Abstract
Nutritional epigenomics is exceptionally important because it describes the complex interactions among food compounds and epigenome modifications. Phytonutrients or bioactive compounds, which are secondary metabolites of plants, can protect against osteoarthritis by suppressing the expression of inflammatory and catabolic mediators, modulating epigenetic changes in DNA methylation, and the histone or chromatin remodelling of key inflammatory genes and noncoding RNAs. The combination of natural epigenetic modulators is crucial because of their additive and synergistic effects, safety and therapeutic efficacy, and lower adverse effects than conventional pharmacology in the treatment of osteoarthritis. In this review, we have summarized the chondroprotective properties of bioactive compounds used for the management, treatment, or prevention of osteoarthritis in both human and animal studies. However, further research is needed into bioactive compounds used as epigenetic modulators in osteoarthritis, in order to determine their potential value for future clinical applications in osteoarthritic patients as well as their relation with the genomic and nutritional environment, in order to personalize food and nutrition together with disease prevention.
Keywords:
nutriepigenomics; osteoarthritis; chondrocyte; cartilage; bioactive compounds; epigenetics 1. Osteoarthritis, a Chronic Disease
Osteoarthritis (OA) is one of the most common disabling chronic progressive diseases in middle-aged and elderly people [1,2], and it is among the main public health problems worldwide, due to its high prevalence [3]. The main characteristics of OA are articular cartilage deterioration, subchondral bone remodelling, the formation of osteophytes, joint space reduction, and synovitis [4]. Symptoms generally include severe joint pain, stiffness, joint contractures, muscle atrophy, reduced movement, swelling, tenderness, and variable degrees of local inflammation, limb deformity and crepitus [5]. There are many etiological factors for OA, including genetic predisposition, dietary intake, obesity, sex, aging, traumatic joint injury, mechanical stress, metabolic disease, and sedentary lifestyle [6]. It is important to highlight the synergistic effects of pathologies such as cardiovascular disease and obesity coexisting with OA [7,8].
Pharmacological treatments such as paracetamol, nonsteroidal anti-inflammatory drugs, tramadol, and opioids are used to reduce pain and inflammation, but do not prevent, reverse or cure OA [9]. However, a long-term use of these drugs to relieve OA is associated with substantial gastrointestinal, renal, hepatic, blood, cardiovascular, and cerebrovascular adverse effects [10,11,12]. In this review, we present the importance of a healthy diet in preventing the development or progression of OA, and summarize chondroprotective properties and beneficial epigenetic modifications of bioactive compounds or nutraceuticals against inflammation and catabolic activity in OA.
2. Epigenetics and Osteoarthritis
Over the last 20 years, the study of epigenetics has expanded (especially in the cancer field). However, studies on the importance of epigenetic mechanisms in OA are only now increasing. Roach and collaborators provided the first evidence of how epigenetic changes, such as DNA methylation, may relate to the pathogenesis of OA and can be potentially reversible [13].
Epigenetics can be defined as heritable changes in gene expression and/or phenotype that can occur without changes in the primary DNA sequence [14]. The epigenome of each cell is unique and can undergo temporal changes in response to environmental factors such as diet, physical activity, smoking, pollutants and disease status [15]. OA is distinguished by the unfavorable dynamic regulation of gene transcription in joint tissues due to environmental disturbances; therefore, epigenetics has developed as a new and important area for OA research [16,17,18]. Candidate gene and epigenome-wide studies have demonstrated their association with OA development and progression through epigenetic modifications, and these epigenetic mechanisms can change in response to stimuli and, in some cases, pass on to future generations [19,20,21]. Given the importance of gene expression or silencing, and associated epigenetic modifications, we will briefly mention various epigenetic mechanisms of pro-inflammatory cytokines and metalloproteinases (MMPs) that contribute to cartilage destruction. Three main mechanisms are implicated in epigenetic regulation: (1) DNA methylation changes that covalently alter chromatin structure. In general, DNA hypomethylation enhances gene transcription, and DNA hypermethylation suppresses gene transcription. (2) The post-translational modification of histones that alters chromatin conformation, including the methylation of arginine and lysine, the acetylation of lysines, the phosphorylation of serine and treonine, and the sumoylation and ubiquitination of lysine. (3) Non-coding RNAs regulate gene expression but do not translate into proteins (i.e., microRNAs (miRNAs), long non-coding RNAs) acting at both transcriptional and post-transcriptional levels [22,23,24].
2.1. DNA Methylation
The DNA methylation process is mediated by DNA methyltransferases (DNMTs), including DNMT1 (maintenance), DNMT3A and DNMT3B (de novo), and involves the addition of a methyl group to the 5′ position of cytosine, which most commonly occurs in CpG dinucleotides, forming 5-methylcytosine. The hypermethylation by DNMTs leads to transcriptional gene silencing and gene inactivation [22,23].
Nakano and collaborators found that DNMT1 and DNMT3A expressions were decreased by IL-1β, while DNMT3A also decreased its expression and activity, caused by the TNF-α in fibroblast-like synoviocytes [25]. Both DNA methylation and histone modification are involved in the control of TNF-α expression [26]. Hashimoto and collaborators found that the methylation of the −115 CpG site enhances MMP13 promoter activity as opposed to the inhibitory effect of −110 CpG methylation; also, the demethylation of the specific CpG sites at the −299 position of the IL1B promoter activity correlates with enhanced IL1B gene expression in human primary chondrocytes [27,28]. Furthermore, Bui and collaborators showed that the −104 CpG site is demethylated in OA cartilage, and this is accompanied by elevated MMP13 expression [29]. In articular cartilage, the methylation of cytosines at positions −1680 and −1674 blocks COL10A1 expression in chondrocytes, while gene expression is activated during chondrogenesis in cells, with the partial methylation of these two specific CpG sites [30]. Cheung and collaborators found that DNA demethylation at one or more specific CpG sites in the ADAMTS4 promoter corresponds to the increased expression of ADAMTS4 in human OA chondrocytes, which plays a role in aggrecan degradation in OA [31]. In addition, Roach and collaborators showed an association between the loss of DNA methylation of CpG sites in the promoters and the abnormal expression of MMP3, MMP9, MMP13, and ADAMTS4 by OA chondrocytes [13]. Besides this, the sclerotin (SOST) mRNA and protein expression levels are increased in OA chondrocytes, suggesting the SOST promoter is hypermethylated in normal chondrocytes and hypomethylated in OA [32]. An interesting study suggests that hip OA is associated with reduced SOX9 gene and protein expression, having showed that that the methylation of the SOX9 promoter was increased in OA cartilage [33]. Imagawa and collaborators reported that COL9A1 promoter activity is significantly decreased by DNA hypermethylation, and could be reversed through the inhibition of DNA methylation. In addition, the abnormal DNA methylation of the CpG sites in the COL9A1 promoter is associated with the decreased expression of SOX9 [34]. Moreover, hypomethylation in the IL8 promoter is correlated with higher IL8 gene expression in OA chondrocytes; a significant increase in IL8 promoter activity by the transcription factors NF-κB, AP-1 and C/EBP was also shown [35]. de Andrés and collaborators demonstrated the association between an increase in inducible nitric oxide synthase (NOS2) gene expression in OA chondrocytes and the demethylation of NF-κB responsive enhancer elements [36]. Furthermore, in OA, synovial fibroblasts showed DNA hypomethylation and histone hyperacetylation in the IL6 promoter [37].
2.2. Histone Modifications
Methylation/demethylation and acetylation/deacetylation are the main and recurrent histone changes in OA [38]. Two families of enzymes catalyze the modification of histones: histone methyltransferases (HMTs) and histone demethylases (HDMTs), or acetyltransferases (HATs) and histone deacetylases (HDACs) [39]. The majority of these modifications take place in lysine, arginine and serine residues within the histone tails, and regulate key cellular processes such as transcription, replication and repair [40]. The hyperacetylation of histone tails induces transcriptional activation, while hypoacetylation is associated with transcriptional repression [41]. HDAC family members have been associated with OA, and HDAC inhibitors (HDACi) can protect chondrocytes and prevent cartilage damage, while possessing therapeutic potential against OA [15,42]. Young and collaborators demonstrated that HDACi decreased the expression and activity of MMPs and ADAMTSs [43]. In addition, histone deacetylase-1 (HDAC1) and HDAC2 levels are elevated in both chondrocytes and synovium from OA patients compared to controls [44,45]. Higashiyama and collaborators demonstrated the increased expression of HDAC7 in human OA cartilage, which was correlated with elevated MMP13 gene expression, contributing to cartilage degradation [46]. Class III HDACs (sirtuins) are a class of NAD+-dependent histone deacetylases that differ from the class I and II HDACs. Sirtuin 1 (SIRT-1) is a positive regulator of cartilage-specific gene expression in chondrocytes [47]. SIRT-1 activation has the potential to prevent cartilage damage and inhibit its destruction [48,49]. SIRT-1 suppresses protein tyrosine phosphatase 1B and activates the insulin-like growth factor (IGF) receptor pathway, enhancing the survival of chondrocytes [50]. Also, the decreased expression of COL2A1 mRNA and type II collagen protein correlates with decreased SIRT1 activity [51]. In addition, in OA cartilage, the overexpression of E74-like factor 3 (ELF3) inhibited Sox9/cAMP-response element-binding (CREB) protein (CBP)-driven HAT activity, and decreased COL2A1 [52]. The disruptor of telomeric silencing, the 1-like (DOT1L) gene (an HMT), is a protector of cartilage health, and as such is reduced in damaged areas of OA joints; the protective function of DOT1L is attributable to Wnt signalling inhibition [53,54].
2.3. Non-Coding RNA (ncRNAs)
ncRNAs, including small non-coding RNAs (miRNA) and long non-coding RNAs (lncRNAs), have the ability to regulate gene expression at both transcriptional (lncRNAs) and post-transcriptional levels (small and lncRNAs) [55]. lncRNAs are key regulators of gene expression; thus, the overexpression of lncRNA-CIR increases the expression of MMPs, whereas collagen and aggrecan expression are reduced in OA cartilage [56]. Small ncRNA mainly includes miRNAs, siRNAs and piRNAs. miRNAs have historically been the most frequently investigated; they are considered an alternative mechanism of post-transcriptional or translational regulation. At the post-transcriptional level, they bind to complementary mRNA, leading to the degradation of mRNA or the prevention of its translation into a protein [55,57,58,59]. Several miRNAs have shown altered expressions in OA, and are involved in various aspects of cartilage homeostasis and OA pathogenesis [60]. Rasheed and collaborators showed that IL-1β-induced iNOS gene expression is correlated with the down-regulation of miR-26a-5p in human OA chondrocytes [61]. Furthermore, miRNAs such as miR-320, miR-381, miR-9, miR-602, miR-608, miR-127-5p, miR-140, miR-27b, miR-98 and miR-146 play a significant role in the regulation of genes relevant to OA pathogenesis [59]. In another study, the overexpression of miR-27b inhibited IL-1β-stimulated MMP13 gene and protein expression in human OA chondrocytes [62]. Moreover, the overexpression of miR-558 directly inhibited COX2 mRNA and protein expression [63]. Also, miR-199a levels are inversely correlated with COX2 mRNA and protein levels in IL-1β-stimulated human chondrocytes [64]. There is a relationship between HDACs and miRNA in OA; thus, the overexpression of miR-92a-3p suppressed HDAC2 production and increased the level of histone H3 acetylation of the COMP/ACAN/COL2A1 promoter [65]. The overexpression of miR-193b-3p inhibited HDAC3 expression, enhanced histone H3 hyperacetylation, and increased the expressions of SOX9, COL2A1, ACAN, and COMP in chondrocytes [66]. Guan and collaborators showed that miR-146a protects against OA, inhibiting inflammatory factors [67]. In addition, a study demonstrated the significant increase in miR-146a expression that was induced by the HDAC inhibitors in OA-fibroblast-like synoviocytes [68]. Another study demonstrated that miR-146b is downregulated in the chondrogenic differentiation of human stem cells, and upregulated in OA [69]. The overexpression of miR193b-5p inhibited HDAC7 expression and decreased MMP3 and MMP13 expression [70]. Both miR-199a-3p and miR-193b expressions are upregulated with age, and may be involved in chondrocyte senescence by downregulating anabolic factors such as type 2 collagen, aggrecan, and SOX9; therefore, they may be involved in cartilage degeneration [71]. In addition, the increases in TNFA, IL1B and IL6 gene expression were correlated with miR-149 downregulation through the inhibition of post-transcriptional control in human OA chondrocytes [72]. miR-140, the most well-studied miRNA in OA, plays a protective role in OA development. It is important for chondrogenesis and osteogenesis, and is highly expressed in normal cartilage, but its expression levels are decreased in OA chondrocytes; its overexpression could inhibit inflammation and cartilage degradation [73,74,75,76,77]. A study showed that miR-140 is specifically expressed in cartilage tissues during mouse embryonic development, and that siRNA-140 significantly downregulated the accumulation of the Hdac4 protein in fibroblast cells [78]. Further, miR-140-3p and its isomiRs (miR-140-3p.1 and miR-140-3p.2) are abundantly expressed in cartilage [79]. Decreased miR-let7e expression has been suggested as a potential predictor of hip OA [57,80]. The increase in miR-145 levels directly represses SOX9 expression, resulting in the inhibition of COL2A1 and ACAN, with increased expressions of RUNX2 and MMP13 in human chondrocytes [81].
3. Inflammation and Diet
Inflammation is a complex biological response of the immune system to pathogens, damaged cells, injury, toxic compounds, and infection. The immune system utilizes a large number of specialized cells, such as lymphocyte, monocytes and macrophages, to restore homeostasis [82,83,84]. Inflammation is an important pathway in OA pathogenesis and development [85,86]. Inflammation in OA joints is chronic and low-grade, and involves the interplay of the innate immune system and inflammatory mediators [85,87,88]. These include cytokines, chemokines, growth factors, adipokines, prostaglandins, leukotrienes, nitric oxide, and neuropeptides [87,89]. Strikingly, reductions in this low-grade inflammation are closely linked with a greater adherence to healthier diets, such us the Mediterranean diet [90,91,92].
Diet plays an important role in the development or prevention of many chronic diseases [93,94], and may regulate chronic inflammation, improving quality of life [95,96,97]. Thus, dietary composition is able to modulate epigenetic markers such as changes in DNA methylation, the histone or chromatin remodelling of key inflammatory genes, and ncRNAs that may be causal for the development of chronic diseases or beneficial against inflammation; in this way, it can block, retard, or reverse pathologic processes [98,99,100,101,102].
A diet with high a dietary inflammatory index (DII) score has been associated with severe pain and lower quality of life in patients with knee OA [103,104]. Another study showed that the energy-adjusted DII (E-DII) score was associated with a high risk of knee OA in the osteoarthritis initiative (OAI) cohort [105]. The DII has been used to predict inflammatory biomarkers [103,106]. Biomarkers of inflammation, especially serum C-reactive protein (CRP), IL-6, TNF-α and MMPs, have been associated with pain and the progression of OA [107,108,109,110]. Dyer and collaborators showed that biomarkers of inflammation and cartilage degradation related to OA were lower with greater uptake of the Mediterranean diet [111]. In addition, several studies have found that a better quality of life is associated with a higher adherence to this diet [112,113,114,115]. Veronesse and collaborators, in a large cohort of North Americans from the OAI database, demonstrated that a greater adherence to the Mediterranean diet is associated with better quality of life, which is correlated with less pain, disability and depression, better cognitive performance, and better physical functioning [116]. The adherence to the Mediterranean diet was assessed in these studies according to the Mediterranean diet score by established Panagiotakos [117], based on a food frequency questionnaire [118]. Strikingly, greater adherence to the Mediterranean diet is associated with a lower prevalence of knee OA [119]. A high adherence to this diet increases the antioxidant levels in serum samples, with a reduction in oxidative stress biomarkers levels [120,121], such as F2-isoprostane, an indicator of oxidative stress in plasma [122]. Moreover, Martín-Núñez and collaborators found a correlation between lower adherence to the Mediterranean diet pattern and changes in DNA methylation levels and diseases [123].
4. Bioactive Compounds: Health-Protective Benefits
The complex biological activities of plants can promote their abundance in secondary metabolites or bioactive compounds, and they are also known as phytonutrients or nutraceuticals. These bioactive compounds are widely known for their unique medicinal properties; they possess antimicrobial [124], anti-inflammatory [125], antiviral [126,127], cardioprotective [128], neuroprotective [129], chemopreventive [130], phytohormone [131], and antioxidant properties [132]. Multiple pathological processes are involved in the pathogenesis of OA, such as inflammation, oxidative stress, apoptosis, autophagy and senescence; hence, phytochemical or bioactive compounds have been used as therapeutic and nutraceutical agents, showing their antiarthritic potential. They mainly exert anti-inflammatory effects through the blockade of pro-inflammatory cytokines (IL1-β, IL-6, IL-8, TNF-α), the inhibition of the NF-κB pathway, antiapoptotic effects, the prevention of oxidative damage to proteins and DNA (reduction in both reactive oxygen species (ROS) and reactive nitrogen species), suppression of the production of prostaglandins and leukotrienes, and reductions in levels of MMPs [133,134,135,136,137].
Bioactive phytochemicals feature a wide variety of compounds, and are classified into phenolics, alkaloids, organosulfur compounds, terpenes and terpenoids, among others, with each class divided into further classes (Figure 1). They are present in fruits, vegetables and spices, and can modify metabolic, cellular, molecular, and epigenetic processes [138]. Polyphenols represent the largest and most ubiquitous group of natural phytochemicals structures; these compounds are present in fruits, vegetables, cereals, tea, dark chocolate, cocoa powder, coffee, extra virgin oil, and wine [139,140,141]. The main groups of polyphenols are flavonoids, phenolic acids, and secoiridoids, among others. Flavonoids a lone comprise more than 10,000 natural compounds, including anthocyanidins, proanthocyanidins flavones, flavanones, flavonols, isoflavones and flavan-3-ols [142,143,144,145].
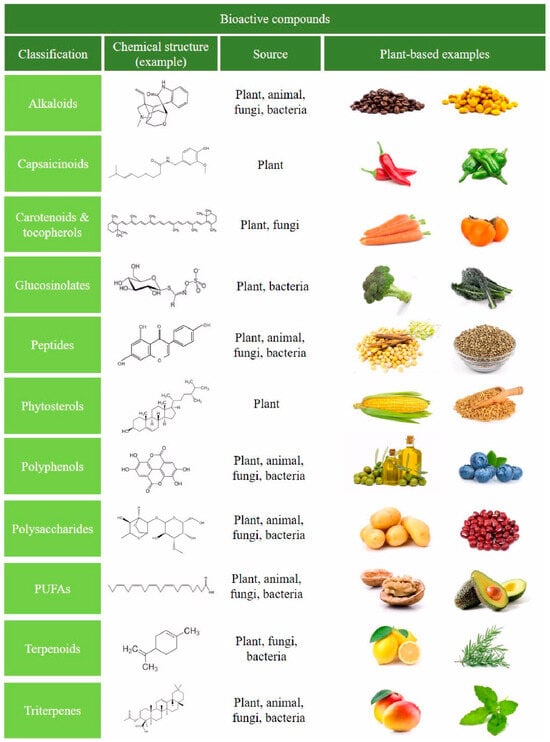
Figure 1.
Schematic representation of the classification of the main bioactive compounds in foods. Representative plant-based foods are shown, as well as sources and an illustrative chemical structure example.
In this review, a total of 85 bioactive compounds and nutraceuticals with potential anti-OA properties were analysed for use in the management, treatment, or prevention of OA in both humans (Table 1) and animals (Table 2).

Table 1.
Bioactive compounds and nutraceuticals used for the management, treatment, or prevention of OA in humans.

Table 2.
Bioactive compounds and nutraceuticals for the management, treatment, or prevention of OA in animals.
In OA, most studied bioactive compounds are curcuminoids [164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,274,275,276,277,278,279], epigallocatechin-3-O-gallate [187,188,189,190,191,192,193], hydroxytyrosol [208,209,210,296], icariin [298,299,300,301,302], oleuropein [223,224], resveratrol [228,229,230,231,232,233,234,321,322,323,324] and sulfuronate [238,239,240,241,242,243]. The most common effects founded in vitro are related to decreased inflammatory and cartilage degradation markers, like MMPs, NO, PGE2 or ROS. On the other hand, in vivo effects observed in OA-induced animal models are critically linked to the reduction in symptoms at the joint level (cartilage, synovium and subchondral bone). Finally, case studies were carried out in humans, showing alleviated pain and enhanced quality of life among other symptoms. Several case studies showed interesting results compared to the conventional analgesic therapy taken by OA patients, especially curcuminoids. It has been proven that they can be as efficacious as ibuprofen [168,169], show potential beneficial effects when used as an adjuvant therapy with diclofenac [170] and meloxican [229] and an alternative therapy for those intolerants to diclofenac’s side effects [171], reduce the use of NSAIDs and gastrointestinal complications [178], and lower adverse effects compared to diacerhein [205,206].
Regarding bioactive compounds’ applications, there are important considerations to take into account: (i) it will be crucial to increase their stability and bioavailability, especially for clinical applications; (ii) a deep understanding must be developed of the underlying molecular mechanisms to increase their bioactivity; and (iii) we must investigate their long-term toxicity and possible side effects.
5. Nutritional Epigenomics: Bioactive Compounds in Dietary Balance and Health
Nutritional epigenomics is exceptionally important because it holds great potential in the prevention, suppression and therapy of a wide variety of diseases by altering various epigenetic factors. This novel field involves the lifelong remodelling of our epigenomes, even during cellular differentiation in embryonic and foetal development, by nutritional factors; it also describes how the bioactive molecules can influence and modify gene expression at the transcriptional level [336,337,338,339]. For example, DNA methylation depends on the methyl group donors and cofactors found in foods, thus dietary excess or deficiencies in a critical and sensitive period like embryogenesis can alter the methylation process and gene expression, and therefore the metabolism and physiology of the individual, programming pathologic processes during a lifetime [340,341]. Jirtle and Skinner observed that hypermethylating dietary compounds could reduce the effects of environmental toxicants that cause DNA hypomethylation [342]. An interesting study on Apis mellifera, into the different honeybee phenotypes, demonstrated that silencing Dnmt3 gene expression decreased methylation in the dynactin p62 gene in larval heads, which led to an increase in the number of queens and a reduction in the number of workers; these epigenetic changes in DNA methylation depended on whether they were fed royal jelly or beebread [343].
Wolff and collaborators provided some of the first evidence that maternal nutrition can impact the epigenome and phenotype of the offspring of dams fed with folate-supplemented diets; this nutrition affected agouti gene expression in Avy/a mice and caused a wide variation in coat colour, ranging from yellow (unmethylated) to light brown (methylated). Pseudoagouti Avy/a brown mice were lean, healthy, and longer-lived than their yellow phenotype siblings (larger, obese, hyperinsulinemic, more susceptible to cancer) [344]. Furthermore, in macaques that were fed a high-fat diet during pregnancy (predisposing offspring to metabolic syndrome), foetal offspring had increased H3 acetylation and decreased Hdac1 gene expression in the liver compared to macaques fed with a low-fat diet [345]. An experimental study in Agouti Avy/a mice fed with genistein (a soy polyphenol), which acts during early embryonic development, showed that genistein-induced hypermethylation persisted into adulthood, by altering the epigenome, decreasing ectopic agouti expression, and protecting offspring from obesity, diabetes, and cancer across multiple generations [346]. In addition, experimental data have shown that the maternal consumption of dietary polyphenols such as resveratrol during preconception, gestation and lactation ameliorated metabolic programming. Resveratrol reduced renal oxidative stress, activated nutrient-sensing signals, modulated gut microbiota, and prevented associated high-fructose-intake-induced programmed hypertension in the rat offspring [347].
The four primary targets for epigenetic therapy are DNMTs, HDACs, HATs and miRNA; thereby, numerous bioactive compounds such as sulforaphane, tea polyphenols, ellagic acid, genistein, curcumin, hydroxytyrosol, resveratrol, organosulfur compound, oleanolic acid, and alkaloids have been studied as potent agents for regulating epigenetic mechanisms [102,339,348]. Bioactive compounds can influence epigenetic processes through different mechanisms that interfere with the 1-carbon metabolism and affect S-adenosyl methionine (SAM) levels, meaning they are able to modulate DNA and histone methylation [349]. Many polyphenols, such as quercetin, fisetin, and myricetin, inhibit DNMT by decreasing SAM and increasing S-adenosyl-L-homocysteine (SAH) and homocysteine levels [350].
Global DNA hypomethylation has been associated with the hypermethylation and inactivation of specific genes [351], thus the hypermethylation of cytidine by DNMTs usually results in transcriptional gene silencing and gene inactivation, including of tumour-suppressor genes, while promoters of transcriptionally active genes typically remain hypomethylated [352]. Genes such as O6-methylguanine methyltransferase, retinoic acid receptor β (RARB), the tumour-suppressor p16INK4a, and the DNA repair gene human mutL homologue 1 (hMLH1) were shown to be frequently inactivated by hypermethylation, and polyphenols such as epigallocatechin-3-gallate and genistein from soybean were demonstrated to be strong DNMT-inhibitors, leading to the demethylation and reactivation of methylation-silenced genes [353]. DNMTs do not act alone, and they also recruit HDACs to synergistically repress gene transcription [354].
The combination of bioactive compounds acting as DNMT inhibitors, together with phytochemicals that can alter histone modifications, and those that can influence miRNAs expression in OA, are all potentially more synergistic and significant approaches when used as therapeutical strategies to prevent and treat various diseases, including cancer [355,356]. In this context of nutriepigenomics, we have specifically analysed the epigenetic mechanisms related to 12 bioactive compounds, focusing on the prevention or treatment of OA in both humans (Table 3) and animals (Table 4).

Table 3.
Bioactive compounds as epigenetic modulators for the management, treatment, or prevention of OA in humans.

Table 4.
Bioactive compounds as epigenetic modulators for the management, treatment, or prevention of OA in animals.
Few (but insightful) studies have shown the epigenetic effects of bioactive compounds in OA. The majority of studies are focused on curcuminoids [377,378,379], epigallocatechin-3-O-gallate [361,362,363], hydroxytyrosol [365,366,367,368,381], oleanoic acid [369,370] and resveratrol [372,373,374,375,385,386,387]. By far, the most studied epigenetic mechanisms are miRNAs, which are generally linked to the regulation of inflammatory and cartilage degradation markers. Sirtuins are also well explored in the context of OA.
6. Conclusions
In this review, we analysed the importance of bioactive compounds as epigenetic modulators in the prevention and treatment of OA. The reduction in inflammation, as well as catabolic and oxidative activity, is essential in OA treatment. Bioactive compounds or nutraceuticals can directly protect and repair DNA damage, modulating signalling pathways and genes implicated in OA pathogenesis or modifying intra- and extracellular activities. Bioactive compounds are potentially capable of reversing the phenotype of OA chondrocytes. Moreover, the combination of bioactive compounds that act as DNMT inhibitors together with HDAC inhibitors, HAT inhibitors or activators, and miRNA regulators offer more synergistic potential approaches with significance in preventing and treating OA (Figure 2).
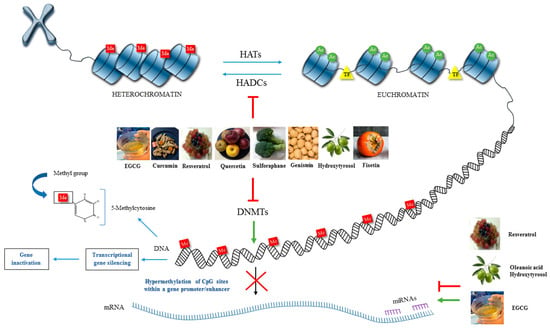
Figure 2.
Schematic representation of the impact of bioactive compounds on the main epigenetic mechanisms happening in OA. Several nutraceuticals have been considered as natural epigenetic modulators that can modify the activity of various epigenetic factors (DNA methylation, HATs, HDACs and miRNA) and, altering the expression of genes related to inflammation and cartilage destruction, being potentially able to reverse the phenotype of OA chondrocytes.
Several mixtures have also demonstrated the additive and synergistic potential of bioactive compounds; these mixtures enhanced their chondroprotective properties via anti-inflammatory mechanisms, and reducing oxidative stress. Bioactive compounds are also effective in reducing pain and decreasing the need for NSAIDs, with fewer adverse effects that provide safety and therapeutic efficacy in OA treatment. In addition, new formulations of bioactive compounds have been developed for example with nanoparticles; these phytonutraceuticals possess higher absorption and bioavailability and, could serve as a therapeutic strategy in the prevention and treatment of OA. However, the potential of bioactive compounds as epigenetic regulators in OA has been little studied; further research is needed towards this promising area of research. For this reason, the proposal nutriepigenomic arises and focusses on the ability of numerous bioactive compounds as an alternative to prevent or treat OA.
Future perspectives of bioactive dietary compounds in OA are mainly preventive more than therapeutic. Mostly because the effects of these natural products probably are very small during short periods of time; however, they could be effective when consumed continuously as part of the diet. This indeed could be crucial for a disease like OA, where prevention before symptoms appear is key to stop the progression of the disease. Finally, it will be critical to identify biomarkers to test the efficacy of bioactive compounds at both inter-individual and population levels.
Author Contributions
Conceptualisation, K.M.V.-A. and M.C.d.A.; methodology, K.M.V.-A. and C.N.-C.; investigation, K.M.V.-A. and C.N.-C.; resources, F.J.B.; writing—original draft preparation, K.M.V.-A.; writing—review and editing, K.M.V.-A., C.N.-C., F.J.B. and M.C.d.A.; supervision, M.C.d.A.; project administration, M.C.d.A.; funding acquisition, M.C.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by Instituto de Salud Carlos III (ISCIII) through the projects “PI19/01213” and “RICORSREI-RD21/0002/0009”, and co-funded by the European Union; and grants IN607D2022/12 and IN607A2021/07 from Xunta de Galicia, Axencia Galega de Innovación GAIN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ACAN | aggrecan |
| ACTL | anterior cruciate ligament transection |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AKT | a serine/threonine protein kinase |
| ALP | alkaline phosphatase |
| AP-1 | activator protein 1 |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B cell lymphoma-2 |
| BMSC | bone marrow stromal cells |
| C/EBP | CCAAT/enhancer-binding protein |
| CASP | caspase |
| c-FOS | fos proto-oncogene |
| COL | collagen |
| COMP | cartilage oligomeric matrix protein |
| COX2 | clyclooxygenase 2 |
| CRP | C-reactive protein |
| DHA | docosahexaenoic acid |
| DMSO | dimethylsulphoxide |
| DNMT | DNA methyltransferase |
| DOT1L | disruptor of telomeric silencing 1-like |
| ECM | extracellular matrix |
| EPA | eicosapentaenoic acid |
| ER | endoplasmatic reticulum |
| ERK | extracellular signal-regulated kinase |
| FLS | fibroblast-like synoviocytes |
| FOXO | forkhead box O |
| GAG | glycosaminoglycan |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylases |
| HDMT | histone demethylase |
| HIF | hypoxia Inducible factor |
| hMSCs | human mesenchymal stem cells |
| HO-1 | heme oxygenase 1 |
| HSP90B | heat shock protein 90-beta |
| HT | hydroxytyrosol |
| HTM | histone methyltransferase |
| IKK | ikappaB kinase |
| IL | interleuquin |
| iNOS | inducible nitric oxyde synthase |
| JNK | jun N-terminal kinase |
| lncRNA | long non-coding RNA |
| LPS | lipopolysaccharides |
| MAPK | mitogen-activated protein kinases |
| MIA | monosodium iodoacetate |
| miRNA | microRNA |
| MMP | metalloproteinase |
| NF-ĸB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NRF2 | nuclear factor erythroid 2–related factor 2 |
| NSAID | non-steroidal anti-inflammatory drugs |
| OA | osteoarthritis |
| OAC | OA chondrocyte |
| OARSI | osteoarthritis research society international |
| OCN | osteocalcin |
| OPN | osteopontin |
| OSM | oncostatin M |
| PARP | poly-ADP ribose polymerase |
| PG | proteoglycan |
| PGE2 | prostaglandin E2 |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| PTEN | phosphatase and tensin homolog |
| PUFA | polyunsaturated fatty acids |
| RANKL | receptor activator of nuclear factor kappa beta ligand |
| RES | resveratrol |
| ROS | Reactive oxygen species |
| RUNX2 | receptor activator of nuclear factor kappa-Β ligand |
| SIRT | sirtuin |
| SOST | sclerostin |
| SOX9 | SRY-Box Transcription Factor 9 |
| SSD | saikosaponin D |
| STAT | signal transducer and activator of transcription |
| TGF-β1 | transforming growth factor beta-1 |
| TIMP | tissue inhibitor of metalloproteinase |
| TLR4 | toll-like receptor 4 |
| TNF-α | tumoral necrosis factor alpha |
| VAS | visual analog scale |
| VEGF | vascular endothelial growth factor |
| WOMAC | Western Ontario and McMaster Universities Arthritis Index |
References
- Buckwalter, J.A.; Martin, J.A. Osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 150–167. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.M. Impact of osteoarthritis on individuals and society: How much disability? Social consequences and health economic implications. Curr. Opin. Rheumatol. 2002, 14, 573–577. [Google Scholar] [CrossRef]
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef]
- Malemud, C.J. Biologic basis of osteoarthritis: State of the evidence. Curr. Opin. Rheumatol. 2015, 27, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Dieppe, P.A.; Lohmander, L.S. Pathogenesis and management of pain in osteoarthritis. Lancet 2005, 365, 965–973. [Google Scholar] [CrossRef]
- Mobasheri, A.; Fonseca, J.E.; Gualillo, O.; Henrotin, Y.; Largo, R.; Herrero-Beaumont, G.; Rocha, F.A.C. Editorial: Inflammation and Biomarkers in Osteoarthritis. Front. Med. 2021, 8, 727700. [Google Scholar] [CrossRef] [PubMed]
- Kanthawang, T.; Bodden, J.; Joseph, G.B.; Lane, N.E.; Nevitt, M.; McCulloch, C.E.; Link, T.M. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: Data from the osteoarthritis initiative. Skeletal Radiol. 2021, 50, 217–229. [Google Scholar] [CrossRef]
- Fernandes, G.S.; Valdes, A.M. Cardiovascular disease and osteoarthritis: Common pathways and patient outcomes. Eur. J. Clin. Investig. 2015, 45, 405–414. [Google Scholar] [CrossRef]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Expert. Rev. Clin. Pharmacol. 2011, 4, 605–621. [Google Scholar] [CrossRef]
- Cheng, D.S.; Visco, C.J. Pharmaceutical therapy for osteoarthritis. PM&R 2012, 4, S82–S88. [Google Scholar] [CrossRef]
- O’Neil, C.K.; Hanlon, J.T.; Marcum, Z.A. Adverse effects of analgesics commonly used by older adults with osteoarthritis: Focus on non-opioid and opioid analgesics. Am. J. Geriatr. Pharmacother. 2012, 10, 331–342. [Google Scholar] [CrossRef]
- Roach, H.I.; Yamada, N.; Cheung, K.S.; Tilley, S.; Clarke, N.M.; Oreffo, R.O.; Kokubun, S.; Bronner, F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005, 52, 3110–3124. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Khan, N.M.; Haqqi, T.M. Epigenetics in osteoarthritis: Potential of HDAC inhibitors as therapeutics. Pharmacol. Res. 2018, 128, 73–79. [Google Scholar] [CrossRef]
- Ramos, Y.F.; Meulenbelt, I. The role of epigenetics in osteoarthritis: Current perspective. Curr. Opin. Rheumatol. 2017, 29, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I.; Choi, Y.J. Epigenetics in osteoarthritis and its implication for future therapeutics. Expert. Opin. Biol. Ther. 2013, 13, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2017, 58, 49–63. [Google Scholar] [CrossRef]
- Rogers, E.L.; Reynard, L.N.; Loughlin, J. The role of inflammation-related genes in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1933–1938. [Google Scholar] [CrossRef]
- Simon, T.C.; Jeffries, M.A. The Epigenomic Landscape in Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 30. [Google Scholar] [CrossRef]
- van Meurs, J.B. Osteoarthritis year in review 2016: Genetics, genomics and epigenetics. Osteoarthr. Cartil. 2017, 25, 181–189. [Google Scholar] [CrossRef]
- Barter, M.J.; Bui, C.; Young, D.A. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthr. Cartil. 2012, 20, 339–349. [Google Scholar] [CrossRef]
- Chatterjee, A.; Eccles, M.R. DNA methylation and epigenomics: New technologies and emerging concepts. Genome Biol. 2015, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, J. Epigenetics and Osteoarthritis. Genes. Dis. 2015, 2, 69–75. [Google Scholar] [CrossRef]
- Nakano, K.; Boyle, D.L.; Firestein, G.S. Regulation of DNA methylation in rheumatoid arthritis synoviocytes. J. Immunol. 2013, 190, 1297–1303. [Google Scholar] [CrossRef]
- Sullivan, K.E.; Reddy, A.B.; Dietzmann, K.; Suriano, A.R.; Kocieda, V.P.; Stewart, M.; Bhatia, M. Epigenetic regulation of tumor necrosis factor alpha. Mol. Cell Biol. 2007, 27, 5147–5160. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Otero, M.; Imagawa, K.; de Andrés, M.C.; Coico, J.M.; Roach, H.I.; Oreffo, R.O.C.; Marcu, K.B.; Goldring, M.B. Regulated transcription of human matrix metalloproteinase 13 (MMP13) and interleukin-1β (IL1B) genes in chondrocytes depends on methylation of specific proximal promoter CpG sites. J. Biol. Chem. 2013, 288, 10061–10072. [Google Scholar] [CrossRef]
- Hashimoto, K.; Oreffo, R.O.; Gibson, M.B.; Goldring, M.B.; Roach, H.I. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009, 60, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Bui, C.; Barter, M.J.; Scott, J.L.; Xu, Y.; Galler, M.; Reynard, L.N.; Rowan, A.D.; Young, D.A. cAMP response element-binding (CREB) recruitment following a specific CpG demethylation leads to the elevated expression of the matrix metalloproteinase 13 in human articular chondrocytes and osteoarthritis. FASEB J. 2012, 26, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Boeuf, S.; Dickhut, A.; Boehmer, S.; Olek, S.; Richter, W. Correlation of COL10A1 induction during chondrogenesis of mesenchymal stem cells with demethylation of two CpG sites in the COL10A1 promoter. Arthritis Rheum. 2008, 58, 2743–2753. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hashimoto, K.; Yamada, N.; Roach, H.I. Expression of ADAMTS-4 by chondrocytes in the surface zone of human osteoarthritic cartilage is regulated by epigenetic DNA de-methylation. Rheumatol. Int. 2009, 29, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, I.; Kostopoulou, F.; Malizos, K.N.; Tsezou, A. DNA methylation regulates sclerostin (SOST) expression in osteoarthritic chondrocytes by bone morphogenetic protein 2 (BMP-2) induced changes in Smads binding affinity to the CpG region of SOST promoter. Arthritis Res. Ther. 2015, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Park, Y.S.; Im, G.I. Changes in the epigenetic status of the SOX-9 promoter in human osteoarthritic cartilage. J. Bone Miner. Res. 2013, 28, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Imagawa, K.; de Andrés, M.C.; Hashimoto, K.; Itoi, E.; Otero, M.; Roach, H.I.; Goldring, M.B.; Oreffo, R.O. Association of reduced type IX collagen gene expression in human osteoarthritic chondrocytes with epigenetic silencing by DNA hypermethylation. Arthritis Rheumatol. 2014, 66, 3040–3051. [Google Scholar] [CrossRef]
- Takahashi, A.; de Andrés, M.C.; Hashimoto, K.; Itoi, E.; Oreffo, R.O. Epigenetic regulation of interleukin-8, an inflammatory chemokine, in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1946–1954. [Google Scholar] [CrossRef]
- de Andrés, M.C.; Imagawa, K.; Hashimoto, K.; Gonzalez, A.; Roach, H.I.; Goldring, M.B.; Oreffo, R.O. Loss of methylation in CpG sites in the NF-κB enhancer elements of inducible nitric oxide synthase is responsible for gene induction in human articular chondrocytes. Arthritis Rheum. 2013, 65, 732–742. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, S.; Wang, C.; Huang, Y.; Li, H.; Wang, Y.; Zhu, Z.; Tang, J.; Yan, M. Epigenetic modifications of interleukin-6 in synovial fibroblasts from osteoarthritis patients. Sci. Rep. 2017, 7, 43592. [Google Scholar] [CrossRef]
- Kim, H.; Kang, D.; Cho, Y.; Kim, J.H. Epigenetic Regulation of Chondrocyte Catabolism and Anabolism in Osteoarthritis. Mol. Cells 2015, 38, 677–684. [Google Scholar] [CrossRef]
- Choudhuri, S.; Cui, Y.; Klaassen, C.D. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol. Appl. Pharmacol. 2010, 245, 378–393. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- de Ruijter, A.J.; van Gennip, A.H.; Caron, H.N.; Kemp, S.; van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, L.; Yang, Y.; Zhang, X.; Gang, Y.; Bai, L. The Role of HDACs and HDACi in Cartilage and Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 560117. [Google Scholar] [CrossRef] [PubMed]
- Young, D.A.; Lakey, R.L.; Pennington, C.J.; Jones, D.; Kevorkian, L.; Edwards, D.R.; Cawston, T.E.; Clark, I.M. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res. Ther. 2005, 7, R503–R512. [Google Scholar] [CrossRef]
- Hong, S.; Derfoul, A.; Pereira-Mouries, L.; Hall, D.J. A novel domain in histone deacetylase 1 and 2 mediates repression of cartilage-specific genes in human chondrocytes. FASEB J. 2009, 23, 3539–3552. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.C.; Brock, M.; Hemmatazad, H.; Giger, O.T.; Moritz, F.; Trenkmann, M.; Distler, J.H.; Gay, R.E.; Kolling, C.; Moch, H.; et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007, 56, 1087–1093. [Google Scholar] [CrossRef]
- Higashiyama, R.; Miyaki, S.; Yamashita, S.; Yoshitaka, T.; Lindman, G.; Ito, Y.; Sasho, T.; Takahashi, K.; Lotz, M.; Asahara, H. Correlation between MMP-13 and HDAC7 expression in human knee osteoarthritis. Mod. Rheumatol. 2010, 20, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Gagarina, V.; Lee, E.J.; Hall, D.J. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2008, 283, 36300–36310. [Google Scholar] [CrossRef] [PubMed]
- Dvir-Ginzberg, M.; Mobasheri, A.; Kumar, A. The Role of Sirtuins in Cartilage Homeostasis and Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 43. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Gagarina, V.; Gabay, O.; Dvir-Ginzberg, M.; Lee, E.J.; Brady, J.K.; Quon, M.J.; Hall, D.J. SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway. Arthritis Rheum. 2010, 62, 1383–1392. [Google Scholar] [CrossRef]
- Oppenheimer, H.; Kumar, A.; Meir, H.; Schwartz, I.; Zini, A.; Haze, A.; Kandel, L.; Mattan, Y.; Liebergall, M.; Dvir-Ginzberg, M. Set7/9 impacts COL2A1 expression through binding and repression of SirT1 histone deacetylation. J. Bone Miner. Res. 2014, 29, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Peng, H.; Hachem, K.E.; Culley, K.L.; Wondimu, E.B.; Quinn, J.; Asahara, H.; Tsuchimochi, K.; Hashimoto, K.; Goldring, M.B. ELF3 modulates type II collagen gene (COL2A1) transcription in chondrocytes by inhibiting SOX9-CBP/p300-driven histone acetyltransferase activity. Connect. Tissue Res. 2017, 58, 15–26. [Google Scholar] [CrossRef]
- Monteagudo, S.; Cornelis, F.M.F.; Aznar-Lopez, C.; Yibmantasiri, P.; Guns, L.A.; Carmeliet, P.; Cailotto, F.; Lories, R.J. DOT1L safeguards cartilage homeostasis and protects against osteoarthritis. Nat. Commun. 2017, 8, 15889. [Google Scholar] [CrossRef]
- Castaño Betancourt, M.C.; Cailotto, F.; Kerkhof, H.J.; Cornelis, F.M.; Doherty, S.A.; Hart, D.J.; Hofman, A.; Luyten, F.P.; Maciewicz, R.A.; Mangino, M.; et al. Genome-wide association and functional studies identify the DOT1L gene to be involved in cartilage thickness and hip osteoarthritis. Proc. Natl. Acad. Sci. USA 2012, 109, 8218–8223. [Google Scholar] [CrossRef]
- Peschansky, V.J.; Wahlestedt, C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.; Dai, L.; Hu, X.; Zhu, J.; Li, L.; Zhou, C.; Ao, Y. Long noncoding RNA related to cartilage injury promotes chondrocyte extracellular matrix degradation in osteoarthritis. Arthritis Rheumatol. 2014, 66, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Zampetaki, A.; Lin, N.Y.; Kleyer, A.; Perricone, C.; Iagnocco, A.; Distler, A.; Langley, S.R.; Gelse, K.; Sesselmann, S.; et al. Signature of circulating microRNAs in osteoarthritis. Ann. Rheum. Dis. 2015, 74, e18. [Google Scholar] [CrossRef]
- Miyaki, S.; Asahara, H. Macro view of microRNA function in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 543–552. [Google Scholar] [CrossRef]
- Sondag, G.R.; Haqqi, T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef]
- Swingler, T.E.; Niu, L.; Smith, P.; Paddy, P.; Le, L.; Barter, M.J.; Young, D.A.; Clark, I.M. The function of microRNAs in cartilage and osteoarthritis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 120), 40–47. [Google Scholar]
- Rasheed, Z.; Al-Shobaili, H.A.; Rasheed, N.; Mahmood, A.; Khan, M.I. MicroRNA-26a-5p regulates the expression of inducible nitric oxide synthase via activation of NF-κB pathway in human osteoarthritis chondrocytes. Arch. Biochem. Biophys. 2016, 594, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Rasheed, Z.; Ramamurthy, S.; Anbazhagan, A.N.; Voss, F.R.; Haqqi, T.M. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010, 62, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Cheon, E.J.; Kim, H.A. MicroRNA-558 regulates the expression of cyclooxygenase-2 and IL-1β-induced catabolic effects in human articular chondrocytes. Osteoarthr. Cartil. 2013, 21, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann. Rheum. Dis. 2012, 71, 1073–1080. [Google Scholar] [CrossRef]
- Mao, G.; Zhang, Z.; Huang, Z.; Chen, W.; Huang, G.; Meng, F.; Kang, Y. MicroRNA-92a-3p regulates the expression of cartilage-specific genes by directly targeting histone deacetylase 2 in chondrogenesis and degradation. Osteoarthr. Cartil. 2017, 25, 521–532. [Google Scholar] [CrossRef]
- Meng, F.; Li, Z.; Zhang, Z.; Yang, Z.; Kang, Y.; Zhao, X.; Long, D.; Hu, S.; Gu, M.; He, S.; et al. MicroRNA-193b-3p regulates chondrogenesis and chondrocyte metabolism by targeting HDAC3. Theranostics 2018, 8, 2862–2883. [Google Scholar] [CrossRef]
- Guan, Y.J.; Li, J.; Yang, X.; Du, S.; Ding, J.; Gao, Y.; Zhang, Y.; Yang, K.; Chen, Q. Evidence that miR-146a attenuates aging- and trauma-induced osteoarthritis by inhibiting Notch1, IL-6, and IL-1 mediated catabolism. Aging Cell 2018, 17, e12752. [Google Scholar] [CrossRef]
- Wang, J.H.; Shih, K.S.; Wu, Y.W.; Wang, A.W.; Yang, C.R. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1β signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthr. Cartil. 2013, 21, 1987–1996. [Google Scholar] [CrossRef]
- Budd, E.; de Andrés, M.C.; Sanchez-Elsner, T.; Oreffo, R.O.C. MiR-146b is down-regulated during the chondrogenic differentiation of human bone marrow derived skeletal stem cells and up-regulated in osteoarthritis. Sci. Rep. 2017, 7, 46704. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Chang, Z.; Mao, G.; Hu, S.; Zeng, A.; Fu, M. miR-193b-5p regulates chondrocytes metabolism by directly targeting histone deacetylase 7 in interleukin-1β-induced osteoarthritis. J. Cell Biochem. 2019, 120, 12775–12784. [Google Scholar] [CrossRef]
- Ukai, T.; Sato, M.; Akutsu, H.; Umezawa, A.; Mochida, J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism. J. Orthop. Res. 2012, 30, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Santini, P.; Politi, L.; Vedova, P.D.; Scandurra, R.; Scotto d’Abusco, A. The inflammatory circuitry of miR-149 as a pathological mechanism in osteoarthritis. Rheumatol. Int. 2014, 34, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, S.; Nakasa, T.; Otsuki, S.; Grogan, S.P.; Higashiyama, R.; Inoue, A.; Kato, Y.; Sato, T.; Lotz, M.K.; Asahara, H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009, 60, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Araldi, E.; Schipani, E. MicroRNA-140 and the silencing of osteoarthritis. Genes. Dev. 2010, 24, 1075–1080. [Google Scholar] [CrossRef]
- Si, H.B.; Yang, T.M.; Li, L.; Tian, M.; Zhou, L.; Li, D.P.; Huang, Q.; Kang, P.D.; Yang, J.; Zhou, Z.K.; et al. miR-140 Attenuates the Progression of Early-Stage Osteoarthritis by Retarding Chondrocyte Senescence. Mol. Ther. Nucleic Acids 2020, 19, 15–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, J.; Zhou, X.; Chen, X.; Chen, A.C.; Pi, B.; Pan, G.; Pei, M.; Yang, H.; Liu, T.; et al. Melatonin Prevents Osteoarthritis-Induced Cartilage Degradation via Targeting MicroRNA-140. Oxid. Med. Cell Longev. 2019, 2019, 9705929. [Google Scholar] [CrossRef]
- Karlsen, T.A.; de Souza, G.A.; Ødegaard, B.; Engebretsen, L.; Brinchmann, J.E. microRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1β-induced Osteoarthritis. Mol. Ther. Nucleic Acids 2016, 5, e373. [Google Scholar] [CrossRef]
- Tuddenham, L.; Wheeler, G.; Ntounia-Fousara, S.; Waters, J.; Hajihosseini, M.K.; Clark, I.; Dalmay, T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006, 580, 4214–4217. [Google Scholar] [CrossRef]
- Woods, S.; Charlton, S.; Cheung, K.; Hao, Y.; Soul, J.; Reynard, L.N.; Crowe, N.; Swingler, T.E.; Skelton, A.J.; Piróg, K.A.; et al. microRNA-seq of cartilage reveals an overabundance of miR-140-3p which contains functional isomiRs. RNA 2020, 26, 1575–1588. [Google Scholar] [CrossRef]
- Feng, L.; Feng, C.; Wang, C.X.; Xu, D.Y.; Chen, J.J.; Huang, J.F.; Tan, P.L.; Shen, J.M. Circulating microRNA let-7e is decreased in knee osteoarthritis, accompanied by elevated apoptosis and reduced autophagy. Int. J. Mol. Med. 2020, 45, 1464–1476. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Dudek, K.A.; Murphy, C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J. Biol. Chem. 2012, 287, 916–924. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Tsuchimochi, K.; Ijiri, K.; Li, Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann. Rheum. Dis. 2008, 67 (Suppl. 3), iii75–iii82. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Peter, P.; Davis, C.R.; Crowell, J.A.; Mantzoros, C.S. Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metabolism 2014, 63, 233–241. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.D.M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J.A. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Romagnolo, D.F.; Selmin, O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today 2017, 52, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Martínez-González, M.A.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N.G. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Graffigna, G.; Bosio, C.; Donini, L.M.; Marangoni, F. The complex relationship between diet, quality of life and life expectancy: A narrative review of potential determinants based on data from Italy. Eat. Weight. Disord. 2019, 24, 411–419. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Graves, K.Y.; Sumerau, J.E. Mediation analysis of relationships between chronic inflammation and quality of life in older adults. Health Qual. Life Outcomes 2016, 14, 46. [Google Scholar] [CrossRef]
- McKay, J.A.; Mathers, J.C. Diet induced epigenetic changes and their implications for health. Acta Physiol. 2011, 202, 103–118. [Google Scholar] [CrossRef]
- Milagro, F.I.; Mansego, M.L.; De Miguel, C.; Martínez, J.A. Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol. Aspects Med. 2013, 34, 782–812. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.D.; Uthus, E.O. DNA methylation, cancer susceptibility, and nutrient interactions. Exp. Biol. Med. 2004, 229, 988–995. [Google Scholar]
- Widiker, S.; Karst, S.; Wagener, A.; Brockmann, G.A. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J. Appl. Genet. 2010, 51, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Szarc vel Szic, K.; Declerck, K.; Vidaković, M.; Vanden Berghe, W. From inflammaging to healthy aging by dietary lifestyle choices: Is epigenetics the key to personalized nutrition? Clin. Epigenetics 2015, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Toopchizadeh, V.; Dolatkhah, N.; Aghamohammadi, D.; Rasouli, M.; Hashemian, M. Dietary inflammatory index is associated with pain intensity and some components of quality of life in patients with knee osteoarthritis. BMC Res. Notes 2020, 13, 448. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Shivappa, N.; Stubbs, B.; Smith, T.; Hébert, J.R.; Cooper, C.; Guglielmi, G.; Reginster, J.Y.; Rizzoli, R.; Maggi, S. The relationship between the dietary inflammatory index and prevalence of radiographic symptomatic osteoarthritis: Data from the Osteoarthritis Initiative. Eur. J. Nutr. 2019, 58, 253–260. [Google Scholar] [CrossRef]
- Liu, Q.; Hebert, J.R.; Shivappa, N.; Guo, J.; Tao, K.; Zeng, C.; Lei, G.; Lin, J.; Zhang, Y. Inflammatory potential of diet and risk of incident knee osteoarthritis: A prospective cohort study. Arthritis Res. Ther. 2020, 22, 209. [Google Scholar] [CrossRef]
- Cavicchia, P.P.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Hébert, J.R. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J. Nutr. 2009, 139, 2365–2372. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Chandran, V.; Power, J.D.; Kapoor, M.; Mahomed, N.N.; Gandhi, R. Systemic inflammation and painful joint burden in osteoarthritis: A matter of sex? Osteoarthr. Cartil. 2017, 25, 53–59. [Google Scholar] [CrossRef]
- Larsson, S.; Englund, M.; Struglics, A.; Lohmander, L.S. Interleukin-6 and tumor necrosis factor alpha in synovial fluid are associated with progression of radiographic knee osteoarthritis in subjects with previous meniscectomy. Osteoarthr. Cartil. 2015, 23, 1906–1914. [Google Scholar] [CrossRef]
- Jin, X.; Beguerie, J.R.; Zhang, W.; Blizzard, L.; Otahal, P.; Jones, G.; Ding, C. Circulating C reactive protein in osteoarthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 703–710. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Raynauld, J.P.; Caron, J.; Mineau, F.; Abram, F.; Dorais, M.; Haraoui, B.; Choquette, D.; Martel-Pelletier, J. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 2095–2101. [Google Scholar] [CrossRef]
- Dyer, J.; Davison, G.; Marcora, S.M.; Mauger, A.R. Effect of a Mediterranean Type Diet on Inflammatory and Cartilage Degradation Biomarkers in Patients with Osteoarthritis. J. Nutr. Health Aging 2017, 21, 562–566. [Google Scholar] [CrossRef]
- Henríquez Sánchez, P.; Ruano, C.; de Irala, J.; Ruiz-Canela, M.; Martínez-González, M.A.; Sánchez-Villegas, A. Adherence to the Mediterranean diet and quality of life in the SUN Project. Eur. J. Clin. Nutr. 2012, 66, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Bonanni, A.; Costanzo, S.; De Lucia, F.; Pounis, G.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; et al. Adherence to a Mediterranean diet is associated with a better health-related quality of life: A possible role of high dietary antioxidant content. BMJ Open 2013, 3, e003003. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Struijk, E.A.; Fransen, H.P.; Onland-Moret, N.C.; de Wit, G.A.; Boer, J.M.; van der Schouw, Y.T.; Hoekstra, J.; Bueno-de-Mesquita, H.B.; Peeters, P.H.; et al. The impact of a healthy lifestyle on Disability-Adjusted Life Years: A prospective cohort study. BMC Med. 2015, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tasigchana, R.F.; León-Muñoz, L.M.; López-García, E.; Banegas, J.R.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. Mediterranean Diet and Health-Related Quality of Life in Two Cohorts of Community-Dwelling Older Adults. PLoS ONE 2016, 11, e0151596. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Maggi, S. Adherence to the Mediterranean diet is associated with better quality of life: Data from the Osteoarthritis Initiative. Am. J. Clin. Nutr. 2016, 104, 1403–1409. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Block, G.; Hartman, A.M.; Naughton, D. A reduced dietary questionnaire: Development and validation. Epidemiology 1990, 1, 58–64. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Smith, T.O.; Cooper, C.; Guglielmi, G.; Reginster, J.Y.; Rizzoli, R.; et al. Adherence to a Mediterranean diet is associated with lower prevalence of osteoarthritis: Data from the osteoarthritis initiative. Clin. Nutr. 2017, 36, 1609–1614. [Google Scholar] [CrossRef]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef]
- Chatzianagnostou, K.; Del Turco, S.; Pingitore, A.; Sabatino, L.; Vassalle, C. The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging. Antioxidants 2015, 4, 719–736. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C.; Cano, M.P.; de Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Martín, A. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, prostaglandin E2 and monocyte chemotactic protein-1 in healthy humans. J. Nutr. Biochem. 2006, 17, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Martín-Núñez, G.M.; Cabrera-Mulero, R.; Rubio-Martín, E.; Rojo-Martínez, G.; Olveira, G.; Valdés, S.; Soriguer, F.; Castaño, L.; Morcillo, S. Methylation levels of the SCD1 gene promoter and LINE-1 repeat region are associated with weight change: An intervention study. Mol. Nutr. Food Res. 2014, 58, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Gabrić, D.; Roohinejad, S.; Barba, F.J.; Granato, D.; Mallikarjunan, K.; Lorenzo, J.M.; Bursać Kovačević, D. An overview of organosulfur compounds from Allium spp.: From processing and preservation to evaluation of their bioavailability, antimicrobial, and anti-inflammatory properties. Food Chem. 2019, 276, 680–691. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Resveratrol as a Novel Anti-Herpes Simplex Virus Nutraceutical Agent: An Overview. Viruses 2018, 10, 473. [Google Scholar] [CrossRef]
- Annunziata, G.; Sanduzzi Zamparelli, M.; Santoro, C.; Ciampaglia, R.; Stornaiuolo, M.; Tenore, G.C.; Sanduzzi, A.; Novellino, E. May Polyphenols Have a Role Against Coronavirus Infection? An Overview of. Front. Med. 2020, 7, 240. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential Use in the Prevention and Treatment of Cardiovascular Diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Toda, T.; Sugioka, Y.; Koike, T. Soybean isoflavone can protect against osteoarthritis in ovariectomized rats. J. Food Sci. Technol. 2020, 57, 3409–3414. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and Molecular Mechanism of Action of Phytonutraceuticals on Osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; Sun, H.B. Nutraceuticals: Potential for chondroprotection and molecular targeting of osteoarthritis. Int. J. Mol. Sci. 2013, 14, 23063–23085. [Google Scholar] [CrossRef]
- Chin, K.Y.; Pang, K.L. Therapeutic Effects of Olive and Its Derivatives on Osteoarthritis: From Bench to Bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Zhang, S.N. Recent advance in treatment of osteoarthritis by bioactive components from herbal medicine. Chin. Med. 2020, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.A.R.; Pinheiro-Castro, N.; Novaes, G.M.; Pascoal, G.F.L.; Ong, T.P. Bioactive food compounds, epigenetics and chronic disease prevention: Focus on early-life interventions with polyphenols. Food Res. Int. 2019, 125, 108646. [Google Scholar] [CrossRef]
- Crascì, L.; Lauro, M.R.; Puglisi, G.; Panico, A. Natural antioxidant polyphenols on inflammation management: Anti-glycation activity vs metalloproteinases inhibition. Crit. Rev. Food Sci. Nutr. 2018, 58, 893–904. [Google Scholar] [CrossRef]
- Kinger, M.; Kumar, S.; Kumar, V. Some Important Dietary Polyphenolic Compounds: An Anti-inflammatory and Immunoregulatory Perspective. Mini Rev. Med. Chem. 2018, 18, 1270–1282. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Choi, D.J.; Choi, S.I.; Choi, B.R.; Lee, Y.S.; Lee, D.Y.; Kim, G.S. Cartilage protective and anti-analgesic effects of ALM16 on monosodium iodoacetate induced osteoarthritis in rats. BMC Complement. Altern. Med. 2019, 19, 325. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Rasheed, Z.; Akhtar, N.; Haqqi, T.M. Pomegranate extract inhibits the interleukin-1β-induced activation of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human osteoarthritis chondrocytes. Arthritis Res. Ther. 2010, 12, R195. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Hafeez, B.B.; Cheruvu, V.K.; Haqqi, T.M. Punica granatum L. extract inhibits IL-1beta-induced expression of matrix metalloproteinases by inhibiting the activation of MAP kinases and NF-kappaB in human chondrocytes in vitro. J. Nutr. 2005, 135, 2096–2102. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, W.; Zhong, X.; Xu, J.; Huang, H.; Zheng, X.; Zhang, J.; Yang, S.; Shang, P.; Tang, Q.; et al. Arctigenin prevents the progression of osteoarthritis by targeting PI3K/Akt/NF-κB axis: In vitro and in vivo studies. J. Cell Mol. Med. 2020, 24, 4183–4193. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Piao, T.; Wang, Y.; Liu, J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. Int. Immunopharmacol. 2015, 25, 83–87. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Sanchez, C.; Deberg, M.A.; Piccardi, N.; Guillou, G.B.; Msika, P.; Reginster, J.Y. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J. Rheumatol. 2003, 30, 1825–1834. [Google Scholar] [PubMed]
- Henrotin, Y.E.; Deberg, M.A.; Crielaard, J.M.; Piccardi, N.; Msika, P.; Sanchez, C. Avocado/soybean unsaponifiables prevent the inhibitory effect of osteoarthritic subchondral osteoblasts on aggrecan and type II collagen synthesis by chondrocytes. J. Rheumatol. 2006, 33, 1668–1678. [Google Scholar] [PubMed]
- Au, R.Y.; Al-Talib, T.K.; Au, A.Y.; Phan, P.V.; Frondoza, C.G. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 2007, 15, 1249–1255. [Google Scholar] [CrossRef]
- Goudarzi, R.; Taylor, J.F.; Yazdi, P.G.; Pedersen, B.A. Effects of Arthrocen, an avocado/soy unsaponifiables agent, on inflammatory mediators and gene expression in human chondrocytes. FEBS Open Bio 2017, 7, 187–194. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, C.; Cai, L.; Xie, H.; Hu, W.; Wang, T.; Lu, D.; Chen, H. Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models. Int. Immunopharmacol. 2017, 52, 218–226. [Google Scholar] [CrossRef]
- Liu, S.C.; Lee, H.P.; Hung, C.Y.; Tsai, C.H.; Li, T.M.; Tang, C.H. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharmacol. 2015, 289, 20–29. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Jin, Y.; Wang, Q.; Chen, L.; Feng, Z.; Chen, H.; Wu, Y. Butein inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Mu, Y.; Hao, W.; Li, S. Casticin protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes. Eur. J. Pharmacol. 2019, 842, 314–320. [Google Scholar] [CrossRef]
- Ding, Q.H.; Cheng, Y.; Chen, W.P.; Zhong, H.M.; Wang, X.H. Celastrol, an inhibitor of heat shock protein 90β potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2013, 708, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Hsiao, G.; Lin, K.H.; Hsieh, M.S.; Jayakumar, T.; Wu, T.S.; Sheu, J.R. Cinnamophilin isolated from Cinnamomum philippinense protects against collagen degradation in human chondrocytes. Phytother. Res. 2013, 27, 892–899. [Google Scholar] [CrossRef]
- Feng, Z.; Zheng, W.; Li, X.; Lin, J.; Xie, C.; Li, H.; Cheng, L.; Wu, A.; Ni, W. Cryptotanshinone protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 50, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Tanzil, G.; Mobasheri, A.; Sendzik, J.; John, T.; Shakibaei, M. Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin-1beta-stimulated chondrocytes. Ann. N. Y. Acad. Sci. 2004, 1030, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Schulze-Tanzil, G.; John, T.; Mobasheri, A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: An immunomorphological study. Ann. Anat. 2005, 187, 487–497. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef]
- Mathy-Hartert, M.; Jacquemond-Collet, I.; Priem, F.; Sanchez, C.; Lambert, C.; Henrotin, Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm. Res. 2009, 58, 899–908. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Thanakhumtorn, S.; Chinswangwatanakul, P.; Wattanamongkonsil, L.; Thamlikitkul, V. Efficacy and safety of Curcuma domestica extracts in patients with knee osteoarthritis. J. Altern. Complement. Med. 2009, 15, 891–897. [Google Scholar] [CrossRef]
- Pinsornsak, P.; Niempoog, S. The efficacy of Curcuma Longa L. extract as an adjuvant therapy in primary knee osteoarthritis: A randomized control trial. J. Med. Assoc. Thai 2012, 95 (Suppl. 1), S51–S58. [Google Scholar]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress. Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes. Nutr. 2011, 6, 171–179. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Murata, S.; Yabumoto, H.; Maeda, T.; Akamatsu, S. The Efficacy and Safety of Highly-Bioavailable Curcumin for Treating Knee Osteoarthritis: A 6-Month Open-Labeled Prospective Study. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2020, 13, 1179544120948471. [Google Scholar] [CrossRef]
- Chopra, A.; Lavin, P.; Patwardhan, B.; Chitre, D. A 32-week randomized, placebo-controlled clinical evaluation of RA-11, an Ayurvedic drug, on osteoarthritis of the knees. J. Clin. Rheumatol. 2004, 10, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Cesarone, M.R.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.G.; Togni, S.; Appendino, G. Efficacy and safety of Meriva®, a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010, 15, 337–344. [Google Scholar]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1β via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2019, 28, 547–553. [Google Scholar] [CrossRef]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator’s synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V.; et al. Exploiting Curcumin Synergy With Natural Products Using Quantitative Analysis of Dose-Effect Relationships in an Experimental. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef] [PubMed]
- Kare, S.K.; Vinay, V.; Maresz, K.; Prisk, V.; Vik, H. Seed Extract-Based Botanical Compositions Alleviate Knee Pain and Improve Joint Function in Mild-to-Moderate Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Clinical Study. Evid. Based Complement. Alternat Med. 2022, 2022, 2226139. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lee, H.J.; Lee, D.R.; Choi, B.K.; Yang, S.H. Anti-osteoarthritic Effects of an Herbal Composition LI73014F2 on Interleukin-1β-induced Primary Human Articular Chondrocytes. Molecules 2020, 25, 2033. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Chen, D.; Haqqi, T.M. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology 2013, 52, 998–1008. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, C.; Fu, C.; Lu, H.; Jin, H.; Chen, Q.; Pan, J. The protective effect of Ellagic acid (EA) in osteoarthritis: An in vitro and in vivo study. Biomed. Pharmacother. 2020, 125, 109845. [Google Scholar] [CrossRef]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [Google Scholar] [CrossRef]
- Huang, G.S.; Tseng, C.Y.; Lee, C.H.; Su, S.L.; Lee, H.S. Effects of (-)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE(2), and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol. Int. 2010, 30, 1197–1203. [Google Scholar] [CrossRef]
- Singh, R.; Ahmed, S.; Islam, N.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: Suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002, 46, 2079–2086. [Google Scholar] [CrossRef]
- Ahmed, S.; Rahman, A.; Hasnain, A.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic. Biol. Med. 2002, 33, 1097–1105. [Google Scholar] [CrossRef]
- Singh, R.; Ahmed, S.; Malemud, C.J.; Goldberg, V.M.; Haqqi, T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. 2003, 21, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar] [CrossRef]
- Sakata, S.; Hayashi, S.; Fujishiro, T.; Kawakita, K.; Kanzaki, N.; Hashimoto, S.; Iwasa, K.; Chinzei, N.; Kihara, S.; Haneda, M.; et al. Oxidative stress-induced apoptosis and matrix loss of chondrocytes is inhibited by eicosapentaenoic acid. J. Orthop. Res. 2015, 33, 359–365. [Google Scholar] [CrossRef]
- Hooshmand, S.; Soung, D.Y.; Lucas, E.A.; Madihally, S.V.; Levenson, C.W.; Arjmandi, B.H. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J. Nutr. Biochem. 2007, 18, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective Effects of Genistein against Osteoarthritis Induced Joint Inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liu, Q.; Guo, P.; Huang, Y.; Ye, Z.; Hu, J. Anti-chondrocyte apoptosis effect of genistein in treating inflammation-induced osteoarthritis. Mol. Med. Rep. 2020, 22, 2032–2042. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Naderi, Z.; Mozaffari-Khosravi, H.; Dehghan, A.; Nadjarzadeh, A.; Huseini, H.F. Effect of ginger powder supplementation on nitric oxide and C-reactive protein in elderly knee osteoarthritis patients: A 12-week double-blind randomized placebo-controlled clinical trial. J. Tradit. Complement. Med. 2016, 6, 199–203. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Degli Agosti, I.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef]
- Amorndoljai, P.; Taneepanichskul, S.; Niempoog, S.; Nimmannit, U. A Comparative of Ginger Extract in Nanostructure Lipid Carrier (NLC) and 1% Diclofenac Gel for Treatment of Knee Osteoarthritis (OA). J. Med. Assoc. Thai 2017, 100, 447–456. [Google Scholar] [PubMed]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The Use of a New Food-Grade Lecithin Formulation of Highly Standardized Ginger (Zingiber officinale) and Acmella Oleracea Extracts for the Treatment of Pain and Inflammation in a Group of Subjects with Moderate Knee Osteoarthritis. J. Pain. Res. 2020, 13, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Di Maio, V.; Gullì, M.; Dalla Vedova, P.; Ammendola, S.; Scotto d’Abusco, A. Antiarthritic Effects of a Root Extract from. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. Harpagoside suppresses IL-6 expression in primary human osteoarthritis chondrocytes. J. Orthop. Res. 2017, 35, 311–320. [Google Scholar] [CrossRef]
- Chantre, P.; Cappelaere, A.; Leblan, D.; Guedon, D.; Vandermander, J.; Fournie, B. Efficacy and tolerance of Harpagophytum procumbens versus diacerhein in treatment of osteoarthritis. Phytomedicine 2000, 7, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Leblan, D.; Chantre, P.; Fournié, B. Harpagophytum procumbens in the treatment of knee and hip osteoarthritis. Four-month results of a prospective, multicenter, double-blind trial versus diacerhein. Joint Bone Spine 2000, 67, 462–467. [Google Scholar]
- Schulze-Tanzil, G.; Hansen, C.; Shakibaei, M. Effect of a Harpagophytum procumbens DC extract on matrix metalloproteinases in human chondrocytes in vitro. Arzneimittelforschung 2004, 54, 213–220. [Google Scholar] [CrossRef]
- Takeda, R.; Koike, T.; Taniguchi, I.; Tanaka, K. Double-blind placebo-controlled trial of hydroxytyrosol of Olea europaea on pain in gonarthrosis. Phytomedicine 2013, 20, 861–864. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Colakoglu, M.; Banerjee, S.; Bitik, B.; Aktekin, C.N.; Goker, B.; Karasu, C. AB0090 Verbascoside and hydroxytyrosol downregulate stress-related pathways in human osteoarthritic articular chondrocytes. Ann. Rheum. Dis. 2018, 77, 1241. [Google Scholar]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, Y.; Chen, N.; Yang, L. Icariin Regulates Cellular Functions and Gene Expression of Osteoarthritis Patient-Derived Human Fibroblast-Like Synoviocytes. Int. J. Mol. Sci. 2017, 18, 2656. [Google Scholar] [CrossRef]
- Zeng, L.; Rong, X.F.; Li, R.H.; Wu, X.Y. Icariin inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017, 15, 2853–2858. [Google Scholar] [CrossRef]
- Zuo, S.; Zou, W.; Wu, R.M.; Yang, J.; Fan, J.N.; Zhao, X.K.; Li, H.Y. Icariin Alleviates IL-1β-Induced Matrix Degradation By Activating The Nrf2/ARE Pathway In Human Chondrocytes. Drug Des. Devel Ther. 2019, 13, 3949–3961. [Google Scholar] [CrossRef] [PubMed]
- Piscoya, J.; Rodriguez, Z.; Bustamante, S.A.; Okuhama, N.N.; Miller, M.J.; Sandoval, M. Efficacy and safety of freeze-dried cat’s claw in osteoarthritis of the knee: Mechanisms of action of the species Uncaria guianensis. Inflamm. Res. 2001, 50, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yu, X.; Hu, Z.; Tang, S.; Zhong, X.; Xu, J.; Shang, P.; Huang, Y.; Liu, H. Isofraxidin targets the TLR4/MD-2 axis to prevent osteoarthritis development. Food Funct. 2018, 9, 5641–5652. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Qi, W.; Yan, Y.; Chen, K.; Xue, X.; Xu, X.; Feng, Z.; Pan, X. Isofraxidin inhibits interleukin-1β induced inflammatory response in human osteoarthritis chondrocytes. Int. Immunopharmacol. 2018, 64, 238–245. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Wang, X.; Huo, S. Juglanin inhibits IL-1β-induced inflammation in human chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3614–3620. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Qiao, J.; Guan, D.; Chen, T. Anti-Inflammatory Effects of Licochalcone A on IL-1β-Stimulated Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1894–1902. [Google Scholar] [CrossRef]
- de Andrés, M.C.; Meiss, M.S.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C.; Oreffo, R.O. Osteoarthritis treatment with a novel nutraceutical acetylated ligstroside aglycone, a chemically modified extra-virgin olive oil polyphenol. J. Tissue Eng. 2020, 11, 2041731420922701. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Sousa, C.; Judas, F.; Salgueiro, L.; Cavaleiro, C.; Mendes, A.F. Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur. J. Pharmacol. 2015, 750, 141–150. [Google Scholar] [CrossRef]
- Pan, X.; Chen, T.; Zhang, Z.; Chen, X.; Chen, C.; Chen, L.; Wang, X.; Ying, X. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Conde, J.; Abella, V.; López, V.; Francisco, V.; Ruiz, C.; Campos, V.; Lago, F.; Gomez, R.; Pino, J.; et al. Oleocanthal Inhibits Catabolic and Inflammatory Mediators in LPS-Activated Human Primary Osteoarthritis (OA) Chondrocytes Through MAPKs/NF-κB Pathways. Cell Physiol. Biochem. 2018, 49, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Lin, J.; Zheng, W.; Hu, Z.; Xuan, J.; Ni, W.; Pan, X. Oleuropein inhibits the IL-1β-induced expression of inflammatory mediators by suppressing the activation of NF-κB and MAPKs in human osteoarthritis chondrocytes. Food Funct. 2017, 8, 3737–3744. [Google Scholar] [CrossRef] [PubMed]
- Varela-Eirín, M.; Carpintero-Fernández, P.; Sánchez-Temprano, A.; Varela-Vázquez, A.; Paíno, C.L.; Casado-Díaz, A.; Continente, A.C.; Mato, V.; Fonseca, E.; Kandouz, M.; et al. Senolytic activity of small molecular polyphenols from olive restores chondrocyte redifferentiation and promotes a pro-regenerative environment in osteoarthritis. Aging 2020, 12, 15882–15905. [Google Scholar] [CrossRef] [PubMed]
- Elmazoglu, Z.; Bek, Z.A.; Sarıbaş, G.S.; Özoğul, C.; Goker, B.; Bitik, B.; Aktekin, C.N.; Karasu, Ç. TLR4, RAGE, and p-JNK/JNK mediated inflammatory aggression in osteoathritic human chondrocytes are counteracted by redox-sensitive phenolic olive compounds: Comparison with ibuprofen. J. Tissue Eng. Regen. Med. 2020, 14, 1841–1857. [Google Scholar] [CrossRef]
- Peng, L.; Xie, Z.; Pei, J.; Wang, B.; Gao, Y.; Qu, Y. Puerarin alters the function of monocytes/macrophages and exhibits chondroprotection in mice. Mol. Med. Rep. 2019, 19, 2876–2882. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yu, T.; Peng, L.; Wang, L.; Liao, Z.; Xu, W. PIM1, CYP1B1, and HSPA2 Targeted by Quercetin Play Important Roles in Osteoarthritis Treatment by. Evid. Based Complement. Alternat Med. 2019, 2019, 1205942. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Gu, H.; Jiang, M.; Huang, Y.; Liu, L. Resveratrol inhibits the development of obesity-related osteoarthritis via the TLR4 and PI3K/Akt signaling pathways. Connect. Tissue Res. 2019, 60, 571–582. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Attur, M.; Palmer, G.; Al-Mussawir, H.E.; Kennish, L.; Patel, J.; Abramson, S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008, 58, 2786–2797. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Jiang, M.; Huang, Y.; Liu, L.; Gu, H. Resveratrol Exerts Anti-Osteoarthritic Effect by Inhibiting TLR4/NF-κB Signaling Pathway via the TLR4/Akt/FoxO1 Axis in IL-1β-Stimulated SW1353 Cells. Drug Des. Devel Ther. 2020, 14, 2079–2090. [Google Scholar] [CrossRef]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, X.; Huang, K.; Shen, S.; Lin, X.; Xie, Z.; Wang, J.; Fan, S.; Ma, J.; Zhao, X. Sanguinarine protects against osteoarthritis by suppressing the expression of catabolic proteases. Oncotarget 2017, 8, 62900–62913. [Google Scholar] [CrossRef]
- Liao, S.; Zhou, K.; Li, D.; Xie, X.; Jun, F.; Wang, J. Schisantherin A suppresses interleukin-1β-induced inflammation in human chondrocytes via inhibition of NF-κB and MAPKs activation. Eur. J. Pharmacol. 2016, 780, 65–70. [Google Scholar] [CrossRef]
- Phitak, T.; Pothacharoen, P.; Settakorn, J.; Poompimol, W.; Caterson, B.; Kongtawelert, P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry 2012, 80, 77–88. [Google Scholar] [CrossRef]
- Kim, H.A.; Yeo, Y.; Jung, H.A.; Jung, Y.O.; Park, S.J.; Kim, S.J. Phase 2 enzyme inducer sulphoraphane blocks prostaglandin and nitric oxide synthesis in human articular chondrocytes and inhibits cartilage matrix degradation. Rheumatology 2012, 51, 1006–1016. [Google Scholar] [CrossRef]
- Kim, H.A.; Yeo, Y.; Kim, W.U.; Kim, S. Phase 2 enzyme inducer sulphoraphane blocks matrix metalloproteinase production in articular chondrocytes. Rheumatology 2009, 48, 932–938. [Google Scholar] [CrossRef][Green Version]
- Facchini, A.; Stanic, I.; Cetrullo, S.; Borzì, R.M.; Filardo, G.; Flamigni, F. Sulforaphane protects human chondrocytes against cell death induced by various stimuli. J. Cell Physiol. 2011, 226, 1771–1779. [Google Scholar] [CrossRef]
- Davidson, R.K.; Jupp, O.; de Ferrars, R.; Kay, C.D.; Culley, K.L.; Norton, R.; Driscoll, C.; Vincent, T.L.; Donell, S.T.; Bao, Y.; et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013, 65, 3130–3140. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Gardner, S.; Jupp, O.; Bullough, A.; Butters, S.; Watts, L.; Donell, S.; Traka, M.; Saha, S.; Mithen, R.; et al. Isothiocyanates are detected in human synovial fluid following broccoli consumption and can affect the tissues of the knee joint. Sci. Rep. 2017, 7, 3398. [Google Scholar] [CrossRef]
- Ko, J.Y.; Choi, Y.J.; Jeong, G.J.; Im, G.I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials 2013, 34, 5359–5368. [Google Scholar] [CrossRef]
- Piao, T.; Ma, Z.; Li, X.; Liu, J. Taraxasterol inhibits IL-1β-induced inflammatory response in human osteoarthritic chondrocytes. Eur. J. Pharmacol. 2015, 756, 38–42. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J.W.; Lee, D.S.; Yim, M.J.; Lee, J.M.; Han, M.H.; Kim, S.; Kim, H.S.; Kim, G.Y.; Park, E.K.; et al. Extract Attenuates Interleukin-1β-Induced Oxidative Stress and Inflammatory Response in Chondrocytes by Suppressing the Activation of NF-κB, p38 MAPK, and PI3K/Akt. Int. J. Mol. Sci. 2018, 19, 2308. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qiao, J.; Zhao, X.; Chen, T.; Guan, D. Thymoquinone Inhibits IL-1β-Induced Inflammation in Human Osteoarthritis Chondrocytes by Suppressing NF-κB and MAPKs Signaling Pathway. Inflammation 2015, 38, 2235–2241. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci. Rep. 2017, 7, 43789. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Ansari, M.Y.; Haqqi, T.M. Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1β stimulated osteoarthritis chondrocytes. Chem. Biol. Interact. 2017, 274, 13–23. [Google Scholar] [CrossRef]
- Ma, P.; Yue, L.; Yang, H.; Fan, Y.; Bai, J.; Li, S.; Yuan, J.; Zhang, Z.; Yao, C.; Lin, M.; et al. Chondroprotective and anti-inflammatory effects of amurensin H by regulating TLR4/Syk/NF-κB signals. J. Cell Mol. Med. 2020, 24, 1958–1968. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Q.; Wang, K. Artesunate attenuates ACLT-induced osteoarthritis by suppressing osteoclastogenesis and aberrant angiogenesis. Biomed. Pharmacother. 2017, 96, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Guo, X.H.; Tang, C.; Yue, S.T.; Shi, L.; Qiang, B. Effects of Artesunate on the Expressions of Insulin-Like Growth Factor-1, Osteopontin and C-Telopeptides of Type II Collagen in a Rat Model of Osteoarthritis. Pharmacology 2018, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, K.; Felisaz, N.; Bogdanowicz, P.; Galera, P.; Guillou, G.B.; Pujol, J.P. Avocado/soya unsaponifiables enhance the expression of transforming growth factor beta1 and beta2 in cultured articular chondrocytes. Arthritis Rheum. 1999, 42, 148–156. [Google Scholar] [CrossRef]
- Al-Afify, A.S.A.; El-Akabawy, G.; El-Sherif, N.M.; El-Safty, F.E.A.; El-Habiby, M.M. Avocado soybean unsaponifiables ameliorates cartilage and subchondral bone degeneration in mono-iodoacetate-induced knee osteoarthritis in rats. Tissue Cell 2018, 52, 108–115. [Google Scholar] [CrossRef]
- Heinecke, L.F.; Grzanna, M.W.; Au, A.Y.; Mochal, C.A.; Rashmir-Raven, A.; Frondoza, C.G. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthr. Cartil. 2010, 18, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ownby, S.L.; Fortuno, L.V.; Au, A.Y.; Grzanna, M.W.; Rashmir-Raven, A.M.; Frondoza, C.G. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J. Inflamm. 2014, 11, 8. [Google Scholar] [CrossRef]
- Frondoza, C.G.; Fortuno, L.V.; Grzanna, M.W.; Ownby, S.L.; Au, A.Y.; Rashmir-Raven, A.M. α-Lipoic Acid Potentiates the Anti-Inflammatory Activity of Avocado/Soybean Unsaponifiables in Chondrocyte Cultures. Cartilage 2018, 9, 304–312. [Google Scholar] [CrossRef]
- Grzanna, M.W.; Secor, E.J.; Fortuno, L.V.; Au, A.Y.; Frondoza, C.G. Anti-Inflammatory Effect of Carprofen Is Enhanced by Avocado/Soybean Unsaponifiables, Glucosamine and Chondroitin Sulfate Combination in Chondrocyte Microcarrier Spinner Culture. Cartilage 2020, 11, 108–116. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, P.; Liu, R.; Meng, Q.; Li, S. Baicalin promotes extracellular matrix synthesis in chondrocytes via the activation of hypoxia-inducible factor-1α. Exp. Ther. Med. 2020, 20, 226. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, D.; Lu, Q.; Liu, L.; Li, X.; Li, Z. Baicalin prevents the apoptosis of endplate chondrocytes by inhibiting the oxidative stress induced by H2O2. Mol. Med. Rep. 2017, 16, 2985–2991. [Google Scholar] [CrossRef]
- Moon, P.D.; Jeong, H.S.; Chun, C.S.; Kim, H.M. Baekjeolyusin-tang and its active component berberine block the release of collagen and proteoglycan from IL-1β-stimulated rabbit cartilage and down-regulate matrix metalloproteinases in rabbit chondrocytes. Phytother. Res. 2011, 25, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.F.; Chen, W.P.; Tang, J.L.; Bao, J.P.; Wu, L.D. Protective effects of berberine in an experimental rat osteoarthritis model. Phytother. Res. 2011, 25, 878–885. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Xia, C.; Shi, L.; Wang, S.; Zheng, X.; Hu, T.; Zhang, B. Berberine ameliorates cartilage degeneration in interleukin-1β-stimulated rat chondrocytes and in a rat model of osteoarthritis via Akt signalling. J. Cell Mol. Med. 2014, 18, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.Q.; Yu, L.; He, B.; Wu, S.H.; Zhao, Q.; Xia, S.Q.; Mei, H.J. Berberine prevents nitric oxide-induced rat chondrocyte apoptosis and cartilage degeneration in a rat osteoarthritis model via AMPK and p38 MAPK signaling. Apoptosis 2015, 20, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, H.; Li, Y.; Deng, M.; He, B.; Xia, S.; Zhang, C.; Liu, S. Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur. J. Pharmacol. 2016, 789, 109–118. [Google Scholar] [CrossRef]
- Li, X.; He, P.; Hou, Y.; Chen, S.; Xiao, Z.; Zhan, J.; Luo, D.; Gu, M.; Lin, D. Berberine inhibits the interleukin-1 beta-induced inflammatory response via MAPK downregulation in rat articular chondrocytes. Drug Dev. Res. 2019, 80, 637–645. [Google Scholar] [CrossRef]
- Feng, K.; Chen, H.; Xu, C. Chondro-protective effects of celastrol on osteoarthritis through autophagy activation and NF-κB signaling pathway inhibition. Inflamm. Res. 2020, 69, 385–400. [Google Scholar] [CrossRef]
- Wang, W.; Ha, C.; Lin, T.; Wang, D.; Wang, Y.; Gong, M. Celastrol attenuates pain and cartilage damage via SDF-1/CXCR4 signalling pathway in osteoarthritis rats. J. Pharm. Pharmacol. 2018, 70, 81–88. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, B.L.; Yang, J.B.; Zhou, K. Celastrol ameliorates endoplasmic stress-mediated apoptosis of osteoarthritis via regulating ATF-6/CHOP signalling pathway. J. Pharm. Pharmacol. 2020, 72, 826–835. [Google Scholar] [CrossRef]
- Jin, T.; Wu, D.; Liu, X.M.; Xu, J.T.; Ma, B.J.; Ji, Y.; Jin, Y.Y.; Wu, S.Y.; Wu, T.; Ma, K. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J. Nanobiotechnology 2020, 18, 94. [Google Scholar] [CrossRef]
- Kang, S.; Siddiqi, M.H.; Yoon, S.J.; Ahn, S.; Noh, H.Y.; Kumar, N.S.; Kim, Y.J.; Yang, D.C. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: An in silico and in vitro study. In Vitro Cell Dev. Biol. Anim. 2016, 52, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, C.; Hua, L.; Xue, S.; Yu, D.; Zhang, C.; Wang, D. Crocin Attenuates Joint Pain and Muscle Dysfunction in Osteoarthritis Rat. Inflammation 2017, 40, 2086–2093. [Google Scholar] [CrossRef]
- Ding, Q.; Zhong, H.; Qi, Y.; Cheng, Y.; Li, W.; Yan, S.; Wang, X. Anti-arthritic effects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm. Res. 2013, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Mobasheri, A.; Shakibaei, M.; Allaway, D.; Harris, P. Interleukin-1beta-induced extracellular matrix degradation and glycosaminoglycan release is inhibited by curcumin in an explant model of cartilage inflammation. Ann. N. Y. Acad. Sci. 2009, 1171, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Clutterbuck, A.L.; Allaway, D.; Harris, P.; Mobasheri, A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1β-treated articular cartilage. F1000Res 2013, 2, 147. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Gu, J.H.; Wang, F.Y.; Shang, X.S.; Tao, H.R.; Wang, X. Regulation of type II collagen, matrix metalloproteinase-13 and cell proliferation by interleukin-1β is mediated by curcumin via inhibition of NF-κB signaling in rat chondrocytes. Mol. Med. Rep. 2017, 16, 1837–1845. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34, e201900604. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, H.J.; Lee, D.R.; Choi, B.K.; Yang, S.H. Herbal Composition LI73014F2 Alleviates Articular Cartilage Damage and Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Molecules 2020, 25, 5467. [Google Scholar] [CrossRef]
- Ding, Q.H.; Ye, C.Y.; Chen, E.M.; Zhang, W.; Wang, X.H. Emodin ameliorates cartilage degradation in osteoarthritis by inhibiting NF-κB and Wnt/β-catenin signaling in-vitro and in-vivo. Int. Immunopharmacol. 2018, 61, 222–230. [Google Scholar] [CrossRef]
- Liu, Z.; Lang, Y.; Li, L.; Liang, Z.; Deng, Y.; Fang, R.; Meng, Q. Effect of emodin on chondrocyte viability in an. Exp. Ther. Med. 2018, 16, 5384–5389. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Song, X.; Li, Y.; Ma, T.; Bai, H.; Zhao, M.; Wang, X.; Liu, L.; Gao, L. Emodin protects knee joint cartilage in rats through anti-matrix degradation pathway: An in vitro and in vivo study. Life Sci. 2021, 269, 119001. [Google Scholar] [CrossRef] [PubMed]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019, 142, 1–8. [Google Scholar] [CrossRef]
- Wann, A.K.; Mistry, J.; Blain, E.J.; Michael-Titus, A.T.; Knight, M.M. Eicosapentaenoic acid and docosahexaenoic acid reduce interleukin-1β-mediated cartilage degradation. Arthritis Res. Ther. 2010, 12, R207. [Google Scholar] [CrossRef]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef]
- Chen, Y.; Shou, K.; Gong, C.; Yang, H.; Yang, Y.; Bao, T. Anti-Inflammatory Effect of Geniposide on Osteoarthritis by Suppressing the Activation of p38 MAPK Signaling Pathway. Biomed. Res. Int. 2018, 2018, 8384576. [Google Scholar] [CrossRef]
- Pan, T.; Shi, X.; Chen, H.; Chen, R.; Wu, D.; Lin, Z.; Zhang, J.; Pan, J. Geniposide Suppresses Interleukin-1β-Induced Inflammation and Apoptosis in Rat Chondrocytes via the PI3K/Akt/NF-κB Signaling Pathway. Inflammation 2018, 41, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Bao, T.Z.; Chen, K.; Zhu, C.M.; Wan, F.; Tan, Y.L.; Yan, F. Effects of geniposide on SNP-induced apoptosis of chondrocyte and cell cycle. Zhongguo Gu Shang 2013, 26, 232–235. [Google Scholar]
- Yuan, J.; Ding, W.; Wu, N.; Jiang, S.; Li, W. Protective Effect of Genistein on Condylar Cartilage through Downregulating NF-. Biomed. Res. Int. 2019, 2019, 2629791. [Google Scholar] [CrossRef]
- Cui, Z.; Crane, J.; Xie, H.; Jin, X.; Zhen, G.; Li, C.; Xie, L.; Wang, L.; Bian, Q.; Qiu, T.; et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann. Rheum. Dis. 2016, 75, 1714–1721. [Google Scholar] [CrossRef]
- Mu, W.; Xu, B.; Ma, H.; Li, J.; Ji, B.; Zhang, Z.; Amat, A.; Cao, L. Halofuginone Attenuates Osteoarthritis by Rescuing Bone Remodeling in Subchondral Bone Through Oral Gavage. Front. Pharmacol. 2018, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Xu, B.; Ma, H.; Ji, B.; Zhang, Z.; Li, J.; Amat, A.; Cao, L. Halofuginone attenuates articular cartilage degeneration by inhibition of elevated TGF-β1 signaling in articular cartilage in a rodent osteoarthritis model. Mol. Med. Rep. 2017, 16, 7679–7684. [Google Scholar] [CrossRef]
- Chrubasik, J.E.; Lindhorst, E.; Neumann, E.; Gerlach, U.; Faller-Marquardt, M.; Torda, T.; Müller-Ladner, U.; Chrubasik, S. Potential molecular basis of the chondroprotective effect of Harpagophytum procumbens. Phytomedicine 2006, 13, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef]
- Takuma, M.; Haruka, K.; Mutsuto, W.; Toshiki, M.; Kenshiro, M.; Akane, T.; Hiroshi, M.; Yoshihiro, N. Olive leaf extract prevents cartilage degeneration in osteoarthritis of STR/ort mice. Biosci. Biotechnol. Biochem. 2018, 82, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Mével, E.; Merceron, C.; Vinatier, C.; Krisa, S.; Richard, T.; Masson, M.; Lesoeur, J.; Hivernaud, V.; Gauthier, O.; Abadie, J.; et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci. Rep. 2016, 6, 33527. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Xiang, W.; Guo, J.; Yao, X.; Liang, S.; Guo, F.; Xu, T. Hyperoside ameliorates the progression of osteoarthritis: An in vitro and in vivo study. Phytomedicine 2021, 80, 153387. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Liu, D.; Jiao, F. Icariin accelerates cartilage defect repair by promoting chondrogenic differentiation of BMSCs under conditions of oxygen-glucose deprivation. J. Cell Mol. Med. 2022, 26, 202–215. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, J.; Wang, R.; Wang, L.; Wang, S.; Wu, Y.; Dong, Y.; Guo, F.; Xu, T. Role of IFT88 in icariin-regulated maintenance of the chondrocyte phenotype. Mol. Med. Rep. 2018, 17, 4999–5006. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.F.; Qin, F.W.; Tang, W.; Liu, D.H.; Wu, P.Y.; Jiao, F. Icariin inhibits chondrocyte apoptosis and angiogenesis by regulating the TDP-43 signaling pathway. Mol. Genet. Genomic Med. 2019, 7, e00586. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B.; Lv, H.; Liu, J.; Xiong, Y.; Hu, L.; Xue, H.; Panayi, A.C.; Liu, G.; Zhou, W. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J. Cell Biochem. 2018, 120, 7741–7750. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tang, W.; Zhang, H.; Huang, H.; Zhang, Z.; Tang, D.; Jiao, F. Icariin protects rabbit BMSCs against OGD-induced apoptosis by inhibiting ERs-mediated autophagy via MAPK signaling pathway. Life Sci. 2020, 253, 117730. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.P.; Hu, Z.N.; Jin, L.B.; Wu, L.D. Licochalcone A Inhibits MMPs and ADAMTSs via the NF-κB and Wnt/β-Catenin Signaling Pathways in Rat Chondrocytes. Cell Physiol. Biochem. 2017, 43, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Qi, W.; Zhan, J.; Lin, Z.; Lin, J.; Xue, X.; Pan, X.; Zhou, Y. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J. Cell Mol. Med. 2020, 24, 13046–13057. [Google Scholar] [CrossRef]
- Hu, S.; Wang, S.; He, J.; Bian, Y. Tetramethylpyrazine alleviates endoplasmic reticulum stress-activated apoptosis and related inflammation in chondrocytes. Mol. Med. Rep. 2022, 25, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Y.; Zhang, Z.; Yang, Z.; Huang, G. Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis. Asian J. Pharm. Sci. 2018, 13, 229–238. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, Y.; Li, C.; Yang, K.; Sun, T.; Xu, L.; Chen, Y.; Yan, C.H.; Lu, W.W.; Chiu, K.Y. Magnoflorine with hyaluronic acid gel promotes subchondral bone regeneration and attenuates cartilage degeneration in early osteoarthritis. Bone 2018, 116, 266–278. [Google Scholar] [CrossRef]
- Cai, Z.; Hong, M.; Xu, L.; Yang, K.; Li, C.; Sun, T.; Feng, Y.; Zeng, H.; Lu, W.W.; Chiu, K.Y. Prevent action of magnoflorine with hyaluronic acid gel from cartilage degeneration in anterior cruciate ligament transection induced osteoarthritis. Biomed. Pharmacother. 2020, 126, 109733. [Google Scholar] [CrossRef]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B.; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B.; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef]
- Aini, H.; Ochi, H.; Iwata, M.; Okawa, A.; Koga, D.; Okazaki, M.; Sano, A.; Asou, Y. Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model. PLoS ONE 2012, 7, e37728. [Google Scholar] [CrossRef] [PubMed]
- Masuda, I.; Koike, M.; Nakashima, S.; Mizutani, Y.; Ozawa, Y.; Watanabe, K.; Sawada, Y.; Sugiyama, H.; Sugimoto, A.; Nojiri, H.; et al. Apple procyanidins promote mitochondrial biogenesis and proteoglycan biosynthesis in chondrocytes. Sci. Rep. 2018, 8, 7229. [Google Scholar] [CrossRef]
- Ma, T.W.; Wen, Y.J.; Song, X.P.; Hu, H.L.; Li, Y.; Bai, H.; Zhao, M.C.; Gao, L. Puerarin inhibits the development of osteoarthritis through antiinflammatory and antimatrix-degrading pathways in osteoarthritis-induced rat model. Phytother. Res. 2020, 35, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, C.; Sun, H.; Hong, H.; Jin, J.; Bei, C.; Lu, Z.; Zhang, X. Puerarin suppresses inflammation and ECM degradation through Nrf2/HO-1 axis in chondrocytes and alleviates pain symptom in osteoarthritic mice. Food Funct. 2021, 12, 2075–2089. [Google Scholar] [CrossRef]
- Wang, L.; Shan, H.; Wang, B.; Wang, N.; Zhou, Z.; Pan, C.; Wang, F. Puerarin Attenuates Osteoarthritis via Upregulating AMP-Activated Protein Kinase/Proliferator-Activated Receptor-γ Coactivator-1 Signaling Pathway in Osteoarthritis Rats. Pharmacology 2018, 102, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Zhang, Y.; Tang, L.; Ji, Y.; Yan, C.; Zhang, X. Protective effects of quercetin against inflammation and oxidative stress in a rabbit model of knee osteoarthritis. Drug Dev. Res. 2019, 80, 360–367. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Zhao, D.; Wang, C.; Zhang, Y.; Wang, Y.; Li, T. Therapeutic effect and mechanism of action of quercetin in a rat model of osteoarthritis. J. Int. Med. Res. 2020, 48, 300060519873461. [Google Scholar] [CrossRef]
- Permatasari, D.A.; Karliana, D.; Iskandarsyah, I.; Arsianti, A.; Bahtiar, A. Quercetin prevent proteoglycan destruction by inhibits matrix metalloproteinase-9, matrix metalloproteinase-13, a disintegrin and metalloproteinase with thrombospondin motifs-5 expressions on osteoarthritis model rats. J. Adv. Pharm. Technol. Res. 2019, 10, 2–8. [Google Scholar] [CrossRef]
- Britti, D.; Crupi, R.; Impellizzeri, D.; Gugliandolo, E.; Fusco, R.; Schievano, C.; Morittu, V.M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. A novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models. BMC Vet. Res. 2017, 13, 229. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef]
- Elmali, N.; Esenkaya, I.; Harma, A.; Ertem, K.; Turkoz, Y.; Mizrak, B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 2005, 54, 158–162. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018, 17, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral Resveratrol Prevents Osteoarthritis Progression in C57BL/6J Mice Fed a High-Fat Diet. Nutrients 2016, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Sanchez, C.; Membrez Scalfo, F.; Drion, P.; Comblain, F.; Taralla, S.; Donneau, A.F.; Offord, E.A.; Henrotin, Y. Oleuropein or rutin consumption decreases the spontaneous development of osteoarthritis in the Hartley guinea pig. Osteoarthr. Cartil. 2015, 23, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Huang, Y.; Santoso, M.B.; Wu, L.D. Sclareol exerts anti-osteoarthritic activities in interleukin-1β-induced rabbit chondrocytes and a rabbit osteoarthritis model. Int. J. Clin. Exp. Pathol. 2015, 8, 2365–2374. [Google Scholar]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef]
- Li, F.; Yin, Z.; Zhou, B.; Xue, F.; Yang, W.; Chang, R.; Ma, K.; Qiu, Y. Shikonin inhibits inflammatory responses in rabbit chondrocytes and shows chondroprotection in osteoarthritic rabbit knee. Int. Immunopharmacol. 2015, 29, 656–662. [Google Scholar] [CrossRef]
- Wang, L.; Gai, P.; Xu, R.; Zheng, Y.; Lv, S.; Li, Y.; Liu, S. Shikonin protects chondrocytes from interleukin-1beta-induced apoptosis by regulating PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 298–308. [Google Scholar]
- Ju, X.D.; Deng, M.; Ao, Y.F.; Yu, C.L.; Wang, J.Q.; Yu, J.K.; Cui, G.Q.; Hu, Y.L. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi 2010, 130, 1053–1060. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Yan, Z.; Wang, Z.; Fu, X.; Yu, K. Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-κB signaling pathways. Int. Immunopharmacol. 2019, 75, 105715. [Google Scholar] [CrossRef]
- Javaheri, B.; Poulet, B.; Aljazzar, A.; de Souza, R.; Piles, M.; Hopkinson, M.; Shervill, E.; Pollard, A.; Chan, B.; Chang, Y.M.; et al. Stable sulforaphane protects against gait anomalies and modifies bone microarchitecture in the spontaneous STR/Ort model of osteoarthritis. Bone 2017, 103, 308–317. [Google Scholar] [CrossRef]
- Sudirman, S.; Chen, C.K.; Long, B.T.; Chang, H.W.; Tsou, D.; Kong, Z.L. Nut Triterpene-Rich Extract Ameliorates Symptoms of Inflammation on Post-Traumatic Osteoarthritis in Obese Rats. J. Pain. Res. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Lee, H.J.; Lee, D.Y.; Jo, H.S.; Jeong, J.H.; Kim, D.H.; Nam, D.C.; Lee, C.J.; Hwang, S.C. Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo. Biomol. Ther. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Smith, J.F.; Starr, E.G.; Goodman, M.A.; Hanson, R.B.; Palmer, T.A.; Woolstenhulme, J.B.; Weyand, J.A.; Marchant, A.D.; Bueckers, S.L.; Nelson, T.K.; et al. Topical Application of Wogonin Provides a Novel Treatment of Knee Osteoarthritis. Front. Physiol. 2020, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Bhawal, S.; Kapila, S.; Yadav, H.; Kapila, R. Health-promoting role of dietary bioactive compounds through epigenetic modulations: A novel prophylactic and therapeutic approach. Crit. Rev. Food Sci. Nutr. 2022, 62, 619–639. [Google Scholar] [CrossRef]
- Vahid, F.; Zand, H.; Nosrat-Mirshekarlou, E.; Najafi, R.; Hekmatdoost, A. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: A review. Gene 2015, 562, 8–15. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Developmental programming of health and disease. Proc. Nutr. Soc. 2006, 65, 97–105. [Google Scholar] [CrossRef]
- Chmurzynska, A. Fetal programming: Link between early nutrition, DNA methylation, and complex diseases. Nutr. Rev. 2010, 68, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Maleszka, J.; Foret, S.; Maleszka, R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 2008, 319, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental origins of disease and determinants of chromatin structure: Maternal diet modifies the primate fetal epigenome. J. Mol. Endocrinol. 2008, 41, 91–102. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef]
- Remely, M.; Lovrecic, L.; de la Garza, A.L.; Migliore, L.; Peterlin, B.; Milagro, F.I.; Martinez, A.J.; Haslberger, A.G. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol. 2015, 172, 2756–2768. [Google Scholar] [CrossRef]
- Montgomery, M.; Srinivasan, A. Epigenetic Gene Regulation by Dietary Compounds in Cancer Prevention. Adv. Nutr. 2019, 10, 1012–1028. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Chen, D.; Yang, C.S. Dietary polyphenols may affect DNA methylation. J. Nutr. 2007, 137, 223S–228S. [Google Scholar] [CrossRef] [PubMed]
- Cameron, E.E.; Bachman, K.E.; Myöhänen, S.; Herman, J.G.; Baylin, S.B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999, 21, 103–107. [Google Scholar] [CrossRef]
- Shankar, S.; Kumar, D.; Srivastava, R.K. Epigenetic modifications by dietary phytochemicals: Implications for personalized nutrition. Pharmacol. Ther. 2013, 138, 1–17. [Google Scholar] [CrossRef]
- Link, A.; Balaguer, F.; Goel, A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem. Pharmacol. 2010, 80, 1771–1792. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Sun, Y.; Wen, J.; Zhou, Q.; Ding, X.; Zhang, X. Correlation analysis of differentially expressed long non-coding RNA HOTAIR with PTEN/PI3K/AKT pathway and inflammation in patients with osteoarthritis and the effect of baicalin intervention. J. Orthop. Surg. Res. 2023, 18, 34. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Liu, J. Baicalin Protects Human OA Chondrocytes Against IL-1β-Induced Apoptosis and ECM Degradation by Activating Autophagy via MiR-766-3p/AIFM1 Axis. Drug Des. Devel Ther. 2020, 14, 2645–2655. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Gao, Z.; Yu, C.; Zhang, L. Baicalin alleviates IL-1β-induced inflammatory injury via down-regulating miR-126 in chondrocytes. Biomed. Pharmacother. 2018, 99, 184–190. [Google Scholar] [CrossRef]
- Ji, Q.; Qi, D.; Xu, X.; Xu, Y.; Goodman, S.B.; Kang, L.; Song, Q.; Fan, Z.; Maloney, W.J.; Wang, Y. Cryptotanshinone Protects Cartilage against Developing Osteoarthritis through the miR-106a-5p/GLIS3 Axis. Mol. Ther. Nucleic Acids 2018, 11, 170–179. [Google Scholar] [CrossRef]
- Yang, D.; Cao, G.; Ba, X.; Jiang, H. Epigallocatechin-3-O-Gallate promotes extracellular matrix and inhibits inflammation in IL-1B stimulated chondrocytes by the PTEN/miRNA-29b pathway. Pharm. Biol. 2022, 60, 589–599. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shaya, O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: Potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur. J. Nutr. 2018, 57, 917–928. [Google Scholar] [CrossRef]
- Rasheed, Z.; Rasheed, N.; Al-Shobaili, H.A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell Mol. Med. 2016, 20, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Chen, H.; Wu, Y. Fisetin inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents chondrocyte death under oxidative stress by inducing autophagy through sirtuin 1-dependent and -independent mechanisms. Biochim. Biophys. Acta 2016, 1860, 1181–1191. [Google Scholar] [CrossRef]
- Facchini, A.; Cetrullo, S.; D’Adamo, S.; Guidotti, S.; Minguzzi, M.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene α. PLoS ONE 2014, 9, e109724. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Borzì, R.M.; Flamigni, F. Hydroxytyrosol modulates the levels of microRNA-9 and its target sirtuin-1 thereby counteracting oxidative stress-induced chondrocyte death. Osteoarthr. Cartil. 2017, 25, 600–610. [Google Scholar] [CrossRef]
- D’Adamo, S.; Cetrullo, S.; Borzì, R.M.; Flamigni, F. Effect of oxidative stress and 3-hydroxytyrosol on DNA methylation levels of miR-9 promoters. J. Cell Mol. Med. 2019, 23, 7885–7889. [Google Scholar] [CrossRef]
- Bao, J.; Yan, W.; Xu, K.; Chen, M.; Chen, Z.; Ran, J.; Xiong, Y.; Wu, L. Oleanolic Acid Decreases IL-1. Oxid. Med. Cell Longev. 2020, 2020, 7517219. [Google Scholar] [CrossRef]
- Li, Y.; Nie, J.; Jiang, P. Oleanolic acid mitigates interleukin-1β-induced chondrocyte dysfunction by regulating miR-148-3p-modulated FGF2 expression. J. Gene Med. 2020, 22, e3169. [Google Scholar] [CrossRef]
- Dong, S.; Xu, G.; Li, X.; Guo, S.; Bai, J.; Zhao, J.; Chen, L. Exosomes Derived from Quercetin-Treated Bone Marrow Derived Mesenchymal Stem Cells Inhibit the Progression of Osteoarthritis Through Delivering miR-124-3p to Chondrocytes. DNA Cell Biol. 2024, 43, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xing, H.; Wang, C.; Xu, X.; Hao, Y.; Qiu, B. Resveratrol Improves the Progression of Osteoarthritis by Regulating the SIRT1-FoxO1 Pathway-Mediated Cholesterol Metabolism. Mediators Inflamm. 2023, 2023, 2936236. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, W.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Identification and validation of key long non-coding RNAs in resveratrol protect against IL-1β-treated chondrocytes via integrated bioinformatic analysis. J. Orthop. Surg. Res. 2021, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Hu, B.; Zhang, M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp. Ther. Med. 2017, 14, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- Abed, É.; Delalandre, A.; Lajeunesse, D. Beneficial effect of resveratrol on phenotypic features and activity of osteoarthritic osteoblasts. Arthritis Res. Ther. 2017, 19, 151. [Google Scholar] [CrossRef]
- Yue, S.; Su, X.; Teng, J.; Wang, J.; Guo, M. Cryptotanshinone interferes with chondrocyte apoptosis in osteoarthritis by inhibiting the expression of miR-574-5p. Mol. Med. Rep. 2021, 23, 424. [Google Scholar] [CrossRef]
- Ye, H.; Long, Y.; Yang, J.M.; Wu, Y.L.; Dong, L.Y.; Zhong, Y.B.; Luo, Y.; Wang, M.Y. Curcumin regulates autophagy through SIRT3-SOD2-ROS signaling pathway to improve quadriceps femoris muscle atrophy in KOA rat model. Sci. Rep. 2024, 14, 8176. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-eIF2. Oxid. Med. Cell Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhou, J.; Lei, Z.; Yang, X. Fisetin suppresses chondrocyte senescence and attenuates osteoarthritis progression by targeting sirtuin 6. Chem. Biol. Interact. 2024, 390, 110890. [Google Scholar] [CrossRef]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef]
- Ruan, H.; Zhu, T.; Wang, T.; Guo, Y.; Liu, Y.; Zheng, J. Quercetin Modulates Ferroptosis via the SIRT1/Nrf-2/HO-1 Pathway and Attenuates Cartilage Destruction in an Osteoarthritis Rat Model. Int. J. Mol. Sci. 2024, 25, 7461. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Luo, Y.; Chen, X. Quercetin attenuates mitochondrial dysfunction and biogenesis via upregulated AMPK/SIRT1 signaling pathway in OA rats. Biomed. Pharmacother. 2018, 103, 1585–1591. [Google Scholar] [CrossRef]
- Liang, C.; Xing, H.; Wang, C.; Xu, X.; Hao, Y.; Qiu, B. Resveratrol protection against IL-1β-induced chondrocyte damage via the SIRT1/FOXO1 signaling pathway. J. Orthop. Surg. Res. 2022, 17, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; You, W.; Tang, X.; Li, X.; Gong, Z. Therapeutic effect of Resveratrol in the treatment of osteoarthritis via the MALAT1/miR-9/NF-κB signaling pathway. Exp. Ther. Med. 2020, 19, 2343–2352. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, J.G.; Xiao, D.M.; Fan, M.; Wang, D.P.; Xiong, J.Y.; Chen, Y.; Ding, Y.; Liu, S.L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Yan, M.; Zhang, D.; Yang, M. Saikosaponin D alleviates inflammatory response of osteoarthritis and mediates autophagy via elevating microRNA-199-3p to target transcription Factor-4. J. Orthop. Surg. Res. 2024, 19, 151. [Google Scholar] [CrossRef]
- Dong, H.C.; Li, P.N.; Chen, C.J.; Xu, X.; Zhang, H.; Liu, G.; Zheng, L.J.; Li, P. Sinomenine Attenuates Cartilage Degeneration by Regulating miR-223-3p/NLRP3 Inflammasome Signaling. Inflammation 2019, 42, 1265–1275. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Liang, W.; Zeng, J.; Shao, X.; Xu, L.; Jia, L.; He, X.; Li, H.; Zheng, C.; et al. Tougu Xiaotong capsules may inhibit p38 MAPK pathway-mediated inflammation: In vivo and in vitro verification. J. Ethnopharmacol. 2020, 249, 112390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).