Abstract
Background/Objective: Bee pollen, a rich nutritional food, was employed to develop a raw material for skin whitening. Methods: The polyphenol profile and antioxidant, antityrosinase, and anti-melanogenesis activities of the ethanol extracts of five species of bee pollens (EEBPs) were determined. Results: The results showed that there were a total of 121 phenolic compounds in these EEBPs. Each type of bee pollen had unique substances. The best anti-melanogenesis activity was observed for sunflower EEBP, about 25% at a concentration of 25 μg/mL BEEP. The anti-melanogenesis activities of EEBPs from high to low were sunflower, apricot, camellia, rapeseed, and lotus EEBPs. The anti-melanogenesis activity in B16F10 cells was positively correlated with the antityrosinase activity and total phenol content, with coefficients of 0.987 and 0.940. The Kyoto Encyclopedia of Genes and Genomes enrichment analysis results of untargeted proteomics revealed that sunflower EEBP inhibited melanogenesis in B16F10 cells by reducing the expression of the proteins MAP2K1, NFKB2, RELB, RPS6KA3, CASP3, TRAF6, MAP2K5, MAPKAPK3, STRADA, CCNA2, and FASN involved in the cAMP, MAPK, and TNF signaling pathways, even though these pathways were not significantly different from the control group. Conclusions: The sunflower EEBP has high inhibition effect on melanogenesis than other species EEBPs. The results provide a basis for the future industrial development of a raw material for skin whitening.
1. Introduction
Bee pollen is composed of copious amounts of pollen grains, which were foraged by workers using the weak electrostatic field generated between the negatively charged flower and the positively charged body, mixed with a small dose of the secretions from salivary glands or nectar, and placed in specific baskets situated on the tibia of their hind legs [1,2]. There are many biological substances, including carbohydrates, proteins, vitamins, minerals, polyphenols, dietary fiber, volatile compounds, and lipids, in bee pollen. The moisture content ranges from 1.82 to 7.33% in commercial bee pollen [3] and 20 to 30% in the original bee pollen [4]. Bee pollen contains approximately 40–85% carbohydrates, including 15.2–22.4% fructose, 7.0–21.9% glucose, 14–19.8% sucrose, and others (W/W, dry weight, DW) [5]. There are approximately 13.6–26.8% (W/W, DW) protein with a total of 20 amino acids, 1–10% total lipids, 0.0019–0.0344% vitamin B, 0.0015% vitamin A, 0.0062% vitamin E (α-tocopherol), and trace amounts of vitamin C in bee pollen [5,6]. It contains rich micronutrients such as potassium (K), sodium (Na), calcium (Ca), magnesium (Mg), phosphorus (P), zinc (Zn), iron (Fe), copper (Cu), and manganese (Mn) [5]. There is also a very diverse group of yellow-to-red-color polyenes, polyphenols, dietary fiber, and volatile compounds, among which polyphenols include 19 phenolic acids, 25 flavonoids, and seven glycosylated flavonoids [3,4,5,6,7,8]. The chemical compositions of diverse bee pollens vary from different botanical origins, geographic origins, entomological origins, processing methods, and storage conditions [2,3,4,5,6,7,8]. These chemical compositions decide the functional effects of bee pollen, such as antioxidant, antifungal, antimicrobial, antiviral, anti-inflammatory, hepatoprotective, anticancer, immunostimulatory, radical-scavenging, gut-microbiota-modulating, and local analgesic activities [5,9,10,11].
The antioxidant capability is one of the significant bioactivities of bee pollen. This primarily depends on the presence of various compounds, including 4-hydroxybenzoic, p-coumaric, phenyllactic, caffeic, transcinnamic, syringic, 3,4 dimethoxycinnamic (acid), chlorogenic, rosmarinic, and vanillic acids; apigenin; caffeic acid phenethyl ester (CAPE); chrysin; epicatechin; hesperetin; taxifolin; myricetin; catechin; genistein; isorhamnetin; kaempferol; luteolin; pinocembrin; quercetin; and rutin [8]. Enzymes like catalase and superoxide dismutase (SOD), along with glutathione (GSH), also play roles in the antioxidant effects of bee pollen [12]. The antioxidant capability is influenced by several factors, including the processing method [13,14,15], botanical origin [8,16], storage [17], geographical origin [18], and extraction solvents [14,19].
In addition to the in vitro antioxidant activity, in vivo studies have reported benefits. Cistaceae bee pollen modulates the expression of antioxidant enzymes in the liver, brain, and lysate of erythrocytes and reduces hepatic lipid peroxidation levels in mice [20]. The water and methanol extracts of bee pollen increase the SOD level and the total antioxidant capacity and significantly reduce the malondialdehyde (MDA) content in mice and rats [12,21]. Bee pollen relieves the oxidative stress on hepatocytes [22], oxidative damage and reactive oxygen species produced by propionic acid [23,24], and NaF-induced oxidative stress in rats [25]. Furthermore, bee pollen alleviates oxidative stress in hepatocytes and both oxidative damage and reactive oxygen species produced by propionic acid and sodium fluoride in rats [26].
Beyond the antioxidant activity, polyphenols also possess the ability to inhibit tyrosinase [27], which is a biofunctional copper-containing enzyme that plays a crucial role in the melanin biosynthesis pathway within melanocytes. Tyrosinase catalyzes the hydroxylation of monophenols and the oxidation of diphenols to form quinines [28]. Melanin is the primary determinant of skin color and a vital substance to prevent skin damage caused by UV radiation and oxidative stress [29]. However, the excessive accumulation of melanin might result in hyperpigmentation diseases, such as lentigo, ephelides, melasma, freckles and nevus, and melanomas, which may increase the risks of both cancer and Parkinson’s disease [30,31,32,33]. Consequently, tyrosinase inhibitors are being widely studied as treatments for pigmentation issues and for the development of skin-whitening agents [27,28,34,35,36,37,38,39]. Examples of natural compounds with antityrosinase activity include a quercetin-loaded olive oil nanoemulsion [34], flavonoids from Trifolium nigrescens subsp. petrisavi [36], citrus essential oils [37], mango seed kernel extract [38], and safflospermidines separated from the bee pollen of Helianthus annuus L. [39]. Notably, safflospermidines exhibit higher antityrosinase activity than kojic acid in vitro. Traditional tyrosinase inhibitors, such as kojic acid, aloesin, hydroquinone, and α- and β-arbutins, have been used as skin-whitening agents [27]. However, there is a need for more effective and safer tyrosinase inhibitors, as cytotoxicity or side effects may be caused by irritation, peeling, redness, or stinging in daily life [40].
There are limited research reports on the antityrosinase and antioxidant activities of bee pollen harvested in China. In this paper, the polyphenol profiles and antioxidant, antityrosinase, and anti-melanogenesis activities of sunflower bee pollen extract in B16F10 melanoma cells were determined to discover an effective and safer tyrosinase inhibitor for further utilization as a raw material for skin-whitening agents.
2. Results
2.1. Components in Ethanol Extracts of Bee Pollens
2.1.1. TPCs and TFCs in Ethanol Extracts of Bee Pollens
There were significant differences among the TPCs and TFCs of ethanol extracts of various species of bee pollen (EEBP) (Table 1). The TPCs and TFCs of bee pollen samples ranged from 5.36 to 132.57 mg GAE/g and 1.99 to 109.06 mg RE/g calculated by the equations of the standard curves y = 0.00567x + 0.07371 (r2 = 0.9929) and y = 0.00148x + 0.05319 (r2 = 0.9929), respectively.

Table 1.
Total phenolic and flavonoid contents of ethanol extracts of bee pollens.
2.1.2. The Polyphenols in Ethanol Extracts of Bee Pollens
The ion spectra of ethanol extracts of bee pollens were shown in Figure 1. A diverse variety of polyphenols has been identified in five species of EEBPs. In total, 71 polyphenolic compounds were found, with significant variation across different bee pollen samples (Table 2).
Figure 1.
UHPLC–MS/MS ion spectra of ethanol extracts of bee pollens: A, B, C, D, and E represent rapeseed, apricot, camellia, lotus, and sunflower bee pollens, respectively. P and N represent positive ion mode and negative ion mode, respectively.

Table 2.
Flavonoids in ethanol extracts of bee pollens.
EEBPs of rapeseed, camellia, sunflower, lotus, and apricot bee pollens contain 56, 46, 45, 42, and 35 polyphenolic compounds, respectively. Certain flavonoids are unique to specific types of bee pollen: orientin, (−)-catechin gallate, methyl hesperidin, hesperidin, and epicatechin are found only in rapeseed bee pollen; chloropelargonidin is exclusive to camellia bee pollen; violacein and tricin-O-malonylhexoside are present only in sunflower bee pollen; and heptamethoxyflavonoids, isovitexin, chlorocyanidin, and baohuoside I are unique to lotus bee pollen. In terms of the relative content, camelliaside A is the most abundant flavonoid in rapeseed bee pollen; rutin is the most prevalent in both lotus and apricot bee pollens; kaempferol has the highest relative content in camellia bee pollen; and quercetin-3β-D-glucoside is the dominant flavonoid in sunflower bee pollen.
2.2. Antioxidant and Tyrosinase Inhibitory Capacities of Ethanol Extracts of Bee Pollens
The antioxidant capacities of EEBPs are shown in Table 3. The antioxidant capacities of sunflower EEBP were highest among those of the other four species EEBPs determined by the DPPH, FRAP, and ABTS assays.

Table 3.
Antioxidant capacities of ethanol extracts of bee pollens.
The tyrosinase inhibitory capacities of EEBP are shown in Figure 2. The five EEBPs inhibited the activity of tyrosinase in a concentration-dependent manner. There were significant differences in the inhibition rate among different species of EEBPs. The sunflower EEBP had much higher inhibition rates for tyrosinase activity than the EEBPs of apricot, rapeseed, camellia, and lotus. At a concentration of 50 μg/mL, the inhibition rate of the sunflower EEBP was higher than 50%, while those of the other EEBPs were lower than 50%.
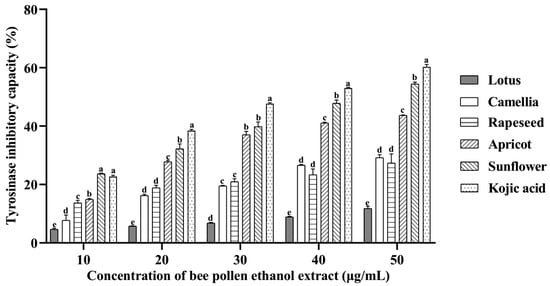
Figure 2.
The antityrosinase activities of ethanol extracts of bee pollens. Note: Different lowercase characters above the same concentration mean significant differences (p < 0.05).
2.3. Anti-Melanogenesis Activity of Ethanol Extracts of Bee Pollens on Melanin Production in Mouse B16F10 Melanoma Cells
The relative melanin contents in B16F10 cells treated with EEBPs decreased in a concentration-dependent manner (Figure 3). The highest anti-melanogenesis activity was observed for sunflower EEBP, which was about 25% at 25 μg/mL BEEP, followed by apricot, camellia, rapeseed, and lotus EEBPs.
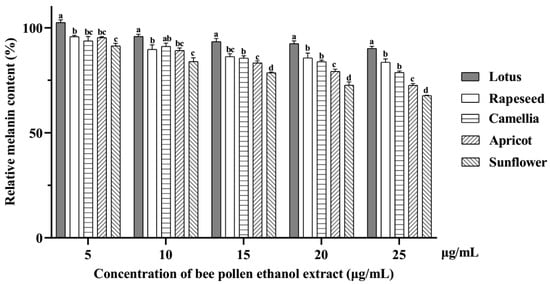
Figure 3.
The relative melanin content in B16F10 cells treated with ethanol extracts of bee pollens. Note: Different lowercase characters above the same concentration mean significant differences (p < 0.05).
The correlation coefficients among DPPH, FRAP, ABTS, TPC, TFC, tyrosinase inhibitory, and anti-melanogenesis activities showed that the anti-melanogenesis activity was highly dependent on the TPCs in the EEBPs (p < 0.05, Table 4).

Table 4.
The correlation coefficients among DPPH, FRAP, ABTS, TPC, TFC, tyrosinase inhibitory, and anti-melanogenesis activities.
2.4. Anti-Melanogenesis Mechanism of the Ethanol Extract of Sunflower Bee Pollen on Melanin Production in Mouse B16F10 Melanoma Cells
The DIA quantitative proteomics analysis identified a total of 7555 proteins. Compared to the control group, 272 proteins were differentially expressed, consisting of 171 up-regulated and 101 down-regulated differentially expressed proteins (DEPs). The volcano map of differentially expressed proteins in B16F10 cells (sunflower EEBP vs. control) is shown in Figure S2. Analyzing the subcellular localization of these differentially expressed proteins revealed that 28.35% were located in the cytoplasm, 27.84% in the nucleus, and 11.34% in the mitochondria. The Gene Ontology (GO) enrichment analysis indicated that the differentially expressed proteins primarily participated in metabolic processes, catalytic activities, redox processes, and calcium ion binding. Additionally, the KEGG enrichment analysis suggested that the sunflower EEBP inhibited melanogenesis in B16F10 cells by reducing the expression of proteins associated with the cAMP, MAPK, and TNF signaling pathways (Table 5), which are highly related to melanogenesis, even though these pathways were not significantly different from the control group.

Table 5.
The differentially expressed proteins enriched in pathways highly related to melanogenesis in mouse B16F10 melanoma cells treated with the ethanol extract of sunflower bee pollen compared with the control group.
3. Discussion
The polyphenol components varied mainly depending on the botanic source. In this experiment, the TPC of bee pollen samples ranged from 5.39 to 132.51 mg GAE/g EEBP. This range is similar to other research if the extraction rate was included, which were 9.41 to 27.49 [41], 4.64 to 17.93 [42], and 12.60 to 84.22 mg GAE/g bee pollen [43], and 15.73 to 26.92 [44], 4.2 to 29.6 GAE mg/g extract of bee pollen [45]. The EEBP with the highest TPC was sunflower bee pollen, which was different from previous reports, which were 12.29 mg GAE/g dried bee pollen from Hungary [41], 7.56 mg GAE/g dried bee pollen from Romania [42], and 2.907 mg GAE/g of dried bee pollen harvested from Serbia [46]. Meanwhile, the EEBP with the lowest TPC was lotus bee pollen, which was similar to the reported values [47] of 1.81 mg PAE/g bee pollen from China extracted with 75% ethanol/water solvents and determined by protocatechuic acid (PA) as the standard solution. The EEBP with a medium TPC was rapeseed, 12.57 mg GAE/g dried bee pollen from China [48] and calculated by free and bound phenolic extracts and measured by the same method as this paper. There was 12.90 mg PAE/g bee pollen from China extracted by 75% ethanol/water solvents [47]. This difference in the TPC may be related to the different geographic origins, extraction solvents, and storage conditions of bee pollen.
The TPC mainly depends on the polyphenols in bee pollen. EEBPs of rapeseed, camellia, sunflower, lotus, and apricot bee pollens contain 56, 46, 45, 42, and 35 polyphenols. Reports showed that there were 37 phenolic compounds in sunflower bee pollen from Serbia [46], 35 phenolic compounds in eight bee pollen samples from Morocco [49], and 258 phenolic compounds in thirty-two bee pollen samples from Italy [44]. The different phenolic compounds in bee pollen depend on the geographic and botanical origins, storage conditions, extraction solvent, and determination methods of bee pollen.
The antioxidant activities of various species of bee pollen are mostly attributed to their polyphenols. Consistency exists in the antioxidant activities from the DPPH, FRAP, and ABTS assays. There is a consistency in the DPPH (IC50) and ABTS assays [47]. EEBPs from five of a total of fourteen species from high to low were rapeseed = camellia > apricot > sunflower > lotus [47], which were similar to sunflower > rapeseed > camellia > apricot > lotus in this experiment. This different result was caused by the different expression method, TE per bee pollen or EEBPs. The phenolic compounds can directly participate in antioxidant activity [50]. This is consistent with the results of this study, where the correlation coefficients between the phenolic content and antioxidant activity were higher than 0.858.
Tyrosinase is the key enzyme for melanin production in the human body. Inhibiting tyrosinase can effectively reduce melanin synthesis, thereby achieving a whitening effect on the skin [51]. Some studies have found that phenolamide in bee pollen is a component that affects the tyrosinase activity [52]. In this study, the five EEBPs inhibited tyrosinase in a concentration-dependent manner. The inhibitory activity of sunflower EEBP toward tyrosinase was the highest. Sunflower bee pollen from Thailand also showed inhibition of tyrosinase activity after extraction with methanol [39], which is consistent with the results of this study. However, some studies have shown that apricot bee pollen, after extraction with ethyl acetate, has a better inhibitory effect on tyrosinase activity than sunflower bee pollen [52]. This difference may be the difference in extraction methods and solvents. The bee pollen in this study was extracted with ethanol, while it was first extracted with ethanol followed by ethyl acetate in the previous studies. A study analyzed the tyrosinase inhibitory properties of 14 types of single-flower bee pollens from China. The results showed that almost all the samples had different tyrosinase inhibitory activities due to the plant sources of bee pollen and extraction solvents. The apricot, camellia, and sunflower extracts showed excellent tyrosinase inhibitory activity [47]. In this study, the five EEBPs inhibited melanin synthesis in the mouse B16F10 melanoma cells within the concentration ranges that did not affect cellular activity. Some studies have also shown that the phenolic substances extracted from rapeseed bee pollen inhibited tyrosinase activity, thereby inhibiting melanin production [48]. DIA quantitative proteomics was used to detect the in vitro inhibitory effect of sunflower EEBP on melanin production in B16F10 cells. DEPs STAT1, caspase-3, FASN, and METTL3 were significantly up-regulated, and Heme oxygenase 1 (HO-1), Cytochrome c, and Melanophilin (MLPH) were significantly down-regulated. They were enriched in pathways related to melanin production.
The protein STAT1 usually exists in the form of a functional dimer. When STAT1 is up-regulated, it may be closely related to tumorigenesis. The biological forms of STAT1 include activated phosphorylated forms and the less active non-phosphorylated form [53]. The expression of phosphorylation STAT1 increased when normal human melanocytes (NHMs) [54] and B16F10 cells [55] were treated with the cytokine IFN-γ. In this study, expression of STAT1 in B16F10 cells was also significantly increased. Sunflower EEBP inhibits melanin production through the up-regulation of STAT1.
Caspase-3 is a cysteine–aspartic protease commonly involved in pyroptosis and apoptosis [56], but it is also involved in the processing and production of melanin. Rhododendrol inhibits tyrosinase with a decrease in melanin synthesis in mouse B16 melanoma cells and an increase in caspase-3 levels [57]. In the study of epidermal functional melanocytes and melanin loss, caffeic acid derivatives down-regulated caspase-3 in PIG1 cells, thereby protecting against melanin loss [58]. In this study, the caspase-3 protein was up-regulated after EEBP acted on B16F10 cells. Sunflower EEBP destroyed the protection of melanin loss, thereby inhibiting melanin production.
Fatty acid synthase (FASN) is an important protein that can synthesize fatty acids in cells [59]. During this process, the overexpression of METTL3 can increase the expression of FASN [60]. Different types of fatty acids can inhibit melanin production [61,62,63,64]. Both FASN and METTL3 were significantly up-regulated in this study. Sunflower EEBP increased the synthesis of fatty acids in cells and further inhibited melanin production.
Heme oxygenase 1 (HO-1) is a ubiquitous protein in most human tissues and is involved in many physiological processes [65]. Kaempferol can promote melanin synthesis and the expression of HO-1 in PIG1 cells [66] and reduce the production of ROS in the cells and the damage caused by H2O2. HO-1 was significantly down-regulated in B16F10 cells treated with EEBP. Sunflower EEBP decreased HO-1 levels and blocked the melanin synthesis pathway.
Cytochrome c is an intermembrane mitochondrial protein that interacts in the peroxidation process of various molecules. Cytochrome c can oxidize catecholamines and their S-cysteinyl derivatives, producing melanin as the final product [67]. Cytochrome c was down-regulated by the sunflower EEBP thereby reducing the synthesis of the final product melanin.
Melanophilin (MLPH) is a protein involved in the formation and transfer of melanin [68]. MLPH expression in B16F10 cells was significantly reduced after wogonin treatment [69], consistent with the down-regulation of MLPH in this study when B16F10 cells were treated with the sunflower EEBP.
4. Materials and Methods
4.1. Samples and Chemical Reagents
Rapeseed bee pollen, apricot bee pollen, camellia bee pollen, lotus bee pollen, and sunflower bee pollen were produced in Luoping County of Yunnan Province, Qinglong County of Hebei Province, Fuzhou City of Fujian Province, Jianyang County of Fujian Province, and Xuchang County of Henan Province, China, respectively. All bee pollen samples were harvested in 2023 and stored at −30 °C. The identification of the botanical origins and purities of bee pollen samples was determined by counting the percent of the matching types of pollen grains using a conventional inverted microscope (Nikon TS-100f, Tokyo, Japan) [3]. The purities of bee pollen samples are shown in Table S1. The morphologies of pollen gains are shown in Figure S1.
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, double antibiotics, and serum-free non-programmed cell freezing solution were purchased from Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). LC-MS-grade methanol and formic acid were purchased from Thermo Fisher Scientific (Waltham, MA, USA). LC-MS-grade water was purchased from Merck, Germany. Analytical grade anhydrous ethanol and ferric chloride were purchased from Aladdin Scientific (Riverside, CA, USA). Trolox, 1,1-diphenyl-2-trinitrophenylhydrazine (DPPH), 2,2-azino-bis(3-ethyl-benzothiazole-6-sulfonic acid) diammonium salt (ABTS), petroleum ether, 2,4,6-tripyridyltriazine (TPTZ), PBS powder, mushroom tyrosinase, kojic acid, anhydrous ethanol, and sodium acetate were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Acetic acid, PBS (pH 7.2–7.4), and hydrochloric acid were purchased from Sinopharm (Beijing, China). Trypan blue dye (0.4%) was purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). The CCK-8 kit was purchased from DOJINDO (Kumamoto, Japan). Trypsin was purchased from Hyclone Co., Logan, UT, USA. The B16F10 cell line was purchased from the China Center for Type Culture Collection (Wuhan, China).
4.2. Methods
4.2.1. Extraction of Bee Pollen
EEBPs were prepared according to reference [52], with some modifications. Bee pollen (50 g) was crushed. The lipids of the powder were removed with 100 mL of petroleum ether at 25 °C for 30 min with ultrasonic assistance (40 kHz) two times. Then, the residue was extracted with 200 mL of 80% ethanol at 25 °C for 30 min with ultrasonic assistance two times. Subsequently, the mixture was concentrated using a rotary evaporator after filtration. It was then dried using a vacuum freeze dryer (DGJ-500H, Shanghai Boden Biotechnology Co., Ltd., Shanghai, China) to obtain the EEBP, which was then stored at −30 °C (Haier Biomedical, Qingdao, China) for further experiments.
4.2.2. Determination of the Components in the Ethanol Extracts of Bee Pollens
The total phenolic contents (TPCs) of EEBPs were determined by the Folin–Ciocalteu (FC) colorimetric method [70]. Briefly, the EEBP was dissolved in ethanol and diluted to a concentration of 500 μg/mL. Then, 0.5 mL of the EEBP solution was mixed thoroughly with 2.5 mL of a 10% Folin phenol reagent in a 10 mL centrifuge tube. After 5 min, 2 mL of a 7.5% sodium carbonate solution (Na2CO3) was added to the mixture. The tube was placed in the dark at 25 °C for 60 min. The absorbance at 765 nm was measured by an ultraviolet spectrophotometer (T6, Beijing Puxi General Instrument Co., Ltd., Beijing, China). Gallic acid solutions (0.5 mL) of 10, 20, 40, 80, and 160 μg/mL were employed to establish a standard curve. The TPC is expressed as the gallic acid equivalent (mg GAE/g) contained in the EEBP.
The total flavone contents (TFCs) of EEBPs were determined using rutin standards and the NaNO2-Al(NO3)3-NaOH colorimetric method [43]. The EEBP was dissolved in anhydrous ethanol and diluted to 500 μg/mL. The EEBP solution (1 mL) was added to a 10 mL centrifuge tube, 0.25 mL of a 5% NaNO2 solution was added to mix and react for 6 min, then 0.25 mL of a 10% Al(NO3)3 solution was added to mix and react for 6 min, and finally, 2 mL of a 4% NaOH solution and 1.5 mL of ethanol were added. After standing for 15 min, the absorbance was measured at 510 nm. Rutin working solutions (1 mL) at 10, 20, 40, 80, and 160 μg/mL were used to establish the standard curve. The total flavonoid content is expressed as the rutin equivalent (mg RE/g) contained in the EEBP.
The chemical components of EEBPs were determined using a UHPLC-MS/MS system (Vanquish UHPLC system (Thermo Fisher Scientific Inc., Germering, Germany) coupled with an Orbitrap Q ExactiveTM HF-X mass spectrometer (ThermoFisher, Germering, Germany) by an untargeted metabolomic method performed by Novogene Co., Ltd. (Beijing, China) [71]. EEBP (25 mg) and 5000 μL of an 80% methanol aqueous solution were added to a 10 mL EP tube. It was whirled and centrifuged at 15,000× g at 4 °C for 20 min. A certain amount of the supernatant was diluted with mass spectrometry-grade water to a methanol content of 53%. It was centrifuged again for the UHPLC-MS/MS analysis. The chromatographic column was a Hypesil Gold column (C18) at 40 °C. Solutions of 0.1% formic acid and methanol were mobile phases A and B, respectively. The chromatographic gradient elution program was 0–1.5 min, 98% A; 3 min 15% A; 10 min 0% A; and 10.1–12 min 2% A at 0.2 mL/min. The mass spectrometry conditions were as follows: scan range of 100–1500 m/z; ESI source spray voltage of 3.5 kV; sheath gas flow rate of 35 psi; aux gas flow rate of 10 L/min; capillary temperature of 320 °C; s-lens RF level of 60; aux gas heater temperature of 350 °C; positive and negative polarity; and data-dependent MS/MS secondary scans.
4.2.3. Antioxidant and Tyrosinase Inhibitory Capacities of Ethanol Extracts of Bee Pollens
The DPPH radical scavenging activity, FRAP total antioxidant capacity, and ABTS cation radical scavenging activity of EEBPs were determined at 517, 593, and 734 nm using an ultraviolet spectrophotometer [72]. The results are shown in Trolox equivalents (TE), μg TE/mg EEBP.
The tyrosinase inhibitory capacities were determined according to reference [3], with some modifications. EEBPs were dissolved in ethanol (2 mg/mL) and then diluted to different concentrations (10–50 μg/mL) with PBS buffer (pH 6.8). A sample solution of 25 μL and 25 μL of tyrosinase solution (200 U/mL) were added to a 96-well plate and pre-incubated at 37 °C for 2 min, and then 200 μL of L-DOPA (0.5 mM) was added to each well for the reaction. The absorbance of the mixture was measured at 475 nm every 20 s at 37 °C for a total of 20 times. The inhibition rate was calculated as follows:
where k1 is the slope of the kinetic equation of the sample group and the positive control group, and k0 is the slope of the kinetic equation of the blank control group.
Inhibition rate (%) = (1 − k1/k0) × 100
4.2.4. Anti-Melanogenesis Activities of Ethanol Extracts of Bee Pollens on Melanin Production in Mouse B16F10 Melanoma Cells
Mouse B16F10 melanoma cells were cultured in high-glucose DMEM (containing 10% FBS fetal bovine serum and 1% double antibiotics) in a 5% CO2 incubator at 37 °C (C150, Binder, Tuttlingen, Germany). The cell number in suspension (100 μL) was counted with the assistance of trypan blue staining (100 μL). The concentration of cells was diluted to 5 × 104 cells/mL with the culture medium. Add 100 μL of cell suspension to each well of the 96-well plate. These cells were cultured for 24 h. The culture medium was removed and 100 μL of sterile PBS was added to each well for washing. Culture medium (100 μL) containing 5, 10, 15, 20, or 25 μg/mL EEBPs were added to 6 wells for each dose. Wells containing whole culture medium were the control group. Cells were continuously cultured in the incubator for 48 h. These cells were washed with sterile PBS. And then, 110 μL of culture medium containing the CCK-8 solution was added in a dark place. The cells were cultured for 2 h. Then, the absorbance at 450 nm was measured.
Cell proliferation activity (%) = [(OD of the experimental group − OD of the blank group)/(OD of the control group − OD of the blank group)] × 100
The determination of the anti-melanogenesis effect of EEBPs was performed according to the method of Sim et al. [73], with slight changes. Cells at a concentration of 1 × 104 cells/mL were transferred into 6-well plates. After treatment with 5, 10, 15, 20, or 25 μg/mL EEBP for 48 h, the cells in each group were collected into centrifuge tubes and counted. After centrifugation, 0.5 mL of a 1 mM NaOH solution (containing 10% DMSO) was added and heated in a water bath at 80 °C for 2 h. The absorbance at 405 nm was measured using an ELISA reader.
Relative melanin content (%) = (OD of experimental group/number of cells in the experimental group)/(OD of blank group/number of blank cells) × 100
The correlations among DPPH, FRAP, ABTS, TPC, TFC, antityrosinase, and anti-melanogenesis activities were analyzed.
4.2.5. Analysis of the Anti-Melanogenesis Mechanism of the Ethanol Extract of Sunflower Bee Pollen on Melanin Production in Mouse B16F10 Melanoma Cells by Label-Free Proteomics
Differentially expressed proteins in mouse melanoma cells (B16F10) treated with sunflower EEBP, compared to a control group, were determined using DIA quantitative proteomics. In brief, 2 mL of cells at a concentration of 1 × 105 cells/mL were added to a 6-well plate and cultured for 24 h. After removing the culture medium, the cells were rewashed with 2 mL of PBS (pH 7.2–7.4). The cells were then incubated with 2 mL of culture medium containing 25 μg/mL sunflower EEBP (resulting in a final concentration of 25 μg/mL) for 48 h. Following this, the cells were washed twice with pre-cooled PBS at 4 °C and then digested with trypsin (0.25%). The collected cells were washed again with pre-cooled PBS and centrifuged (TDZ4K, Hunan Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, China) at 137× g for 5 min twice. The cells were frozen in liquid nitrogen for 15 min and stored at −80 °C in a Haier Biomedical refrigerator (Qingdao, China). The extraction and determination of the total protein concentration, as well as the protein spectral analysis, were performed as outlined in a previous report [71].
4.2.6. Statistical Analysis
All experiments except for the chemical substance determination were performed in triplicate. Except for the proteomic experiment, one-way ANOVA was used to analyze the differences using GraphPad Prism 8.4.3 for Windows (GraphPad Software, Inc. San Diego, CA, USA), where p < 0.01 means an extremely statistically significant difference between the treatment group and the control group, and p < 0.05 means a statistically significant difference. The results are expressed as means ± standard errors.
The offline data files for the chemical substance determination were imported into CD 3.3 library search software for processing. Each metabolite was screened by parameters such as the retention time and mass-to-charge ratio. Then, the peak area was corrected with the first QC to make the identification more accurate. Then, the mass deviation was set to 5 ppm, the signal intensity deviation was 30%, and the minimum signal intensity, the adduct ion, and other information were set for peak extraction. At the same time, the peak area was quantified, and the target ion was integrated. The molecular formula was predicted by the molecular ion peak and fragment ion and compared with the mzCloud (https://www.mzcloud.org/, accessed on 10 August 2024), mzVault, and Masslist databases. A blank sample was employed to remove the background ions, and the original quantitative results were standardized to obtain the relative peak area. The compounds with a relative peak area CV higher than 30% in the QC sample were deleted, and, finally, the metabolite identification and relative quantitative values were obtained using the following formula: sample metabolite original quantitative value/(sum of sample metabolite quantitative values/sum of QC1 sample metabolite quantitative values). The data processing was based on the Linux operating system (CentOS v6.6) and R (R-3.4.3) and Python (Python-3.5.0) software.
The spectra obtained from LC-MS/MS were analyzed as described in a previous report [74]. The proteins whose quantities were significantly different between the experimental and control groups (p ≤ 0.05 and fold change (FC) > 1.2 or FC < 0.83) were defined as differentially expressed proteins (DEPs).
5. Conclusions
Each type of bee pollen has unique substances. The antityrosinase activity of sunflower EEBP and anti-melanogenesis activity in B16F10 cells were the highest among the rapeseed, apricot, camellia, lotus, and sunflower EEBPs. The anti-melanogenesis activities of bee pollens were positively correlated with their antityrosinase activities and total phenol contents. The sunflower EEBP inhibited melanogenesis in B16F10 cells by reducing the expression of related proteins in the cAMP, MAPK, and TNF signaling pathways. This result provides a basis for the future industrial development of a raw material for skin whitening using sunflower bee pollen.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph17121634/s1: Table S1. The purity of bee pollen samples. Figure S1. Morphology of bee pollen grains. Figure S2. Volcano map of proteins in B16F10 cells (sunflower EEBP vs. control).
Author Contributions
Conceptualization, methodology, software, W.Y.; data curation, Q.H., J.W. and J.L.; writing—original draft preparation, Q.H. and J.W.; writing—review and editing, W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clarke, D.; Morley, E.; Robert, D. The bee, the flower, and the electric field: Electric ecology and aerial electroreception. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2017, 203, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G.; Frigerio, C.; Lopes, J.; Bogdanov, S. What is the future of Bee-Pollen? J. ApiProd. ApiMed. Sci. 2010, 2, 131–144. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Campos, M.G.; Bogdanov, S.; de Almeida-Muradian, L.B.; Szczęsna, T.; Mancebo, Y.; Frigerio, C.; Ferreira, F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008, 47, 154–161. [Google Scholar] [CrossRef]
- Li, Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.; Hu, L.; Xue, X.; Wu, L.; Hu, F. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef]
- Qiao, J.; Feng, Z.; Zhang, Y.; Xiao, X.; Dong, J.; Haubruge, E.; Zhang, H. Phenolamide and flavonoid glycoside profiles of 20 types of monofloral bee pollen. Food Chem. 2023, 405, 134800. [Google Scholar] [CrossRef] [PubMed]
- Çobanoğlu, D.N. Assessing monofloral bee pollens from Türkiye: Palynological verification, phenolic profile, and antioxidant activity. J. Food Sci. 2024, 89, 1711–1726. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolaylı, S.; Erim, F.B. Characterization of Turkish honeybee pollens by principal component analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT–Food Sci. Technol. 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Cheng, N.; Chen, S.; Liu, X.; Zhao, H.; Cao, W. Impact of schisandrachinensis bee pollen on nonalcoholic fatty liver disease and gut microbiota in highfat diet induced obese mice. Nutrients 2019, 11, 346. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, E.; Li, F.; Meng, L.; Li, Q.; Wu, L. Allergenicity alleviation of bee pollen by enzymatic hydrolysis: Regulation in mice allergic mediators, metabolism, and gut microbiota. Food 2022, 11, 3454. [Google Scholar] [CrossRef] [PubMed]
- Khalil, F.; El-Sheikh, N.M. The effects of dietary Egyptian propolis and bee pollen supplementation against toxicity if sodium fluoride in rats. Vet. Med. J. Giza 2010, 11, 310–316. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaskeviciute, V.; Kaškonas, P.; Mickienė, R.; Maruška, A.S. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Lawag, I.L.; Yoo, O.; Lim, L.Y.; Hammer, K.; Locher, C. Optimisation of bee pollen extraction to maximise extractable antioxidant constituents. Antioxidants 2021, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- De-Melo, A.A.; Estevinho, M.L.; Sattler, J.A.; Souza, B.R.; Freitas, A.D.; Barth, O.M.; Almeida-Muradian, L.B. Effect of processing conditions on characteristics of dehydrated bee-pollen and correlation between quality parameters. LWT–Food Sci. Technol. 2016, 65, 808–815. [Google Scholar] [CrossRef]
- Gercek, Y.C.; Celik, S.; Bayram, S. Screening of plant pollen sources, polyphenolic compounds, fatty acids and antioxidant/antimicrobial activity from bee pollen. Molecules 2022, 27, 117. [Google Scholar] [CrossRef] [PubMed]
- Orvalho, T.; Vaz, D.C.; Ribeiro, V.; Campos, M.J. Storage procedures influence the antioxidant capacity of bee pollen. In Proceedings of the 1st International Conference on Water Energy Food and Sustainability (ICoWEFS 2021), Leiria, Portugal, 10–12 May 2021. [Google Scholar] [CrossRef]
- Leblanc, B.; Davis, O.K.; Boue, S.M.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Babiker, E.E.; Uslu, N.; Ceylan, D.A.; Ghafoor, K.; Özcan, M.M.; Dursun, N.; Ahmed, I.A.; Jamiu, F.G.; et al. Determination of antioxidant activity, phenolic compound, mineral contents and fatty acid compositions of bee pollen grains collected from different locations. J. Apic. Sci. 2019, 63, 69–79. [Google Scholar] [CrossRef]
- Sarić, A.; Balog, T.; Sobocanec, S.; Kusić, B.; Sverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D.; et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- Jin, T.; Saravanakumar, K.; Wang, M. In vitro and in vivo antioxidant properties of water and methanol extracts of linden bee pollen. Biocatal. Agric. Biotechnol. 2018, 13, 186–189. [Google Scholar] [CrossRef]
- Yıldız, O.; Can, Z.; Saral, O.; Yuluğ, E.; Oztürk, F.; Aliyazıcıoğlu, R.; Canpolat, S.; Kolaylı, S. Hepatoprotective potential of chestnut bee pollen on carbon tetrachloride-induced hepatic damages in rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 461478. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, H.S.; Al-Yousef, H.M.; Ashour, A.E.; Ahmed, A.F.; Amina, M.; Issa, I.S.; Bhat, R.S. Antioxidant and hepatorenal protective effects of bee pollen fractions against propionic acid-induced autistic feature in rats. Food Sci. Nutr. 2020, 8, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Aabed, K.; Shafi Bhat, R.; Moubayed, N.; Al-Mutiri, M.; Al-Marshoud, M.; Al-Qahtani, A.; Ansary, A. Ameliorative effect of probiotics (Lactobacillus paracaseii and Protexin®) and prebiotics (propolis and bee pollen) on clindamycin and propionic acid-induced oxidative stress and altered gut microbiota in a rodent model of autism. Cell. Mol. Biol. 2019, 65, 1–7. [Google Scholar] [CrossRef]
- Al-daihan, S.; Bhata, R.S. Protective effect of bee pollen against sodium fluoride induced hepatonephrotoxicity and serum electrolyte changes in rats. Fluoride 2019, 52, 9–17. [Google Scholar]
- Oyarzún, J.E.; Andia, M.E.; Uribe, S.; Núñez Pizarro, P.; Núñez, G.; Montenegro, G.; Bridi, R. Honeybee pollen extracts reduce oxidative stress and steatosis in hepatic cells. Molecules 2020, 26, 6. [Google Scholar] [CrossRef]
- Pham, T.N.; Cazier, E.A.; Gormally, E.; Lawrence, P. Valorization of biomass polyphenols as potential tyrosinase inhibitors. Drug Discov. Today 2024, 29, 103843. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Lee, J.; Hao, H.; Park, Y.D.; Zhan, Y.; Lü, Z.R. The effect of oxaloacetic acid on tyrosinase activity and structure: Integration of inhibition kinetics with docking simulation. Int. J. Biol. Macromol. 2017, 101, 59–66. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigm. Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef]
- Saud, A.; Sagineedu, S.R.; Ng, H.S.; Stanslas, J.; Lim, J.C.W. Melanoma metastasis: What role does melanin play? Oncol. Rep. 2022, 48, 217. [Google Scholar] [CrossRef]
- Bose, A.; Petsko, G.A.; Eliezer, D. Parkinson’s disease and melanoma: Co-occurrence and mechanisms. J. Park. Dis. 2018, 8, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Krainc, T.; Monje, M.H.G.; Kinsinger, M.; Bustos, B.I.; Lubbe, S.J. Melanin and neuromelanin: Linking skin pigmentation and Parkinson’s disease. Mov. Disord. 2023, 38, 185–195. [Google Scholar] [CrossRef]
- Silva, C.C.; Benati, R.B.; Massaro, T.N.; Pereira, K.D.; Gaspar, L.R.; Marcato, P.D. Antioxidant and anti-tyrosinase activities of quercetin-loaded olive oil nanoemulsion as potential formulation for skin hyperpigmentation. J. Dispers. Sci. Technol. 2022, 44, 2628–2638. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef]
- Demirkiran, O.; Sabudak, T.; Ozturk, M.; Topcu, G. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens Subsp. petrisavi. J. Agric. Food Chem. 2013, 61, 12598–12603. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Maisuthisakul, P.; Gordon, M.H. Antioxidant and tyrosinase inhibitory activity of mango seed kernel by product. Food Chem. 2009, 117, 332–341. [Google Scholar] [CrossRef]
- Khongkarat, P.; Ramadhan, R.; Phuwapraisirisan, P.; Chanchao, C. Safflospermidines from the bee pollen of Helianthus annuus L. exhibit a higher in vitro antityrosinase activity than kojic acid. Heliyon 2020, 6, e03638. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, J.L. Hydroquinone and its analogues in dermatology-a risk-benefit viewpoint. J. Cosmet. Dermatol. 2006, 5, 196–203. [Google Scholar] [CrossRef]
- Végh, R.; Sipiczki, G.; Csóka, M. Investigating the antioxidant and color properties of bee pollens of various plant sources. Chem. Biodivers. 2023, 20, e202300126. [Google Scholar] [CrossRef]
- Oroian, M.; Dranca, F.; Ursachi, F. Characterization of romanian bee pollen—An important nutritional source. Foods 2022, 11, 2633. [Google Scholar] [CrossRef] [PubMed]
- De Arruda, V.A.S.; dos Santos, A.V.; Sampaio, D.F.; Araújo, E.D.S.; Peixoto, A.L.D.C.; Estevinho, L.M.; de Almeida-Muradian, L.B. Brazilian bee pollen: Phenolic content, antioxidant properties and antimicrobial activity. J. Apic. Res. 2021, 60, 775–783. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. LWT–Food Sci. Technol. 2021, 142, 110973. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2018, 54, 335–346. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT 2019, 112, 108244. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Wang, K.; Li, C. Antioxidant and tyrosinase inhibitory properties of aqueous ethanol extracts from monofloral bee pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in b16 mouse melanoma cells. Front. Pharmacol. 2017, 8, 104. [Google Scholar] [CrossRef]
- Aylanc, V.; Larbi, S.; Calhelha, R.; Barros, L.; Rezouga, F.; Rodríguez-Flores, M.S.; Seijo, M.C.; El Ghouizi, A.; Lyoussi, B.; Falcão, S.I.; et al. Evaluation of antioxidant and anticancer activity of mono- and polyfloral moroccan bee pollen by characterizing phenolic and volatile compounds. Molecules 2023, 28, 835. [Google Scholar] [CrossRef]
- Duh, P.D.; Tu, Y.Y.; Yen, G.C. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat). Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, X.; Huang, Q.; Zhang, L.; Liu, X.; Liu, R.; Lu, Q. Antioxidant and anti-inflammatory activities of rape bee pollen after fermentation and their correlation with chemical components by ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry-based untargeted metabolomics. Food Chem. 2023, 409, 135342. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, M.; Zhu, X.; Liu, R.; Lu, Q. Metabolomics reveals that phenolamides are the main chemical components contributing to the anti-tyrosinase activity of bee pollen. Food Chem. 2022, 389, 133071. [Google Scholar] [CrossRef] [PubMed]
- Bonham, A.J.; Wenta, N.; Osslund, L.M.; Prussin, A.J., 2nd; Vinkemeier, U.; Reich, N.O. STAT1: DNA sequence-dependent binding modulation by phosphorylation, protein: Protein interactions and small-molecule inhibition. Nucleic Acids Res. 2013, 41, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, H.; Han, J.; Jin, S.H.; Park, J.Y.; Shin, D.W.; Lee, T.R.; Kim, K.; Lee, A.Y.; Noh, M. IL-4 inhibits the melanogenesis of normal human melanocytes through the JAK2-STAT6 signaling pathway. J. Investig. Dermatol. 2013, 133, 528–536. [Google Scholar] [CrossRef]
- Han, N.R.; Park, H.J.; Ko, S.G.; Moon, P.D. Stigmasterol exerts an anti-melanoma property through down-regulation of reactive oxygen species and programmed cell death ligand 1 in melanoma cells. Antioxidants 2024, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.A.; Thapa, R.; Afzal, O.; Agrawal, N.; Almalki, W.H.; Kazmi, I.; Alzarea, S.I.; Altamimi, A.S.A.; Prasher, P.; Singh, S.K.; et al. The pyroptotic role of Caspase-3/GSDME signalling pathway among various cancer: A Review. Int. J. Biol. Macromol. 2023, 242, 124832. [Google Scholar] [CrossRef]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigm. Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef]
- Jin, R.; Hu, W.; Zhou, M.; Lin, F.; Xu, A. Caffeic acid derivative WSY6 protects melanocytes from oxidative stress by reducing ROS production and MAPK activation. Heliyon 2024, 10, e24843. [Google Scholar] [CrossRef]
- Günenc, A.N.; Graf, B.; Stark, H.; Chari, A. Fatty acid synthase: Structure, function, and regulation. In Macromolecular Protein Complexes IV; Springer: Cham, Switzerland, 2022; Volume 99, pp. 1–33. [Google Scholar] [CrossRef]
- Xie, W.; Ma, L.L.; Xu, Y.Q.; Wang, B.H.; Li, S.M. METTL3 inhibits hepatic insulin sensitivity via N6-methyladenosine modification of Fasn mRNA and promoting fatty acid metabolism. Biochem. Biophys. Res. Commun. 2019, 518, 120–126. [Google Scholar] [CrossRef]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef]
- Kose, A. Chemical composition and tyrosinase inhibitory activities of fatty acids obtained from Heterotrophic Microalgae, S. limacinum and C. cohnii. Appl. Biochem. Biotechnol. 2023, 195, 369–385. [Google Scholar] [CrossRef]
- Diwakar, G.; Rana, J.; Saito, L.; Vredeveld, D.; Zemaitis, D.; Scholten, J. Inhibitory effect of a novel combination of Salvia hispanica (chia) seed and Punica granatum (pomegranate) fruit extracts on melanin production. Fitoterapia 2014, 97, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Okadaic acid suppresses melanogenesis via proteasomal degradation of tyrosinase. Biol. Pharm. Bull. 2013, 36, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme Oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mei, X.; Shi, W. Kaempferol promotes melanogenesis and reduces oxidative stress in PIG1 normal human skin melanocytes. J. Cell. Mol. Med. 2023, 27, 982–990. [Google Scholar] [CrossRef]
- Rosei, M.A.; Blarzino, C.; Coccia, R.; Foppoli, C.; Mosca, L.; Cini, C. Production of melanin pigments by cytochrome c/H2O2 system. Int. J. Biochem. Cell Biol. 1998, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, W.; Fan, D.; Hu, J.; An, X.; Wang, Z. The biochemistry of melanogenesis: An insight into the function and mechanism of melanogenesis-related proteins. Front. Mol. Biosci. 2024, 11, 1440187. [Google Scholar] [CrossRef]
- Kudo, M.; Kobayashi-Nakamura, K.; Tsuji-Naito, K. Bifunctional effects of O-methylated flavones from Scutellaria baicalensis Georgi on melanocytes: Inhibition of melanin production and intracellular melanosome transport. PLoS ONE 2017, 12, e0171513. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, Y.; Yang, A.; Tan, W.; Liu, X.; Yang, W. Antitumor effect of poplar propolis on human cutaneous squamous cell carcinoma A431 cells. Int. J. Mol. Sci. 2023, 24, 16753. [Google Scholar] [CrossRef]
- Tan, W.; Tian, Y.; Zhang, Q.; Miao, S.; Wu, W.; Miao, X.; Kuang, H.; Yang, W. Antioxidant and antibacterial activity of Apis laboriosa honey against Salmonella enterica serovar Typhimurium. Front. Nutr. 2023, 10, 1181492. [Google Scholar] [CrossRef]
- Sim, M.; Choi, I.; Cho, J.; Shin, H.M.; Cho, H. Anti-melanogenesis and anti-oxidant of Salix pseudo-lasiogyne water extract in α-MSH-induced B16F10 melanoma cells. Food Agric. Immunol. 2017, 28, 1003–1016. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Yang, A.; Zhang, C.; Miao, X.; Yang, W. Antitumor effects of poplar propolis on DLBCL SU-DHL-2 Cells. Foods 2023, 12, 283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).