Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Photosensitizer
4.2. Cell Lines
4.3. Photodynamic Treatment and Cell Viability Assays
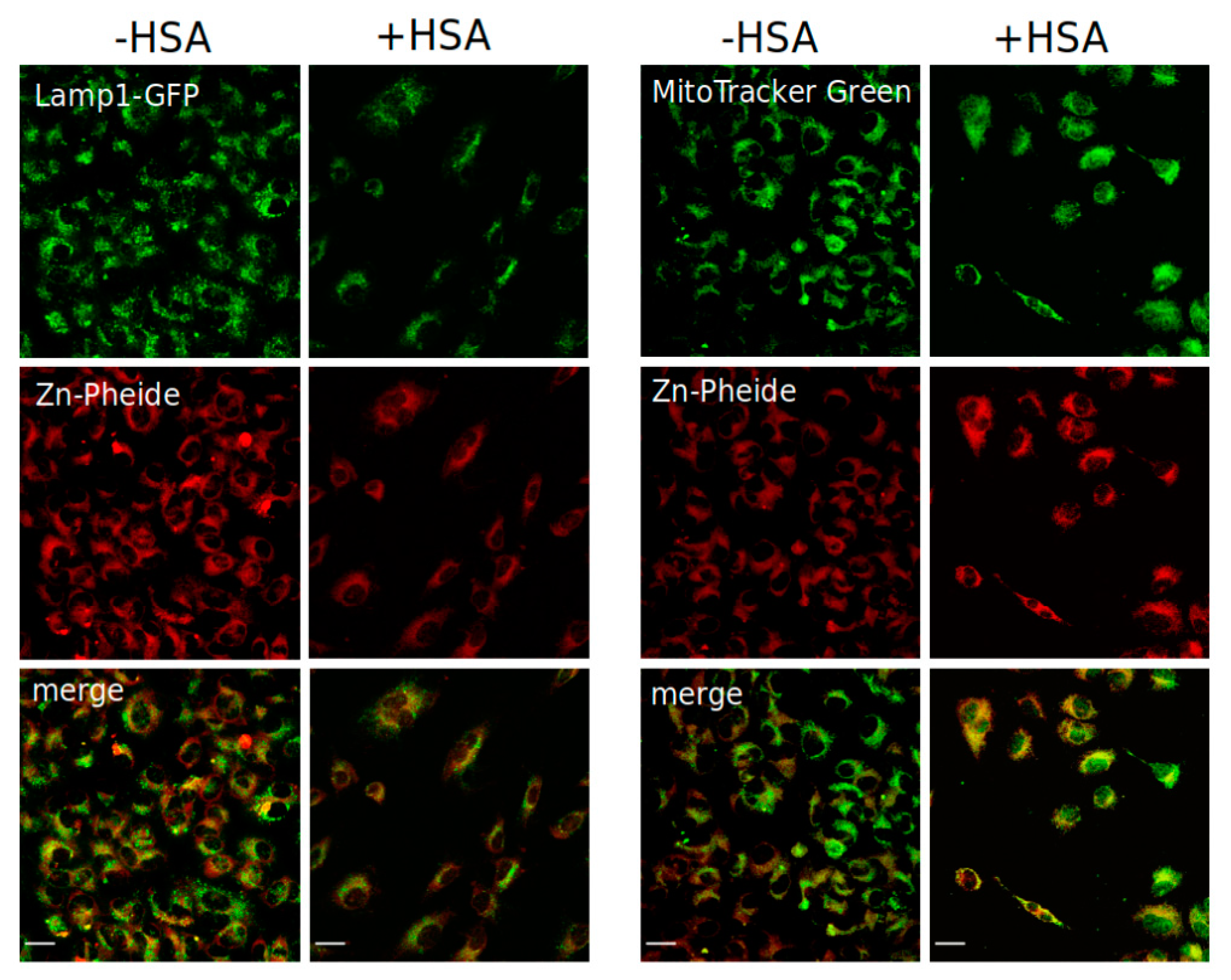
4.4. Cellular Localization of Zn-Pheide
4.5. Efflux Assay
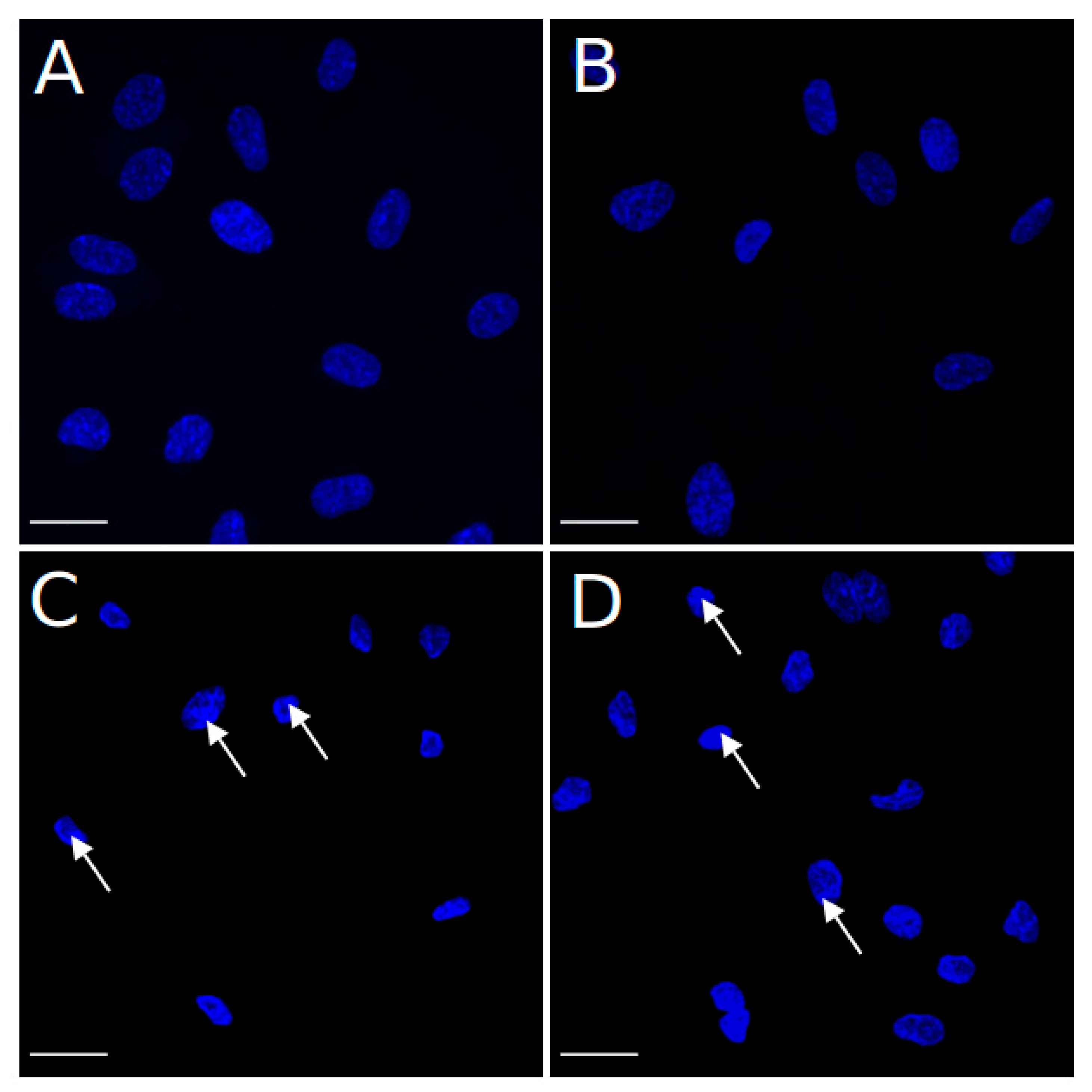
4.6. Hoechst Staining Assay
4.7. Analysis of Cell Death Mode by Flow Cytometry
4.8. Quantitative Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. An Update on Photodynamic Therapy Applications. J. Clin. Laser Med. Surg. 2002, 20, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, V.; Op’t Hoog, C.; Oliveira, S. Vascular targeted photodynamic therapy: A review of the efforts towards molecular targeting of tumor vasculature. J. Porphyr. Phtalocyanines 2019, 23, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Ezleen, E.; Benachour, H.; Barberi-Heyob, M.; Frochot, C.A.H.; Guillemin, F.; Vanderesse, R. Vascular-Targeted Photodynamic Therapy (VTP). Adv. Cancer Ther. 2011, 10, 681–688. [Google Scholar] [CrossRef][Green Version]
- Azzouzi, A.R.; Barret, E.; Moore, C.M.; Villers, A.; Allen, C.; Scherz, A.; Muir, G.; De Wildt, M.; Barber, N.J.; Lebdai, S.; et al. TOOKAD® Soluble vascular-targeted photodynamic (VTP) therapy: Determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013, 112, 766–774. [Google Scholar] [CrossRef]
- Mellish, K.J.; Brown, S.B. Verteporfin: A milestone in opthalmology and photodynamic therapy. Expert Opin. Pharmacother. 2001, 2, 351–361. [Google Scholar] [CrossRef]
- Olivo, M.; Lucky, R.B.S.S.; Dendukuri, N.; Thong, P.S.-P. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals 2010, 3, 1507–1529. [Google Scholar] [CrossRef]
- Gerola, A.P.; Tsubone, T.M.; Santana, A.; De Oliveira, H.P.M.; Hioka, N.; Caetano, W. Properties of chlorophyll and derivatives in homogeneous and microheterogeneous systems. J. Phys. Chem. B 2011, 115, 7364–7373. [Google Scholar] [CrossRef]
- Gerola, A.P.; Semensato, J.; Pellosi, D.S.; Batistela, V.R.; Rabello, B.R.; Hioka, N.; Caetano, W. Chemical determination of singlet oxygen from photosensitizers illuminated with LED: New calculation methodology considering the influence of photobleaching. J. Photochem. Photobiol. A Chem. 2012, 232, 14–21. [Google Scholar] [CrossRef]
- Jakubowska, M.; Szczygieł, M.; Michalczyk-Wetula, D.; Susz, A.; Stochel, G.; Elas, M.; Fiedor, L.; Urbanska, K. Zinc-pheophorbide a-Highly efficient low-cost photosensitizer against human adenocarcinoma in cellular and animal models. Photodiagn. Photodyn. Ther. 2013, 10, 266–277. [Google Scholar] [CrossRef]
- Szczygieł, M.; Boroń, B.; Szczygieł, D.; Szafraniec, M.; Susz, A.; Matuszak, Z.; Urbańska, K.; Fiedor, L. Real-time Non-invasive Transdermal Monitoring of Photosensitizer Level in vivo for Pharmacokinetic Studies and Optimization of Photodynamic Therapy Protocol. J. Anal. Bioanal. Technol. 2014, 5. [Google Scholar] [CrossRef]
- Gerola, A.P.; Costa, P.F.A.; de Morais, F.A.P.; Tsubone, T.M.; Caleare, A.O.; Nakamura, C.V.; Brunaldi, K.; Caetano, W.; Kimura, E.; Hioka, N. Liposome and polymeric micelle-based delivery systems for chlorophylls: Photodamage effects on Staphylococcus aureus. Colloids Surf. B Biointerfaces 2019, 177, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, M.J.; Fiedor, L. One ring is not enough to rule them all. Albumin-dependent ABCG2-mediated transport of chlorophyll-derived photosensitizers. Eur. J. Pharm. Sci. 2021, 167, 106001. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, M.J. Interactions of chlorophyll-derived photosensitizers with human serum albumin are determined by the central metal ion. J. Biomol. Struct. Dyn. 2021. [Google Scholar] [CrossRef]
- Vogel, S.M.; Minshall, R.D.; Pilipović, M.; Tiruppathi, C.; Malik, A.B. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 281, L1512–L1522. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ben-Josef, E.; Robb, R.; Vedaie, M.; Seum, S.; Thirumoorthy, K.; Palanichamy, K.; Harbrecht, M.; Chakravarti, A.; Williams, T.M. Caveolae-mediated endocytosis is critical for albumin cellular uptake and response to albumin-bound chemotherapy. Cancer Res. 2017, 77, 5925–5937. [Google Scholar] [CrossRef]
- Taverna, M.; Marie, A.-L.; Mira, J.-P.; Guidet, B. Specific antioxidant properties of human serum albumin. Ann. Intensive Care 2013, 3, 4. [Google Scholar] [CrossRef]
- Jerjes, W.; Upile, T.; Hamdoon, Z.; Mosse, C.A.; Akram, S.; Morley, S.; Hopper, C. Interstitial PDT for vascular anomalies. Lasers Surg. Med. 2011, 43, 357–365. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.H.; Li, Y.Y.; Shi, S.J.; Zhou, S.W.; Fu, Y.Y.; Zhang, Q.; Yang, X.; Fu, R.Q.; Lu, L.C. Hypericin-photodynamic therapy induces human umbilical vein endothelial cell apoptosis. Sci. Rep. 2015, 5, 18398. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Finnegan, A.; Malik, A.B. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Chanthick, C.; Kanlaya, R.; Kiatbumrung, R.; Pattanakitsakul, S.N.; Thongboonkerd, V. Caveolae-mediated albumin transcytosis is enhanced in dengue-infected human endothelial cells: A model of vascular leakage in dengue hemorrhagic fever. Sci. Rep. 2016, 6, 31855. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, H.; Höfler, M.; Beckmann, R.; Hufnagl, P.; Vanyek, E.; Bielek, E.; Wojta, J.; Fabry, A.; Lockie, S.; Binder, B.R. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J. Cell Sci. 1996, 109, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.D.; Hamilton, K.D. Nutrient deprivation increases vulnerability of endothelial cells to proinflammatory insults. Free Radic. Biol. Med. 2014, 67, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.B.; Xiao, Z.; Tulip, J.; Chapman, J.D. A comparison of susceptibility to photodynamic treatment between endothelial and tumor cells in vitro and in vivo. Photodiagn. Photodyn. Ther. 2007, 4, 160–169. [Google Scholar] [CrossRef]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef]
- Essmann, F.; Engels, I.H.; Totzke, G.; Schulze-Osthoff, K.; Jänicke, R.U. Apoptosis resistance of MCF-7 breast carcinoma cells to ionizing radiation is independent of p53 and cell cycle control but caused by the lack of caspase-3 and a caffeine-inhibitable event. Cancer Res. 2004, 64, 7065–7072. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Engels, I.H.; Dunkern, T.; Kaina, B.; Schulze-Osthoff, K.; Porter, A.G. Ionizing radiation but not anticancer drugs causes cell cycle arrest and failure to activate the mitochondrial death pathway in MCF-7 breast carcinoma cells. Oncogene 2001, 20, 5043–5053. [Google Scholar] [CrossRef]
- Jänicke, R.U.; Sprengart, M.L.; Wati, M.R.; Porter, A.G. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 1998, 273, 9357–9360. [Google Scholar] [CrossRef]
- Jänicke, R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009, 117, 219–221. [Google Scholar] [CrossRef]
- Yang, X.H.; Sladek, T.L.; Liu, X.; Butler, B.R.; Froelich, C.J.; Thor, A.D. Reconstitution of caspase 3 sensitizes MCF-7 breast cancer cells to doxorubicin- and etoposide-induced apoptosis. Cancer Res. 2001, 61, 348–354. [Google Scholar] [PubMed]
- Yang, S.; Zhou, Q.; Yang, X. Caspase-3 status is a determinant of the differential responses to genistein between MDA-MB-231 and MCF-7 breast cancer cells. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C.K. The role of subcellular localization in initiation of apoptosis by photodynamic therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D.; Luo, Y. Mitochondrial photodamage and PDT-induced apoptosis. J. Photochem. Photobiol. B Biol. 1998, 42, 89–95. [Google Scholar] [CrossRef]
- Schnitzer, J.E. Update on the cellular and molecular basis of capillary permeability. Trends Cardiovasc. Med. 1993, 3, 124–130. [Google Scholar] [CrossRef]
- Prinsen, B.H.C.M.T.; De Sain-Van Der Velden, M.G.M. Albumin turnover: Experimental approach and its application in health and renal diseases. Clin. Chim. Acta 2004, 347, 1–14. [Google Scholar] [CrossRef]
- Soeters, P.B.; Wolfe, R.R.; Shenkin, A. Hypoalbuminemia: Pathogenesis and Clinical Significance. J. Parenter. Enter. Nutr. 2019, 43, 181–193. [Google Scholar] [CrossRef]
- Scherz, A.; Salomon, Y.; Brandis, A.; Scheer, H. Palladium-Substituted Bacteriochlorophyll Derivatives and Use Thereof. International Patent Application No. PCT/IL1999/000673, 15 June 2000. [Google Scholar]
- Clement, M.; Daniel, G.; Trelles, M. Optimising the design of a broad-band light source for the treatment of skin. J. Cosmet. Laser Ther. 2005, 7, 177–189. [Google Scholar] [CrossRef]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): Successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef]
- Mazor, O.; Brandis, A.; Plaks, V.; Neumark, E.; Rosenbach-Belkin, V.; Salomon, Y.; Scherz, A. WST11, A Novel Water-soluble Bacteriochlorophyll Derivative; Cellular Uptake, Pharmacokinetics, Biodistribution and Vascular-targeted Photodynamic Activity Using Melanoma Tumors as a Model. Photochem. Photobiol. 2005, 81, 342. [Google Scholar] [CrossRef]
- Szczygieł, M.; Urbańska, K.; Jurecka, P.; Stawoska, I.; Stochel, G.; Fiedor, L. Central metal determines pharmacokinetics of chlorophyll-derived xenobiotics. J. Med. Chem. 2008, 51, 4412–4418. [Google Scholar] [CrossRef]
- Vakrat-Haglili, Y.; Weiner, L.; Brumfeld, V.; Brandis, A.; Salomon, Y.; Mcllroy, B.; Wilson, B.C.; Pawlak, A.; Rozanowska, M.; Sarna, T.; et al. The Microenvironment Effect on the Generation of Reactive Oxygen Species by Pd−Bacteriopheophorbide. J. Am. Chem. Soc. 2005, 127, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Ashur, I.; Goldschmidt, R.; Pinkas, I.; Salomon, Y.; Szewczyk, G.; Sarna, T.; Scherz, A. Photocatalytic generation of oxygen radicals by the water-soluble bacteriochlorophyll derivative WST1l, noncovalently bound to serum albumin. J. Phys. Chem. A 2009, 113, 8027–8037. [Google Scholar] [CrossRef]
- Gerola, A.P.; Santana, A.; França, P.B.; Tsubone, T.M.; De Oliveira, H.P.M.; Caetano, W.; Kimura, E.; Hioka, N. Effects of metal and the phytyl chain on chlorophyll derivatives: Physicochemical evaluation for photodynamic inactivation of microorganisms. Photochem. Photobiol. 2011, 87, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Szczygieł, M. Characteristics of Metallochlorophyllides Interactions with Tumor Cells and Animal Organism in the Context of Photodynamic Therapy; Jagiellonian University: Kraków, Poland, 2009. [Google Scholar]
- Handoko, Y.A.; Rondonuwu, F.S.; Limantara, L. The Photosensitizer Stabilities of Tookad® on Aggregation, Acidification, and Day-light Irradiation. Procedia Chem. 2015, 14, 474–483. [Google Scholar] [CrossRef]
- Sułkowski, L.; Matyja, A.; Osuch, C.; Matyja, M. Stability of spectrofluorimetric spectra of hematoporphyrin–serum albumin complexes: In Vitro study. Arch. Med. Sci.-Civiliz. Dis. 2021, 6, 18–21. [Google Scholar] [CrossRef]
- Weersink, R.A.; Bogaards, A.; Gertner, M.; Davidson, S.R.H.; Zhang, K.; Netchev, G.; Trachtenberg, J.; Wilson, B.C. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: Clinical experience and practicalities. J. Photochem. Photobiol. B Biol. 2005, 79, 211–222. [Google Scholar] [CrossRef]
- Yang, C.H.; Huang, K.S.; Wang, Y.T.; Shaw, J.F. A review of bacteriochlorophyllides: Chemical structures and applications. Molecules 2021, 26, 1293. [Google Scholar] [CrossRef]
- Origin, Version 2021; OriginLab Corporation: Northampton, MA, USA, 2021.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Castillo-Hair, S.M.; Sexton, J.T.; Landry, B.P.; Olson, E.J.; Igoshin, O.A.; Tabor, J.J. FlowCal: A User-Friendly, Open Source Software Tool for Automatically Converting Flow Cytometry Data from Arbitrary to Calibrated Units. ACS Synth. Biol. 2016, 5, 774–780. [Google Scholar] [CrossRef] [PubMed]








| Gene | Forward Primer Sequence (5′→3′) | Reverse Primer Sequence (5′→3′) |
|---|---|---|
| BAX | AGTGGCAGCTGACATGTTTT | GGAGGAAGTCCAATGTCCAG |
| BCL2 | GCCCTGTGGATGACTGAGTA | GGCCGTACAGTTCCACAAAG |
| CASP3 | TGTGAGGCGGTTGTGGAAGAGT | AATGGGGGAAGAGGCAGGTGCA |
| GAPDH | CGGAGTCAACGGATTTGGTCGTAT | AGCCTTCTCCATGGTGGTGAAGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec, M.J.; Toporkiewicz, M.; Gamian, A. Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent. Pharmaceuticals 2022, 15, 235. https://doi.org/10.3390/ph15020235
Szafraniec MJ, Toporkiewicz M, Gamian A. Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent. Pharmaceuticals. 2022; 15(2):235. https://doi.org/10.3390/ph15020235
Chicago/Turabian StyleSzafraniec, Milena J., Monika Toporkiewicz, and Andrzej Gamian. 2022. "Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent" Pharmaceuticals 15, no. 2: 235. https://doi.org/10.3390/ph15020235
APA StyleSzafraniec, M. J., Toporkiewicz, M., & Gamian, A. (2022). Zinc-Substituted Pheophorbide A Is a Safe and Efficient Antivascular Photodynamic Agent. Pharmaceuticals, 15(2), 235. https://doi.org/10.3390/ph15020235







