Exploration of the Antioxidant and Anti-inflammatory Potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, Traditionally Used in the Treatment of Hepatitis in the Hauts-Bassins Region of Burkina Faso
Abstract
1. Introduction
2. Results
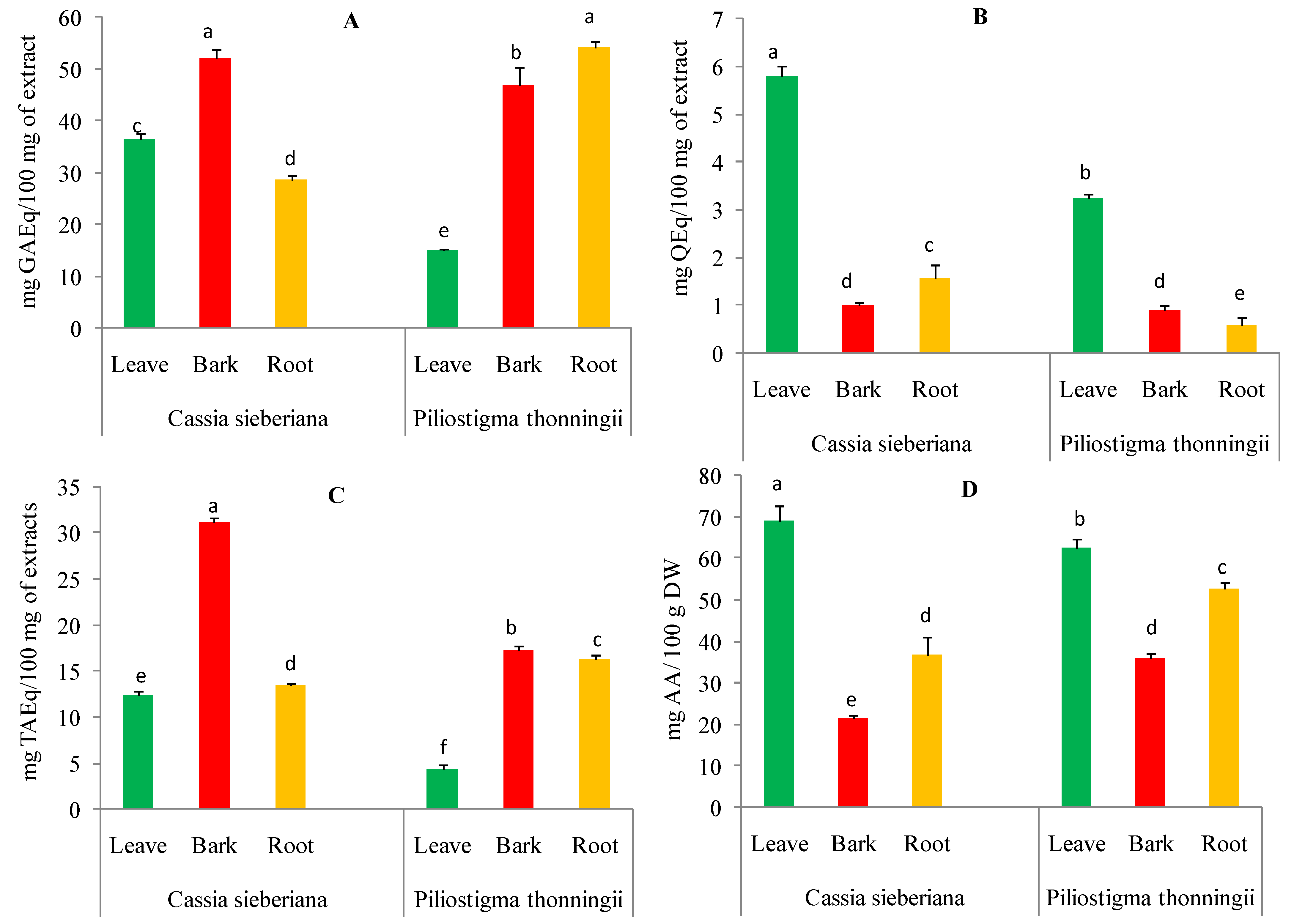
2.1. Determination of Phytochemical Constituents of C. Sieberiana and P. Thonningii Extracts
2.2. HPLC and UHPLC-MS Analysis of C. Sieberiana and P. Thonningii Extracts
2.3. In Vitro Antioxidant Activities
2.4. In Vitro Anti-Inflammatory Activities
2.5. Correlations between Antioxidant and Anti-Inflammatory with Phytochemicals
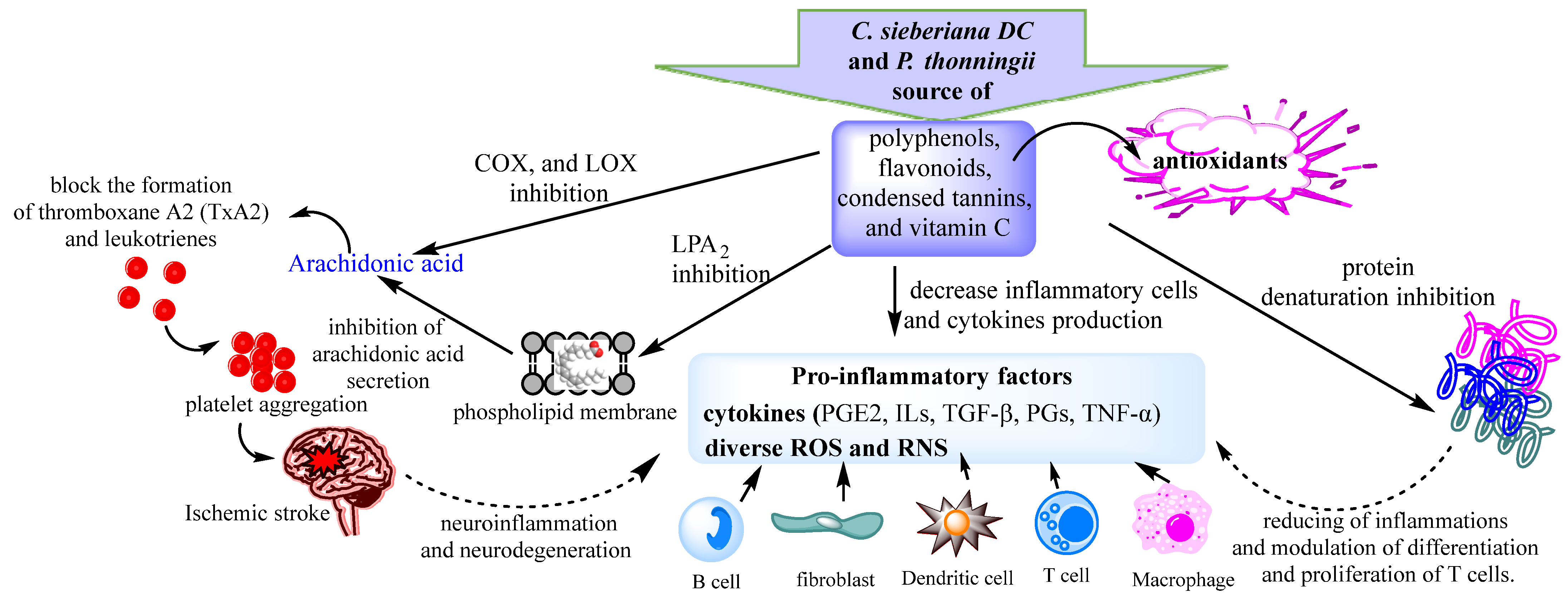
3. Discussion
3.1. In Vitro Antioxidant Activities
3.2. Anti-Inflammatory Activities in Vitro
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Preparation of Aqueous Extracts
4.2.1. Plant Material
4.2.2. Preparation of Aqueous Extracts
4.3. Determination of Phytochemical Constituents of Aqueous Extracts
4.3.1. Determination of Total Polyphenols
4.3.2. Determination of Total Flavonoids
4.3.3. Quantification of Condensed Tannins
4.3.4. Quantification of Ascorbic Acid
4.4. HPLC and UHPLC-MS Analysis of Aqueous Extracts
4.5. In Vitro Antioxidant Activities
4.5.1. Free Radical Scavenging Activities of 2,2-Diphenyl-1 Picrylhydrazyl (DPPH)
4.5.2. ABTS Free Radical Scavenging Activities
4.5.3. Total Antioxidant Capacity (TAC)
4.6. In Vitro Anti-Inflammatory Activities
4.6.1. Anti-Lipoxygenase Activity
4.6.2. Anti-Proteinase Action
4.7. Membrane Stabilization Test
4.8. Statistical Analyzes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. From Oxidative Stress to Inflammation: Redox Balance and Immune System. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef]
- Tsermpini, E.E.; Glamočlija, U.; Ulucan-Karnak, F.; Redenšek Trampuž, S.; Dolžan, V. Molecular Mechanisms Related to Responses to Oxidative Stress and Antioxidative Therapies in COVID-19: A Systematic Review. Antioxidants 2022, 11, 1609. [Google Scholar] [CrossRef]
- Rodrigues, E.B.; Farah, M.E.; Bottós, J.M.; Aggio, F.B. Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Retinal Diseases. Dev. Ophthalmol. 2015, 55, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sriuttha, P.; Sirichanchuen, B.; Permsuwan, U. Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials. Int. J. Hepatol. 2018, 2018, 5253623. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Mokuda, S.; Matoba, K.; Yamada, A.; Jouyama, K.; Murata, Y.; Ozaki, Y.; Ito, T.; Nomura, S.; Okuda, Y. The Prevalence of Endoscopic Gastric Mucosal Damage in Patients with Rheumatoid Arthritis. PLoS ONE 2018, 13, e0200023. [Google Scholar] [CrossRef]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; da Silva, G.B., Jr. Pathophysiological Aspects of Nephropathy Caused by Non-Steroidal Anti-Inflammatory Drugs. J. Bras. Nefrol. 2019, 41, 124–130. [Google Scholar] [CrossRef]
- Archer, M.-A.; Agyei, A.T.; Mintah, S.O.; Adjei, P.A.; Kumadoh, D.; Asiedu-Larbi, J. Medicinal Uses of Cassia Sieberiana; A Review. Int. J. Sci. Basic Appl. Res. 2019, 48, 161–180. [Google Scholar]
- Cyril, O.; Jonathan, E.C.; Chiedu, O.F.B. Piliostigma Thonningii ( Fabaceae ): A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemistry, Pharmacology and Toxicology. Sch. Int. J. Biochem. 2021, 4, 66–81. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; Abd El-Hack, M.E.; Taha, A.E.; Algammal, A.M.; Ali Elewa, Y.H. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Krithiga, N.; Jayachitra, A. Antioxidant Activity of the Chitosan Extracted from Shrimp Exoskeleton. Middle East J. Sci. Res. 2013, 16, 1446–1451. [Google Scholar] [CrossRef]
- Tanobe, V.O.A.; Sydenstricker, T.H.D.; Munaro, M.; Amico, S.C. Corrigendum to “A Comprehensive Characterization of Chemically Treated Brazilian Sponge-Gourds (Luffa Cylindrica)”. Polym. Test. 2010, 29, 288–289. [Google Scholar] [CrossRef]
- Hendra, R.; Ahmad, S.; Oskoueian, E.; Sukari, A.; Shukor, M.Y. Antioxidant, Anti-Inflammatory and Cytotoxicity of Phaleria Macrocarpa (Boerl.) Scheff Fruit. BMC Complement. Altern. Med. 2011, 11, 1–10. [Google Scholar] [CrossRef]
- Ibewuike, J.C.; Ogungbamila, F.O.; Ogundaini, A.O.; Okeke, I.N.; Bohlin, L. Antiinflammatory and Antibacterial Activities of C-Methylflavonols from Piliostigma Thonningii. Phyther. Res. 1997, 11, 281–284. [Google Scholar] [CrossRef]
- Macedo, T.; Ferreres, F.; Pereira, D.M.; Oliveira, A.P.; Gomes, N.G.M.; Gil-Izquierdo, Á.; Valentão, P.; Araújo, L.; Andrade, P.B. Cassia Sieberiana DC. Leaves Modulate LPS-Induced Inflammatory Response in THP-1 Cells and Inhibit Eicosanoid-Metabolizing Enzymes. J. Ethnopharmacol. 2021, 269, 113746. [Google Scholar] [CrossRef]
- Sawadogo, M.; Bangou, M.J.; Dakio, B.; Lema, A.; Thiombiano, H.M.; Ouoba, B.; Ouoba, H.Y.; Ouedraogo, G.A. Ethnobotanical Survey on Medicinal Plants (Carica Papaya L. and Agelanthus Dodoneifolius (DC.) Polhill & Wiens ) Used in the Treatment of Hepatitis in Burkina Faso, Phytochemistry and Antioxidant Activity. World J. Adv. Pharm. Life Sci. 2021, 1, 23–34. [Google Scholar] [CrossRef]
- Zongo, E.; Meda, R.N.-T.; Kam, S.E.; Koama, B.K.; Ouoba, H.Y.; Ouedraogo, G.A. Ethnobotanical Study of Medicinal Plants Used for Viral Hepatitis Treatment in Hauts-Bassins Areas of Burkina Faso. World J. Pharm. Pharm. Sci. 2021, 10, 76–92. [Google Scholar] [CrossRef]
- Okpoko, C.; Ezenyi, I.; Adzu, B.; Salawu, O. Evaluation of Two Medicinal Plants Used for Arthritis in Northern Nigeria with Focus on Terminalia Avicennioides Guill. & Perr. and Its Mechanism of Action. Sci. Afr. 2020, 8, e00357. [Google Scholar] [CrossRef]
- Babajide, O.J.; Babajide, O.O.; Daramola, A.O.; Mabusela, W.T. Flavonols and an Oxychromonol from Piliostigma Reticulatum. Phytochemistry 2008, 69, 2245–2250. [Google Scholar] [CrossRef]
- Gebre, T.; Haile, M.; Birhane, E.; Tewolde-Berhan, S.; Girmay, Z. Multipurpose Benefits and Scaling-up Strategies for Bauhinia Thonningii Schumacher: A Review. Agrofor. Syst. 2022, 96, 265–280. [Google Scholar] [CrossRef]
- Jimoh, F.O.; Oladiji, A.T. Preliminary Studies on Piliostigma Thonningii Seeds: Proximate Analysis, Mineral Composition and Phytochemical Screening. Afr. J. Biotechnol. 2005, 4, 1439–1442. [Google Scholar]
- Bvenura, C.; Afolayan, A.J. The Role of Wild Vegetables in Household Food Security in South Africa: A Review. Food Res. Int. 2015, 76, 1001–1011. [Google Scholar] [CrossRef]
- Kpabi, I.; Munsch, T.; Agban, A.; Théry-Koné, I.; Dorat, J.; Boudesocque-Delaye, L.; Delaye, P.O.; Neveu, C.; Lanoue, A.; Enguehard-Gueiffier, C. Cassia Sieberiana Root Bark Used in Traditional Medicine in Togo: Anthelmintic Property against Haemonchus Contortus and Tannins Composition. South Afr. J. Bot. 2022, 151, 549–558. [Google Scholar] [CrossRef]
- Asuzu, I.U.; Gray, A.I.; Waterman, P.G. The Anthelmintic Activity of D -3- O -Methylchiroinositol Isolated from Piliostigma Thonningii Stem Bark. Fitoterapia 1999, 70, 77–79. [Google Scholar] [CrossRef]
- Ibe, C.I.; Ajaegbu, E.E.; Ajaghaku, A.A.; Eze, P.M.; Onyeka, I.P.; Ezugwu, C.O.; Okoye, F.B.C. In Vitro and in Vivo Antioxidant Potential of the Methanol Extract, Its Fractions and Isolated Compounds of Piliostigma Thonningi. Phytomedicine Plus 2022, 2, 100335. [Google Scholar] [CrossRef]
- Dougnon, V.; Hounsa, E.; Koudokpon, H.; Legba, B.B.; Fabiyi, K.; Sintondji, K.; Afaton, A.; Akouta, M.; Klotoe, J.R.; Bankole, H.; et al. A Literature Review—Khaya Senegalensis, Anacardium Ouest L., Cassia Sieberiana DC., Pterocarpus Erinaceus, Diospyros Mespiliformis, Ocimum Gratissimum, Manihot Esculenta, Vernonia Amygdalina Delile, Pseudocedrela Kotschyi and Daniellia Oliveri Possess Pro. Adv. Biosci. Biotechnol. 2020, 11, 457–473. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Agunbiade, S.O.; Omole, J.O.; Kuti, O.A. Evaluation of the Chemical, Nutritional, Antimicrobial and Antioxidant-Vitamin Profiles of Piliostigma Thonningii Leaves (Nigerian Species). Res. J. Med. Plant 2012, 6, 537–543. [Google Scholar] [CrossRef]
- Moriasi, G.; Ireri, A.; Ngugi, M.P. In Vitro Antioxidant Activities of the Aqueous and Methanolic Stem Bark Extracts of Piliostigma Thonningii (Schum.). J. Evid.-Based Integr. Med. 2020, 25, 2515690X20937988. [Google Scholar] [CrossRef] [PubMed]
- Sospeter, N.N.; Meshack, A.O.; Silas, M.N.; Samwel, N.O.; John, M.N.; Paul, K.K. Antituberculous, Antimicrobial, Cytotoxicity and Phytochemical Activity Study of Piliostigma Thonningii Extract Fractions. J. Med. Plants Res. 2015, 9, 655–663. [Google Scholar] [CrossRef][Green Version]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of Chlorogenic Acids and Related Cinnamic Acid Derivatives from Nicotiana Tabacum Tissues with the Aid of UPLC-QTOF-MS/MS Based on the in-Source Collision-Induced Dissociation Method. Chem. Cent. J. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Cuong, D.M.; Sathasivam, R.; Park, C.H.; Yeo, H.J.; Park, Y.E.; Kim, J.K.; Park, S.U. Analysis of Triterpenoids, Carotenoids, and Phenylpropanoids in the Flowers, Leaves, Roots, and Stems of White Bitter Melon (Cucurbitaceae, Momordica Charantia). Trop. J. Pharm. Res. 2021, 20, 155–160. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the Effects of Vitexin in Oxidative Stress-Related Diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef]
- Lema, A.; Bangou, M.J.; Sawadogo, M.; Thiombiano, H.M.; Ouoba, H.Y. Medicinal Plant Recipes Used in the Management of Peptic Ulcers in Burkina Faso: Ethnobotanical Study. Int. J. Sci. Res. Arch. 2022, 6, 263–278. [Google Scholar] [CrossRef]
- Boualam, K.; Ndiaye, B.; Harhar, H.; Tabyaoui, M.; Ayessou, N.; Taghzouti, K. Study of the Phytochemical Composition, the Antioxidant and the Anti-Inflammatory Effects of Two Sub-Saharan Plants: Piliostigma Reticulatum and Piliostigma Thonningii. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5549478. [Google Scholar] [CrossRef]
- Pitchakarn, P.; Ogawa, K.; Suzuki, S.; Takahashi, S.; Asamoto, M.; Chewonarin, T.; Limtrakul, P.; Shirai, T. Momordica Charantia Leaf Extract Suppresses Rat Prostate Cancer Progression in Vitro and in Vivo. Cancer Sci. 2010, 101, 2234–2240. [Google Scholar] [CrossRef]
- Kolarov, R.; Tukuljac, M.P.; Kolbas, A.; Kolbas, N.; Barać, G.; Ognjanov, V.; Ljubojević, M.; Prvulović, D. Antioxidant Capacity of Wild-Growing Bilberry, Elderberry, and Strawberry Fruits. Acta Hortic. Regiotect. 2021, 24, 119–126. [Google Scholar] [CrossRef]
- Evenamede, K.S.; Kpegba, K.; Simalou, O.; Boyode, P.; Agbonon, A.; Gbeassor, M. Etude Comparative Des Activités Antioxydantes d’extraits Éthanoliques de Feuilles, d’écorces et de Racines de Cassia Sieberiana. Int. J. Biol. Chem. Sci. 2018, 11, 2924. [Google Scholar] [CrossRef][Green Version]
- Arroyave-ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Shahamat, Z.; Abbasi-Maleki, S.; Mohammadi Motamed, S. Evaluation of Antidepressant-like Effects of Aqueous and Ethanolic Extracts of Pimpinella Anisum Fruit in Mice. Avicenna J. Phytomed. 2016, 6, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Eltamany, E.E.; Goda, M.S.; Nafie, M.S.; Abu-Elsaoud, A.M.; Hareeri, R.H.; Aldurdunji, M.M.; Elhady, S.S.; Badr, J.M.; Eltahawy, N.A. Comparative Assessment of the Antioxidant and Anticancer Activities of Plicosepalus Acacia and Plicosepalus Curviflorus: Metabolomic Profiling and In Silico Studies. Antioxidants 2022, 11, 1249. [Google Scholar] [CrossRef]
- Nobossé, P.; Fombang, E.N.; Mbofung, C.M.F. Effects of Age and Extraction Solvent on Phytochemical Content and Antioxidant Activity of Fresh Moringa Oleifera L. Leaves. Food Sci. Nutr. 2018, 6, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Fidrianny, I.; Budiana, W.; Ruslan, K. Antioxidant Activities of Various Extracts from Ardisia Sp Leaves Using Dpph and Cuprac Assays and Correlation with Total Flavonoid, Phenolic, Carotenoid Content. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 859–865. [Google Scholar]
- Kpegba, K.; Eloh, K.; Evenamede, K.S.; Afanyibo, Y.-G.; Elomri, A.; Simalou, O.; Boyode, P.; Agbonon, A.; Seguin, E. A Comparative Study of the Chemical Composition of the Extracts from Leaves, Stem Bark, and Root Bark of Cassia Sieberiana: Antibacterial Activities. Orient. J. Chem. 2019, 35, 1678–1689. [Google Scholar] [CrossRef]
- Traore, L.; Bekro, Y.; Pirat, J.; Mamyrbeva-bekro, J.A. Study of Crude Extracts from Cassia Sieberiana Root Bark and Khaya Grandifoliola Trunk Bark: Phytochemical Screening, Quantitative Analysis and Radical Scavenging Activity. Int. J. Curr. Pharm. Res. 2015, 7, 22–26. [Google Scholar]
- De Lopes, S.C.A.; Novais, M.V.M.; Teixeira, C.S.; Honorato-Sampaio, K.; Pereira, M.T.; Ferreira, L.A.M.; Braga, F.C.B.; Oliveira, M.C. Preparation, Physicochemical Characterization, and Cell Viability Evaluation of Long-Circulating and PH-Sensitive Liposomes Containing Ursolic Acid. Biomed Res. Int. 2013, 2013, 467147. [Google Scholar] [CrossRef]
- Sombié, P.A.E.D.; Hilou, A.; Mounier, C.; Coulibaly, A.Y.; Kiendrebeogo, M.; Millogo, J.F.; Nacoulma, O.G. Antioxidant and Anti-Inflammatory ACtivities from Galls of Guiera Senegalensis J.F Gmel (Combretaceae). Res. J. Med. Plant 2011, 5, 448–461. [Google Scholar] [CrossRef]
- Chen, G.L.; Mutie, F.M.; Xu, Y.B.; Saleri, F.D.; Hu, G.W.; Guo, M.Q. Antioxidant, Anti-Inflammatory Activities and Polyphenol Profile of Rhamnus Prinoides. Pharmaceuticals 2020, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein-Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- De Franco, C.J.P.; Ferreira, O.O.; de Moraes, Â.A.B.; Varela, E.L.P.; do Nascimento, L.D.; Percário, S.; de Oliveira, M.S.; de Andrade, E.H.A.A. Chemical Composition and Antioxidant Activity of Essential Oils from Eugenia Patrisii Vahl, E. Punicifolia (Kunth) DC., and Myrcia Tomentosa (Aubl.) DC., Leaf of Family Myrtaceae. Mol. Artic. 2021, 26, 3292. [Google Scholar] [CrossRef]
- Elgorashi, E.E.; McGaw, L.J. African Plants with in Vitro Anti-Inflammatory Activities: A Review. South Afr. J. Bot. 2019, 126, 142–169. [Google Scholar] [CrossRef]
- Derouich, M.; Bouhlali, E.D.T.; Hmidani, A.; Bammou, M.; Bourkhis, B.; Sellam, K.; Alem, C. Assessment of Total Polyphenols, Flavonoids and Anti-Inflammatory Potential of Three Apiaceae Species Grown in the Southeast of Morocco. Sci. Afr. 2020, 9, e00507. [Google Scholar] [CrossRef]
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase Inhibition by Plant Extracts. Biomolecules 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Engeu, P.O.; Omujal, F.; Agwaya, M.; Kyakulaga, H.; Obua, C. Variations in Antimalarial Components of Artemisia Annua Linn from Three Regions of Uganda. Afr. Health Sci. 2015, 15, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Agbangnan, D.C.P.; Noudogbessi, J.P.; Chrostowska, A.; Tachon, C.; Fouquet, E.; Sohounhloue, D.C.K. Phenolic Compound of Benin’s Red Sorghum and Their Antioxidant Properties. Asian J. Pharm. Clin. Res. 2013, 6, 277–280. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of Phenolics, Carotenoids, and Antioxidant Capacity Following Simulated Gastrointestinal Digestion and Dialysis of Selected Edible Green Leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Li, Y.; Yuan, B.; Xiao, W.; Liu, Z.; Zhang, S.; Lin, H. Development and Validation of an Analytical Method Based on HPLC-ELSD for the Simultaneous Determination of Rosmarinic Acid, Carnosol, Carnosic Acid, Oleanolic Acid and Ursolic Acid in Rosemary. Molecules 2019, 24, 323. [Google Scholar] [CrossRef] [PubMed]
- Deghima, A.; Righi, N.; Rosales-Conrado, N.; León-González, M.E.; Baali, F.; Gómez-Mejía, E.; Madrid, Y.; Bedjou, F. Anti-Inflammatory Activity of Ethyl Acetate and n-Butanol Extracts from Ranunculus Macrophyllus Desf. and Their Phenolic Profile. J. Ethnopharmacol. 2021, 265, 113347. [Google Scholar] [CrossRef]
- Yeo, D.; Dinica, R.; Yapi, H.F.; Furdui, B.; Praisler, M.; Djaman, A.J.; N’Guessan, J.D. Évaluation de l’activité Anti-Inflammatoire et Screening Phytochimique Des Feuilles de Annona Senegalensis. Therapie 2011, 66, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.; Hashim, Y.Z.H.Y.; El-Kersh, D.M.; Abd-Azziz, S.S.S.; Salleh, H.M.; Isa, M.L.M.; Warif, N.M.A. Metabolite Profiling of Aquilaria Malaccensis Leaf Extract Using Liquid Chromatography-Q-TOF-Mass Spectrometry and Investigation of Its Potential Antilipoxygenase Activity in-Vitro. Processes 2020, 8, 202. [Google Scholar] [CrossRef]
- Dadoriya, P.; Dey, Y.N.; Sharma, D.; Yadav, M.; Wanjari, M.M.; Gaidhani, S.N.; Subhose, V. In-Vitro Anti-Inflammatory and Antioxidant Activities of an Ayurvedic Formulation –Trayodashang Guggulu. J. Herb. Med. 2020, 23, 100366. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Ghinea, I.O.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.E.; Urlan, F.; Dinica, R.M. Exploring New Antioxidant and Mineral Compounds from Nymphaea Alba Wild-Grown in Danube Delta Biosphere. Molecules 2018, 23, 1247. [Google Scholar] [CrossRef]
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Composición de Isoflavonas, Contenido de Polifenoles y Actividad Antioxidante de Las Semillas de Soja Durante Fermentación de Tempeh. CYTA-J. Food 2017, 15, 27–33. [Google Scholar] [CrossRef]
- Marquardt, P.; Vissiennon, C.; Schubert, A.; Birkemeyer, C.; Ahyi, V.; Fester, K. Phytochemical Analysis, in Vitro Anti-Inflammatory and Antimicrobial Activity of Piliostigma Thonningii Leaf Extracts from Benin. Planta Med. 2020, 86, 1269–1277. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Ghinea, I.O.; Mihaila, M.D.I.; Blaga, G.V.; Avramescu, S.M.; Cudalbeanu, M.; Isticioaia, S.F.; Dinica, R.M.; Furdui, B. Hplc-Dad Polyphenolic Profiling and Antioxidant Activities of Sorghum Bicolor during Germination. Agronomy 2021, 11, 417. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Stämpfli, R.; Brühwiler, P.; Mourad, S.; Verdejo, R.; Shaffer, M. Development and Characterisation of Carbon Nanotube-Reinforced Polyurethane Foams. EMPA Act. 2007, 26, 51. [Google Scholar]
- Carac, A.; Boscencu, R.; Patriche, S.; Dinica, R.M.; Carac, G.; Gird, C.E. Antioxidant and Antimicrobial Potential of Extracts from Aloe Vera Leaves. Rev. Chim. 2016, 67, 654–658. [Google Scholar]
- Balanescu, F.; Busuioc, A.C.; Botezatu, A.V.D.; Gosav, S.; Avramescu, S.M.; Furdui, B.; Dinica, R.M. Comparative Study of Natural Antioxidants from Glycine Max, Anethum Graveolensand Pimpinella Anisum Seed and Sprout Extracts Obtained by Ultrasound-Assisted Extraction. Separations 2022, 9, 152. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. In Vitro Anti-Inflammatory Properties of Selected Green Leafy Vegetables. Biomedicines 2018, 6, 107. [Google Scholar] [CrossRef]
| Compounds | RT * | C. sieberiana | P. thonningii | ||||
|---|---|---|---|---|---|---|---|
| Root | Bark | Leaves | Root | Bark | Leaves | ||
| Hydroxybenzoic acids | |||||||
| Gallic acid | 5.9 | n.d. | n.d. | 18.029 ± 0.41 | 26.744 ±1.04 | 216.069 ± 1.59 | n.d. |
| Epicatechin | 23.9 | 27.393 ± 0.78 | 26.867 ± 0.59 | n.d. | n.d. | n.d. | n.d. |
| Tannic acid | 24.3 | n.d. | 1077.675 ± 1.60 | 12.27 ± 1.33 | 10.478 ± 0.81 | 11.638 ± 0.55 | n.d. |
| Flavons | |||||||
| Rutin | 31.4 | n.d. | 623.84 ± 0.70 | 490.517 ± 1.04 | 31.138 ± 0.66 | n.d. | n.d. |
| Naringin | 11.3 | 27.67 ± 0.96 | 107.352 ± 0.26 | 816.968 ± 1.11 | 545.117 ± 1.95 | n.d. | 457.14 ± 1.04 |
| Genistein | 22.7 | 421.376 ± 1.23 | n.d | n.d. | n.d. | n.d. | n.d. |
| Daidzein | 33.6 | 148.362 ± 2.45 | n.d | n.d. | n.d. | 118.029 ± 0.38 | n.d. |
| Hyperoside | 6.9 | 80.296 ± 0.77 | 484.07 ± 0.84 | n.d. | n.d. | n.d. | n.d. |
| Hydrocinnamic acids | |||||||
| Chlorogenic acid | 22.3 | n.d. | 705.412 ± 2.67 | n.d. | n.d. | n.d. | 23.435 ± 0.59 |
| p-Coumaric acid | 28.9 | 1.757 ± 0.29 | 56.36 ± 1.01 | n.d. | n.d. | n.d. | 0.217 ± 0.92 |
| Total | 706.854 ± 6.48 | 3081.576 ± 7.67 | 1337.784 ± 3.89 | 613.477 ± 4.45 | 345.736 ± 2.52 | 480.792 ± 2.55 | |
| Plants | Parts of the Plant | DPPH (IC50 µg/mL) | ABTS (IC50 µg/mL) | TAC (mg AAE/g DW) |
|---|---|---|---|---|
| Cassia sieberiana | Leaves | 21.98 ± 0.19 d | 5.96 ± 1.20 b | 25.63 ± 0.58 c |
| Bark | 22.20 ± 0.35 d | 1.91 ± 0.26 b | 27.55 ± 1.89 bc | |
| Root | 31.00 ± 0.38 c | 1.83 ± 0.34 b | 18.90 ± 0.76 d | |
| Piliostigma thonningii | Leaves | 72.48 ± 0.19 a | 26.90 ± 4.41 a | 14.12 ± 0.30 e |
| Bark | 13.45 ± 0.10 e | 6.36 ± 0.20 b | 28.03 ± 0.25 bc | |
| Root | 55.99 ± 0.49 b | 5.96 ± 0.28 b | 29.68 ± 1.48 b | |
| Trolox | 10.57 ± 0.0019 | 32.56 ± 0.0016 | - | |
| Vitamin C | - | - | 44.95 ± 0.002 a |
| Plants | Parts of the Plant | Anti-Lipoxygenase Activity (IC50 µg/mL) | Protease Inhibitory Activity (IC50 µg/mL) | Membrane Stabilization Activity (IC50 µg/mL) |
|---|---|---|---|---|
| Cassia sieberiana | Leaves | 31.53 ± 7.93 a | 75.74 ± 1.07 e | 48.32 ± 6.39 c |
| Bark | 13.04 ± 1.99 d | 200.16 ± 2.63 a | 51.10 ± 0.97 bc | |
| Root | 38.07 ± 2.62 a | 188.02 ± 0.85 c | 50.15 ± 5.23 c | |
| Piliostigma thonningii | Leaves | 16.20 ± 1.82 cd | 78.07 ± 0.05 e | 53.80 ± 0.75 bc |
| Bark | 24.79 ± 2.61 b | 195.87 ± 0.83 b | 67.43 ± 7.36 a | |
| Root | 22.01 ± 1.36 bc | 117.77 ± 3.63 d | 58.87 ± 1.80 b | |
| Aspirin (acetylsalicylic acid) | - | 160.20 ± 0.02 | - | |
| Indomethacin Diclofenac sodium | 45.12 ± 0.014 | - | - | |
| - | - | 68.78 ± 0.07 |
| DPPH | ABTS | TAC | Anti-Lipoxygenase Activity | Protease Inhibitory Activity | Membrane Stabilization Activity | ||
|---|---|---|---|---|---|---|---|
| Polyphenols | r | −0.481 | −0.679 | 0.960 | −0.199 | 0.455 | 0.326 |
| P | 0.043 | 0.015 | 0.000 | 0.429 | 0.058 | 0.187 | |
| Flavonoids | r | 0.016 | 0.312 | −0.317 | 0.269 | −0.729 | −0.475 |
| P | 0.949 | 0,324 | 0.316 | 0.284 | 0.001 | 0.046 | |
| Tannins | r | −0.585 | −0.702 | 0.701 | −0.310 | 0.698 | 0.032 |
| P | 0.011 | 0.011 | 0.011 | 0.210 | 0.001 | 0.900 | |
| Vitamin C | r | 0.487 | 0.583 | −0.324 | 0.173 | −0.953 | −0.187 |
| P | 0.040 | 0.047 | 0.305 | 0.493 | 0.000 | 0.481 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zongo, E.; Busuioc, A.; Meda, R.N.-T.; Botezatu, A.V.; Mihaila, M.D.; Mocanu, A.-M.; Avramescu, S.M.; Koama, B.K.; Kam, S.E.; Belem, H.; et al. Exploration of the Antioxidant and Anti-inflammatory Potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, Traditionally Used in the Treatment of Hepatitis in the Hauts-Bassins Region of Burkina Faso. Pharmaceuticals 2023, 16, 133. https://doi.org/10.3390/ph16010133
Zongo E, Busuioc A, Meda RN-T, Botezatu AV, Mihaila MD, Mocanu A-M, Avramescu SM, Koama BK, Kam SE, Belem H, et al. Exploration of the Antioxidant and Anti-inflammatory Potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, Traditionally Used in the Treatment of Hepatitis in the Hauts-Bassins Region of Burkina Faso. Pharmaceuticals. 2023; 16(1):133. https://doi.org/10.3390/ph16010133
Chicago/Turabian StyleZongo, Eliasse, Anna Busuioc, Roland Nâg-Tiero Meda, Andreea Veronica Botezatu, Maria Daniela Mihaila, Ana-Maria Mocanu, Sorin Marius Avramescu, Benjamin Kouliga Koama, Sami Eric Kam, Hadidiatou Belem, and et al. 2023. "Exploration of the Antioxidant and Anti-inflammatory Potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, Traditionally Used in the Treatment of Hepatitis in the Hauts-Bassins Region of Burkina Faso" Pharmaceuticals 16, no. 1: 133. https://doi.org/10.3390/ph16010133
APA StyleZongo, E., Busuioc, A., Meda, R. N.-T., Botezatu, A. V., Mihaila, M. D., Mocanu, A.-M., Avramescu, S. M., Koama, B. K., Kam, S. E., Belem, H., Somda, F. L. S., Ouedraogo, C., Ouedraogo, G. A., & Dinica, R. M. (2023). Exploration of the Antioxidant and Anti-inflammatory Potential of Cassia sieberiana DC and Piliostigma thonningii (Schumach.) Milne-Redh, Traditionally Used in the Treatment of Hepatitis in the Hauts-Bassins Region of Burkina Faso. Pharmaceuticals, 16(1), 133. https://doi.org/10.3390/ph16010133








