Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
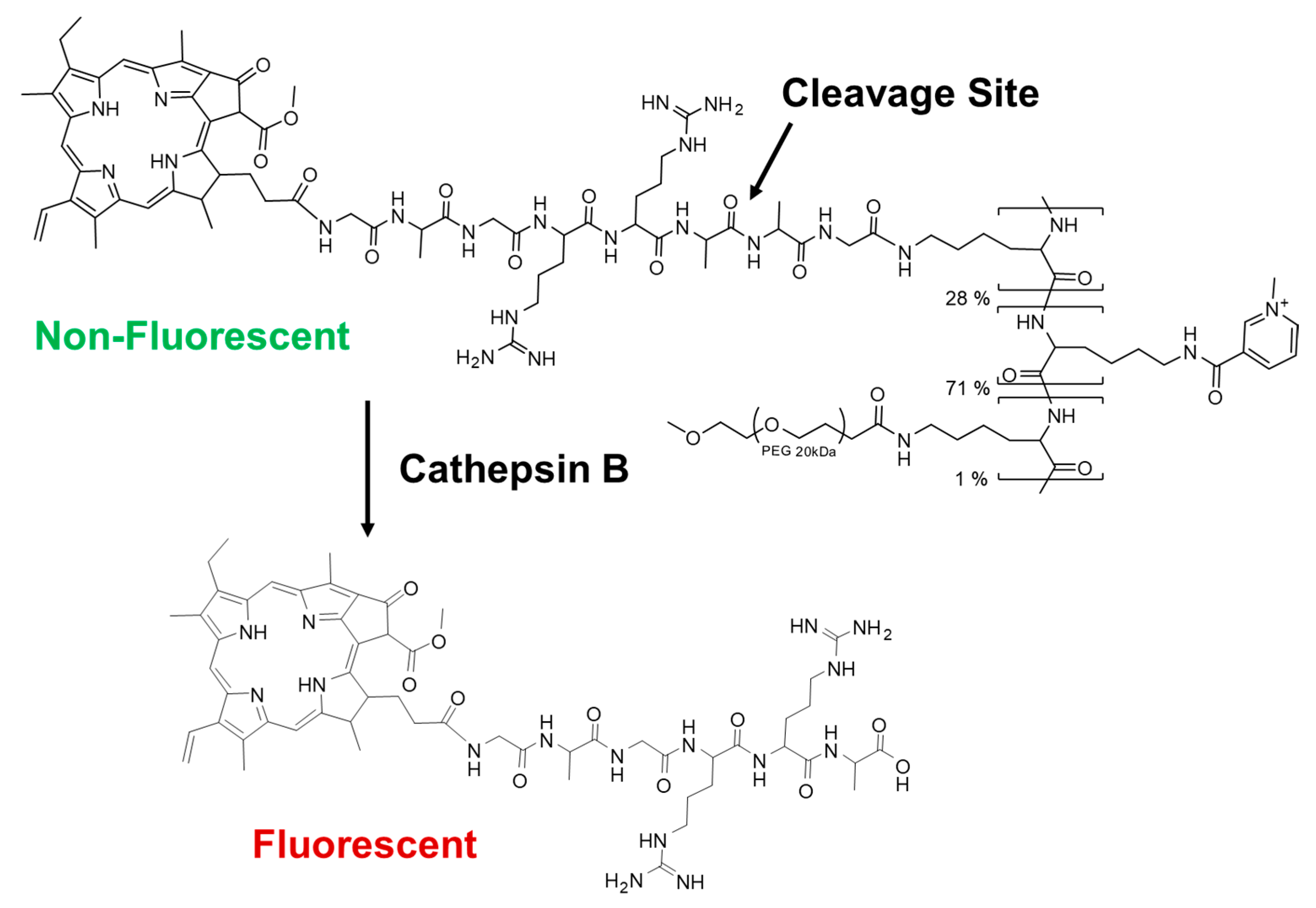
2.2. Synthesis and Characterization of CTSB-PPP
2.2.1. Synthesis of Pha-CTSB
2.2.2. Synthesis of CTSB-PPP
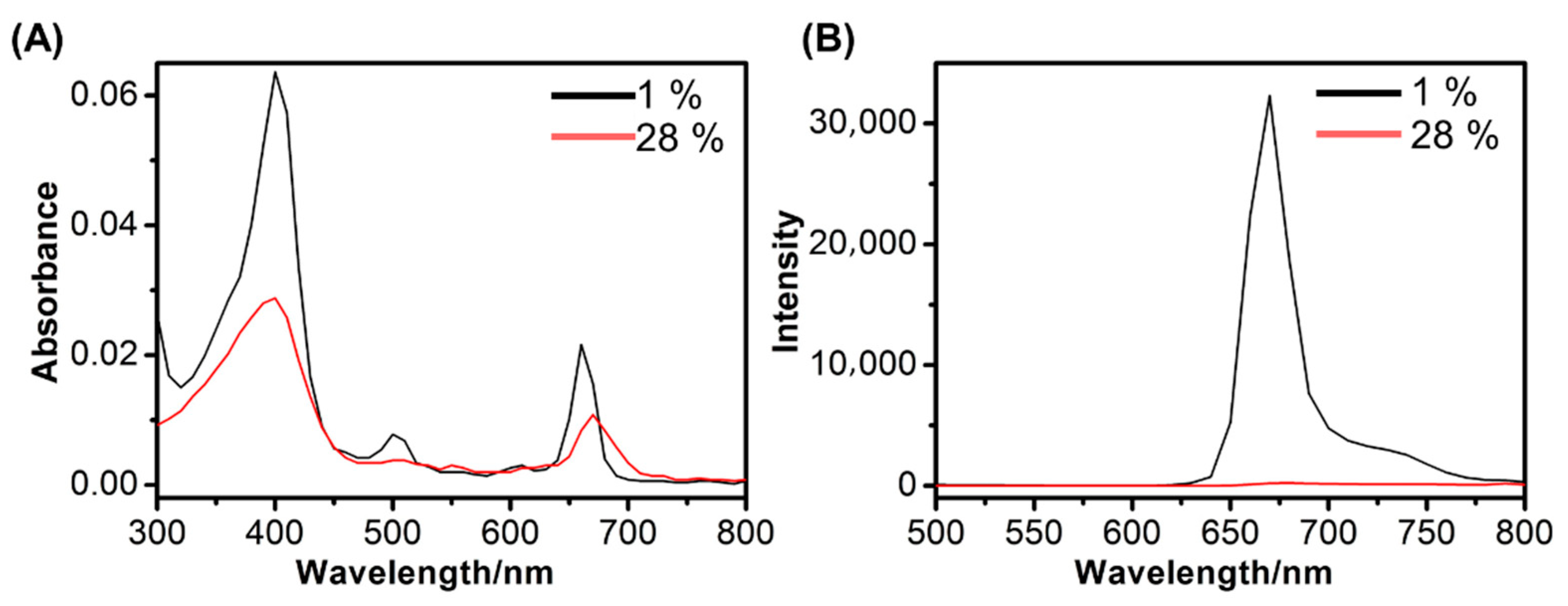
2.3. Quenching Efficiency
2.4. Spectral Characterization
2.5. Animal Experimentation
2.6. Cell Culture Conditions
2.7. In Vitro Activation of CTSB-PPP
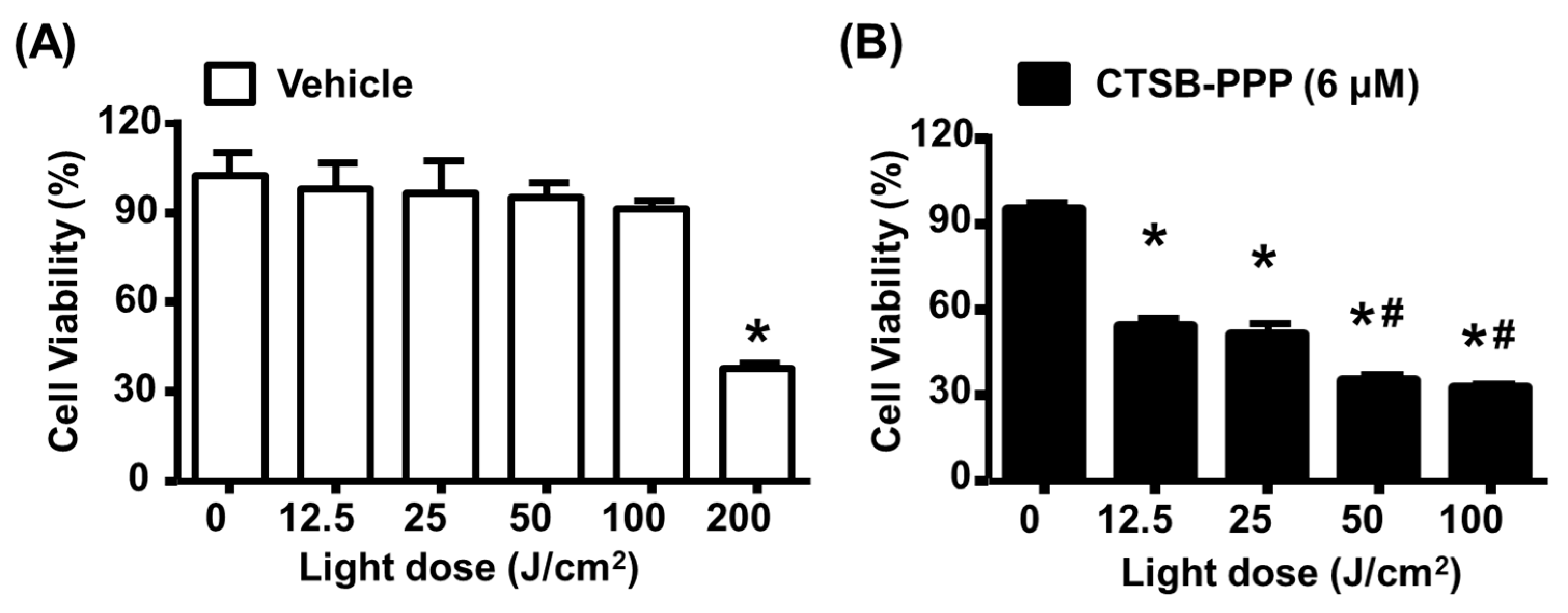
2.8. MTT Assay
2.9. Light Source and PDT
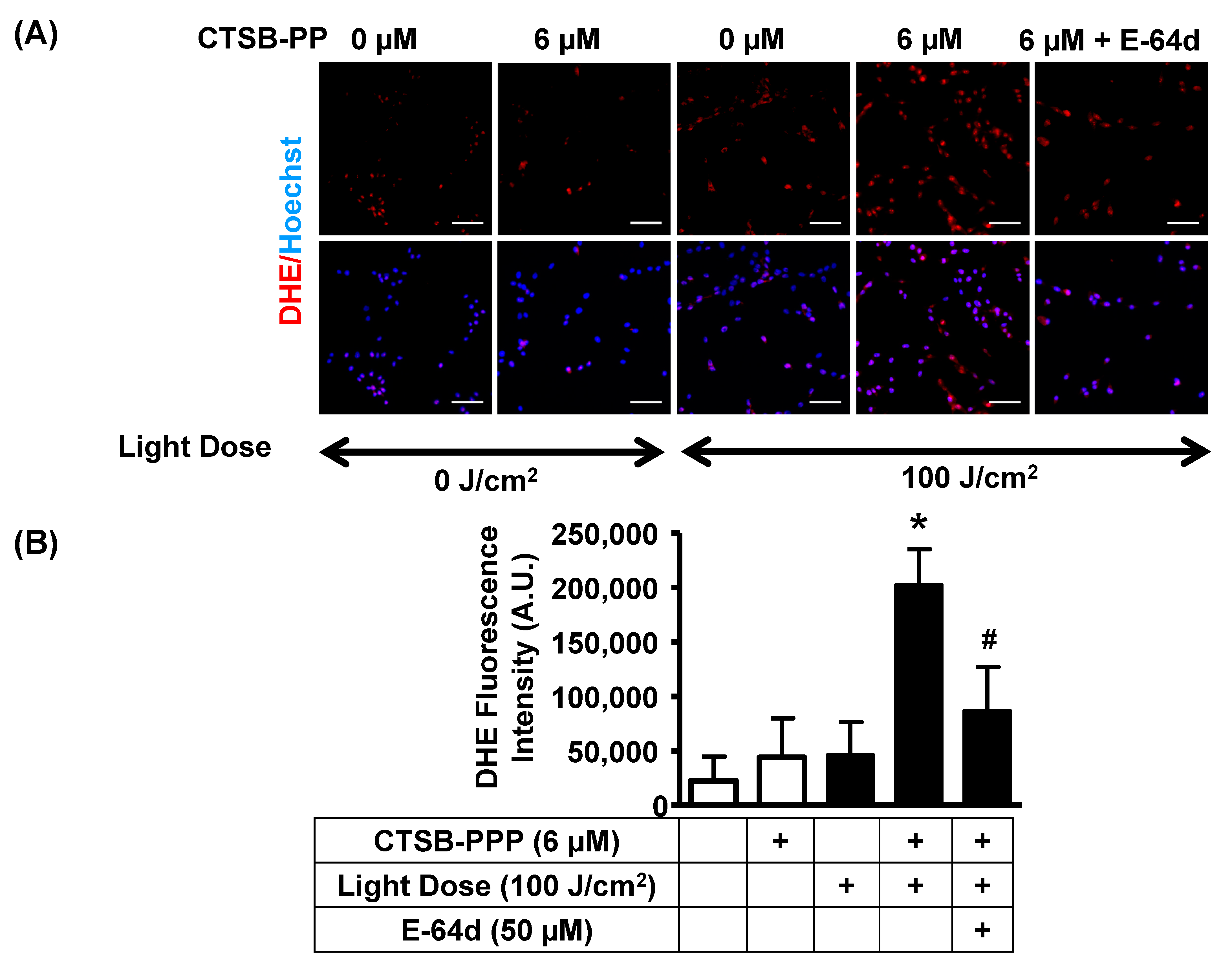
2.10. Detection of ROS
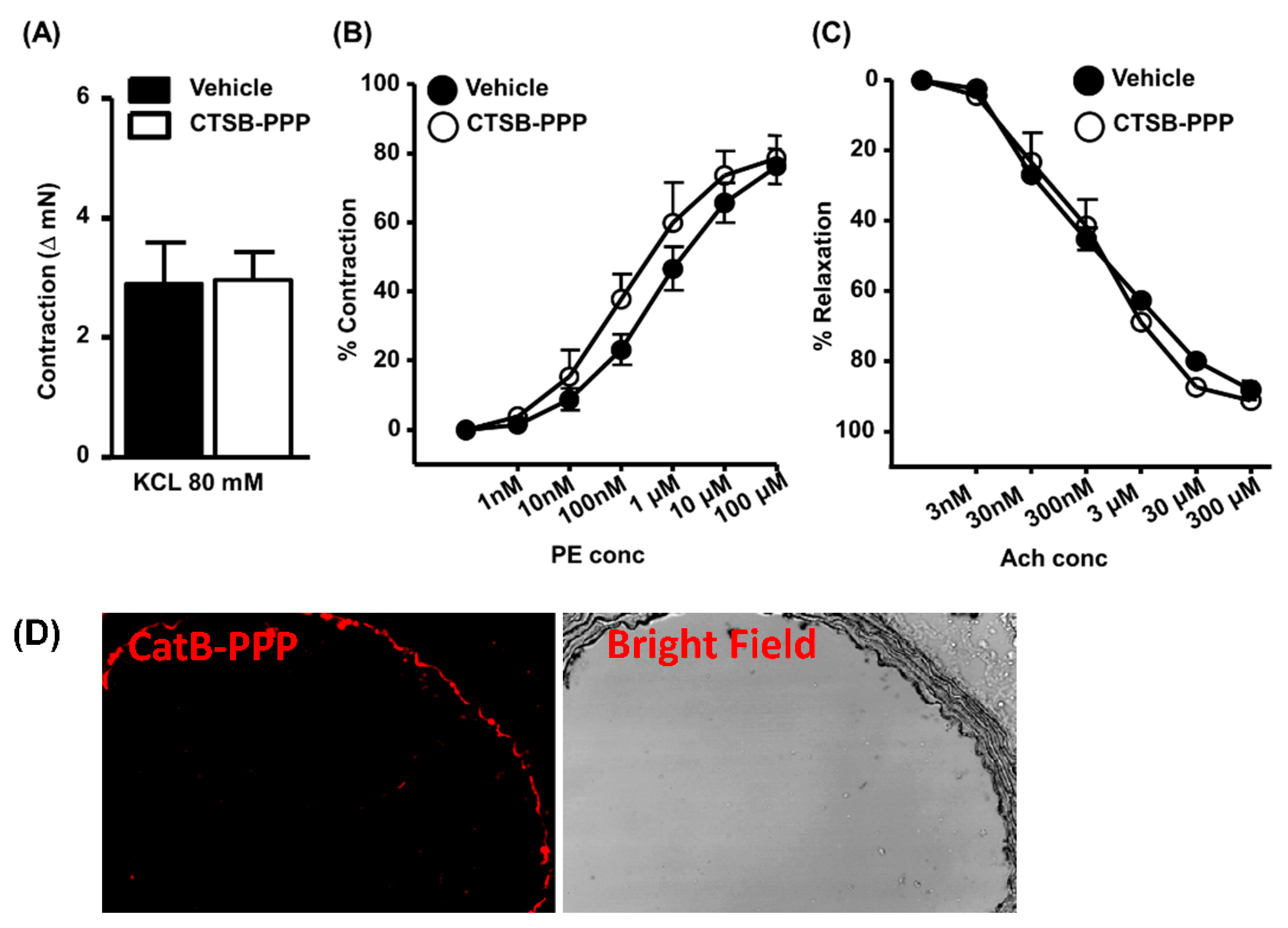
2.11. Assessment of Vascular Function
2.12. Statistical Analysis
3. Results
3.1. Preparation and Characterization of CTSB-PPP
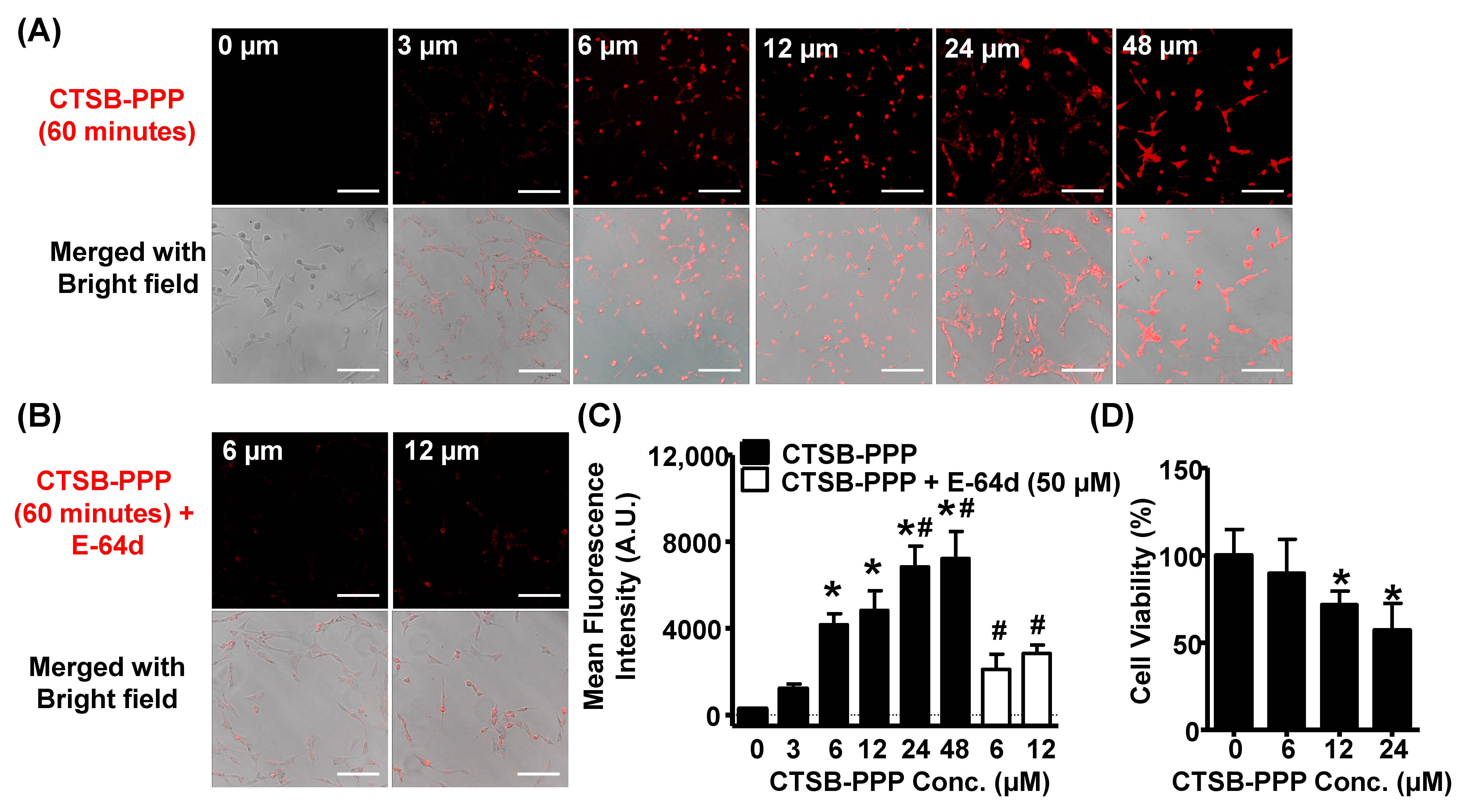
3.2. Cellular Uptake, Specificity, and Dark Toxicity of CTSB-PPP
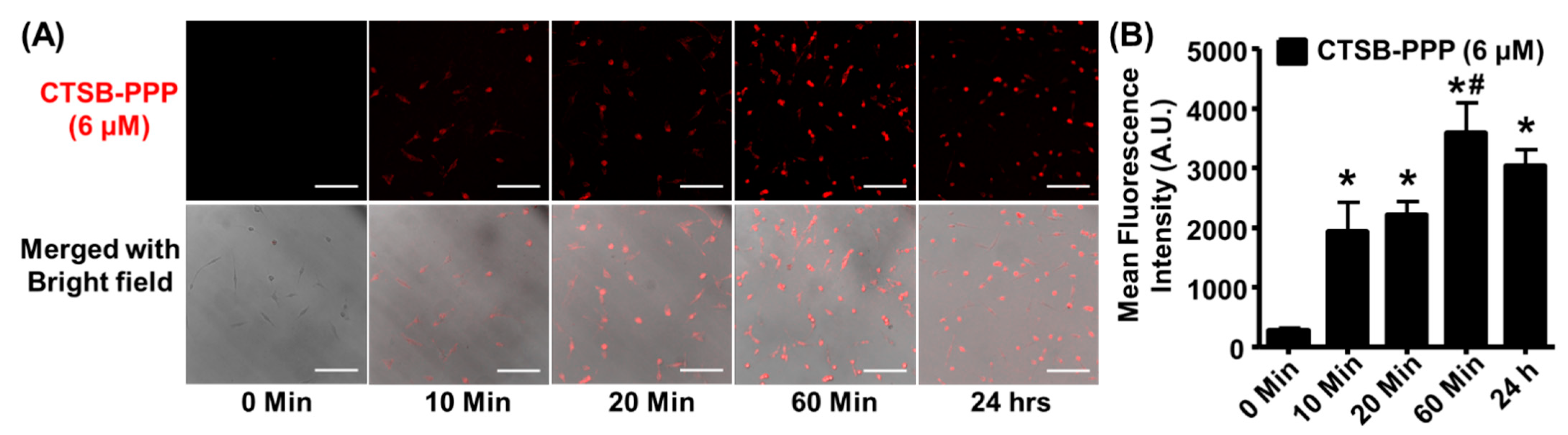
3.3. Time-Dependent CTSB-PPP Accumulation
3.4. Ex Vivo Toxicity
3.5. CTSB-PPP Based PDT Efficacy
3.6. PDT with CTSB-PPP Stimulated ROS Generation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Nair, S. Proteases in cardiometabolic diseases: Pathophysiology, molecular mechanisms and clinical applications. Biochim. Biophys. Acta 2015, 1852, 195–208. [Google Scholar] [CrossRef]
- Hook, V.; Yoon, M.; Mosier, C.; Ito, G.; Podvin, S.; Head, B.P.; Rissman, R.; O’Donoghue, A.J.; Hook, G. Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim. Biophys. Acta Proteins Proteom 2020, 1868, 140428. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Ye, N.; Qiu, M.; Guo, H.; Li, J.; Zhou, X.; Yang, M.; Xi, J.; Liang, Y.; Gong, Y.; et al. Cathepsin B is a potential therapeutic target for coronavirus disease 2019 patients with lung adenocarcinoma. Chem. Biol. Interact. 2022, 353, 109796. [Google Scholar] [CrossRef]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The Ins and Outs of Cathepsins: Physiological Function and Role in Disease Management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.T.; Meroueh, S.O.; Lee, M.; Heeg, M.J.; Mobashery, S. Strategy in inhibition of cathepsin B, a target in tumor invasion and metastasis. J. Am. Chem. Soc. 2004, 126, 10271–10277. [Google Scholar] [CrossRef]
- Ward, C.; Kuehn, D.; Burden, R.E.; Gormley, J.A.; Jaquin, T.J.; Gazdoiu, M.; Small, D.; Bicknell, R.; Johnston, J.A.; Scott, C.J.; et al. Antibody targeting of cathepsin S inhibits angiogenesis and synergistically enhances anti-VEGF. PLoS ONE 2010, 5, e12543. [Google Scholar] [CrossRef]
- Schenker, P.; Alfarano, P.; Kolb, P.; Caflisch, A.; Baici, A. A double-headed cathepsin B inhibitor devoid of warhead. Protein Sci. 2008, 17, 2145–2155. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Sosic, I.; Mirkovic, B.; Arenz, K.; Stefane, B.; Kos, J.; Gobec, S. Development of new cathepsin B inhibitors: Combining bioisosteric replacements and structure-based design to explore the structure-activity relationships of nitroxoline derivatives. J. Med. Chem. 2013, 56, 521–533. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Zheng, L.; Zhong, Z.; Li, X.; Zhao, J.; Kou, J.; Jiang, Y.; Zheng, X.; Liu, Z.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway. Int. J. Nanomedicine 2015, 10, 3719–3736. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Chen, Z.; Woodburn, K.W.; Shi, C.; Adelman, D.C.; Rogers, C.; Simon, D.I. Photodynamic therapy with motexafin lutetium induces redox-sensitive apoptosis of vascular cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Zellweger, M.; Wagnieres, G.; van den Bergh, H.; Cook, S.; Giraud, M.N. Photodynamic therapy for the treatment of atherosclerotic plaque: Lost in translation? Cardiovasc. Ther. 2017, 35, e12238. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Hajri, A.; Wack, S.; Meyer, C.; Smith, M.K.; Leberquier, C.; Kedinger, M.; Aprahamian, M. In vitro and in vivo efficacy of photofrin and pheophorbide a, a bacteriochlorin, in photodynamic therapy of colonic cancer cells. Photochem. Photobiol. 2002, 75, 140–148. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Xodo, L.E.; Rapozzi, V.; Zacchigna, M.; Drioli, S.; Zorzet, S. The chlorophyll catabolite pheophorbide a as a photosensitizer for the photodynamic therapy. Curr. Med. Chem. 2012, 19, 799–807. [Google Scholar] [CrossRef]
- Djalil, A.D.; Nurulita, N.A.; Limantara, W.L.; Ibrahim, S.; Tjahjono, D. Biological evaluations of protoporphyrin ix, pheophorbide a, and its 1hydroxyethyl derivativess for application in photodynamic therapy. Int. J. Pharm. Pharm. Sci. 2012, 4, 741–746. [Google Scholar]
- Radestock, A.; Elsner, P.; Gitter, B.; Hipler, U.C. Induction of apoptosis in HaCaT cells by photodynamic therapy with chlorin e6 or pheophorbide a. Skin. Pharmacol. Physiol. 2007, 20, 3–9. [Google Scholar] [CrossRef]
- Tung, C.H.; Mahmood, U.; Bredow, S.; Weissleder, R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000, 60, 4953–4958. [Google Scholar]
- Choi, Y.; Weissleder, R.; Tung, C.H. Selective antitumor effect of novel protease-mediated photodynamic agent. Cancer Res. 2006, 66, 7225–7229. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Campo, M.A.; Gurny, R.; Lange, N. Tailoring protease-sensitive photodynamic agents to specific disease-associated enzymes. Bioconjug Chem. 2007, 18, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Herceg, V.; Bouilloux, J.; Janikowska, K.; Allemann, E.; Lange, N. Cathepsin B-Cleavable Cyclopeptidic Chemotherapeutic Prodrugs. Molecules 2020, 25, 4285. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, Q.; Pan, W.; Li, N.; Tang, B. A Near-Infrared Triggered Nanophotosensitizer Inducing Domino Effect on Mitochondrial Reactive Oxygen Species Burst for Cancer Therapy. ACS Nano 2015, 9, 11064–11074. [Google Scholar] [CrossRef]
- Tedjamulia, M.L.; Srivastava, P.C.; Knapp, F.F., Jr. Evaluation of the brain-specific delivery of radioiodinated (iodophenyl)alkyl-substituted amines coupled to a dihydropyridine carrier. J. Med. Chem. 1985, 28, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.A.; Gabriel, D.; Kucera, P.; Gurny, R.; Lange, N. Polymeric photosensitizer prodrugs for photodynamic therapy. Photochem. Photobiol. 2007, 83, 958–965. [Google Scholar] [CrossRef]
- Ruzza, P.; Quintieri, L.; Osler, A.; Calderan, A.; Biondi, B.; Floreani, M.; Guiotto, A.; Borin, G. Fluorescent, internally quenched, peptides for exploring the pH-dependent substrate specificity of cathepsin B. J. Pept. Sci. 2006, 12, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, C. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J. Vis. Exp. 2010, 37, e1852. [Google Scholar] [CrossRef]
- Zuluaga, M.F.; Sekkat, N.; Gabriel, D.; van den Bergh, H.; Lange, N. Selective photodetection and photodynamic therapy for prostate cancer through targeting of proteolytic activity. Mol. Cancer Ther. 2013, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Lange, N.; Chobaz-Peclat, V.; Zuluaga, M.F.; Gurny, R.; van den Bergh, H.; Busso, N. Thrombin-sensitive dual fluorescence imaging and therapeutic agent for detection and treatment of synovial inflammation in murine rheumatoid arthritis. J. Control Release 2012, 163, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. (Hoboken) 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Harris-Love, M.O.; Seamon, B.A.; Teixeira, C.; Ismail, C. Ultrasound estimates of muscle quality in older adults: Reliability and comparison of Photoshop and ImageJ for the grayscale analysis of muscle echogenicity. PeerJ 2016, 4, e1721. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Singh, A.; Singh, V.; Barthwal, M.K. Involvement of interleukin-1 receptor-associated kinase-1 in vascular smooth muscle cell proliferation and neointimal formation after rat carotid injury. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1445–1455. [Google Scholar] [CrossRef]
- Jain, M.; Dhanesha, N.; Doddapattar, P.; Nayak, M.K.; Guo, L.; Cornelissen, A.; Lentz, S.R.; Finn, A.V.; Chauhan, A.K. Smooth Muscle Cell-Specific PKM2 (Pyruvate Kinase Muscle 2) Promotes Smooth Muscle Cell Phenotypic Switching and Neointimal Hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1724–1737. [Google Scholar] [CrossRef]
- Borle, F.; Radu, A.; Fontolliet, C.; van den Bergh, H.; Monnier, P.; Wagnieres, G. Selectivity of the photosensitiser Tookad for photodynamic therapy evaluated in the Syrian golden hamster cheek pouch tumour model. Br. J. Cancer 2003, 89, 2320–2326. [Google Scholar] [CrossRef][Green Version]
- Rapozzi, V.; Zorzet, S.; Zacchigna, M.; Drioli, S.; Xodo, L.E. The PDT activity of free and pegylated pheophorbide a against an amelanotic melanoma transplanted in C57/BL6 mice. Investig. New Drugs 2013, 31, 192–199. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Missinato, M.A.; Onuoha, E.; Perkins, L.A.; Watkins, S.C.; St Croix, C.M.; Tsang, M.; Bruchez, M.P. A genetically targetable near-infrared photosensitizer. Nat. Methods 2016, 13, 263–268. [Google Scholar] [CrossRef]
- Jain, M.; Zellweger, M.; Frobert, A.; Valentin, J.; van den Bergh, H.; Wagnieres, G.; Cook, S.; Giraud, M.N. Intra-Arterial Drug and Light Delivery for Photodynamic Therapy Using Visudyne(R): Implication for Atherosclerotic Plaque Treatment. Front. Physiol. 2016, 7, 400. [Google Scholar] [CrossRef]
- Jain, M.; Singh, A.; Singh, V.; Maurya, P.; Barthwal, M.K. Gingerol Inhibits Serum-Induced Vascular Smooth Muscle Cell Proliferation and Injury-Induced Neointimal Hyperplasia by Suppressing p38 MAPK Activation. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Barthwal, M.K.; Haq, W.; Katti, S.B.; Dikshit, M. Synthesis and pharmacological evaluation of novel arginine analogs as potential inhibitors of acetylcholine-induced relaxation in rat thoracic aortic rings. Chem. Biol. Drug Des. 2012, 79, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.F.; Gabriel, D.; Lange, N. Enhanced prostate cancer targeting by modified protease sensitive photosensitizer prodrugs. Mol. Pharm. 2012, 9, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.; Liu, X.Z.; Zhang, D.M.; Fong, W.P.; Fung, K.P. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial-mediated pathway in human uterine carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.H.; Ryoo, I.G.; Kang, H.C.; Kwak, M.K. The sensitivity of cancer cells to pheophorbide a-based photodynamic therapy is enhanced by Nrf2 silencing. PLoS ONE 2014, 9, e107158. [Google Scholar] [CrossRef]
- Gabriel, D.; Busso, N.; So, A.; van den Bergh, H.; Gurny, R.; Lange, N. Thrombin-sensitive photodynamic agents: A novel strategy for selective synovectomy in rheumatoid arthritis. J. Control Release 2009, 138, 225–234. [Google Scholar] [CrossRef]
- Maurer, M.H. Proteomic definitions of mesenchymal stem cells. Stem Cells Int. 2011, 2011, 704256. [Google Scholar] [CrossRef]
- Sasnouski, S.; Pic, E.; Dumas, D.; Zorin, V.; D’Hallewin, M.A.; Guillemin, F.; Bezdetnaya, L. Influence of incubation time and sensitizer localization on meta-tetra(hydroxyphenyl)chlorin (mTHPC)-induced photoinactivation of cells. Radiat. Res. 2007, 168, 209–217. [Google Scholar] [CrossRef]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C.K. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef]
- Jespersen, B.; Tykocki, N.R.; Watts, S.W.; Cobbett, P.J. Measurement of smooth muscle function in the isolated tissue bath-applications to pharmacology research. J. Vis. Exp. 2015, 95, e52324. [Google Scholar] [CrossRef]
- Wunder, A.; Tung, C.H.; Muller-Ladner, U.; Weissleder, R.; Mahmood, U. In vivo imaging of protease activity in arthritis: A novel approach for monitoring treatment response. Arthritis. Rheum. 2004, 50, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Kim, S.A.; Koo, H.; Son, S.; Ryu, J.H.; Youn, I.C.; Choi, K.; Kwon, I.C.; Kim, K. Optical imaging of cancer-related proteases using near-infrared fluorescence matrix metalloproteinase-sensitive and cathepsin B-sensitive probes. Theranostics 2012, 2, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Calfon, M.A.; Rosenthal, A.; Mallas, G.; Mauskapf, A.; Nudelman, R.N.; Ntziachristos, V.; Jaffer, F.A. In vivo near infrared fluorescence (NIRF) intravascular molecular imaging of inflammatory plaque, a multimodal approach to imaging of atherosclerosis. J. Vis. Exp. 2011, 54, e2257. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Man, X.X.; Yao, H.X.; Tan, Y.Y. Effects of pheophorbide a-mediated photodynamic therapy on proliferation and metastasis of human prostate cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5571–5579. [Google Scholar] [CrossRef]
- Yoon, H.E.; Oh, S.H.; Kim, S.A.; Yoon, J.H.; Ahn, S.G. Pheophorbide a-mediated photodynamic therapy induces autophagy and apoptosis via the activation of MAPKs in human skin cancer cells. Oncol. Rep. 2014, 31, 137–144. [Google Scholar] [CrossRef]
- Satoh, K.; Nigro, P.; Berk, B.C. Oxidative stress and vascular smooth muscle cell growth: A mechanistic linkage by cyclophilin A. Antioxid. Redox. Signal 2010, 12, 675–682. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, M.; Bouilloux, J.; Borrego, I.; Cook, S.; van den Bergh, H.; Lange, N.; Wagnieres, G.; Giraud, M.-N. Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies. Pharmaceuticals 2022, 15, 564. https://doi.org/10.3390/ph15050564
Jain M, Bouilloux J, Borrego I, Cook S, van den Bergh H, Lange N, Wagnieres G, Giraud M-N. Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies. Pharmaceuticals. 2022; 15(5):564. https://doi.org/10.3390/ph15050564
Chicago/Turabian StyleJain, Manish, Jordan Bouilloux, Ines Borrego, Stéphane Cook, Hubert van den Bergh, Norbert Lange, Georges Wagnieres, and Marie-Noelle Giraud. 2022. "Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies" Pharmaceuticals 15, no. 5: 564. https://doi.org/10.3390/ph15050564
APA StyleJain, M., Bouilloux, J., Borrego, I., Cook, S., van den Bergh, H., Lange, N., Wagnieres, G., & Giraud, M.-N. (2022). Cathepsin B-Cleavable Polymeric Photosensitizer Prodrug for Selective Photodynamic Therapy: In Vitro Studies. Pharmaceuticals, 15(5), 564. https://doi.org/10.3390/ph15050564






