Phenylethanol Glycoside from Cistanche tubulosa Attenuates BSA-Induced Liver Fibrosis in Rats by Modulating the Gut Microbiota–Liver Axis
Abstract
1. Introduction
2. Results
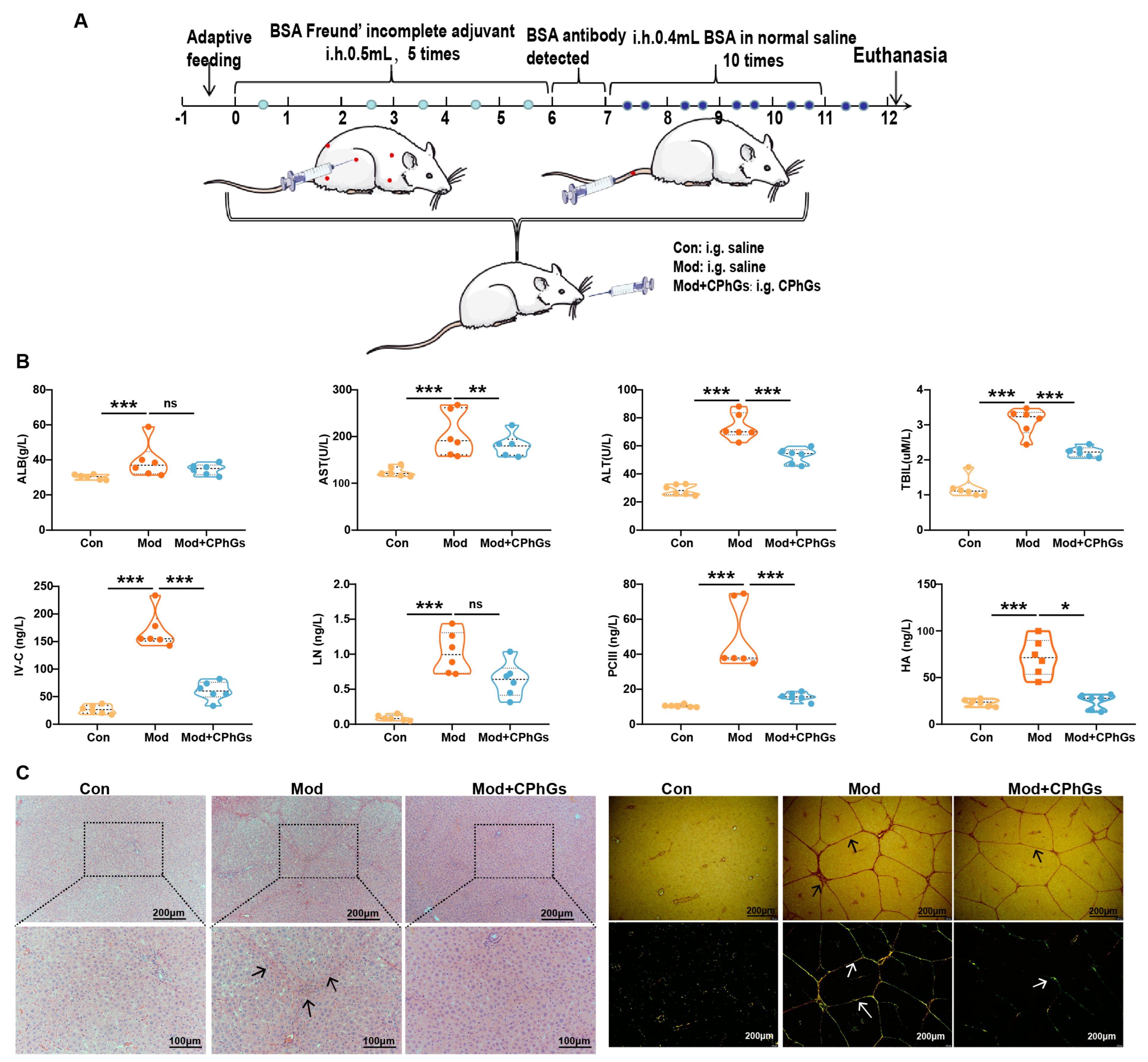
2.1. CPhGs Enhances Liver Function and Alleviates BSA-Induced Hepatic Fibrosis in Rats
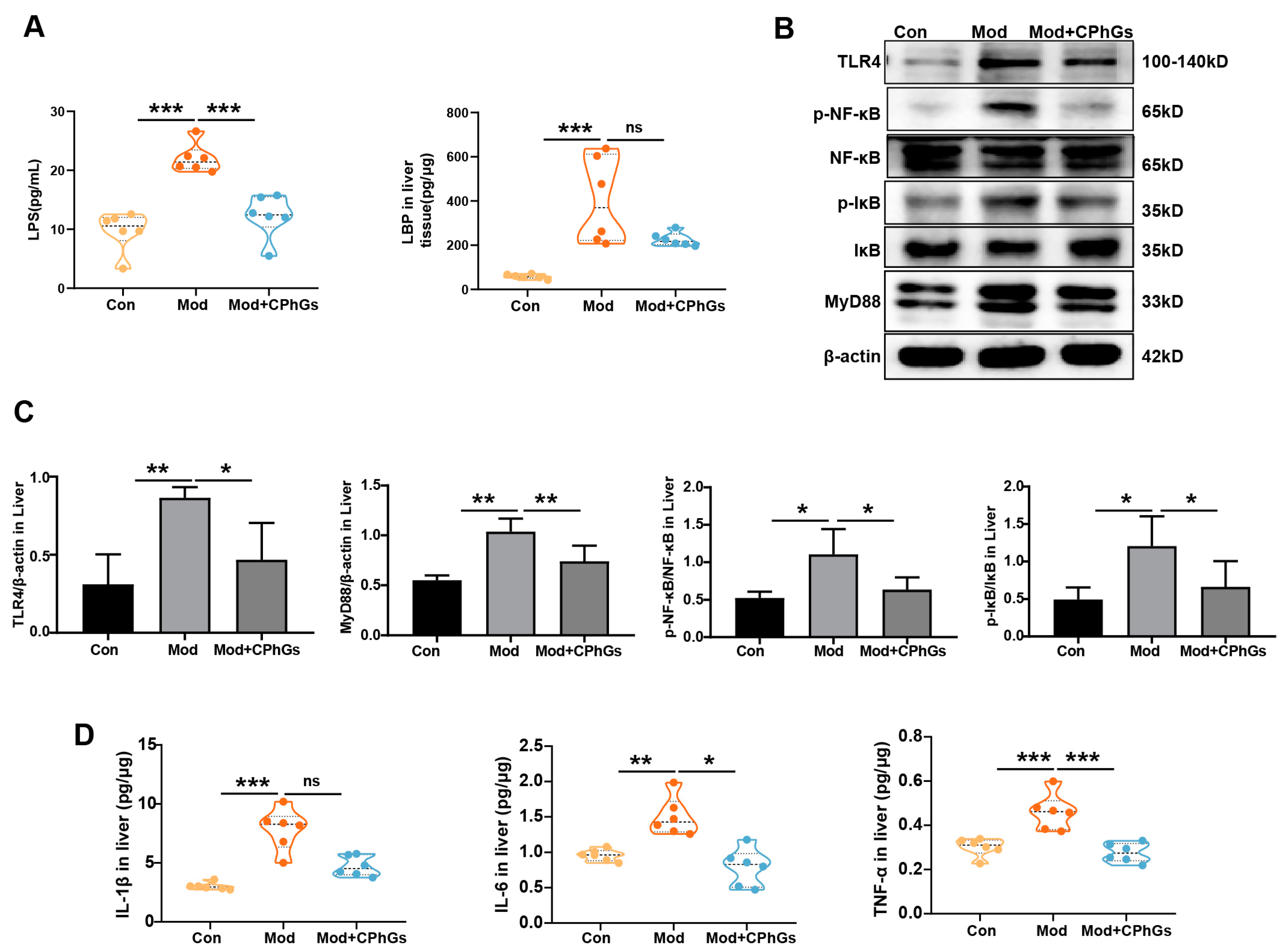
2.2. CPhGs Improves Abnormal Inflammatory Responses via the TLR4/NF-κB Signaling Pathway
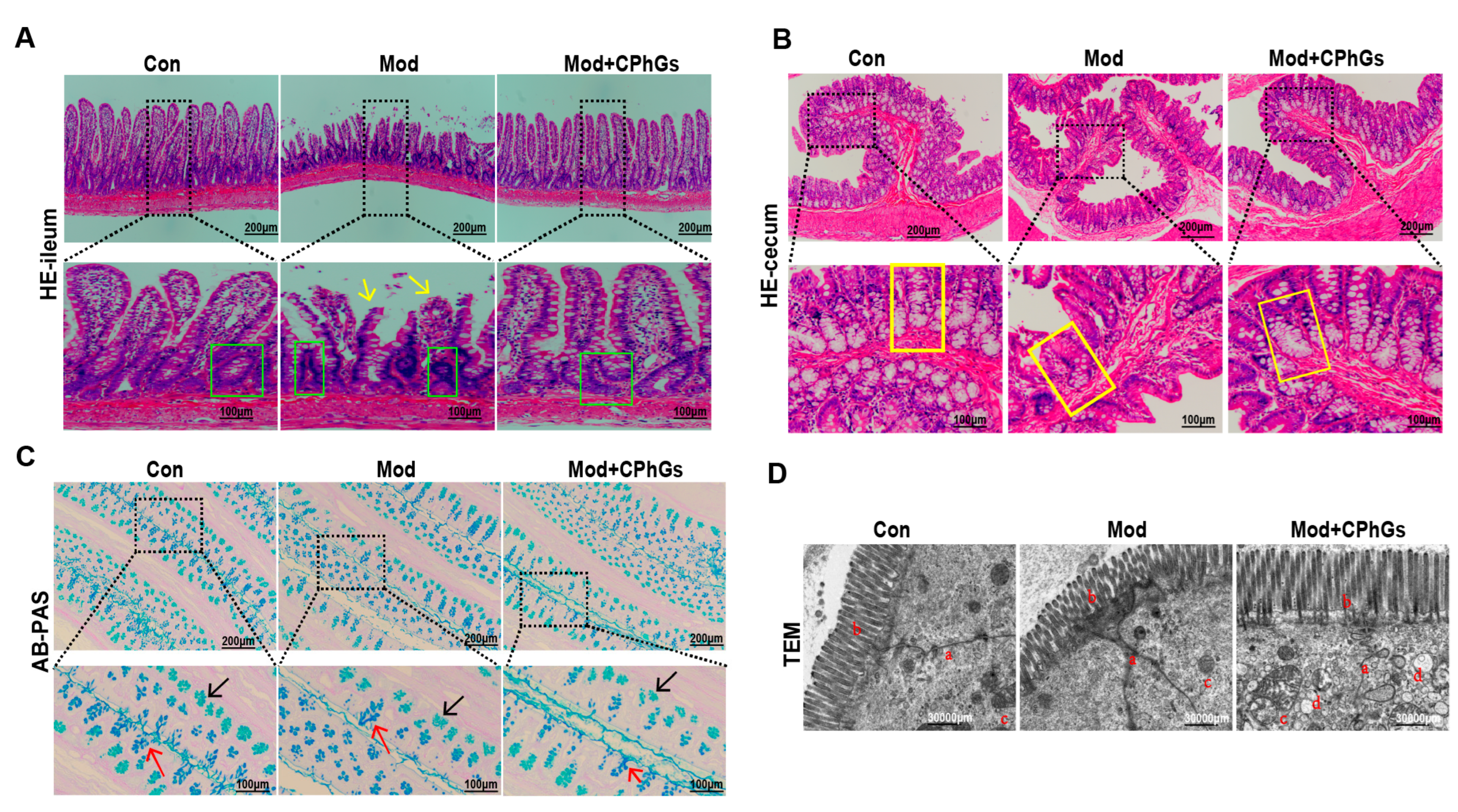
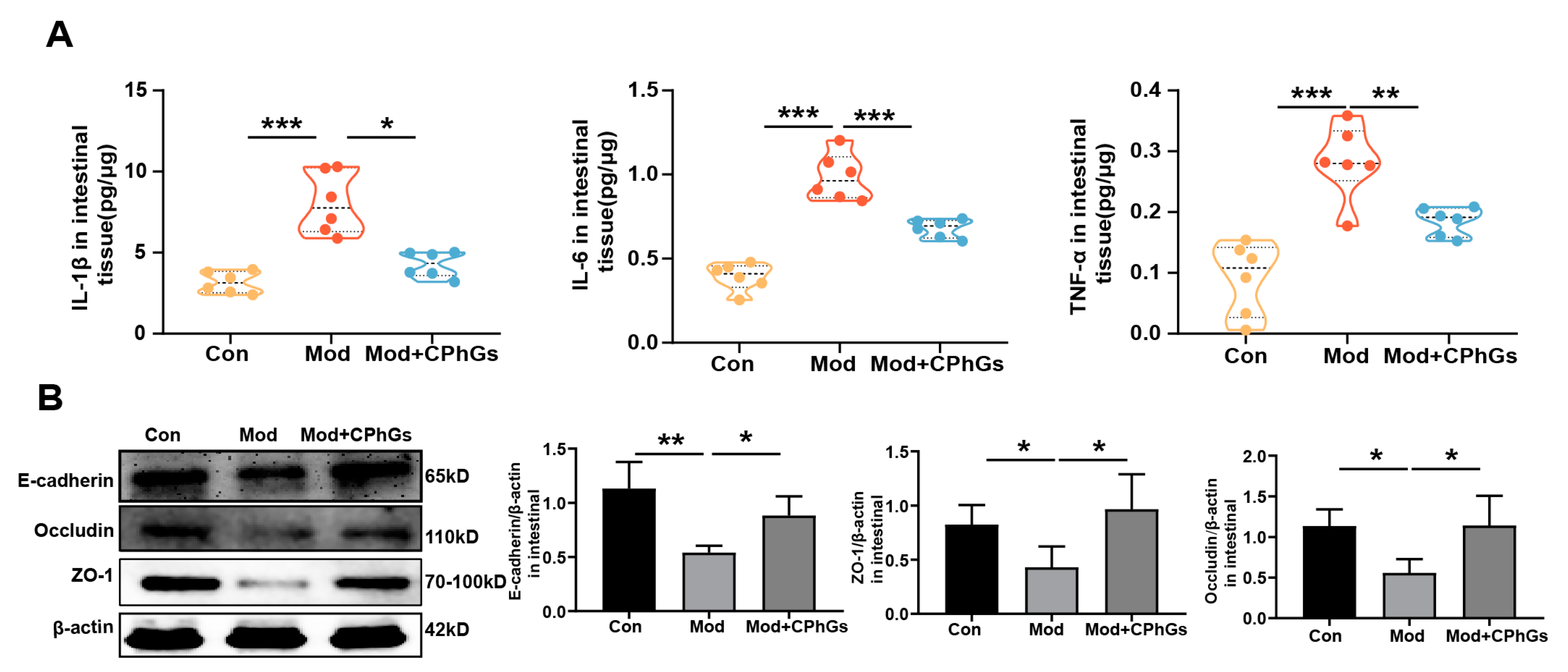
2.3. CPhGs Attenuates Gut Barrier Injury
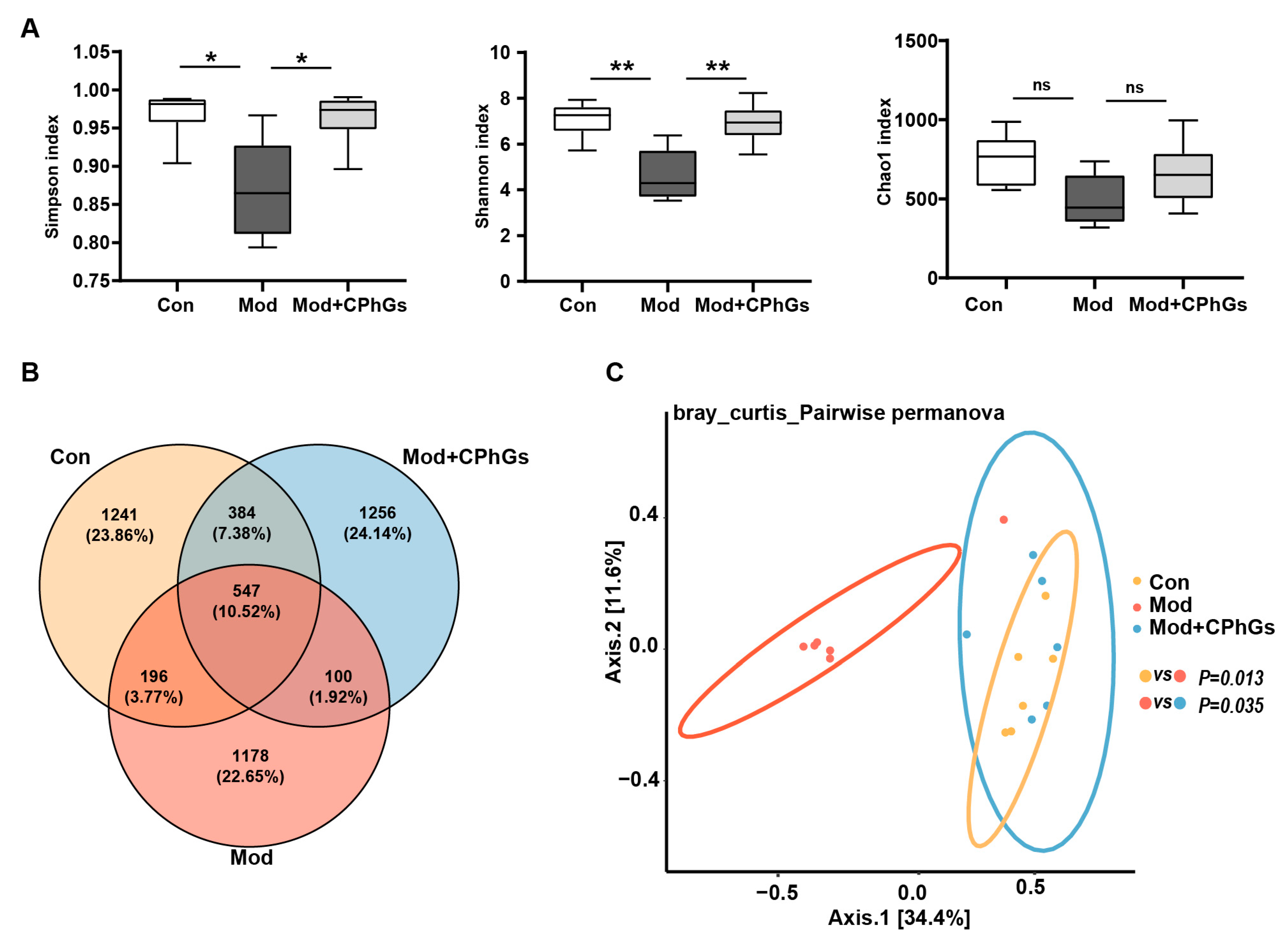
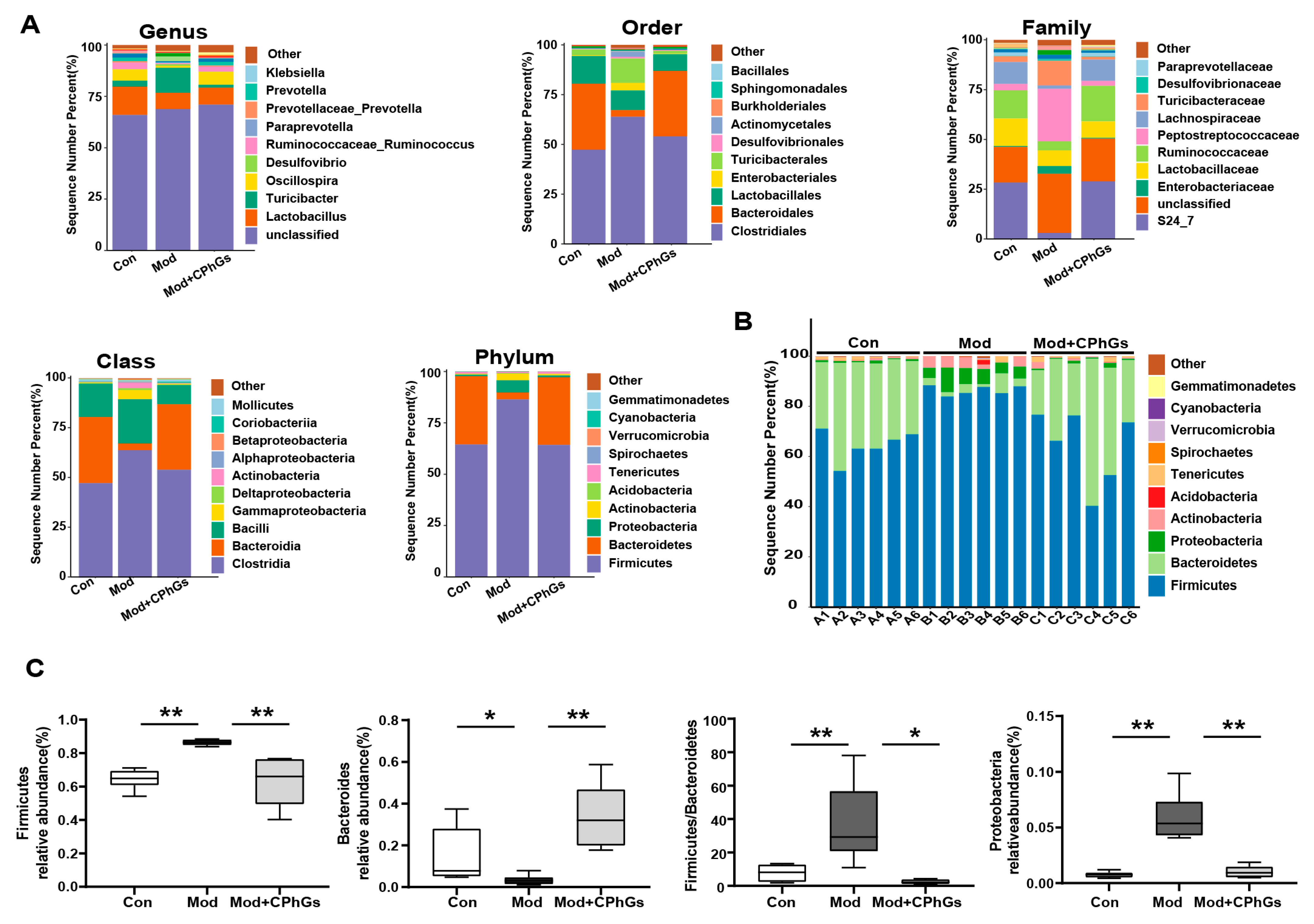
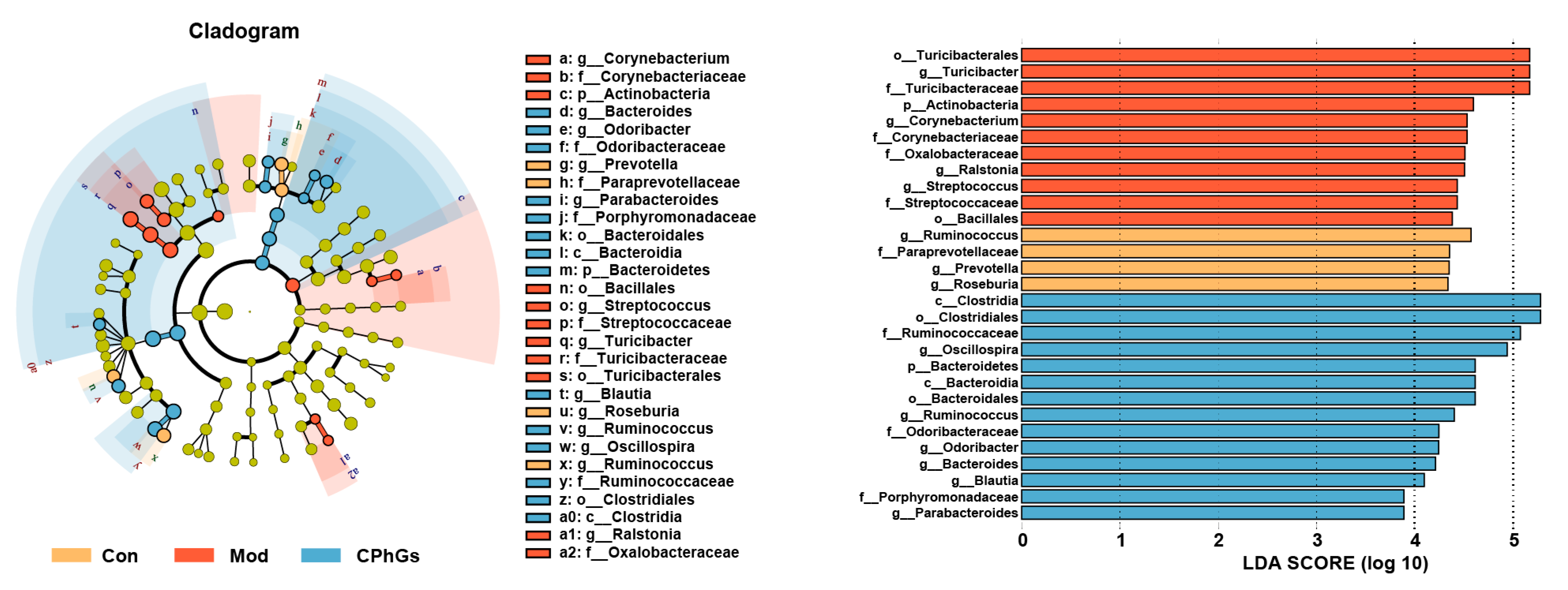
2.4. Gut Microbiota Analysis
2.5. Preventive Effect of CPhGs on Liver Fibrosis
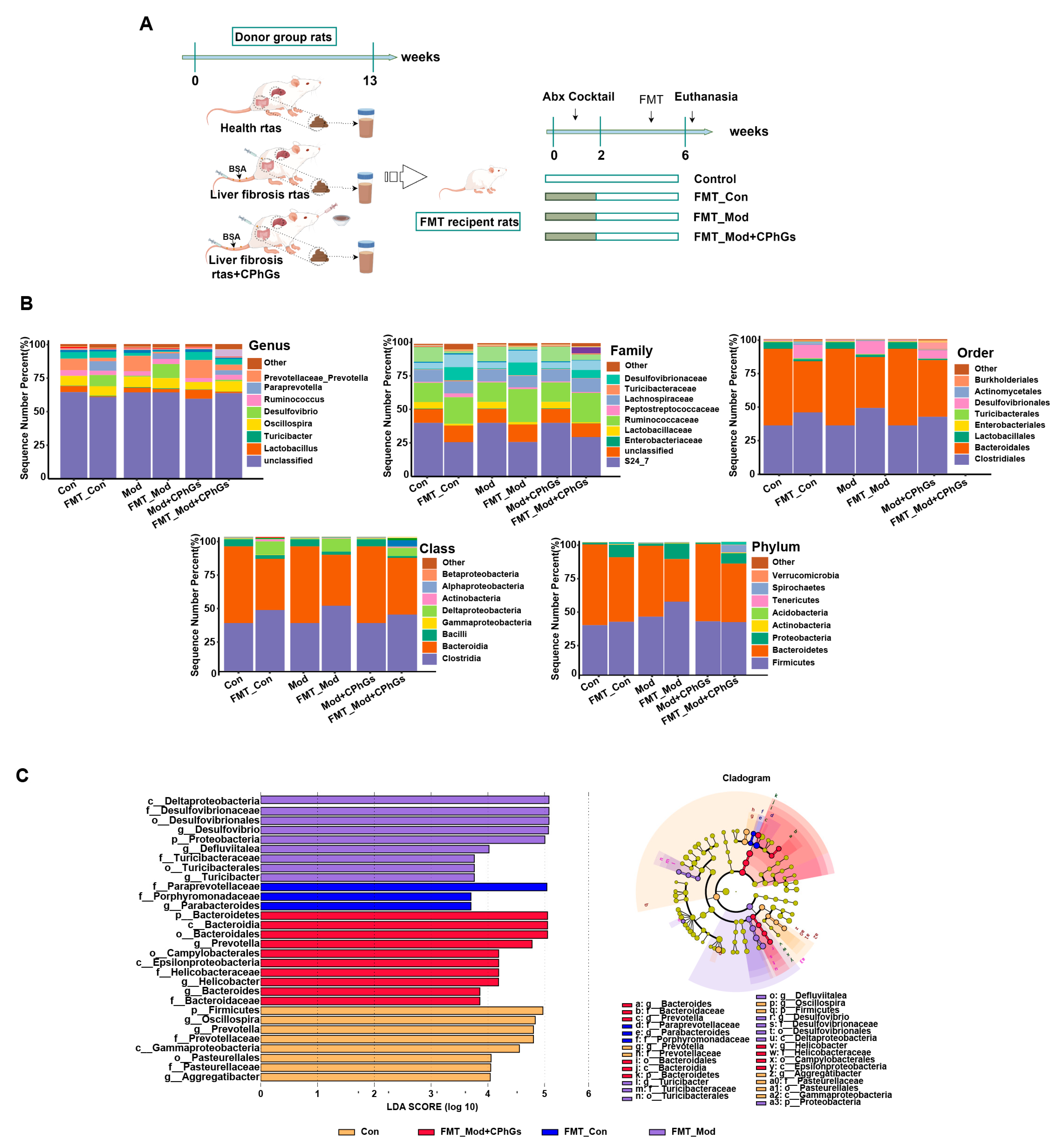
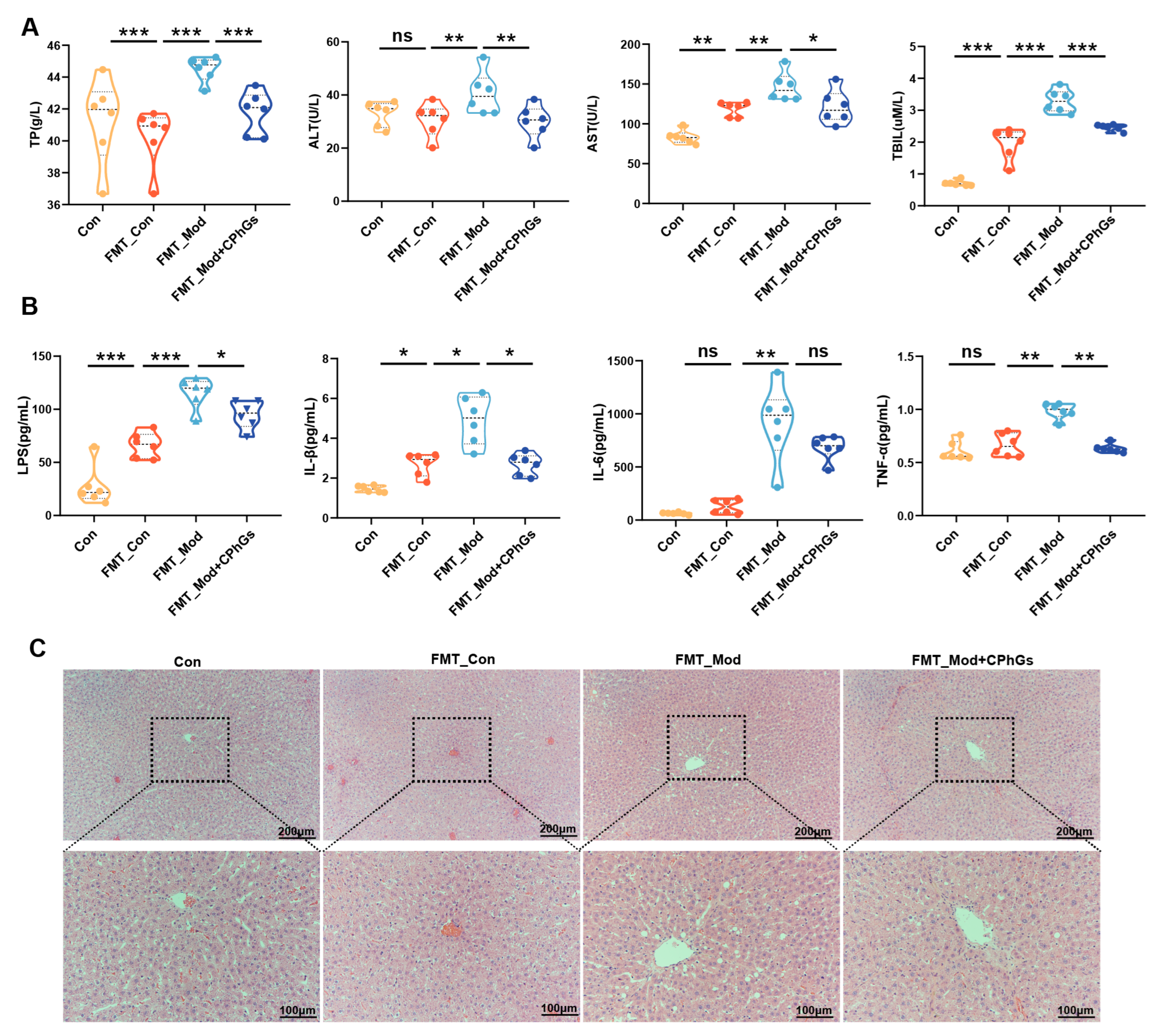
2.6. Gut Microbiota of Rats with Liver Fibrosis Transmit the Inflammatory Response and Induce Liver Injury
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Experimental Animals
4.3. BSA-Induced Hepatic Fibrosis Rat Model and CPhGs Treatments
4.4. The Fecal Microbiota Transplantation (FMT) Design for Investigation
4.5. Western Blotting
4.6. Biochemical Assays
4.7. Histopathological Analysis
4.8. Ultrastructural Analysis
4.9. 16S rDNA
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sharma, S.; Le Guillou, D.; Chen, J.Y. Cellular stress in the pathogenesis of nonalcoholic steatohepatitis and liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 662–678. [Google Scholar] [CrossRef]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Puengel, T.; Loomba, R.; Friedman, S.L. An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J. Hepatol. 2023, 79, 552–566. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, b. The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. 2023, 21, 719–733. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhou, R.; Xie, X.; Zhu, A.; Nan, Y.; Wu, T.; Hu, X.; Cao, Z.; Ju, D.; Fan, J. A Novel Bifunctional Fusion Protein (Anti-IL-17A-sST2) Protects against Acute Liver Failure, Modulating the TLR4/MyD88 Pathway and NLRP3 Inflammasome Activation. Biomedicines 2024, 12, 1118. [Google Scholar] [CrossRef]
- Lei, H.; Wang, X.; Zhang, Y.; Cheng, T.; Mi, R.; Xu, X.; Zu, X.; Zhang, W. Herba Cistanche (Rou Cong Rong): A Review of Its Phytochemistry and Pharmacology. Chem. Pharm. Bull. 2020, 68, 694–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, Y.; Chen, J.; Yu, M.; Zhao, Q.; Jing, B.; Yang, N.; Ma, X.; Wang, Y. Chemical composition, pharmacological effects, and parasitic mechanisms of Cistanche deserticola: An update. Phytomedicine 2024, 132, 155808. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Yang, H.; Jiang, J.; Wang, C.; Lv, B.; Feng, Y. A α-L-rhamnosidase from Echinacea purpurea endophyte Simplicillium sinense EFF1 and its application in production of Calceorioside B. Int. J. Biol. Macromol. 2024, 270, 132090. [Google Scholar] [CrossRef]
- Xiong, W.L.; Sun, Y.; Ma, T.C.; Zhang, X.Y.; Wang, J.Y.; Du, Y.Y.; Wu, B.; Yan, T.X.; Jia, Y. A pair of novel phenylethanol glycosides from Cistanche tubulosa (Schenk) Wight. Fitoterapia 2022, 160, 105227. [Google Scholar] [CrossRef]
- Qi, X.; Hou, X.; Su, D.; He, Z.; Zhao, J.; Liu, T. Effect of PhenylEthanol Glycosides from Cistanche Tubulosa on Autophagy and Apoptosis in H22 Tumor-Bearing Mice. Evid. Based Complement. Altern. Med. 2022, 9, 3993445. [Google Scholar] [CrossRef]
- Salvoza, N.; Bedin, C.; Saccani, A.; Tiribelli, C.; Rosso, N. The Beneficial Effects of Triterpenic Acid and Acteoside in an In Vitro Model of Nonalcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2022, 23, 3562. [Google Scholar] [CrossRef]
- Zhang, S.L.; Ma, L.; Zhao, J.; You, S.P.; Ma, X.T.; Ye, X.Y.; Liu, T. The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway. Molecules 2019, 24, 3282. [Google Scholar] [CrossRef]
- Zeng, H.; Huang, L.; Zhou, L.; Wang, P.; Chen, X.; Ding, K. A galactoglucan isolated from of Cistanche deserticola Y. C. Ma. and its bioactivity on intestinal bacteria strains. Carbohydr. Polym. 2019, 223, 115038. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Loucks, K.; Waddell, D.; Ross, C. Lipopolysaccharides elicit an oxidative burst as a component of the innate immune system in the seagrass Thalassia testudinum. Plant Physiol. Biochem. 2013, 70, 295–303. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Yan, L.; Sun, C.; Miao, Q.; Wang, Q.; Xiao, X.; Lian, M.; Li, B.; Chen, Y.; et al. Alterations of gut microbiome in autoimmune hepatitis. Gut 2020, 69, 569–577. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, L.; Yu, Y.; Liu, S.; Xu, H.; Xu, Z.; Yang, C.; Liu, C. Comparison of Deep and Moderate Neuromuscular Blockade on Intestinal Mucosal Barrier in Laparoscopic Gastrectomy: A Prospective, Randomized, Double-Blind Clinical Trial. Front. Med. 2021, 8, 789597. [Google Scholar] [CrossRef]
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Advances in anti-hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J. Ethnopharmacol. 2020, 251, 112442. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Wang, K.; Liu, Y.; Liu, S.; Wu, K.; Wang, X.; Haider, A.; Liu, Y.; Zhou, Q.; et al. The gut-liver axis perspective: Exploring the protective potential of polysaccharides from Cistanche deserticola against alcoholic liver disease. Int. J. Biol. Macromol. 2024, 256, 128394. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.; Trauner, M.J.C. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Guo, L.; Liu, J.J.; Zhao, H.P.; Zhang, J.; Wang, J.H. Alteration of the esophageal microbiota in Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2019, 25, 2149–2161. [Google Scholar] [CrossRef]
- Ran, Z.; Ju, B.; Cao, L.; Hou, Q.; Wen, L.; Geng, R.; Liao, Y.; Hu, J.; Yang, J. Microbiome-metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 2023, 14, 3488–3508. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Wang, J.; Chai, X.Q.; Li, Z.C.; Jiang, Y.H.; Li, J.; Liu, X.; Fan, J.; Cai, J.B.; Liu, F. The Intratumoral Bacterial Metataxonomic Signature of Hepatocellular Carcinoma. Microbiol. Spectr. 2022, 10, e0098322. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiu, C.C.; Hung, S.W.; Huang, W.C.; Lee, Y.P.; Liu, J.Y.; Huang, Y.T.; Chen, T.H.; Chuang, H.L. Gnotobiotic mice inoculated with Firmicutes, but not Bacteroidetes, deteriorate nonalcoholic fatty liver disease severity by modulating hepatic lipid metabolism. Nutr. Res. 2019, 69, 20–29. [Google Scholar] [CrossRef]
- Chen, J.; Ding, X.; Wu, R.; Tong, B.; Zhao, L.; Lv, H.; Meng, X.; Liu, Y.; Ren, B.; Li, J.; et al. Novel Sesquiterpene Glycoside from Loquat Leaf Alleviates Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease by Improving Insulin Resistance, Oxidative Stress, Inflammation, and Gut Microbiota Composition. J. Agric. Food Chem. 2021, 69, 14176–14191. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Borody, T.J.; Zhang, F. Encyclopedia of fecal microbiota transplantation: A review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 2022, 135, 1927–1939. [Google Scholar] [CrossRef]
- Kim, G.; Yoo, R.N.; So, H.; Lee, J.Y.; Kim, M.N.; Kim, S.H.; Jhang, W.K.; Park, S.J.; Lee, J. Clinical Manifestation of Ralstonia mannitolilytica Infection in Pediatric Patients and Epidemiological Investigation of Outbreaks. J. Korean Med. Sci. 2023, 38, e252. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.F.; Gogokhia, L.; Viladomiu, M.; Chou, L.; Putzel, G.; Jin, W.B.; Pires, S.; Guo, C.J.; Gerardin, Y.; Crawford, C.V.; et al. Transferable Immunoglobulin A-Coated Odoribacter splanchnicus in Responders to Fecal Microbiota Transplantation for Ulcerative Colitis Limits Colonic Inflammation. Gastroenterology 2022, 162, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Dai, M.Y.; Huang, R.Y.; Duan, J.Y.; Zhang, T.; Bao, W.M.; Zhang, J.Y.; Gui, S.Q.; Xia, S.M.; Dai, C.T.; et al. Parabacteroides distasonis ameliorates hepatic fibrosis potentially via modulating intestinal bile acid metabolism and hepatocyte pyroptosis in male mice. Nat. Commun. 2023, 14, 1829. [Google Scholar] [CrossRef]
- Delannoy-Bruno, O.; Desai, C.; Raman, A.S.; Chen, R.Y.; Hibberd, M.C.; Cheng, J.; Han, N.; Castillo, J.J.; Couture, G.; Lebrilla, C.B.; et al. Evaluating microbiome-directed fibre snacks in gnotobiotic mice and humans. Nature 2021, 595, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef]
- Shtossel, O.; Turjeman, S.; Riumin, A.; Goldberg, M.R.; Elizur, A.; Bekor, Y.; Mor, H.; Koren, O.; Louzoun, Y. Recipient-independent, high-accuracy FMT-response prediction and optimization in mice and humans. Microbiome 2023, 11, 181. [Google Scholar] [CrossRef]
- Pabst, O.; Hornef, M.W.; Schaap, F.G.; Cerovic, V.; Clavel, T.; Bruns, T. Gut-liver axis: Barriers and functional circuits. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 447–461. [Google Scholar] [CrossRef]
- You, S.P.; Zhao, J.; Ma, L.; Tudimat, M.; Zhang, S.L.; Liu, T. Preventive effects of phenylethanol glycosides from Cistanche tubulosa on bovine serum albumin-induced hepatic fibrosis in rats. Daru 2015, 23, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Sun, H.; Liu, J.; Cong, M.; Zhang, X.; Yan, Y.; Xia, Z.; Liu, T.; Zhao, J. Phenylethanol Glycoside from Cistanche tubulosa Attenuates BSA-Induced Liver Fibrosis in Rats by Modulating the Gut Microbiota–Liver Axis. Pharmaceuticals 2024, 17, 1149. https://doi.org/10.3390/ph17091149
Qi X, Sun H, Liu J, Cong M, Zhang X, Yan Y, Xia Z, Liu T, Zhao J. Phenylethanol Glycoside from Cistanche tubulosa Attenuates BSA-Induced Liver Fibrosis in Rats by Modulating the Gut Microbiota–Liver Axis. Pharmaceuticals. 2024; 17(9):1149. https://doi.org/10.3390/ph17091149
Chicago/Turabian StyleQi, Xinxin, Hongguang Sun, Jincun Liu, Meili Cong, Xinxuan Zhang, Yuxin Yan, Zhaolin Xia, Tao Liu, and Jun Zhao. 2024. "Phenylethanol Glycoside from Cistanche tubulosa Attenuates BSA-Induced Liver Fibrosis in Rats by Modulating the Gut Microbiota–Liver Axis" Pharmaceuticals 17, no. 9: 1149. https://doi.org/10.3390/ph17091149
APA StyleQi, X., Sun, H., Liu, J., Cong, M., Zhang, X., Yan, Y., Xia, Z., Liu, T., & Zhao, J. (2024). Phenylethanol Glycoside from Cistanche tubulosa Attenuates BSA-Induced Liver Fibrosis in Rats by Modulating the Gut Microbiota–Liver Axis. Pharmaceuticals, 17(9), 1149. https://doi.org/10.3390/ph17091149





