Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances
Abstract
1. Introduction
- -
- -
- -
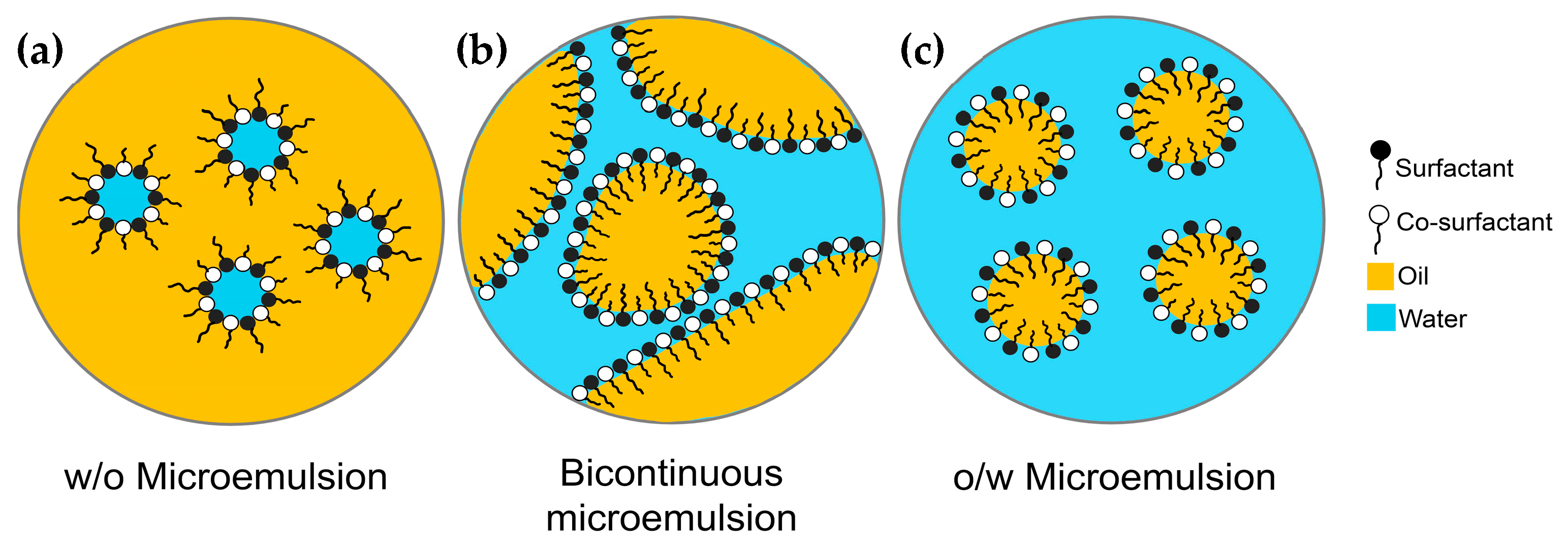
- Conductivity measurement serves as a means to differentiate ME types. The o/w MEs, with water as the external phase, exhibit conductivity, whereas the w/o MEs, with oil as the external phase, act as insulators. This technique is useful for investigating ME structure and phase transitions with increasing water content [47,48].
- -
- Electron microscopy techniques such as transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are commonly used to assess ME morphology [49,50,51,52], providing higher resolution than optical microscopes [39,53]. TEM images are two-dimensional, generated by transmitting electrons through the sample. These images often require staining with heavy metal compounds, such as uranyl acetate or phosphotungstic acid, as contrast agents and the samples are dried before measurement [54,55,56]. SEM is employed to directly visualize the surface features of samples by generating three-dimensional images through the detection of backscattered and secondary electrons [39,53]. Cryo- and freeze-fracture techniques have been developed to minimize the drying artifacts when investigating ME structures using these methods [52,57,58,59,60,61].
- -
- -
- DLS is employed to analyze droplet size and polydispersity index (PDI) of MEs by detecting scattered light intensity as particles undergo Brownian motion. The hydrodynamic radius was calculated using the Stokes-Einstein equation [65]. PDI values range from 0 to 1, with values below 0.1 indicating high monodispersity, 0.1 to 0.4 indicating moderate polydispersity, and values above 0.4 indicating high polydispersity [65,66].
- -
- Zeta potential (ZP) is utilized to predict colloidal dispersion stability, with its value dependent on pH, ionic strength, and particle concentration. ZP measurement is common for o/w MEs; however, some studies have reported ZP values for w/o MEs [54,59,67,68,69]. ZP values greater than ±30 mV indicate excellent stability [65]. Other factors, such as steric hindrance, also contribute to the stability of colloidal dispersions stabilized by nonionic surfactants [70].
- -
- DSC is employed to characterize MEs and differentiate between “bulk” and “bound” water [40,71,72]. Water in o/w MEs exhibits properties similar to those of pure water, while bound water in w/o MEs exhibits different characteristics [40,43], resulting in a lower freezing peak of water in w/o MEs compared to o/w MEs [73,74]. However, the addition of co-solvents such as pentylene glycol or propylene glycol (PG) may affect the freezing peak in the DSC thermogram [73,74].
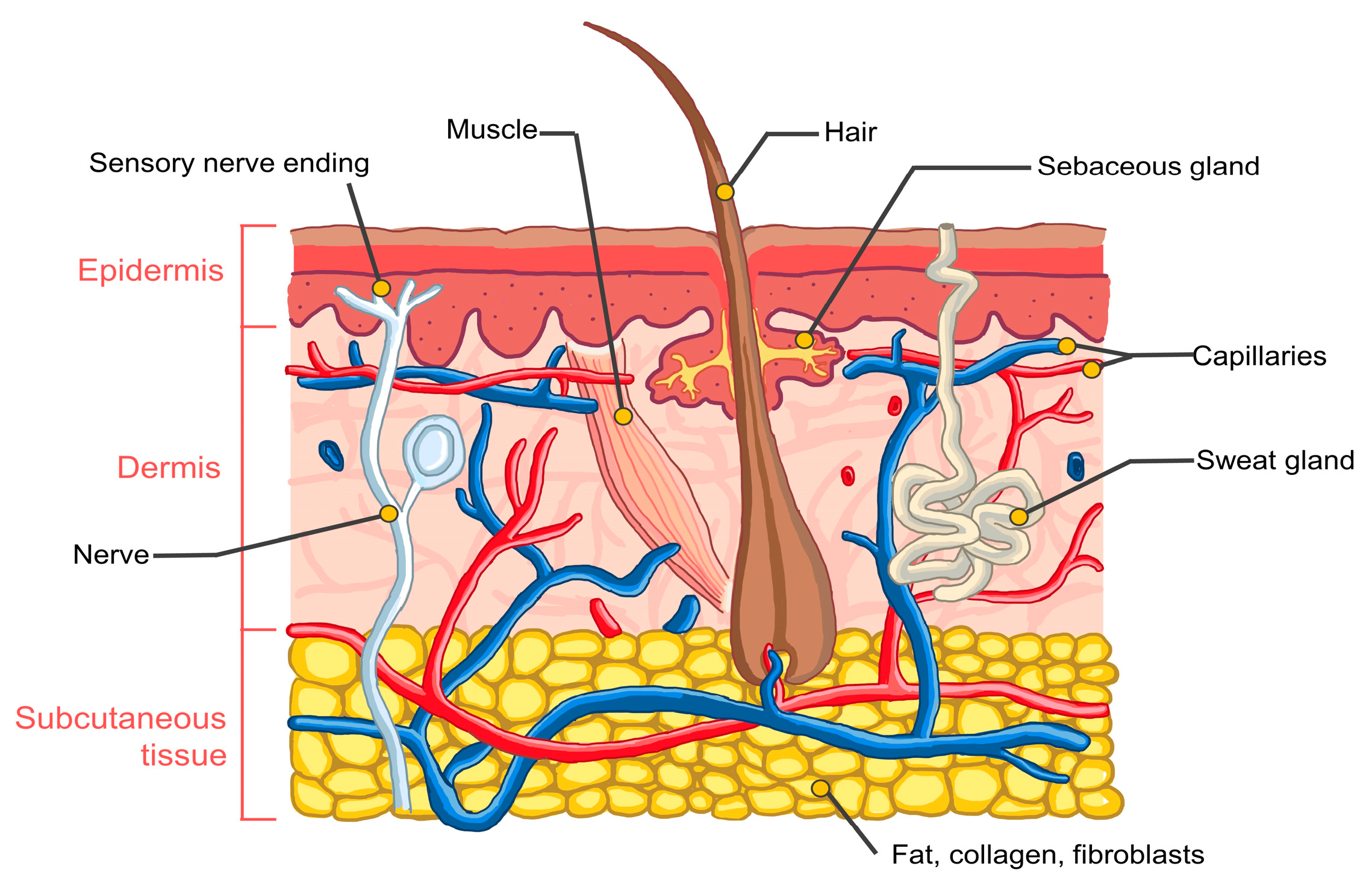
2. Human Skin Structure
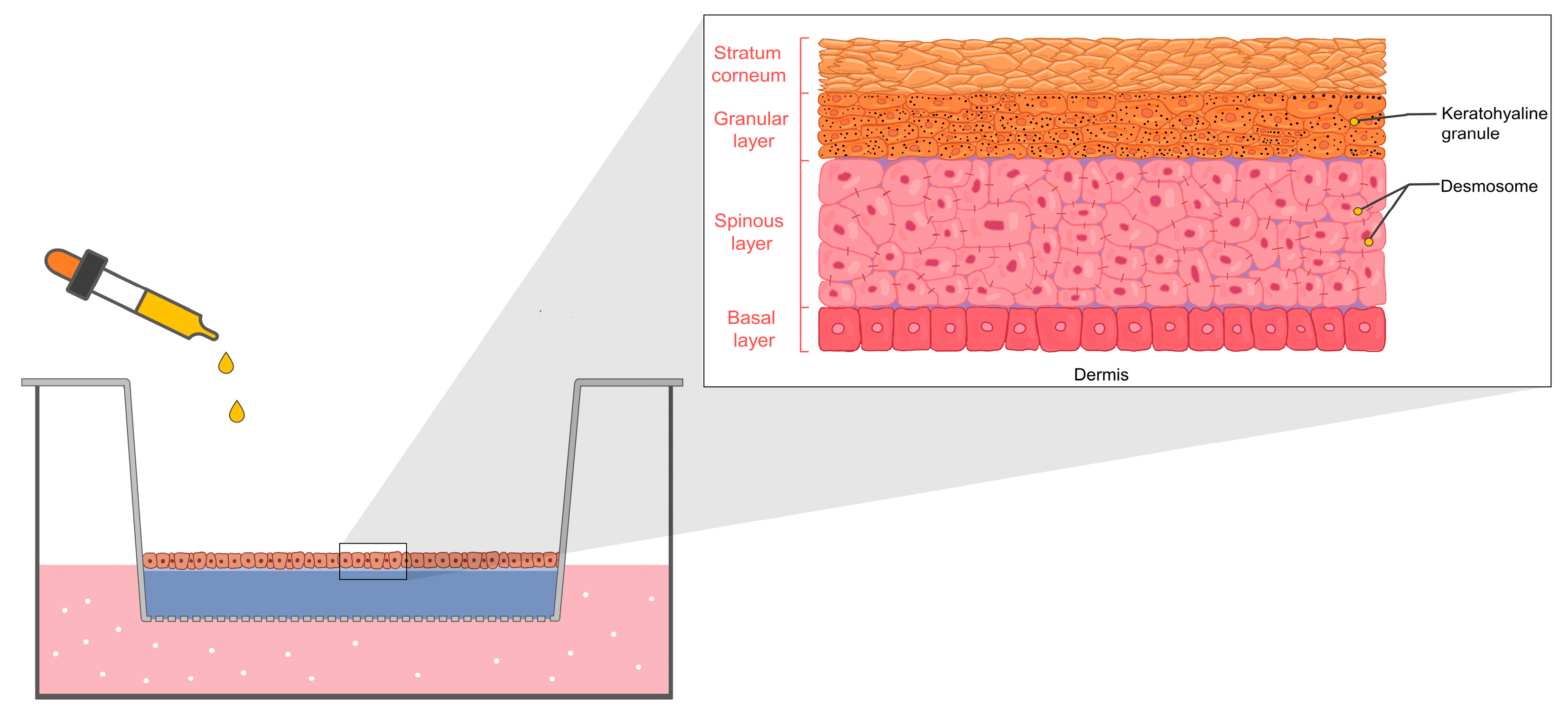
3. Skin Irritation and Mechanism
4. Skin Irritation Potential of MEs Containing Herbal Extracts
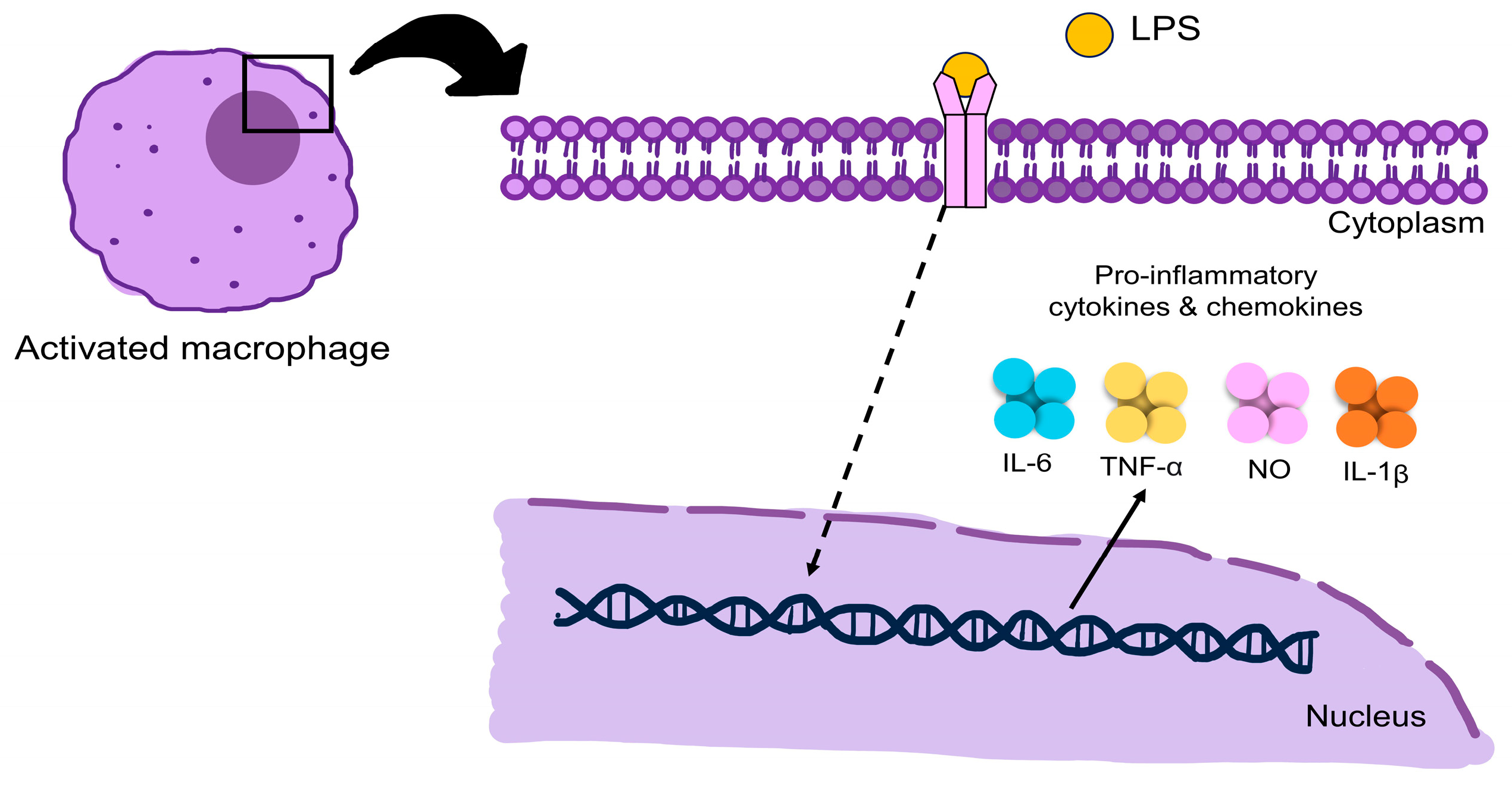
5. Inflammation and Pathways
6. Anti-Inflammatory of MEs Containing Herbal Substances
7. Future Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoar, T.P.; Schulman, J.H. Transparent water-in-oil dispersions: The oleopathic hydro-micelle. Nature 1943, 152, 102–103. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of formation and structure of micro emulsions by electron microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Gogarty, W.B.; Tosch, W.C. Miscible-type waterflooding: Oil recovery with micellar solutions. J. Pet. Technol. 1968, 20, 1407–1414. [Google Scholar] [CrossRef]
- Holm, L.W. Use of soluble oils for oil recovery. J. Pet. Technol. 1971, 23, 1475–1483. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Kanwar, R.; Rathee, J.; Patil, M.T.; Mehta, S.K. Microemulsions as nanotemplates: A soft and versatile approach. In Microemulsion—A Chemical Nanoreactor; Mejuto, J., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Akter, N.; Radiman, S.; Mohamed, F.; Reza, M.I.H. Self-assembled potential bio nanocarriers for drug delivery. Mini Rev. Med. Chem. 2013, 13, 1327–1339. [Google Scholar] [CrossRef] [PubMed]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Ma, Q.; Zhong, Q. Incorporation of soybean oil improves the dilutability of essential oil microemulsions. Food Res. Int. 2015, 71, 118–125. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Fu, Y.; Xiao, S.; Liu, S.; Chang, Y.; Ma, R.; Zhang, Z.; He, J. Atomistic insights into the droplet size evolution during self-microemulsification. Langmuir 2022, 38, 3129–3138. [Google Scholar] [CrossRef]
- Thakore, S.D.; Patel, R.B.; Patel, M. Nanoemulsion or microemulsion? Understanding the differences and similarities. Pharma Rev. 2014, 136–142. [Google Scholar]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical nano and microemulsions for skin delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Boonme, P.; Boonthongchuay, C.; Amnuaikit, T.; Wongpoowarak, W. Effects of components on and predictive modeling of microemulsion phase behavior in nonionic systems. J. App. Pharm. Sci. 2013, 3, 001–006. [Google Scholar] [CrossRef]
- Mitra, D. Microemulsion and its application: An inside story. Mater. Today Proc. 2023, 83, 75–82. [Google Scholar] [CrossRef]
- Suhail, N.; Alzahrani, A.K.; Basha, W.J.; Kizilbash, N.; Zaidi, A.; Ambreen, J.; Khachfe, H.M. Microemulsions: Unique properties, pharmacological applications, and targeted drug delivery. Front. Nanotechnol. 2021, 3, 754889. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A. Microemulsions: A novel approach to enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Boonme, P. Applications of microemulsions in cosmetics. J. Cosmet. Dermatol. 2007, 6, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Tenjarla, S. Microemulsions: An overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef] [PubMed]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Szumała, P.; Macierzanka, A. Topical delivery of pharmaceutical and cosmetic macromolecules using microemulsion systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Meng, S.; Zhao, X.; Wang, H.; Ning, Y.; Li, Y.; Chen, Z. Development and in vitro and in vivo evaluations of a microemulsion formulation for the oral delivery of oxaprozin. Curr. Drug Deliv. 2022, 19, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Chaurawal, N.; Misra, C.; Barkat, H.A.; Jatyan, R.; Chitkara, D.; Barkat, M.A.; Sharma, T.; Singh, B.; Raza, K. Oral sorafenib-loaded microemulsion for breast cancer: Evidences from the in-vitro evaluations and pharmacokinetic studies. Sci. Rep. 2022, 12, 13746. [Google Scholar] [CrossRef]
- Koli, A.R.; Ranch, K.M.; Patel, H.P.; Parikh, R.K.; Shah, D.O.; Maulvi, F.A. Oral bioavailability improvement of felodipine using tailored microemulsion: Surface science, ex vivo and in vivo studies. Int. J. Pharm. 2021, 596, 120202. [Google Scholar] [CrossRef] [PubMed]
- Daryab, M.; Faizi, M.; Mahboubi, A.; Aboofazeli, R. Preparation and characterization of lidocaine-loaded, microemulsion-based topical gels. Iran. J. Pharm. Res. 2022, 21, e123787. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Amirimoghadam, S.; Bagheri, F. Preparation, optimization, and investigation of naringenin-loaded microemulsion for topical application. Iran. Biomed. J. 2022, 26, 366–373. [Google Scholar] [CrossRef]
- Zhang, J.; Froelich, A.; Michniak-Kohn, B. Topical delivery of meloxicam using liposome and microemulsion formulation approaches. Pharmaceutics 2020, 12, 282. [Google Scholar] [CrossRef]
- Oliveira, D.A.J.; Amaral, J.G.; Garcia, L.B.; Dos Santos, M.S.; Silva, L.A.O.; Almeida, M.P.; Gomes, A.F.; Barros, D.R.P.; Lopes, N.P.; Pereira, G.R.; et al. Associating chitosan and microemulsion as a topical vehicle for the administration of herbal medicines. Carbohydr. Polym. 2021, 255, 117482. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Che, X.; Zhao, M.; Wang, Y.; Liu, Y.; Schwendeman, A.; Li, S. Development of cyclosporine A microemulsion for parenteral delivery. J. Microencapsul. 2015, 32, 273–280. [Google Scholar] [CrossRef]
- Okur, N.U.; Özdemir, D.I.; Kahyaoğlu, S.G.; Şenyiğit, Z.A.; Aşıkoğlu, M.; Genç, L.; Karasulu, H.Y. Assessment of aprotinin loaded microemulsion formulations for parenteral drug delivery: Preparation, characterization, in vitro release and cytotoxicity studies. Curr. Drug Deliv. 2015, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Lamaisakul, S.; Tantituvanont, A.; Lipipun, V.; Ritthidej, G. Development of novel cationic microemulsion as parenteral adjuvant for influenza vaccine. Asian J. Pharm. Sci. 2020, 15, 591–604. [Google Scholar] [CrossRef]
- Li, Y.; Angelova, A.; Liu, J.; Garamus, V.M.; Li, N.; Drechsler, M.; Gong, Y.; Zou, A. In situ phase transition of microemulsions for parenteral injection yielding lyotropic liquid crystalline carriers of the antitumor drug bufalin. Colloids Surf. B Biointerfaces 2019, 173, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bushettii, S.S.; Raju, A.S.; Ahmad, R.; Singh, M.; Bisht, A. Microemulsions as promising delivery systems: A review. Ind. J. Pharm. Edu. Res. 2011, 45, 392–401. [Google Scholar]
- Sharma, A.K.; Garg, T.; Goyal, A.K.; Rath, G. Role of microemulsions in advanced drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1177–1185. [Google Scholar] [CrossRef]
- Acharya, D.P.; Hartley, P.G. Progress in microemulsion characterization. Curr. Opin. Colloid Interface Sci. 2012, 17, 274–280. [Google Scholar] [CrossRef]
- Boonme, P.; Krauel, K.; Graf, A.; Rades, T.; Junyaprasert, V.B. Characterization of microemulsion structures in the pseudoternary phase diagram of isopropyl palmitate/water/Brij 97:1-butanol. AAPS PharmSciTech 2006, 7, E45. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet radiation protective and anti-inflammatory effects of Kaempferia galanga L. rhizome oil and microemulsion: Formulation, characterization, and hydrogel preparation. Gels 2022, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Sanguansajapong, V.; Sakdiset, P.; Puttarak, P. Development of oral microemulsion spray containing pentacyclic triterpenes-rich Centella asiatica (L.) Urb. extract for healing mouth ulcers. Pharmaceutics 2022, 14, 2531. [Google Scholar] [CrossRef] [PubMed]
- Chaiyana, W.; Rades, T.; Okonogi, S. Characterization and in vitro permeation study of microemulsions and liquid crystalline systems containing the anticholinesterase alkaloidal extract from Tabernaemontana divaricata. Int. J. Pharm. 2013, 452, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Patel, M.R.; Bhatt, K.K.; Patel, B.G. Formulation consideration and characterization of microemulsion drug delivery system for transnasal administration of carbamazepine. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 243–253. [Google Scholar] [CrossRef]
- Nouraei, M.; Acosta, E.J. Predicting solubilisation features of ternary phase diagrams of fully dilutable lecithin linker microemulsions. J. Colloid Interface Sci. 2017, 495, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.V.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P.D. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef]
- Kupper, S.; Kłosowska-Chomiczewska, I.; Szumała, P. Collagen and hyaluronic acid hydrogel in water-in-oil microemulsion delivery systems. Carbohydr. Polym. 2017, 175, 347–354. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, J.; Zheng, M.; Zheng, Y.; Xu, X.; Liu, Y.; Wang, X. Co-surfactant free microemulsions: Preparation, characterization and stability evaluation for food application. Food Chem. 2016, 204, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Shen, L.-N.; Zhang, Y.-T.; Wang, Q.; Xu, L.; Feng, N.-P. Preparation and evaluation of microemulsion-based transdermal delivery of total flavone of rhizoma arisaematis. Int. J. Nanomed. 2014, 9, 3453–3464. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Leis, F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv. Pharm. Bull. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Muller, F.; Dégousée, T.; Degrouard, J.; Brûlet, A.; Salonen, A. Probing structure in submicronic aqueous assemblies of emulsified microemulsions and charged spherical colloids using SANS and cryo-TEM. J. Colloid Interface Sci. 2015, 446, 114–121. [Google Scholar] [CrossRef]
- Imae, T. Overview of nanolayers: Formulation and characterization methods. In Nanolayer Research–Methodology and Technology for Green Chemistry; Imae, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–34. [Google Scholar]
- Liu, Q.; Lin, Y.; Li, J.; Wang, Y.; Li, S.; Bian, H.; Zhang, W.; Wu, Z. Microemulsion-based lutein extraction from marigold petals: Process optimization and stabilization evaluation. Ind. Crops Prod. 2023, 195, 116454. [Google Scholar] [CrossRef]
- Durgapal, S.; Goswami, L.; Nair, A.B.; Juyal, V.; Verma, A. Enhanced anti-cataract effect of microemulsion containing Cineraria maritima: Formulation, optimization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2022, 77, 103872. [Google Scholar] [CrossRef]
- Kaur, A.; Kamalpreet; Sharma, G.; Verma, S.; Goindi, S.; Katare, O.P. Oral microemulsion of phytoconstituent found in licorice as chemopreventive against benzo(a)pyrene induced forestomach tumors in experimental mice model. J. Drug Deliv. Sci. Technol. 2017, 39, 523–530. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Guo, R. Interaction of methemoglobin with GDA/n-C5H11OH/water assemblies. J. Colloid Interface Sci. 2008, 317, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Wellert, S.; Karg, M.; Imhof, H.; Steppin, A.; Altmann, H.J.; Dolle, M.; Richardt, A.; Tiersch, B.; Koetz, J.; Lapp, A.; et al. Structure of biodiesel based bicontinuous microemulsions for environmentally compatible decontamination: A small angle neutron scattering and freeze fracture electron microscopy study. J. Colloid Interface Sci. 2008, 325, 250–258. [Google Scholar] [CrossRef] [PubMed]
- López-Cano, J.J.; González-Cela-Casamayor, M.A.; Andrés-Guerrero, V.; Vicario -de-la-Torre, M.; Benítez del Castillo, J.M.; Herrero-Vanrell, R.; Molina-Martínez, I.T. Development of an osmoprotective microemulsion as a therapeutic platform for ocular surface protection. Int. J. Pharm. 2022, 623, 121948. [Google Scholar] [CrossRef]
- Lu, X.; Xie, S.; Kuang, J.; Xie, H. Microstructures of the Gemini surfactant microemulsion system 14-4-14/1-propanol/n-heptane/water. J. Mol. Liq. 2020, 320, 114485. [Google Scholar] [CrossRef]
- Martín, R.F.; Prietzel, C.; Koetz, J. Template-mediated self-assembly of magnetite-gold nanoparticle superstructures at the water-oil interface of AOT reverse microemulsions. J. Colloid Interface Sci. 2021, 581, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.A.; Nour, S.A.; Sakran, W.S.; El-Mancy, S.M.S. Preparation and evaluation of microemulsion systems containing salicylic acid. AAPS PharmSciTech 2009, 10, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- El Maghraby, G.M. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: Effects of cosurfactants. Int. J. Pharm. 2008, 355, 285–292. [Google Scholar] [CrossRef]
- Numin, M.S.; Jumbri, K.; Ramli, A.; Borhan, N. Microemulsion rheological analysis of alkaline, surfactant, and polymer in oil-water interface. Processes 2020, 8, 762. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Takechi-Haraya, Y.; Ohgita, T.; Demizu, Y.; Saito, H.; Izutsu, K.I.; Sakai-Kato, K. Current status and challenges of analytical methods for evaluation of size and surface modification of nanoparticle-based drug formulations. AAPS PharmSciTech 2022, 23, 150. [Google Scholar] [CrossRef]
- Mandal, S.; Das Mandal, S.; Surti, N.; Patel, V. Development of microemulsion formulation for the solubility enhancement of flunarizine. Der Pharm. Lett. 2010, 2, 227–236. [Google Scholar]
- Khichariya, A.; Jeswani, G.; Choudhary, R.; Alexander, A.; Nakhate, K.T.; Ramchandra Badwaik, H. Formulation of plumbagin-loaded microemulsion: Evaluation of anti-rheumatoid efficacy in Wistar rat model. J. Mol. Liq. 2022, 363, 119851. [Google Scholar] [CrossRef]
- Gundogdu, E.; Alvarez, I.G.; Karasulu, E. Improvement of effect of water-in-oil microemulsion as an oral delivery system for fexofenadine: In vitro and in vivo studies. Int. J. Nanomed. 2011, 6, 1631–1640. [Google Scholar] [CrossRef]
- Paramashivaiah, B.M.; Rajashekhar, C.R. Studies on effect of various surfactants on stable dispersion of graphene nano particles in simarouba biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012083. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A.; Ezrahi, S.; Tiunova, I.; Berkovic, G. Water behavior in nonionic surfactant systems I: Subzero temperature behavior of water in nonionic microemulsions studied by DSC. J. Colloid Interface Sci. 1996, 178, 60–68. [Google Scholar] [CrossRef]
- Moghimipour, E.; Salimi, A.; Changizi, S. Preparation and microstructural characterization of griseofulvin microemulsions using different experimental methods: SAXS and DSC. Adv. Pharm. Bull. 2017, 7, 281–289. [Google Scholar] [CrossRef]
- Savić, V.; Todosijević, M.; Ilić, T.; Lukić, M.; Mitsou, E.; Papadimitriou, V.; Avramiotis, S.; Marković, B.; Cekić, N.; Savić, S. Tacrolimus loaded biocompatible lecithin-based microemulsions with improved skin penetration: Structure characterization and in vitro/in vivo performances. Int. J. Pharm. 2017, 529, 491–505. [Google Scholar] [CrossRef]
- Goebel, A.S.; Neubert, R.H.; Wohlrab, J. Dermal targeting of tacrolimus using colloidal carrier systems. Int. J. Pharm. 2011, 404, 159–168. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Schauber, J. Skin: Architecture and function. In Dermal Replacements in General, Burn, and Plastic Surgery–Tissue Engineering in Clinical Practice; Kamolz, L.P., Lumenta, D., Eds.; Springer: Vienna, Austria, 2013; pp. 1–11. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Böhling, A.; Bielfeldt, S.; Himmelmann, A.; Keskin, M.; Wilhelm, K.-P. Comparison of the stratum corneum thickness measured in vivo with confocal Raman spectroscopy and confocal reflectance microscopy. Skin Res. Technol. 2014, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic structure and function. In Pathobiology of Human Disease—A Dynamic Encyclopedia of Disease Mechanisms; McManus, L.M., Mitchell, R.N., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1134–1144. [Google Scholar] [CrossRef]
- West, H.C.; Bennett, C.L. Redefining the role of Langerhans cells as immune regulators within the skin. Front. Immunol. 2017, 8, 1941. [Google Scholar] [CrossRef]
- Bray, N. Merkel cells touch a nerve. Nat. Rev. Neurosci. 2019, 20, 4. [Google Scholar] [CrossRef]
- Basketter, D.; Jírova, D.; Kandárová, H. Review of skin irritation/corrosion hazards on the basis of human data: A regulatory perspective. Interdiscip. Toxicol. 2012, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Novak-Bilić, G.; Vučić, M.; Japundžić, I.; Meštrović-Štefekov, J.; Stanić-Duktaj, S.; Lugović-Mihić, L. Irritant and allergic contact dermatitis—Skin lesion characteristics. Acta Clin. Croat. 2018, 57, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.H.; Maibach, H.I. Allergic contact dermatitis. In Allergy and Asthma: The Basics to Best Practices, 1st ed.; Mahmoudi, M., Ledford, D.K., Craig, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 245–272. [Google Scholar]
- Schliemann, S.; Breternitz, M.; Elsner, P. Principles and mechanisms of skin irritation. In Handbook of Cosmetic Science and Technology, 3rd ed.; Barel, A.O., Paye, M., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 443–454. [Google Scholar]
- Fluhr, J.W.; Darlenski, R.; Angelova-Fischer, I.; Tsankov, N.; Basketter, D. Skin irritation and sensitization: Mechanisms and new approaches for risk assessment: 1. Skin irritation. Skin Pharmacol. Physiol. 2008, 21, 124–135. [Google Scholar] [CrossRef]
- Smith, J.S.; Rajagopal, S.; Atwater, A.R. Chemokine signaling in allergic contact dermatitis: Toward targeted therapies. Dermatitis 2018, 29, 179–186. [Google Scholar] [CrossRef]
- Basketter, D.; Darlenski, R.; Fluhr, J.W. Skin irritation and sensitization: Mechanisms and new approaches for risk assessment: 2. Skin sensitization. Skin Pharmacol. Physiol. 2008, 21, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T. Surfactants. In Encyclopedia of Colloid and Interface Science; Tadros, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1242–1290. [Google Scholar]
- Salomon, G.; Giordano-Labadie, F. Surfactant irritations and allergies. Eur. J. Dermatol. 2022, 32, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Effendy, I.; Maibach, H.I. Surfactants and experimental irritant contact dermatitis. Contact Derm. 1995, 33, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development (OECD). Test No. 404: Acute Dermal Irritation/Corrosion; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Miles, A.; Berthet, A.; Hopf, N.B.; Gilliet, M.; Raffoul, W.; Vernez, D.; Spring, P. A new alternative method for testing skin irritation using a human skin model: A pilot study. Toxicol. In Vitro 2014, 28, 240–247. [Google Scholar] [CrossRef]
- Pedrosa, T.D.N.; Catarino, C.M.; Pennacchi, P.C.; Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; Barros, S.B.M.; Maria-Engler, S.S. A new reconstructed human epidermis for in vitro skin irritation testing. Toxicol. In Vitro 2017, 42, 31–37. [Google Scholar] [CrossRef]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Vibhute, S.; Kasture, V.; Kasture, S.; Kendre, P.; Rupnar, S.; Vishal, P. Design and characterization of Moringa oleifera seed oil impregnated anti-inflammatory topical micro-dispersion. Der Pharm. Lett. 2015, 7, 7–16. [Google Scholar]
- Guo, J.-W.; Cheng, Y.-P.; Liu, C.-Y.; Thong, H.-Y.; Huang, C.-J.; Lo, Y.; Wu, C.-Y.; Jee, S.-H. Salvianolic acid B in microemulsion formulation provided sufficient hydration for dry skin and ameliorated the severity of imiquimod-induced psoriasis-like dermatitis in mice. Pharmaceutics 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Marsup, P.; Sirilun, S.; Prommaban, A.; Sri, S.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-anun, C.; Chaiyana, W. Potential of topical microemulsion serum formulations to enhance in vitro and clinical anti-skin wrinkle benefits of Cordyceps militaris extracts. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Parveen, R.; Akhtar, N.; Mahmood, T. Topical microemulsion containing Punica granatum extract: Its control over skin erythema and melanin in healthy Asian subjects. Postȩpy Dermatol. Alergol. 2014, 31, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Tsai, M.-J.; Fang, Y.-P.; Fu, Y.-S.; Huang, Y.-B.; Wu, P.-C. Microemulsion formulation design and evaluation for hydrophobic compound: Catechin topical application. Colloids Surf. B Biointerfaces 2018, 161, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Kaur, A.; Raza, K.; Goindi, S.; Katare, O.P. Development and evaluation of topical microemulsion of dibenzoylmethane for treatment of UV induced photoaging. J. Drug Deliv. Sci. Technol. 2017, 37, 1–12. [Google Scholar] [CrossRef]
- Gandhi, J.; Suthar, D.; Patel, H.; Shelat, P.; Parejiya, P. Development and characterization of microemulsion based topical gel of essential oil of clove (Syzygium aromaticum) for superficial fungal infections. Orient. Pharm. Exp. Med. 2021, 21, 519–534. [Google Scholar] [CrossRef]
- Brathwaite, A.C.N.; Alencar-Silva, T.; Carvalho, L.A.C.; Branquinho, M.S.F.; Ferreira-Nunes, R.; Cunha-Filho, M.; Gelfuso, G.M.; Maria-Engler, S.S.; Carvalho, J.L.; Silva, J.K.R.; et al. Pouteria macrophylla fruit extract microemulsion for cutaneous depigmentation: Evaluation using a 3D pigmented skin model. Molecules 2022, 27, 5982. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Abla, K.K.; Elmaradny, H.A. Tailored limonene-based nanosized microemulsion: Formulation, physicochemical characterization and in vivo skin irritation assessment. Adv. Pharm. Bull. 2021, 11, 274–285. [Google Scholar] [CrossRef]
- Prommaban, A.; Chaiyana, W. Microemulsion of essential oils from citrus peels and leaves with anti-aging, whitening, and irritation reducing capacity. J. Drug Deliv. Sci. Technol. 2022, 69, 103188. [Google Scholar] [CrossRef]
- Nasr, M.; Abdel-Hamid, S.; Moftah, N.H.; Fadel, M.; Alyoussef, A.A. Jojoba oil soft colloidal nanocarrier of a synthetic retinoid: Preparation, characterization and clinical efficacy in psoriatic patients. Curr. Drug Deliv. 2017, 14, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Abdel-Hamid, S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev. Ind. Pharm. 2016, 42, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ramez, S.A.; Soliman, M.M.; Fadel, M.; El-Deen, F.N.; Nasr, M.; Youness, E.R.; Aboel-Fadl, D.M. Novel methotrexate soft nanocarrier/fractional erbium YAG laser combination for clinical treatment of plaque psoriasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, 12–26. [Google Scholar] [CrossRef]
- Hannoodee, S.; Nasuruddin, D.N. Acute inflammatory response. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556083/ (accessed on 24 May 2023).
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Jakopin, Ž.; Corsini, E. THP-1 cells and pro-inflammatory cytokine production: An in vitro tool for functional characterization of NOD1/NOD2 antagonists. Int. J. Mol. Sci. 2019, 20, 4265. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, H.; Li, Y.; Meng, X.; Yan, L.; Dan, Z.; Wu, S.; Zhou, H.; Peng, L.; Xie, Q.; et al. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3 pathways. Sci. Rep. 2016, 6, 34611. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr, E.T.B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Mahajan, U.B.; Unger, B.S.; Goyal, S.N.; Belemkar, S.; Surana, S.J.; Ojha, S.; Patil, C.R. Animal models of inflammation for screening of anti-inflammatory drugs: Implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019, 20, 4367. [Google Scholar] [CrossRef]
- Eze, F.; Uzor, P.; Peter, I.; Obi, B.; Osadebe, P. In vitro and in vivo models for anti-inflammation: An evaluative review. INNOSC Theranostics Pharmacol. Sci. 2019, 2, 3–15. [Google Scholar] [CrossRef]
- Burodom, A.; Itharat, A. Inflammatory suppressive effect of Benjakul, a Thai traditional medicine on intestinal epithelial cell line. J. Med. Plant Res. 2013, 7, 3286–3291. [Google Scholar] [CrossRef]
- Kuropakornpong, P.; Itharat, A.; Ooraikul, B.; Loebenberg, R.; Davies, N.M. Development and optimization of Benjakul microemulsion formulations for enhancing topical anti-inflammatory effect and delivery. Res. Pharm. Sci. 2022, 17, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.; Rasul, A.; Waqas, M.K.; Irfan, M.; Khalid, S.H.; Aamir, M.N.; Murtaza, G.; Rehman, K.U.; Iqbal, M.; Khan, B.A. Development, characterization and evaluation of in-vitro anti-inflammatory activity of ginger extract based micro emulsion. Pak. J. Pharm. Sci. 2019, 32, 1327–1332. [Google Scholar] [PubMed]
- Reis, M.Y.F.A.; Santos, S.M.D.; Silva, D.R.; Silva, M.V.; Correia, M.T.S.; Navarro, D.M.A.F.; Santos, G.K.N.; Hallwass, F.; Bianchi, O.; Silva, A.G.; et al. Anti-inflammatory activity of babassu oil and development of a microemulsion system for topical delivery. Evid. Based Complement. Alternat. Med. 2017, 2017, 3647801. [Google Scholar] [CrossRef]
- Pascoa, H.; Diniz, D.G.A.; Florentino, I.F.; Costa, E.A.; Bara, M.T.F. Microemulsion based on Pterodon emarginatus oil and its anti-inflammatory potential. Braz. J. Pharm. Sci. 2015, 51, 117–126. [Google Scholar] [CrossRef]
- Chaiyana, W.; Anuchapreeda, S.; Leelapornpisid, P.; Phongpradist, R.; Viernstein, H.; Mueller, M. Development of microemulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech 2017, 18, 1332–1342. [Google Scholar] [CrossRef]
- Triastuti, A.; Indrati, O.; Hayati, F. Development of microemulsion containing Plantago major extracts: Formulation and evaluation of topical anti-inflammatory activities. In Proceedings of the Fifth International Mediterranean Symposium on Medicinal and Aromatic Plants, Cappadocia, Turkey, 24–26 April 2019; pp. 211–215. [Google Scholar]
- De Oliveira Neves, J.K.; Apolinário, A.C.; Alcantara Saraiva, K.L.; da Silva, D.T.C.; de Freitas Araújo Reis, M.Y.; de Lima Damasceno, B.P.G.; Pessoa, A.; Moraes Galvão, M.A.; Soares, L.A.L.; da Veiga Júnior, V.F.; et al. Microemulsions containing Copaifera multijuga Hayne oil-resin: Challenges to achieve an efficient system for β-caryophyllene delivery. Ind. Crops Prod. 2018, 111, 185–192. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Araujo, G.A.; Ferreira, P.S.; Victorelli, F.D.; Pironi, A.M.; Araújo, V.H.S.; Carvalho, S.G.; Chorilli, M. Design and characterization of lipid-surfactant-based systems for enhancing topical anti-inflammatory activity of ursolic acid. Pharmaceutics 2023, 15, 366. [Google Scholar] [CrossRef] [PubMed]

| Topical MEs | Indications | ME Components | Physicochemical Properties | Main Results | References |
|---|---|---|---|---|---|
| Moringa oleifera seed oil-loaded ME | Anti-inflammatory activity | M. oleifera seed oil, Tween® 80, Span® 80, water | Size: 167 nm PDI: 0.30 ZP: –35.8 mV pH: 7.1 RI: 1.469 η: 88.7 mPa.s |
| [97] |
| Salvianolic acid B-loaded ME | Anti-psoriatic effects | Silicon oil AR200, squalene, triglyceride, PEG-40 castor oil, Tween® 80, PEG 400, 1,2-propylene glycol, sorbitol, glycerol, phosphate-buffered saline | σ: 24 μS/cm Size: 696 nm PDI: 0.44 ZP: –15.0 mV η: 3112.3 mPa.s |
| [98] |
| Cordyceps militaris extract-loaded ME serum | Anti-skin wrinkle properties | Sugar squalane, Tween® 85, propylene glycol, water | Size: 146 nm PDI: 0.50 |
| [99] |
| Punica granatum extract-loaded ME | Control of skin erythema and melanin | Palm oil, Tween® 80, propylene glycol, water | σ: 6 μS/cm Size: 8 nm PDI: 0.36 pH: 5.7 |
| [100] |
| Catechin-loaded ME | Not specified | Isopropyl myristate, Brij 35, Brij 30, ethanol, water | Size: 369 nm η: 9.3 mPa.s |
| [101] |
| Dibenzoylmethane-loaded ME | Treatment of UV-induced photoaging | Captex 300, Tween® 80, n-butanol, menthol, water | Size: 36 nm PDI: 0.28 ZP: 0.4 mV pH: 6.8 η: 45.9 mPa.s |
| [102] |
| Clove oil-loaded ME gel | Treatment of superficial fungal infections | Clove oil, Tween® 80, isopropyl alcohol, water | σ: 143 μS/cm Size: 14 nm PDI: 0.01 ZP: –0.7 mV pH: 6.0 |
| [103] |
| Pouteria macrophylla fruit extract-loaded ME | Treatment of cutaneous depigmentation | Ethyl oleate, Cremophor® ELP, Span® 80, HEPES buffer (pH 4.5) | Size: 49 nm PDI: 0.35 ZP: –25.0 mV pH: 4.0 |
| [104] |
| Topical MEs | Anti-Inflammatory Substances | ME Components | Physicochemical Properties | Main Results | References |
|---|---|---|---|---|---|
| Kaempferia galanga oil-loaded ME | K. galanga rhizome oil | K. galanga oil, Tween® 80, water | σ: 54,400 μS/cm Size: 216 nm PDI: 0.40 ZP: –13.8 mV pH: 6.7 |
| [41] |
| Benjakul-loaded ME | Benjakul: A combination of five botanical herbal constituents (Piper chaba, Piper sarmentosum, Piper interuptum, Plumbago indica, and Zingiber officinale) | Isopropyl myristate, Labrasol®, Transcutol®, water | σ: 46 μS/cm Size: 279 nm PDI: 0.39 pH: 3.8 |
| [122] |
| Ginger-loaded ME | Zingiber officinale rhizome extract | Isopropyl myristate, Tween® 80, polyethylene glycol 400, water | σ: 207 μS/cm Size: 22 nm PDI: 0.16 ZP: –22.8 mV pH: 5.9 RI: 1.397 η: 26.0 mPa.s |
| [123] |
| Babassu oil-loaded ME | Babassu oil | Babassu oil, Span® 80, Kolliphor® EL, propylene glycol, water | σ: 21 μS/cm Size: 5–15 nm η: 300.0 mPa.s |
| [124] |
| Pterodon emarginatus oil-loaded ME | P. emarginatus oil | P. emarginatus oil, Ultramone R-540®, propylene glycol, water | Size: 57 nm PDI: <0.20 ZP: –11.3 mV pH: 5.7 |
| [125] |
| Zingiber cassumunar oil-loaded ME | Z. cassumunar rhizome oil | Z. cassumunar oil, Tween® 20, propylene glycol, water | Size: 212–367 nm PDI: <0.38 η: 0.7–0.8 mPa.s |
| [126] |
| Plantago major extract-loaded ME | P. major extract | Isopropyl myristate, Span® 80, Tween® 80, propylene glycol, propylparaben, methylparaben, water | Size: 54 nm PDI: 0.40 pH: 6.9 η: 122.8 mPa.s |
| [127] |
| Copaifera multijuga oil-resin-loaded ME | C. multijuga (copaiba) oil-resin | Copaiba oil-resin, Plurol® Oleique 5203, Labrasol®, water | σ: 121 μS/cm (ME-I), 79 μS/cm (ME-II) Size: 165 nm (ME-I), 199 nm (ME-II) ZP: –2.6 mV (ME-I), –3.6 mV (ME-II) pH: 5.3 (ME-I), 5.7 (ME-II) RI: 1.412 (ME-I), 1.426 (ME-II) η: 64.4 mPa.s (ME-I), 118.8 mPa.s (ME-II) |
| [128] |
| Ursolic acid-loaded ME | Ursolic acid | Oleic acid, Procetyl® AWS, water | Yield shear stress: 1.0245 Pa (ME-A), 0.0000 Pa (ME-B) Consistency index: 0.0890 Pa.sn (ME-A), 0.3777 Pa.sn (ME-B) Flow index: 1.0628 (ME-A), 0.9077 (ME-B) S constant (relating to gel strength): 0.0023 (ME-A), 1182.0945 (ME-B) Viscoelastic exponent: 2.4691 (ME-A), 0.2531 (ME-B) |
| [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanpolchareanchai, J.; Teeranachaideekul, V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals 2023, 16, 999. https://doi.org/10.3390/ph16070999
Leanpolchareanchai J, Teeranachaideekul V. Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals. 2023; 16(7):999. https://doi.org/10.3390/ph16070999
Chicago/Turabian StyleLeanpolchareanchai, Jiraporn, and Veerawat Teeranachaideekul. 2023. "Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances" Pharmaceuticals 16, no. 7: 999. https://doi.org/10.3390/ph16070999
APA StyleLeanpolchareanchai, J., & Teeranachaideekul, V. (2023). Topical Microemulsions: Skin Irritation Potential and Anti-Inflammatory Effects of Herbal Substances. Pharmaceuticals, 16(7), 999. https://doi.org/10.3390/ph16070999










