Abstract
A series of novel 3-(prop-1-en-2-yl)azetidin-2-one, 3-allylazetidin-2-one and 3-(buta-1,3-dien-1-yl)azetidin-2-one analogues of combretastatin A-4 (CA-4) were designed and synthesised as colchicine-binding site inhibitors (CBSI) in which the ethylene bridge of CA-4 was replaced with a β-lactam (2-azetidinone) scaffold. These compounds, together with related prodrugs, were evaluated for their antiproliferative activity, cell cycle effects and ability to inhibit tubulin assembly. The compounds demonstrated significant in vitro antiproliferative activities in MCF-7 breast cancer cells, particularly for compounds 9h, 9q, 9r, 10p, 10r and 11h, with IC50 values in the range 10–33 nM. These compounds were also potent in the triple-negative breast cancer (TBNC) cell line MDA-MB-231, with IC50 values in the range 23–33 nM, and were comparable with the activity of CA-4. The compounds inhibited the polymerisation of tubulin in vitro, with significant reduction in tubulin polymerization, and were shown to interact at the colchicine-binding site on tubulin. Flow cytometry demonstrated that compound 9q arrested MCF-7 cells in the G2/M phase and resulted in cellular apoptosis. The antimitotic properties of 9q in MCF-7 human breast cancer cells were also evaluated, and the effect on the organization of microtubules in the cells after treatment with compound 9q was observed using confocal microscopy. The immunofluorescence results confirm that β-lactam 9q is targeting tubulin and resulted in mitotic catastrophe in MCF-7 cells. In silico molecular docking supports the hypothesis that the compounds interact with the colchicine-binding domain of tubulin. Compound 9q is a novel potent microtubule-destabilising agent with potential as a promising lead compound for the development of new antitumour agents.
1. Introduction
Breast cancer is the most commonly reported cancer among women in developed countries. []. Over the last decade, the clinical treatment options for breast cancer patients have significantly improved with the approval of multiple drugs for various indications [,]. The hormone receptor (HR) positive/human epidermal growth factor receptor 2 (HER2)-negative subtype is the most commonly identified subtype and is found in approximately 70% of breast cancers diagnosed. Endocrine therapies (tamoxifen, fulvestrant, anastrozole, exemestane and letrozole) are conventionally used for treatment of this HR+/HER2- breast cancer subtype. Targeted therapies such as CDK4/6 inhibitors palbociclib, ribociclib and abemaciclib [] in combination with endocrine therapies show improved therapeutic effects in HR+/HER2- and metastatic breast cancer (MBC) []. Alpelisib, a PI3K inhibitor, is approved for patients with HR+, HER2- and PIK3CA-mutated cancers in combination with fulvestrant [], while the PARP inhibitor olaparib targets BRCA mutations in early breast cancer []. Recently approved breast cancer drugs include the antibody–drug conjugate (ADC) trastuzumab deruxtecan [] and the estrogen receptor degrader (SERD) elacestrant [].
Microtubule-targeting agents (MTAs) such as paclitaxel and docetaxel and the vinca alkaloid vinorelbine are widely used in breast cancer chemotherapy. [,] MTAs interact with tubulin at eight major binding sites: the binding sites for taxane and vinca alkaloid [], gatorbulin [], laulimalide/peloruside [], maytansine [], pironetin [], cevipabulin [] and colchicine 1a (Figure 1) []. Eribulin, a potent inhibitor of microtubule dynamics, is used to treat refractory breast cancers [,]. Although colchicine (1a) inhibits mitosis, the clinical value of the drug is limited by its low therapeutic index (Figure 1) []. Many colchicine-type binding site inhibitors (CBSI) act as angiogenesis inhibitors and vasculature-disrupting agents (VDAs). Combretastatins CA-1 (2a) and CA-4 (2c) and structural analogues demonstrate potent cytotoxicity against human cancer cells (Figure 1) []. The water-soluble CA-1 diphosphate (2b), CA-4 phosphate (2d), CA-4 serine prodrug ombrabulin (2e) [] and CA-4 phosphoramidate [] have been explored as novel anticancer prodrugs.
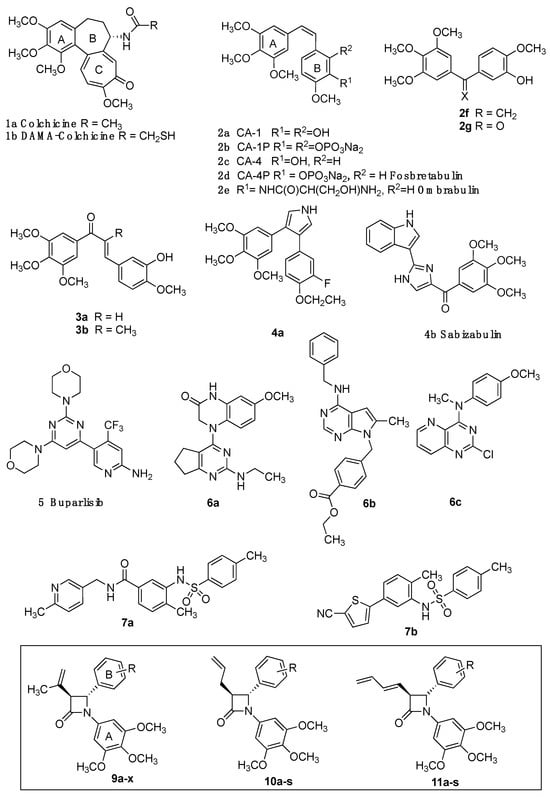
Figure 1.
Small molecules targeting the colchicine-binding site of tubulin. Colchicine 1a, DAMA-colchicine 1b, combretastatins 2a-c, fosbretabulin 2d, ombrabulin 2e, isocombretastatin 2f, phenstatin 2g, chalcones 3a, 3b; colchicine-binding site inhibitors 4a, sabizabulin 4b, buparlisib 5, dihydroquinoxalinone 6a, pyrrolopyrimidine 6b, pyridopyrimidine 6c, sulfonamides 7a, 7b and β-lactam target structures 9a-x, 10a-s, 11a-s.
Isocombretastatin 2f [], dihydronaphthalene OXi6196 [,], phenstatin 2g [] and chalcones 3a and 3b display comparable biological activity to CA-4 []; however, poor water solubility and cis/trans isomerization of combretastatins remain problematic []. CA-4 analogues are reported in which a heterocycle replaces the olefinic bond of CA-4 and prevents cis/trans isomerization [,,,]. The pyrrole 4a induces significant apoptosis [], while the bis-indole sabizabulin 4b is effective in metastatic breast [,] and prostate cancers []. The 4-aryl-4H-chromene crolibulin [], diketopiperazine plinabulin [] and pyrimidine PI3K inhibitor buparlisib 5 inhibit tubulin polymerization, with promising activity in metastatic triple-negative breast cancer (TNBC) (Figure 1) [].
Among recently reported colchicine-binding site inhibitors are dihydroquinoxalinone 6a (SB226) [], pyrrolopyrimidine 6b [] and sulfonamides 7a and 7b [,]. Interestingly 5, 6a, 6b, 7a and 7b, together with the recently reported quinazoline 6c [], lack the characteristic 3,4,5-trimethoxyaryl pharmacophore.
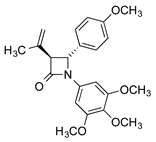
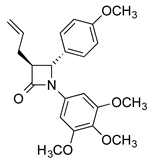
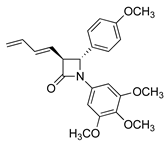
CA-4 analogues containing the β-lactam heterocycle have been reported [,,], while chiral azetidin-2-ones disrupt tubulin polymerization and suppress angiogenesis [,,]. We previously reported the antimitotic properties of novel β-lactam analogues of CA-4, which were synthesised using optimised Staudinger and Reformatsky chemistry, with preferred trans stereochemistry for the C3 and C4 ring substituents [,,]. These β-lactam compounds are distinguished by introduction of the 3,4,5-trimethoxyphenyl ring A (as in CA-4), required for activity, a β-lactam ring as the linking group to replace the double bond of CA-4 (thus preventing E/Z isomerisation in aqueous conditions) and a substituted aryl ring at C-4 as ring B.
To progress our understanding of effects of C-3 substitution on the antiproliferative activity of these β-lactam compounds, we synthesised a series of azetidin-2-ones having prop-1-en-2-yl, allyl and buta-1,3-dien-1-yl substituents at C3 of the azetidin-2-one ring (see Figure 1 target structures). The cytotoxic effects of the novel compounds and prodrugs in ER+ human MCF-7 breast cancer cells and triple-negative MDA-MB-231 breast cancer cells were determined together with their pro-apoptotic and tubulin-targeting effects.
2. Results and Discussion
2.1. Chemistry: Synthesis of β-Lactams
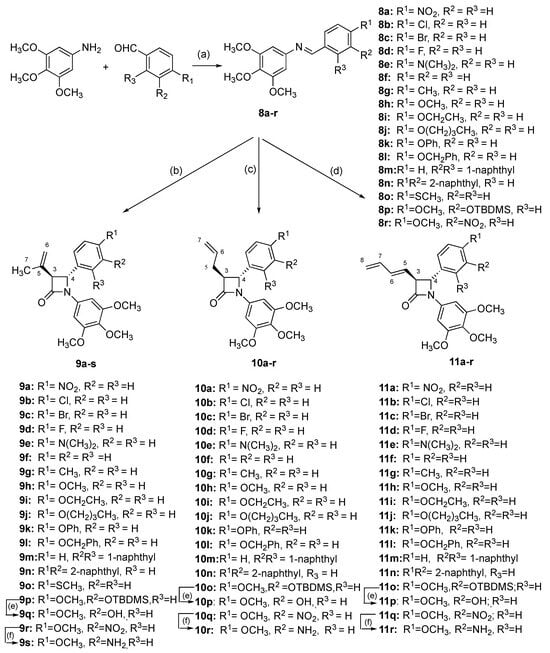
β-Lactams are the most widely used group of antibiotic drugs for bacterial infections []; β-lactam-containing molecules are also useful as intermediates in organic synthesis, and many synthetic routes are available for the construction of the β-lactam ring [,,], including the recently reported carbonylative formal cycloaddition of alkylarenes with imines []. In the present work, the required series of 3-isopropenyl β-lactams with a variety of ring B aryl substituents located at C-4 of the β-lactam ring were obtained by Staudinger reaction of imines with 3,3-dimethylacryloyl chloride. Schiff bases 8a-8r were prepared by the condensation of the appropriately substituted arylaldehyde with the 3,4,5-trimethoxyaniline catalysed by sulphuric acid (Scheme 1). The imines contained the C-4 aryl ring B substituents e.g., nitro (8a), halogen (8b-8d), alkyl (8g), amino (8e), ethers (8h-8l, 8p), thioether (8o) and naphthyl (8m and 8n). The Schiff bases (8s-8u) where ring B is located at C-1 of the imine were obtained by reaction of 3,4,5-trimethoxybenzaldehyde with appropriate anilines (Scheme 2), together with additional imines 8v-w. The 3,4,5-trimethoxy-substituted A-ring of CA-4 provides important interactions with the colchicine-binding site residues of tubulin []. The crystal structures of the imines 8h and 8i demonstrated the E configuration of the imines (Supplementary Materials Tables S1 and S13).
Scheme 1.
Synthesis of β-lactam compounds 9a-s, 10a-r, 11a-r. Scheme reagents and conditions: (a) EtOH, conc. H2SO4, reflux, 4 h; (b) 3,3-dimethylacryloyl chloride, triethylamine, dry DCM, reflux, 5 h, N2 (7–51%); (c) 4-pentenoyl chloride, triethylamine, dry DCM, reflux, 5 h, N2 (11–96%); (d) (i) sorbic acid, tripropylamine, dry DCM, 2-chloro-1-methylpyridinium iodide, reflux, 12 h, N2; (ii) imine, DCM, 12 h reflux (16–59%); (e) TBAF, dry THF, 0 °C, 30 min, N2 (22–37%); (f) Zn dust, acetic acid, 7 days, N2, 20 °C (48–96%). Products obtained as a mixture of enantiomers; one enantiomer illustrated.
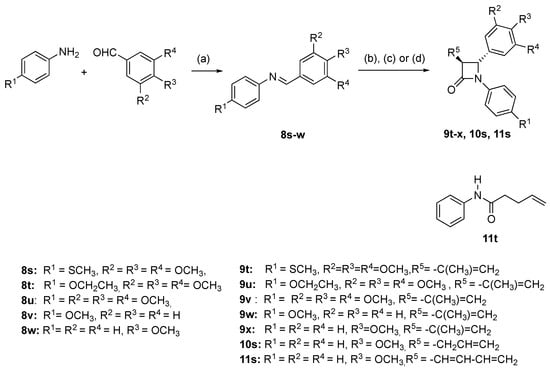
Scheme 2.
Synthesis of compounds 9t-x, 10s, 11s, 11t. Reagents and conditions: (a) EtOH, reflux, 4-5 h. (b) For compounds 9t-9x: 3,3-dimethylacryloyl chloride, triethylamine, dry DCM, reflux, 5 h, N2 (4.6–45%). (c) For compound 10s: 4-pentenoyl chloride, triethylamine, dry DCM, reflux, 5 h, N2 (40%). (d) For compound 11s, 11t: (i) sorbic acid, tripropylamine, dry DCM, 2-chloro-1-methylpyridinium iodide, reflux, 12 h, N2; (ii) imine, DCM, 12 h reflux (45%). β-lactam products obtained as a mixture of enantiomers; one enantiomer illustrated.
The novel 3-(prop-1-en-2-yl)azetidin-2-ones (9a-p, 9r) were obtained by reaction of imines 8a-8r with 3,3-dimethylacryloyl chloride using the Staudinger reaction conditions with triethylamine as the base [,,] (Scheme 1; Scheme 2). The β-lactam compounds (9t-v), were also prepared containing the 3,4,5-trimethoxyphenyl substituent (ring A) at the C-4 position, together with structurally related β-lactams 9w and 9x (Scheme 2). All products are obtained as a racemic mixture, and one enantiomer is illustrated. β-Lactam 9q containing the ring B phenol was obtained by reaction of the tertbutylsilyloxy imine 8p and 3,3-dimethylacryloyl chloride to afford the silyl ether 9p, which was subsequently deprotected with tBAF to afford 9q (Scheme 1). Interesting biochemical activity has been demonstrated by synthetic CA-4 analogues in which the phenol on ring B is replaced by the amino substituent []. The amino-substituted β-lactam 9s was obtained by reduction of the nitro compound 9r using zinc dust in the presence of acetic acid (Scheme 1).
The β-lactams 9a-9x were obtained with exclusively trans configuration with coupling constant J = 1–3 Hz for H-3 and H-4 protons (J = 5–6 Hz are usually observed for β-lactams with cis stereochemistry []). For compound 9q, the aliphatic signal at δ 1.88 pm was assigned to the methyl group of the C-3 isopropenyl substituent, with the corresponding carbon observed at δ 20.17 ppm in 13C NMR spectrum. H-3 was identified as a doublet at δ 3.72 coupled with H-4 at δ 4.71 (J = 2.52 Hz). The corresponding C-3 and C4 carbons appear at 60.50 and 66.37 ppm, respectively, in the 13C NMR spectrum. The geminal protons of H-6 appear in the 1H NMR spectrum at δ 5.02 and 5.07 as broad singlet signals, and C-6 is observed as a negative signal in the DEPT 90 spectrum at δ 113.93 ppm. The β-lactam carbonyl C-2 appeared furthest downfield in the 13C NMR spectrum at δ 164.81 ppm. (See Supplementary Materials Figures S1–S20). The β-lactams 9a-9x demonstrated the characteristic β-lactam IR absorption at 1750 cm−1.
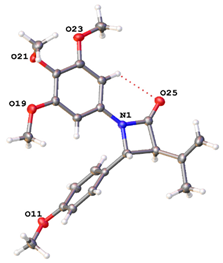
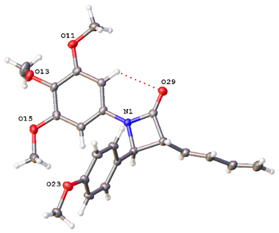
The X-ray crystal structure of compound 9h (Table 1) confirmed the trans configuration for H-3 and H-4 and is consistent with data previously reported for colchicine [], combretastatins [,] and monocyclic β-lactams [,] (Table S12). Hydrogen bonding is observed between the β-lactam carbonyl oxygen and the ortho hydrogen of ring A [].

Table 1.
X-ray crystal structure of compounds 9h, 10h and 11h a,b.
A series of trans-3-allyl-β-lactams, 10a-s, with a variety of substituents at C4 of the β-lactam ring were next synthesised as CA-4 analogues from imines 8a-r by Staudinger reaction using 4-pentenoyl chloride (Scheme 1 and Scheme 2). The compounds were obtained exclusively with trans stereochemistry in moderate yields (11–96%) after chromatographic purification. In the 1H NMR spectrum for 10h, H-5 appeared as a multiplet (δ 2.55–2.74), with H-3 as a multiplet at δ 3.19–3.23. The doublet at δ 4.63 ppm was assigned to H-4 (J = 2.48 Hz), indicating the trans isomer was isolated. Two alkene methylene protons (H-7) were observed as a multiplet signal at δ 5.13–5.19, with the alkene methine proton (H-6) as a multiplet at δ 5.83–5.89. In the 13C NMR spectrum, the signals at 32.34 and 117.17 ppm (negative in DEPT-90 spectrum) are assigned to C-5 and C-7, respectively, and C-3 and C-4 of the β-lactam ring are observed at 60.06 and 60.49 ppm, respectively (see Supplementary Materials Figures S1–S20). The trans stereochemistry at C3 and C4 for 10h was confirmed by X-ray crystallography analysis (Table 1 and Supplementary Materials, Tables S1 and S12). The phenolic compound 10p was obtained in 22% overall yield on treatment of the silyl ether 10o with TBAF, while the ring B amino compound 10r was obtained by reduction of the nitro compound 10q (96%).
The buta-1,3-dienyl-containing azetidinones 11a-o, 11q and 11s were next prepared in moderate yield (16–55%) by direct reaction of the carboxylic acid (sorbic acid) with the panel of imines 8a-8r using 2-chloro-N-methylpyridinium iodide as an acid-activating agent and tripropylamine as the base (Scheme 1 and Scheme 2) []. Exclusively trans isomer products were obtained. Additionally, 11s could also be obtained by reaction of sorbyl chloride with imine 8w using triethylamine as base []. Deprotection of the silyl ether 11o with TBAF afforded the phenol 11p, while reduction (Zn/acetic acid) of the 4-nitrophenyl-β-lactam 11q yielded the amine 11r.
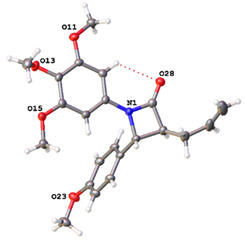
The 1H NMR spectrum of the β-lactam 11c confirmed the assigned structure, with the multiplet signal at δ 3.76–3.77 assigned to H-3 and the doublet at δ 4.74 (J = 2.00 Hz) assigned to H-4, confirming the trans isomer. The H-8 alkene protons were observed as multiplet signals (δ 5.17–5.29) and the alkene H-5 as a double doublet at δ 5.87 (J5,6 = 14.06 Hz and J5,3 = 8.02 Hz), while the multiplet signals δ 6.31-6.40 were diagnostic for the alkenes H-6 and H-7. In the 13C NMR spectrum, the β-lactam C-3 and C-4 signals are observed at 55.63 and 62.75 ppm, respectively; the signal at 118.46 ppm (negative in DEPT-90 spectrum) was assigned to the methylene C-8. The X-ray structure of compound 11h demonstrates the trans configuration for H-3 and H-4 (Table 1 and Supplementary Materials, Tables S1 and S12). The amide 11t [], obtained by acylation of the sorbic acid, was isolated as a minor product in the preparation of 11s (see X-ray crystal structure in Supplementary Materials Table S13).
Prodrugs of amino-containing azetidinones 9s, 10r and 11r were prepared by reaction with the Fmoc-protected amino acids L-phenylalanine, L-valine and glycine using HOBt and the coupling reagent DCC (Scheme 3) []. The Fmoc protecting group was removed from products 12a-e under mild basic conditions with aqueous sodium hydroxide in methanol (Scheme 3) to afford the β-lactam conjugates (13a-e) in 38–59% yield following purification by flash chromatography. The 1H NMR spectra of products 12a-e and 13a-e are complex, possibly due to the presence of rotamers arising from the amino acid moieties []. The 1H NMR spectrum of the diastereomeric prodrug 13e confirmed the trans stereochemistry with H-4 as a doublet, δ 5.32 (J 3,4 = 1.48 Hz), while an additional amide carbonyl signal in the region 1680–1690 cm−1 is observed in the IR spectra of these prodrugs (see Supplementary Materials Figures S1–S20).
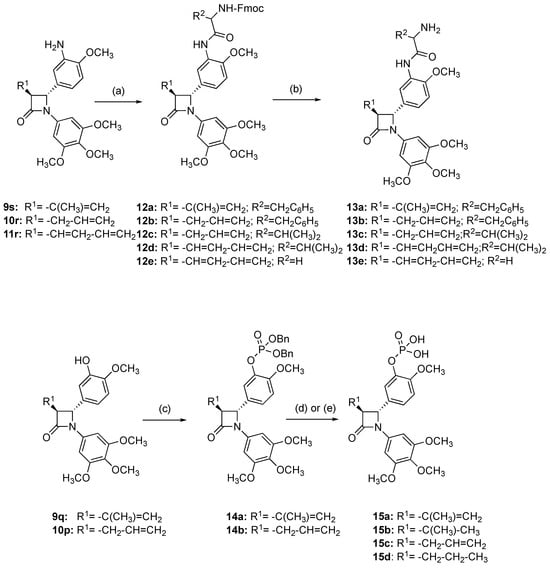
Scheme 3.
Synthesis of β-lactam prodrugs 13a-e, 15a-d. Scheme reagents and conditions: Amino acid prodrugs 13a-e: (a) Fmoc amino acid phenylalanine, glycine or alanine, anhydrous DMF, DCC, HOBt.H2O, 20 °C, 24 h (18–85%); (b) 2N NaOH aq, MeOH, 20 °C, 24 h (38–59%). Phosphate prodrugs 15a-d: (c) dibenzyl phosphite, DIPEA, DMAP, CCl4, CH3CN, −10 °C (27–45%); (d) for compounds 15a and 15c, (i) bromotrimethylsilane, dry DCM, 45 min, (ii) Na2S2O3 (57–96%); (e) for compounds 15b and 15d, Pd/C (10%), EtOAc, 3 h (88–98%). Products obtained as a mixture of diastereomers (12a-d, 13a-d) and enantiomers (12e, 13e, 14a, 14b and 15a); one enantiomer illustrated.
Phosphate ester prodrugs of the phenols 9q and 10p were prepared by controlled esterification [] to afford the dibenzyl phosphate esters (14a, 14b) (Scheme 3). In the 1H NMR spectrum of 14a, the H-4 signal was observed at δ 4.68 as a doublet (J4,3 = 2.52 Hz) and demonstrated trans stereochemistry with H-3 (δ 3.68, J3,4 = 2.04 Hz). The methyl protons were identified at δ 1.86; the alkene protons were assigned as broad singlets at δ 5.02 and δ 5.05, while the benzylic methylene signals occurring as a multiplet at δ 5.13-5.17 were confirmed with negative signals in the DEPT experiment (69.48, 69.50 ppm).
Treatment of the phosphate esters 14a and 14b with bromotrimethylsilane afforded the desired phosphates 15a and 15c. Reduction of 14a and 14b with hydrogen and palladium/carbon catalyst also allowed removal of the dibenzyl protecting groups; however, reduction of the C-3 alkene occurred to afford compounds 15b and 15d (without decomposition of the main four-member ring). The 1H NMR spectrum of compound 15a confirms the removal of the benzylic group protons, with the isopropyl methyl signal at δ 1.86, and the β−lactam and vinylic protons overlapping as a multiplet at δ 4.91–5.06 (H-3, H-4, H6). In the reduced product 15b, the methyl (δ 1.00–1.08) and methine (δ 2.06) signals for the C-3 isopropyl substituent are clearly identified together with the β-lactam H-3 and H-4 signals at δ 2.99 and δ 4.64, respectively.
The synthesis of structurally related β-lactams that contain alkene and ester substituents at C-3 was investigated (Scheme 4). Lithium enolates of 3-unsubstituted β-lactams react with numerous electrophiles to provide 3-substituted compounds []. In the present work, alkylation of the enolate of 3-unsubstituted β-lactams 16a-c with cinnamyl bromide cleanly afforded the 3-substituted β-lactams (17a-c) (Scheme 4). Similarly, treatment of the enolates of compounds 16a and 16b with ethyl bromoacetate afforded the ester products 17f and 17g, respectively. The enolate chemistry is stereoselective, and compounds 17a-c, 17f and 17g were obtained with exclusively trans stereochemistry. The structural assignment for compound 17a was confirmed from the 1H NMR,13C NMR and HH COSY spectra (see Supplementary Materials, Figures S1–S20). The multiplet signals δ 2.69–2.93 were assigned as H-5 with coupling to H-3, H-6 and H-5, while the corresponding carbon appears as a negative signal at 31.68 ppm in the DEPT 90 spectrum. The multiplet signal δ 3.28–3.33 was attributed to H-3. The doublet at δ 4.69 (J = 2.28 Hz) was assigned to H-4 of the β-lactam ring. The multiplet (δ 6.24–6.31) was assigned to the alkene H-6 with coupling to H-5 and H-7. The signal at δ 6.51 is diagnostic for the alkene H-7 with a trans vicinal coupling constant of 15.8 Hz.
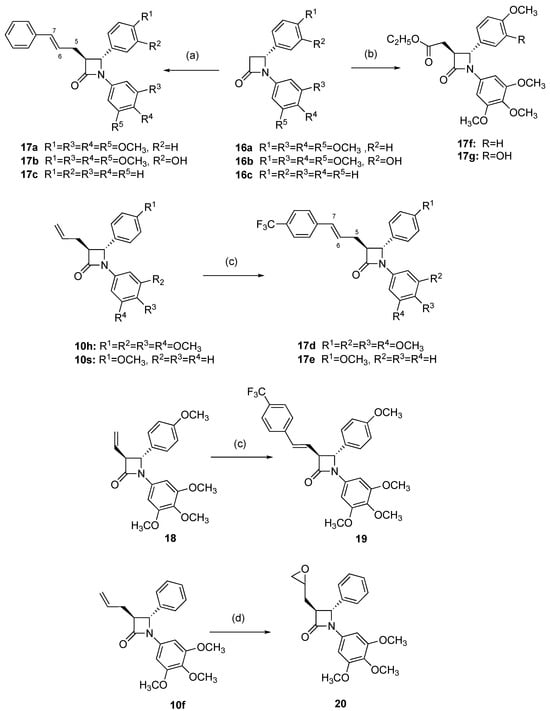
Scheme 4.
Synthesis of β-lactam compounds 17a-g, 19 and 21. Scheme reagents and conditions: (a) LDA, −78 °C, THF followed by 3-bromo-1-phenylpropene, 30 min, then 20 °C for 5 min (14–90%); (b) LDA, −78 °C, THF followed by ethyl bromoacetate, 30 min, then 20 °C for 5 min (60–77%); (c) 1-Bromo-4-(trifluoromethyl)benzene, Pd(II)(OAc)2, PPh3, KOAc, Bu4NCl, DMF, N2, 80 °C, 18 h (19–72%); (d) mCPBA, CH2Cl2, 24 h, 20 °C (67%). Products obtained as a mixture of enantiomers; one enantiomer illustrated.
To explore the biological effects of additional structural modifications of the 3-(prop-1-en-2-yl), 3-allyl and 3-(buta-1,3-dien-1-yl)-1,4-diarylazetidin-2-ones, the alkenes 10s, 10h and 18 were successfully coupled with 4-trifluoromethylphenyl bromide using the catalyst PdOAc2/PPh3 to obtain the products 17d, 17e and 19 with retention of the trans β-lactam stereochemistry (Scheme 4). Introduction of the trifluoromethyl substituent may be useful for exploring potential protein ligand interactions for these compounds []. Treatment of the 3-allyl-β-lactam 10f with meta-chloroperbenzoic acid (mCPBA) afforded the epoxide 20 (67%).
In a further series of structural modifications, complex β-lactams were obtained by Diels–Alder cycloaddition reactions of 3-butadienylazetidiones 11b, 11c, 11j and 11p with maleic anhydride and N-phenylmaleimide as dienophiles to afford products 21a-d, in moderate yields (Scheme 5). Diels–Alder cycloaddition reactions of 3-butadienylazetidinones with dienophiles such as acrylic acid and benzoquinone have been reported [,,]. The structure of 21d was confirmed from 1H, 13C and HMBC NMR spectra (see Supplementary Materials, Figures S1–S20). H-3 was observed as a double doublet (δ 3.94, J 2.00 Hz), with coupling to H-5 and H-4, while H-4 was identified as a doublet (δ 4.64) with trans coupling (J3,4 2.00 Hz). The β-lactams 11m and 11n reacted with dimethylacetylene dicarboxylate to afford products 22a, 22b and 22c, 22d as diastereoisomeric mixtures in low yield (22–23%) (Scheme 5). Separation by flash chromatography afforded each of the pure diastereomeric β-lactams (22a, 22b and 22c, 22d), and the diastereomeric ratio was determined for the isolated products to be 1.75:1 for 22a:22b and 1.3:1 for 22c:22d. The trans stereochemistry of the β-lactam products was confirmed from the 1H NMR spectrum, with J 3,4 = 2 Hz.
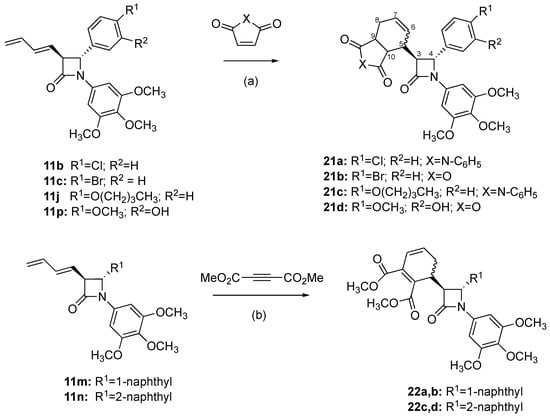
Scheme 5.
Synthesis of maleimide, maleic anhydride and dimethylacetylene dicarboxylate adducts 21a-d, 22a-d. Scheme reagents and conditions: (a) maleic anhydride or N-phenylmaleimide, toluene, reflux, 1 h (24–70%); (b) toluene, reflux, 6–7 h, N2 (8–14%).
2.2. Stability Study of β-Lactams
Stability evaluation of compounds was carried out to avoid subsequent significant loss of pharmacological activity in vivo. The stability of representative β-lactams 9q, 10h, 10q, 10p, 10r, 15a and CA-4 was evaluated by HPLC at relevant in vivo acidic, neutral and basic conditions (pH 4, 7.4 and 9) and in plasma (see Supplementary Materials, Tables S2 and S3 and Figure S21). The half-life (t½) for 9q (the most potent compound in the series, containing the phenolic ring B) was determined to be 13 h at both pH 4 and pH 7.4 and 6 h at pH 9. However, the t½ in plasma for compound 9q was greater than 24 h. The t½ of the corresponding phosphate prodrug phosphate ester 15a was greater than 24 h at pH 4 and pH 7.4 and in human blood plasma. CA-4 was stable at pH 4.0, 7.4 and 9.0 and in human plasma for more than 7 h. Based on this stability study, β-lactam 9q and the phosphate 15a would be suitable for further development. The 3-allyl β-lactam compounds 10h and 10q demonstrated poor stability over the pH range studied. The 3-allyl β-lactam phenolic compound 10p demonstrated superior stability at all pH values, with over 95% remaining after 11 days, compared to 10h (24–41%) and 10q (22–26%).
Compound 10r (containing the 3-amino-4-methoxyphenyl ring A) showed similar results to phenolic compound 10p (t½ > 24h). The resilience of 10p and 10r against ring opening degradation may be due to the additional electron-donating hydroxyl and amino substituent groups in ring B. Compound 10h was the most stable of the 3-allyl compounds in plasma, with 100% recovery after 3 days, while compounds 10p, 10q and 10r showed 97%, 89% and 96% recovery respectively. Based on these stability studies, β-lactams 10p and 10r would be suitable for further development and compared well with CA-4. Clearly, the 3-allylic β-lactam compound 10p is superior in stability to the corresponding isomeric 3-(prop-1-en-2-yl)-β-lactam 9q. In an additional study, the stability of compound 10p was determined under degradation conditions and was stable after 4 h treatment in acidic (0.1 M HCl), alkaline (0.1M NaOH), oxidative (3% H2O2), heat (60 °C) and UV light conditions, with 91%, 95%, 79%, 100% and 100% of the compound remaining, respectively (Supplementary Materials, Tables S2 and S3 and Figure S21).
2.3. Biological Results and Discussion
2.3.1. In Vitro Antiproliferative Activity of 3-(Prop-1-en-2-yl)azetidinones 9a-9x, 3-Allylazetidinones 10a-10s and 3-butadienyl-β-lactams 11a-11s in the MCF-7 Breast Cancer Cell Line
The antiproliferative activity of the novel 3-(prop-1-en-2-yl)azetidinones 9a-9x, 3-allylazetidinones 10a-10s and 3-butadienyl-β-lactams 11a-11s was initially examined in the human breast cancer cell line MCF-7 (ER positive); CA-4 was used as the reference compound (IC50 = 3.9 nM in MCF-7 cells). The results are displayed in Table 2, Table 3 and Table 4. The β-lactams were evaluated as the trans isomers, which were isolated exclusively in these synthetic reactions as enantiomeric mixtures. The 3-(prop-1-en-2-yl)azetidinones 9a-9s contain the characteristic 3,4,5-trimethoxyphenyl ring A at the N1 position of the azetidinone, while compounds 9t, 9u and 9v contain the 3,4,5-trimethoxyphenyl substituent (ring A of CA-4) at the C4 position. Compounds 9x, 10s and 11s contain a single aryl para-methoxy substituent at the C4 position, while compound 9w contains a single aryl methoxy substituent at the N1 position.
The phenolic β-lactam compound 9q containing the 3-hydroxy-4-methoxyphenyl ring B, designed as a direct analogue of CA-4, was identified as the most potent in the series, with an IC50 value of 10 nM (Table 2). Compound 9s containing the ring B 3-amino-4-methoxyphenyl substitution pattern demonstrated notable potency, with IC50 = 0.033 mM; such amino ring B substitution is reported to contribute to antitubulin activity in many CA-4 analogues []. Compounds 9h and 9i, having methoxy and ethoxy electron-releasing substituents at C-4 of ring B, displayed potent antiproliferative effects, with IC50 = 33 nM and 81 nM, respectively. Among the other members of this series displaying sub-micromolar antiproliferative activity in the MCF-7 cell line were 9o (4-thiomethyl), 9g (4-methyl), 9e (4-dimethylamino), 9b (4-chloro) and 9c (4-bromo) compounds. Interestingly, the 2-naphthyl compound 9n (IC50 = 139 nM) was 58-fold more potent than the 1-naphthyl compound 9m, as previously observed for stilbene CA-4 analogues []. Antiproliferative activity was minimal for compounds 9t, 9u and 9v containing the 3,4,5-trimethoxyphenyl ring A at the C-4 position, thus confirming the optimal N-1 position for activity.

Table 2.
Antiproliferative activities of β-lactams 9a-9o, 9q-9x, 13a, 14b, 15a and 15b in MCF-7 human breast cancer cells.
Table 2.
Antiproliferative activities of β-lactams 9a-9o, 9q-9x, 13a, 14b, 15a and 15b in MCF-7 human breast cancer cells.
| Compound Number | Antiproliferative Activity IC50 (µM) a | cLogP b |
|---|---|---|
| 9a | 4.957 ± 0.69 | 3.06 |
| 9b | 0.630 ± 0.07 | 4.03 |
| 9d | 0.691 ± 0.10 | 4.18 |
| 9e | 5.950 ± 0.92 | 3.46 |
| 9f | 0.494 ± 0.14 | 3.32 |
| 9h c | 4.892 ± 0.53 | 3.49 |
| 9j | 0.161 ± 0.010 | 3.82 |
| 9k | 0.033 ± 0.005 | 3.24 |
| 9l | 0.081 ± 0.009 | 3.77 |
| 9m | 44.1 ± 2.57 | 4.83 |
| 9n | 78.1 ± 5.47 | 5.42 |
| 9o c | 271.9 ± 26.4 | 5.01 |
| 9r | 8.066 ± 0.93 | 4.50 |
| 9s | 0.139 ± 0.04 | 4.50 |
| 9t d | 0.131 ± 0.04 | 3.88 |
| 9u d | 0.010 ± 0.001 | 2.32 |
| 9v d | 1.165 ± 0.12 | 2.70 |
| 9w | 0.033 ± 0.005 | 2.19 |
| 9x | <10% inhibition | 3.88 |
| 13a | <10% inhibition | 3.77 |
| 15a | <10% inhibition | 3.24 |
| 15b | 144.6 ± 7.76 | 4.18 |
| CA-4 e | 3.567 ± 0.32 | 4.18 |
a IC50 values are half-maximal inhibitory concentrations required to inhibit growth stimulation of MCF-7 cells. Values represent the mean ± SEM (error values × 10−6) for at least three experiments performed in triplicate. Treatment at eight different concentrations in the range 1–50 μM over 72 h was used for determination of the IC50 values for each compound in comparison to the control compound CA-4. bclogP values were calculated with ChemBioDraw 13.0 and are documented in the table. c [] d IC50 values were not calculated; the percentage shown is the inhibition of cell viability at a concentration of 10 μM. e IC50 value determined for CA-4 (0.0039 μM) in MCF-7 cells is in agreement with reported values [,].
The series of β-lactams with the 3-allyl substituent at C-3 position of the β-lactam ring was next investigated (compounds 10a-10s), and the results are displayed in Table 3, together with the cLogP values. As observed for the 3-(prop-1-en-2-yl)azetidinones series, the most potent compounds in the 3-allyl series were identified as 10p (3-hydroxy-4-methoxyphenyl ring B, IC50 = 31 nM), 10h (4-methoxyphenyl ring B, IC50 = 35 nM) and 10r (3-amino-4-methoxyphenyl ring B, IC50 = 35 nM). The 4-ethoxyphenyl compound 10i also displayed excellent antiproliferative activity against MCF-7 cells, with an IC50 value of 91 nM. The remaining compounds displayed reduced activity.
The 3-Butadienyl β-lactam compounds 11a-11s were also evaluated for antiproliferative activity in MCF-7 human breast cancer cells and showed trends that were consistent with the 3-propenyl and 3-allyl β-lactam series (Table 4). The most active compounds in the series were again identified as the ring B para-methoxy 11h (IC50 = 33 nM), the ring B 3-amino-4-methoxyphenyl compound 11r (IC50 = 36 nM) and the ring B 3-hydroxy-4-methoxyphenyl compound 11p (IC50 = 61 nM). The remaining compounds showed reduced antiproliferative activities against MCF-7 cells.

Table 3.
Antiproliferative activities of β-lactams 10a-10n, 10p-10s, 13c, 13d and 15d in MCF-7 human breast cancer cells.
Table 3.
Antiproliferative activities of β-lactams 10a-10n, 10p-10s, 13c, 13d and 15d in MCF-7 human breast cancer cells.
| Compound Number | Antiproliferative Activity IC50 (µM) a | cLogP c |
|---|---|---|
| 10a | 4.128 ± 0.62 | 2.99 |
| 10b | 0.621 ± 0.095 | 3.96 |
| 10c | 0.944 ± 0.10 | 4.11 |
| 10d | 33.80 ± 9.04 | 3.39 |
| 10e | 4.46 ± 0.11 | 3.42 |
| 10f | 9.609 ± 1.20 | 3.25 |
| 10g | 0.199 ± 0.03 | 3.75 |
| 10h | 0.035 ± 0.005 | 3.17 |
| 10i | 0.091 ± 0.01 | 3.7 |
| 10j | 17.41 ± 1.23 | 4.76 |
| 10k | 7.494 ± 0.60 | 5.35 |
| 10l | 7.388 ± 0.67 | 4.94 |
| 10m | 0.210 ± 0.026 | 4.43 |
| 10n | 0.318 ± 0.03 | 4.43 |
| 10p | 0.031 ± 0.002 | 2.25 |
| 10q | 0.619 ± 0.07 | 2.63 |
| 10r | 0.035 ± 0.004 | 2.12 |
| 10s | 84.50 ± 8.10 | 4.11 |
| 13b | 10.67 ± 1.94 | 2.45 |
| 13c | 13.02 ± 1.25 | 1.93 |
| 15c | 0.094 ± 0.015 | 1.17 |
| 15d | 0.032 ± 0.005 | 1.65 |
| CA-4 b | 0.0039 ± 0.00032 | 3.32 |
a IC50 values are half-maximal inhibitory concentrations required to block the growth stimulation of MCF-7 cells. Treatment at eight different concentrations [0.001–50 μM] was used for the determination of the IC50 values for each compound in comparison to the control compound CA-4. Values represent the mean ± SEM (error values × 10−6) for at least three experiments performed in triplicate. b IC50 value determined for CA-4 (0.0039 μM) in MCF-7 cells is in agreement with reported values [,]. c clogP values were calculated using ChemBioDraw 13.0 and are documented in the table.

Table 4.
Antiproliferative activities of β-lactams 11a-n, 11p-s, 13d and 13e in MCF-7 human breast cancer cells.
Table 4.
Antiproliferative activities of β-lactams 11a-n, 11p-s, 13d and 13e in MCF-7 human breast cancer cells.
| Compound Number | Antiproliferative Activity IC50 (µM) a | cLogP c |
|---|---|---|
| 11a | 0.784 ± 0.13 | 3.3 |
| 11b | 0.315 ± 0.04 | 4.27 |
| 11c | 0.428 ± 0.042 | 4.42 |
| 11d | 3.214 ± 0.62 | 3.7 |
| 11e | 1.929 ± 0.34 | 3.72 |
| 11f | 19.4 ± 4.03 | 3.56 |
| 11g | 0.200 ± 0.04 | 4.06 |
| 11h | 0.033 ± 0.005 | 3.48 |
| 11i | 0.261 ± 0.05 | 4 |
| 11j | 182.4 ± 19.4 | 5.06 |
| 11k | 37.89 ± 3.80 | 5.65 |
| 11l | 144.9 ± 11.66 | 5.24 |
| 11m | 0.576 ± 0.10 | 4.73 |
| 11n | 0.296 ± 0.05 | 4.73 |
| 11p | 0.061 ± 0.008 | 2.56 |
| 11q | 0.511 ± 0.09 | 2.94 |
| 11r | 0.036 ± 0.005 | 2.03 |
| 11s | 15.21 ± 1.41 | 4.42 |
| 13d | 24.16 ± 3.91 | 2.23 |
| 13e | 9.359 ± 1.21 | 0.99 |
| CA-4 b | 0.0039 ± 0.00032 | 3.32 |
a IC50 values are half-maximal inhibitory concentrations required to block the growth stimulation of MCF-7 cells. Treatment at eight different concentrations [0.001–50 μM] was used for the determination of the IC50 values for each compound in comparison to the control compound CA-4. Values represent the mean ± SEM (error values × 10−6) for at least three experiments performed in triplicate b IC50 value determined for CA-4 (0.0039 μM) in MCF-7 cells is in agreement with reported values [,]. c clogP values were calculated using ChemBioDraw 13.0.
The antiproliferative activity of the amino acid and phosphate prodrugs synthesised was next evaluated in MCF-7 cells. The antiproliferative activity for the phenylalanine prodrug 13a (IC50 = 4.915 μM) was low, indicating that in vivo hydrolysis of the amide is required to release the active amine 9s (Table 2) []. Reduced activity was also observed for amino acid prodrugs 13b-e (Table 3 and Table 4). However, the 3-isopropenyl phosphate ester 15a displayed impressive antiproliferative activity, with an IC50 value of 19 nM (Table 2), compared with parent phenol 9q (IC50 = 10 nM), suggesting that this compound is a useful prodrug, as in vivo dephosphorylation to produce 9q would be expected to occur as observed for CA-4P []. The 3-isopropyl group in compound 15b resulted in reduced activity (Table 2). However, the phosphates 15c and 15d prepared from the 3-allyl phenolic 10p retained excellent activity, with IC50 values of 94 nM and 32 nM, respectively (Table 3).
2.3.2. In Vitro Antiproliferative Activity of Structurally Modified β-Lactams 17a-g, 19, 20, 21a-d, 22a-d in the MCF-7 Breast Cancer Cell Line
To determine the effect of modification of the alkene substituent at C-3 of the β-lactam ring of the designed compounds, a number of structurally related β-lactams were synthesised for evaluation. Compounds 17a-e were designed to determine the effect of the introduction of an aryl substituent at the C-3 allylic position of potent 3-allyl-β-lactam compounds 10h and 10p, while all aryl ring A and ring B methoxy substituents were removed in compound 17c. In compounds 17f and 17g, an ethyl ester group was introduced to replace the C-3 alkene substituent (isopropenyl, allyl and butadiene). In compounds 17d, 17e and 19, a trifluoromethylstyryl substituent was located at C-3 in place of the 3-(prop-1-en-2-yl) and 3-butadienyl series compounds.
The compounds were evaluated in the MCF-7 cell line, and the results are displayed in Table 5. Compounds 17a, 17b and 17c demonstrated reduced antiproliferative effects, compared with the parent compounds 10h and 10p. Interestingly, the ester compounds 17f and 17g demonstrated the most potent antiproliferative effects of the modified series in MCF-7 cells, with IC50 values of 35 nM and 75 nM, respectively. Compound 19, having a trifluoromethylstyryl substituent at C-3 to replace the alkene, and 17e, having a trifluoroaryl substituent at the C-3 allylic position, both displayed moderate activity, while activity was poor for the ring A unsubstituted compound 17f and the epoxide 20.

Table 5.
Antiproliferative activities of β-lactams 17a-g, 19, 20, 21a-d, 22a-d in human MCF-7 breast cancer cells.
The antiproliferative activity of modified compounds 21a-d and 22a-d is presented in Table 5. A significant decrease in activity for all β-lactam compounds was observed compared with the parent 3-butadienyl β-lactam compounds and CA-4, which indicated that reduction in antiproliferative activity may result from increasing the substituent group size at the C-3 position of β-lactam ring, in line with our previous observations []. The most potent compound of the series was the trans diastereoisomer 22d with IC50 = 5.33 µM, which was 3-fold more potent than the diastereomer 22c (IC50 = 15.33 µM).
2.3.3. Evaluation of Antiproliferative Activity of Selected β-Lactam Compounds in the MDA-MB-231, HL60, HT29 and SW60 Cell Lines
Selected examples of the more potent compounds were evaluated in the triple-negative MDA-MB-231 breast cancer cell line (Table 6). Triple-negative MDA-MB-231 breast cancer cells do not express the ER, progesterone (PR) or HER2 receptors and possess mutant p53 []. Triple-negative breast cancers (TNBCs) account for 10–15% of breast cancers diagnosed, have poor prognosis and are not responsive to hormone therapies, e.g., tamoxifen, to aromatase inhibitors such as anastrozole or to the HER2-receptor-targeting antibody Herceptin.

Table 6.
Antiproliferative activities of selected β-lactams in MDA-MB-231 human breast cancer cellsa.
Of the 3-isopropenyl series, the phenolic compound 9q was found to be the most effective, with an IC50 value of 23.9 nM in the triple-negative MDA-MB-231 cell line. It compared favourably with the positive control CA-4 (IC50 = 43 nM) in this cell line [,] and also with the result for 9q in the MCF-7 cell line (IC50 = 10 nM). The p-methoxy compound 9h and the phosphate ester 15a were also very effective in the MDA-MB-231 cell line, with IC50 values of 31.3 nM and 32 nM, respectively. Of the 3-allyl and butadienyl compounds evaluated in the MDA-MB-231 cell line, the phenolic 10p was notable, with IC50 = 27 nM, together with the amine 10r (IC50 = 35 nM), the methoxy 11h (IC50 = 31 nM) and the amine derivative 11r (IC50 = 33 nM). It is notable that the compounds retained potency in the MDA-MB-231 cell line, which may indicate their potential in development of therapeutic applications for this group of aggressive breast cancers.
In addition, the 3-isopropenyl compound 9q was found to be particularly effective in the chemoresistant HT-29 human colorectal adenocarcinoma cell line, with an IC50 value of 0.711 μM, in comparison to the control CA-4 (IC50 = 4.165 μM) in this cell line (Figure 2), indicating the potential of these compounds in chemoresistant colon cancers. Furthermore, 9q was also evaluated in the human leukaemia cell line HL-60 (acute myeloid leukaemia) and the colon adenocarcinoma cell line SW-480, with IC50 values of 15 nM and 8 nM, respectively. It compares well with the control compound CA-4 (IC50 = 2 nM and 7 nM, respectively, in these cell lines) (Figure 2). The physicochemical properties of the most potent compounds were next investigated.
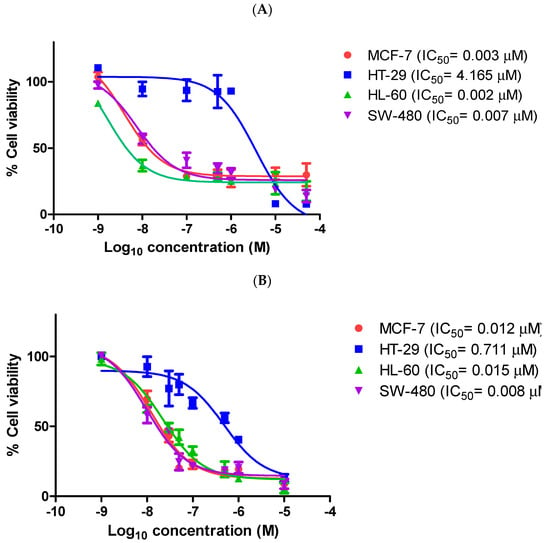
Figure 2.
Antiproliferative activity of β-lactam compound 9q and CA-4 in MCF-7 human breast cancer cells, HL-60 leukaemia cells, HT-29 and SW-480 colon cancer cells.
2.4. Physicochemical Properties
The physicochemical properties and various metabolic properties of eleven selected β-lactam compounds from the panel synthesised were also determined to establish their druggability (see Supplementary Materials Tables S4 and S5). The relevant physicochemical and pharmacokinetic properties of the most potent compounds, 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 15a and 17g, were obtained using Pipeline Pilot Professional []. The physicochemical properties of these compounds were found to comply with the requirements of Lipinski and Veber rules, with molecular weight range 445–457, hydrogen bond acceptor range 5–9, hydrogen bond donor range 0–2, 7–10 rotatable bonds and logP range 2.61–3.63. There is some correlation observed between the log P values calculated for the compounds and the antiproliferative activity determined (see Table 4, Table 5 and Table 6). The most potent compounds, 9q, 9s, 10q, 10r, 11q, 11r and phosphate 15a, with IC50 values in the range 10–61 nM in MCF-7 cells, have a low cLog P values in the range 1.235–2.55. However, the isomeric 1 and 2-naphthyl compounds 9m and 9n, each having higher cLogP values of 4.95, demonstrated a significant difference in potency, with IC50 values of 8.066 μM and 0.139 μM, respectively, in MCF-7 cells, indicating that the 2-naphthyl compound 9n may be a better fit at the colchicine-binding site.
The pKaH value for potent compound 9q was calculated with Marvin as 9.83, while the phosphate 15a was ionised at physiological pH with pKaH values of 1.62 and 6.59. The calculated topological polar surface area (TPSA) of this panel was in the range 57–103 Å2, below the required limit of <140 Å2 for good intestinal absorption and membrane permeability. Additionally, three of the compounds, 9h, 10h and 11h, followed the Pfizer rule and GSK rule (MW ≤ 400, log P ≤ 4), with a low log P value (log P < 3) and a low TPSA value (TPSA <75 Å2). The compounds exhibited good drug-likeness parameters with predicted lipophilic–hydrophilic balance, together with high blood–brain barrier (BBB) absorption and plasma-protein-binding properties (>90%), and were not predicted to inhibit the metabolic activity of CYP2D6 (see Supplementary Materials Tables S4 and S5 for details).
Good aqueous solubility (logSw = −3.4130 mol/L) was predicted for the phenolic ester compound 17g, although lower aqueous solubility was predicted for the remaining panel of β-lactam compounds (see Supplementary Materials, Table S4); phosphate esters such as 15a-d and amino acid prodrugs such as 13a-e may result in increased water solubility, as reported for CA-4 and related compounds [,]. The most potent compounds identified in the preliminary screening panel [9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 17g] were confirmed as free from pan-assay interference compounds (PAINS) alerts [] and were identified as suitable candidate compounds for subsequent in vitro biochemical investigations based on the phenotypic screening and Tier-1 profiling of their drug-like properties (Tables S4 and S5, Supplementary Materials) [].
Cells were grown in 96-well plates and treated with (A) CA-4 or (B) β-lactam compound 9q at 0.001–50 μM for 72 h. Cell viability was expressed as a percentage of vehicle control (ethanol 1% (v/v))-treated cells. The values represent the mean ± S.E.M. for three independent experiments performed in triplicate.
2.4.1. National Cancer Institute 60 Cell Line Screening for Azetidinones 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 15a and 15b
The more potent compounds 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 15a and 15b were selected for screening for antiproliferative activity by the National Cancer Institute (NCI)/Division of Cancer Treatment and Diagnosis (DCTD)/Developmental Therapeutics Program (DTP) [] in their drug screening programme and evaluated using approximately 60 different human cancer cell lines. The initial NCI 60 cell line screen used the sulforhodamine B (SRB) protein assay [], at one dose concentration (10 μM), and subsequently, a five-dose screening in the concentration range 0.01–100 μM was carried out. GI50 (50% growth inhibition), TGI (total growth inhibition) and LC50 (50% lethal concentration) were determined (see Table 7 and Supplementary Materials Tables S6–S8). The compounds demonstrated excellent broad-spectrum antiproliferative activity against a range of tumour cell lines contained in the NCI panel of cancer cell lines, e.g., leukaemia, colon cancer, CNS cancer, melanoma, non-small-cell lung cancer, ovarian cancer, renal cancer, breast cancer and prostate cancer, and the results confirmed our in-house evaluations in the MCF-7 cell line.

Table 7.
Summary of NCI 60 cell line mean screening results (GI50, TGI, LC50) for selected compounds.
The ring B phenolic 3-isopropenyl compound 9q was identified as the most potent compound synthesised in our panel, with a mean GI50 value obtained across the entire NCI panel of cell lines screened of 39.81 nM. (See Figure 3 for a heatmap of the activity of compound 9q across the cell lines in the NCI-60 screen). This result compares favourably with the mean GI50 for CA-4 of 99.30 nM in the NCI-60 cell panel, demonstrating the superior inhibitory potency of the β-lactam analogue (see Table 7 and Supplementary Materials Tables S6–S8 for further details). Significantly, the GI50 values for 9q were in the sub-micromolar range for all cell lines investigated, except for the breast cancer cell line T-47D (ERα+/PR+ and HER2-). Furthermore, 9q displayed significant potency in all other breast cancer cell lines evaluated in the panel (MCF-7, MDA-MB-231, HS 578T, BT-549, MDA-MB-468), with GI50 values in the range <10–16 nM.

Figure 3.
Heatmap of the activity of compound 9q across cell lines in the NCI-60 screen using three different values (growth-inhibitory effect, GI50; drug concentration at which the response is reduced by half, IC50; cytostatic effect, TGI; cytotoxic effect, LC50; concentration in molar).
GI50 values below 50 nM were obtained for compound 9q in 48 of the panel cell lines tested, with activity against non-small-cell lung, colon, CNS, ovarian and prostate cell lines tested. Figure 3 displays a heatmap of the activity of compound 9q across the cell lines in the NCI-60 screen. In addition, compounds 9s, 11h and 11r were found to be particularly effective in the chemoresistant HT-29 human colorectal adenocarcinoma cell line, with IC50 values of 34, 33 and 34 nM, in comparison to the control compound CA-4 (IC50 = 4.165 μM). Compounds 9h, 9q, 9s, 10p, 11h, 11r and 15a displayed significant potency when evaluated in the leukaemia cell line HL-60 (acute myeloid leukaemia), with GI50 values of < 34 nM.
The mean GI50 values for the panel of 60 cell lines for the most potent compounds evaluated (9h, 9q, 9s, 10h, 10p, 10r, 11h and 11r) were determined to be <91.20 nM, as shown in Table 7. These results compare very favourably with the mean GI50 value determined for CA-4 of 99.3 nM in this 60-cell-line panel.
The COMPARE programme developed by the NCI was used to further investigate the mechanism of action of the β-lactam series []. The antiproliferative profiles of potent compounds 9q and 9s were compared with compounds possessing known mechanisms of antiproliferative action in the NCI Standard Agents Database. Based on GI50, TGI and LC50, the compounds 9q and 9s demonstrated a good correlation to tubulin-targeting agents such as maytansine (r = 0.741) and also to the clinically utilised tubulin-targeting anticancer drugs vincristine (r = 0.664), vinblastine (r = 0.632) and paclitaxel (r = 0.768) (see Supplementary Materials, Tables S9 and S10).
2.4.2. Effect of β-Lactam 9q on Non-Carcinogenic HEK-293T Cells
The 3-(prop-1-en-2-yl)azetidin-2-one compounds 9h and 9q were next examined for cytotoxic effects in MCF-7 cells at 10 μM concentration using the lactate dehydrogenase (LDH) assay, in which the release of cytoplasmic LDH is used as a measure of cell lysis []. The b-lactams 9h and 9q resulted in 30% and 32% cell death, respectively. CA-4 was used as the positive control in this assay, with 12% cell death at 10 μM.
The cytotoxicity of the most potent example of the 3-isopropenyl series, 9q, was also investigated in the non-tumorigenic cell line HEK-293T (normal human epithelial embryonic kidney cells). The viability of the normal HEK-293T cells was demonstrated to be significantly higher than the treated MCF-7 cells following exposure to compound 9q for 72 h. The cell viabilities observed in the HEK-293T cells were 83%, 60% and 50% at the concentrations of 0.1 μM, 1 μM and 10 μM, respectively (Figure 4); this result compares very favourably with the viabilities obtained in MCF-7 cells of 22%, 21% and 11% at the concentrations of 0.1 μM, 1 μM and 10 μM, respectively, with IC50 = 10 nM in the MCF-7 cell line, demonstrating that β-lactam 9q was selectively less toxic to human normal cells (HEK-293T) than cancer cells (MCF-7).
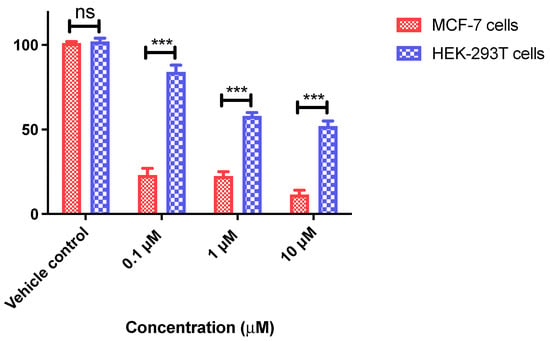
Figure 4.
Antiproliferative activity of β-lactam compound 9q in human breast cancer MCF-7 cells and non-tumorigenic human embryonic kidney HEK-293T cells. MCF-7 and HEK-293T cells were grown in 96-well plates and treated with compound 9q at 0.1, 1 and 10 μM for 72 h. Cell viability (as a percentage of vehicle control (ethanol 1% (v/v) treated cells) was determined in an AlamarBlue assay. Values represent the mean ± SEM (error values × 10−6) obtained for at least three independent experiments. Two-way ANOVA (Bonferroni post-test) was used to test for statistical significance (***, p < 0.05). Cell cycle and pro-apoptotic effects of 3-(prop-1-en-2-yl)azetidinone 9q.
These results indicate that compound 9q is suitable for further development as an anticancer agent for breast cancers with selective lower cytotoxicity to normal cells. Additionally, the mean TGI (total growth inhibition) value for the potent compound 9q over the NCI-60 cancer cell line panel was 32 mM (compared with TGI for CA-4 of 10.30 μM), with TGI values >100 μM for 34 of the cell lines tested indicating a wide therapeutic window for the compound (Table 7).
LC50 values obtained from the NCI screen over the 60 cancer cell lines were also useful in assessing the cytotoxicity of these compounds (Table 7). For compound 9q, LC50 values were greater than 100 μM in all but two of the cell lines tested (melanoma M14 and colon COLO 205), which indicated minimal toxicity and suggested the potential for this compound in a number of therapeutic applications (Tables S6–S8 Supporting Information). For compound 9h, the LC50 values were greater than 100 μM in all but one of the cell lines tested (melanoma SK-MEL-5). Similarly low cytotoxicity was also obtained for the related compounds evaluated in the series, e.g., compounds 9s, 15a, 15b, 10r, 10p, 11r and 11p. The antiproliferative activity determined for 9q (IC50 <10 nM against the MCF-7 cell line) demonstrated a significant therapeutic window between the drug concentration required for cancer cell growth inhibition (GI50) and the concentration that is toxic to these MCF-7 cells, LC50 (>100 μM). For compound 9q, the mean GI50 value over all 60 cell lines was 39.81 nM, while the mean LC50 value was 81.28 μM.
2.4.3. Cell Cycle and Pro-Apoptotic Effects of 3-(Prop-1-en-2-yl)azetidinone 9q
The effects of the β-lactam 9q on the cell cycle in MCF-7 cells were next explored in flow cytometry studies. Tubulin-destabilising agents such as colchicine and CA-4 arrest the cell cycle in the G2/M phase, promoting depolymerisation of microtubules and disruption of the cytoskeleton, while the antimitotic action of paclitaxel is to stabilise the microtubule polymer and prevent disassembly. MCF-7 cells were treated with 9q and analysed by propidium iodide staining (Figure 5). The effect of compound 9q on the mitotic phase G2/M (50 μM and 500 μM) at 24 h, 48 h and 72 h was first determined (Figure 5B). For the 50 μM concentration, the G2/M population increased at each of the time points examined to 72% (24 h), 69% (48 h) and 70% (72 h), while this cell population also increased when treated at 500 nM to 85% (24 h), 81% (48 h) and 70% (72 h). In contrast, the G0G1 cell population decreased (10% and 7% (24 h), 10% and 7% (48h) and 8% and 8% (72 h)) when treated with 9q at 50 nM and 500 nM, respectively (Figure 5A). The vehicle control (ethanol 0.1% v/v) is shown for comparison (Figure 5). A proapoptotic effect (sub-G0G1) was evident for compound 9q (10% and 19% at 50 nM and 500 nM, respectively) at 72 h, compared with vehicle control (6%) (Figure 5C).
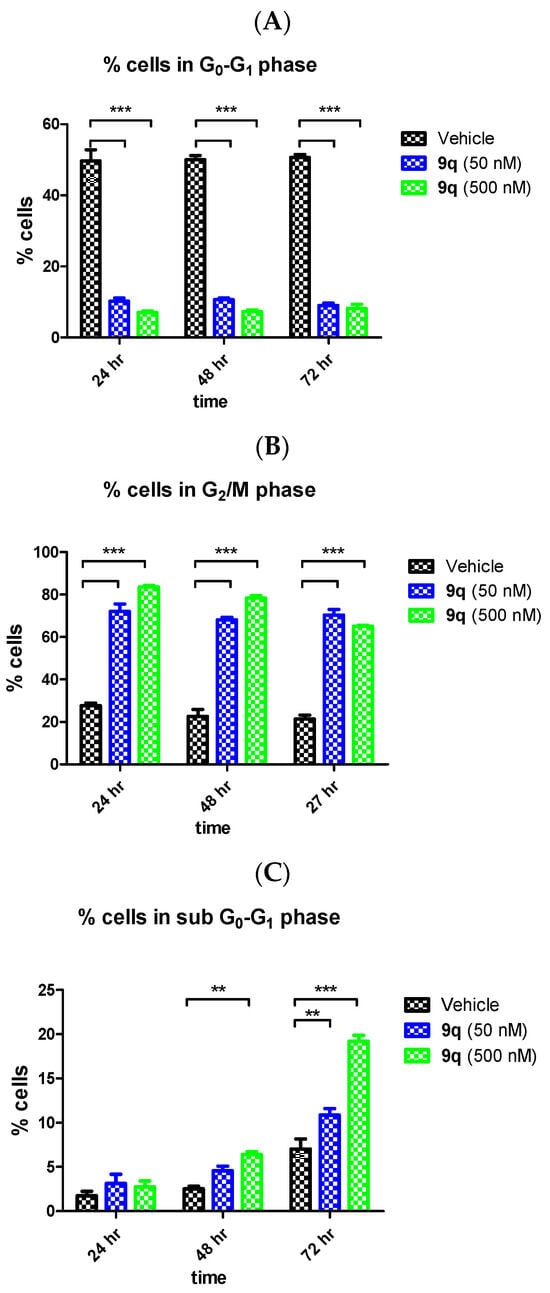
Figure 5.
Effect of β-lactam compound 9q on the cell cycle and apoptosis in MCF-7 cells. MCF-7 cells were treated with either vehicle (0.1% ethanol (v/v)) or 9q (50 nM and 500 nM) for 24 h, 48 h and 72 h. The MCF-7 cells were then fixed and stained with propidium iodide, and the distribution was analysed by flow cytometry. Cell cycle analysis was obtained from histograms of gated counts per DNA area (FL2-A). The number of cells with <2N (sub-G1) (A), 2N (G0G1) (B) and 4N (G2/M) (C) DNA content was determined using CellQuest software. The results obtained represent the mean of three independent experiments performed. Two-way ANOVA (Bonferroni post-test) was used to test for statistical significance (**, p < 0.05; ***, p < 0.001).
To further investigate and confirm the induction of apoptosis by 9q in MCF-7 cells, a dual staining with annexin-V and with propidium iodide (PI) was performed (Figure 6). Live cells (annexin-V-/PI-), early apoptotic cells (annexin-V+/PI-), late apoptotic cells (annexin-V+/PI+) and necrotic cells (annexin-V-/PI+) can be identified. Compound 9q induced an increase in apoptosis (observed as annexin-V-positive cells) in a concentration-dependent manner when compared to CA-4 (Figure 6) when examined at 72 h, with 27% of cells undergoing apoptosis (early+late) at 50 nM concentration of 9q, and 38.9% at 500 nM. CA-4 (50 nM) induced apoptosis in 34.6% of the MCF-7 cells. Cell death may also be due to autophagy following treatment with 9q []. Collectively, these findings indicate that the β-lactam compound 9q demonstrated tubulin-targeting effects, e.g., G2/M arrest followed by apoptosis, on cell cycle in MCF-7 cells. The effects of the compounds on tubulin polymerisation were further examined.
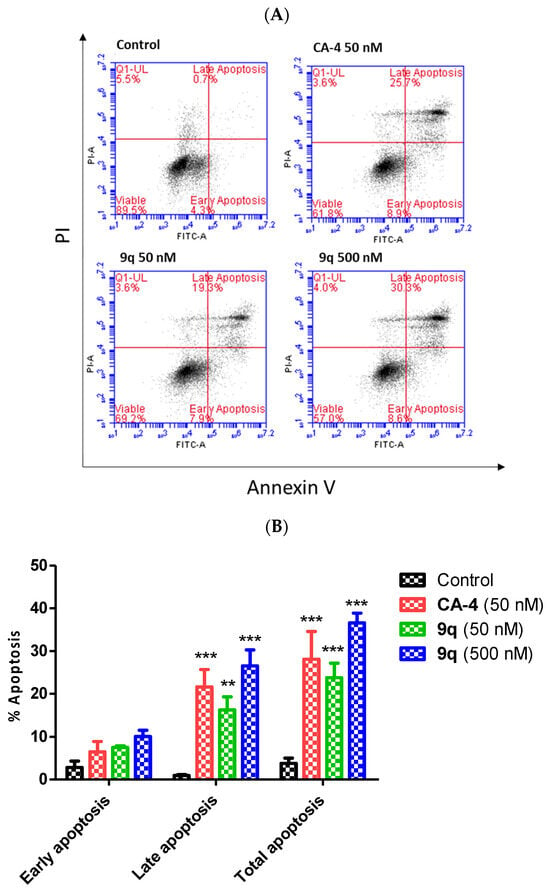
Figure 6.
β-Lactam compound 9q induces apoptosis in MCF-7 cells (annexin-V / PI FACS). (A) The effect of β-lactam compound 9q on the induction of apoptosis in MCF-7 cells was analysed by flow cytometry following double staining of the MCF-7 cells with annexin-V-FITC and PI. The MCF-7 cells were treated with 50 and 500 nM concentrations of β-lactam compound 9q and CA-4 (50 nM) for 72 h (control 0.1% (v/v) ethanol). Cells were then collected and processed for analysis. (B) The percentage of cells in each phase of the cell cycle in MCF-7 cells treated with the indicated concentration of 9q is shown. Two-way ANOVA (Bonferroni post-test) was used to test for statistical significance (**, p < 0.05; ***, p < 0.001).
2.4.4. Tubulin Polymerisation Effects of 3-(Prop-1-en-2-yl)azetidinones, 3-Allylazetidinones and 3-butadienylazetidinones
A panel of β-lactam compounds that demonstrated the most potent antiproliferative effects in vitro were selected for study of their inhibitory effect on tubulin assembly using a light-scattering assay. CA-4 was used as the positive control for the tubulin polymerization experiment, which effectively inhibits the assembly of bovine tubulin; paclitaxel was the positive control for polymerisation, with ethanol and DMSO as the solvent vehicles. (Figure 7). The Vmax value for the polymerization reaction for each compound was determined (Table 8), in addition to the fold-changes in the Vmax values for the polymerisation reaction [].
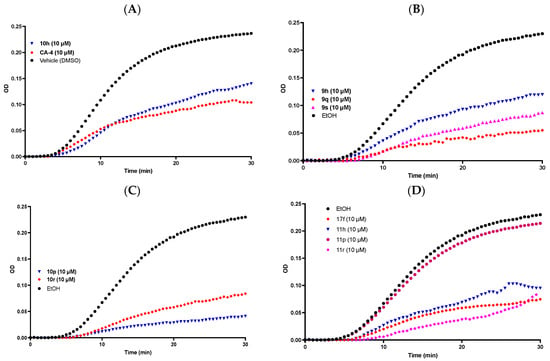
Figure 7.
β-Lactam compounds 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 17f induce tubulin polymerization in vitro. (A) Compounds CA-4, 10h (10 μM) and vehicle DMSO (1% v/v); (B) compounds 10h, 10q, 10s (10 μM) and vehicle ethanol (1%v/v); (C) compounds 10p, 10r (10 μM) and vehicle ethanol (1% v/v); (D) compounds 17f, 11h, 11p, 11r (10 μM) and vehicle ethanol (1% v/v). The tubulin polymerization reaction was initiated by gently warming a solution of purified bovine tubulin and GTP (mixed in a 96-well plate) from 4 °C to 37 °C. Ethanol or DMSO (1% v/v) was used as a vehicle control. CA-4 (10 μM) was used as a reference. β-Lactam compounds were tested at a final concentration of 10 μM. Tubulin polymerisation was monitored at 37 °C with a Spectramax 340PC spectrophotometer at 340 nm for 30 min at 30 s time intervals. The inhibition (fold) of the tubulin polymerization reaction was calculated using the Vmax value for each reaction. The representative results of three separate experiments are shown.

Table 8.
Inhibition of tubulin polymerisation for compounds 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 17d.
The ring B phenolic 3-allyl 10p was identified as the most potent polymerisation inhibitor in the series (Vmax 0.0015 mOD/min), with 4.5-fold reduction in Vmax value at 10 μM concentration. This result compares favourably to CA-4 (10 μM), for which a 6.3-fold inhibition in the Vmax for polymerisation was observed. These Vmax results show correlation with the antiproliferative data recorded for both CA-4 (IC50 = 4.2 nM) and 10p (IC50 = 31 nM) in the MCF-7 line and indicate that tubulin is the molecular target for this series of 3-allyl-β-lactams. The most potent antiproliferative compound, 3-(prop-1-en-2-yl)azetidinone 9q (IC50 = 10 nM), was also found to be a potent inhibitor of tubulin polymerization, demonstrating a 4.27-fold reduction in Vmax value at 10 μM concentration, together with the ring B amino 3-allyl compound 10r (3.4-fold reduction in Vmax value) and the ring B methoxy-3-butadienyl-β-lactam 11h (2.9-fold reduction in Vmax value).
In a further investigation of the mechanism of action of the series of β-lactams, the interaction of the most potent antiproliferative compound, 3-(prop-1-en-2-yl)azetidinone 9q, at the colchicine-binding site of tubulin was examined. The colchicine-binding site of tubulin is characterised by the Cys239 and Cys354 thiol-containing residues. The alkylating reagent N,N’-ethylene-bis(iodoacetamide) (EBI) reacts with the thiol-containing amino acid residues of cysteine 239 and cysteine 354 to crosslink [,].
In the present work, the β-lactam 9q (10 μM) or CA-4 was used to treat MCF-7 cells for 2 h; EBI was then added, and the cells were incubated for 15 h (Figure 8). Vehicle-treated control samples were observed at a lower position on the gel, confirming the formation of the β-tubulin-EBI adduct and demonstrating that the alkylating reagent EBI had formed cross links on β-tubulin with the cysteine thiol residues Cys239 and Cys354. The formation of the EBI adduct was prevented when the MCF-7 cells were treated with β-lactam 9q and also with CA-4, demonstrating that the β-lactam 9q interacts with tubulin at the colchicine site of tubulin.
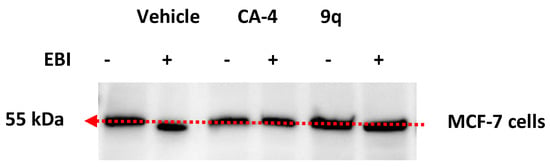
Figure 8.
Colchicine-binding assay. Effect of β-lactam compound 9q on the inhibition of bisthioalkylation of β-tubulin amino acids Cys239 and Cys354 by N,N’-ethylene-bis(iodoacetamide) (EBI). MCF-7 cells were treated with vehicle control (ethanol 0.1% (v/v)), CA-4 and β-lactam 9q (10 μM) for 2 h; samples were then treated with EBI for an additional 1.5 h. Cells were harvested, lysed and then analysed using sedimentation followed by Western blotting to identify β-tubulin and β-tubulin-EBI adduct.
Evidence of the effects of the β-lactam 9q on the molecular structure of the target tubulin was investigated using immunofluorescence and confocal microscopy studies, through which the changes in the microtubule network structure produced by β-lactam 9q in MCF-7 cells can be observed. MCF-7 cells demonstrated an organised microtubular network structure following staining with α-tubulin mAb (Figure 9) in the presence of the vehicle control (1% ethanol (v/v)). The cells were also treated with paclitaxel, which acts as a microtubule-stabilising agent []; paclitaxel clearly induced the formation of microtubule bundles and pseudo-asters. When the MCF-7 cells were treated with CA-4 or β-lactam 9q for 16 h, microtubule formation was inhibited and resulted in depolymerised microtubules (Figure 9), with multiple micronuclei present in these cells. Mitotic catastrophe, characterised by the appearance of multinucleated cells, is a type of programmed cell death occurring during mitosis. It results from a combination of deficient cell-cycle checkpoints (in particular, the DNA structure checkpoints and the spindle assembly checkpoint) and also cellular damage []. Induction of mitotic catastrophe by CA-4 was previously reported in non-small-cell lung cancer and breast cancer cells (MCF-7) []. The confocal imaging results support the proposed tubulin-targeting action of the 3-(prop-1-en-2-yl)azetidinone 9q.
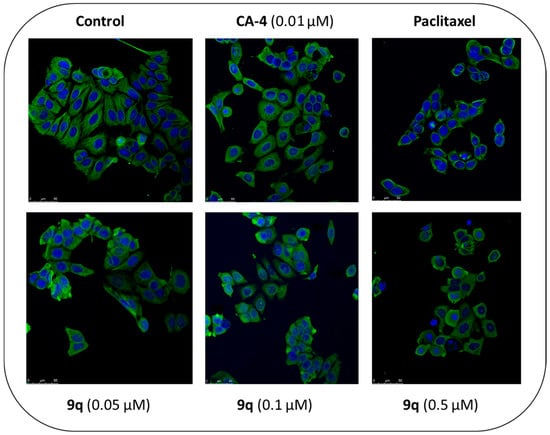
Figure 9.
β-lactam compound 9q induces depolymerization of the microtubule network in MCF-7 breast cancer cells.
CA-4 and β-lactam 9q depolymerise the microtubule network in MCF-7 cells. The MCF-7 cells were treated with vehicle control (1% ethanol (v/v)), paclitaxel (1 μM), CA-4 (10 nM) top panels and β-lactam 9q (0.05, 0.1 and 0.5 μM) bottom panels. After 16 h, the cells were fixed in 4% paraformaldehyde and stained with mouse monoclonal anti-α-tubulin−FITC antibody (clone DM1A) (green), Alexa Fluor 488 dye, and counterstained with DAPI (blue). The confocal images were obtained by Leica SP8 confocal microscopy with Leica application suite X software. Representative confocal micrograph images of three separate experiments are shown above [scale bar: 50 μM].
2.5. Computational Modelling of β-Lactam Compounds
The 3-(prop-1-en-2-yl)azetidin-2-one compound 9q was identified as the most potent compound synthesised in this study (IC50 = 10 nM in MCF-7 breast cancer cells) and also as an inhibitor of tubulin polymerisation. The tubulin-binding and related immunofluorescence studies of 9q indicated that the colchicine-binding site of tubulin is the target for the series of compounds. The structural similarity of the β-lactam compounds with CA-4 was demonstrated by X-ray studies revealing the similar torsional angle observed between rings A and B and a flexible alignment of the 3-butadienyl β-lactam compound 11p with CA-4 (Supplementary Figure S22), which showed excellent overlap with the 3,4,5-trimethoxyphenyl ring A and phenolic ring B of both compounds. In a further comparison of the energy-minimised structures of compound 11p and CA-4, the inter-atomic distances of the methoxy oxygens of ring A and ring B is 9.16 Å, while for CA-4, this distance is slightly longer (9.27 Å) (Supplementary Figure S23).
Computational docking calculations using MOE 2022.02 [] were undertaken for the most potent compounds identified (9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r and 17d) using the X-ray structure of bovine tubulin co-crystallised with N-deacetyl-N-(2-mercaptoacetyl)-colchicine (DAMA-colchicine) 1b 1SA0 [] (see Figure 10). 1H NMR spectroscopy confirmed that only the trans isomers of the compounds were obtained in the synthesis. All trimethoxy compounds overlaid their B-rings on the C-ring of DAMA-colchicine (forming HBA interactions with Lys352 and with the 4-methoxy group close to Thr353), co-located the 3,4,5-trimethoxyphenyl-substituted A-rings (with interactions possible with Cys241 and Ala326, Ala317 and Val318) and positioned the 3-alkenyl or ester side chain in an open region of the tubulin-binding site at the dimer interface (all amino acid residues refer to α-tubulin). The predicted docking ranking from best to worst was 17d, 11p, 9q, 11h, 9s, 9h, 10p, 10h, 11r and 10r (see Supplementary Materials, Table S11).
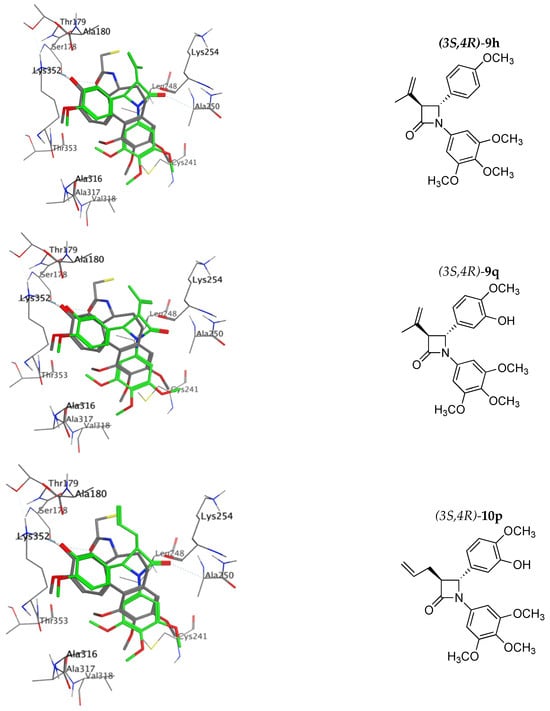
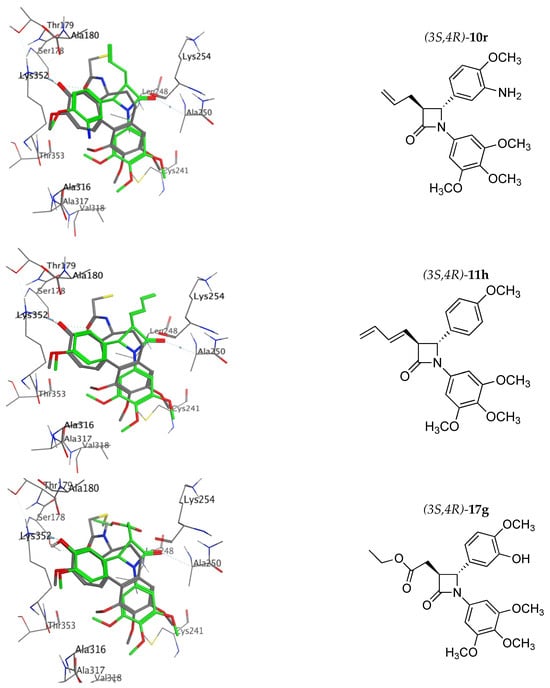
Figure 10.
Overlay of the X-ray structure of tubulin co-crystallised with DAMA-colchicine (PDB entry 1SA0) on the best-ranked docked pose of the 3S,4R enantiomer of 9h, 9q, 10p, 10r, 11h, 17g. Ligands are rendered as tubes and amino acids as lines. Tubulin amino acids and DAMA-colchicine are coloured by atom type: carbon = grey, hydrogen = white, oxygen = red, nitrogen = blue. The beta-lactam is depicted with a green backbone. The atoms are coloured by element type, key amino acid residues are labelled, and multiple residues are hidden to enable a clearer view.
Although the compounds were biologically evaluated as racemates, it was interesting that the 3S,4R enantiomer of each compound was found to be ranked at lower energy in the docking study than the corresponding 3R,4S enantiomer. We had previously reported stereochemical selectivity in docking energy calculated for related β-lactam compounds [,]. However, a very small difference was observed in the cellular efficacy of this series of compounds in the modelling study (e.g., IC50 values in the range 10–61 nM), so it would not be expected to see a large difference in ranking. Indeed, the docking scores only differ by <0.8 from best- to worst-ranked (see Supplementary Materials, Table S11). Thus, docking studies are not ideal for studying changes in cellular efficacy associated with small changes in the β-lactam scaffold substituents located at C-3.
The top three ranked compounds, 3-acetate ester 17e, 3-butadienyl-β-lactam 11p and 3-(prop-1-en-2-yl)-β-lactam 9q, all contained a meta-hydroxyl substituent on the B ring. Figure 10 shows the best docking pose of the top ranked phenolic compounds, 9q, 10p, 11p and 17e. For the 3-(prop-1-en-2-yl) compound 9s, the HBA interaction of the ring B amino group with Lys252 is clearly observed, while for the ring B methoxy compound 11h, the interaction of this methoxy group with the Thr353 residue is also illustrated, together with the β-lactam carbonyl interaction with Ala250 and Leu β248 (hydrophobic), which are residues of the T7 loop H8 helix, observed for all β-lactam compounds and also for colchicine. Although all molecules are located slightly deeper in the binding pocket than DAMA-colchicine, nevertheless, they demonstrate the observed ligand–protein interactions. (See Supplementary Materials, Figures S24–S26 for additional molecular modelling illustrations for compounds 9h, 9q, 9s, 10h, 11p and 11r).
We recently developed a novel pharmacophore generation tool, MoPBS []. In brief, MoPBS floods the protein-binding site with fragments describing classic molecular interaction features (acetate ion, benzene, methane and methylammonium) and independently minimising their positions within the binding site. Each fragment clusters in the region of complementary amino acids, thereby mapping out preferred interaction locations. By applying K-means clustering algorithms, we, in this case, reduce the clusters to eight pharmacophore features. Application of this tool to the 1SA0 binding site yielded a pharmacophore shown in Figure 11. As expected, many of the features mapped to those present in DAMA-colchicine and 17g, such as the two aromatic cores and the hydrogen bond acceptor (HBA) interacting with Lys352. Interestingly, a unique feature is present in the β-lactam, in that the carbonyl oxygen atom maps to a HBA feature. The pharmacophores also point towards future synthetic possibilities, such as introducing a hydrogen bond donor (F3 HBD) in place of the methoxy group on the compound 17g B-ring, including more hydrophobicity off the trimethoxy phenyl ring (F5) or extending the compound towards the region of space occupied by the F1 HBD feature.
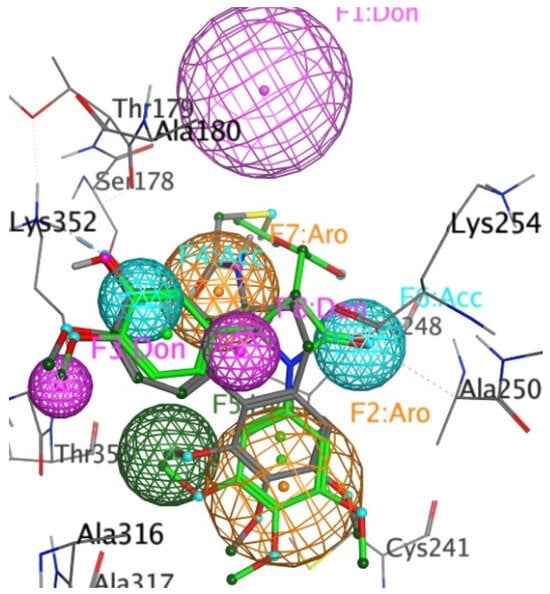
Figure 11.
Mapping of the X-ray structure of tubulin co-crystallised with DAMA-colchicine (PDB entry 1SA0) and the best ranked docked pose of the 3S,4R enantiomer 17g, with a pharmacophore created by MoPBS. The ligand and protein colouring is explained in the legend above. The pharmacophore feature colours are HBA, light blue; HBD, pink; aromatic, orange; and hydrophobic, green.
3. Experimental Section
3.1. Materials and Methods: Chemistry
Melting points were measured on a Gallenkamp SMP 11 melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded as thin film on NaCl plates or as potassium bromide discs on a Perkin Elmer FT-IR Spectrum 100 spectrometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 27 °C on a Bruker Avance DPX 400 spectrometer (400.13 MHz, 1H; 100.61 MHz, 13C) at 20 °C in CDCl3 (internal standard tetramethylsilane TMS) or DMSO-d6 by Dr. John O’Brien and Dr. Manuel Ruether, School of Chemistry, Trinity College Dublin. For CDCl3, 1H-NMR spectra were assigned relative to the TMS peak at 0.00 δ and 13C-NMR spectra were assigned relative to the middle CDCl3 triplet at 77.00 ppm. Electrospray ionisation mass spectrometry (ESI-MS) on a liquid chromatography time-of-flight (TOF) mass spectrometer (Micromass LCT, Waters Ltd., Manchester, UK) equipped with electrospray ionization (ES) interface operated in the positive ion mode with high-resolution mass measurement accuracies of <±5 ppm. Rf values are quoted for thin-layer chromatography on silica gel Merck F-254 plates. Flash column chromatography was carried out on Merck Kieselgel 60 (particle size 0.040–0.063 mm) and also on Biotage SP4 instruments. All products isolated were homogenous on TLC. Analytical high-performance liquid chromatography (HPLC) for purity determination of products was performed using a Waters 2487 Dual Wavelength Absorbance detector, Waters 1525 binary HPLC pump, Waters In-Line Degasser AF, Waters 717plus Autosampler and Varian Pursuit XRs C18 reverse-phase 150 × 4.6 mm chromatography column with detection at 254 nm. Imines 8a-w and azetidine-2-ones 16a-c and 18 were prepared following the reported procedures [,].
3.2. General Method I: Preparation of 3-(Prop-1-en-2-yl)-2-azetidinones (9a-x)
To a stirring, refluxing solution of imine (5 mmol) and triethylamine (6 mmol) in anhydrous dichloromethane (40 mL), a solution of 3,3-dimethylacryloyl chloride (6 mmol) in anhydrous dichloromethane (10 mL) was added dropwise over 45 min under nitrogen. The reaction was then heated at reflux for 5–8 h and stirred at 20 °C for 16 h. The reaction mixture was washed with water (2 × 100 mL), organic extract was dried over anhydrous sodium sulphate, and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography over silica gel (eluent: n-hexane:ethyl acetate, 4:1).
- 4-(4-Nitrophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9a)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-nitrobenzylidene)-3,4,5-trimethoxyphenylamine (8a) afforded the product as a yellow solid, yield 24%, Mp 129–130 °C (HPLC: 80.0%). IR (KBr) νmax: 2952 (C-H), 1754 (C=O, β-lactam), 1587 (C=C), 1506 (NO2), 1344 (NO2), 1236 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.92 (s, 3H), 3.72 (d, J = 2.00 Hz, 1H), 3.74 (s, 6H), 3.79 (s, 3H), 4.95 (d, J = 2.48 Hz, 1H), 5.09 (br s, 1H), 5.10 (br s, 1H), 6.52 (s, 2H), 7.57 (d, J = 9.04 Hz, 2H), 8.20–8.28 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 19.99, 55.73, 60.52, 60.61, 66.64, 94.16, 115.08, 124.17, 126.24, 132.68, 134.45, 136.81, 146.18, 148.79, 153.29, 153.24, 163.76 (C=O). HRMS (m/z) calculated for C21H23N2O6 (M++H): 399.1556, found 399.1556.
- 4-(4-Chlorophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidine-2-one (9b)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-chlorobenzylidene)-(3,4,5-trimethoxyphenyl)amine (8b) afforded the product as a yellow solid, yield 24%, Mp 137–138 °C (HPLC: 97.9%). IR (KBr) νmax: 2987 (C-H), 1746 (C=O, β-lactam), 1586 (C=C), 1503 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.90 (s, 3H), 3.70 (d, J = 2.00 Hz, 1H), 3.74 (s, 6H), 3.79 (s, 3H), 4.80 (d, J = 2.48 Hz, 1H), 5.05 (br s, 1H), 5.09 (br s, 1), 6.54 (s, 2H), 7.33 (d, J = 8.52 Hz, 2H), 7.40 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.54, 55.60, 59.38, 60.52, 66.51, 94.16, 114.41, 126.77, 129.07, 133.03, 133.89, 134.05, 135.81, 137.30, 153.12, 164.39. HRMS (m/z) calculated for C21H2235ClNO4Na (M++Na): 410.1135, found 410.1133.
- 4-(4-Bromophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidine-2-one (9c)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-bromobenzylidene)-3,4,5-trimethoxyphenylamine (8c) afforded the product as yellow solid, yield 19%, Mp 130–131 °C (HPLC: 97.6%). IR (KBr) νmax: 2940 (C-H), 1749 (C=O, β-lactam), 1585 (C=C), 1502 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.90 (s, 3H), 3.70 (br s, 1H), 3.75 (s, 6H), 3.79 (s, 3H), 4.80 (d, J = 1.84 Hz, 1H), 5.03 (br s, 1H), 5.09 (br s, 1H), 6.54 (s, 2H), 7.28 (d, J = 7.92 Hz, 2H), 7.56 (d, J = 8.56 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.54, 56.07, 59.88, 60.97, 66.94, 94.65, 114.89, 122.58, 127.54, 132.48, 133.48, 134.62, 136.82, 137.76, 153.60, 164.83. HRMS (m/z) calculated for C21H2379BrNO4 (M++H): 432.0810, found 432.0832.
- 4-(4-Fluorophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9d)
Preparation as described in the general method I above from 3,3-dimethylacryloyl chloride and (4-fluorobenzylidene)-3,4,5-trimethoxyphenylamine (8d) afforded the product as colourless crystals, yield 14%, Mp 99–100 °C (HPLC: 97.9%). IR (KBr) νmax: 2941 (C-H), 1746 (C=O, β-lactam), 1585 (C=C), 1508 (C=C), 1228 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.89 (s, 3H), 3.71 (d, J = 2.00 Hz, 1H), 3.73 (s, 6H), 3.78 (s, 3H), 4.81 (d, J = 2.52 Hz, 1H), 5.04 (br s, 1H), 5.09 (br s, 1H), 6.54 (s, 2H), 7.09–7.13 (m, 2H), 7.36–7.39 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 20.10, 55.56, 59.42, 60.50, 66.55, 94.17, 114.27, 115.77, 115.98, 127.14, 127.23, 133.03, 133.10, 134.04, 137.41, 153.09, 161.05, 164.50. HRMS (m/z) calculated for C21H22FNO4Na (M++Na): 394.1431, found 394.1443.
- 4-(4-Dimethylaminophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidine-2-one (9e)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-(dimethylamino)benzylidene)-3,4,5-trimethoxyphenylamine (8e) afforded the product as a brown oil, yield 34% (HPLC: 92.4%). IR (NaCl) νmax: 2942 (C-H), 1742 (C=O, β-lactam), 1597 (C=C), 1586 (C=C), 1506 (C=C), 1234 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.88 (s, 3H), 2.98 (s, 6H), 3.74 (s, 6H), 3.75 (d, J = 2.00 Hz, 1H), 3.77 (s, 3H), 4.73 (d, J = 2.44 Hz, 1H), 5.00 (br s, 1H), 5.07 (br s, 1H), 6.61 (s, 2H), 6.74 (d, J = 6.84 Hz, 2H), 7.27 (d, J = 9.28 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.17, 40.05, 55.53, 60.22, 60.48, 66.28, 94.23, 112.34, 113.70, 126.64, 133.51, 133.73, 134.78, 137.95, 140.56, 152.95, 165.19. HRMS (m/z) calculated for C23H29N2O4 (M++H): 397.2127; found 397.2124.
- 4-Phenyl-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9f)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and benzylidene-(3,4,5-trimethoxyphenyl)amine (8f) afforded the product as colourless crystals, yield 24%, Mp 108–109 °C (HPLC: 99.9%). IR (KBr) νmax: 2934 (C-H), 1746 (C=O, β-lactam), 1587 (C=C), 1506, 1237 cm−1. 1H NMR (400 MHz, CDCl3): δ 1.91 (s, 3H), 3.72 (s, 6H), 3.76 (d, J = 2.48 Hz, 1H), 3.78 (s, 3H), 4.82 (d, J = 2.48 Hz, 1H), 5.04 (br s, 1H), 5.10 (br s, 1H), 6.57 (s, 2H), 7.35–7.44 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 20.60, 55.99, 60.64, 60.94, 66.84, 94.70, 114.55, 125.97, 128.70, 129.27, 133.76, 134.33, 137.70, 138.10, 153.51, 165.16. HRMS (m/z) calculated for C21H23NO4Na (M++Na): 376.1525, found 376.1522.
- 4-p-Tolyl-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidine-2-one (9g)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-methylbenzylidene)-(3,4,5-trimethoxyphenyl)amine (8g) afforded the product as a colourless solid, yield 12%, Mp 102–104 °C (HPLC: 99.9%). IR (KBr) νmax: 2938 (C-H), 1746 (C=O, β-lactam), 1587 (C=C), 1505 (C=C), 1236 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.89 (s, 3H), 2.38 (s, 3H), 3.73 (s, 7H), 3.78 (s, 3H), 4.78 (d, J = 2.52 Hz, 1H), 5.02 (br s, 1H), 5.08 (br s, 1H), 6.57 (s, 2H), 7.22 (d, J = 8.04 Hz, 2H), 7.29 (d, J = 8.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.15, 20.77, 55.54, 60.04, 60.49, 66.40, 94.20, 113.97, 125.45, 129.46, 133.34, 133.87, 134.16, 137.71, 138.10, 153.01, 164.83. HRMS (m/z) calculated for C22H25NO4Na (M++Na): 390.1681, found 390.1680.
- 4-(4-Methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9h)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-methoxybenzylidene)-3,4,5-trimethoxyphenylamine (8h) afforded the product as a yellow solid, yield 40%, Mp 103–105 °C (HPLC: 87.1%). [] IR (KBr) νmax: 2995 (C-H), 1744 (C=O, β-lactam), 1588 (C=C), 1508 (C=C), 1249 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.89 (s, 3H), 3.73 (s, 7H, OCH3), 3.78 (s, 3H), 3.83 (s, 3H), 4.77 (d, J = 2.52 Hz, 1H), 5.02 (br s, 1H), 5.08 (br s, 1H), 6.57 (s, 2H), 6.94 (d, J = 8.52 Hz, 2H), 7.32 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.14, 54.88, 55.53, 59.85, 60.49, 66.46, 94.19, 113.94, 114.15, 126.80, 129.04, 133.33, 133.85, 137.71, 153.00, 159.36, 164.87 (C=O). HRMS (m/z) calculated for C22H25NO5Na (M++Na): 406.1630, found 406.1618.
- 4-(4-Ethoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9i)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-ethoxybenzylidene)-(3,4,5-trimethoxyphenyl)amine (8i) afforded the product as a colourless solid, yield 12%, Mp 100–102 °C [] (HPLC: 83.9%). IR (KBr) νmax: 2984 (C-H), 1745 (C=O, β-lactam), 1591 (C=C), 1587 (C=C), 1509 (C=C), 1240 (C-O) −1. 1H NMR (400 MHz, CDCl3): δ 1.44 (t, J = 4.64 Hz, 3H), 1.90 (s, 3H), 3.74 (s, 7H), 3.78 (s, 3H), 4.05 (q, J = 4.88 Hz, 2H), 4.77 (d, J = 1.48 Hz, 1H), 5.03 (br s, 1H), 5.09 (br s, 1H), 6.58 (s, 2H), 6.93 (d, J = 5.88 Hz, 2H), 7.29–7.32 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 14.62, 20.43, 55.86, 60.24, 60.76, 63.42, 66.77, 94.65, 114.14, 115.00, 127.08, 129.23, 133.68, 134.31, 138.07, 153.34, 159.10, 165.16. HRMS (m/z) calculated for C23H27NO5Na (M++Na): 420.1787, found 420.1772.
- 4-(4-Butoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidine-2-one (9j)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-butoxybenzylidene)-3,4,5-trimethoxyphenylamine (8j) afforded the product as an off-white solid, yield 7%, Mp 55–57 °C (HPLC: 99.9%). IR (KBr) νmax: 2936 (C-H), 1747 (C=O, β-lactam), 1603 (C=C), 1587 (C=C), 1509 (C-C), 1243 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.99 (t, J = 7.28Hz, 3H), 1.49–1.51 (m, 2H), 1.76–1.80 (m, 2H), 1.89 (s, 3H), δ 3.73 (s, 7H), 3.78 (s, 3H), 3.97 (t, J = 6.28 Hz, 2H), 4.76 (d, J = 2.48Hz, 1H), 5.02 (br s, 1H), 5.08 (br s, 1H), 6.57 (s, 2H), 6.92 (d, J = 8.52 Hz, 2H), 7.31 (d, J = 9.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 13.40, 18.78, 20.15, 30.80, 55.54, 59.90, 60.49, 66.45, 67.33, 94.20, 113.91, 114.67, 126.77, 128.78, 133.36, 133.84, 137.75, 153.30, 158.98, 164.90. HRMS (m/z) calculated for C25H31NO5Na (M++Na): 448.2100, found 448.2106.
- 4-(4-Phenoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9k)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-phenoxylbenzylidene)-(3,4,5-trimethoxyphenyl)amine (8k) afforded the product as a brown solid, yield 20%, Mp 126–127 °C (HPLC: 89.1%). IR (KBr) νmax: 2938 (C-H), 1748 (C=O, β-lactam), 1587 (C=C), 1507 (C=C), 1239 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.91 (s, 3H), 3.75 (s, 7H), 3.79 (s, 3H), 4.81 (d, J = 2.52 Hz, 1H), 5.04 (br s, 1H), 5.10 (br s, 1H), 6.58 (s, 2H), 7.02–7.05 (m, 4H), 7.14–7.17 (m, 1H), 7.35–7.39 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 20.15, 55.57, 59.69, 60.52, 66.45, 94.24, 114.13, 118.72, 118.80, 123.38, 127.00, 129.44, 131.64, 133.24, 133.99, 137.59, 153.07, 156.09, 157.36, 164.71. HRMS (m/z) calculated for C27H27NO5Na (M++Na): 468.1787, found 468.1786.
- 4-(4-Benzyloxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9l)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and (4-benzyloxybenzylidene)-3,4,5-trimethoxyphenylamine (8l) afforded the product as a yellow green resin, yield 35% (HPLC: 82.4%). IR (NaCl) νmax: 2976 (C-H), 1744 (C=O, β-lactam), 1587 (C=C), 1508 (C=C), 1275 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.89 (s, 3H), 3.73 (s, 7H), 3.78 (s, 3H), 4.78 (d, J = 2.48 Hz, 1H), 5.03 (br s, 1H), 5.09 (br s, 3H), 6.57 (s, 2H), 7.02 (d, J = 8.56 Hz, 2H), 7.31–7.43 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 20.16, 55.55, 59.84, 60.51, 66.43, 69.61, 94.20, 113.98, 115.09, 126.85, 127.03, 127.68, 128.19, 129.33, 133.33, 133.86, 136.14, 137.70, 153.01, 158.53, 164.85. HRMS (m/z) calculated for C28H29NO5Na (M++Na): 482.1943, found 482.1937.
- 4-Naphthalen-1-yl-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9m)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and naphthalen-1-ylmethylene-(3,4,5-trimethoxyphenyl)amine (8m) afforded the product as a brown oil, yield 51% (HPLC: 86.8%). IR (NaCl) νmax: 2984(C-H), 1749 (C=O, β-lactam), 1588 (C=C), 1506 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.02 (s, 3H), 3.70 (s, 6H), 3.81 (s, 4H), 5.11 (br s, 1H), 5.18 (br s, 1H), 5.66 (d, J = 2.00 Hz, 1H), 6.65 (s, 2H), 7.45–8.03 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 19.62, 55.47, 55.66, 60.52, 66.59, 94.40, 116.16, 122.20, 122.58, 124.44, 125.11, 125.70, 126.25, 128.19, 128.73, 129.99, 133.41, 133.47, 134.06, 137.75, 153.15, 164.88. HRMS (m/z) calculated for C25H25NO4Na (M++Na): 426.1681, found 426.1687.
- 4-Naphthalen-2-yl-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9n)
Preparation as described in general method I above from 3,3-dimethylacryloyl chloride and naphthalen-2-ylmethylene-(3,4,5-trimethoxyphenyl)amine (8n) afforded the product as colourless solid, yield 15%, Mp 123–124 °C (HPLC: 98.8%). IR (KBr) νmax: 2978 (C-H), 1746 (C=O, β-lactam), 1586 (C=C), 1505 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.94 (s, 3H), 3.69 (s, 6H), 3.77 (s, 3H), 3.83 (d, J = 2.00 Hz, 1H), 5.00 (d, J = 3.00 Hz, 1H), 5.06 (br s, 1H), 5.12 (br s, 1H), 6.63 (s, 2H), 7.48–7.87 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 20.17, 55.56, 60.33, 60.49, 66.47, 94.23, 114.25, 122.51, 124.95, 126.11, 126.32, 127.39, 127.42, 129.02, 132.88, 132.92, 133.39, 134.00, 134.73, 137.59, 153.08, 164.75. HRMS (m/z) calculated for C25H25NO4Na (M++Na): 426.1681, found 426.1674.
- 4-(4-(Methylthio)phenyl)-3-(prop-1-en-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (9o)
Preparation as described in general method I above from imine (8o) and 3,3-dimethylacryloyl chloride afforded the product as a yellow powder, yield 56%, Mp 99–100 °C [] (HPLC: 99.1%). IR νmax: 1741.7 (C=O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.85 (s, 3 H), 2.46 (s, 3 H), 3.66–3.69 (m, 1 H), 3.69 (s, 6 H), 3.75 (s, 3 H), 4.74 (d, J=2.44 Hz, 1 H), 4.99–5.07 (m, 2 H), 5.60–5.91 (m, 1 H), 6.53 (s, 2 H), 7.24–7.29 (m, 4 H). 13C NMR (100 MHz, CDCl3): δ 15.09, 20.13, 55.60, 59.78, 60.51, 66.44, 94.22, 114.16, 125.44, 126.47, 133.81, 137.56, 138.93, 153.07, 164.68. HRMS (m/z) calculated for C22H25KNO4S [M++K]: 438.1141; found 438.1141.
- 4-(3-Hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9q)
Following the general method I above, a solution of the protected TBDMS imine 8p (5 mmol) and triethylamine (6 mmol) in anhydrous dichloromethane (40 mL) was treated with a solution of 3,3-dimethylacryloyl chloride (6 mmol) in anhydrous dichloromethane (10 mL) to afford the intermediate β-lactam (9p), which was immediately deprotected. To a stirring solution of 9p (5 mmol) under nitrogen at 0 0C in dry THF was added dropwise t-BAF solution (1.0 M) in hexanes (5 mL, 5 mmol). The resulting solution was left to stir at 0 °C until reaction was completed by TLC. The reaction mixture was then diluted with ethyl acetate (75 mL) and washed with aqueous HCl (0.1M, 100 mL). Following repeated extraction with ethyl acetate (2 × 25 mL), the organic layers were combined and washed with water (100 mL) and saturated brine (100 mL) and dried over Na2SO4. The solvent was removed under reduced pressure to yield the desired product as a brown solid, which was recrystallised from ethanol, yield 22%, Mp 82–84 °C (HPLC: 88.9%). IR (KBr) νmax: 3390 (OH), 1740 (C=O, β-lactam), 1593 (C=C), 1508 (C=C), 1275 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.88 (s, 3H), 3.72 (d, J = 2.00 Hz, 1H), 3.74 (s, 6H), 3.78 (s, 3H), 3.91 (s, 3H), 4.71 (d, J = 2.52 Hz, 1H), 5.01 (br s, 1H), 5.07 (br s, 1H), 5.76 (br s, 1H), 6.58 (s, 2H), 6.85–6.96 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 20.17, 55.55, 55.58, 59.87, 60.50, 66.37, 94.24, 110.52, 111.54, 113.93, 117.28, 130.30, 133.32, 133.88, 137.70, 145.81, 146.31, 153.01, 164.81. HRMS (m/z) calculated for C22H25NO6Na (M++Na): 422.1580, found 422.1592.
- 4-(4-Methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9r)
Preparation as described following the general method I above from 3,3-dimethylacryloyl chloride and (4-methoxy-3-nitrobenzylidene)-(3,4,5-trimethoxyphenyl) amine (8r) afforded the product as a brown oil, yield 19% (HPLC: 75.5%). IR (NaCl) νmax: 2940 (C-H), 1751 (C=O, β-lactam), 1535 (NO2), 1507 (C=C), 1281 (NO2) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.90 (s, 3H), 3.73 (d, J = 2.24 Hz, 1H), 3.76 (s, 6H), 3.79 (s, 3H), 4.00 (s, 3H), 4.84 (d, J = 2.76 Hz, 1H), 5.07 (br s, 1H), 5.09 (br s, 1H), 6.54 (s, 2H), 7.16 (d, J = 8.76 Hz, 1H), 7.55–7.58 (m, 1H), 7.91 (d, J = 2.48 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 20.00, 55.70, 56.26, 58.54, 60.50, 66.56, 94.31, 114.17, 114.74, 123.12, 129.69, 130.67, 132.74, 134.45, 136.96, 139.42, 152.60, 153.26, 164.11. HRMS (m/z) calculated for C22H24N2O7Na (M++Na): 451.1481, found 451.1481.
- 4-(3-Amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(prop-1-en-2-yl)azetidin-2-one (9s)
To a mixture of 3-(prop-1-en-2-yl)-4-(4-methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (9r) (0.25 mmol) and zinc powder 10 μm (2.5 mmol) was added acetic acid (15 mL), and the reaction mixture was stirred for 7 days at 20 °C under nitrogen. The mixture was filtered through a celite pad, and solvent was removed under reduced pressure. Purification by flash chromatography over silica gel (elutant ethyl acetate-n-hexane, 1:1) yielded the title compound as a yellow oil, yield 48% (HPLC: 91.6%). IR (NaCl) νmax: 3376 (NH2), 1726 (C=O, β-lactam), 1594 (C=C), 1509 (C=C), 1237 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.87 (s, 3H), 3.72 (br s, 1H), 3.74 (s, 6H), 3.78 (s, 3H), 3.87 (s, 3H), 4.67 (d, J = 2.52 Hz, 1H), 5.00 (br s, 1H), 5.06 (br s, 1H), 6.60 (s, 2H), 6.74–6.78 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 20.16, 55.11, 55.56, 60.11, 60.49, 66.31, 94.21, 109.99, 111.31, 113.82, 116.09, 129.66, 133.47, 133.80, 136.13, 137.84, 147.14, 152.98, 165.02. HRMS (m/z) calculated for C22H27N2O5 (M++H): 399.1920, found 399.1900.
- 1-(4-(Methylthio)phenyl)-3-(prop-1-en-2-yl)-4-(3,4,5-trimethoxyphenyl)azetidin-2-one (9t)
Preparation following the general method I above from imine 8s and dimethylacryloyl chloride afforded the product as a yellow powder, yield 45%, Mp 113–115 °C (HPLC: 96%). IRνmax (ATR): 1737.1 (C=O, β-lactam) cm−1. 1H NMR (400 MHz, CDCl3): δ ppm 1.90 (s, 3 H), 2.45 (s, 3 H), 3.72–3.77 (m, 1 H), 3.81–3.89 (m, 9 H), 4.74 (d, J = 2.51 Hz, 1 H), 5.04 (s, 1 H), 5.09 (s, 1 H), 6.55 (s, 2 H), 7.19 (d, J = 8.53 Hz, 2 H), 7.28 (d, J = 8.53 Hz, 2 H). 13C NMR (100 MHz, CDCl3): δ 16.08, 20.07, 55.78, 60.12, 60.41, 66.67, 102.03, 114.37, 117.17, 125.44, 127.45, 133.06, 134.66, 137.52, 153.51, 164.78. HRMS (m/z) calculated for C22H25NNaO4S [M + Na]+: 422.1402, found 422.1413.
- 1-(4-Ethoxyphenyl)-3-(prop-1-en-2-yl)-4-(3,4,5-trimethoxyphenyl)azetidin-2-one (9u)
Preparation following the general method I above from imine 8t and dimethylacryloyl chloride afforded the product as yellow powder, yield 38%, Mp 106–108 °C (HPLC: 98%). IRνmax (ATR): 1729.4 (C=O, β-lactam) cm−1. 1H NMR (400 MHz, CDCl3): δ ppm 1.40 (t, J = 7.03 Hz, 3 H), 1.91 (s, 3 H), 3.73 (s, 1 H), 3.80 (s, 6 H), 3.89 (s, 3 H), 3.94–4.04 (m, 2 H) 4.73 (d, J = 2.51 Hz, 1 H), 5.04 (s, 1 H), 5.09 (s, 1 H), 6.55 (s, 2 H), 6.82 (d, J = 9.03 Hz, 2 H), 7.27 (d, J = 9.03 Hz, 2 H). 13C NMR (100 MHz, CDCl3): δ 14.38, 20.06, 55.76, 60.14, 60.43, 63.22, 66.60, 94.65, 102.07, 114.30, 117.93, 130.52, 133.07, 137.73, 153.46, 164.41. HRMS (m/z) calculated for C23H27NNaO5 [M + Na]+: 420.1787, found 420.1787.
- 1-(4-Methoxyphenyl)-3-(prop-1-en-2-yl)-4-(3,4,5-trimethoxyphenyl)azetidin-2-one (9v)
Preparation following the general method I above from imine 8u and dimethylacryloyl chloride afforded the product as a pale yellow powder; yield 35%, Mp 128–130 °C (HPLC: 99%). IRνmax (ATR): 1727.5 (C=O, β-lactam) cm-1. 1H NMR (400 MHz, CDCl3): δ ppm 1.91 (s, 3 H, H-7), 3.74 (s, 1 H, H-3), 3.78 (s, 3 H, OCH3), 3.83 (s, 6 H, OCH3), 3.87 (s, 3 H, OCH3), 4.73 (d, J = 2.01 Hz, 1 H, H-4), 5.04 (s, 1 H, H-6), 5.09 (s, 1 H, H-6), 6.56 (s, 2 H, ArH), 6.83 (d, J = 9.03 Hz, 2 H, ArH), 7.26–7.35 (m, 2 H, ArH). 13C NMR (100 MHz, CDCl3): δ 20.06, 55.00, 55.76, 60.16, 60.43, 66.60, 102.09, 113.85, 114.30, 117.95, 130.66, 133.06, 137.46, 137.73, 153.48, 155.68, 164.43 (C2, C=O). HRMS (m/z) calculated for C22H25NNaO5 [M + Na]+: 406.1630, found 406.1631.
- 1-(4-Methoxyphenyl)-4-phenyl-3-(prop-1-en-2-yl)azetidin-2-one (9w)
Preparation followed the general method I above from dimethylacryloyl chloride and imine 8v. The product was obtained as a colourless solid, yield 4.6%, 68 mg, melting point 111–113 °C (HPLC: 100.0%). IR (KBr) νmax: 1727 (C=O, β-lactam), 1624 (C=C), 1506 (C=C), 1253 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.90 (s, 3H, H-7), 3.72 (s, 1H, H-3), 3.76 (s, 3H, OCH3), 4.82 (d, J = 2.52 Hz, 1H, H-4), 5.03 (br s, 1H, H-6), 5.09 (br s, 1H, H-6), 6.81 (d, J = 9.04 Hz, 2H, H-3′, H-5′), 7.27 (d, J = 9.00 Hz, 2H, H-2′, H-6′), 7.36–7.39 (m, 5H, H-2c″, H-3″, H-4″, H-5″, H-6″). 13C NMR (100MHz, CDCl3): δ 20.45 (C-7), 55.35 (OCH3), 60.19 (C-3), 66.95 (C-4), 114.22 (C-3′, C-5′), 114.40 (C-6), 118.28 (C-2′, C-6′), 125.79, 128.41, 129.10 (C-2″, C-3″, C-4″, C-5″, C-6″), 131.00 (C-1′), 137.72 (C-1″), 138.16 (C-5), 155.96 (C-4′), 164.67 (C-2). HRMS (m/z) calculated for C19H20NO2 [M+H]+: 294.1494, found 294.1500.
- 4-(4-Methoxyphenyl)-1-phenyl-3-(prop-1-en-2-yl)azetidin-2-one (9x)
Preparation following the general method I above from dimethylacryloyl chloride and imine 8w afforded the product as a colourless solid, yield 34%, 497 mg, melting point 104–105 °C (HPLC: 99.7%). IR (KBr) νmax: 2928 (C-H), 1749 (C=O, β-lactam), 1635 (C=C), 1513 (C=C), 1250 (C-O), 1087 (C-O) cm−1. 1H NMR (400MHz, CDCl3): δ 1.89 (s, 3H, H-7), 3.72 (d, J = 2.00 Hz, 1H, H-3), 3.82 (s, 3H, OCH3), 4.82 (d, J = 2.48 Hz, 1H, H-4), 5.02 (br s, 1H, H-6), 5.08 (br s, 1H, H-6), 6.92–6.94 (m, 2H, H-3″ H-5″), 7.04–7.08 (m, 1H, H-4′), 7.25–7.34 (m, 6H, H-2″, H-6″, H-2′, H-3′, H-5′, H-6′). 13C NMR (100 MHz, CDCl3): δ 20.12 (C-7), 54.87 (OCH3), 59.44 (C-3), 66.67 (C-4), 114.00 (C-6), 114.15 (C-’’, C-5″), 116.63 (C-2′, C6′), 123.43 (C-4′), 126.70 (C-2″, C-6″), 128.59 (C-3′, C-5′), 129.12 (C-1″), 137.10 (C-1′), 137.74 (C-5), 159.30 (C-4″), 165.03 (C-2). HRMS (m/z) calculated for C19H19NO2Na [M+Na]+: 316.1313, found 316.1311.
3.3. General Method II: Preparation of β-Lactams (10a-s)
To a stirring, refluxing solution of the appropriate imine (5 mmol) and triethylamine (6 mmol) in anhydrous dichloromethane (40 mL), a solution of 4-pentenoyl chloride (6 mmol) in anhydrous dichloromethane (10 mL) was added dropwise over 45 min under nitrogen. The reaction mixture was heated at reflux for 5 h, and then stirred at 20 °C for 20 h. The reaction mixture was washed with water (2 × 100 mL), the organic layer was dried (Na2SO4), and the solvent was removed under reduced pressure. The crude product was purified by flash chromatography over silica gel (eluent: 4:1 n-hexane: ethyl acetate).
- 3-Allyl-4-(4-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10a)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-nitrobenzylidene)-3,4,5-trimethoxyphenylamine (8a). The product was isolated as a yellow oil (yield 16%) (HPLC: 98.9%). IR (NaCl) νmax: 2942 (C-H), 1751 (C=O, β-lactam), 1585 (C=C), 1521 (NO2), 1344 (NO2), 1232 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.57–2.65 (m, 1H), 2.75–2.82 (m, 1H), 3.19–3.24 (m, 1H), 3.74 (s, 6H), 3.79 (s, 3H), 4.80 (d, J = 2.00 Hz, 1H), 5.20–5.26 (m, 2H) 5.83–5.93 (m, 1H), 6.49 (s, 2H), 7.54 (d, J = 9.04 Hz, 2H), 8.28 (d, J = 9.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 32.40, 55.67, 58.85, 59.27, 60.52, 94.07, 117.89, 124.07, 126.40, 132.85, 133.18, 134.36, 135.92, 144.84, 153.26, 165.51. HRMS (m/z) calculated for C21H22N2O6Na [M++Na]: 421.1376, found 421.1397.
- 3-Allyl-4-(4-chlorophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10b)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-chlorobenzylidene)-(3,4,5-trimethoxyphenyl)amine (8b). The product was isolated as a yellow oil (yield 21%) (HPLC: 98.1%). IR (NaCl) νmax: 2994 (C-H), 1749 (C=O, β-lactam), 1586 (C=C), 1503 (C=C), 1233 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.54–2.61 (m, 1H), 2.71–2.77 (m, 1H), 3.17–3.21 (m, 1H), 3.73 (s, 6H), 3.78 (s, 3H), 4.66 (d, J = 2.04 Hz, 1H), 5.15–5.21 (m, 2H), 5.82–5.92 (m, 1H), 6.51 (s, 2H), 7.30 (d, J = 8.04 Hz, 2H), 7.38 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 32.35, 55.58, 58.82, 59.65, 60.51, 94.08, 117.46, 126.93, 128.96, 133.20, 133.42, 133.86, 134.02, 135.91, 153.10, 166.19. HRMS (m/z) calculated for C21H2235ClNO4Na [M++Na]: 410.1135, found 410.1139.
- 3-Allyl-4-(4-bromophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10c)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-bromobenzylidene)-3,4,5-trimethoxyphenylamine (8c). The product was isolated as a yellow oil (yield 21%) (HPLC: 98.9%). IR (NaCl) νmax: 2997 (C-H), 1746 (C=O, β-lactam), 1643 (C=C), 1587 (C=C), 1503 (C=C), 1234 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.53–2.61 (m, 1H), 2.71–2.77 (m, 1H), 3.17–3.20 (m, 1H), 3.74 (s, 6H), 3.78 (s, 3H), 4.64 (d, J = 1.52 Hz, 1H), 5.15–5.21 (m, 2H), 5.82–5.92 (m, 1H), 6.51 (s, 2H), 7.25 (d, J = 8.56 Hz, 2H), 7.53 (d, J = 8.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 32.35, 55.59, 58.79, 59.68, 60.51, 94.07, 117.17, 121.94, 127.23, 131.91, 133.18, 133.41, 134.03, 136.45, 153.11, 166.17. HRMS (m/z) calculated for C21H2280BrNO4Na [M++Na]: 454.0630, found 454.0645.
- 3-Allyl-4-(4-fluorophenyl)-1-(3,4,5-trimethoxyphenyl) azetidin-2-one (10d)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-fluorobenzylidene)-3,4,5-trimethoxyphenylamine (8d). The product was isolated as colourless crystals (yield 13%); mp: 89–90 °C (HPLC: 100.0%). IR (KBr) νmax: 2947 (C-H), 1748 (C=O, β-lactam), 1585 (C=C), 1508 (C=C), 1228 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.54–2.62 (m, 1H), 2.70–2.77 (m, 1H), 3.18–3.22 (m, 1H), 3.72 (s, 6H), 3.78 (s, 3H), 4.66 (d, J = 2.00 Hz, 1H), 5.15–5.21 (m, 2H), 5.83–5.93 (m, 1H), 6.51 (s, 2H), 7.07–7.11 (m, 2H), 7.33–7.36 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 32.34, 55.54, 58.82, 59.68, 60.51, 94.07, 115.65, 115.87, 117.38, 127.25, 127.34, 133.09, 133.26, 133.48, 133.93, 153.07, 160.20, 166.33. HRMS (m/z) calculated for C21H22FNO4Na [M++Na]: 394.1431, found 394.1435.
- 3-Allyl-4-(4-dimethylaminophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10e)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-(dimethylamino)benzylidene)-3,4,5-trimethoxyphenylamine (8e). The product was isolated as a yellow oil (yield 11%) (HPLC: 94.7%). IR (NaCl) νmax: 2991 (C-H), 1741 (C=O, β-lactam), 1656 (C=C), 1587 (C=C), 1504 (C=C), 1231 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.52–2.60 (m, 1H), 2.67–2.74 (m, 2H), 2.97 (s, 6H), 3.21–3.25 (m, 1H), 3.73 (s, 6H), 3.77 (s, 3H), 4.58 (d, J = 2.52 Hz, 1H), 5.11–5.18 (m, 2H), 5.84–5.94 (m, 1H), 6.58 (s, 2H), 6.72 (d, J = 7.52 Hz, 2H), 7.24 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 32.35, 40.00, 55.52, 58.47, 60.39, 60.48, 94.15, 112.19, 117.01, 124.24, 126.70, 133.71, 133.75, 135.01, 150.13, 152.92, 167.06. HRMS (m/z) calculated for C23H29N2O4 [M++H]: 397.2127, found 397.2133.
- 3-Allyl-4-phenyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10f)
Preparation followed the general method II above from 4-pentenoyl chloride and benzylidene-(3,4,5-trimethoxyphenyl)amine (8f). The product was obtained as a yellow resin (yield 35%) (HPLC: 100.0%). IR (NaCl) νmax: 2979 (C-H), 1748 (C=O, β-lactam), 1588 (C=C), 1505 (C=C), 1236 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.56–2.64 (m, 1H), 2.71–2.78 (m, 1H), 3.23–3.27 (m, 1H), 3.72 (s, 6H), 3.78 (s, 3H), 4.68 (d, J = 1.96 Hz, 1H), 5.15–5.22 (m, 2H), 5.86–5.95 (m, 1H), 6.54 (s, 2H), 7.35–7.38 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 32.39, 55.50, 58.68, 60.39, 60.50, 94.10, 117.31, 125.63, 126.99, 128.09, 128.71, 133.46, 133.54, 136.33, 137.31, 153.02, 166.55. HRMS (m/z) calculated for C21H23NO4Na [M++Na]: 376.1525, found 376.1524.
- 3-Allyl-4-p-tolyl-1-olyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10g)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-methylbenzylidene)-(3,4,5-trimethoxyphenyl)amine (8g). The product was obtained as a yellow oil (yield 28%) (HPLC: 92.8%). IR (NaCl) νmax: 2979 (C-H), 1747 (C=O, β-lactam), 1588 (C=C), 1506 (C=C), 1236 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.37 (s, 3H), 2.54–2.61 (m, 1H), 2.69–2.76 (m, 1H), 3.20–3.24 (m, 1H), 3.72 (s, 6H), 3.77 (s, 3H), 4.64 (d, J = 2.48 Hz, 1H), 5.13–5.20 (m, 2H), 5.85–5.95 (m, 1H), 6.54 (s, 2H), 7.20 (d, J = 8.00 Hz, 2H), 7.26 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.75, 32.37, 55.52, 58.68, 60.24, 60.49, 94.12, 117.21, 125.57, 129.36, 133.50, 133.59, 133.77, 134.26, 137.93, 152.99, 166.67. HRMS (m/z) calculated for C22H26NO4 [M++H]: 368.1862, found 368.1869.
- 3-Allyl-4-(4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10h)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-methoxybenzylidene)-3,4,5-trimethoxyphenylamine (8h). The product was obtained as a yellow solid (yield 36%); mp: 86–88 °C (HPLC: 98.95%). IR (KBr) νmax: 2984 (C-H), 1746 (C=O, β-lactam), 1604 (C=C), 1508 (C=C), 1250 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.55–2.61 (m, 1H), 2.69–2.74 (m, 1H), 3.19–3.23 (m, 1H), 3.72 (s, 6H), 3.77 (s, 3H), 3.82 (s, 3H), 4.63 (d, J = 2.48 Hz, 1H), 5.13–5.19 (m, 2H), 5.85–5.89 (m, 1H), 6.55 (s, 2H), 6.92 (d, J = 8.52 Hz, 2H), 7.30 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 32.34, 54.87, 55.53, 58.69, 60.06, 60.49, 94.12, 114.04, 117.17, 126.92, 129.18, 133.50, 133.61, 133.78, 153.00, 159.25, 166.71. HRMS (m/z) calculated for C22H25NO5Na [M++Na]: 406.1630, found 406.1638.
- 3-Allyl-4-(4-ethoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10i)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-ethoxybenzylidene)-(3,4,5-trimethoxyphenyl)amine (8i). The product was isolated as a yellow oil (yield 41%) (HPLC 94.8%). IR (NaCl) νmax: 2983 (C-H), 1746 (C=O, β-lactam), 1588 (C=C), 1508 (C=C), 1239 (C-O) cm−1 1H NMR (400 MHz, CDCl3): δ 1.43 (t, J = 7.02 Hz, 3H), 2.53–2.59 (m, 1H), 2.61–2.75 (m, 1H), 3.19–3.24 (m, 1H), 3.72 (s, 6H), 3.77 (s, 3H), 4.04 (q, J = 7.02 Hz, 2H), 4.62 (d, J = 2.52 Hz, 1H), 5.13–5.19 (m, 2H), 5.84–5.95 (m, 1H), 6.55 (s, 2H), 6.91 (d, J = 8.56 Hz, 2H), 7.28 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 14.34, 32.34, 55.52, 58.66, 60.09, 60.50, 63.07, 94.10, 114.55, 117.16, 126.90, 128.98, 133.52, 133.62, 133.87, 152.98, 158.63, 166.72. HRMS (m/z) calculated for C23H27NO5Na [M++Na]: 420.1787, found 420.1783.
- 3-Allyl-4-(4-butoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10j)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-butoxybenzylidene)-3,4,5-trimethoxyphenylamine (8j). The product was isolated as a yellow oil (yield 46%) (HPLC: 96.2%). IR (NaCl) νmax: 2940 (C-H), 1747 (C=O, β-lactam), 1602 (C=C), 1599 (C=C) 1507 (C=C), 1241 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.99 (t, J = 7.28 Hz, 3H), 1.47–1.53 (m, 2H), 1.76–1.80 (m, 2H), 2.53–2.61 (m, 1H), 2.69–2.75 (m, 1H), 3.19–3.24 (m, 1H), 3.72 (s, 6H), 3.77 (s, 3H), 3.97 (t, J = 6.54 Hz, 2H), 4.62 (d, J = 2.00 Hz, 1H), 5.13–5.19 (m, 2H), 5.83–5.93 (m, 1H), 6.55 (s, 2H), 6.91 (d, J = 8.56 Hz, 2H), 7.28 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 13.40, 18.78, 30.81, 32.34, 55.52, 58.67, 60.10, 60.50, 67.32, 94.11, 114.58, 117.15, 126.88, 128.91, 133.52, δ133.63, 133.75, 152.98, 158.85, 166.74. HRMS (m/z) calculated for C25H31NO5Na [M++Na] 448.2100, found 448.2090.
- 3-Allyl-4-(4-phenoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10k)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-phenoxylbenzylidene)-(3,4,5-trimethoxyphenyl)amine (8k). The product was obtained as a yellow oil (yield 31%) (HPLC: 100.0%). IR (NaCl) νmax: 2978 (C-H), 2941 (C-H), 1748 (C=O, β-lactam), 1587 (C=C), 1506 (C=C), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.55–2.63 (m, 1H, CH2), 2.71–2.78 (m, 1H, CH2), 3.22–3.26 (m, 1H), 3.74 (s, 6H), 3.79 (s, 3H), 4.66 (d, J = 2.52 Hz, 1H), 5.15–5.22 (m, 2H), 5.85–5.96 (m, 1H), 6.55 (s, 2H), 7.01–7.03 (m, 4H), 7.13–7.17 (m, 1H), 7.32–7.39 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 32.37, 55.55, 58.72, 59.91, 60.52, 94.14, 117.31, 118.67, 118.74, 123.32, 127.11, 129.42, 131.78, 133.41, 133.55, 133.88, 153.05, 156.14, 157.19, 166.52. HRMS (m/z) calculated for C27H27NO5Na [M++Na]: 468.1787, found 468.1786.
- 3-Allyl-4-(4-benzyloxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10l)
Preparation followed the general method II above from 4-pentenoyl chloride and (4-benzyloxybenzylidene)-3,4,5-trimethoxyphenylamine (8l). The product was isolated as a grey solid (yield 34.7%); mp: 140–141 °C (HPLC: 99.8%). IR (KBr) νmax: 2827 (C-H), 1744 (C=O, β-lactam), 1605 (C=C), 1642 (C=C), 1508 (C=C), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.53–2.61 (m, 1H), 2.69–2.76 (m, 1H), 3.20–3.24 (m, 1H), 3.72 (s, 6H), 3.78 (s, 3H), 4.63 (d, J = 2.00 Hz, 1H), 5.08 (s, 2H), 5.13–5.20 (m, 2H), 5.84–5.94 (m, 1H), 6.54 (s, 2H), 7.00 (d, J = 8.52 Hz, 2H), 7.30 (d, J = 8.52 Hz, 2H), 7.35–7.45 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 32.35, 55.53, 58.67, 60.05, 60.51, 69.60, 94.12, 115.01, 117.20, 126.95, 127.02, 127.66, 128.18, 129.48, 133.49, 133.61, 136.18, 139.82, 153.00, 158.42, 166.68. HRMS (m/z) calculated for C28H29NO5Na [M+Na]+: 482.1943, found 482.1941.
- 3-Allyl-4-naphthalen-1-yl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10m)
Preparation followed the general method II above from 4-pentenoyl chloride and naphthalen-1-ylmethylene-(3,4,5-trimethoxyphenyl)amine (8m). The product was isolated as yellow solid (yield 41.7%); mp: 139–140 °C (HPLC: 98.7%). IR (KBr) νmax: 2990 (C-H), 1747 (C=O, β-lactam), 1641 (C=C), 1587 (C=C), 1505 (C=C), 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.82–2.85 (m, 2H), 3.29–3.33 (m, 1H), 3.66 (s, 6H), 3.79 (s, 3H), 5.21–5.36 (m, 2H), 5.53 (d, J = 2.00 Hz, 1H), 5.91–6.02 (m, 1H), 6.59 (s, 2H), 7.44–8.31 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 32.55, 55.57, 56.16, 58.71, 60.51, 94.30, 117.94, 121.76, 122.79, 125.26, 125.62, 126.21, 128.24, 128.87, 130.03, 132.57, 133.36, 133.47, 133.92, 153.09, 166.61. HRMS (m/z) calculated for C25H25NO4Na [M+Na]+: 426.1681, found 426.1685.
- 3-Allyl-4-naphthalen-2-yl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10n)
Preparation followed the general method II above from 4-pentenoyl chloride and naphthalen-2-ylmethylene-(3,4,5-trimethoxyphenyl)amine (8n). The product was isolated as a yellow oil (yield 36%) (HPLC: 98.9%). IR (NaCl) νmax: 2999 (C-H), 1746 (C=O, β-lactam), 1587 (C=C), 1505 (C=C), 1236 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.60–2.68 (m, 1H), 2.75–2.81 (m, 1H), 3.30–3.34 (m, 1H), 3.68 (s, 6H), 3.76 (s, 3H), 4.85 (d, J = 2.00 Hz, 1H), 5.16–5.24 (m, 2H), 5.86–6.00 (m, 1H), 6.60 (s, 2H), 7.46–7.90 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 32.41, 55.54, 58.76, 60.49, 60.52, 94.13, 117.38, 122.76, 125.00, 126.0, 126.24, 127.38, 127.40, 128.88, 132.86, 133.16, 133.54, 134.84, 135.03, 153.06, 166.58. HRMS (m/z) calculated for C25H25NO4Na [M+Na]+: 426.1681, found 426.1689.
- 4-(3-Hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-allylazetidin-2-one (10p)
Preparation followed the general method II above from 4-pentenoyl chloride and TBDMS-protected imine 8p. The intermediate 4-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-allylazetidin-2-one 10o was obtained as described above for compound 9q. Following deprotection with TBAF, the title compound was purified by flash chromatography over silica gel (eluent, 4:1 n-hexane: ethyl acetate) to afford the product as a grey-green solid (yield 22%); mp: 96–98 °C (HPLC: 98.08%). IR (KBr) νmax: 3400 (OH), 1746 (C=O, β-lactam), 1592 (C=C), 1506 (C=C), 1275 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.51–2.59 (m, 1H), 2.68–2.74 (m, 1H), 3.18–3.22 (m, 1H), 3.73 (s, 6H), 3.77 (s, 3H), 3.91 (s, 3H), 4.57 (d, J = 2.52 Hz, 1H), 5.12–5.19 (m, 2H), 5.71 (br s, 1H), 5.82–5.92 (m, 1H), 6.56 (s, 2H), 6.85–6.94 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 32.34, 55.53, 55.55, 58.64, 60.02, 60.49, 94.13, 110.46, 111.69, 117.20, 117.36, 130.42, 133.49, 133.59, 133.77, 145.71, 146.18, 152.98, 166.64. HRMS (m/z) calculated for C22H25NO6Na [M+Na]+: 422.1580, found 422.1582.
- 3-Allyl-4-(4-methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10q)
Preparation following the general method II above from 4-pentenoyl chloride and (4-methoxy-3-nitrobenzylidene)-(3,4,5-trimethoxyphenyl)amine (8r) afforded the product as a colourless solid (yield 18%); mp: 108–110 °C (HPLC: 99.14%). IR (KBr) νmax: 1750 (C=O, β-lactam), 1587 (C=C), 1504 (NO2), 1280 (NO2) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.55–2.63 (m, 1H), 2.72–2.78 (m, 1H), 3.19–3.24 (m, 1H), 3.76 (s, 6H), 3.79 (s, 3H), 3.99 (s, 3H), 4.68 (d, J = 2.00 Hz, 1H), 5.18–5.23 (m, 2H), 5.82–5.92 (m, 1H), 6.51 (s, 2H), 7.13 (d, J = 8.52 Hz, 1H), 7.52–7.55 (m, 1H), 7.88 (d, J = 2.52 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 32.27, 55.68, 56.25, 58.78, 58.85, 60.52, 94.10, 114.02, 117.77, 123.27, 129.77, 130.85, 132.92, 133.20, 134.24, 139.30, 152.49, 153.21, 165.89. HRMS (m/z) calculated for C22H24N2O7Na [M+Na]+: 451.1481, found 451.1477.
- 3-Allyl-4-(3-amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (10r)
To a mixture of the β-lactam 3-allyl-4-(4-methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 10q (0.25 mmol) and zinc powder (10 μm, 2.5 mmol) was added acetic acid (15 mL) at room temperature under N2, and the reaction was stirred for 7 days. The reaction mixture was filtered through a celite pad, and the solvent was removed under reduced pressure. The residue was purified by flash chromatography over silica gel (eluent, 1:1 ethyl acetate: n-hexane) to afford the title compound. The product was isolated as a brown oil (yield 96%) (HPLC: 97.93%). IR (NaCl) νmax: 3376 (NH2), 1727 (C=O, β-lactam), 1592 (C=C), 1507 (C=C), 1292 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.52–2.57 (m, 1H), 2.67–2.74 (m, 1H), 3.19–3.23 (m, 1H), 3.74 (s, 6H), 3.77 (s, 3H), 3.89 (s, 3H), 4.58 (d, J = 2.00 Hz, 1H), 5.13–5.20 (m, 2H), 5.83–5.93 (m, 1H), 6.56 (s, 2H), 6.85–7.02 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 32.32, 55.28, 55.60, 58.56, 60.05, 60.50, 94.13, 110.30, 113.61, 117.25, 123.85, 129.57, 133.52, 133.58, 133.77, 133.97, 147.80, 152.99, 166.79. HRMS (m/z) calculated for C22H27N2O5 [M+H]+: 399.1920, found 399.1907.
- 3-Allyl-4-(4-Methoxyphenyl)-1-phenylazetidin-2-one (10s)
Preparation following the general method II above from 4-pentenoyl chloride and the imine 8v (4-methoxybenzylidene)(phenyl)amine afforded the product as a yellow oil, yield 40%, 590 mg (HPLC 100.0%). IR (NaCl, film) νmax: 2979 (C-H), 1749 (C=O, β-lactam), 1640 (C=C), 1602 (C=C), 1588 (C=C), 1513 (C=C), 1251 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.53–2.61 (m, 1H), 2.70–2.76 (m, 1H), 3.17–3.22 (m, 1H), 3.82 (s, 3H), 4.67 (d, J = 2.04 Hz, 1H), 5.13–5.20 (m, 2H), 5.84–5.94 (m, 1H), 6.91 (d, J = 8.52 Hz, 2H), 7.02–7.07 (m, 1H), 7.23–7.31 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 32.83, 55.32, 59.35, 60.13, 114.51, 117.02, 117.61, 123.76, 127.29, 129.03, 129.72, 134.16, 137.73, 159.63, 167.32. HRMS (m/z) calculated for C19H19NO2Na [M+Na]+: 316.1313, found 316.1320.
3.4. General Method III: Preparation of β-Lactams (11a-11s)
Sorbic acid (2 mmol) was mixed with 2-chloro-1-methylpyridinium iodide (2.4 mmol) and tripropylamine (6 mmol) in anhydrous dichloromethane (30 mL) under a nitrogen atmosphere at room temperature. The suspension was then heated to reflux for 12 h to afford a clear solution. A solution of the appropriate imine (2 mmol) in anhydrous dichloromethane (10 mL) was added, and reaction mixture was heated at reflux for 24 h. The solution was then cooled and washed with water, HCl (2%, aqueous solution) and water. The organic layer was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography over silica gel (eluent, 4:1 n-hexane and ethyl acetate).
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11a)
Preparation following the general method III above from sorbic acid and (4-nitrobenzylidene)-3,4,5-trimethoxyphenylamine 8a afforded the product as a yellow solid (yield 33%); mp: 133–134 °C (HPLC: 85.8%). IR (KBr) νmax: 2972 (C-H), 1749 (C=O, β-lactam), 1653 (C=C), 1568 (C=C), 1506 (NO2), 1345 (NO2), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 6H), 3.77 (br s, 1H), 3.79 (s, 3H), 4.91 (d, J = 2.00 Hz, 1H), 5.21–5.23 (m, 1H), 5.28–5.32 (m, 1H), 5.89 (dd, J5,6 = 13.54 Hz, J5,3 = 8.54 Hz, 1H), 6.31–6.44 (m, 2H), 6.50 (s, 2H), 7.57 (d, J = 8.52 Hz, 2H), 8.29 (d, J = 8.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 55.69, 60.53, 60.60, 62.91, 94.20, 118.98, 123.98, 124.17, 126.24, 132.72, 134.52, 135.10, 135.90, 144.23, 147.65, 153.29, 163.91. HRMS (m/z) calculated for C22H22N2O6Na [M+Na]+: 433.1376, found 433.1392.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-chlorophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11b)
Preparation following the general method III above from sorbic acid and (4-chlorobenzylidene)-(3,4,5-trimethoxyphenyl)amine 8b afforded the product as a colourless solid (yield 32%); mp: 145–147 °C (HPLC: 95.4%). IR (KBr) νmax: 2971 (C-H), 1748 (C=O, β-lactam), 1655 (C=C), 1584 (C=C), 1506 (C=C), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 6H), 3.77–3.78 (m, 1H), 3.79 (s, 3H), 4.76 (d, J = 2.52 Hz, 1H), 5.18–5.20 (m, 1H), 5.26–5.30 (m, 1H), 5.88 (dd, J5,6 = 14.16 Hz, J5,3 = 8.16 Hz, 1H), 6.31–6.43 (m, 2H), 6.53 (s, 2H), 7.32–7.34 (m, 2H), 7.39–7.41 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 55.62, 60.52, 60.95, 62.79, 94.21, 118.44, 126.79, 128.67, 129.06, 133.06, 134.12, 134.17, 135.31, 135.34, 135.36, 153.12, 164.51. HRMS (m/z) calculated for C22H2235ClNO4Na [M+Na]+: found 422.1130.
- (E)-4-(4-Bromophenyl)-3-(buta-1,3-dien-1-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11c)
Preparation following the general method III above from sorbic acid and (4-bromobenzylidene)-3,4,5-trimethoxyphenylamine 8c afforded the product as a colourless solid (yield 44%); mp: 153–155 °C (HPLC: 93.5%). IR (KBr) νmax: 2969, 1748 (C=O, β-lactam), 1657 (C=C), 1583 (C=C), 1505 (C=C), 1239 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 6H), 3.76–3.77 (m, 1H), 3.79 (s, 3H), 4.74 (d, J = 2.00 Hz, 1H), 5.17–5.20 (m, 1H), 5.25–5.29 (m, 1H), 5.87 (dd, J5,6 = 14.06 Hz, J5,3 = 8.02 Hz, 1H), 6.31–6.40 (m, 2H), 6.52 (s, 2H), 7.27 (d, J = 8.00 Hz, 2H), 7.55 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 55.63, 60.52, 61.00, 62.75, 94.22, 118.46, 122.20, 124.61, 127.08, 132.01, 133.03, 134.20, 135.30, 135.35, 135.89, 153.13, 164.49. HRMS (m/z) calculated for C22H2280BrNO4N [M+Na]+: 466.0630, found 466.0622.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-fluorophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11d)
Preparation following the general method III above from sorbic acid and (4-fluorobenzylidene)-3,4,5-trimethoxyphenylamine (8d) afforded the product as a colourless solid (yield 20%); mp: 137–139 °C (HPLC: 100.0%). IR (KBr). νmax: 2968 (C-H), 1748 (C=O, β-lactam), 1658 (C=C), 1583 (C=C), 1505 (C=C), 1227 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 6H), 3.76–3.77 (m, 1H), 3.79 (s, 3H), 4.77 (d, J = 2.00 Hz, 1H), 5.17–5.19 (m, 1H), 5.25–5.29 (m, 1H), 5.85–5.91 (m, 1H), 6.34–6.40 (m, 2H), 6.53 (s, 2H), 7.09–7.14 (m, 2H), 7.36–7.39 (m, 2H). 13C NMR (100MHz, CDCl3): δ 55.59, 60.52, 61.01, 62.82, 94.22, 115.77, 115.98, 118.36, 124.74, 127.17, 127.24, 132.60, 133.13, 133.94, 135.24, 135.35, 153.10, 163.57, 164.63. HRMS (m/z) calculated for C22H22FNO4Na [M+Na]+: 406.1431, found 406.1421.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-dimethylaminophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11e)
Preparation following the general method III above from sorbic acid and (4-(dimethylamino)benzylidene)-3,4,5-trimethoxyphenylamine 8e afforded the product as a brown oil (yield 16%) (HPLC: 91.1%). IR (NaCl) νmax: 2968 (C-H), 1743 (C=O, β-lactam), 1656 (C=C), 1584 (C=C), 1505 (C=C), 1236 (C=O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.98 (s, 6H), 3.73 (s, 6H), 3.77 (s, 3H), 3.80–3.82 (m, 1H), 4.68 (d, J = 2.52 Hz, 1H), 5.13–5.16 (m, 1H), 5.22–5.26 (m, 1H,), 5.88 (dd, J5,6 = 14.06 Hz, J5,3 = 8.02 Hz, 1H), 6.29–6.42 (m, 2H), 6.60 (s, 2H), 6.74 (d, J = 8.04 Hz, 2H), 7.25–7.27 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 40.02, 55.55, 60.49, 61.74, 62.57, 94.29, 112.22, 117.81, 125.51, 126.67, 133.55, 133.79, 133.89, 134.72, 135.58, 145.67, 152.95, 165.30. HRMS (m/z) calculated for C24H29N2O4 [M+H]+: 409.2127, found 409.2141.
- (E)-3-(Buta-1,3-dien-1-yl)-4-phenyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11f)
Preparation following the general method III above from sorbic acid and benzylidene-(3,4,5-trimethoxyphenyl)amine 8f afforded the product as a yellow solid (yield 37%); mp: 90–93 °C (HPLC: 94.1%). IR (NaCl) νmax: 2969 (C-H), 1747 (C=O, β-lactam), 1660 (C=C), 1585 (C=C), 1505 (C=C), 1239 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.71 (s, 6H), 3.78 (s, 3H), 3.80–3.83 (m, 1H), 4.77 (d, J = 2.52 Hz, 1H), 5.16–5.18 (m, 1H), 5.24–5.28 (m, 1H), 5.89 (dd, J5,6 = 14.06 Hz, J5,3 = 8.02 Hz, 1H), 6.32–6.43 (m, 2H), 6.55 (s, 2H), 7.35–7.39 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 55.54, 60.51, 61.71, 62.65, 94.23, 118.18, 125.02, 125.52, 128.33, 128.81, 133.31, 133.98, 135.10, 135.44, 136.77, 153.04, 164.82. HRMS (m/z) calculated for C22H24NO4 [M+H]+: 366.1705, found 366.1695.
- 3-(Buta-1,3-dienyl)-4-p-tolyl-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11g)
Preparation following the general method III above from sorbic acid and (4-methylbenzylidene)-(3,4,5-trimethoxyphenyl)amine 8g afforded the product as a yellow solid (yield 40%); mp: 88–89 °C (HPLC: 97.0%). IR (KBr) νmax: 2960 (C-H), 1748 (C=O, β-lactam), 1659 (C=C), 1640 (C=C), 1588 (C=C), 1505 (C=C), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.37 (s, 3H), 3.72 (s, 6H), 3.78 (s, 3H), 3.80–3.81 (m, 1H), 4.74 (d, J = 2.48 Hz, 1H), 5.15–5.17 (m, 1H), 5.23–5.27 (m, 1H), 5.88 (dd, J5,6 = 14.28 Hz, J5,3 = 8.28 Hz, 1H), 6.30–6.40 (m, 2H), 6.56 (s, 2H), 7.22 (d, J = 8.04 Hz, 2H), 7.28 (d, J = 8.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 20.77, 55.55, 60.50, 61.56, 62.68, 94.24, 118.08, 125.15, 125.47, 129.45, 133.37, 133.72, 133.94, 134.98, 135.48, 138.21, 153.02, 164.93. HRMS (m/z) calculated for C23H26NO4 [M+H]+: 380.1862, found 380.1862.
- 3-(Buta-1,3-dienyl)-4-(4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11h)
Preparation following the general method III above from sorbic acid and (4-methoxybenzylidene)-3,4,5-trimethoxyphenylamine 8h afforded the product as a colourless solid (yield 31%); mp: 109–110 °C (HPLC: 99.7%). IR (KBr) νmax: 2969 (C-H), 1745 (C=O, β-lactam), 1656 (C=C), 1581 (C=C), 1508 (C=C), 1247 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.73 (s, 6H), 3.78 (s, 3H), 3.80 (m, 1H), 3.83 (s, 3H), 4.73 (d, J = 2.00 Hz, 1H), 5.15–5.18 (m, 1H), 5.24–5.28 (m, 1H), 5.88 (dd, J5,6 = 14.28 Hz, J5,3 = 8.28 Hz, 1H), 6.31–6.43 (m, 2H), 6.56 (s, 2H), 6.94 (d, J = 9.00 Hz, 2H), 7.32 (d, J = 9.04 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 54.90, 55.56, 60.51, 61.38, 62.74, 94.25, 114.15, 118.07, 125.15, 126.84, 128.62, 133.36, 133.94, 134.96, 135.48, 153.02, 159.44, 164.98. HRMS (m/z) calculated for C23H26NO5 [M+H]+: 396.1811, found 396.1800.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-ethoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-azetidin-2-one (11i)
Preparation following the general method III above from sorbic acid and (4-ethoxybenzylidene)-(3,4,5-trimethoxyphenyl)amine 8i afforded the product as a yellow solid (yield 41%); mp: 73–75 °C (HPLC: 99.8%). IR (KBr) νmax: 2969 (C-H), 1747 (C=O, β-lactam), 1658 (C=C), 1584 (C=C), 1511 (C=C), 1244 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.43 (t, J = 7.02 Hz, 3H), 3.73 (s, 6H), 3.78 (s, 3H), 3.79–3.80 (m, 1H), 4.05 (q, J = 7.02 Hz, 2H), 4.72 (d, J = 2.52 Hz, 1H), 5.15–5.17 (m, 1H), 5.24–5.28 (m, 1H), 5.88 (dd, J5,6 = 14.04 Hz, J5,3 = 8.52 Hz, 1H), 6.31–6.42 (m, 2H), 6.56 (s, 2H), 6.91–6.93 (m, 2H), 7.29–7.32 (m, 2H). 13C NMR (100 MHz, CDCl3): δ 14.33, 55.55, 60.51, 61.42, 62.72, 63.11, 94.24, 114.66, 118.04, 125.19, 126.82, 128.42, 133.39, 133.92, 134.93, 135.48, 153.01, 158.83, 165.00. HRMS (m/z) calculated for C24H27NO5Na [M+Na]+: 432.1787, found 432.1792.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-butoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-azetidin-2-one (11j)
Preparation following the general method III above from sorbic acid and (4-butoxybenzylidene)-3,4,5-trimethoxyphenylamine 8j afforded the product as a yellow oil (yield 48%) (HPLC: 94.0%). IR (NaCl) νmax: 2968 (C-H), 1747 (C=O, β-lactam), 1657 (C=C), 1583 (C=C), 1508 (C=C), 1245 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.99 (t, J = 7.54 Hz, 3H), 1.48–1.53 (m, 2H), 1.74–1.81 (m, 2H), 3.73 (s, 6H), 3.78 (s, 3H), 3.79–3.80 (m, 1H), 3.97 (t, J = 6.28Hz, 2H), 4.72 (d, J = 2.52 Hz, 1H), 5.14–5.17 (m, 1H), 5.23–5.27 (m, 1H), 5.88 (dd, J5,6 = 14.30 Hz, J5,3 = 8.26 Hz, 1H), 6.30–6.42 (m, 2H), 6.56 (s, 2H), 6.92 (d, J = 8.52 Hz, 2H), 7.30 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 13.39, 18.77, 30.79, 55.55, 60.50, 61.42, 62.73, 67.34, 94.26, 114.68, 118.03, 125.20, 126.80, 128.35, 133.39, 133.93, 134.92, 135.48, 153.01, 159.04, 165.00. HRMS (m/z) calculated for C26H31NO5Na [M+Na]+: 460.2100, found 460.2103.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-phenoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11k)
Preparation following the general method III above from sorbic acid and (4-phenoxylbenzylidene)-(3,4,5-trimethoxyphenyl)amine 8k afforded the product as a colourless solid (yield 33%); mp: 119–120 °C (HPLC: 90.3%). IR (KBr) νmax: 2973 (C-H), 1748 (C=O, β-lactam), 1654 (C=C), 1587 (C=C), 1507 (C=C), 1239 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 6H), 3.79 (s, 3H), 3.82–3.83 (m, 1H), 4.76 (d, J = 2.48 Hz, 1H), 5.16–5.19 (m, 1H), 5.25–5.29 (m, 1H), 5.89 (dd, J5,6 = 14.06 Hz, J5,3 = 8.02 Hz, 1H), 6.33–6.43 (m, 2H), 6.56 (s, 2H), 7.02–7.05 (m, 4H), 7.14–7.17 (m, 1H), 7.34–7.39 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 55.59, 60.52, 61.27, 62.73, 94.31, 118.19, 118.74, 118.76, 123.37, 124.98 (CH), 127.03, 129.44, 131.22, 133.26, 133.95, 135.10, 135.43, 153.08, 156.11, 157.42, 164.79. HRMS (m/z) calculated for C28H28NO5 [M+H]: 458.1967, found 458.1974.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-benzyloxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11l)
Preparation following the general method III above from sorbic acid and (4-benzyloxybenzylidene)-3,4,5-trimethoxyphenylamine 8l afforded the product as a yellow oil (yield 41%) (HPLC: 94.1%). IR (NaCl) νmax: 2969 (C-H), 1746 (C=O, β-lactam), 1658 (C=C), 1584 (C=C), 1508 (C=C), 1240 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.72 (s, 6H), 3.78 (s, 3H), 3.79–3.80 (m, 1H), 4.72 (d, J = 2.52 Hz, 1H), 5.09 (s, 2H), 5.15–5.18 (m, 1H), 5.24–5.28 (m, 1H), 5.88 (dd, J5,6 = 14.06 Hz, J5,3 = 8.02 Hz, 1H), 6.31–6.42 (m, 2H), 6.56 (s, 2H), 7.01 (d, J = 8.04 Hz, 2H), 7.31–7.45 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 55.56, 60.51, 61.38, 62.7, 69.61, 94.25, 115.10, 118.08, 125.14, 126.87, 127.01, 127.67, 128.19, 128.90, 133.35, 133.95, 134.98, 135.47, 136.13, 153.02, 158.59, 164.96. HRMS (m/z) calculated for C29H29NO5Na [M+Na]+: 494.1943, found 494.1931.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(naphthalen-1-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11m)
Preparation following the general method III above from sorbic acid and naphthalen-1-ylmethylene-(3,4,5-trimethoxyphenyl)amine 8m afforded the product as a grey solid (yield 27%); mp: 113–114 °C (HPLC: 95.4%). IR (KBr) νmax: 2968 (C=C), 1748 (C=O, β-lactam), 1657 (C=C), 1584 (C=C), 1505 (C=C), 1237 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.71 (s, 6H), 3.78–3.81 (m, 1H), 3.82 (s, 3H), 5.20–5.23 (m, 1H), 5.26–5.30 (m, 1H), 5.56 (d, J = 2.00 Hz, 1H), 6.09 (dd, J5,6 = 14.80 Hz, J5,3 = 8.80 Hz, 1H), 6.36–6.52 (m, 2H), 6.66 (s, 2H), 7.43–8.01 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 55.71, 58.95, 60.53, 62.35, 94.56, 118.40, 122.22, 122.39, 125.10, 125.40, 125.44, 125.71, 126.30, 128.21, 128.74, 129.95, 132.14, 133.45, 133.57, 135.48, 136.04, 153.19, 164.88. HRMS (m/z) calculated for C26H25NO4Na [M+Na]+: 438.1681, found 438.1682.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(naphthalen-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11n)
Preparation following the general method III above from sorbic acid and naphthalen-2-ylmethylene-(3,4,5-trimethoxyphenyl)amine 8n afforded the product as a yellow oil (yield 53%) (HPLC: 86.4%). IR (NaCl) νmax: 2968 (C-H), 1747 (C=O, β-lactam), 1659 (C=C), 1585 (C=C), 1506 (C=C), 1238 (C-O) cm−1. 1H NMR (400MHz, CDCl3): δ 3.69 (s, 6H), 3.77 (s, 3H), 3.87–3.90 (m, 1H), 4.95 (d, J = 2.96 Hz, 1H), 5.16–5.19 (m, 1H), 5.25–5.29 (m, 1H), 5.94 (dd, J5,6 = 14.16 Hz, J5,3 = 8.32 Hz, 1H), 6.35–6.42 (m, 2H), 6.62 (s, 2H), 7.47–7.92 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 55.58, 60.48, 61.89, 62.75, 94.33, 118.21, 122.45, 124.98, 126.12, 126.33, 127.39, 127.42, 129.01, 132.87, 132.95, 133.42, 134.13, 134.28, 135.19, 135.43, 153.09, 164.84. HRMS (m/z) calculated for C26H26NO4 [M+H]+: 416.1862, found 416.1863.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11p)
Preparation followed the general method III above from sorbic acid and imine 8p as described above for compound 9q. The intermediate 4-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-3-(buta-1,3-dienyl) azetidin-2-one 11o was isolated as described for compound 9q. Following deprotection with TBAF, the solvent was removed and the crude product was purified by flash chromatography over silica gel (eluent, 4:1 n-hexane: ethyl acetate) to afford the title compound as a grey-brown oil (yield 37%) (HPLC: 97.8%). IR (NaCl) νmax: 3359 (OH), 1748 (C=O, β-lactam), 1594 (C=C), 1509 (C=C), 1280 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.72 (s, 6H), 3.75–3.76 (m, 1H), 3.77 (s, 3H), 3.82 (s, 3H), 4.67 (d, J = 2.36 Hz, 1H), 5.15–5.17 (m, 1H), 5.24–5.28 (m, 1H), 5.85–5.90 (m, 1H), 6.31–6.40 (m, 2H), 6.56 (s, 2H), 6.82–6.96 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 55.06, 55.51, 60.49, 61.34, 62.60, 94.30, 111.89, 117.96, 118.03, 119.06, 125.20, 129.04, 133.30, 133.91, 134.91, 135.50, 145.23, 150.86, 152.99, 164.95. HRMS (m/z) calculated for C23H24NO6 [M-H]-: 410.1604, found 410.1605.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11q)
Preparation following the general method III above from sorbic acid and (4-methoxy-3-nitrobenzylidene)-(3,4,5-trimethoxyphenyl)amine 8r afforded the product as a yellow solid (yield 25%); mp: 88–90 °C (HPLC: 95.7%). IR (KBr) νmax: 2969 (C-H), 1748 (C=O, β-lactam), 1656 (C=C), 1584 (C=C), 1506 (C=C), 1280 (C-O) cm−1 1H NMR (400 MHz, CDCl3): δ 3.76 (s, 6H), 3.79 (s, 3H), 3.84–3.87 (m, 1H), 3.99 (s, 3H), 4.79 (d, J = 2.52 Hz, 1H), 5.18–5.21 (m, 1H), 5.27–5.30 (m, 1H), 5.86 (dd, J5,6 = 14.32 Hz, J5,3 = 8.28 Hz, 1H), 6.34–6.40 (m, 2H), 6.52 (s, 2H), 7.15 (d, J = 8.52 Hz, 1H), 7.55–7.57 (m, 1H), 7.90 (d, J = 2.00 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 55.70, 56.28, 60.18, 60.52, 62.77, 94.27, 114.13, 118.72, 123.16, 124.19, 129.19, 130.72, 132.77, 133.04, 135.18, 135.60, 139.29, 152.65, 153.23, 164.27. HRMS (m/z) calculated for C23H24N2O7Na [M+Na]+: 463.1481, found 463.1465.
- (E)-4-(3-Amino-4-methoxyphenyl)-3-(buta-1,3-dien-1-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (11r)
Preparation from (E)-3-(buta-1,3-dienyl)-4-(4-methoxy-3-nitrophenyl)-1-(3,4,5-trimethoxyphenyl) azetidin-2-one 11q followed the general method III described above for 9s. The product was isolated as a yellow solid (yield 59%); mp: 136–138 °C (HPLC: 98.0%). IR (KBr) νmax: 3376 (NH2), 1741 (C=O, β-lactam), 1593 (C=C), 1504 (C=C), 1292 (C-O), 1234 cm−1. 1H NMR (400 MHz, CDCl3): δ 3.75 (s, 6H), 3.77 (d, J = 2.48 Hz, 1H), 3.78 (s, 3H), 3.89 (s, 3H), 4.66 (d, J = 2.52 Hz, 1H), 5.14–5.17 (m, 1H), 5.23–5.28 (m, 1H), 5.84–5.89 (m, 1H), 6.30–6.42 (m, 2H), 6.59–6.62 (m, 2H), 6.82–6.91 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 55.76, 56.10, 60.94, 61.92, 63.11, 94.82, 110.90, 114.00, 118.45, 125.65, 126.64, 129.84, 133.88, 134.50, 134.89, 135.39, 135.96, 144.75, 153.51, 165.46. HRMS (m/z) calculated for C23H26N2O5Na [M+Na]+: 433.1739, found 433.1729.
- (E)-3-(Buta-1,3-dien-1-yl)-4-(4-methoxyphenyl)-1-phenylazetidin-2-one (11s)
Preparation following the general method III above from sorbic acid and imine 8v afforded the product as a yellow oil, yield 45%, 685 mg [] (HPLC: 87.1%). IR (NaCl, film) νmax: 2969 (C-H), 1749 (C=O, β-lactam), 1656 (C=C), 1602 (C=C), 1574 (C=C), 1513 (C=C), 1250 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.75–3.78 (m, 1H), 3.82 (s, 3H), 4.77 (d, J = 2.52 Hz, 1H), 5.15–5.17 (m, 1H), 5.23–5.27 (m, 1H), 5.89 (dd, J5,6 = 13.54 Hz, J5,3 = 8.02 Hz, 1H), 6.33–6.40 (m, 2H), 6.91–6.94 (m, 2H), 7.02–7.08 (m, 2H), 7.24–7.33 (m, 5H). 13C NMR (100 MHz, CDCl3): δ 54.88, 60.96, 62.99, 114.14, 116.65, 118.01, 123.51, 126.74, 128.61, 128.65, 129.21, 134.98, 135.51, 137.12, 159.36, 165.14. HRMS (m/z) calculated for C20H20NO2 [M+H]+: 306.1494, found 306.1496.
3.5. General Method IV: Preparation of Fmoc-Protected β-Lactams (12a-e)
To a stirred solution of the amino β-lactam (4.76 mmol) in anhydrous DMF (30 mL) were added DCC (5.7 mmol), Fmoc-protected amino acid (5.6 mmol) and HOBt.H2O (7.3 mmol) at room temperature. The mixture was stirred overnight. After 24 h, ethyl acetate (50 mL) was added, and the mixture was then filtered. The DMF was removed by washing with water (5 × 50 mL). The organic solvent was removed in vacuo, and the product was isolated by flash column chromatography over silica gel (eluent, dichloromethane: methanol gradient).
- (1-((2-Methoxy-5-(4-oxo-3-(prop-1-en-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl) phenyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamaic acid 9H-fluoren-9-ylmethyl ester (12a)
Following the general method IV above, to a stirred solution of β-lactam 9s (4.76 mmol) in anhydrous DMF (30 mL) were added DCC (5.7 mmol), Fmoc-phenylalanine (5.6 mmol) and HOBt.H2O (7.3 mmol) at room temperature. The product was isolated by flash column chromatography over silica gel (eluent: dichloromethane: methanol gradient) as a colourless solid, yield 32%, 229 mg, Mp 132–133 °C. IR (KBr) νmax: 3321 (NH), 1725 (C=O), 1650 (C=O), 1598 (C=C), 1507 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.90 (s, 3H), 3.19 (br s, 2H), 3.74 (s, 6H), 3.75 (s, 3H), 3.78 (s, 3H), 3.87 (br s, 1H), 4.22 (t, J = 7.02 Hz, 1H), 4.40–4.46 (m, 2H), 4.61–4.63 (m, 1H), 4.79 (br s, 1H), 5.03 (br s, 1H), 5.09 (br s, 1H), 6.60 (s, 2H), 6.82–7.79 (m, 16H), 8.06 (br s, 1H), 8.48 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 20.12, 38.20, 46.63, 55.35, 55.63, 60.50, 59.84, 61.39, 66.36, 66.83, 94.29, 110.15, 114.12, 117.66, 119.59, 120.65, 124.53, 126.64, 126.75, 127.35, 128.39, 128.80, 128.84, 129.60, 133.30, 133.88, 137.62, 140.85, 143.14, 143.19, 147.53, 153.03, 156.71, 164.86, 168.49. HRMS (m/z) calculated for C46H45N3O8Na (M++Na): 790.3104, found 790.3113.
- (1-((5-(3-Allyl-4-oxo-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl)-2-methoxyphenyl) amino)-1-oxo-3-phenylpropan-2-yl)carbamaic acid 9H-fluoren-9-ylmethyl ester (12b)
Following the general method IV above, the desired product was obtained from 3-allyl-4-(3-amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 10r and Fmoc-phenylalanine as a yellow oil (yield 20%). IR (NaCl) νmax: 3317 (NH), 1738 (C=O, β-lactam), 1693 (C=O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.47–2.63 (m, 2H), 3.19–3.27 (m, 2H), 3.45–3.51 (m, 1H), 3.72 (s, 6H), 3.74 (s, 3H), 3.77 (s, 3H), 4.21–4.59 (m, 3H), 4.64 (br s, 1H), 5.15–5.17 (m, 2H), 5.22 (m, 1H), 5.87–5.93 (m, 1H), 6.57 (s, 2H), 6.80–7.79 (m, 16H), 8.05 (br s, 1H), 8.44 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 32.25, 33.30, 46.63, 48.96, 55.33, 55.61, 58.59, 59.97, 60.50, 66.82, 94.18, 110.04, 117.42, 119.59, 124.50, 126.64, 126.74, 126.81, 127.34, 128.38, 128.80, 128.84, 129.73, 132.60, 133.36, 133.43, 140.85, 143.19, 143.19, 147.44, 153.00, 166.67, 168.45, 170.76. HRMS (m/z) calculated for C46H45N3O8Na [M+Na]+: 790.3104, found 790.3094.
- (1-((5-(3-Allyl-4-oxo-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl)-2-methoxyphenyl) amino)-3-methyl-1-oxo-butan-2-yl) carbamaic acid 9H-fluoren-9-ylmethyl ester (12c)
Following the general method IV above, the desired product was obtained from 3-allyl-4-(3-amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 10r and Fmoc valine as a yellow oil (yield 18%). IR (NaCl) νmax: 3336 (NH), 2935 (C-H), 1724 (C=O, β-lactam), 1669 (C=O), 1607 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.02–1.04 (m, 6H), 2.23–2.62 (m, 2H), 2.69–2.72 (m, 1H), 3.25–3.27 (m, 1H), 3.73 (s, 6H), 3.77 (s, 3H), 3.86 (s, 3H), 4.24–4.43 (m, 4H), 4.65 (br s, 1H), 5.14–5.21 (m, 2H), 5.85–5.92 (m, 1H), 6.58 (s, 2H), 6.86–7.79 (m, 11H), 8.19 (br s, 1H), 8.48 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 18.78, 32.21, 33.20, 46.72, 55.47, 55.60, 58.56, 59.98, 60.48, 62.43, 65.58, 66.76, 94.21, 110.15, 117.46, 119.58, 120.74, 124.53, 124.65, 126.63, 127.25, 127.32, 128.43, 129.90, 132.60, 133.36, 133.46, 140.86, 143.19, 143.30, 147.63, 153.00, 156.05, 166.74, 170.82. HRMS (m/z) calculated for C42H45N3O8Na [M+Na]+: 742.3104, found 742.3100.
- (1-((5-(3-(Buta-1,3-dien-1-yl)-4-oxo-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl)-2-methoxyphenyl)amino)-3-methyl-1-oxo-butan-2-yl) carbamaic acid 9H-fluoren-9-ylmethyl ester (12d)
Following the general method IV above, the desired product was obtained from (E)-3-(buta-1,3-dienyl)-4-(3-amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenylazetidin-2-one 11r and Fmoc valine as a yellow resin (yield 85%); IR (NaCl) νmax: 3400 (NH), 1745 (C=O, β-lactam), 1679 cm−1 (C=O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.02–1.05 (m, 6H), 2.53–2.65 (m, 1H), 3.74 (s, 6H), 3.77 (s, 3H), 3.83 (br s, 1H), 3.86 (s, 3H), 4.25–4.49 (m, 4H), 4.74 (br s, 1H), 5.14–5.17 (m, 1H), 5.23–5.27 (m, 1H), 5.85–5.90 (m, 1H), 6.31–6.39 (m, 2H), 6.59 (s, 2H), 6.84–7.79 (m, 9H), 8.20 (br s, 1H), 8.52 (br s, 1H). 13C NMR (100 MHz, CDCl3): δ 18.80, 30.70, 46.71, 55.62, 55.65, 60.50, 59.97, 60.99, 62.46, 65.89, 66.76, 94.34, 110.21, 117.87, 118.04, 119.58, 124.52, 125.08, 126.64, 127.33, 128.39, 129.26, 132.60, 133.33, 133.95, 134.91, 135.51, 140.86, 143.19, 147.63, 153.03, 155.99, 164.96, 169.06. HRMS (m/z) calculated for C43H45N3O8Na [M+Na]+: 754.3104, found 754.3132.
- (2-((5-((E)-3-(Buta-1,3-dien-1-yl)-4-oxo-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl)-2-methoxyphenyl)amino)-2-oxo-ethyl) carbamaic acid 9H-fluoren-9-ylmethyl ester (12e)
Following the general method IV above, the desired product was obtained from (E)-3-(buta-1,3-dienyl)-4-(3-amino-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11r and Fmoc glycine as a colourless solid (yield 49%); mp: 167–168 °C; IR (NaCl) νmax: 3333 (NH), 1727 (C=O, β-lactam), 1655 (C=O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.74 (s, 3H), 3.79 (s, 6H), 3.85 (s, 3H), 3.80–3.82 (m, 1H), 4.08 (br s, 2H), 4.27–4.29 (m, 1H), 4.47–4.49 (m, 2H), 4.75 (br s, 1H), 5.15–5.17 (m, 1H), 5.23–5.27 (m, 1H), 5.87 (dd, J5,6 = 13.69 Hz, J5,3 = 8.28 Hz, 1H), 6.33–6.39 (m, 2H), 6.58 (s, 2H), 6.88–7.29 (m, 3H), 7.34–7.81 (m, 8H), 8.29 (br s, 1H), 8.49 (s, 1H). 13C NMR (100 MHz, CDCl3): δ 44.65, 46.61, 55.49, 55.65, 60.51, 61.39, 62.62, 67.00, 94.30, 110.21, 118.07, 119.62, 120.66, 124.53, 125.05, 126.66, 127.37, 129.34, 133.31, 133.95, 134.96, 135.49, 140.86, 143.18, 147.52, 153.03, 156.78, 164.93, 166.47. HRMS (m/z) calculated for C40H39N3O8Na [M+Na]+: 712.2635, found 712.2627.
3.6. General Method V: Synthesis of Amino Acid Prodrugs (13a-e)
To the appropriate amino acid amide 12a-e (1.56 mmol) in methanol (10 mL)/CH2Cl2 (10 mL) was added 2N NaOH (3.4 mmol) at room temperature, and the mixture was stirred for 24 h. Saturated aq. NaHCO3 was added, and the mixture was extracted with dichloromethane (25 mL × 3). The organic solution was dried, the solvent was removed, and the crude product was dissolved in diethyl ether and extracted with 2N HCl (5 × 50 mL). Then, 2N NaOH was added to neutralise the mixture, and the mixture was extracted with diethyl ether (5 × 50 mL). The organic solution was dried (Na2SO4), the solvent was removed, and the product was obtained by flash chromatography over silica gel (eluent, dichloromethane-methanol gradient).
- 2-Amino-N-(2-methoxy-5-(1-(3,4,5-trimethoxyphenyl)-4-oxo-3-(prop-1-en-2-yl) azetidin-2-yl)phenyl)-3-phenylpropanamide (13a)
Following the general method V above, to amino acid amide 10 (1.56 mmol) in methanol (10 mL)/CH2Cl2 (10 mL) was added 2N NaOH (3.4 mmol) at room temperature, and the mixture was stirred for 24 h. The product was isolated by flash chromatography over silica gel (eluent, dichloromethane-methanol gradient) as a yellow oil, yield 58% (HPLC: 99.1%). IR (NaCl) νmax: 3318 (NH2), 2917 (C-H), 1733 (C=O, β-lactam), 1678 (C=O), 1617 (C=C), 1598 (C=C), 1512 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.60 (br s, 3H, CH3), 3.42–3.52 (m, 2H), 3.78 (s, 9H), 3.83 (br s, 1H), 3.88 (s, 3H), 3.89 (br s, 1H), 5.32 (d, J = 3.48 Hz, 1H), 6.66 (s, 2H), 6.87–6.89 (m, 1H), 7.10–7.12 (m, 1H), 7.28–7.37 (m, 8H). 13C NMR (100 MHz, CDCl3): δ 19.37, 32.15, 51.31, 55.45, 55.57, 59.95, 60.03, 60.47, 94.16, 110.16, 117.42, 120.07, 125.07, 127.33, 127.43, 129.62, 133.34, 133.53, 133.67, 134.26, 147.83, 152.96, 166.79, 174.18. HRMS (m/z) calculated for C31H34N3O6 (M+-H): 544.2448, found 544.2455.
- N-(5-(3-Allyl-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl)-2-amino-3-phenylpropanamide (13b)
Preparation was as described in the general method V above from the Fmoc-protected β-lactam 12b. The desired product was isolated as a brown oil, yield 37.6%, 139 mg (HPLC: 91.7%). IR (NaCl, film) νmax: 3343 (NH), 1743 (C=O, β-lactam), 1697 (C=O), 1598 (C=C), 1503 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.21–2.51 (m, 2H), 2.72–2.93 (m, 2H), 3.40–3.47 (m, 1H), 3.71 (s, 3H), 3.75 (s, 6H), 3.86 (s, 3H), 3.89 (m, 1H), 4.52 (br s, 1H), 5.02–5.09 (m, 2H), 5.68–5.77 (m, 1H), 5.82 (s, 2H), 6.60–7.37 (m, 8H). 13C NMR (100 MHz, CDCl3): δ 34.23, 34.28, 52.01, 55.32, 55.37, 58.79, 60.49, 65.42, 94.17, 109.43, 116.97, 117.28, 121.13, 126.47, 127.25, 127.28, 128.38, 128.82, 129.00, 133.30, 133.36, 134.26, 137.46, 147.22, 153.19, 165.57, 172.03. HRMS (m/z) calculated for C31H34N3O6 [M-H]-: 544.2448, found 544.2464.
- N-(5-(3-Allyl-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl)-2-amino-3-methylbutanamide (13c)
Preparation was as described in the general method V above from Fmoc-protected β-lactam 12c. The desired product was isolated as a yellow oil, yield 59%, 59 mg (HPLC: 92.3%). IR (NaCl, film) νmax: 3343 (NH), 1746 (C=O, β-lactam), 1674 (C=O), 1607 (C=C), 1523 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.88–1.07 (m, 6H), 2.44–2.63 (m, 2H), 2.67–2.68 (m, 1H), 3.26–3.29 (m, 1H), 3.73 (s, 6H), 3.76 (s, 3H), 3.90 (s, 3H), 4.14 (br s, 1H), 4.65 (br s, 1H), 5.12–5.20 (m, 2H), 5.81–5.94 (m, 1H), 6.58 (s, 2H), 6.79–7.05 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 19.30, 30.43, 32.15, 55.45, 55.57, 58.53, 59.96, 60.03, 60.47, 94.17, 110.16, 117.42, 117.98, 120.07, 127.33, 133.34, 133.40, 133.53, 133.67, 134.26, 147.83, 153.17, 166.75, 174.18. HRMS (m/z) calculated for C27H34N3O6 [M-H]-: 496.2448, found 496.2437.
- 2-Amino-N-(5-(3-((E)-buta-1,3-dienyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl)-3-methylbutanamide (13d)
Preparation was as described in the general method V above from Fmoc-protected β-lactam 12d. The desired product was isolated as a yellow oil, yield 49%, 102 mg (HPLC: 96.9%). IR (NaCl, film) νmax: 3321 (NH), 2931 (C-H), 1734 (C=O, β-lactam), 1681 (C=O), 1612 (C=C), 1598 (C=C), 1513 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.04–1.06 (m, 6H), 2.40–2.41 (m, 1H), 3.76 (br s, 3H), 3.77 (br s, 1H), 3.88 (s, 6H), 3.89 (br s, 1H), 3.90 (s, 3H), 5.44 (br s, 1H), 6.24–6.29 (m, 1H), 6.66 (s, 2H), 6.82–7.42 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 18.67, 30.44, 55.56, 55.63, 60.47, 59.94, 60.61, 62.71, 93.89, 110.47, 119.01, 119.34, 124.90, 126.91, 129.51, 132.60, 133.16, 133.95, 134.87, 135.35, 147.03, 153.29, 161.70, 172.36. HRMS (m/z) calculated for C28H36N3O6 [M+H]+: 510.2604, found 510.2607.
- 2-Amino-N-(5-(3-(buta-1,3-dienyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl)acetamide (13e)
Preparation was as described in the general method V above from Fmoc-protected β-lactam 12e. The desired product was isolated as a yellow oil, yield 56%, 68 mg (HPLC: 90.0%). IR (NaCl, film) νmax: 3335 (NH), 1754 (C=O, β-lactam), 1655 (C=O), 1567 (C=C), 1503 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.48–3.52 (m, 2H), 3.55 (s, 6H), 3.69 (s, 3H), 3.78 (s, 3H), 3.82–3.83 (m, 1H), 5.32 (d, J = 1.48 Hz, 1H), 5.85–6.33 (m, 5H), 6.65 (s, 2H), 6.84–7.42 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 45.20, 55.41, 55.62, 60.49, 60.62, 62.65, 94.31, 110.46, 117.44, 119.01, 120.97, 125.73, 133.20, 133.73, 134.97, 135.45, 147.90, 152.99, 161.70, 167.02. HRMS (m/z) calculated for C25H28N3O6 [M-H]-: 466.1978, found 466.1971.
3.7. 2-Methoxy-5-(1-(3,4,5-trimethoxyphenyl)-4-oxo-3-(prop-1-en-2-yl)azetidin-2-yl) phenyl dibenzyl phosphate (14a)
To a solution of phenol 9q (17 mmol) in acetonitrile (100 mL), cooled to 0 °C, was added carbon tetrachloride (85 mmol). The solution was stirred for 10 min prior, and then diisopropylethylamine (35 mmol) and dimethylaminopyridine (1.7 mmol) were added. The dibenzyl phosphate (24.5 mmol) was then added dropwise to the mixture. When the reaction was complete, 0.5M KH2PO4 (aq) was added and the reaction mixture was warmed to room temperature. The mixture was extracted with ethyl acetate (3 × 50 mL), washed with saturated sodium chloride (aqueous, 100 mL) followed by water (100 mL) and dried (Na2SO4). The solvent was reduced in vacuo, and the product was isolated by flash column chromatography over silica gel (n-hexane: ethyl acetate gradient). Yield: 45%, 342 mg, brown oil. IR (NaCl, film) νmax: 2940 (C-H), 1740 (C=O, β-lactam), 1507 (C=C), 1304 (P=O), 1231 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.86 (s, 3H), 3.68 (d, J = 2.04 Hz, 1H), 3.72 (s, 6H), 3.76 (s, 3H), 3.82 (s, 3H), 4.68 (d, J = 2.52 Hz, 1H), 5.02 (br s, 1H), 5.05 (br s, 1H), 5.13–5.17 (m, 4H), 6.54 (s, 2H), 6.94–7.21 (m, 3H), 7.31–7.36 (m, 10H). 13C NMR (100 MHz, CDCl3): δ 20.08, 55.55, 55.60, 59.32, 60.47, 66.37, 69.44, 69.50, 69.55, 94.17, 112.82, 114.22, 119.21, 119.24, 122.70, 127.40, 127.48, 128.15, 128.21, 129.66, 133.17, 133.96, 135.12, 137.43, 139.57, 150.42, 153.07, 164.64. HRMS (m/z) calculated for C36H39NO9P (M++H): 660.2362, found 660.2372.
- 5-(3-Allyl-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl dibenzyl phosphate (14b)
Following the procedure described above for 14a, to a solution of the phenol 3-allyl-4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 10r (17 mmol) in acetonitrile (100 mL), cooled to 0 °C, was added carbon tetrachloride (85 mmol). The mixture was treated with diisopropylethylamine (35 mmol), N,N-dimethylaminopyridine (1.7 mmol) and dibenzyl phosphate (24.5 mmol). The product was isolated by flash column chromatography over silica gel (eluent, n-hexane: ethyl acetate gradient) as a brown oil (yield 27%), 325 mg. IR (NaCl, film) νmax: 1746 (C=O, β-lactam), 1503 (C=C), 1300 (P=O), 1235 (C-O), 1126 cm−1. 1H NMR (400 MHz, CDCl3): δ 2.51–2.58 (m, 1H), 2.65–2.71 (m, 1H), 3.14–3.18 (m, 1H), 3.71 (s, 6H), 3.76 (s, 3H), 3.81 (s, 3H), 4.54 (br s, 1H), 5.12–5.14 (m, 2H), 5.15–5.18 (m, 4H), 5.80–5.88 (m, 1H), 6.53 (s, 2H), 6.92–7.21 (m, 3H), 7.33–7.35 (m, 10H). 13C NMR (100 MHz, CDCl3): δ 32.21, 55.53, 55.58, 58.67, 59.50, 60.47, 69.40, 69.46, 69.52, 94.07, 112.72, 117.43, 119.32, 122.85, 127.38, 127.47, 128.14, 128.19, 129.81, 133.33, 133.37, 133.86, 135.06, 139.51, 139.58, 150.36, 153.05, 166.43. HRMS (m/z) calculated for C36H39NO9P [M+H]+: 660.2362, found 660.2375.
3.8. 2-Methoxy-5-(4-oxo-3-(prop-1-en-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-yl)-phenyl dihydrogen phosphate (15a)
Dibenzyl phosphate ester 14a (0.27 mmol) was dissolved in dry dichloromethane (5 mL) under nitrogen at 0 °C. Bromotrimethylsilane (0.59 mmol) was added to reaction mixture and allowed to stir for 45 min. Sodium thiosulphate solution (10%, 5 mL) was added to the reaction, and stirring was continued for 5 min. The aqueous phase was extracted with ethyl acetate (3 × 25 mL). The combined organic phases were concentrated in vacuo and purified by flash chromatography on silica gel (eluent: n-hexane: ethyl acetate, 1:1) to afford the product as an off-yellow solid, yield 57%, Mp > 300 °C (HPLC: 91.7%). IR (KBr) νmax: 3452 (OH), 1742 (C=O, β-lactam), 1275 (P=O) cm−1. 1H NMR (400 MHz, DMSO-d6): δ 1.86 (s, 3H), 3.52 (s, 3H), 3.59 (s, 6H), 3.68 (s, 3H), 4.91–5.06 (m, 4H, H-3, H-4), 6.52 (s, 2H), 6.90–7.15 (m, 3H). 13C NMR (100 MHz, DMSO-d6): δ 20.53, 55.85, 56.17, 59.17, 60.49, 66.53, 94.93, 111.46, 114.82, 120.31, 129.00, 129.24, 133.89, 134.38, 137.01, 139.42, 152.46, 153.53, 165.52. HRMS (m/z) calculated for C22H25NO9P (M+-H): 478.1267, found 478.1239.
3.9. 5-(3-Isopropyl-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl dihydrogen phosphate (15b)
The dibenzylphosphate ester 14a (2 mmol) was dissolved in ethanol: ethyl acetate (50 mL; 1:1 mixture) and hydrogenated over 1.2 g of 10% palladium on carbon until complete on TLC, typically for less than 3 h. The catalyst was filtered, the solvent was removed in vacuo, and the product was isolated by flash column chromatography over silica gel (eluent, n-hexane: ethyl acetate gradient) to afford the desired product as a brown oil, 239 mg, yield 98% (HPLC: 73.9%). IR (NaCl, film) νmax: 3472 (OH), 2961 (C-H), 1738 (C=O), 1593 (C=C), 1300 (P=O), 1238 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.00–1.08 (m, 6H), 2.06 (br s, 1H), 2.98–2.99 (m, 1H), 3.68 (s, 6H), 3.73 (s, 6H), 4.64 (br s, 1H), 6.53 (s, 2H), 6.86–7.35 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 19.41, 19.77, 28.21, 55.56, 58.20, 60.43, 66.05, 94.44, 112.57, 118.48, 122.60, 130.11, 132.93, 133.95, 137.59, 145.25, 152.97, 170.81. HRMS (m/z) calculated for C22H27NO9P (M+-H): 480.1423, found 480.1419.
3.10. 5-(3-Allyl-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-2-yl)-2-methoxyphenyl dihydrogen phosphate (15c)
The dibenzyl phosphate ester 14b (0.27 mmol) was dissolved in dry dichloromethane (5 mL) under nitrogen at 0 °C. Bromotrimethylsilane (0.59 mmol) was added to reaction mixture and stirred for 45 min. Sodium thiosulphate solution (10%, 5 mL) was added to the reaction, and stirring was continued for 5 min. The aqueous phase was extracted with ethyl acetate (3×25 mL), and the combined organic phases were concentrated in vacuo. Purification by flash chromatography on silica gel (eluent, 1:1 n-hexane: ethyl acetate) afforded the product as brown oil (yield 96%), IR (NaCl) νmax: 3483 (OH), 2938 (C-H), 1743 (C=O, β-lactam), 1592 (C=C), 1302 (P=O), 1237 (C-O) cm−1. 1H NMR (400MHz, CDCl3): δ 2.48–2.61 (m, 2H, CH2), 3.23 (br s, 1H, H-3), 3.68 (s, 6H, OCH3), 3.72 (s, 6H, OCH3), 4.58 (br s, 1H, H-4), 5.05–5.12 (m, 2H, CH2), 5.78–5.80 (m, 1H, CH), 6.51 (s, 2H, ArH), 6.85–7.54 (m, 3H, ArH). 13C NMR (100MHz, CDCl3): δ 32.09, 55.46, 55.55, 58.32 (OCH3), 59.64 (C-3), 60.44 (C-4), 94.30, 112.67, 117.44 (CH2), 119.27, 122.90, 129.18, 133.10, 133.27 (CH), 133.90, 139.86, 150.38, 152.99, 167.37 (C=O). HRMS (m/z) calculated for C22H26NO9PNa [M+Na]+: 502.1243, found 502.1226.
3.11. 2-Methoxy-5-(1-(3,4,5-trimethoxyphenyl)-4-oxo-3-propylazetidin-2-yl)phenyl dihydrogen phosphate (15d)
The dibenzyl phosphate ester 14b (2 mmol) was dissolved in ethanol: ethyl acetate (50 mL; 1:1 mixture) and hydrogenated over 1.2 g of palladium (10%) on carbon until complete as monitored by TLC, approx. 3 h. The catalyst was filtered, the solvent was reduced in vacuo, and the product was isolated by flash column chromatography over silica gel (eluent, n-hexane: ethyl acetate gradient) to afford the product as a brown oil, yield 88%, 106 mg, IR (NaCl, film) νmax: 3492 (OH), 2933 (C-H), 1743 (C=O, β-lactam), 1592 (C=C), 1311 (P=O), 1231 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.94–0.96 (m, 3H), 1.42–1.44 (m, 2H), 1.71–1.81 (m, 2H), 3.15 (br s, 1H), 3.65 (s, 6H), 3.71 (s, 6H), 4.56 (br s, 1H), 6.51 (s, 2H), 6.83–7.40 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 13.46, 19.85, 30.28, 55.41, 55.53, 59.45, 60.37, 60.50, 94.29, 112.40, 118.70, 122.13, 130.15, 133.23, 133.79, 140.59, 149.96, 152.95, 168.23. HRMS (m/z) calculated for C22H28NO9PNa [M+Na]+: 504.1399, found 504.1374.
3.12. General Method VI: Preparation of 3-Substituted β-lactams (17a-c, f, g)
The appropriate 3-unsubstituted β-lactam 16a-c (0.8 mmol) was dissolved in dry THF (7 mL) under N2 atmosphere and cooled to −78 °C (dry ice and acetone). To this stirring solution, LDA 2.0 M solution (1.6 mmol) was added all at once and the reaction left to stir for 5 min prior to the dropwise addition of 3-bromo-1-phenylpropene or ethyl bromoacetate (1.2 mmol) in dry THF (2 mL). The reaction was allowed to stir at −78 °C for 30 min and then allowed to stir at room temperature for 5 min before being poured into saturated brine (50 mL). Following extraction with ethyl acetate (2 × 50 mL), the solution was dried over anhydrous sodium sulphate. Solvent was removed under reduced pressure, and the residue was purified by flash chromatography over silica gel (eluent, n-hexane: ethyl acetate, 4:1) to yield the title compound.
- 3-Cinnamyl-4-(4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (17a)
Preparation was as described in the general method VI above from β-lactam 16a and 3-bromo-1-phenylpropene. The product was obtained as a yellow solid, yield: 39%, 145 mg. Mp 103–104 °C (HPLC: 95.0%). IR (KBr) νmax: 2937 (OH), 1747 (C=O, β-lactam), 1610 (C=C), 1590 (C=C), 1504 (C=C), 1247 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.69–2.77 (m, 1H), 2.87–2.93 (m, 1H), 3.28–3.32 (m, 1H), 3.73 (s, 6H), 3.78 (s, 3H), 3.83 (s, 3H), 4.69 (d, J = 2.28 Hz, 1H), 6.24–6.31 (m, 1H), 6.51 (d, J = 15.80 Hz, 1H), 6.55 (s, 2H), 6.92 (d, J = 8.56 Hz, 2H), 7.29–7.34 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 31.68, 54.88, 55.53, 59.06, 60.16, 60.51, 94.16, 114.10, 125.15, 125.74, 126.89, 127.01, 128.14, 129.15, 132.07, 133.49, 133.80, 136.48, 153.00, 159.27, 166.65. HRMS (m/z) calculated for C28H30NO5 [M+H]+: 460.2124, found 460.2115.
- 3-Cinnamyl-4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one (17b)
Preparation was as described in the general method VI above from β-lactam 16b and 3-bromo-1-phenylpropene. The product was afforded as a yellow oil, yield 14%, 33 mg (HPLC: 80.9%). IR (NaCl, film) νmax: 3333 (OH), 2934 (C-H), 1737 (C=O, β-lactam), 1650 (C=O), 1598 (C=C), 1502 (C=C), 1267 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.68–2.79 (m, 1H), 2.88–2.94 (m, 1H), 3.60–3.62 (m, 1H), 3.73 (s, 3H), 3.80 (s, 6H), 3.85 (s, 3H), 4.72 (br s, 1H), 6.23–6.34 (m, 1H), 6.57 (s, 2H), 6.68–6.76 (m, 1H), 6.85–7.34 (m, 8H). HRMS (m/z) calculated for C28H29NO6Na [M+Na]+: 498.1893, found 498.1885.
- 3-Cinnamyl-1,4-diphenylazetidin-2-one (17c)
Preparation was as described in the general method VI above from β-lactam 16c and 3-bromo-1-phenylpropene. The product was obtained as a brown oil, yield 90%, 307 mg (HPLC: 76.7%). IR (NaCl, film) νmax: 2932 (C-H), 1747 (C=O, β-lactam), 1507 (C=C), 1234 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.78–3.79 (m, 1H), 4.18–4.20 (m, 1H), 4.78 (br s, 1H), 6.25–6.27 (m, 1H), 6.65–6.69 (m, 1H), 7.29–7.40 (m, 15H). HRMS (m/z) calculated for C24H22NO [M+H]+: 340.1701, found 340.1696.
- Ethyl-2-(1-(3,4,5-trimethoxyphenyl)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)acetate (17f)
Preparation was as described in the general method VI above from β-lactam 16a and ethyl bromoacetate. The product was obtained as a brown oil, yield 64%, 131 mg (HPLC: 93.4%). IR (NaCl, film) νmax: 2945 (C-H), 1746 (C=O, β-lactam and acetate), 1589 (C=C), 1507 (C=C), 1245 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.18–1.30 (m, 3H), 2.91–2.95 (m, 2H), 3.48–3.53 (m, 1H), 3.70 (s, 6H), 3.74 (s, 3H), 3.79 (s, 3H), 4.16–4.24 (m, 2H), 4.91 (br s, 1H), 6.53 (s, 2H), 6.90 (d, J = 8.52 Hz), 7.31 (d, J = 8.52 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 13.53 (CO2CH2CH3), 46.85 (C-5), 53.62, 54.84, 55.50, 60.52, 60.91, 66.57, 93.96, 114.06, 126.84, 129.51, 133.62, 133.73, 152.96, 159.33, 164.16, 170.28. C23H27NO7Na [M+Na]+: 452.1685, found 452.1688.
- Ethyl-2-(2-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-3-yl)acetate (17g)
Preparation was as described in the general method VI above from β-lactam 16b and ethyl bromoacetate. The product was isolated as a brown oil, yield 77%, 275 mg (HPLC: 72.3%). IR (NaCl, film) νmax: 3300 (OH), 2938 (C-H), 1747 (C=O, β-lactam and acetate), 1591 (C=C), 1507 (C=C), 1237 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 1.23–1.28 (m, 3H), 2.90–2.96 (m, 1H), 3.50–3.55 (m, 1H), 3.73 (s, 6H), 3.77 (s, 3H), 3.89 (s, 3H), 4.10–4.18 (m, 3H, H3), 4.89–4.91 (m, 1H), 5.78 (br s, 1H), 6.53 (s, 2H), 6.84–7.02 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 14.19, 46.85, 54.11, 54.18, 56.04, 60.40, 60.91, 66.53, 94.52, 111.99, 112.38, 120.24, 130.50, 134.02, 134.37, 147.83, 149.97, 153.48, 164.52, 168.52. HRMS (m/z) calculated for C23H27NO8Na [M+Na]+: 468.1634, found 468.1654.
3.13. General Method VII: Preparation of Compounds 17d, 17e and 19
A solution of 1-bromo-4-(trifluoromethyl)benzene (2.75 mmol), palladium acetate (0.38 mmol), triphenylphosphine (0.41 mmol), potassium acetate (oven dried, 11.0 mmol), anhydrous tetrabutylammonium chloride (2.75 mmol) and appropriate β-lactam 10f, 10s and 18 (2 mmol) in DMF (50 mL) under N2 was heated to 80 °C for 18 h. The solution was cooled, diluted with ethyl acetate, washed with water (3 × 50 mL), dried and concentrated in vacuo. The crude product mixture was purified by flash chromatography (eluent, n-hexane: ethyl acetate, 4:1).
- 3-(4-(Trifluoromethyl)cinnamyl)-4-(4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl) azetidin-2-one (17d)
Preparation as described in the general method VII above from β-lactam 10f and 1-bromo-4-(trifluoromethyl)benzene afforded the product as a brown oil, yield 72%, 754 mg (HPLC: 81.8%). IR (NaCl, film) νmax: 1747 (C=O, β-lactam), 1677 (C=C), 1507 (C=C), 1320 (C-CF3), 1248 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.76–2.84 (m, 1H), 2.93–3.00 (m, 1H), 3.33–3.37 (m, 1H), 3.76 (s, 6H), 3.82 (s, 3H), 3.87 (s, 3H), 4.71 (d, J = 2.00 Hz, 1H), 6.39–6.46 (m, 1H), 6.55 (br s, 1H), 6.59 (s, 2H), 6.96 (d, J = 9.04 Hz, 2H), 7.33 (d, J = 8.00 Hz, 2H), 7.46 (d, J = 8.52 Hz, 2H), 7.61 (d, J = 8.56 Hz, 2H). 13C NMR (100 MHz, CDCl3): δ 31.72, 54.89, 55.53, 58.82, 60.26, 60.50, 94.16, 114.16, 125.08, 125.12, 125.86, 126.74, 126.85, 128.04, 128.97, 130.78, 133.40, 133.89, 139.88, 153.03, 159.37, 166.36. HRMS (m/z) calculated for C29H28F3NO5Na [M+Na]+: 550.1817, found 550.1816.
- 3-(4-(Trifluoromethyl)cinnamyl)-4-(4-methoxyphenyl)-1-phenylazetidin-2-one (17e)
Preparation as described in the general method VII above from β-lactam 10s and 1-bromo-4-(trifluoromethyl)benzene afforded the product as a brown oil, yield 28%, 246 mg (HPLC: 92.1%). IR (NaCl, film) νmax: 1740 (C=O, β-lactam), 1589 (C=C), 1507 (C=C), 1339 (C-CF3), 1278 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.72–2.80 (m, 1H), 2.90–2.96 (m, 1H), 3.28–3.32 (m, 1H), 3.82 (s, 3H), 4.71 (d, J = 2.48 Hz, 1H), 6.35–6.42 (m, 1H), 6.54 (d, J = 16.80 Hz, 1H), 6.91 (d, J = 8.52 Hz, 2H), 7.04–7.08 (m, 2H), 7.24–7.60 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 31.70, 54.87, 58.96, 59.87, 114.04, 114.14, 116.58, 123.46, 125.08, 125.86, 126.76, 127.69, 128.11, 128.61, 128.70, 130.77, 137.14, 139.89, 159.28, 166.55. HRMS (m/z) calculated for C26H22F3NO2Na [M+Na]+: 460.1500, found 460.1519.
- 3-(4-(Trifluoromethyl)styryl)-1-(3,4,5-trimethoxyphenyl)-4-(4-methoxyphenyl)azetidin-2-one (19)
Preparation as described in the general method VII above from β-lactam 18 and 1-bromo-4-(trifluoromethyl)benzene afforded the product as a yellow oil, yield 19%, 191 mg (HPLC: 82.6%). IR (NaCl, film) νmax: 1737 (C=O, β-lactam), 1588 (C=C), 1506 (C=C), 1326 (C-CF3), 1279 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.76 (s, 6H), 3.78 (s, 3H), 3.85 (s, 3H), 4.56–4.57 (m, 1H), 4.79 (d, J = 2.00 Hz, 1H), 6.60–6.64 (m, 1H), 6.75 (s, 2H), 6.93–6.96 (m, 1H), 7.18–7.49 (m, 8H). HRMS (m/z) calculated for C28H27F3NO5 [M+H]+: 514.1841, found 514.1845.
3.14. 1-(3,4,5-Trimethoxyphenyl)-3-((oxiran-2-yl)methyl)-4-phenylazetidin-2-one (20)
To a stirred solution of β-lactam 10f (0.18 mmol, 1 eq) in DCM (1.3 mL) was added a solution of mCPBA (3 eq) dissolved in DCM (0.5 mL). The resulting mixture was stirred at room temperature for 24 h. The reaction was diluted with DCM (10 mL) and washed with 50 mL each of saturated NaHCO3 and H2O. The organic layer was collected and dried over anhydrous Na2SO4, and the solvent removed under reduced pressure. Further purification was performed by flash column chromatography over silica gel eluted with 2:1 n-hexane: ethyl acetate. The product was obtained as a brown oil, yield 13%, 24 mg (HPLC: 67%); IR (NaCl, film) νmax: 1747 (C=O, β-lactam), 1620 (C=C), 1256 (epoxy C-O), 917 (epoxy C-O), 747 (epoxy C-O) cm−1. HRMS (m/z) calculated for C21H23NO5Na [M+Na]+: 392.1474, found 392.1488.
3.15. General Method VIII: Maleic Anhydride and N-Phenylmaleimide adducts (21a-d)
A solution of the appropriate β-lactam (1.10 mmol) and maleic anhydride (1.11 mmol) or N-phenylmaleimide (1.12 mmol) in toluene (4 mL) was refluxed for 1 h. The solvent was removed under reduced pressure, and the crude product was purified by recrystallization from dichloromethane.
- 4-(2-(4-Chlorophenyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-3-yl)-3a,4,7,7a-tetrahydro-2-phenyl-2H-isoindole-1,3-dione (21a)
Preparation was as described in the general method VIII above from (E)-3-(buta-1,3-dienyl)-4-(4-chlorophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11b and N-phenylmaleimide. The product was obtained as a colourless solid (yield 70%); mp: 208–210 °C (HPLC: 84.5%). IR (KBr) νmax: 2950 (C-H), 1744 (C=O, β-lactam), 1708 (C=O), 1595 (C=C), 1503 (C=C) 1235 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.27–2.34 (m, 1H), 2.88–2.96 (m, 2H), 3.36–3.40 (m, 1H), 3.73 (s, 6H), 3.78 (s, 3H), 3.87–3.91, 4.12–4.17 (m, 2H), 4.72 (d, J = 2.48 Hz, 1H), 5.82–5.84, 6.12–6.16 (m, 2H), 6.53 (s, 2H), 7.18–7.46 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 24.61, 36.65, 39.47, 41.60, 55.56, 59.34, 60.49, 60.52, 94.17, 125.95, 127.14, 128.23, 128.61, 129.12, 129.40, 129.50, 130.90, 131.18, 132.97, 134.21, 135.56, 153.11, 165.71, 176.16, 178.01. HRMS (m/z) calculated for C32H2935ClN2O6Na [M+Na]+: 595.1612, found 595.1602.
- 4-(2-(4-Bromophenyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-3-yl)-3a,4,7,7a-tetrahydroisobenzofuran-1,3-dione (21b)
Preparation was as described in the general method VIII above from (E)-3-(buta-1,3-dienyl)-(4-(4-bromophenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11c and maleic anhydride. The product was isolated as a colourless solid (yield 24%); mp: 242–244 °C (HPLC: 97.9%). IR (KBr) νmax: 2950 (C-H), 1776 (C=O, β-lactam), 1746 (C=O), 1592 (C=C), 1507 (C=C), 1239 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.26–2.32 (m, 1H), 2.81–2.90 (m, 2H), 3.50–3.55 (m, 1H), 3.73 (s, 6H), 3.78 (s, 3H), 3.92–3.95, 3.98–4.02 (m, 2H), 4.72 (d, J = 2.00 Hz, 1H), 5.85–5.88, 6.14–6.18 (m, 2H), 6.51 (s, 2H), 7.34 (d, J = 8.56 Hz, 2H), 7.56 (d, J = 8.52 Hz, 2h). 13C NMR (100 MHz, CDCl3): δ 24.24, 35.84, 39.99, 42.53, 55.59, 58.75, 60.29, 60.52, 94.20, 122.53, 127.41, 129.67, 129.72, 130.78, 132.20, 133.60, 133.80, 152.99, 164.11, 171.11, 173.76. HRMS (m/z) calculated for C26H2580BrNO7 [M+H]+: 542.0814, found 542.0811.
- 4-(2-(4-Butoxyphenyl)-4-oxo-1-(3,4,5-trimethoxyphenyl)azetidin-3-yl)-2-phenyl-3a,4,7,7a-tetrahydro-1H-isoindole-1,3(2H)-dione (21c)
Preparation was as described in the general method VIII above from (E)-3-(buta-1,3-dienyl)4-(4-butoxyphenyl)-1-(3,4,5-trimethoxyphenyl) azetidin-2-one 11j and N-phenylmaleimide. The product was obtained as colourless solid (yield 45%); mp: 163–164 °C (HPLC: 76.1%). IR (KBr) νmax: 2958 (C-H), 1745 (C=O, β-lactam), 1710 (C=O), 1591 (C=C), 1506 (C=C), 1245 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 0.98 (t, J = 7.54 Hz, 3H), 1.47–1.53 (m, 2H), 1.75–1.79 (m, 2H), 2.30–2.32 (m, 1H), 2.87–2.94 (m, 2H), 3.35–3.39 (m, 1H), 3.72 (s, 6H), 3.78 (s, 3H), 3.89–3.93, 4.16–4.20 (m, 2H), 3.96 (t, J = 6.52 Hz, 2H), 4.67 (d, J = 2.52 Hz, 1H), 5.83–5.86, 6.10–6.14 (m, 2H), 6.58 (s, 2H), 7.18–7.45 (m, 9H). 13C NMR (100 MHz, CDCl3): δ 13.39, 18.77, 24.59, 30.78, 36.76, 39.51, 41.52, 55.50, 59.10, 60.50, 61.03, 67.33, 94.20, 114.75, 125.98, 127.05, 128.16, 128.50, 128.57, 129.02, 129.95, 131.25, 133.36, 133.87, 153.00, 159.06, 166.19, 176.15, 178.11. HRMS (m/z) calculated for C36H38N2O7Na [M+Na]+: 633.2577, found 633.2571.
- 3a,4,7,7a-Tetrahydro-4-(2-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)-4-oxoazetidin-3-yl)isobenzofuran-1,3-dione (21d)
Preparation was as described in the general method VIII above from (E)-3-(buta-1,3-dienyl)-4-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11p and maleic anhydride. The product was obtained as a colourless solid (yield 41%); mp: 224–225 °C (HPLC: 82.9%). IR (KBr) νmax: 3300 (OH), 2932 (C-H), 1778 (C=O, β-lactam), 1747 (C=O), 1591 (C=C), 1508 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 2.26–2.33 (m, 1H), 2.80–2.86 (m, 2H), 3.51 (t, J = 8.52 Hz, 1H), 3.71 (s, 6H), 3.77 (s, 3H), 3.83 (s, 3H), 3.92–3.96 (m, 1H), 4.01–4.05 (m, 1H), 4.64 (d, J = 2.00 Hz, 1H), 5.35 (bs, 1H), 5.87–5.89, 6.12–6.16 (m, 2H), 6.55 (s, 2H), 6.88–7.02 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 24.23, 35.96, 40.03, 42.43, 55.10, 55.49, 60.50, 58.54, 60.75, 94.29, 112.15, 118.34, 119.09, 128.72, 129.31, 130.21, 133.03, 134.03, 145.28, 151.02, 153.03, 165.42, 170.90, 173.31. HRMS (m/z) calculated for C27H26NO9 [M-H]-: 508.1608, found 508.1589.
3.16. General Method IX: Adducts with Dimethylacetylene Dicarboxylate (22a-d)
Equivalent amounts of β-lactam and dimethylacetylene dicarboxylate (DMAD) were refluxed in dry toluene (5 mL) for 6–7 h under N2. The reaction mixture was then purified by flash chromatography over silica gel (eluent, 4:1 n-hexane: ethyl acetate).
- Dimethyl-6-(1-(3,4,5-trimethoxyphenyl)-2-(naphthalen-1-yl)-4-oxoazetidin-3-yl)cyclohexa-1,3-diene-1,2-dicarboxylate (22a)
Preparation was as described in the general method IX above from (E)-3-(buta-1,3-dienyl)-4-(naphthalen-1-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11m. The product was obtained as a yellow solid (yield 14%); mp: 120–122 °C (HPLC: 94.2%). IR (KBr) νmax: 2954 (C-H), 1750 (C=O, β-lactam), 1726 (C=O), 1587 (C=C), 1507 (C=C), 1267 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.01–3.09 (m, 1H), 3.25–3.33 (m, 1H), 3.50–3.51 (m, 1H), 3.57 (s, 6H), 3.61 (s, 3H), 3.76 (s, 3H), 3.81 (s, 3H), 4.01–4.03 (m, 1H), 5.71 (br s, 1H), 5.91–5.93 (m, 2H), 6.52 (s, 2H), 7.45–8.10 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 27.79, 35.85, 52.00, 55.50, 59.96, 60.46, 62.95, 94.28, 121.56, 123.03, 123.25, 125.25, 125.65, 126.19, 128.44, 128.90, 130.12, 132.21, 133.18, 133.41, 133.46, 133.87, 134.41, 153.01, 164.46, 167.26, 167.48. HRMS (m/z) calculated for C32H31NO8Na [M+Na]+: 580.1947, found 580.1940.
- Dimethyl-6-(1-(3,4,5-trimethoxyphenyl)-2-(naphthalen-1-yl)-4-oxoazetidin-3-yl)cyclohexa-1,3-diene-1,2-dicarboxylate (22b)
Preparation was as described in the general method IX above from (E)-3-(buta-1,3-dienyl)-4-(naphthalen-1-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11m. The product was obtained as a yellow oil (yield 8%) (HPLC: 96.4%). IR (KBr) νmax: 2954 (C-H), 1746 (C=O, β-lactam), 1727 (C=O), 1590 (C=C), 1507 (C=C) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.13–3.14 (m, 1H), 3.18 (s, 3H), 3.43–3.51 (m, 1H), 3.60 (s, 7H), 3.75 (s, 6H), 4.00 (br s, 1H), 5.50 (br s, 1H), 5.92–5.95 (m, 1H), 6.12–6.15 (m, 1H), 6.50 (s, 2H), 7.41–8.01 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 27.64, 35.53, 51.27, 51.87, 55.49, 59.95, 60.45, 62.07, 94.25, 121.54, 122.33, 125.04, 125.54, 125.70, 126.16, 128.44, 128.57, 130.14, 131.96, 132.96, 133.29, 133.44, 133.72, 133.99, 153.04, 164.61, 166.62, 167.12. HRMS (m/z) calculated for C32H31NO8Na [M+Na]+: 580.1947, found 580.1950.
- Dimethyl-6-(1-(3,4,5-trimethoxyphenyl)-2-(naphthalen-2-yl)-4-oxoazetidin-3-yl)cyclohexa-1,3-diene-1,2-dicarboxylate (22c)
Preparation was as described in the general method IX above from (E)-3-(buta-1,3-dienyl)-4-(naphthalen-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11n. The product was obtained as a yellow solid (yield 13%); mp: 146–147 °C (HPLC: 98.9%). IR (KBr) νmax: 2958 (C-H), 1748 (C=O, β-lactam), 1726 (C=O), 1594 (C=C), 1506 (C=C), 1287 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.01–3.07 (m, 1H), 3.22–3.29 (m, 1H), 3.42–3.44 (m, 1H), 3.66 (s, 9H), 3.75 (s, 3H), 3.79 (s, 3H), 3.93–3.94 (m, 1H), 5.02 (d, J = 2.00 Hz, 1H), 5.89–5.91 (m, 1H), 5.95–5.98 (m, 1H), 6.56 (s, 2H), 7.40–7.88 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 27.52, 36.00, 51.97, 52.05, 55.51, 58.18, 60.45, 63.10, 94.22, 122.76, 123.60, 124.95, 125.24, 126.09, 126.28, 127.35, 127.40, 128.88, 132.80, 132.91, 133.22, 133.85, 133.91, 134.17, 134.53, 152.98, 164.24, 167.12, 167.72. HRMS (m/z) calculated for C32H31NO8Na [M+Na]+: 580.1947, found 580.1948.
- Dimethyl-6-(1-(3,4,5-trimethoxy-phenyl)-2-(naphthalen-2-yl)-4-oxo-azetidin-3-yl)-cyclohexa-1,3-diene-1,2-dicarboxylate (22d)
Preparation was as described in the general method IX above from (E)-3-(buta-1,3-dienyl)-4-(naphthalen-2-yl)-1-(3,4,5-trimethoxyphenyl)azetidin-2-one 11n. The product was isolated as a yellow solid (yield 10%); mp: 144–146 °C. (HPLC: 98.2%). IR (KBr) νmax: 2955 (C-H), 1746 (C=O, β-lactam), 1727 (C=O), 1591 (C=C), 1507 (C=C), 1278 (C-O) cm−1. 1H NMR (400 MHz, CDCl3): δ 3.12–3.19 (m, 1H), 3.33 (s, 3H), 3.35–3.42 (m, 1H), 3.58–3.60 (m, 1H), 3.68 (s, 6H), 3.77 (s, 3H), 3.86 (s, 3H), 3.95–3.96 (m, 1H), 4.79 (d, J = 2.00 Hz, 1H), 5.91–5.93 (m, 1H), 6.06–6.09 (m, 1H), 6.58 (s, 2H), 7.36–7.83 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 28.13, 34.70, 51.53, 52.07, 55.59, 56.62, 60.48, 62.12, 94.19, 122.60, 122.68, 124.97, 125.49, 125.88, 126.09, 127.33, 127.43, 128.58, 131.39, 132.74, 132.85, 133.20, 134.03, 134.18, 135.81, 153.08, 164.29, 166.03, 168.01. HRMS (m/z) calculated for C32H31NO8Na [M+Na]+: 580.1947, found 580.1951.
3.17. Biochemical Evaluation
3.17.1. Cell Culture
All biochemical assays were performed in triplicate for the determination of mean values reported. The human breast carcinoma cell line MCF-7 was purchased from the European Collection of Animal Cell Cultures (ECACC) and was cultured in Eagle’s minimum essential medium with 10% fetal bovine serum, 2 mM L-glutamine and 100 μg/mL penicillin/streptomycin. The medium was supplemented with 1% non-essential amino acids. The human breast carcinoma cell line MDA-MB-231 was purchased from the ECACC. MDA-MB-231 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine and 100 μg/mL penicillin/streptomycin (complete medium). HEK-293T normal epithelial embryonic kidney cells were cultured in DMEM with GlutaMAXTM-I in the absence of non-essential amino acids. HL-60 cells were originally obtained from the ECACC. SW-480 cells were a kind gift from Dr. Emma Creagh, School of Biochemistry and Immunology, Trinity College Dublin. SW-480 cells were cultured in DMEM with GlutaMAX-I, with the same supplement in the absence of non-essential amino acids. HL-60 cells were cultured in RPMI-1640 Glutamax 1 medium supplemented with 10% FBS media, and 100 μg/mL penicillin/streptomycin. HT-29 cells originate from a human adenocarcinoma of the colon, were originally obtained from the ECACC and were grown in DMEM Glutamax media. HT-29 media were supplemented with 10% foetal bovine serum (FBS). Cells were maintained at 37 °C in 5% CO2 in a humidified incubator. All cells were sub-cultured 3 times/week by trypsinisation.
3.17.2. Cell Viability Assay
Cells were seeded at a density of 5 × 103 cells/well (MCF-7) in triplicate in 96-well plates. After 24 h, cells were then treated with medium alone, with vehicle (1% ethanol (v/v)) or with selected dilutions of control CA-4 or the synthesised azetidinone compounds in the concentration range 1–50 μM. Cell proliferation for MCF-7 and MDA-MB-231 cells was analysed using the Alamar Blue assay (Invitrogen Corp.). After 72 h, Alamar Blue (10% (v/v)) was added to the contents of each well, and plates were then incubated in the dark for 3–5 h at 37 °C. Fluorescence results were obtained with a 96-well fluorimeter operating with excitation (530 nm) and emission (590 nm), and the results were expressed as viability (%) relative to vehicle control (100%). IC50 values (concentration of drug resulting in 50% reduction in cell survival) were obtained from the dose response curves using Prism (GraphPad Software, Inc., La Jolla, CA, USA). Experiments were performed in triplicate on at least three separate occasions.
3.17.3. Lactate Dehydrogenase Assay for Cytotoxicity
The cytotoxic effects of the compounds were determined using the CytoTox 96 non-radioactive cytotoxicity assay (Promega) [] as previously reported []. The MCF-7 cells were seeded in 96-well plates and incubated for 24 h. Cells were treated with test compounds 16, 26 and 30 at 10 μM concentration as described in the cell viability assay above. At 72 hr, 20 μL of ‘lysis solution (10X)’ was added to the control wells to ensure 100% death. The cells were incubated for a further 1 hr. Supernatant (50 μL) was removed from each well and transferred to a 96-well plate. Cytotox 96R Reagent (50 μL) was added to each well, and the plate was retained in darkness at 20 °C for 30 min. 50 μL of ‘stop solution’ was then added to each well, and the absorbance was determined at 490 nm using a Dynatech MR5000 plate reader. The cell death (%) at 10 μM was determined.
3.17.4. Cell Cycle Analysis
MCF-7 cells were treated with compound 9q (at concentration of 50 nM and 500 nM) and incubated for 72 h. Cells (adherent and detached) were collected, trypsinised and centrifuged (800× g, 15 min). Cells were washed with ice-cold PBS (x2) and fixed in ice-cold 70% ethanol overnight at −20 °C. Fixed cells were centrifuged (800× g, 15 min) and stained with PI (50 μg/mL) containing DNase-free RNase A (50 μg/mL) at 37 °C for 30 min. The DNA content of the cells (10,000 cells/experimental group) was recorded by flow cytometry at 488 nm using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analysed using cellQuest software (BD Biosciences, San Jose, CA, USA).
3.17.5. Annexin-V/PI Apoptosis Assay
Flow cytometry using annexin-V and propidium iodide (PI) was used to detect apoptotic cell death in MCF-7 cells. Cells were seeded in 6-well plates (density 1 × 105 cells/mL) and treated with either vehicle (0.1% (v/v) EtOH), CA-4 or β-lactam compound 9q at concentrations of 50 nM and 500 nM for 72 h. Cells were then harvested and washed in 1X binding buffer (20X binding buffer: 0.1M HEPES, pH 7.4; 1.4 M NaCl; 25 mM CaCl2 diluted in dH2O). Cells were then incubated on ice in darkness for 30 min in annexin-V-containing binding buffer [1:100]. Cells were washed once in binding buffer and then re-suspended in propidium iodide containing binding buffer [1:1000]. Cells were analysed directly using the BD Accuri flow cytometer and Prism software. Four cell populations are produced in this assay: annexin-V- and PI-negative (Q4, healthy cells), annexin-V-positive and PI-negative (Q3, early apoptosis), annexin-V- and PI-positive (Q2, late apoptosis) and annexin-V-negative and PI-positive (Q1, necrosis). CA-4 (50 nM) was used as the positive control for apoptosis.
3.17.6. Tubulin Polymerization Assay
Tubulin polymerization was monitored using the BK006 kit obtained from Cytoskeleton Inc. (Denver, CO, USA). [] The values reported represent the average values determined from two independent assays. Purified bovine brain tubulin (>99%, 3 mg/mL) in buffer containing PIPES (80 mM, pH 6.9), EGTA (0.5 mM), MgCl2 (2 mM), GTP (1 mM) and glycerol (10%) was incubated at 37 °C in vehicle (1% DMSO or EtOH (v/v) in dH2O) or with compounds 9h, 9q, 9s, 10h, 10p, 10r, 11h, 11p, 11r, 17f (all at 10 µM concentration); CA-4 (10 μM) was used as control. In this assay, light scattering is related to the concentration of polymerised microtubules produced. Tubulin assembly was monitored turbidimetrically at 340 nm at 37 °C in a Spectramax 340 PC spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The absorbance was measured at 30 s intervals over 30–60 min, and the Vmax and fold reduction in Vmax were determined.
3.17.7. Colchicine-Binding Site Assay
MCF-7 cells were seeded at density of 5 × 104 cells/well in 6-well plates and incubated overnight. Cells were treated with vehicle control (ethanol (0.1% v/v)) or compound 9q (10 μM) for 2 h, followed by treatment with N,N’-ethylene-bis(iodoacetamide)(EBI) (100 μM solution in ethanol) (Santa Cruz Biotechnology, Dallas, TX, USA), for 1.5 h. Cells were then washed with ice-cold PBS (x2) and lysed by addition of Laemmli buffer. Samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes and examined with β-tubulin antibodies (Sigma-Aldrich, Milwaulkee, WI, USA) [].
3.17.8. Immunofluorescence Microscopy
The effects of β-lactam 9q on MCF-7 cytoskeleton were determined using confocal microscopy as previously described. [] MCF-7 cells were seeded at 1 × 105 cells/mL in glass slides with eight chambers (BD Biosciences, San Jose, CA, USA). Cells were treated with vehicle (1% ethanol (v/v)), paclitaxel (1 μM), combretastatin A-4 (50 nM) or 9q (10 nM, 100 nM and 500 nM) for 16 h. Following treatment, cells were gently washed in PBS, fixed for 20 min with 4% paraformaldehyde in PBS and permeabilised in 0.5% Triton X-100. The cells were then washed with PBS-T (PBS containing 0.1% Tween), blocked in 5% bovine serum albumin diluted in PBS-T and incubated with mouse monoclonal anti-α-tubulin-FITC antibody (clone DM1A) (Sigma, Milwaulkee, WI, USA) (1:100) for 2 h at 20 °C. The cells were washed again with PBS-T and incubated with Alexa Fluor 488 dye (1:450) for 1 h at 20 °C. Following washes in PBST, the cells were mounted in Ultra Cruz Mounting Media (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which contained 4,6-diamino-2-phenolindol dihydrochloride (DAPI). The immunofluorescence cell images were captured by Leica SP8 confocal microscopy and analysed with Leica application suite X software. Microscopy experiments were performed on three independent occasions, and the images for these experiments were obtained on the same day using identical conditions.
3.17.9. Stability Study for Compounds 9q, 15a, 10h, 10q, 10p, 10r and CA-4
Analytical high-performance liquid chromatography (HPLC) stability studies were performed as follows using Symmetry® column (C18, 5 µm, 4.6 × 150 mm), Waters 2487 Dual Wavelength Absorbance detector, Waters 1525 binary HPLC pump and Waters 717 plus Autosampler (Waters Corporation, Milford, MA, USA) with detection at 254 nm as previously described []. Samples were analysed using mobile phase acetonitrile (80%): water (20%), flow rate (1 mL/min) over 10 min. Stock solutions of compounds 9q, 15a, 10h, 10q, 10p, 10r and CA-4 (5 mg) in mobile phase (10 mL) were prepared (with and without the addition of 0.5% Tween) to aid solubility. (i) Stability in phosphate buffers: Phosphate buffers at the desired pH values (4, 7.4 and 9) were prepared as described in the European Pharmacopoeia (Ph. Eur., 11th edition, 2022). First, 30 µL of compound stock solution was diluted with appropriate buffer (1 mL); sample was shaken and injected immediately. Samples were withdrawn and analysed at the following time points: t = 0 min, 5 min, 30 min, 60 min, 90 min, 120 min, 24 h and 48 h. (ii) Thermal stability: Compound stock solution (1 mL) was placed in a glass vial on a heating block for 4 h at 60 °C. The sample was then cooled, diluted (acetonitrile) and analysed. (ii) Oxidising conditions: H2O2 (3%, 0.2 mL) was added to compound stock solution (0.8 mL). The vial was vortexed and retained at 20 °C, and the sample was analysed at 60, 120 180 and 240 min. (iv) Acidic conditions: HCl (0.1 M, 0.2 mL) was added to stock solutions (0.8 mL). The vial was mixed by vortex and retained at 20 °C. A sample was neutralised with NaOH (0.1 M, 0.2 mL) at 60, 120, 180 and 240 min, followed by HPLC analysis. (v) Alkaline conditions: NaOH (0.1 M, 0.2 mL) was added to stock solution (0.8 mL) of the compound in a vial. The vial was mixed by vortex and retained at 20 °C. A sample was neutralised with HCl (0.1 M, 0.2 mL) at 60, 120, 180 and 240 min followed by HPLC analysis. (vi) Photostability study: Compounds (stock solution, 1 mL) were placed in a vial, exposed to UV light for 4 h and then analysed by HPLC as above. Stability studies in plasma: Approval for this study was obtained from the School of Pharmacy and Pharmaceutical Sciences Trinity College Dublin Research Ethics Committee (2020–06-01-MS). Following informed consent, blood was withdrawn from healthy volunteers, and plasma was obtained by centrifugation and kept at −20 °C until further use. Then, 360 µL stock solution of compounds 9q, 15a, 10h, 10q, 10p, 10r or CA-4 was added to buffered plasma (plasma: buffer = 1:9, 4 mL total volume) at 37 °C in HPLC vial. A sample (250 µL) was then added to the Eppendorf tube containing ZnSO4.7H2O solution (500 µL) (2% w/v ZnSO4 solution in acetonitrile:water, 1:1). The samples were centrifuged (10,000 rpm, 3 min), filtered (0.2 micron filter) and analysed by HPLC as above. Further samples were taken at one-hour intervals.
3.18. X-ray Crystallography
Data for 8h and 8i were measured on a Bruker APEX DUO, and data for 10h-11t were measured on a Bruker D8 Quest ECO, using Mo Kα radiation (λ = 0.71073 Å). Each sample was mounted on a MiTeGen cryoloop, and data were collected at 100(2) K using an Oxford Cryosystems Cobra (8h, 8i) or Cryostream (10h-11t) low-temperature device. Bruker APEX [,] software was used to collect and reduce data. Absorption corrections were applied using SADABS []. Structures were solved with the SHELXT structure solution program [] using intrinsic phasing. All were refined using least-squares method on F2 with SHELXL []. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were assigned to calculated positions using a riding model with appropriately fixed isotropic thermal parameters. Molecular graphics were generated using OLEX2 []. Crystal data and details of data collection and refinement are provided in Table S1, Supplementary Materials. In 10h, there are two independent molecules in the asymmetric unit. Part of one beta-lactam ring (C32/C32b) and the vinylic substituent are disordered and modelled in two positions with 49:51% occupancy with displacement restraints (SIMU). In 11t, the donor hydrogens were located and refined, and in 11h, the sample was weakly diffracting, with weak high-angle data resulting in a high R(int). Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications No. 1820359 [], 2241430, 2241431, 2241432, 2241433, 2241434, 2241435, 2241436, 2241437, 2241438. Copies of the data can be obtained, free of charge, upon application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax, +44-(0)1223–336033, or e-mail: deposit@ccdc.cam.ac.uk).
3.19. Computational Procedure: Molecular Docking Study
3.19.1. Computational Procedure for Molecular Docking
The 1SA0 X-ray structure of bovine tubulin co-crystallised with N-deacetyl-N-(2-mercaptoacetyl)-colchicine (DAMA-colchicine) was downloaded from the PDB website []. A UniProt Align analysis confirmed a 100% sequence identity between human and bovine β tubulin. The crystal structure was prepared using QuickPrep (minimised to a gradient of 0.001 kcal/mol/Å), Protonate 3D, Residue pKa and Partial Charges protocols in MOE 2022 with the MMFF94x force field. Each compound was drawn in MOE, saved as an mdb and processed in MOE []. 3S,4R trans enantiomers of the compounds were examined. For each compound, MMFF94x partial charges were calculated, and each was energy-minimised to a gradient of 0.001 kcal/mol/Å. Default parameters were used for docking, except that 300 poses were sampled for each compound, and the top 50 docked poses generated for each compound were retained for subsequent analysis.
3.19.2. Mapping of Protein-Binding Sites (MoPBS) Algorithm
The 1SA0 X-ray structure of bovine tubulin co-crystallised with N-deacetyl-N-(2-mercaptoacetyl)colchicine (DAMA-colchicine) [] was used after the processing above was completed. As described above and in our recent paper [], the binding site was flooded with 100 copies of each fragment—in this case, a more focused selection of four fragments (acetate ion, benzene, methane, methylammonium) compared to the nine fragments used in the original report. The DBSCAN K-means algorithm was used to cluster eight pharmacophore features from the 400 minimised fragments.
4. Conclusions
Microtubule-targeting agents (MTA) such as paclitaxel and docetaxel and the vinca alkaloids vincristine, vinblastine and vinorelbine are widely used in chemotherapy for a variety of different cancers. Extensive preclinical studies have shown that CBSIs are promising drug candidates for cancer therapy. CA-4-based codrugs (or mutual prodrugs) have recently been demonstrated to improve the therapeutic profile of clinically used drugs such as doxorubicin, floxuridine and tegafur, and they have achieved targeted delivery of drugs to cancer tissues []. Colchicine-binding-site-targeting compounds are of interest not only for their potent antimitotic effects [,,,]; in a recent study, the potent antimitotic sabizabulin 5 was evaluated in a clinical trial in metastatic breast cancer and also as an antiviral agent for the treatment of hospitalised COVID-19 patients at high risk for acute respiratory distress syndrome []. Sabizabulin binds to viral tubulin at the colchicine-binding site and disrupts the intracellular transport of the virus. Therefore, investigation of the non-covalent tubulin-targeting b-lactam compounds developed in the present work as potential antibacterial agents is also of current interest.
Increasing evidence is also available in relation to the non-mitotic effects of tubulin-targeting compounds, e.g., as vascular disrupting agents (VDA) and inhibitors of tumour angiogenesis []. Many small-molecule dual-targeting tubulin inhibitors have been reported with applications for cancer therapy []; e.g., novel CA-4 sulphamate derivatives identified for dual tubulin polymerization and arylsulphatase inhibitors having potential for applications in cancer therapy []. Dual CBSIs and Src kinase inhibitors are reported to be effective in regulating the overexpression of tubulin isotypes and can inhibit drug resistance, which is mediated by P-glycoprotein (P-gp), and other multidrug-resistance-associated proteins (MRP1, MRP2) []. Interestingly, it is suggested that microtubules may modulate immune responses, and therefore, the use of microtubule inhibitors together with immune therapy may offer a highly effective option for selected cancer treatments [].
In this study, a series of ninety-six compounds based on the 2-azetidinone scaffold structure were designed and synthesised as CBSIs. These novel 3-(prop-1-en-2-yl)azetidin-2-one, 3-allylazetidin-2-one and 3-(buta-1,3-dien-1-yl)azetidin-2-one analogues of CA-4 were prepared using an efficient Staudinger procedure, and they allowed introduction of the alkene, allyl and diene substituents at the C-3 position of the β-lactam, together with structurally varied electron-releasing or electron-withdrawing substituent groups on the ring B pharmacophore. These compounds, together with some amino acid and phosphate prodrugs and their Diels–Alder adducts, were evaluated for their antiproliferative activity, cell cycle effects and inhibition of tubulin assembly.
The compounds were shown to have significant in vitro antiproliferative activities in MCF-7 breast cancer cells, particularly compounds 9h, 9q, 9r, 10p 10r, 11h, with IC50 values in the range 10–33 nM. The phenolic 3-(prop-1-en-2-yl) β-lactam compound 9q was identified as the most potent in MCF-7 breast cancer cells (IC50 = 10 nM, with drug-like properties. Significant antiproliferative effects were also demonstrated for the compounds in the TNBC cell line MDA-MB-231, with IC50 values in the range 23–33 μM, which were comparable with the activity of CA-4. The biological target of these compounds was identified as tubulin. They demonstrated significant reduction in tubulin polymerisation in vitro and were shown to interact non-covalently at the colchicine-binding site on tubulin. Cell cycle analysis through flow cytometry demonstrated that compound 9q arrested MCF-7 cells in the G2/M phase, resulting in cellular apoptosis.
Microtubule depolymerisation was confirmed by confocal microscopy, and the immunofluorescence results confirm the antimitotic properties of β-lactam 9q, which was observed to target tubulin and result in mitotic catastrophe. In addition, in silico molecular docking results indicate that the β-lactam compounds can interact with the colchicine-binding site of tubulin, further supporting the observed inhibitory effects of these compounds on tubulin polymerisation. Tubulin-targeted chemotherapy has been clinically successful against a wide spectrum of solid tumours and blood cancers. Compound 9q is representative of a novel class of potent microtubule-destabilising agents having low toxicity with promising small-molecule, drug-like properties and translational potential for the development of new molecules with potent tubulin-inhibitory activity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph16071000/s1. Table S1: Crystal data and structure refinement for 8h, 8i, 9h, 10f, 10h, 11b-d, 11h, 11t; Table S2: Stability study for compounds 9q, 15a, 10h, 10q, 10p, 10r and CA-4 at pH 4.0, pH 7.5, pH 9.0 and in plasma; Table S3: Stability study for compounds 10h, 10q, 10p and 10r (HCl, NaOH, heat (80 °C), Light, H2O2); Table S4: Tier-1 Profiling Screen of Selected β-Lactams; Table S5: ADMET and Lipinski Properties for Selected β-Lactams; Table S6: Antitumour evaluations of compound 9q in the NCI60 cell line in vitro primary one-dose screen; Table S7: Comparative antitumour evaluations of compounds 9h, 9q, 9s, 10p, 10h, 10r, 15a, 15b in the NCI60 leukaemia, non-small-cell lung cancer, colon cancer and CNS cancer cell lines in vitro primary screen; Table S8: Comparative antitumour evaluations of compounds 9h, 9q, 9s, 10p, 10h, 10r, 15a, 15b in the NCI60 melanoma, ovarian cancer, renal cancer and breast cancer cell lines in vitro primary screen; Table S9: Standard COMPARE analysis of β-lactam 9q; Table S10: Standard COMPARE analysis of β-lactam 9s; Table S11: Docking scores for selected β-lactams; Table S12: X-ray data and torsional angles for compounds 9h, 10h, 10f, 11a-c, 11h; Table S13. X-ray crystal structure of compounds 8h, 8i and 11t; Table S14: X-ray crystal structure of compounds 10f, 11b, 11c, 11d; Figures S1–S20: 1H NMR and 13C NMR spectra; Figure S21: Stability study for compounds 9q, 15a, 10h, 10q, 10p, 10r and CA-4 at pH 4.0, pH 7.5, pH 9.0 and in plasma; Figure S22: Flexible alignment of 11p and combretastatin A-4; Figure S23: Inter-atomic distances between oxygen atoms for compounds 25, 11p and CA-4; Figure S24: Protein ligand interactions for β-Lactam 11p docked in the colchicine-binding site of tubulin; Figure S25: Overlay of the X-ray structure of tubulin crystallised with DAMA colchicine with β-lactams; Figure S26: β-Lactams 9s, 10h, 11p and 11r docked in the colchicine-binding site of tubulin.
Author Contributions
Conceptualisation, T.F.G., S.W., A.M.M. and M.J.M.; formal analysis, S.W., A.M.M., B.T., S.M.N., D.F., N.M.O., T.F.G., S.K. and M.J.M.; funding acquisition, A.M.M. and M.J.M.; investigation, S.W., T.F.G., A.M.M., S.M.N., D.F. and S.K.; methodology, S.W., A.M.M., D.F., B.T., S.K. and S.M.N.; supervision, M.J.M., D.F. and D.M.Z.; writing: original draft, M.J.M., S.W., A.M.M., B.T. and D.F.; writing: review and editing, M.J.M., S.W., A.M.M., N.M.O., D.M.Z., S.M.N., T.F.G. and D.F. All authors have read and agreed to the published version of the manuscript.
Funding
Research conducted in this publication was funded by the Irish Research Council Postdoctoral Fellowship (GOIPD/2013/188; NMO′B), Irish Research Council Postgraduate Fellowship (GOIPG/2021/954; SK and DF) and Trinity College Dublin Postgraduate research scholarships (TG, SW).
Institutional Review Board Statement
Approval for this study was obtained from the School of Pharmacy and Pharmaceutical Sciences Trinity College Dublin Research Ethics Committee (2020-06-01-MS).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Postgraduate research scholarships from Trinity College Dublin (TG, SW), the Irish Research Council Postdoctoral Fellowship (GOIPD/2013/188; NMO′B) and the Irish Research Council Postgraduate Fellowship (GOIPG/2021/954; SK and DF) are gratefully acknowledged. We thank John O’Brien and Manuel Ruether for NMR spectroscopy. We thank Gavin McManus for assistance with confocal microscopy, Barry Moran for assistance with flow cytometry and Kathy Dillon for analytical contribution to the stability study. The authors are deeply grateful to the authority of the National Cancer Institute, Biological Testing Branch, Bethesda, MD, USA, for the cancer cell line screening. The Trinity Biomedical Sciences Institute (TBSI) is supported by a capital infrastructure investment from Cycle 5 of the Irish Higher Education Authority’s Programme for Research in Third Level Institutions (PRTLI). This study was also co-funded under the European Regional Development Fund. DF thanks the software vendors for their continuing support of academic research efforts, in particular the contributions of the Chemical Computing Group, Biovia and OpenEye Scientific. The support and provisions of Dell Ireland, the Trinity Centre for High Performance Computing (TCHPC) and the Irish Centre for High-End Computing (ICHEC) are also gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ADC | Antibody–drug conjugate |
| BRCA | Breast cancer gene |
| CA-4 | Combretastatin A-4 |
| CBSI | Colchicine-binding site inhibitor |
| CCDC | Cambridge Crystallographic Data Centre |
| CDK | Cyclin-dependent kinase |
| DAMA | N-deacetyl-N-(2-mercaptoacetyl)-colchicine |
| DCC | N,N’-Dicyclohexylcarbodiimide |
| DCM | Dichloromethane |
| DCTD | Division of Cancer Treatment and Diagnosis |
| DEPT | Distortionless enhancement by polarization transfer |
| DIPEA | N,N-diisopropylethylamine |
| DMAP | 4-Dimethylaminopyridine |
| DMF | Dimethylformamide |
| DTP | Development Therapeutics Program |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EBI | N,N′-Ethylene-bis(iodoacetamide) |
| ECACC | European Collection of Animal Cell Cultures |
| EI | Electron Impact |
| EMA | European Medicines Agency |
| ER | Estrogen receptor |
| FDA | United States Food and Drug Administration |
| Fmoc | Fluorenylmethyloxycarbonyl |
| GI50 | 50% growth inhibition |
| HER2 | Human epidermal growth factor receptor 2 |
| HRMS | High-resolution mass spectrometry |
| IC | Inhibitory concentration |
| IR | Infrared |
| LC50 | 50% lethal concentration |
| LDH | Lactate dehydrogenase |
| MBC | Metastatic breast cancer |
| m-CPBA | meta-Chloroperoxybenzoic acid |
| MDR | Multidrug resistance |
| MRP1, MRP2 | Multidrug-resistance-associated proteins |
| MTD | Maximum tolerated dose |
| MTA | Microtubule-targeting agent |
| MS | Mass spectrometry |
| NCI | National Cancer Institute |
| NIH | National Institute of Health |
| NMR | Nuclear magnetic resonance |
| PAINS | Pan-assay interference compounds |
| PARP | Poly(adenosine diphosphate–ribose) polymerase |
| PBS | Phosphate buffer saline |
| P-gp | P-glycoprotein |
| PI | Propidium iodide |
| PI3K | Phosphoinositide 3-kinase |
| PR | Progesterone receptor |
| SAR | Structure–activity relationship |
| SERD | Selective estrogen receptor degrader |
| TBA | Tubulin-binding agent |
| TBAF | Tetrabutylammonium fluoride |
| TBDMS | tert-Butyldimethylchlorosilane |
| TEA | Triethylamine |
| TGI | Total growth inhibition |
| TLC | Thin-layer chromatography |
| TMS | Tetramethylsilane |
| TMCS | Trimethylchlorosilane |
| UV | Ultraviolet |
| VDA | Vascular disrupting agent |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Narayan, P.; Osgood, C.L.; Wedam, S.; Prowell, T.M.; Gao, J.J.; Shah, M.; Krol, D.; Wahby, S.; Royce, M.; et al. U.S. FDA drug approvals for breast cancer: A decade in review. Clin. Cancer Res. 2022, 28, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Duranti, S.; Fabi, A.; Filetti, M.; Falcone, R.; Lombardi, P.; Daniele, G.; Franceschini, G.; Carbognin, L.; Palazzo, A.; Garganese, G.; et al. Breast cancer drug approvals issued by EMA: A review of clinical trials. Cancers 2021, 13, 5198. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Beeram, M.; Beck, J.T.; Conlin, A.; Dees, E.C.; Puhalla, S.L.; Rexer, B.N.; Burris, H.A.; Jhaveri, K.; Helsten, T.; et al. Abemaciclib in combination with endocrine therapy for patients with hormone receptor-positive, HER2-negative metastatic breast cancer: A phase Ib study. Front. Oncol. 2021, 11, 810023. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Zangardi, M.; Bardia, A. CDK4/6 inhibitors in breast cancer: Current controversies and future directions. Curr. Oncol. Rep. 2019, 21, 25. [Google Scholar] [CrossRef]
- Narayan, P.; Prowell, T.M.; Gao, J.J.; Fernandes, L.L.; Li, E.; Jiang, X.; Qiu, J.; Fan, J.; Song, P.; Yu, J.; et al. FDA approval summary: Alpelisib plus fulvestrant for patients with HR-positive, HER2-negative, PIK3CA-mutated, advanced or metastatic breast cancer. Clin. Cancer Res. 2021, 27, 1842–1849. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: Results from the randomized phase III emerald trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, X.; Feng, B. A review of research progress of antitumor drugs based on tubulin targets. Transl. Cancer Res. 2020, 9, 4020–4027. [Google Scholar] [CrossRef]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorg. Med. Chem. 2014, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-targeting agents: Strategies to hijack the cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Chen, Q.Y.; Ratnayake, R.; Fermaintt, C.S.; Lucena-Agell, D.; Bonato, F.; Prota, A.E.; Lim, S.T.; Wang, X.; Diaz, J.F.; et al. Gatorbulin-1, a distinct cyclodepsipeptide chemotype, targets a seventh tubulin pharmacological site. Proc. Natl. Acad. Sci. USA 2021, 118, e2021847118. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Bargsten, K.; Northcote, P.T.; Marsh, M.; Altmann, K.H.; Miller, J.H.; Diaz, J.F.; Steinmetz, M.O. Structural basis of microtubule stabilization by laulimalide and peloruside a. Angew. Chem. Int. Ed. Engl. 2014, 53, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Bargsten, K.; Diaz, J.F.; Marsh, M.; Cuevas, C.; Liniger, M.; Neuhaus, C.; Andreu, J.M.; Altmann, K.H.; Steinmetz, M.O. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc. Natl. Acad. Sci. USA 2014, 111, 13817–13821. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Wang, T.; Jiang, J.; Botting, C.H.; Liu, H.; Chen, Q.; Yang, J.; Naismith, J.H.; Zhu, X.; et al. Pironetin reacts covalently with cysteine-316 of alpha-tubulin to destabilize microtubule. Nat. Commun. 2016, 7, 12103. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.; Li, Y.; Yan, W.; Ye, H.; Niu, L.; Tang, M.; Wang, Z.; Yang, Z.; Pei, H.; et al. Cevipabulin-tubulin complex reveals a novel agent binding site on alpha-tubulin with tubulin degradation effect. Sci. Adv. 2021, 7, eabg4168. [Google Scholar] [CrossRef]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Cortes, J.; Schoffski, P.; Littlefield, B.A. Multiple modes of action of eribulin mesylate: Emerging data and clinical implications. Cancer Treat Rev. 2018, 70, 190–198. [Google Scholar] [CrossRef]
- Shingaki, S.; Kogawa, T.; Shimokawa, M.; Harano, K.; Naito, Y.; Kusuhara, S.; Fujimoto, Y.; Matsubara, N.; Hosono, A.; Mukai, H.; et al. Use of eribulin as an earlier-line chemotherapy for patients with HER2-negative metastatic breast cancer. J. Cancer 2020, 11, 4099–4105. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-binding site inhibitors from chemistry to clinic: A review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef]
- Krajewska, J.J.B. Fosbretabulin tromethamine in the treatment of thyroid cancer. Expert Opin. Orphan Drugs 2016, 4, 555–561. [Google Scholar] [CrossRef]
- Zhang, S.; Li, T.; Pang, W.; Wu, J.; Wu, F.; Liu, Y.; Wu, F. Synthesis, biological evaluation and molecular docking studies of combretastatin A-4 phosphoramidates as novel anticancer prodrugs. Med. Chem. Res. 2020, 29, 2192–2202. [Google Scholar] [CrossRef]
- Hamze, A.; Alami, M.; Provot, O. Developments of isocombretastatin A-4 derivatives as highly cytotoxic agents. Eur. J. Med. Chem. 2020, 190, 112110. [Google Scholar] [CrossRef]
- Liu, L.; Schuetze, R.; Gerberich, J.L.; Lopez, R.; Odutola, S.O.; Tanpure, R.P.; Charlton-Sevcik, A.K.; Tidmore, J.K.; Taylor, E.A.; Kapur, P.; et al. Demonstrating tumor vascular disrupting activity of the small-molecule dihydronaphthalene tubulin-binding agent Oxi6196 as a potential therapeutic for cancer treatment. Cancers 2022, 14, 4208. [Google Scholar] [CrossRef]
- Le-Vinh, B.; Akkus-Dagdeviren, Z.B.; Le, N.M.N.; Nazir, I.; Bernkop-Schnurch, A. A reliable endogenous partner for drug delivery and diagnostics. Adv. Ther. 2022, 5, 2100219. [Google Scholar] [CrossRef]
- Pettit, G.R.; Toki, B.; Herald, D.L.; Verdier-Pinard, P.; Boyd, M.R.; Hamel, E.; Pettit, R.K. Antineoplastic agents. 379. Synthesis of phenstatin phosphate. J. Med. Chem. 1998, 41, 1688–1695. [Google Scholar] [CrossRef]
- Ducki, S.; Rennison, D.; Woo, M.; Kendall, A.; Chabert, J.F.; McGown, A.T.; Lawrence, N.J. Combretastatin-like chalcones as inhibitors of microtubule polymerization. Part 1: Synthesis and biological evaluation of antivascular activity. Bioorg. Med. Chem. 2009, 17, 7698–7710. [Google Scholar] [CrossRef]
- Gaspari, R.; Prota, A.E.; Bargsten, K.; Cavalli, A.; Steinmetz, M.O. Structural basis of cis- and trans-combretastatin binding to tubulin. Chem 2017, 2, 102–113. [Google Scholar] [CrossRef]
- Xia, L.Y.; Zhang, Y.L.; Yang, R.; Wang, Z.C.; Lu, Y.D.; Wang, B.Z.; Zhu, H.L. Tubulin inhibitors binding to colchicine-site: A review from 2015 to 2019. Curr. Med. Chem. 2020, 27, 6787–6814. [Google Scholar] [CrossRef] [PubMed]
- La Regina, G.; Coluccia, A.; Naccarato, V.; Silvestri, R. Towards modern anticancer agents that interact with tubulin. Eur. J. Pharm. Sci. 2019, 131, 58–68. [Google Scholar] [CrossRef]
- Romagnoli, R.; Oliva, P.; Prencipe, F.; Manfredini, S.; Budassi, F.; Brancale, A.; Ferla, S.; Hamel, E.; Corallo, D.; Aveic, S.; et al. Design, synthesis and biological investigation of 2-anilino triazolopyrimidines as tubulin polymerization inhibitors with anticancer activities. Pharmaceuticals 2022, 15, 1031. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, R.; Oliva, P.; Salvador, M.K.; Manfredini, S.; Padroni, C.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; et al. A facile synthesis of diaryl pyrroles led to the discovery of potent colchicine site antimitotic agents. Eur. J. Med. Chem. 2021, 214, 113229. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Krutilina, R.I.; Wang, Q.; Lin, Z.; Parke, D.N.; Playa, H.C.; Chen, H.; Miller, D.D.; Seagroves, T.N.; Li, W. An orally available tubulin inhibitor, VERU-111, suppresses triple-negative breast cancer tumor growth and metastasis and bypasses taxane resistance. Mol. Cancer Ther. 2020, 19, 348–363. [Google Scholar] [CrossRef] [PubMed]
- Krutilina, R.I.; Hartman, K.L.; Oluwalana, D.; Playa, H.C.; Parke, D.N.; Chen, H.; Miller, D.D.; Li, W.; Seagroves, T.N. Sabizabulin, a potent orally bioavailable colchicine binding site agent, suppresses HER2+ breast cancer and metastasis. Cancers 2022, 14, 5336. [Google Scholar] [CrossRef]
- Dreicer, R.C.; Chu, F.; Cahn, D.J.; Getzenberg, R.H.; Rodriguez, D.; Barnette, K.G.; Steiner, M.S.; Saltzstein, D.R.; Tutrone, R.F.; Shore, N.D. Phase 3 veracity clinical study of sabizabulin in men with metastatic castrate resistant prostate cancer who have progressed on an androgen receptor targeting agent. J. Clin. Oncol. 2022, 40, TPS217 suppl. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Ma, L.; Jiang, X.; Wu, C.; Wang, Y.; Jiang, Y.; Zheng, W.; Yang, Y.; Ma, Y.; et al. Molecular mechanism of crolibulin in complex with tubulin provides a rationale for drug design. Biochem. Biophys. Res. Commun. 2019, 511, 381–386. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Gigant, B.; Yu, Y.; Wu, Y.; Chen, X.; Lai, Q.; Yang, Z.; Chen, Q.; Yang, J. Structures of a diverse set of colchicine binding site inhibitors in complex with tubulin provide a rationale for drug discovery. FEBS J. 2016, 283, 102–111. [Google Scholar] [CrossRef]
- Bohnacker, T.; Prota, A.E.; Beaufils, F.; Burke, J.E.; Melone, A.; Inglis, A.J.; Rageot, D.; Sele, A.M.; Cmiljanovic, V.; Cmiljanovic, N.; et al. Deconvolution of buparlisib’s mechanism of action defines specific PI3K and tubulin inhibitors for therapeutic intervention. Nat. Commun. 2017, 8, 14683. [Google Scholar] [CrossRef]
- Deng, S.; Banerjee, S.; Chen, H.; Pochampally, S.; Wang, Y.; Yun, M.K.; White, S.W.; Parmar, K.; Meibohm, B.; Hartman, K.L.; et al. SB226, an inhibitor of tubulin polymerization, inhibits paclitaxel-resistant melanoma growth and spontaneous metastasis. Cancer Lett. 2023, 555, 216046. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Josa-Prado, F.; Beauvineau, C.; Naud-Martin, D.; Vanwonterghem, L.; Mahuteau-Betzer, F.; Moreno, A.; Falson, P.; Lafanechere, L.; Frachet, V.; et al. Identification of pyrrolopyrimidine derivative PP-13 as a novel microtubule-destabilizing agent with promising anticancer properties. Sci. Rep. 2017, 7, 10209. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Du, T.; Zhang, J.; Wu, D.; Tian, H.; Zhang, K.; Jiang, L.; Lu, D.; Sheng, L.; Li, Y.; et al. Optimization of benzamide derivatives as potent and orally active tubulin inhibitors targeting the colchicine binding site. J. Med. Chem. 2022, 65, 16372–16391. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.H.; Ma, L.L.; Xian, J.H.; Chen, H.; Zhou, J.J.; Chen, H.; Lei, Q.; Li, Y.Y.; Wang, Y.Y.; Wang, Y.X. Structural insights into targeting of the colchicine binding site by ELR510444 and parbendazole to achieve rational drug design. RSC Adv. 2021, 11, 18938–18944. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wu, C.; Zhang, J.; Yu, Q.; Wang, X.; Zhang, L.; Ge, M.; Wang, Z.; Ouyang, L.; Wang, Y. Design, synthesis, and biological evaluation of heterocyclic-fused pyrimidine chemotypes guided by x-ray crystal structure with potential antitumor and anti-multidrug resistance efficacy targeting the colchicine binding site. J. Med. Chem. 2023, 66, 3588–3620. [Google Scholar] [CrossRef]
- Fu, D.J.; Zhang, Y.F.; Chang, A.Q.; Li, J. Beta-lactams as promising anticancer agents: Molecular hybrids, structure activity relationships and potential targets. Eur. J. Med. Chem. 2020, 201, 112510. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.P.; Pathak, P.; Grishina, M.; et al. Nitrogen containing heterocycles as anticancer agents: A medicinal chemistry perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef]
- Tripodi, F.; Pagliarin, R.; Fumagalli, G.; Bigi, A.; Fusi, P.; Orsini, F.; Frattini, M.; Coccetti, P. Synthesis and biological evaluation of 1,4-diaryl-2-azetidinones as specific anticancer agents: Activation of adenosine monophosphate activated protein kinase and induction of apoptosis. J. Med. Chem. 2012, 55, 2112–2124. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, Y.; Zhou, L.; Zhu, K.; Feng, K.; Zhang, H.; Liang, Y.; Jiang, H.; Luo, C.; Liu, M.; et al. Potent antitumor activities and structure basis of the chiral beta-lactam bridged analogue of combretastatin A4 binding to tubulin. J. Med. Chem. 2016, 59, 10329–10334. [Google Scholar] [CrossRef]
- Zhou, P.; Liang, Y.; Zhang, H.; Jiang, H.; Feng, K.; Xu, P.; Wang, J.; Wang, X.; Ding, K.; Luo, C.; et al. Design, synthesis, biological evaluation and cocrystal structures with tubulin of chiral beta-lactam bridged combretastatin A-4 analogues as potent antitumor agents. Eur. J. Med. Chem. 2018, 144, 817–842. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, J.; Liang, Y.; Wang, Y. Discovery of a chiral fluorinated azetidin-2-one as a tubulin polymerisation inhibitor with potent antitumour efficacy. Eur. J. Med. Chem. 2020, 197, 112323. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Carr, M.; Greene, L.M.; Bergin, O.; Nathwani, S.M.; McCabe, T.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Synthesis and evaluation of azetidinone analogues of combretastatin A-4 as tubulin targeting agents. J. Med. Chem. 2010, 53, 8569–8584. [Google Scholar] [CrossRef] [PubMed]
- Greene, T.F.; Wang, S.; Greene, L.M.; Nathwani, S.M.; Pollock, J.K.; Malebari, A.M.; McCabe, T.; Twamley, B.; O’Boyle, N.M.; Zisterer, D.M.; et al. Synthesis and biochemical evaluation of 3-phenoxy-1,4-diarylazetidin-2-ones as tubulin-targeting antitumor agents. J. Med. Chem. 2016, 59, 90–113. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Duffy Morales, G.; Twamley, B.; Fayne, D.; Khan, M.F.; McLoughlin, E.C.; O’Boyle, N.M.; Zisterer, D.M.; Meegan, M.J. Synthesis, characterisation and mechanism of action of anticancer 3-fluoroazetidin-2-ones. Pharmaceuticals 2022, 15, 1044. [Google Scholar] [CrossRef]
- Mora-Ochomogo, M.L.; Lohans, C.T. Β-lactam antibiotic targets and resistance mechanisms: From covalent inhibitors to substrates. RSC Med.Chem. 2021, 12, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I.; Delaloge, F. Asymmetric synthesis of building-blocks for peptides and peptidomimetics by means of the beta-lactam synthon method. Chem. Soc. Rev. 1997, 26, 377–386. [Google Scholar] [CrossRef]
- Singh, G.S.; Sudheesh, S. Advances in synthesis of monocyclic beta-lactams. Arkivoc 2014, 337–385. [Google Scholar] [CrossRef]
- Kamath, A.; Ojima, I. Advances in the chemistry of beta-lactam and its medicinal applications. Tetrahedron 2012, 68, 10640–10664. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, J.; Huang, H. Carbonylative formal cycloaddition between alkylarenes and aldimines enabled by palladium-catalyzed double c-h bond activation. J. Am. Chem. Soc. 2023, 145, 4982–4988. [Google Scholar] [CrossRef]
- Zamboni, R.; Just, G. Beta-lactams.7. Synthesis of 3-vinyl and 3-isopropenyl 4-substituted azetidinones. Can. J. Chem. 1979, 57, 1945–1948. [Google Scholar] [CrossRef]
- Wang, S.; Malebari, A.M.; Greene, T.F.; O’Boyle, N.M.; Fayne, D.; Nathwani, S.M.; Twamley, B.; McCabe, T.; Keely, N.O.; Zisterer, D.M.; et al. 3-vinylazetidin-2-ones: Synthesis, antiproliferative and tubulin destabilizing activity in MCF-7 and MDA-MB-231 breast cancer cells. Pharmaceuticals 2019, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Malebari, A.M.; Wang, S.; Meegan, M.J. Synthesis and biological evaluation of novel 3-isopropenyl-β- lactams: Heterocyclic bridged analogues of combretastatin A-4 as novel antimitotic agents in breast cancer . Med. Sci. Forum 2022, 14, 72. [Google Scholar]
- Ohsumi, K.; Nakagawa, R.; Fukuda, Y.; Hatanaka, T.; Morinaga, Y.; Nihei, Y.; Ohishi, K.; Suga, Y.; Akiyama, Y.; Tsuji, T. Novel combretastatin analogues effective against murine solid tumors: Design and structure-activity relationships. J. Med. Chem. 1998, 41, 3022–3032. [Google Scholar] [CrossRef] [PubMed]
- Combes, S.; Barbier, P.; Douillard, S.; McLeer-Florin, A.; Bourgarel-Rey, V.; Pierson, J.T.; Fedorov, A.Y.; Finet, J.P.; Boutonnat, J.; Peyrot, V. Synthesis and biological evaluation of 4-arylcoumarin analogues of combretastatins. Part 2. J. Med. Chem. 2011, 54, 3153–3162. [Google Scholar] [CrossRef]
- Lara-Ochoa, F.; Espinosa-Perez, G. A new synthesis of combretastatins A-4 and AVE-8062A. Tetrahedron Lett. 2007, 48, 7007–7010. [Google Scholar] [CrossRef]
- Spek, A.L.; Vandersteen, F.H.; Jastrzebski, J.T.B.H.; Vankoten, G. Trans-3-amino-1-methyl-4-phenyl-2-azetidinone, C10H12N2O. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1994, 50, 1933–1935. [Google Scholar] [CrossRef]
- Kabak, M.; Senoz, H.; Elmali, A.; Adar, V.; Svoboda, I.; Dusek, M.; Fejfarova, K. Synthesis and x-ray crystal structure determination of N-p-methylphenyl-4-benzoyl-3,4-diphenyl-2-azetidinone. Crystallogr. Rep. 2010, 55, 1220–1222. [Google Scholar] [CrossRef]
- Wang, S.; Malebari, A.M.; Greene, T.F.; O’Boyle, N.M.; Fayne, D.F.; Nathwani, S.M.; Twamley, B.; McCabe, T.; Keely, N.O.; Zisterer, D.M.; et al. CCDC 1820359: Experimental Crystal Structure Determination. 2020. Available online: https://10.5517/ccdc.csd.cc1z378l (accessed on 4 July 2023).
- Georg, G.I.; Mashava, P.M.; Guan, X. An improved method for the stereoselective synthesis of β-lactams from carboxylic acids and imines. Tetrahedron Lett. 1991, 32, 581–584. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mazumdar, S.N.; Mahajan, M.P. A convenient trans diastereoselective synthesis of 3-butadienylazetidinones and their diels-alder cycloaddition reactions. J. Org. Chem. 1996, 61, 5506–5509. [Google Scholar] [CrossRef]
- Li, Z.; Song, L.; Li, C. Silver-catalyzed radical aminofluorination of unactivated alkenes in aqueous media. J. Am. Chem. Soc. 2013, 135, 4640–4643. [Google Scholar] [CrossRef]
- Ohsumi, K.; Hatanaka, T.; Nakagawa, R.; Fukuda, Y.; Morinaga, Y.; Suga, Y.; Nihei, Y.; Ohishi, K.; Akiyama, Y.; Tsuji, T. Synthesis and antitumor activities of amino acid prodrugs of amino-combretastatins. Anticancer Drug Des. 1999, 14, 539–548. [Google Scholar] [PubMed]
- McLoughlin, E.C.; O’Brien, J.E.; Trujillo, C.; Meegan, M.J.; O’Boyle, N.M. Application of 2D EXSY and qNMR spectroscopy for diastereomeric excess determination following chiral resolution of beta-lactams. ChemistryOpen 2023, 12, e202200119. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Lippert, J.W. Antineoplastic agents 429. Syntheses of the combretastatin A-1 and combretastatin B1 prodrugs. Anti-Cancer Drug Des. 2000, 15, 203–216. [Google Scholar]
- Shankar, B.B.; Kirkup, M.P.; McCombie, S.W.; Clader, J.W.; Ganguly, A.K. Synthesis of an optically 3-unsubstituted β-lactam using an asymmetric Reformasky reaction and its conversion to cholesterol absorption inhibitors. Tetrahedron Lett. 1996, 37, 4095–4098. [Google Scholar] [CrossRef]
- Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W.; Aisa, H.A. Substitution effect of the trifluoromethyl group on the bioactivity in medicinal chemistry: Statistical analysis and energy calculations. J. Chem. Inf. Model 2020, 60, 6242–6250. [Google Scholar] [CrossRef]
- Anand, A.; Bhargava, G.; Singh, P.; Mehra, S.; Kumar, V.; Mahajan, M.P.; Singh, P.; Bisetty, K. Regio- and diastereoselective nitroso diels-alder cycloaddition reactions of 3-dienyl-2-azetidinones with nitrosoarenes. Lett. Org. Chem. 2012, 9, 411–421. [Google Scholar] [CrossRef]
- Bhargava, G.; Mahajan, M.P.; Saito, T.; Otani, T.; Takashi, O.; Kurashima, M.; Sakai, K. Highly diastereoselective and remarkably π-facially selective lewis acid-catalyzed diels-alder cycloaddition reactions: Access to novel 1,3,4-trisubstituted 2-azetidinones. Eur. J. Org. Chem. 2005, 2005, 2397–2405. [Google Scholar] [CrossRef]
- Medarde, M.; Maya, A.B.; Perez-Melero, C. Naphthalene combretastatin analogues: Synthesis, cytotoxicity and antitubulin activity. J. Enzym. Inhib. Med. Chem. 2004, 19, 521–540. [Google Scholar] [CrossRef]
- Malebari, A.M.; Greene, L.M.; Nathwani, S.M.; Fayne, D.; O’Boyle, N.M.; Wang, S.; Twamley, B.; Zisterer, D.M.; Meegan, M.J. Beta-lactam analogues of combretastatin A-4 prevent metabolic inactivation by glucuronidation in chemoresistant HT-29 colon cancer cells. Eur. J. Med. Chem. 2017, 130, 261–285. [Google Scholar] [CrossRef]
- Cushman, M.; Nagarathnam, D.; Gopal, D.; He, H.M.; Lin, C.M.; Hamel, E. Synthesis and evaluation of analogues of (z)-1-(4-methoxyphenyl)-2-(3,4,5-trimethoxyphenyl)ethene as potential cytotoxic and antimitotic agents. J. Med. Chem. 1992, 35, 2293–2306. [Google Scholar] [CrossRef]
- Flynn, B.L.; Flynn, G.P.; Hamel, E.; Jung, M.K. The synthesis and tubulin binding activity of thiophene-based analogues of combretastatin A-4. Bioorg. Med. Chem. Lett. 2001, 11, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Devkota, L.; Lin, C.M.; Strecker, T.E.; Wang, Y.; Tidmore, J.K.; Chen, Z.; Guddneppanavar, R.; Jelinek, C.J.; Lopez, R.; Liu, L.; et al. Design, synthesis, and biological evaluation of water-soluble amino acid prodrug conjugates derived from combretastatin, dihydronaphthalene, and benzosuberene-based parent vascular disrupting agents. Bioorg. Med. Chem. 2016, 24, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Rustin, G.J.; Galbraith, S.M.; Anderson, H.; Stratford, M.; Folkes, L.K.; Sena, L.; Gumbrell, L.; Price, P.M. Phase I clinical trial of weekly combretastatin A4 phosphate: Clinical and pharmacokinetic results. J. Clin. Oncol. 2003, 21, 2815–2822. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Greene, L.M.; Bergin, O.; Fichet, J.B.; McCabe, T.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Synthesis, evaluation and structural studies of antiproliferative tubulin-targeting azetidin-2-ones. Bioorg. Med. Chem. 2011, 19, 2306–2325. [Google Scholar] [CrossRef] [PubMed]
- WHO Breast Cancer. 2023. Available online: https://www.Who.Int/news-room/fact-sheets/detail/breast-cancer (accessed on 23 February 2023).
- Messaoudi, S.; Treguier, B.; Hamze, A.; Provot, O.; Peyrat, J.F.; De Losada, J.R.; Liu, J.M.; Bignon, J.; Wdzieczak-Bakala, J.; Thoret, S.; et al. Isocombretastatins A versus combretastatins A: The forgotten isoCA-4 isomer as a highly promising cytotoxic and antitubulin agent. J. Med. Chem. 2009, 52, 4538–4542. [Google Scholar] [CrossRef] [PubMed]
- Mousset, C.; Giraud, A.; Provot, O.; Hamze, A.; Bignon, J.; Liu, J.M.; Thoret, S.; Dubois, J.; Brion, J.D.; Alami, M. Synthesis and antitumor activity of benzils related to combretastatin A-4. Bioorg. Med. Chem. Lett. 2008, 18, 3266–3271. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.3ds.Com/fileadmin/products-services/biovia/pdf/biovia-pipeline%20pilot-pipeline-pilot-overview.Pdf (accessed on 4 May 2023).
- Baell, J.B.; Nissink, J.W.M. Seven year itch: Pan-assay interference compounds (PAINS) in 2017-utility and limitations. ACS Chem. Biol. 2018, 13, 36–44. [Google Scholar] [CrossRef]
- Davis, A.; Ward, S.E. The Handbook of Medicinal Chemistry: Principles and Practice; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- National Cancer Institute, DCTD Division of Cancer Treatment & Diagnosis, DTP Developmental Therapeutics Program. Available online: https://dtp.Cancer.Gov (accessed on 4 May 2023).
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- National Cancer Institute Biological Testing Branch; National Cancer Institute; Bethesda, MD. Available online: https://dtp.Nci.Nih.Gov/branches/btb/hfa.Html (accessed on 4 May 2023).
- Compare Analysis. Available online: https://dtp.Cancer.Gov/databases_tools/compare.Htm (accessed on 4 May 2023).
- Parhamifar, L.; Andersen, H.; Moghimi, S.M. Lactate dehydrogenase assay for assessment of polycation cytotoxicity. Methods Mol. Biol. 2013, 948, 13–22. [Google Scholar]
- Greene, L.M.; O’Boyle, N.M.; Nolan, D.P.; Meegan, M.J.; Zisterer, D.M. The vascular targeting agent combretastatin-A4 directly induces autophagy in adenocarcinoma-derived colon cancer cells. Biochem. Pharmacol. 2012, 84, 612–624. [Google Scholar] [CrossRef]
- Tubulin Polymerization Assay Kit Manual (CDS03 and BK006); Cytoskeleton: Denver, CO, USA, 2009; pp. 1–18.
- Fortin, S.; Lacroix, J.; Cote, M.F.; Moreau, E.; Petitclerc, E.C.; Gaudreault, R. Quick and simple detection technique to assess the binding of antimicrotubule agents to the colchicine-binding site. Biol. Proced. Online 2010, 12, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Canela, M.D.; Perez-Perez, M.J.; Noppen, S.; Saez-Calvo, G.; Diaz, J.F.; Camarasa, M.J.; Liekens, S.; Priego, E.M. Novel colchicine-site binders with a cyclohexanedione scaffold identified through a ligand-based virtual screening approach. J. Med. Chem. 2014, 57, 3924–3938. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Tsvetkov, P.O.; Breuzard, G.; Devred, F. Deciphering the molecular mechanisms of anti-tubulin plant derived drugs. Phytochem. Rev. 2014, 13, 157–169. [Google Scholar] [CrossRef]
- Castedo, M.; Perfettini, J.-L.; Roumier, T.; Andreau, K.; Medema, R.; Kroemer, G. Cell death by mitotic catastrophe: A molecular definition. Oncogene 2004, 23, 2825–2837. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Carr, M.; Greene, L.M.; Knox, A.J.S.; Lloyd, D.G.; Zisterer, D.M.; Meegan, M.J. Synthesis, biochemical and molecular modelling studies of antiproliferative azetidinones causing microtubule disruption and mitotic catastrophe. Eur. J. Med. Chem. 2011, 46, 4595–4607. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE) Version 2022.02; Chemical Computing Group Inc.: Montreal, QC, Canada, 2022.
- Malebari, A.M.; Wang, S.; Greene, T.F.; O’Boyle, N.M.; Fayne, D.; Khan, M.F.; Nathwani, S.M.; Twamley, B.; McCabe, T.; Zisterer, D.M.; et al. Synthesis and antiproliferative evaluation of 3-chloroazetidin-2-ones with antimitotic activity: Heterocyclic bridged analogues of combretastatin A-4. Pharmaceuticals 2021, 14, 1119. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Fayne, D. Mapping of protein binding sites using clustering algorithms—Development of a pharmacophore based drug discovery tool. J. Mol. Graph Model 2022, 115, 108228. [Google Scholar] [CrossRef]
- Promega Corporation, Cytotox 96® Non-Radioactive Cytotoxicity Assay; Promega Cytotox 96 Nonradioactive Cytotoxicity Assay Protocol.Pdf. Available online: https://worldwide.promega.com/products/cell-health-assays/cell-viability-and-cytotoxicity-assays/cytotox-96-non_radioactive-cytotoxicity-assay/?catNum=G1780 (accessed on 11 July 2022).
- Bruker, APEX3; Bruker AXS Inc.: Madison, WI, USA, 2017.
- Bruker, APEX4; Bruker AXS Inc.: Madison, WI, USA, 2021.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus x-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Guo, K.; Ma, X.; Li, J.; Zhang, C.; Wu, L. Recent advances in combretastatin A-4 codrugs for cancer therapy. Eur. J. Med. Chem. 2022, 241, 114660. [Google Scholar] [CrossRef]
- Tozer, G.M.; Kanthou, C.; Baguley, B.C. Disrupting tumour blood vessels. Nat. Rev. Cancer 2005, 5, 423–435. [Google Scholar] [CrossRef]
- Greene, L.M.; Meegan, M.J.; Zisterer, D.M. Combretastatins: More than just vascular targeting agents? J. Pharmacol. Exp. Ther. 2015, 355, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2017, 83, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Sabizabulin for COVID-19. Available online: https://verupharma.Com/pipeline/veru-111-for-covid-19/ (accessed on 18 January 2023).
- Furst, R.; Vollmar, A.M. A new perspective on old drugs: Non-mitotic actions of tubulin-binding drugs play a major role in cancer treatment. Pharmazie 2013, 68, 478–483. [Google Scholar]
- Arnst, K.E.; Banerjee, S.; Chen, H.; Deng, S.; Hwang, D.J.; Li, W.; Miller, D.D. Current advances of tubulin inhibitors as dual acting small molecules for cancer therapy. Med. Res. Rev. 2019, 39, 1398–1426. [Google Scholar] [CrossRef]
- Huang, L.; Huang, J.; Nie, H.; Li, Y.; Song, L.; Wu, F. Design, synthesis and biological evaluation of combretastatin A-4 sulfamate derivatives as potential anti-cancer agents. RSC Med. Chem. 2021, 12, 1374–1380. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Q.; Li, W. Recent advances in heterocyclic tubulin inhibitors targeting the colchicine binding site. Anticancer Agents Med. Chem. 2016, 16, 1325–1338. [Google Scholar] [CrossRef]
- Fong, A.; Durkin, A.; Lee, H. The potential of combining tubulin-targeting anticancer therapeutics and immune therapy. Int. J. Mol. Sci. 2019, 20, 586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).