Abstract
Background: Trichomonas vaginalis is the causative agent of human trichomoniasis, the most common non-viral sexually transmitted infection. This disease is associated with an increased susceptibility to HIV and HPV infections. Currently, resistance to metronidazole (MTZ), the main drug used for treatment, has been reported in up to 9.6% of cases; additionally, the compound is also associated with adverse side effects. Therefore, it is urgent to identify new treatment options. Objective: In this study, we investigated for the first time the in vitro and in silico activity against T. vaginalis of betulin and stigmasterol isolated from Tagetes nelsonii Greenm, as well as their hemolytic activity. Methods: Plant specimen was collected in Chiapas, Mexico. Hexane and methanol extracts were prepared through sonication-assisted maceration. The antiprotozoal and hemolytic activities were evaluated in vitro against Trichomonas vaginalis trophozoites and human erythrocytes. The most active extract was fractionated using chromatographic techniques in a bioassay-guided study. The active metabolites were identified by 1H and 13C-NMR spectroscopy, and their biological activity was further assessed in silico against lactate dehydrogenase (LDH), pyruvate ferredoxin oxidoreductase (PFOR) methionine gamma-lyase (MGL) and purine nucleoside phosphorylase (PNP) T. vaginalis enzymes. Results: Both triterpenes showed anti-trichomonal activity and no hemolytic activity at 100 µg/mL. Molecular docking studies predicted promising interactions of triterpenes with T. vaginalis drug target proteins, TvpFOR and TvLDH. Conclusions: Our results revealed that betulin and stigmasterol are potential molecules for the development of new trichomonacidal therapies against T. vaginalis.
1. Introduction
Trichomonas vaginalis is an anaerobic, flagellated protozoan parasite that infects the human urogenital tract, causing trichomoniasis, the most common non-viral sexually transmitted infection globally, with approximately 156 million cases per year [1]. Trichomoniasis represents a serious global reproductive health concern. Approximately 30% of infected individuals, predominantly women, develop symptoms such as genital discomfort, itching, burning, odor and discharge [2,3,4]. Chronic or untreated infections have been associated with an increased risk of human papillomavirus (HPV) and human immunodeficiency virus (HIV) infections, pelvic inflammatory disease, cervical neoplasia, adverse pregnancy outcomes, and infertility [5,6,7].
Current treatment for Trichomonas vaginalis infection relies almost exclusively on 5-nitroimidazole (5-NMZ) derivatives, mainly metronidazole (MTZ) and tinidazole (TNZ). Nevertheless, the emergence of resistant strains, reported in up to 9.6% of clinical isolates [8], together with documented genotoxic and neurotoxic side effects of MTZ, including encephalopathy [9,10,11,12], highlight the urgent need for novel and safer therapeutic alternatives to manage trichomoniasis.
Natural products represent a prolific source of structurally diverse metabolites capable of modulating multiple cellular processes in protozoa, including redox homeostasis, membrane integrity, and mitochondrial/hydrogenosomal metabolism. In this context, medicinal plants offer key advantages such as well-established safety profiles, potent pharmacological effects, cost-effectiveness, and extensive traditional use in humans and animals. Consequently, the World Health Organization (WHO) has endorsed the integration of herbal medicines within traditional healthcare systems [13].
In recent years, multiple studies have revealed the potential of natural products as promising alternative treatments for trichomoniasis, especially those derived from ethnomedicinal plants [14]. Furthermore, several anti-T. vaginalis compounds have also been isolated from microorganisms and other natural sources [15].
Bioactive metabolites with reported trichomonacidal activity include ajoene and allicin from Allium sativum, berberine from Berberis aristata, resveratrol and catechins from grapes and Camellia sinensis, as well as cinnamaldehyde, carvacrol, and thymol from Cinnamomum zeylanicum [16,17,18,19,20,21,22]. In addition, fungal metabolites such as phomasetin and pyrrolocin A—particularly tetramate derivatives—have also shown promising in vitro antiparasitic effects [23]. Vaginal Lactobacillus species also inhibit T. vaginalis through the production of lactic acid, hydrogen peroxide, and bacteriocin-like peptides [24].
Despite extensive research into natural antiparasitic agents, their translation into clinically approved drugs remains limited. Since 1981, only about twenty natural products or derivatives have been approved by the U.S. Food and Drug Administration (FDA) [25]. Therefore, the continued exploration of natural sources for antiparasitic drug discovery remains a relevant and promising strategy.
The genus Tagetes (Asteraceae) comprises a diverse group of herbaceous plants commonly known as “cempasúchil,” “clavel de muerto,” or “marigold.” Native to the Americas, Mexico is recognized as the center of origin and diversity for these aromatic and visually striking species. Several Tagetes species are cultivated worldwide and used in traditional medicine for their wide range of therapeutic properties including applications in skin care, anti-inflammatory, analgesic, antispasmodic, antipyretic, dental healing, antidiarrheal, anti-abortifacient, and vaginal antiseptic treatments [26,27,28,29,30]. In particular, our research group has previously reported anti-Trichomonas tenax activity in Tagetes nelsonii, a protozoan associated with periodontal disease [31].
Phytochemical analyses of Tagetes species have revealed a rich diversity of metabolites with pharmacological potential, notably monoterpenes, phenylpropanoids, carotenoids, flavonoids, and thiophenes [32]. The biological activities of these constituents have been extensively documented, including antimicrobial effects against bacteria, fungi, and protozoa [33,34,35], as well as insecticidal [36,37,38], nematicidal [39,40], antioxidant, antineoplastic [41], and cytotoxic properties [42,43].
Ethnomedicinal knowledge has proven valuable tool for guiding the systematic screening of plants with antiprotozoal potential. Building upon previous evidence of biological activity in Tagetes species, this study explored the in vitro and in silico anti-Trichomonas vaginalis activity of betulin and stigmasterol isolated from Tagetes nelsonii, a medicinal plant traditionally used in southeastern Mexico.
2. Results
2.1. Bioguided Isolation of Compounds from Tagetes nelsonii
The methanolic extract of Tagetes nelsonii was partitioned using dichloromethane (CH2Cl2), yielding a non-polar fraction that exhibited anti-T. vaginalis activity, suggesting that the active compounds were of low polarity. Subsequent fractionation of the hexanic extract by Sephadex column chromatography yielded fraction 2 with the best anti-trichomonal activity. Further purification of this active fraction by silica gel column chromatography led to the isolation of two known compounds, betulin (1) and stigmasterol (2), obtained as white powders, Figure 1.
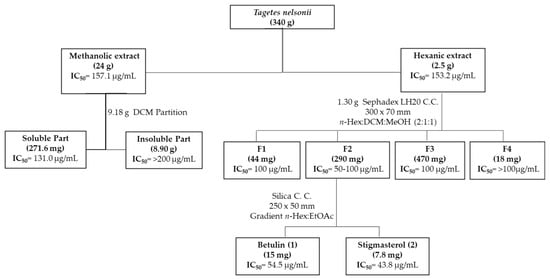
Figure 1.
General scheme of the bioguided isolation of compounds with anti-T. vaginalis activity from Tagetes nelsonii.
2.2. Structural Elucidation of Isolated Compounds from Tagetes nelsonii
1D and 2D NMR spectra were recorded for the isolated compounds using deuterated chloroform (Table 1). The 1H-NMR spectra of compound 1 showed the presence of six methyl singlets at δH 1.03 (CH3, C-23), 0.94 (CH3, C-24), 0.83 (CH3, C-25), 1.01 (CH3, C-26), 0.97 (CH3, C-27) and 1.65 (CH3, C-30); the proton signal at δH 2.37 (CH, C-19, td, J = 5.9, 11.1, 11.1); one oxymethine proton at C-3 as a broad double doublet (δH 3.18, J = 4.7, 11.6 Hz) and proton doublet at δH 4.69 (CH2, C-29, J = 2.5 Hz) and double doublet 4.57 (CH2, C29, J = 1.5, 2.7 Hz) suggested a lupine-type triterpene. The 13C-NMR indicated the presence of an isopropenyl group with an olefinic quaternary carbon at δC 150.9 (C-20) and methylene carbon at δC 109.2 (C-29); and a characteristic signal at δC 78.9 corresponded to a hydroxyl-bearing carbon (C-3).

Table 1.
1H- and 13C- NMR spectral data of terpenes isolated from Tagetes nelsonii.
Regarding compound 2, the 1H-NMR spectra exhibited six methyl groups at δH 0.69 (CH3, C-18), 1.01 (CH3, C-19), 1.02 (CH3, C-21), 0.85 (CH3, C-26), 0.84 (CH3, C-27) and 0.80 (CH3, C-29), a double doublets signal for a methine proton (CH, C-6) was observed at δH 5.36 (J = 2.3, 5.1 Hz); two signals corresponding to olefinic protons (CH, C-22, C-23) of a double bond were observed at δH 5.15 (dd, J = 8.7, 15.2 Hz) and δH 5.02 (dd, J = 8.7, 15.2 Hz); and a multiplet signal at δH 3.52 was assigned to an oxymethine proton (CH, C-3). The 13C-NMR showed a characteristic signal at δC 71.7 corresponded to a carbon attached to hydroxyl group (C-3); two signals at δC 129.2 and 138.2 were assigned to the olefinic carbons C-23 and C-22, respectively. Signals attributable to sp2 carbons of the endocyclic double bond were observed in the downfield region at δC 121.6 (C-6) and 140.7 (C-5).
1H- and 13C-chemicals shifts of compounds 1 and 2 isolated from Tagetes nelsonii (Table 1) were in accordance with those reported for betulin (C30H50O2) [44,45] and stigmasterol (C29H48O) [46,47], respectively (Figure 2).
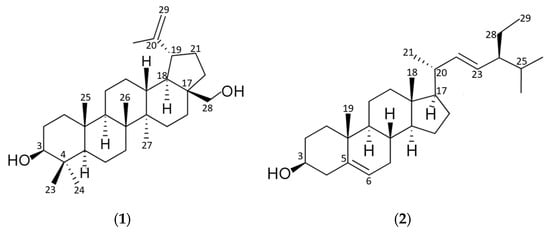
Figure 2.
Molecular structure of triterpenoids isolated from Tagetes nelsonii: (1) betulin; (2) stigmasterol.
2.3. Anti-Trichomonas Vaginalis and Hemolytic Activity
According to previously established criteria for evaluating antiprotozoal activity, extracts and compounds with IC50 < 5 µg/mL are considered highly active, those with IC50 = 5–15 μg/mL exhibit promising activity, IC50 = 15–50 μg/mL indicate moderate activity, and IC50 > 50 μg/mL correspond to low or weak activity [48,49].
As shown in Table 2, methanolic and hexanic extracts from Tagetes nelsonii exhibited low anti-Trichomonas vaginalis activity, with no significate differences (Z = −0.535, p > 0.05). In contrast, the isolated compounds from hexanic extract showed moderate antiprotozoal activity (equal significance, Z = −1.604, p > 0.05), with stigmasterol exhibiting the strongest trichomonacidal activity (IC50 = 43.8 ± 4.3 μg/mL).

Table 2.
Anti- T. vaginalis and hemolytic activities of extracts and isolated compounds from Tagetes nelsonii.
Regarding hemolytic activity, neither the extracts nor the isolated compounds induced erythrocyte lysis at the evaluated concentration.
2.4. In Silico Anti-Trichomonas Vaginalis Activity
A molecular docking study was carried out for betulin and stigmasterol to explore their potential mechanism of action based on predicted interactions with lactate dehydrogenase (LDH), pyruvate ferredoxin oxidoreductase (PFOR) methionine gamma-lyase (MGL) and purine nucleoside phosphorylase (PNP), all considered potential drug targets.
TvLDH and TvPFOR catalyze the oxidation of lactate and pyruvate, respectively, contributing to energy conservation through the generation of NADH molecules. Both enzymes play key roles in the carbohydrate metabolism of T. vaginalis. Notably, TvLDH exhibits low sequence similarity to human LDH, making it an attractive selective target [10,50]. The TvMGL enzyme catalyzes the catabolism of sulfur-containing amino acids and is essential for regulating their intracellular levels. Methionine γ-lyase (MGL) has been characterized in several bacterial species and in the parasitic protozoan Entamoeba histolytica, but it is absent in mammals [51]. In contrast, TvPNP catalyzes the interconversion between purine bases and purine nucleosides, functioning within the purine salvage pathway that is crucial for the survival of obligate parasitic protozoa [52].
Computational docking analysis revealed that both triterpenes interact favorably with TvPFOR, TvLDH, and TvPNP, displaying binding energies lower than −6.58 kcal/mol (Table 3) [53].

Table 3.
Predicted binding energies and molecular interactions between triterpenoids from T. nelsonii and T. vaginalis protein targets.
Docking analyses revealed that triterpenes, especially stigmasterol, interact favorably with TvLDH and TvpFOR, showing binding energies of −7.83 and −8.07 kcal/mol, respectively. These interactions are mainly hydrophobic in nature [54,55,56]. Betulin, however, may additionally form four conventional hydrogen bonds with key residues in the TvpFOR active domain (Figure 3b,d and Figure 4b,d).
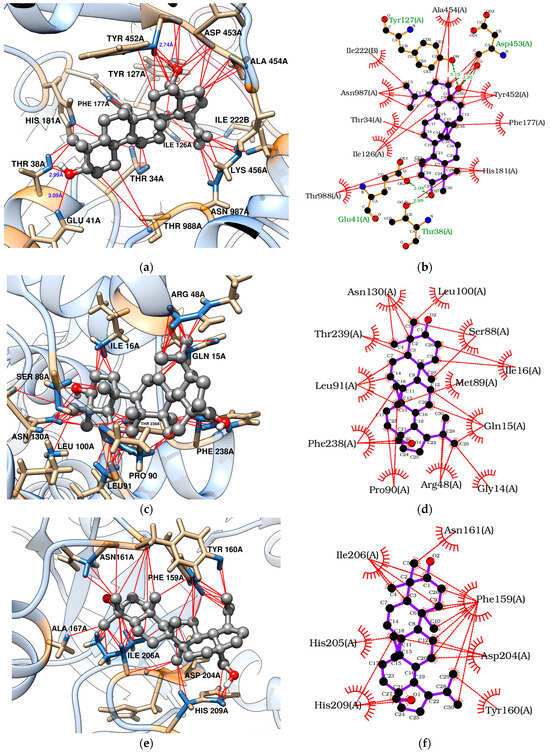
Figure 3.
Molecular docking of betulin with T. vaginalis proteins: (a,b) TvpFOR; (c,d) TvLDH; (e,f) TvPNP.
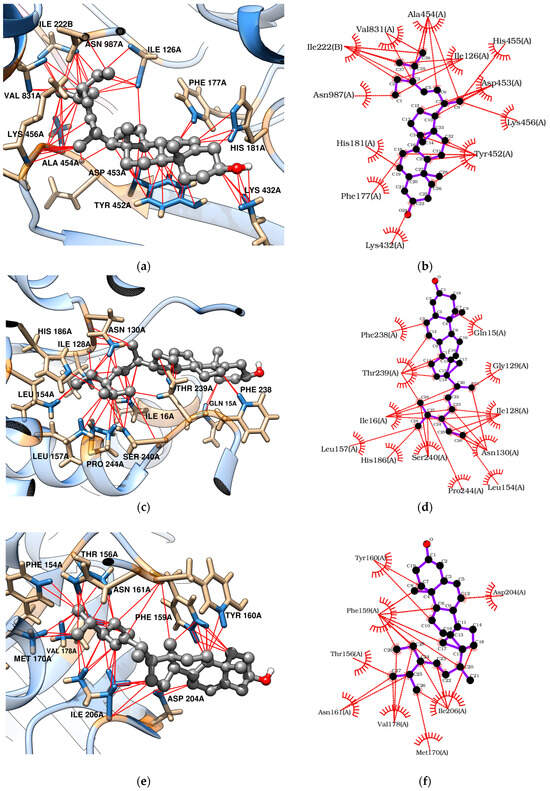
Figure 4.
Molecular docking of stigmasterol with T. vaginalis proteins: (a,b) TvpFOR; (c,d) TvLDH; (e,f) TvPNP.
In silico results also indicated that betulin and stigmasterol interact with Phe159, Val178, and Ile206 key residues located within the active site of the TvPNP enzyme (Figure 3f and Figure 4f) [52]. However, non-phytochemical ligands exhibited more exothermic docking scores, with values exceeding −7.19 kcal/mol. In contrast, TvMGL does not appear to represent a promising molecular target, as none of the triterpenes evaluated in this study displayed binding poses within its active site.
3. Discussion
To contribute to the understanding and documentation of Mexican ethnopharmacognosy and the development of new antiprotozoal agents, the present study evaluated the anti-Trichomonas vaginalis activity of Tagetes nelsonii. In this context, Tagetes species have been traditionally used to treat parasitic infections: decoctions of aerial parts of T. lucida, T. filifolia and, T. minuta are employed against gastrointestinal diseases caused by helminths and protozoa [57,58,59]. Moreover, T. minuta infusions are traditionally used as vaginal washes for infected discharge [26]. Research on therapeutic activities of T. nelsonii is limited [60], although antimicrobial, antifungal, cytotoxic and wound healing activities have been reported [61,62,63].
To our knowledge, this study represents the first report of antiprotozoal activity for Tagetes nelsonii crude extracts. The extracts exhibited weak activity against T. vaginalis (IC50 = 153.2–157.1 μg/mL). Previous studies have demonstrated antiprotozoal activity in other Tagetes species: the ethyl acetate fraction exhibited moderate to low antiplasmodial efficacy (IC50 = 20.0 and 70.0 μg/mL against chloroquine-sensitive and resistant strains of Plasmodium falciparum, respectively) [64]. Methanol extract of T. minuta showed promising activity against Trypanosoma brucei and Trypanosoma cruzi trypomastigotes (IC50 = 2.2 ± 1.5 and 9.2 ± 1.9 μg/mL, respectively) and moderate activity against Plasmodium falciparum (IC50 = 14.0 ± 2.8 μg/mL) and Leishmania infantum amastigotes (IC50 = 30.1 ± 4.6 μg/mL) [65]. Petroleum ether, hidrolated ethyl acetate and dichloromethane extracts of T. mendocina completely lysed Leishmania amazonensis and L. brasiliensis promastigotes at 100 µg/mL [66]. In the presented in this study, bioassay-guided fractionation od T. nelsonii stem bark led to the isolation of two triterpenes with moderate anti-trichomonacidal activity.
Triterpenes are naturally occurring 30-carbon compounds formed by isoprene units, present as carboxylic acids, alcohols, or aldehydes. The main natural triterpenoids are cyclic derivatives [67]. Polycyclic triterpenes have been reported to exhibit pharmacological properties such as anticancer, anti-inflammatory, antioxidant, antimicrobial biofilm, antiviral, anti-hypertensive, anti-atherosclerotic, anti-insulin resistance hepatoprotective, and immunomodulatory activity [68,69,70,71,72,73,74,75,76]. To date, at least 258 steroids and triterpenes from natural and synthetic sources have demonstrated antiprotozoal or anthelmintic activity [77]. In this study, betulin and stigmasterol exhibited moderate anti-T. vaginalis activity, with IC50 values of 54.5 and 43.8 μg/mL, respectively.
Betulin is a pentacyclic triterpene alcohol of the lupane series, characterized by a five- membered ring and an α-isopropenyl group at C-19 [44]. In contrast, stigmasterol is a tetracyclic unsaturated sterol (stigmasta 5,22-diene-3β-ol) with an ethyl group at C-24 of the side chain [78,79]. Both, stigmasterol and lupane-type triterpenes, have been found in Tagetes species [42,80,81]. Previous reports indicate antiprotozoal activity for botulin, with IC50 < 26.6 µg/mL againststandard and resistant strains of Trypanosoma brucei and Trypanosoma congolense, 35% inhibition at 22 µg/mL against Leishmania donovani axenic amastigotes, and IC50 = 58.8 µg/mL against Leishmania infantum promastigotes [82,83,84]. Stigmasterol has shown weak anti-trypanosomal activity (IC50 > 100 µg/mL) and promising activity (IC50 < 15 µg/mL) against T. brucei and L. donovani in a β-sitosterol mixture [46,49,85]. The lipophilicity of triterpenes may facilitate interactions with cell membranes and intracellular targets, inducing morphological changes on mitochondrial membrane, decreased membrane potential, which could explain the lack of hemolytic activity observed in this work [83].
To explore the putative mechanism of action of triterpenes, molecular docking was performed with enzymes required for T. vaginalis survival [10,50,51,52]. Both betulin and stigmasterol bound strongly to the TvpFOR substrate/cofactor pocket, predominantly via hydrophobic interactions. Stigmasterol interacts mainly with residues Ile126, Phe177, His181, Tyr452, Ala454, Val831, and Asn987 of chain A, whereas betulin additionally formed hydrophobic contacts with Thr34 and Arg117 and hydrogen bonds with Thr38, Glu41, Tyr127, and Asp453 (Figure 3b and Figure 4b). Structural characterization of a homologous TPP enzyme–pyruvate complex [55] shows electrostatic interactions of the pyruvate carboxylate with the guanidinium group of Arg114, hydrogen bonding of its oxygen atoms with Thr31, Asn996, and the TPP cofactor, and hydrophobic contacts between the substrate’s methyl group and the side chains of Ile123. The equivalent residues in TvpFOR (Thr34, Asn987 and Ile126) are involved in betulin interactions, suggesting that this triterpene interacts within the conserved catalytic pocket.
The hydrogenosome harbors an electron transport protein complex functionally linked to the TvPFOR-Ferredoxin system [10,86]. Previous studies have shown that compounds like metronidazole typically interact with Ferredoxin protein residues near the [2Fe–2S] cluster, such as Thr37, Leu31, and Lys46 to achieve proper alignment for electron transfer [86]. In contrast, stigmasterol in this study appears to adopt a different binding orientation, this alternative mode suggests that this triterpene may modulate enzyme activity through steric or allosteric effects rather than mimicking the electron-accepting behavior of nitro groups.
Regarding TvLDH, the aliphatic chain of stigmasterol appears to serve as the main contact region within the enzyme active site [87]. Hydrophobic interactions were observed between Ile16 and carbons C-20 to C-24, as well as between Ile128 and carbons C-23 to C-26. Additional contacts were identified involving Pro244 with C-27, the amino group of Asn130 with C-21, C-27 and C-28, and the imidazole group of His186 and the oxygen of hydroxyl group of Ser240 residue with C-25 to C-27, respectively (Figure 4c,d). Considering the central roles of PFOR and LDH in energy metabolism, these docking profiles support the hypothesis that disruption of these pathways contributes to the reduced T. vaginalis viability, especially by stigmasterol.
Key interactions were also observed between the triterpenes and the hydrophobic purine-binding site of TvPNP enzyme. Betulin engaged the A, B, and C rings, as well as the C-23/C-24 and C-30 positions, established hydrophobic interactions primarily with Phe159, while Ile206 interacted with the A ring and the C-23/C-24 carbons. In contrast, stigmasterol showed hydrophobic contacts between its B ring and C-19 with Phe159, whereas the aliphatic side chain interacted with Ile206 residues within the active pocket (Figure 4e,d). In TvPNP, the purine base is stabilized within the active site by four hydrophobic residues Phe159, Val178, Met180, and Ile206. The binding profiles coincide with those described in previous docking studies of TvPNP [87]. Among them, Phe159 and Val178 establish hydrophobic interactions with the purine ring, while the side chains of Ile206 and Met180 contribute additional van der Waals contacts near the 6-amino group of the base [52]. These findings suggest that triterpenes may modulate the purine salvage pathway, which is essential for maintaining purine nucleotide pools required for parasite survival.
Finally, the in silico interactions of the triterpenes with TvPNP and TvLDH were more favorable than those observed with metronidazole [87]. In addition, previous studies with natural oleanane-type inhibitors of T. vaginalis enzymes TvLDH and TvPNP showed binding scores higher than those observed in this study. However, aurones and lignans have demonstrated more promising docking affinities with TvPNP [88,89].
4. Materials and Methods
4.1. General Experimental Procedures
NMR spectra were obtained on Bruker AVANCE 500/600 MHz spectrometers (Bruker, Billerica, MA, USA) using standard pulse sequences, with CDCl3 as solvent and TMS as internal standard. TLC analyses were visualized under UV light or with cobalt chloride reagent after heating.
4.2. Plant Material
Mature stems of Tagetes nelsonii were collected in winter 2021 from Zinacantán, Chiapas, Mexico, identified botanically, and a voucher specimen (025883) was filed at the FCB Herbarium, Universidad Autónoma de Nuevo León. The plant material was cleaned, air-dried, and cut into small pieces.
4.3. Extraction and Isolation
Extracts were prepared for the evaluation of anti-Trichomonas vaginalis activity and hemolytic activities, 340 g of mature stems of T. nelsonii were sequentially macerated with n-hexane and methanol for three weeks at 25 °C. The solvent was replaced weekly (3 × 800 mL). Before filtration, each macerate was subjected to sonication in a low-power ultrasonic bath (Ney Ultrasonik (Cincinnati, OH, USA), 60 Hz, 15 min). The n-hexane and methanolic extracts were then concentrated under reduced pressure using a rotary evaporator.
Based on extraction yield and bioactivity, the methanolic extract (9.18 g) was partitioned with dichloromethane (CH2Cl2), yielding an active soluble fraction (271.6 mg) and an inactive insoluble fraction (8.90 g). In parallel, 1.30 g of the n-hexane extract was chromatographed on a Sephadex LH-20 column (Sigma, 30 × 7 cm) using n-hexane:CH2Cl2:methanol (2:1:1, v/v/v) eluent, affording four fractions. The most active fraction, with the highest yields (F2) was further separated on a normal-phase silica gel column (25 × 5 cm) with an ethyl acetate/n-hexane gradient, affording two pure compounds: compound 1 (15 mg) and compound 2 (7.8 mg). The remaining fractions were discarded either due to their low activity or low yield.
4.4. In Vitro Anti-Trichomonas Vaginalis Assays
T. vaginalis GT15 (CIBIN-IMSS) trophozoites were cultured axenically in TYI-S-33 medium with 10% bovine serum and used in the exponential growth phase. For in vitro antiprotozoal assays, 1 × 105 trophozoites were incubated for 24 h at 37 °C with varying concentrations of extracts or fractions (17.5–300 μg/mL) dissolved in DMSO.
Pure compounds were tested at 50, 35, 25, 15, and 10 μg/mL, dissolved in absolute ethanol. Metronidazole (analytical grade, Sigma-Aldrich Corp., St. Louis, MO, USA) was included as positive control, while the blank consisted of culture medium containing trophozoites only. After incubation, trophozoites were detached by chilling, and a 1:10 dilution was prepared in formalin. Trophozoite density was measured using a hemocytometer, and IC50 values were calculated by probit analysis. Two independent experiments were performed in triplicate.
4.5. Hemolytic Activity of Extracts, Partitions and Compounds
Hemolytic activity was evaluated by monitoring the lysis of a 5% (v/v) erythrocyte suspension prepared in PBS solution (0.01 M, pH 7.4). Human erythrocytes were collected from healthy donors using EDTA, washed, and incubated with extracts, fractions, or pure compounds (100–500 μg/mL) for 30 min at 37 °C. Hemolysis was assessed spectrophotometrically at 540 nm, with distilled water as a positive control. All procedures were conducted under an approved ethical protocol by the Department of Chemistry, FCB/UANL (protocol No. ATL-06-2022, May 2022), with informed consent and strict confidentiality.
4.6. Target Proteins
In the absence of an experimentally determined T. vaginalis pyruvate:ferredoxin oxidoreductase (TvPFOR) crystal structure, a 3D model of the enzyme was generated using the AlphaFold3 server [90], based on the UniProt entry Q27088 (TvpFORA) [91]. The resulting model was subjected to structural optimization and validation using the MolProbity server [92]. The model showed a MolProbity score of 1.37 (98th percentile), a clashscore of 6.72 (88th percentile), with 98.35% of residues in favored regions and only 0.17% in disallowed regions of the Ramachandran plot. Additional validation metrics included 0.58% poor rotamers, no Cβ deviations, and 0.05% bad angles, indicating excellent stereochemical and geometric quality suitable for subsequent structural and functional analyses. The predicted protein model was further confirmed using the SWISS-MODEL v.2024-10.3 server [93]. For comparative docking analyses, the crystallographic structures of T. vaginalis lactate dehydrogenase (TvLDH, PDB ID: 5A1T), methionine gamma lyase (TvMGL, PDB ID: 1E5E), and purine nucleoside phosphorylase (TvPNP, PDB ID: 1Z36) were retrieved from the Protein Data Bank [94].
4.7. Molecular Docking
Molecular docking studies were conducted using the CB-DOCK2 server [95] and UCSF Chimera v.1.18 software [96]. The phytochemical ligands were retrieved from the ZINC20 database [97] (ZINC3978650, ZINC4096712) and the PubChem database [98] (CID 5280794, CID 72326). Two-dimensional ligand structures were converted into three-dimensional formats and geometrically optimized using Avogadro2 v.1.99.0 software [99]. Ligand preparation included the merging of non-polar hydrogen atoms and the assignment of Gasteiger partial charges using AutoDock Tools v.1.5.7p1 [100].
Target protein structures were cleaned by removing ligands, ions, and water before docking. The docking grid was defined based on the coordinates of the co-complexed ligands or active site residues. Docking conformations were ranked according to their binding energy scores and the best docking poses were chosen based on the lowest binding energy and an RMSD cutoff of 2.0 Å. Protein–ligand interactions were visualized and analyzed using LigPlot+ v.2.3.1 software [101].
To validate the molecular docking protocol, redocking assays were conducted using co-crystallized ligands from three Trichomonas vaginalis enzymes: oxamate (OXM) with lactate dehydrogenase (TvLDH, PDB: 5AT1), formycin (FMC) with purine nucleoside phosphorylase (TvPNP, PDB: 1Z36), and the PLP–PPG adduct (PPJ) with methionine γ-lyase (TvMGL, PDB: 1E5E). The predicted binding affinities were −5.09, −7.69, and −8.7 kcal/mol, with RMSD values of 1.20, 1.29–1.33, and 1.13 Å, respectively, compared to their crystallographic poses. For pyruvate:ferredoxin oxidoreductase (TvPFOR), whose structure was modeled via AlphaFold3, docking was validated using the thiamine pyrophosphate (TPP) cofactor from a homologous PFOR (PDB: 1B0P), yielding a binding affinity of −9 kcal/mol while preserving key catalytic interactions.
4.8. Statical Analysis
Anti-T. vaginalis activity was expressed as mean ± SD from two independent assays. To measure anti-T.vaginalis activity, the concentration of extract at which the parasite growth was inhibited by 50% (IC50) was calculated by Probit regression, and statistical differences among extracts, partitions, and compounds were analyzed using the paired Wilcoxon test with a significance level of p < 0.05.
5. Conclusions
This study reports, for the first time, the antiprotozoal potential of Tagetes nelsonii and its metabolites. Two triterpenes, betulin and stigmasterol, were identified with promising in vitro and in silico activity against T. vaginalis, with negligible hemolytic activity at 100 μg/mL. Altogether, these findings expand the structural framework of potential TvpFOR, TvLDH, and TvPNP inhibitors, and support the further optimization of triterpenoid compounds for the development of novel anti-trichomonal agents.
Author Contributions
Bioassays, M.A.H.-T.; conceptualization and supervision, E.V.-V.; antiparasit methodology and supervision, M.E.H.-G.; isolation compounds and supervision, S.G.-D.; Chemical structure assignation and supervision, A.R.D.-M. and J.J.F.; in silico assay, I.E.C.-T., Hemolytic assay and project administration, E.V.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Government of the Canary Islands ProID202401003 (BIMICMAR) and PROMOTUR Turismo de Canarias (Actuaciones de Cohesión de Destino—Canarias Ecoínsulas II), and specifically PROMOTUR_SP3: “Islas Canarias, naturaleza marina singular: Salud y bienestar”, co-funded by the European Union NextGenerationEU/PRTR.
Institutional Review Board Statement
This study complied with the principles of the Declaration of Helsinki and received approval from the Institutional Review Board of the Department of Chemistry, FCB/UANL (protocol ATL-06-2022, approved 1 May 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Author M.A.H.-T. acknowledges CONACyT (Mexico) for the doctoral scholarship No. 809104. (CVU 706423), MEXICO; to CIBIN-IMSS and IUBO-ULL for the attention and infrastructure provided for the proper development of this research. S.G.-D. acknowledges funds of María Zambrano Programme (Programme of Requalification of the Spanish University System from Spanish Ministry for Universities, ULL, Next-Generation EU Funds).
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; World Health Organization: Geneva, Switzerland, 2021; pp. 8–10. Available online: https://www.who.int/publications/i/item/9789240027077 (accessed on 17 October 2025).
- Santos Diéguez, I. Tricomoniasis: Una visión amplia. Iatreia 2014, 27, 198–205. [Google Scholar] [CrossRef]
- Cancelo Hidalgo, M.J.; Cancelo Hidalgo, C.; Chavida García, F. Vaginitis por Trichomonas. Med. De Fam. Semer. 2005, 31, 121–124. [Google Scholar] [CrossRef]
- Poole, D.N.; McClelland, R.S. Global epidemiology of Trichomonas vaginalis. Sex. Transm. Infect. 2013, 89, 418–422. [Google Scholar] [CrossRef]
- Silver, B.J.; Guy, R.J.; Kaldor, J.M.; Jamil, M.S.; Rumbold, A.R. Trichomonas vaginalis as a Cause of Perinatal Morbidity: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2014, 41, 369–376. [Google Scholar] [CrossRef]
- McClelland, R.S.; Sangare, L.; Hassan, W.M.; Lavreys, L.; Mandaliya, K.; Kiarie, J.; Ndinya-Achola, J.; Jaoko, W.; Baeten, J.M. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 2007, 195, 698–702. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, W.; Wang, H.; Wang, Y.; Li, J.; Wu, X. Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Barrientes, F.J. Prevalence of Trichomonas vaginalis isolates with resistance to metronidazole and tinidazole. Antimicrob. Agents Chemother. 2006, 50, 4209–4210. [Google Scholar] [CrossRef] [PubMed]
- López Nigro, M.M.; Carballo, M.A. Los nitroimidazoles como modelo de mutagénesis química y muerte celular. Theoria 2008, 17, 47–62. Available online: https://www.redalyc.org/articulo.oa?id=29911533005 (accessed on 17 October 2025).
- Upcroft, P.; Upcroft, J.A. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clini Microbiol. Rev. 2001, 14, 150–164. [Google Scholar] [CrossRef]
- Seña, A.C.; Bachmann, L.H.; Hobbs, M.M. Persistent and recurrent Trichomonas vaginalis infections: Epidemiology, treatment and management considerations. Expert. Rev. Anti Infect. Ther. 2018, 12, 673–685. [Google Scholar] [CrossRef]
- Roy, U.; Panwar, A.; Pandit, A.; Das, S.K.; Joshi, B. Clinical and Neuroradiological Spectrum of Metronidazole Induced Encephalopathy: Our Experience and the Review of Literature. J. Clin. Diagn. Res. 2016, 10, OE01–OE9. [Google Scholar] [CrossRef]
- Shang, X.; Dai, L.; Cao, X.; Ma, Y.; Gulnaz, I.; Miao, X.; Li, X.; Yang, X. Natural products in antiparasitic drug discovery: Advances, opportunities and challenges. Nat. Prod. Rep. 2025, 42, 1419–1458. [Google Scholar] [CrossRef]
- Hashemi, N.; Ommi, D.; Kheyri, P.; Khamesipour, F.; Setzer, W.N.; Benchimol, M. A review study on the anti-trichomonas activities of medicinal plants. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 92–104. [Google Scholar] [CrossRef]
- Friedman, M.; Tam, C.C.; Cheng, L.W.; Land, K.M. Anti-trichomonad activities of different compounds from foods, marine products, and medicinal plants: A review. BMC Complement. Med. Ther. 2020, 20, 271. [Google Scholar] [CrossRef] [PubMed]
- Millet, C.O.; Lloyd, D.; Williams, C.; Williams, D.; Evans, G.; Saunders, R.A.; Cable, J. Effect of garlic and allium-derived products on the growth and metabolism of Spironucleus vortens. Exp. Parasitol. 2011, 127, 490–499. [Google Scholar] [CrossRef]
- Ibrahim, A.N. Comparison of in vitro activity of metronidazole and garlic-based product (Tomex®) on Trichomonas vaginalis. Parasitol. Res. 2013, 112, 2063–2067. [Google Scholar] [CrossRef]
- Soffar, S.A.; Metwali, D.M.; Abdel-Aziz, S.S.; el-Wakil, H.S.; Saad, G.A. Evaluation of the effect of a plant alkaloid (berberine derived from Berberis aristata) on Trichomonas vaginalis in vitro. J. Egypt. Soc. Parasitol. 2001, 31, 893–904 + 1p plate. [Google Scholar]
- Elizondo-Luevano, J.H.; Verde-Star, J.; González-Horta, A.; Castro-Ríos, R.; Hernández-García, M.E.; Chávez-Montes, A. In Vitro Effect of Methanolic Extract of Argemone mexicana against Trichomonas vaginalis. Korean J. Parasitol. 2020, 58, 135–145. [Google Scholar] [CrossRef]
- Mallo, N.; Lamas, J.; Leiro, J.M. Hydrogenosome metabolism is the key target for antiparasitic activity of resveratrol against Trichomonas vaginalis. Antimicrob. Agents Chemother. 2013, 57, 2476–2484. [Google Scholar] [CrossRef]
- Noritake, S.M.; Liu, J.; Kanetake, S.; Levin, C.E.; Tam, C.; Cheng, L.W.; Land, K.M.; Friedman, M. Phytochemical-rich foods inhibit the growth of pathogenic trichomonads. BMC Complement. Altern. Med. 2017, 17, 461. [Google Scholar] [CrossRef]
- Özel, Y.; Çavuş, İ.; Ünlü, G.; Ünlü, M.; Özbilgin, A. Investigation of the Antitrichomonal Activity of Cinnamaldehyde, Carvacrol and Thymol and Synergy with Metronidazole. Turkiye Parazitol. Derg. 2024, 48, 72–76. [Google Scholar] [CrossRef]
- Peramuna, T.; Wood, G.E.; Hu, Z.; Wendt, K.L.; Aguila, L.K.T.; Kim, C.M.; Duerfeldt, A.S.; Cichewicz, R.H. Semisynthetic Tetramate-Containing Fungal Metabolites with Activity against Trichomonas vaginalis and Mycoplasma genitalium. ACS Med. Chem. Lett. 2024, 15, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Pradines, B.; Domenichini, S.; Lievin-Le Moal, V. Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment. Pharmaceuticals 2022, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Alonso, J.; Desmarchelier, C. Plantas Medicinales Autóctonas de la Argentina. Bases Científicas para su Aplicación en Atención Primaria de la Salud, 1st ed.; Lola: Buenos Aires, Argentina, 2005; pp. 249–252. Available online: https://www.researchgate.net/publication/299371108_Plantas_Medicinales_Autoctonas_de_la_Argentina_-_Bases_Cientificas_para_su_Aplicacion_en_Atencion_Primaria_de_la_Salud (accessed on 17 October 2025).
- Serrato, C.M.A. El recurso genético cempoa lxóchitl (Tagetes spp.) de México (Diagnóstico), 1st ed.; Universidad Autónoma Chapingo: Texcoco, Mexico, 2014; p. 49. Available online: https://www.gob.mx/cms/uploads/attachment/file/225091/El_recurso_gen_tico_del_cempoalxochitl__tagetes_spp__de_mexico__diagnostico_.pdf (accessed on 17 October 2025).
- Rahman, I.U.; Ijaz, F.; Iqbal, Z.; Afzal, A.; Ali, N.; Afzal, M.; Khan, M.A.; Muhammad, S.; Qadir, G.; Asif, M. A novel survey of the ethno medicinal knowledge of dental problems in Manoor Valley (Northern Himalaya), Pakistan. J. Ethnopharmacol. 2016, 194, 877–894. [Google Scholar] [CrossRef]
- Riaz, M.; Ahmad, R.; Rahman, N.U.; Khan, Z.; Dou, D.; Sechel, G.; Manea, R. Traditional uses, Phyto-chemistry and pharmacological activities of Tagetes Patula L. J. Ethnopharmacol. 2020, 255, 112718. [Google Scholar] [CrossRef]
- Bohatu, S.I.; Prystupa, B.V.; Rozhkovskyi, Y.V. Phytochemistry analysis and medicinal use of plants of the genus Tagetes. Farmatsevtychnyi Zhurnal 2024, 2, 73–91. [Google Scholar] [CrossRef]
- Hernández Torres, M.A.; Hernández García, M.E.; Carranza Torres, I.E.; Viveros Valdez, J.E. Actividad antiprotozoaria de plantas de la etnomedicina mexicana frente a Trichomonas tenax, protozoario asociado a enfermedad periodontal. Espacio I+D Innovación Desarro. 2025, 14, 42. Available online: https://www.espacioimasd.unach.mx/index.php/Inicio/article/view/478 (accessed on 17 October 2025).
- Marotti, M.; Piccaglia, R.; Biavati, B.; Marotti, I. Characterization and yield evaluation of essential oils from different Tagetes species. J. Essent. Oil Res. 2004, 16, 440–444. [Google Scholar] [CrossRef]
- Igwaran, A.; Iweriebor, B.C.; Ofuzim Okoh, S.; Uchechukwu Nwodo, U.; Chikwelu Obi, L.; Ifeanyi Okoh, A. Chemical constituents, antibacterial and antioxidant properties of the essential oil flower of Tagetes minuta grown in Cala community Eastern Cape, South Africa. BMC Complement. Altern. Med. 2017, 17, 351. [Google Scholar] [CrossRef]
- Monzote, L.; Gutiérrez, Y.; Machin, L.; Staniek, K.; Scull, R.; Satyal, P.; Gille, L.; Setzer, W.N. Antileishmanial Activity and Influence on Mitochondria of the Essential Oil from Tagetes lucida Cav. and Its Main Component. Sci. Pharm. 2020, 88, 31. [Google Scholar] [CrossRef]
- Rodríguez-Juárez, M.I.; Arjona-Suárez, E.; Cadena-Íñiguez, J.; Guevara-Olivar, B.K.; Ruiz-Posadas, L.d.M. Antifungal potential and chemical composition of Tagetes lunulata Ort. essential oil for the control of Trichophyton rubrum Malmsten. Agro Productividad 2023, 16, 119–126. [Google Scholar] [CrossRef]
- Mendoza-García, E.E.; Ortega-Arenas, L.D.; Serrato-Cruz, M.A.; Díaz-Cedillo, F.; Villanueva-Jiménez, J.A.; López-Arroyo, J.I.; Pérez-Pacheco, R. Efecto biológico del aceite de Tagetes coronopifolia (Asteraceae) contra Diaphorina citri (Hemiptera: Liviidae). R. Rev. Colomb. Entomol. 2015, 41, 157–162. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-04882015000200001&lng=en&tlng=es (accessed on 17 October 2025).
- López, S.B.; López, M.L.; Aragón, L.M.; Tereschuk, M.L.; Slanis, A.C.; Feresin, G.E.; Zygadlo, J.; Tapia, A.A. Composition and anti-insect activity of essential oils from Tagetes L. species (Asteraceae, Helenieae) on Ceratitis capitata Wiedemann and Triatoma infestans Klug. J. Agric. Food Chem. 2011, 59, 5286–5292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Liu, Q.Y.; Li, D.W.; Zhang, Z.M.; You, C.X. Antagonistic storage potential of Tagetes minuta, Eupatorium fortunei and Ocimum basilicum oils with volatile secondary metabolites against Tribolium castaneum and Lasioderma serricorne. Ind. Crop. Prod. 2022, 187, 115502. [Google Scholar] [CrossRef]
- Álvarez, S.; Botina, D.E.; Ortiz, J.A.; Jarminton, A.; Botina, L.L. Evaluación nematicida del aceite esencial de Tagetes zypaquirensis en el manejo del nematodo Meloidogyne spp. Rev. Cienc. Agric. 2016, 33, 22–33. [Google Scholar] [CrossRef]
- Zarate-Escobedo, J.; Castañeda-González, E.L.; Cuevas-Sánchez, J.A.; Carrillo-Fonseca, C.L.; Mendoza-García, E.E.; Serrato-Cruz, M.A. Concentraciones e intervalos de aplicación del aceite esencial de Tagetes lucida Cav. contra Nacobbus aberrans. Rev. Mex. Cienc. Agrícolas 2018, 9, 589–600. [Google Scholar] [CrossRef]
- Kashif, M.; Bano, S.; Naqvi, S.; Faizi, S.; Lubna; Mesaik, M. A.; Shamsuddin Azeemi, K.; Farooq, A.D. Cytotoxic and antioxidant properties of phenolic compounds from Tagetes patula flower. Pharm. Biol. 2014, 53, 672–681. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Thiotagetin A, a new cytotoxic thiophene from Tagetes minuta. Nat. Prod. Res. 2016, 31, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.A. Tagetones A and B, new cytotoxic monocyclic diterpenoids from flowers of Tagetes minuta. Chin. J. Nat. Med. 2017, 15, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Takibayeva, A.T.; Zhumabayeva, G.K.; Bakibaev, A.A.; Demets, O.V.; Lyapunova, M.V.; Mamaeva, E.A.; Yerkassov, R.S.; Kassenov, R.Z.; Ibrayev, M.K. Methods of Analysis and Identification of Betulin and Its Derivatives. Molecules 2023, 28, 5946. [Google Scholar] [CrossRef]
- Tijjani, A.; Ndukwe, I.; Ayo, R. Isolation and Characterization of Lup-20(29)-ene-3, 28- diol (Betulin) from the Stem-Bark of Adenium obesum (Apocynaceae). Trop. J. Pharm. Res. 2012, 11, 259–262. [Google Scholar] [CrossRef]
- Kamal, N.; Clements, C.; Gray, A.I.; Edrada-Ebel, R. Anti-infective Activities of Secondary Metabolites from Vitex pinnata. J. Appl. Pharm. Sci. 2016, 6, 102–106. [Google Scholar] [CrossRef]
- Erwin, U.; Pusparohmana, W.R.; Safitry, R.D.; Marliana, E.; Usman, E.; Kusuma, I.W. Isolation and characterization of stigmasterol and β-sitosterol from wood bark extract of Baccaurea macrocarpa Miq. Mull. Arg. Rasayan J. Chem. 2020, 13, 2552–2558. [Google Scholar] [CrossRef]
- Calzada, F.; Yépez-Mulia, L.; Tapia-Contreras, A. Effect of Mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalis trophozoites. J. Ethnopharmacol. 2007, 113, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Amang à Ngnoung, G.A.; Nganso Ditchou, Y.O.; Leutcha, P.B.; Dize, D.; Tatsimo, S.J.N.; Tchokouaha, L.R.Y.; Kowa, T.K.; Tembeni, B.; Mamoudou, H.; Poka, M.; et al. Antiplasmodial and Antileishmanial Activities of a New Limonoid and Other Constituents from the Stem Bark of Khaya senegalensis. Molecules 2023, 28, 7227. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fiser, A.; ter Kuile, B.; Sali, A.; Müller, M. Convergent evolution of Trichomonas vaginalis lactate dehydrogenase from malate dehydrogenase. Proc. Natl. Acad. Sci. USA 1999, 96, 6285–6290. [Google Scholar] [CrossRef]
- Sato, D.; Nozaki, T. Methionine gamma-lyase: The unique reaction mechanism, physiological roles, and therapeutic applications against infectious diseases and cancers. IUBMB Life 2009, 61, 1019–1028. [Google Scholar] [CrossRef]
- Rinaldo-Matthis, A.; Wing, C.; Ghanem, M.; Deng, H.; Wu, P.; Gupta, A.; Tyler, P.C.; Evans, G.B.; Furneaux, R.H.; Almo, S.C.; et al. Inhibition and structure of Trichomonas vaginalis purine nucleoside phosphorylase with picomolar transition state analogues. Biochemistry 2007, 46, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zothantluanga, J.; Chetia, D. A Beginner’s Guide to Molecular Docking. Sci. Phytochem. 2022, 1, 90–93. [Google Scholar] [CrossRef]
- Steindel, P.A.; Chen, E.H.; Wirth, J.D.; Theobald, D.L. Gradual neofunctionalization in the convergent evolution of trichomonad lactate and malate dehydrogenases. Protein Sci. 2016, 25, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Chabrière, E.; Charon, M.H.; Volbeda, A.; Pieulle, L.; Hatchikian, E.C.; Fontecilla-Camps, J.C. Crystal structures of the key anaerobic enzyme pyruvate:ferredoxin oxidoreductase, free and in complex with pyruvate. Nat. Struct. Biol. 1999, 6, 182–190. [Google Scholar] [CrossRef]
- Katsyv, A.; Schoelmerich, M.C.; Basen, M.; Müller, V. The pyruvate:ferredoxin oxidoreductase of the thermophilic acetogen, Thermoanaerobacter kivui. FEBS Open Bio 2021, 11, 1332–1342. [Google Scholar] [CrossRef]
- Leonti, M.; Vibrans, H.; Sticher, O.; Heinrich, M. Ethnopharmacology of the Popoluca, Mexico: An evaluation. J. Pharm. Pharmacol. 2001, 53, 1653–1669. [Google Scholar] [CrossRef]
- Moreno-Salazar, S.F.; Robles-Zepeda, R.E.; Johnson, D.E. Plant folk medicines for gastrointestinal disorders among the main tribes of Sonora, Mexico. Fitoterapia 2008, 79, 132–141. [Google Scholar] [CrossRef]
- Ijaz, F.; Iqbal, Z.; Rahman, I.U.; Alam, J.; Khan, S.M.; Shah, G.M.; Khan, K.; Afzal, A. Investigation of traditional medicinal floral knowledge of Sarban Hills, Abbottabad, KP, Pakistan. J. Ethnopharmacol. 2016, 179, 208–233. [Google Scholar] [CrossRef]
- Serrato-Cruz, M.A. Extracción de Aceite Esencial de la ‘Chik Chawua’ (Tagetes nelsonii Greenm.), Endémica de los Altos de Chiapas, para Obtención de Bioplaguicidas y de Medicamento y Como Parte de una Estrategia para la Conservación in situ en la Región: Propuesta, 1st ed.; Universidad Autónoma Chapingo: Texcoco, Mexico, 2014; p. 3. Available online: https://www.gob.mx/cms/uploads/attachment/file/225090/Extraccion_de_aceite_esencial_de_la_chik_chawua.pdf (accessed on 17 October 2025).
- Espinoza, R.M.; Palomeque, R.M.A.; Salazar, S.I.; Domínguez, A.S.; Canseco, A.L.M. Análisis preliminar de la actividad antimicrobiana de la planta medicinal Chik chawa (Tagetes nelsonii Greenm.). Rev. Cuba. Plantas Med. 2009, 14, 4. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962009000400007&lng=es&tlng=es (accessed on 17 October 2025).
- De La Cruz-Jiménez, L.; Hernández-Torres, M.A.; Monroy-García, I.N.; Rivas-Morales, C.; Verde-Star, M.J.; González-Villasana, V.; Viveros-Valdez, E. Biological Activities of Seven Medicinal Plants Used in Chiapas, Mexico. Plants 2022, 11, 1790. [Google Scholar] [CrossRef]
- Olán-Jiménez, K.A.; Cruz-Rodríguez, R.I.; Couder-García, B.d.C.; Jacobo-Herrera, N.; Ruiz-Lau, N.; Hernández-Cruz, M.d.C.; Ruíz-Valdiviezo, V.M. Antibacterial and Wound Healing Activity In Vitro of Individual and Combined Extracts of Tagetes nelsonii Greenm, Agave americana and Aloe vera. Sci. Pharm. 2024, 92, 41. [Google Scholar] [CrossRef]
- Gupta, P.; Vasudeva, N. In vitro antiplasmodial and antimicrobial potential of Tagetes erecta roots. Pharm. Biol. 2010, 48, 1218–1223. [Google Scholar] [CrossRef]
- Al-Musayeib, N.M.; Mothana, R.A.; Matheeussen, A.; Cos, P.; Maes, L. In vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complement. Altern. Med. 2012, 12, 49. [Google Scholar] [CrossRef]
- Lima, B.; Agüero, M.B.; Zygadlo, J.A.; Tapia, A.; Solís, C.; Arias, A.R.; Yaluff, G.; Zacchino, S.; Feresin, G.E.; Schmeda-Hirschmann, G. Antimicrobial activity of extracts, essential oil and metabolites obtained from Tagetes mendocina. J. Chil. Chem. Soc. 2009, 54, 68–72. [Google Scholar] [CrossRef]
- Noushahi, H.A.; Khan, A.H.; Noushahi, U.F.; Hussain, M.; Javed, T.; Zafar, M.; Batool, M.; Ahmed, U.; Liu, K.; Harrison, M.T.; et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022, 97, 439–454. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. FBL 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Liaw, C.C.; Chen, Y.C.; Huang, G.J.; Tsai, Y.C.; Chien, S.C.; Wu, J.H.; Wang, S.Y.; Chao, L.K.; Sung, P.J.; Huang, H.C.; et al. Anti-inflammatory lanostanoids and lactone derivatives from Antrodia camphorata. J. Nat. Prod. 2013, 76, 489–494. [Google Scholar] [CrossRef]
- De Silva, M.d.L.; David, J.P.; Silva, L.C.; Santos, R.A.; David, J.M.; Lima, L.S.; Reis, P.S.; Fontana, R. Bioactive oleanane, lupane and ursane triterpene acid derivatives. Molecules 2012, 17, 12197–12205. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Propiedades antiplanctónicas y antibiofilm de los triterpenos pentacíclicos: Ácido asiático y ácido ursólico como prometedores fármacos antibacterianos para el futuro. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Giniyatullina, G.V.; Yamansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorganic Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Monserrat-Mesquida, M.; Pinya, S.; Ferriol, P.; Tejada, S. Hypotensive Effects of the Triterpene Oleanolic Acid for Cardiovascular Prevention. Curr. Mol. Pharmacol. 2021, 14, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305. [Google Scholar] [CrossRef]
- Gao, J.; Tang, X.; Dou, H.; Fan, Y.; Zhao, X.; Xu, Q. Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. J. Pharm. Pharmacol. 2004, 56, 1449–1455. [Google Scholar] [CrossRef]
- Renda, G.; Gökkaya, İ.; Şöhretoğlu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef]
- Kuzminac, I.; Savić, M.P.; Ajduković, J.J.; Nikolić, A.R. Steroid and Triterpenoid Compounds with Antiparasitic Properties. Curr. Top. Med. Chem. 2023, 23, 791–815. [Google Scholar] [CrossRef]
- Struijs, K.; Lampi, A.M.; Ollilainen, V.; Piironen, V. Dimer formation during the thermo-oxidation of stigmasterol. Eur. Food Res. Technol. 2010, 231, 853–863. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.; Kadam, A.; Padole, A.S. HPTLC method development and validation of stigmasterol from different extracts of Tagetes erecta and Capsicum annuum. J. Drug Deliv. Ther. 2019, 9, 169–172. [Google Scholar] [CrossRef]
- Xu, L.W.; Wang, G.Y.; Shi, Y.P. Chemical constituents from Tagetes erecta flowers. Chem. Nat. Compd. 2011, 47, 281–283. [Google Scholar] [CrossRef]
- Alakurtti, S.; Bergström, P.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Yli-Kauhaluoma, J. Anti-leishmanial activity of betulin derivatives. J. Antibiot. 2010, 63, 123–126. [Google Scholar] [CrossRef]
- De Jesus, J.A.; Laurenti, M.D.; Antonangelo, L.; Faria, C.S.; Lago, J.H.G.; Passero, L.F.D. Related Pentacyclic Triterpenes Have Immunomodulatory Activity in Chronic Experimental Visceral Leishmaniasis. J. Immunol. Res. 2021, 2021, 6671287. [Google Scholar] [CrossRef]
- Alenezi, S.S.; Alenezi, N.D.; Ebiloma, G.U.; Natto, M.J.; Ungogo, M.A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Fearnley, J.; de Koning, H.P.; et al. The Antiprotozoal Activity of Papua New Guinea Propolis and Its Triterpenes. Molecules 2022, 27, 1622. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Bianco, A.; Serafini, M.; Pitorri, M.; Sciubba, F.; Di Cocco, M.E.; Spinozzi, E.; Cappellacci, L.; Hofer, A.; et al. Phytochemical Analysis and Trypanocidal Activity of Marrubium incanum Desr. Molecules 2020, 25, 3140. [Google Scholar] [CrossRef]
- Rocha-Garduño, G.; Hernández-Martínez, N.A.; Colín-Lozano, B.; Estrada-Soto, S.; Hernández-Núñez, E.; Prieto-Martinez, F.D.; Medina-Franco, J.L.; Chale-Dzul, J.B.; Moo-Puc, R.; Navarrete-Vázquez, G. Metronidazole and Secnidazole Carbamates: Synthesis, Antiprotozoal Activity, and Molecular Dynamics Studies. Molecules 2020, 25, 793. [Google Scholar] [CrossRef]
- Alves, M.S.D.; das Neves, R.N.; Sena-Lopes, Â.; Dimingues, M.; Casaril, A.M.; Vieira, N.; Mendoca, T.; de Souza, N.; Savegnano, L.; Kömmling, F.; et al. Antiparasitic activity of furanyl N-acylhydrazone derivatives against Trichomonas vaginalis: In vitro and in silico analyses. Parasites Vectors 2020, 13, 59. [Google Scholar] [CrossRef]
- Setzer, M.S.; Byler, K.G.; Ogungbe, I.V.; Setzer, W.N. Natural Products as New Treatment Options for Trichomoniasis: A Molecular Docking Investigation. Sci. Pharm. 2017, 85, 5. [Google Scholar] [CrossRef]
- Mata-Cárdenas, B.D.; Vargas-Villarreal, J.; González-Salazar, F.; Palacios-Corona, R.; Salvador, S.-F. A new vial microassay to screen antiprotozoal drugs. Pharmacologyonline 2008, 1, 529–537. [Google Scholar]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. A Publ. Protein Soc. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Berman, H.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.; Bourne, P. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Liu, Y.; Grimm, M.; Dai, W.T.; Hou, M.C.; Xiao, Z.X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein-ligand blind docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Irwin, J.J.; Tang, K.G.; Young, J.; Dandarchuluun, C.; Wong, B.R.; Khurelbaatar, M.; Moroz, Y.S.; Mayfield, J.; Sayle, R.A. ZINC20—A Free Ultralarge-Scale Chemical Database for Ligand Discovery. J. Chem. Inf. Model. 2020, 60, 6065–6073. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res 2025, 53, D1516–D1525. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).