Chiral Hydroxy Metabolite of Mebendazole: Analytical and Semi-Preparative High-Performance Liquid Chromatography Resolution and Chiroptical Properties
Abstract
1. Introduction
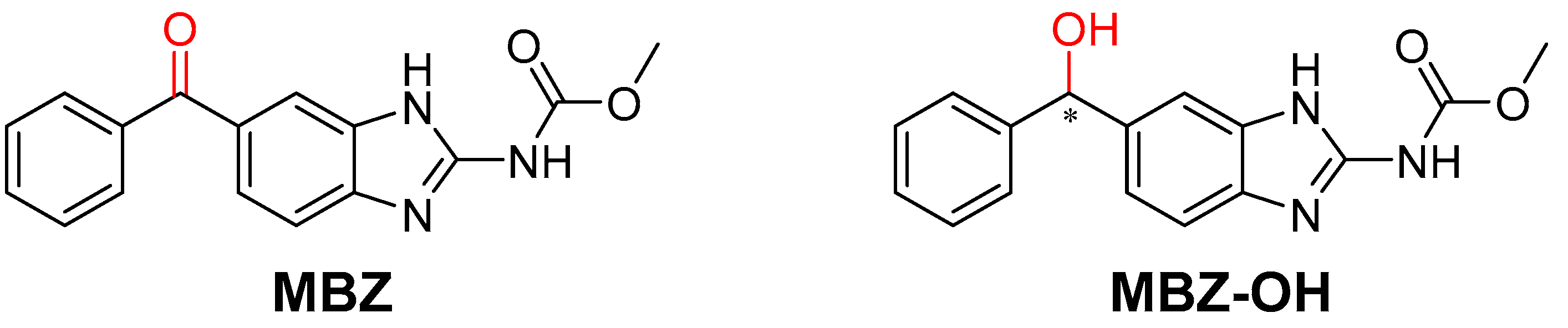
2. Results and Discussion
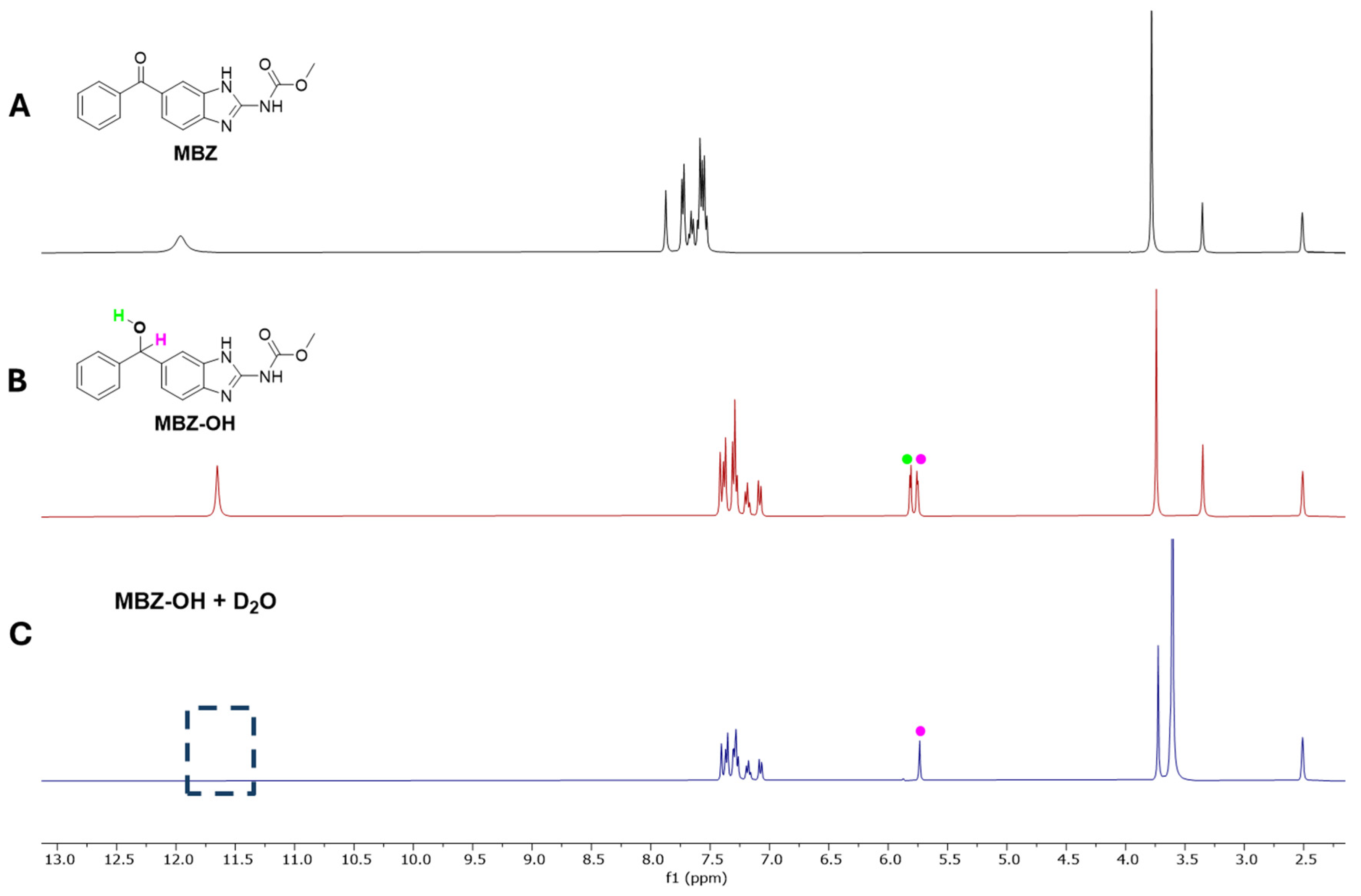
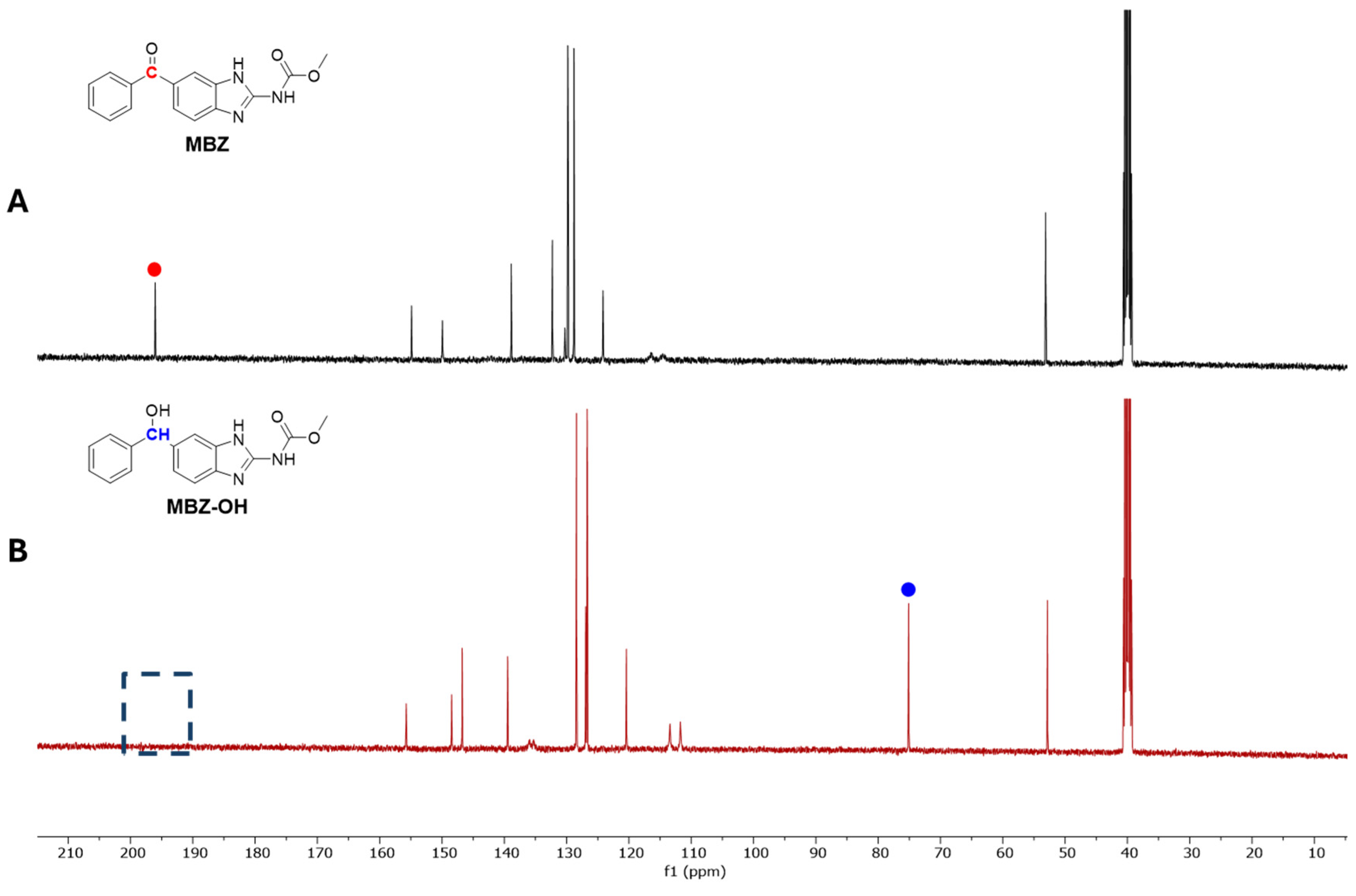
2.1. Synthesis and Chemical Characterization of MBZ-OH
2.2. Analytical HPLC Enantioseparation under Polar Organic Conditions
2.3. Analytical HPLC Enantioseparation under Organic–Aqueous Conditions
2.4. Semi-Preparative Enantioseparation
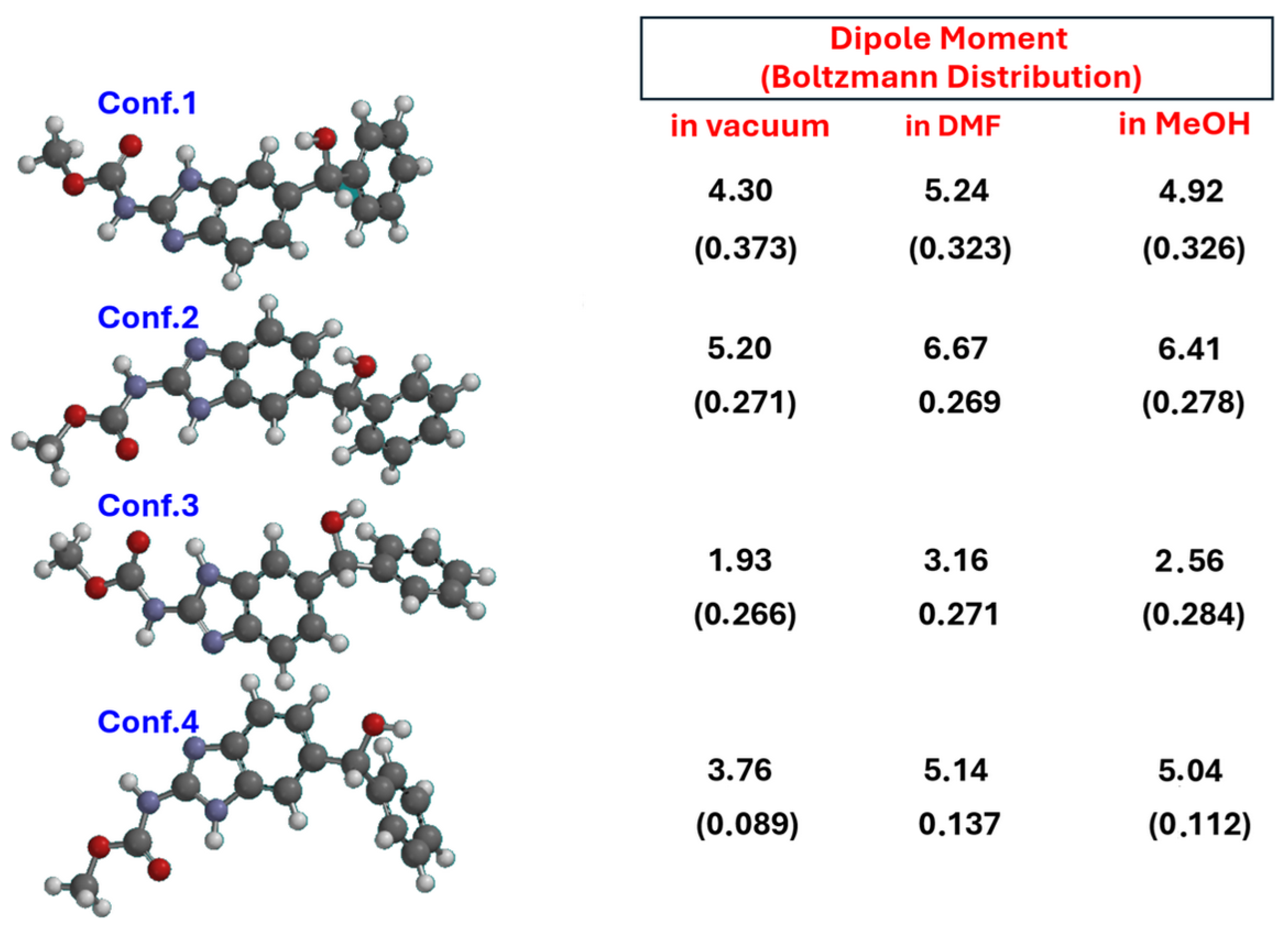
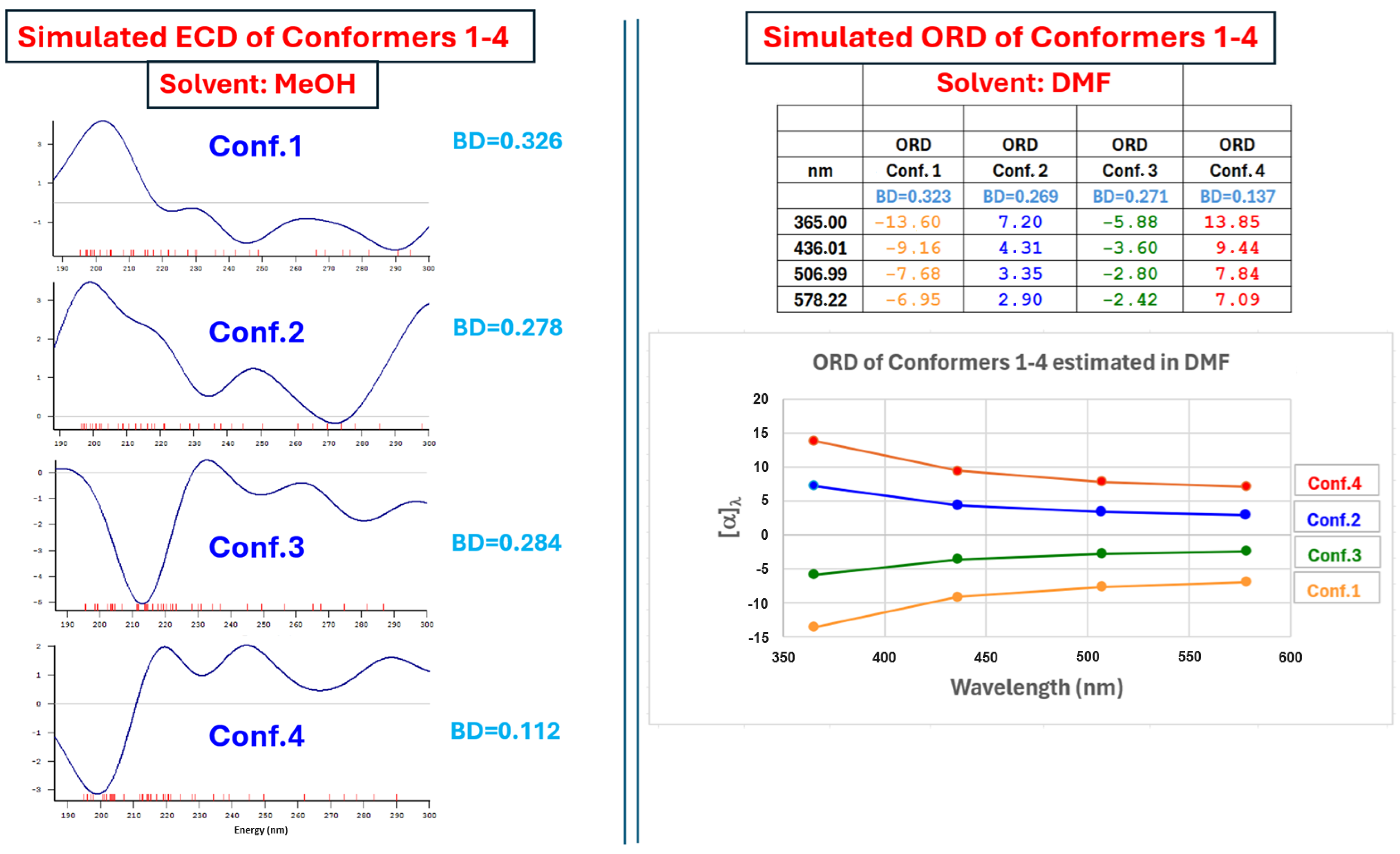
2.5. Chiroptical Properties and Absolute Configuration Assignment
3. Materials and Methods
3.1. Chemistry
3.2. Synthesis of MBZ-OH
3.3. Instruments and Chromatographic Conditions
3.4. Semi-Preparative Separation of MBZ-OH Enantiomers
3.5. Conformational Analysis and Prediction of Chiroptical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Silva, N.; Guyatt, H.; Bundy, D. Anthelmintics A comparative review of their clinical pharmacology. Drugs 1997, 53, 769–788. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, T.; Sasaki, J.i.; Ramesh, R.; Roth, J.A. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 2002, 8, 2963–2969. [Google Scholar] [PubMed]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P. Repurposing drugs in oncology (ReDO)—Mebendazole as an anti-cancer agent. eCancermedicalscience 2014, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Doudican, N.; Rodriguez, A.; Osman, I.; Orlow, S.J. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol. Cancer Res. 2008, 6, 1308–1315. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Khattab, M. Molecular modelling of mebendazole polymorphs as a potential colchicine binding site inhibitor. New J. Chem. 2020, 44, 13990–13996. [Google Scholar] [CrossRef]
- Elmaaty, A.A.; Darwish, K.M.; Chrouda, A.; Boseila, A.A.; Tantawy, M.A.; Elhady, S.S.; Shaik, A.B.; Mustafa, M.; Al-Karmalawy, A.A. In silico and in vitro studies for benzimidazole anthelmintics repurposing as VEGFR-2 antagonists: Novel mebendazole-loaded mixed micelles with enhanced dissolution and anticancer activity. ACS Omega 2022, 7, 875–899. [Google Scholar] [CrossRef] [PubMed]
- Larsen, A.R.; Bai, R.-Y.; Chung, J.H.; Borodovsky, A.; Rudin, C.M.; Riggins, G.J.; Bunz, F. Repurposing the antihelmintic mebendazole as a Hedgehog inhibitor. Mol. Cancer Ther. 2015, 14, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P.; Fryknäs, M.; Ågerup, B.; Larsson, R. Repositioning of the anthelmintic drug mebendazole for the treatment for colon cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Gottmannst, H.; Krokerts, R.; Ungemach, F.R. Investigations on the biotransformation of mebendazole using an isolated perfused rat gut system. Xenobiotica 1991, 21, 1431–1439. [Google Scholar] [CrossRef]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The Significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yashima, E. Polysaccharide derivatives for chromatographic separation of enantiomers. Angew. Chem. Int. Ed. 1998, 37, 1020–1043. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent trends in preparation, investigation and application of polysaccharide-based chiral stationary phases for separation of enantiomers in high-performance liquid chromatography. Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Immobilized polysaccharide CSPs: An advancement in enantiomeric separations. Curr. Pharm. Anal. 2007, 3, 71–82. [Google Scholar] [CrossRef]
- Ianni, F.; Cerra, B.; Shandiz, S.T.; Di Michele, A.; Saluti, G.; Galarini, R.; Gioiello, A.; Sardella, R.; Carotti, A. Integrating experimental and computational techniques to study chromatographic enantioresolutions of chiral tetrahydroindazole derivatives. J. Chromatogr. A 2020, 16, 461310. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Levent, S.; Ianni, F.; Caliskan, B.; Gerstmeier, J.; Pergola, C.; Oliver Werz, O.; Banoglu, E.; Natalini, B. Chromatographic separation and biological evaluation of benzimidazole derivative enantiomers as inhibitors of leukotriene biosynthesis. J. Pharm. Biomed. Anal. 2014, 89, 88–92. [Google Scholar] [CrossRef]
- Vázquez, J.T. Features of electronic circular dichroism and tips for its use in determining absolute configuration. Tetrahedron Asymmetry 2017, 28, 1199–1211. [Google Scholar] [CrossRef]
- Ikai, T.; Okamoto, Y. Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chem. Rev. 2009, 109, 6077–6101. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, B.; Yashima, E.; Okamoto, Y. Chloromethylphenylcarbamate derivatives of cellulose as chiral stationary phases for high-performance liquid chromatography. J. Chromatogr. A 1994, 670, 39–49. [Google Scholar] [CrossRef]
- Chankvetadze, B.; Yashima, E.; Okamoto, Y. Dimethyl-, dichloro- and chloromethylphenylcarbamates of amylose as chiral stationary phases for high-performance liquid chromatography. J. Chromatogr. A 1995, 694, 101–109. [Google Scholar] [CrossRef]
- Ghanem, A.; Wang, C. Enantioselective separation of racemates using CHIRALPAK IG amylose-based chiral stationary phase under normal standard, non-standard and reversed phase high performance liquid chromatography. J. Chromatogr. A 2018, 1532, 89–97. [Google Scholar] [CrossRef]
- Cantatore, C.; Korb, M.; Lang, H.; Cirilli, R. ON/OFF receptor-like enantioseparation of planar chiral 1,2-ferrocenes on an amylose-based chiral stationary phase: The role played by 2-propanol. Anal. Chim. Acta 2022, 1211, 339880. [Google Scholar] [CrossRef] [PubMed]
- Díaz Merino, M.E.; Lancioni, C.; Padró, J.M.; Castells, C.B. Study of enantioseparation of β-blockers using amylose tris(3-chloro-5-methylphenylcarbamate) as chiral stationary phase under polar-organic, reversed-phase and hydrophilic interaction liquid chromatography conditions. J. Chromatogr. A 2020, 1634, 461685. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Ferretti, R.; Gallinella, B.; Zanitti, L. Retention behavior of proton pump inhibitors using immobilized polysaccharide-derived chiral stationary phases with organic-aqueous mobile phases. J. Chromatogr. A 2013, 1304, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R.; Carradori, S.; Casulli, A.; Pierini, M. A chromatographic study on the retention behavior of the amylose tris(3-chloro-5-methylphenylcarbamate) chiral stationary phase under aqueous conditions. J. Separ. Sci. 2018, 41, 4014–4021. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.-A.; Hancu, G.; Szabó, Z.-I. Simultaneous determination of enantiomeric and organic impurities of vildagliptin on a cellulose tris(3-chloro-4-methylphenylcarbamate) column under revered-phase conditions. J. Pharm. Biomed. Anal. 2023, 234, 115495. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, R. HPLC enantioseparations with polysaccharide-based chiral stationary phases in HILIC conditions. Methods Mol. Biol. 2019, 1985, 127–146. [Google Scholar] [PubMed]
- Gumustasa, M.; Ozkanb, S.A.; Chankvetadze, B. Analytical and preparative scale separation of enantiomers of chiral drugs by chromatography and related methods. Curr. Med. Chem. 2018, 25, 4152–4188. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ikai, T. Chiral HPLC for efficient resolution of enantiomers. Chem. Soc. Rev. 2008, 37, 2593–2608. [Google Scholar] [CrossRef]
- Bléhaut, J.; Franco, P.; Zhang, T.; Lang, E.; Valéry, E.; Marcoux, J.-F. Industrial applications of chiral chromatography. Compr. Chirality 2012, 9, 400–456. [Google Scholar]
- Rosetti, A.; Apolloni, G.; Villani, C.; Benincori, T.; Cirilli, R. A new C2-symmetric atropisomeric thiophene-based monomer for inherently chiral electroactive materials: Synthesis, HPLC resolution, and absolute configuration assignment. Appl. Sci. 2023, 13, 1407. [Google Scholar] [CrossRef]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and metalloporphyrins: Versatile circular dichroic reporter groups for structural studies. Chirality 2000, 12, 237–250. [Google Scholar] [CrossRef]
- Polavarapu, P.L. Optical rotation: Recent advances in determining the absolute configuration. Chirality 2002, 14, 768–781. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, B.; Dukor, R.K.; Nafie, L.A. Determination of absolute configuration of chiral molecules using vibrational optical activity: A review. Appl. Spectrosc. 2011, 65, 699–723. [Google Scholar] [CrossRef]
- Bertucci, C.; Guimarães, L.F.L.; Bonato, P.S.; Borges, K.B.; Okano, L.T.; Mazzeo, G.; Rosini, C. Assignment of the absolute configuration at the sulfur atom of thioridazine metabolites by the analysis of their chiroptical properties: The case of thioridazine 2-sulfoxide. J. Pharm. Biomed. Anal. 2010, 52, 796–801. [Google Scholar] [CrossRef] [PubMed]
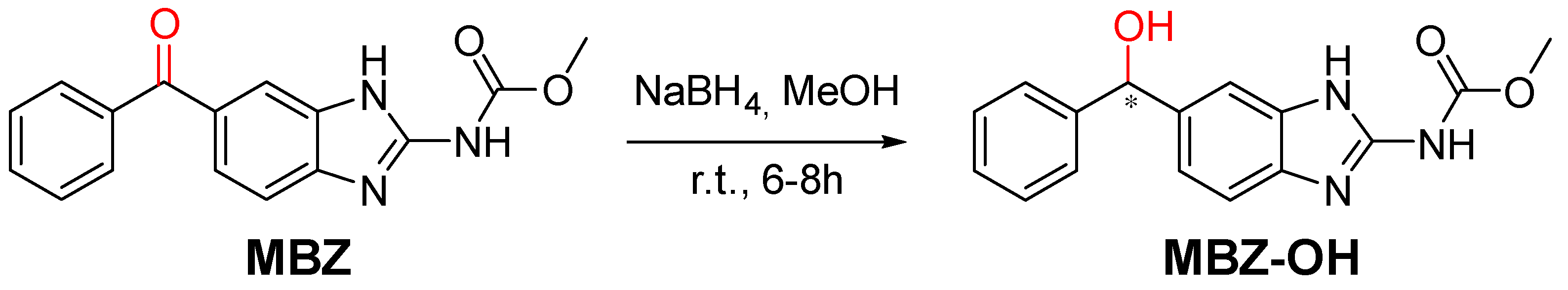
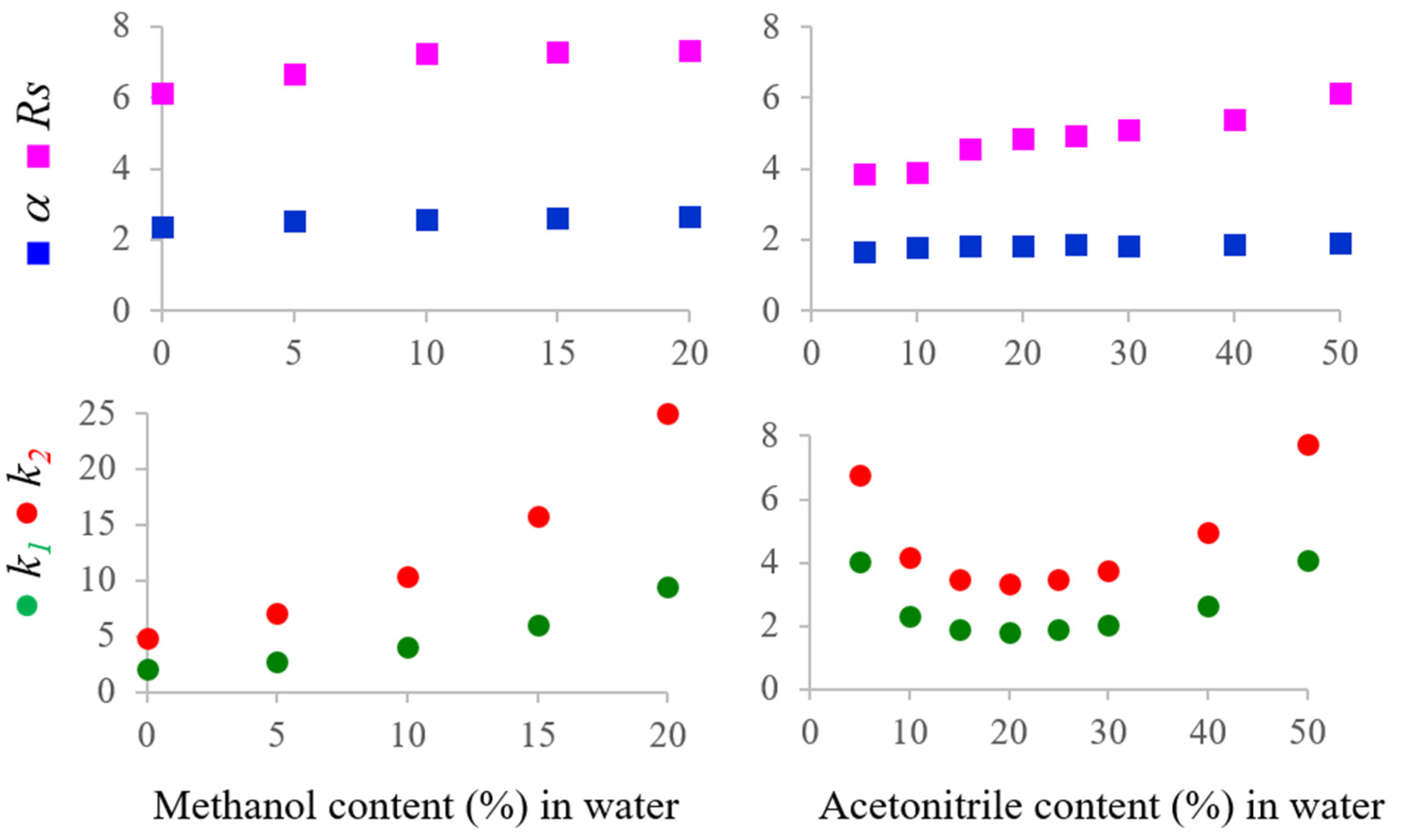
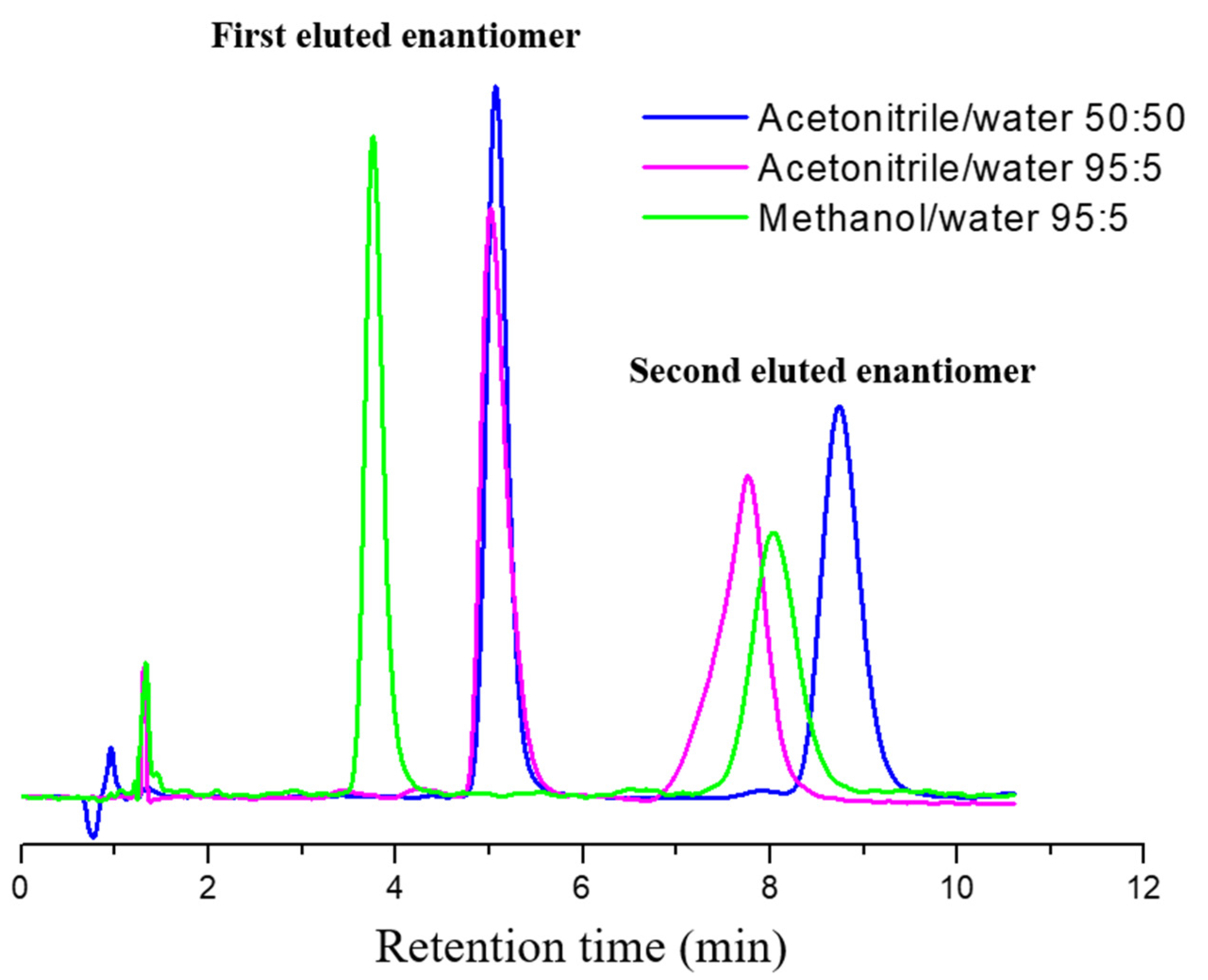
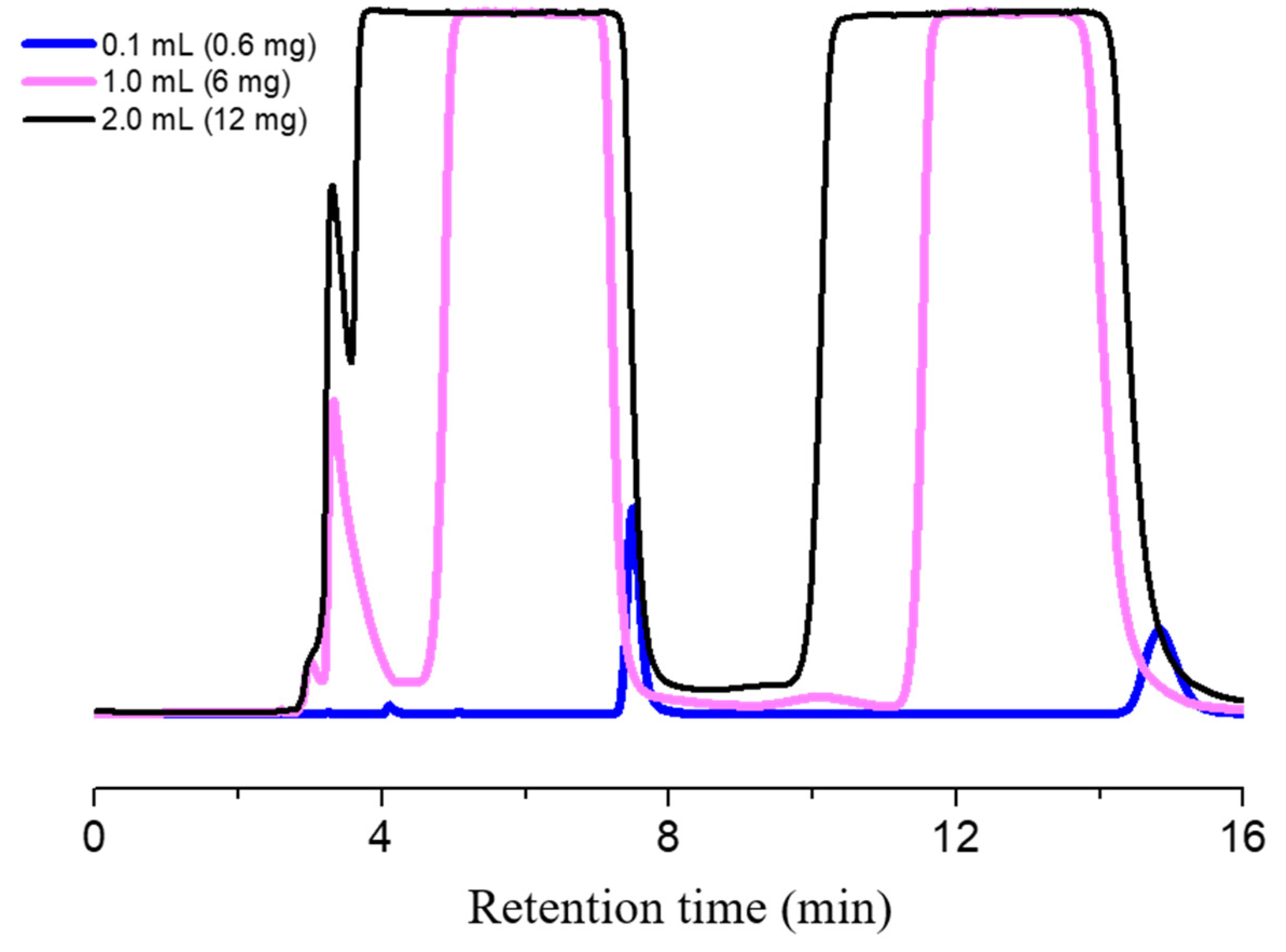

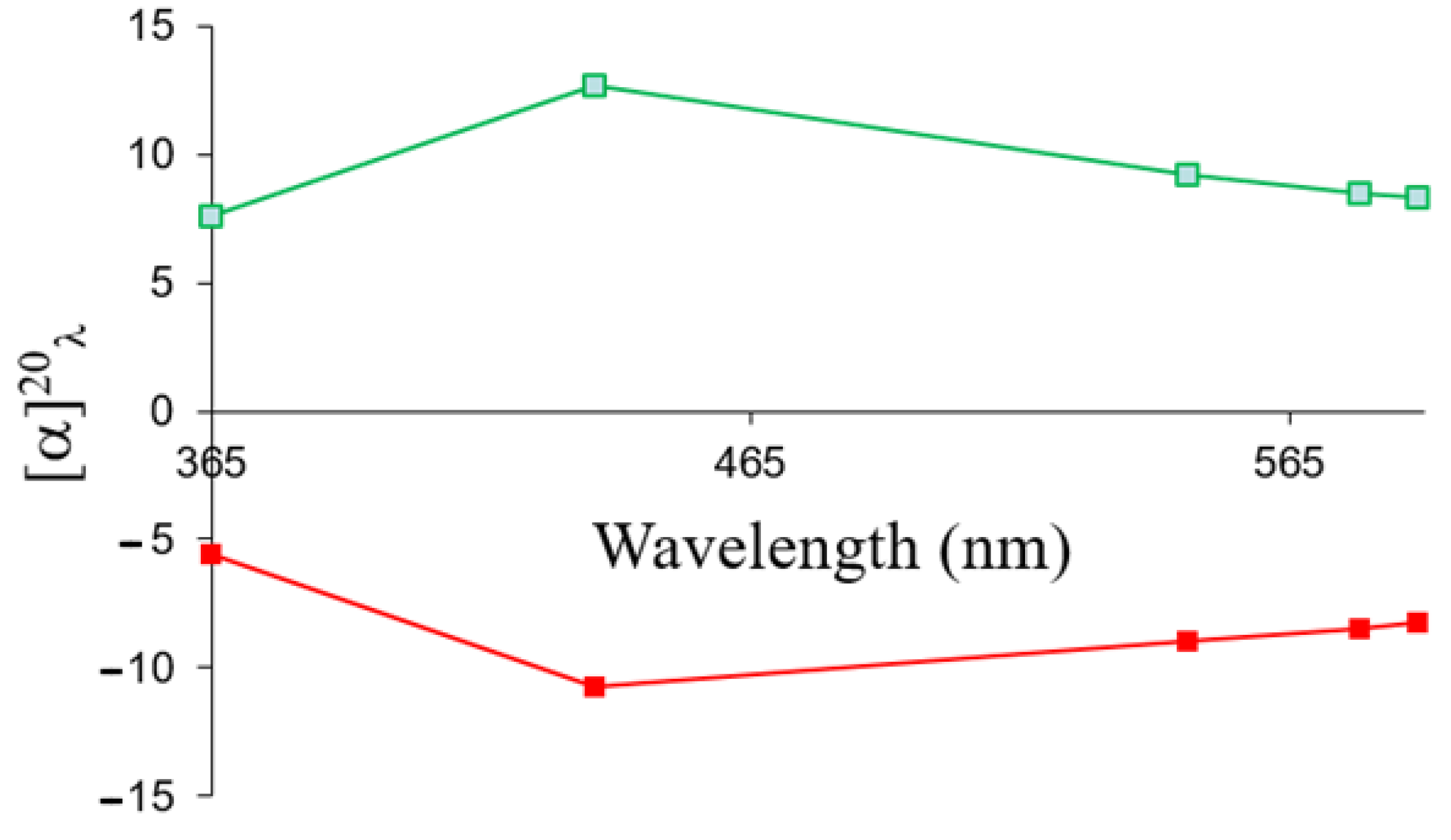

| Mobile Phase | Temperature (°C) | k1 | α | Rs |
|---|---|---|---|---|
| Ethanol | 20 | 1.96 | 2.55 | 4.84 |
| 25 | 1.86 | 2.44 | 5.26 | |
| 30 | 1.75 | 2.33 | 5.60 | |
| 35 | 1.69 | 2.19 | 5.73 | |
| 40 | 1.55 | 2.13 | 5.88 | |
| Methanol | 20 | 2.07 | 2.51 | 5.79 |
| 25 | 2.01 | 2.38 | 6.13 | |
| 30 | 1.92 | 2.28 | 5.07 | |
| 35 | 1.85 | 2.18 | 5.08 | |
| 40 | 1.77 | 2.10 | 5.01 | |
| Acetonitrile | 25 | Not eluted after 30 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guglielmi, P.; Pulitelli, G.; Arrighi, F.; Secci, D.; Pierini, M.; Cirilli, R. Chiral Hydroxy Metabolite of Mebendazole: Analytical and Semi-Preparative High-Performance Liquid Chromatography Resolution and Chiroptical Properties. Pharmaceuticals 2024, 17, 696. https://doi.org/10.3390/ph17060696
Guglielmi P, Pulitelli G, Arrighi F, Secci D, Pierini M, Cirilli R. Chiral Hydroxy Metabolite of Mebendazole: Analytical and Semi-Preparative High-Performance Liquid Chromatography Resolution and Chiroptical Properties. Pharmaceuticals. 2024; 17(6):696. https://doi.org/10.3390/ph17060696
Chicago/Turabian StyleGuglielmi, Paolo, Gaia Pulitelli, Francesca Arrighi, Daniela Secci, Marco Pierini, and Roberto Cirilli. 2024. "Chiral Hydroxy Metabolite of Mebendazole: Analytical and Semi-Preparative High-Performance Liquid Chromatography Resolution and Chiroptical Properties" Pharmaceuticals 17, no. 6: 696. https://doi.org/10.3390/ph17060696
APA StyleGuglielmi, P., Pulitelli, G., Arrighi, F., Secci, D., Pierini, M., & Cirilli, R. (2024). Chiral Hydroxy Metabolite of Mebendazole: Analytical and Semi-Preparative High-Performance Liquid Chromatography Resolution and Chiroptical Properties. Pharmaceuticals, 17(6), 696. https://doi.org/10.3390/ph17060696











