3 | |||
| Chung et al. [16] | 12 | 6 | 50 | - | - | - | 1 | 8.3 | 1 | 8.3 | 4 | 33.3 | ||||
| Kang et al. [17] | 35 | 14 | 40 | 2 | 5.7 | - | - | 2 | 5.7 | 1 | 2.9 | 16 | 45.7 | |||
| Chae et al. [18] | 60 | 23 | 38.3 | 3 | 5 | 1 | 1.7 | - | 5 | 8.3 | 8 | 13.3 | 20 | 33.3 | ||
| Chopra et al. [19] | 3 | - | - | - | 2 | 66.7 | - | - | 1 | 33.3 | ||||||
| Kim et al. [20] | 23 | 10 | 43.5 | 3 | 13 | - | - | 2 | 8.7 | 2 | 8.7 | 6 | 26.1 | |||
| Dunne et al. [21] | 23 | 8 | 34.8 | 2 | 8.7 | 1 | 4.3 | 4 | 17.4 | 2 | 8.7 | 4 | 17.4 | 2 | 8.7 | |
| Lim et al. [22] | 17 | 5 | 29.4 | 3 | 17.6 | - | 1 | 5.9 | - | 2 | 11.8 | 6 | 35.3 | |||
| Benveniste et al. [23] | 55 | 19 | 34.5 | 1 | 1.8 | 1 | 1.8 | 4 | 7.3 | 12 | 21.8 | - | 18 | 32.7 | ||
| Bertagna et al. [24] | 35 | 17 | 48.6 | 2 | 5.7 | 4 | 11.4 | - | 2 | 5.7 | 9 | 25.7 | 1 | 2.9 | ||
| Minamimoto et al. [25] | 161 | 95 | 59 | 27 | 16.8 | - | 39 | 24.2 | - | - | - | |||||
| Shin et al. [26] | 60 | 21 | 35 | 1 | 1.7 | 1 | 1.7 | 2 | 3.3 | 2 | 3.3 | 10 | 16.7 | 23 | 38.3 | |
| Falomo et al. [27] | 11 | - | - | - | - | - | - | - | ||||||||
| Moletta et al. [28] | 1 | 1 | 100 | - | - | - | - | - | - | |||||||
| Bakhshayeshkaram et al. [29] | 22 | 6 | 27.3 | - | 3 | 13.6 | - | 1 | 4.5 | 4 | 18.2 | 8 | 36.4 | |||
| Andersen et al. [30] | 40 | 26 | 65 | - | 1 | 2.5 | 3 | 7.5 | 4 | 10 | 3 | 7.5 | 2 | 5 | ||
| Panareo et al. [31] | 22 | 9 | 40.9 | - | - | 3 | 13.6 | 4 | 18.2 | 6 | 27.3 | 1 | 4.5 | |||
| Wakfie-Corieh et al. [32] | 23 | - | - | 1 | 4.3 | 8 | 34.8 | - | - | 14 | 60.9 | |||||
| Menon et al. [33] | 26 | 10 | 38.5 | 2 | 7.7 | 1 | 3.8 | 3 | 11.5 | 2 | 7.7 | 4 | 15.4 | 4 | 15.4 | |
| Kayadibi et al. [34] | 63 | 10 | 15.9 | - | 2 | 3.2 | 3 | 4.8 | 5 | 7.9 | 21 | 33.3 | 22 | 34.9 | ||
| Pooled values (95%CI) | 42.2% (34–50.5) | 7.1% (3.4–11.8) | 3.5% (1.7–5.8) | 11.1% (4.8–19) | 8.7% (6–11.6) | 14.8% (10.3–19.9) | 25.3% (17.3–34) | |||||||||
| Radiolabeled Somatostatin analogues | Elgeti et al. [35] | 4 | 2 | 50 | - | - | - | 2 | 50 | - | - | |||||
| Kuyumcu et al. [36] | - | - | - | - | - | - | - | - | ||||||||
| Cavicchioli et al. [37] | 1 | - | - | - | - | 1 | 100 | - | - | |||||||
| [18F]F-choline | Broos et al. [38] | 7 | 3 | 42.9 | 1 | 14.3 | 2 | 28.6 | 1 | 14.3 | - | - | - |
Legend: BIU-H = number of breast incidentalomas that underwent histology, DC = ductal carcinoma, DCIS = ductal carcinoma in situ, LC = lobular carcinoma, OUM = other or unspecified primary malignant lesions, MET = metastatic lesion, FA = fibroadenoma, OBL = other or unspecified benign lesion.
3.3.2. Risk of Malignancy
Among histologically verified BIUs (BIU-H),
- −
- For [18F]FDG PET/CT, the pooled malignancy risk was 60.9% (95% CI 52.5–69.0; I2 = 59.5%) (Figure 4);
- −
- For SSA PET/CT, the malignancy risk was 100% (95% CI 66.8–100; I2 = 0%), acknowledging very small numbers;
- −
- For [18F]fluorocholine PET/CT, the malignancy risk was 100% (7/7 malignant lesions in a single study).
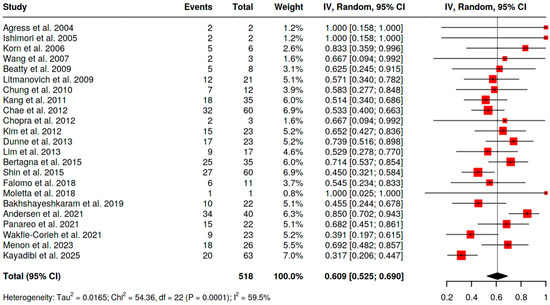
Figure 4.
Pooled prevalence of MBIU among BIU-H [10,11,12,13,14,15,16,17,18,19,20,21,22,24,26,34].
Overall malignancy risk among BIUs in women (i.e., malignant BIUs/total BIUs in female cohorts) was as follows:
- −
- For [18F]FDG PET/CT, 33.5% (95% CI 25.2–42.3; I2 = 87.9%) (Figure 5);
- −
- For SSA PET/CT, 86.4% (95% CI 72.0–100; I2 = 50.7%);
- −
- For [18F]fluorocholine PET/CT, 70% (single study).
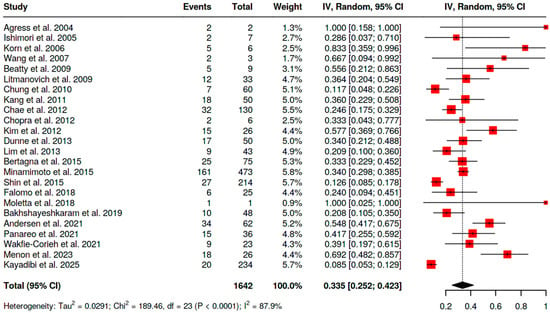
Figure 5.
Overall risk of malignancy of BIU in female patients [10,11,12,13,14,15,16,17,18,19,20,21,22,24,25,26,27,28,29,30,31,32,33,34].
The overall risk of malignancy in BIU in both genders (Figure 6) was similar to that observed in the female cohort, but the analysis had reduced statistical power due to limited data. For [18F]FDG PET/CT this value was 32.5% (95% CI 22.2–43.4; I2 = 88%).
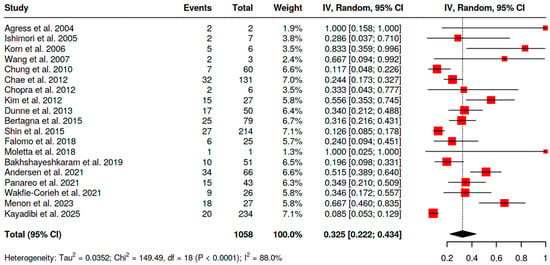
Figure 6.
Overall risk of malignancy of BIU in both genders [10,11,12,13,16,18,19,20,21,24,26,27,28,29,30,31,32,33,34].
3.3.3. Histopathology
Where pooling histology was possible (Table 2), in particular with [18F]FDG PET/CT for which more data were available, malignant BIUs were predominantly ductal carcinoma (42.2%), with DCIS (7.1%) and LC (3.5%) being less frequent; other/unspecified primary malignancies comprised 11.1%, and metastases were 8.7%. Among benign lesions, fibroadenoma accounted for 14.8% and other/unspecified benign lesions for 25.3%.
3.3.4. Heterogeneity, Sensitivity Analyses, and Potential Biases
Across prevalence and malignancy outcomes, statistical heterogeneity was frequent, especially for [18F]FDG prevalence (I2 > 90%) and for the overall risk of malignancy among BIUs in women. The main contributors were differences in population mix (oncologic or non-oncologic indications; screening backgrounds), sex-specific denominators (often incomplete), verification bias (variable histology rates), and heterogeneity in data reporting (e.g., counts given per scan vs. per patient). Excluding the selection-biased [18F]FDG study (Benveniste et al. [23]) did not materially change the pooled estimates.
Funnel plots showed asymmetry for the [18F]FDG “overall risk of malignancy” (Figure 7 and Figure 8). Egger’s test was significant in women (intercept 2.14, 95% CI 0.30–3.97; t = 2.286; p = 0.032) and in both genders (intercept 3.58, 95% CI 1.76–5.40; t = 3.848; p = 0.001), suggesting small-study effects/publication bias. Given the high heterogeneity and likely verification/selection bias in several retrospective studies, the funnel asymmetry should be interpreted with caution. It may reflect small-study effects/publication bias, but it could also come from center- and protocol-specific case-mix/work-up variation.
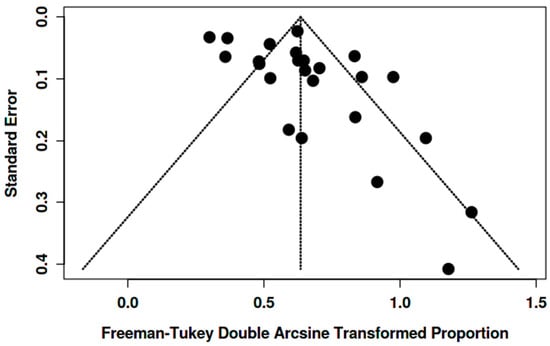
Figure 7.
Funnel plot for overall risk of malignancy among BIUs (women).
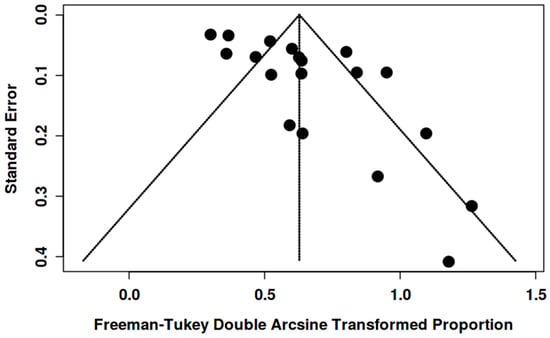
Figure 8.
Funnel plot for overall risk of malignancy among BIUs (both genders).
Taken together, BIU is uncommon on PET/CT but not rare, and a substantial fraction is malignant with tracer-dependent differences that likely mirror biological targets.
4. Discussion
4.1. Literature Data
To our knowledge, this is the most up-to-date systematic review with meta-analysis on breast incidental uptake (BIU) across multiple PET radiopharmaceuticals, extending earlier syntheses centered on [18F]FDG alone. We excluded case reports to minimize reporting/selection biases and because they typically lack an extractable denominator; consistent with methodological guidance for diagnostic/prognostic meta-analyses, we included only studies providing sufficient outcome data to compute summary estimates [8]. Our pooled results confirm that [18F]FDG-BIUs are uncommon but clinically meaningful: prevalence was 0.5% on all scans and 1.0% among women, while about 1/3 of BIUs in women proved malignant, consistent with prior meta-analytic results [5,6].
A key message from this multi-tracer review is the tracer-dependent difference. Although non-[18F]FDG evidence remains limited, somatostatin receptor (SSA) and [18F]fluorocholine series tended to show higher BIU prevalence and, among biopsied cases, higher malignancy proportions than [18F]FDG. For [18F]fluorocholine, the single eligible study in our dataset [38] reported BIU in 3.4% of women and malignancy among all biopsied BIUs (7/7); for SSAs, this systematic review highlights a higher malignancy risk for BIU than that for [18F]FDG. These results plausibly reflect biological targeting (receptor expression or phospholipid turnover) and background biodistribution. However, confidence intervals are wide given the small number of studies and events, so estimates should be interpreted with caution.
From a biological standpoint, lesion visibility on PET is influenced by histology and metabolic phenotype. [18F]FDG uptake is typically higher in ductal than in lobular carcinomas and correlates with tumor aggressiveness, which helps explain [18F]FDG-poor malignant BIUs in certain histotypes [39,40,41]. Our pooled histology mirrors prior reviews, with invasive ductal carcinoma predominating among malignant BIUs and fibroadenoma among benign outcomes [5]. Regarding SSTRs, they are overexpressed in many cancers, and increased choline uptake, driven by choline kinase activity, is also common in cancer cells. These differences in receptor expression help explain higher malignancy rates with SSA and [18F]fluorocholine tracers [4].
The literature on incidental second tumors also underscores that additional, clinically unsuspected malignancies are not rare in oncology populations, reinforcing the value of adequately investigating focal incidental uptake when encountered [42,43]. In practice, an incidental focal breast uptake on whole-body PET/CT should lead to targeted diagnostic breast imaging (mammography/ultrasound) and tissue sampling when indicated. Moreover, while some studies explore SUV cut-offs, SUV alone should not be used to differentiate between malignant and benign BIUs because SUV values for benign and malignant lesions often overlap and are affected by patient- and scanner-related factors, so SUV alone cannot accurately distinguish malignancy with the data currently available [5].
4.1.1. Selection Bias in the Outlier Study
One notable outlier (Benveniste et al. [23]) reported BIU in nearly 1/4 [18F]FDG PET/CT examinations. This overestimation is due to the way the cohort was assembled: cases were identified by searching [18F]FDG PET/CT reports for the word “breast” instead of considering all scans. This keyword-based selection introduces bias, affecting prevalence estimates. For this reason, we included the paper in the qualitative discussion but excluded it from the pooled analysis [6,23].
4.1.2. What to Do with an Incidental Focus
Because we found a meaningful risk of malignancy among BIUs, particularly with SSAs and fluorocholine, a structured management approach is warranted. In line with previous reviews, BIU should be further evaluated with mammography/breast ultrasound and with biopsy when indicated, while avoiding exclusive use of PET SUV-values alone [5].
4.2. Limitations and Suggestions for Future Research
This study has limitations. First, beyond [18F]FDG, the evidence base is small (only four SSA studies and one [18F]fluorocholine study), yielding wide CIs and susceptibility to small-study effects. Therefore, given the limited number of studies, these estimates should be interpreted cautiously and cannot yet inform clinical guidelines. Second, heterogeneity was substantial for several endpoints (e.g., BIU prevalence with [18F]FDG, overall malignancy risk), driven by differences in clinical indications (oncologic or non-oncologic indications, screening backgrounds), sex-specific denominators (often incomplete), verification bias (variable histology rates), and heterogeneity in data reporting (e.g., counts given per scan vs. per patient), and these aspects could explain the high I2. Third, verification bias: histology was available in a part of BIUs and loss to follow-up was common in retrospective designs, as also emphasized in prior BIU syntheses [5,6]. Fourth, selection biases (e.g., report-keyword filters) can markedly distort prevalence and malignancy proportions if included uncritically [23]. Fifth, evidence of funnel-plot asymmetry for [18F]FDG “overall risk of malignancy” suggests small-study effects or publication bias; this result should be interpreted cautiously. It may reflect small-study effects/publication bias, but it could also come from center- and protocol-specific case-mix/work-up variation.
Future work should ideally prospectively report PET/CT cohorts by tracer, with prospective registries and multicenter collaborations; use sex-specific denominators; adopt standardized BIU verification pathways; report histology for all worked-up BIUs (with reasons for non-verification); and integrate correlative pathology to clarify tracer-specific biology (e.g., SSTR expression, choline metabolism) and to assess subsequent management impact.
5. Conclusions
In clinical practice, [18F]FDG-BIUs are rare but meaningful, with malignancy found in about 1/3 of BIUs in women. Non-[18F]FDG tracers (SSAs and [18F]fluorocholine) show higher BIU prevalence and, among biopsied cases, higher malignancy proportions, but evidence is limited and confidence intervals are wide. BIU should lead to correlation with dedicated breast imaging and consideration of tissue sampling, while SUV-based discrimination is unreliable with the data currently available. Larger, standardized prospective studies, especially for SSAs and [18F]fluorocholine, are needed to refine prevalence and malignancy risk estimates and to define tracer-specific management pathways.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph18121831/s1, Figure S1: Flow-chart about the selection of studies according to PRISMA 2020. Table S1: PRISMA 2020 checklist.
Author Contributions
Conceptualization, G.T.; methodology, G.T.; software, G.T.; validation, A.M.; formal analysis, C.M.I.; resources, C.M.I., A.M., and G.T.; data curation, C.M.I., A.M., and G.T.; writing—original draft preparation, C.M.I. and G.T.; writing—review and editing, A.M., S.T., C.M., M.C., G.P., A.R., and D.A.; supervision, G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This scientific project was conducted in the framework of the EANM mentorship program (mentees: A.M. and S.T.; mentors: G.T. and D.A.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Sullivan, J.W.; Muntinga, T.; Grigg, S.; Ioannidis, J.P.A. Prevalence and outcomes of incidental imaging findings: Umbrella review. BMJ 2018, 361, k2387. [Google Scholar] [CrossRef]
- Iacovitti, C.M.; Albano, D.; Rizzo, A.; Piccardo, A.; Cuzzocrea, M.; Paone, G.; Trimboli, P.; Treglia, G. Meta-Analysis on the Prevalence and Significance of Incidental Findings in the Thyroid Gland Using Other PET Radiopharmaceuticals Beyond [18F]FDG. Pharmaceuticals 2025, 18, 723. [Google Scholar] [CrossRef]
- Juweid, M.E.; Al-Qasem, S.F.; Khuri, F.R.; Gallamini, A.; Lohmann, P.; Ziellenbach, H.J.; Mottaghy, F.M. Beyond fluorodeoxyglucose: Molecular imaging of cancer in precision medicine. CA Cancer J. Clin. 2025, 75, 226–242. [Google Scholar] [CrossRef]
- Lin, M.; Coll, R.P.; Cohen, A.S.; Georgiou, D.K.; Manning, H.C. PET Oncological Radiopharmaceuticals: Current Status and Perspectives. Molecules 2022, 27, 6790. [Google Scholar] [CrossRef]
- Bertagna, F.; Treglia, G.; Orlando, E.; Dognini, L.; Giovanella, L.; Sadeghi, R.; Giubbini, R. Prevalence and clinical significance of incidental F18-FDG breast uptake: A systematic review and meta-analysis. Jpn. J. Radiol. 2014, 32, 59–68. [Google Scholar] [CrossRef]
- Aarstad, E.M.; Nordhaug, P.; Naghavi-Behzad, M.; Larsen, L.B.; Gerke, O.; Hildebrandt, M.G. Prevalence of focal incidental breast uptake on FDG-PET/CT and risk of malignancy: A systematic review and meta-analysis. Eur. J. Hybrid Imaging 2019, 3, 16. [Google Scholar] [CrossRef]
- Iacovitti, C.M.; Muoio, B.; Albano, D.; Rizzo, A.; Cuzzocrea, M.; Paone, G.; Treglia, G. The Prevalence and Significance of Incidental Positron Emission Tomography Findings in the Brain Using Radiotracers Other than [18F]FDG: A Systematic Review and Meta-Analysis. Diagnostics 2025, 15, 1204. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseerf, L.; Tetzlaff, J.M.; Akli, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Agress, H., Jr.; Cooper, B.Z. Detection of clinically unexpected malignant and premalignant tumors with whole-body FDG PET: Histopathologic comparison. Radiology 2004, 230, 417–422. [Google Scholar] [CrossRef]
- Ishimori, T.; Patel, P.V.; Wahl, R.L. Detection of unexpected additional primary malignancies with PET/CT. J. Nucl. Med. 2005, 46, 752–757. [Google Scholar] [PubMed]
- Korn, R.L.; Yost, A.M.; May, C.C.; Kovalsky, E.R.; Orth, K.M.; Layton, T.A.; Drumm, D. Unexpected focal hypermetabolic activity in the breast: Significance in patients undergoing 18F-FDG PET/CT. AJR Am. J. Roentgenol. 2006, 187, 81–85. [Google Scholar] [CrossRef]
- Wang, G.; Lau, E.W.; Shakher, R.; Rischin, D.; Ware, R.E.; Hong, E.; Binns, D.S.; Hogg, A.; Drummond, E.; Hicks, R.J. How do oncologists deal with incidental abnormalities on whole-body fluorine-18 fluorodeoxyglucose PET/CT? Cancer 2007, 109, 117–124. [Google Scholar] [CrossRef]
- Beatty, J.S.; Williams, H.T.; Gucwa, A.L.; Hughes, M.P.; Vasudeva, V.S.; Aldridge, B.A.; Fields, D.M.; David, G.S.; Lind, D.S.; Kruse, E.J.; et al. The predictive value of incidental PET/CT findings suspicious for breast cancer in women with non-breast malignancies. Am. J. Surg. 2009, 198, 495–499. [Google Scholar] [CrossRef]
- Litmanovich, D.; Gourevich, K.; Israel, O.; Gallimidi, Z. Unexpected foci of 18F-FDG uptake in the breast detected by PET/CT: Incidence and clinical significance. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1558–1564. [Google Scholar] [CrossRef]
- Chung, A.; Schoder, H.; Sampson, M.; Morrow, M.; Port, E. Incidental breast lesions identified by 18F-fluorodeoxyglucose-positron emission tomography. Ann. Surg. Oncol. 2010, 17, 2119–2125. [Google Scholar] [CrossRef]
- Kang, B.J.; Lee, J.H.; Yoo, I.eR.; Kim, S.H.; Choi, J.J.; Jeong, S.H.; Yim, H.W. Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am. J. Roentgenol. 2011, 197, 341–347. [Google Scholar] [CrossRef]
- Chae, E.Y.; Cha, J.H.; Kim, H.H.; Shin, H.J.; Kim, H.J.; Oh, H.Y.; Koh, Y.H.; Moon, D.H. Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: Clinical significance and differential diagnosis. Acta Radiol. 2012, 53, 530–535. [Google Scholar] [CrossRef]
- Chopra, A.; Ford, A.; De Noronha, R.; Matthews, S. Incidental findings on positron emission tomography/CT scans performed in the investigation of lung cancer. Br. J. Radiol. 2012, 85, e229–e237. [Google Scholar] [CrossRef]
- Kim, M.Y.; Cho, N.; Chang, J.M.; Yun, B.L.; Bae, M.S.; Kang, K.W.; Moon, W.K. Mammography and ultrasonography evaluation of unexpected focal 18F-FDG uptakes in breast on PET/CT. Acta Radiol. 2012, 53, 249–254. [Google Scholar] [CrossRef]
- Dunne, R.M.; O’Mahony, D.; Wilson, G.; McDermott, R.; O’Keeffe, S.A. The role of the breast radiologist in evaluation of breast incidentalomas detected on 18-fludeoxyglucose positron emission tomography/CT. Br. J. Radiol. 2013, 86, 20130034. [Google Scholar] [CrossRef]
- Lim, S.; Lee, E.H.; Park, J.M.; Chang, Y.W.; Kim, H.H.; Jeong, S.H. Role of combined BI-RADS assessment using mammography and sonography for evaluation of incidental hypermetabolic lesions in the breast on 18F-FDG PET-CT. Acta Radiol. 2013, 54, 1117–1124. [Google Scholar] [CrossRef]
- Benveniste, A.P.; Marom, E.M.; Benveniste, M.F.; Mawlawi, O.; Fox, P.S.; Yang, W. Incidental primary breast cancer detected on PET-CT. Breast Cancer Res. Treat. 2015, 151, 261–268. [Google Scholar] [CrossRef]
- Bertagna, F.; Evangelista, L.; Piccardo, A.; Bertoli, M.; Bosio, G.; Giubbini, R.; Orlando, E.; Treglia, G. Multicentric study on 18F-FDG-PET/CT breast incidental uptake in patients studied for non-breast malignant purposes. Rev. Esp. Med. Nucl. Imagen Mol. 2015, 34, 24–29. [Google Scholar] [CrossRef]
- Minamimoto, R.; Senda, M.; Jinnouchi, S.; Terauchi, T.; Yoshida, T.; Inoue, T. Detection of breast cancer in an FDG-PET cancer screening program: Results of a nationwide Japanese survey. Clin. Breast Cancer 2015, 15, e139–e146. [Google Scholar] [CrossRef]
- Shin, K.M.; Kim, H.J.; Jung, S.J.; Lim, H.S.; Lee, S.W.; Cho, S.H.; Jang, Y.J.; Lee, H.J.; Kim, G.C.; Jung, J.H.; et al. Incidental Breast Lesions Identified by (18)F-FDG PET/CT: Which Clinical Variables Differentiate between Benign and Malignant Breast Lesions? J. Breast Cancer 2015, 18, 73–79. [Google Scholar] [CrossRef]
- Falomo, E.; Strigel, R.M.; Bruce, R.; Munoz Del Rio, A.; Adejumo, C.; Kelcz, F. Incidence and outcomes of incidental breast lesions detected on cross-sectional imaging examinations. Breast J. 2018, 24, 743–748. [Google Scholar] [CrossRef]
- Moletta, L.; Bissoli, S.; Fantin, A.; Passuello, N.; Valmasoni, M.; Sperti, C. PET/CT incidental detection of second tumor in patients investigated for pancreatic neoplasms. BMC Cancer 2018, 18, 531. [Google Scholar] [CrossRef]
- Bakhshayeshkaram, M.; Salehi, Y.; Abbasi, M.; Hashemi Beni, R.; Seifi, S.; Hassanzad, M.; Jamaati, H.R.; Aghahosseini, F. A preliminary study to propose a diagnostic algorithm for PET/CT-detected incidental breast lesions: Application of BI-RADS lexicon for US in combination with SUVmax. Eur. Radiol. 2019, 29, 5507–5516. [Google Scholar] [CrossRef]
- Andersen, J.D.; Zacho, H.D.; Petersen, L.J. The frequency and malignancy rate of incidental focal breast lesions identified by 18F-fluorodeoxyglucose positron emission tomography. Nucl. Med. Commun. 2021, 42, 93–100. [Google Scholar] [CrossRef]
- Panareo, S.; Urso, L.; Nieri, A.; Caracciolo, M.; Valpiani, G.; Torricelli, P.; Frassoldati, A.; Cittanti, C.; Rollo, M.; Bartolomei, M. Clinical-Diagnostic Relevance of Breast “Incidentaloma” Detected During 18F-Fluoro-2-Deoxy-D-Glucose Positron Emission Tomography/Computed Tomography: Correlation with Radiological Imaging and Histopathology. Indian. J. Nucl. Med. 2021, 36, 385–390. [Google Scholar] [CrossRef]
- Wakfie-Corieh, C.G.; Rodríguez Rey, C.; Ortega Candil, A.; Ferrando-Castagnetto, F.; Valhondo-Rama, R.; Ruiz Tolón, M.; Pascual Martin, A.; Carreras Delgado, J.L. Clinical relevance of incidental focal breast uptake on fluorine-18 fluorodeoxyglucose PET/computed tomography studies: An experience in a high-load center of Spain. Nucl. Med. Commun. 2021, 42, 678–684. [Google Scholar] [CrossRef]
- Menon, P.; Bourke, A. Breast incidentalomas on 18-Fluorodeoxyglucose positron emission tomography-computed tomography performed for a non-mammary cause: Significance and outcomes. J. Med. Imaging Radiat. Oncol. 2023, 67, 357–364. [Google Scholar] [CrossRef]
- Kayadibi, Y.; Karagoz, S.H.; Kurt, S.A.; Kargin, O.A.; Guneren, C.; Sahin, O.E.; Hamid, R.; Yilmaz, M.H. Diagnostic Characteristics and Clinical Relevance of Incidental Hypermetabolic Breast Lesions Detected on 18F-FDG PET-CT: A Retrospective Evaluation. Acad. Radiol. 2025, 32, 1806–1815. [Google Scholar] [CrossRef]
- Elgeti, F.; Amthauer, H.; Denecke, T.; Steffen, I.; Heuck, F.; Stelter, L.; Ruf, J. Incidental detection of breast cancer by 68Ga-DOTATOC-PET/CT in women suffering from neuroendocrine tumours. Nuklearmedizin. 2008, 47, 261–265. [Google Scholar] [PubMed]
- Kuyumcu, S.; Özkan, Z.G.; Sanli, Y.; Yilmaz, E.; Mudun, A.; Adalet, I.; Unal, S. Physiological and tumoral uptake of (68)Ga-DOTATATE: Standardized uptake values and challenges in interpretation. Ann. Nucl. Med. 2013, 27, 538–545. [Google Scholar] [CrossRef]
- Cavicchioli, M.; Bitencourt, A.G.V.; Lima, E.N.P. 68Ga-DOTATATE PET/CT versus 111In-octreotide scintigraphy in patients with neuroendocrine tumors: A prospective study. Radiol. Bras. 2022, 55, 13–18. [Google Scholar] [CrossRef]
- Broos, W.A.M.; Knol, R.J.J.; Zant, F.M.V.; Schaper, N.C.; Wondergem, M. Incidental Findings on 18 F-Fluorocholine PET/CT for Parathyroid Imaging. World J. Nucl. Med. 2022, 21, 192–199. [Google Scholar] [CrossRef]
- Avril, N.; Menzel, M.; Dose, J.; Schelling, M.; Weber, W.; Jänicke, F.; Nathrath, W.; Schwaiger, M. Glucose metabolism of breast cancer assessed by 18F-FDG PET: Histologic and immunohistochemical tissue analysis. J. Nucl. Med. 2001, 42, 9–16. [Google Scholar] [PubMed]
- Buck, A.; Schirrmeister, H.; Kühn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T. FDG uptake in breast cancer: Correlation with biological and clinical prognostic parameters. Eur. J. Nucl. Med. Mol. Imaging 2002, 29, 1317–1323. [Google Scholar] [CrossRef]
- Crippa, F.; Seregni, E.; Agresti, R.; Chiesa, C.; Pascali, C.; Bogni, A.; Decise, D.; De Sanctis, V.; Greco, M.; Daidone, M.G.; et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: A preliminary observation. Eur. J. Nucl. Med. 1998, 25, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Hemminki, K. Second primary neoplasms among 53,159 haematolymphoproliferative malignancy patients in Sweden, 1958-1996: A search for common mechanisms. Br. J. Cancer 2001, 85, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Muto, T.; Oya, M.; Ota, H.; Azekura, K.; Yamaguchi, T. Multiple primary cancer: An experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int. J. Clin. Oncol. 2003, 8, 162–167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).