Abstract
This study presents the evaluation of biological activities and chemical profiling of Oenanthe aquatica (L.) Poir. and Oenanthe silaifolia M. Bieb. The phytochemical profile, antioxidant, enzyme inhibitory, cytotoxic and antiviral activities of the methanolic and aqueous extracts were investigated. The aqueous extract of O. aquatica possessing the highest content of phenolics (60.85 mg gallic acid equivalent/g extract), also exhibited the strongest radical scavenging potential against 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (79.46 and 148.66 mg Trolox equivalent/g extract, respectively), the highest reducing ability (207.59 and 107.27 mg Trolox equivalent/g extract, for cupric reducing antioxidant capacity and ferric reducing antioxidant activity, respectively), metal chelating potential (33.91 mg ethylenediaminetetraacetic acid equivalent/g extract) and total antioxidant ability (1.60 mmol Trolox equivalent/g extract). Liquid chromatography-electrospray ionization-quadrupole time-of-flight-mass spectrometry (LC-ESI-QTOF-MS/MS) permitted tentative identification of compounds from simple organic acids, phenolic acids, coumarins, flavonoids and their glycosides in O. aquatica and O. silaifolia extracts. The methanolic extract of O. aquatica substantially depressed acetylcholinesterase (3.67 mg galantamine equivalent/g extract), tyrosinase (126.66 mg kojic acid equivalent/g extract), and α-amylase (0.83 mmol acarbose equivalent/g extract) enzymes. The methanolic extract of O. silaifolia showed highest enzymatic inhibitory property against butyrylcholinesterase, and its aqueous extract depressed α-glucosidase activity (0.26 mmol acarbose equivalent/g extract). All tested extracts exerted selective toxicity towards cancer cell lines, and the highest anticancer potential was found for O. aquatica aqueous extract on FaDu and HeLa cells with CC50 of 57.36 and 47.16 µg/mL, respectively. Significant antiviral activity against HSV-1 (HHV-1) was found for both aqueous extracts in concentrations of 1000 µg/mL, which inhibited the HSV-1 cytopathic effect (CPE) in virus infected VERO cells and reduced the virus infective titer by more than 3 log (logCCID50/mL). This study has produced critical scientific data on O. aquatica and O. silaifolia, which are potential contenders for the development of novel phyto-pharmaceuticals.
1. Introduction
In our everyday life, the knowledge of traditional medicinal plants is frequently inherited from previous generations and passed down to younger ones. This heirloom of invaluable expertise obtained via ethnobotanical research is essential for conservation and understanding of indigenous and local plant usage []. The literature today is rich with growing data emphasizing the necessity of actively screening medicinal plants to disclose crucial information about their therapeutic qualities. Indeed, the widespread growth of the pharmaceutical, nutraceutical, cosmeceutical, and food sectors has resulted in a significant increase in the market for medicinal plants and their bioactive components over time [].
Research into the phytochemical composition and phytopharmacology of natural products with medicinal properties is an essential stage in evaluating if the product in question shows potential to be successfully introduced into production systems and be used in the pharma industry. Following this line of thought, our aim with this research paper is to screen two medicinally important plants originating from the Apiaceae family and Oenanthe genus, namely Oenanthe aquatica (L.) Poir. (O. aquatica) and Oenanthe silaifolia M. Bieb (O. silaifolia). O. aquatica, also known as fine-leaved water-dropwort, grows mostly in shallow water reservoirs or oxbows with standing water, although it may also be found in field depressions that are temporarily filled with water. It occurs fairly frequently and can develop dense stands, but its communities have a short life period. The morphology of the plant changes in response to changing environmental circumstances []. The fruit of O. aquatica has antiperiodic, diuretic, expectorant, and pectoral properties. It is used to treat chronic pectoral diseases, dyspepsia, intermittent fevers, persistent ulcers, and other conditions. This plant should be handled with extreme caution and only under the guidance of a knowledgeable practitioner. When consumed in excess, the fruits produce vertigo, drunkenness, and other narcotic symptoms. Externally, the roots have been utilized in pile therapy. The fruits are used to create a homeopathic medicine. It is used to treat bronchitis, coughs, and other respiratory ailments.
In Turkey, the leaves of O. silaifolia, also known as narrow leaved water-dropwort, are often eaten raw as a salad or boiled and cooked with rice []. However, this Oenanthe plant has been linked to livestock poisoning in Greece, causing a central nervous system depressive form of poisoning []. We have noticed a shortage of information on the phytopharmacology of O. aquatica and O. silaifolia in the available literature. Instead, O. aquatica species has been studied for its biological or ecological aspects [], seed reproduction [], and essential oil []. More studies were observed to be conducted on other Oenanthe species, notably O. javanica (Blume) DC. for its antidiabetic [], antioxidant and antigenotoxic activities [,], anti-hepatitis [], and phytochemical analysis [,].
O. javanica is a Chinese traditional medicinal plant and was reported to exert hepatoprotective, anti-inflammatory, immune-modulatory, antioxidant, anticancer, and antiviral properties [,,]. Aerial parts of O. javanica were shown to contain biphenyl derivatives exhibiting anti-inflammatory properties by inhibiting cyclooxygenase-2 (COX-2), with 1-(6′-hydroxy-3′-prenyl-phenyl)-10,11-dimethyl-2H-chromen-2-ol possessing the highest activity (IC50 22.18 μM), comparable to celecoxib used as a reference COX-2 inhibitor (IC50 18.08 μM) []. Recently, O. javanica was reported to display photoprotective activity against UVB-induced collagen disruption and inflammation, which warrants possible application for the treatment of photodamaged skin []. Total phenolics isolated from O. javanica were shown to possess dose-dependent anti-hepatitis B virus activity, resulting in inhibition of viral antigen production and release in a cell line model (Hep G2.2.15) with inhibition rates of 70.12% (HBeAg) and 72.61% (HBsAg) on day 9. In the duck hepatitis B virus (DHBV) infection model, the total phenolics fraction reduced the DHBV DNA level in a dose-dependent manner [].
Oenanthe crocata essential oil was found to dose-dependently inhibit (IC50 0.36 mg/mL) HIV-1 RT (reverse transcriptase) RDDP (RNA-dependent DNA polymerase)–associated activity. Importantly, even at 16 mg/mL, no inhibition of HIV-1 RT–associated RNase-H activity was found. Unfortunately, the essential oil obtained from O. crocata was also proven to significantly decrease cellular viability and fractionation provided fractions which were only weakly active in inhibiting HIV-1 RT RDDP activity []. It is also worth mentioning that one of the bioactive constituents in O. crocata, oenanthotoxin, is related to cicutoxin and may induce seizures. That is why O. crocata (water hemlock dropwort) belongs to the category of highly toxic plant species []. There are also reports concerning the presence of potentially toxic substances, such as polyacetylenes (oenanthotoxin) in roots and phenylpropanoids (myristicin) in fruits of O. aquatica. These naturally occurring substances may pose a possible health risk for consumers of food products and dietary supplements []. Until now, only piecemeal studies on different areas have been conducted on a few Oenanthe plants in an unsystematic way while a concise pharmacological validation of these Oenanthe members under one single study is clearly missing. Therefore, to fill the research gap, we have attempted to evaluate the antioxidant potential, influence on selected enzymes, anticancer and antiviral activities of O. aquatica and O. silaifolia, which are two poorly explored Oenanthe species. As far as we know, the current literature data on O. aquatica and O. silaifolia does not provide any information on the influence on clinically important enzymes related to Alzheimer disease (cholinesterases), skin hyperpigmentation (tyrosinase) or diabetes (α-glucosidase and α-amylase). The cytotoxicity of O. aquatica and O. silaifolia was evaluated towards the normal VERO cell line and a panel of cancer cell lines of various origins (FaDu, HeLa, and RKO). Furthermore, we have evaluated the effect of both extracts on the replication of human herpesvirus type 1 (HSV-1, HHV-1) in infected VERO cells, by the means of cytopathic effect (CPE) reduction, and the decrease of infectious titer using end-point virus titration. We anticipate that the data reported here could bridge a research gap and, as a result, open up new research pathways, notably in the creation of medicinal bioproducts.
2. Results and Discussion
2.1. Biologically Active Compounds
In Table 1, the total phenolic (TPC) and total flavonoid contents (TFC) of the methanolic and aqueous extracts of O. aquatica and O. silaifolia are shown. In the present work, methanol and water were selected as solvents. In the literature, methanol is considered to be one of the most effective solvents for the extraction of phenolic compounds, especially flavonoids [,,]. Because water has been used to make traditional mixtures of most plants in ethnobotanical work, water was also chosen. Overall, the methanolic extracts yielded the highest TFC while the aqueous extracts yielded the highest TPC. Solvents of higher polarity (water > MeOH) are more effective in recovering phenolic compounds []. Herein, the aqueous extracts reported higher TPC in contrast to methanol solvent. However, a different behavior was observed with TFC; methanol was a better extraction solvent in extracting flavonoids. The methanolic and aqueous extracts of O. aquatica expressed 42.35 mg RE/g and 60.85 mg GAE/g of TFC and TPC, respectively. The methanolic and aqueous extracts of O. silaifolia contained 27.08 mg RE/g and 46.91 mg GAE/g of TFC and TPC, respectively. From the obtained data, it can be said that upon comparison, O. aquatica possessed more TFC and TPC than O. silaifolia. As far as we know, no previous research has been done on the tested Oenanthe species. In agreement with our results, Bhaigyabati et al. [] reported that the methanolic extract from O. javanica contained a higher content of phenolics and flavonoids than the aqueous extract. When comparing our results with previous studies on several Oenanthe species, we have also found different results. In a recent study conducted by He et al. [], the total phenolic content was found to be 199.17 mg GAE/g in the aqueous extract of O. javanica, which was higher than that reported in our work. In another study on O. javanica [], the total phenolic content was reported as 44.70 mg GAE/g. Similar content was also reported by Rafat et al. [], as 38.78 mg GAE/g.

Table 1.
Extraction yields, amount of biologically active substances and total antioxidant capacity (by PDA assays) of the analyzed extracts.
The extracts were analyzed by LC-ESI-QTOF-MS/MS to elucidate the specific profile of O. aquatica and O. silaifolia extracts. The analysis in negative mode revealed the presence of several dozen compounds belonging to different chemical groups such as simple organic acids, phenolic acids, coumarins, flavonoids and their glycosides. Table 2 shows the differences in the presence of particular compounds in each extract. The highest peak intensity corresponding to the ionization of the molecules was detected for chlorogenic acid, rutin, luteolin, caffeic acid and their derivatives. Only chlorogenic, caffeic and feruoyloquinic, hydroxylinolenic and hydroxylinoleic acids were found in all samples. The exemplary chromatogram is presented in Figure S1. The results of our study are in accordance with literature reports on Oenanthe species. The presence of coumarins, phenylpropanoids, and flavonoids in the extracts obtained from aerial parts was previously detected in these species [,,]. Ferulic acid, p-coumaric acid, and 4-hydroxyphenethyl trans-ferulate were isolated from O. javanica [,]. Methanolic extract of the same species yielded also flavonoids like quercetin, isorhamnetin, nicotiflorin, isoquercitrin, rutin, hyperin, and glycosides []. Similarly, O. fistulosa was abundant in phenolic acids (15 compounds, including phenylpropanoid skeleton) and flavonoids (17 compounds) []. Differences in phytochemical profiles of tested extracts play a significant role in terms of their biological activity and these results should be taken into account in further research on this subject.

Table 2.
The chemical composition of studied extracts.
2.2. Antioxidant Effects
Several recent papers have reported a positive correlation between oxidative stress and the progression of serious health problems []. In this sense, antioxidants can alleviate the stress, and they can use various mechanisms such as radical quenching, electron donation or chelation of transition metals. Thus, we used several assays to evaluate antioxidant properties of O. aquatica and O. silaifolia. The assay results are depicted in Table 1 and Table 3.

Table 3.
Antioxidant activity of the analyzed extracts.
Water used for the extraction of antioxidant compounds recorded a higher antioxidant activity in the ABTS•+, DPPH, FRAP, CUPRAC, and metal chelating tests in comparison to methanol although in some studies it was the methanolic extracts that showed the strongest activity [,,,]. Extracts produced using solvents with high polarity often have greater antioxidant activity because the polar phase of the extract encourages the suppression of ABTS•+ and DPPH radicals via plain electron and proton relocations []. Herein, the aqueous extracts being more polar than methanolic extracts exhibited the highest DPPH and ABTS•+ radical scavenging activities. The antioxidant capacity of the extracts was also estimated with regard to its reducing power with the use of CUPRAC and FRAP techniques. Similar to its scavenging abilities, the aqueous extract of O. aquatica showed the strongest Cu2+ (207.59 mg TE/g) and Fe3+ (107.27 mg TE/g) reducing activities.
Plant secondary metabolites have exhibited different antioxidant effects including radical quenching and electron-donation abilities []. Similar to DPPH, ABTS•+, CUPRAC and FRAP tests, the aqueous extracts (O. aquatica and O. silaifolia: 33.91 and 28.37 mg EDTAE/g, respectively) showed the most effective chelating ability. However, in terms of total antioxidant capacity, a different behavior was reported. For instance, the methanolic extract of O. silaifolia displayed higher activity compared to its aqueous extract. Overall, O. aquatica possessed better antioxidant activity than the other Oenanthe species, namely O. silaifolia, despite originating from the same genus. The reasons why O. aquatica has a better potential for antioxidant activity could be, on the one hand, the complication of the effects of ecological influences and, on the other hand, the evolution of divergent biochemical pathways in the affected plants. which has led to the synthesis of diverse antioxidant compounds in O. aquatica and not in O. silaifolia []. Indeed, compounds such as 2-isopropylmalic acid [], vanillylmandelic acid hexoside [], hydroxybenzoic acid isomer [], ethyl syringate hexoside, kaempferol rutinoside [] and quercetin 7-O-trirhamnoside [] which were present in O. aquatica but found absent in O. silaifolia, were reported by different studies to possess good antioxidant activity.
2.3. Enzyme Inhibitory Activities
In the present study, the ability of O. aquatica and O. silaifolia extracts to inhibit the bioactivity of enzymes linked with Alzheimer’s disease (acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)), diabetes type 2 (α-amylase and α-glucosidase), and skin hyperpigmentation (tyrosinase) was tested and the results are shown in Table 4.

Table 4.
Inhibition of selected enzymes exerted by the analyzed extracts.
Various enzymes in the human organism are involved in the etiology of many diseases, and because of this, inhibiting these enzymes can be a useful method in therapy. Cholinesterase inhibitors, for example, are compounds that prevent the hydrolysis of acetylcholine, a key neurotransmitter for between neural transmission that, when present in lower concentrations, can led to neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases []. In our investigation, we looked for anti-cholinesterase activity in the differently prepared extracts. Relatively similar activity was recorded with both Oenanthe species. High anti-AChE and anti-BChE properties were observed in the methanolic extracts while the aqueous extracts were inactive for both plants (O. aquatica: AChE = 3.67 and BChE = 5.96 mg GALAE/g; O. silaifolia: AChE = 3.35 and BChE = 6.11 mg GALAE/g).
The α-amylase and α-glucosidase inhibitors are important for controlling blood glucose level in diabetes patients []. The methanolic extracts of O. aquatica and O. silaifolia were found to substantially depress α-amylase activity (0.83 and 0.72 mmol ACAE/g, respectively). A different behavior was observed for α-glucosidase inhibitory activity. For instance, the aqueous extract of O. aquatica possessed high anti-glucosidase activity (0.26 mmol ACAE/g) while the methanolic extract of O. silaifolia exhibited the strongest activity (0.40 mmol ACAE/g).
Tyrosinase inhibitors protect the skin and assist in avoiding hyperpigmentation. The pharmaceutical and cosmeceutical industries strongly support them []. The methanolic extracts of O. aquatica and O. silaifolia displayed the strongest anti-tyrosinase (126.66 and 126.60 mg KAE/g, respectively) activities. It is worth pointing out that the methanolic extracts of both plants exerted the highest potential against four tested enzymes, namely AChE, BChE, tyrosinase and α-amylase, except for α-glucosidase, although the methanolic extracts do not have the highest TFC and TPC. It is possible that there is no crucial relationship between TPC or TFC and enzymatic activities, but that they are more directly connected to other bioactive chemicals. In essence, the biological potential of various phenolic and flavonoid groups varies [].
Until now, there were no reports on the ability of members of the genus Oenanthe to inhibit enzyme activity. In this regard, the given work is the first scientific proof on the enzyme inhibitory actions of O. aquatica and O. silaifolia extracts, and it may make a significant addition to the scientific platform.
2.4. Cytotoxicity Evaluation
The cytotoxicity evaluation of O. aquatica and O. silaifolia extracts on normal VERO cells showed that the methanolic extracts from both plants show higher toxicity, with CC50 values of 340.52 and 252.6 µg/mL (Table 5), respectively, than aqueous extracts, for which the exact CC50 values could not be evaluated, but were above 1000 µg/mL. All tested extracts exerted selectivity towards cancer cells, with cells derived from colon carcinoma (RKO) cells being the most resistant ones.

Table 5.
Cytotoxicity of O. aquatica and O. silaifolia extracts.
Methanolic extracts exerted significant anticancer potential showing SI between 1.62 and 3.40, with HeLa cells being the most sensitive, especially to O. silaifolia methanolic extract (OS-M), which showed CC50 of 74.24 µg/mL. Moreover, the OS-M showed the highest toxicity (CC50 135.8 µg/mL) towards RKO amongst all tested extracts. Interestingly, not only were the CC50 values obtained for the OS-M on FaDu and RKO cells comparable (Table 5), but also the dose response curves (Figure 1) were similar. Noticeable anticancer activity and selectivity was found for aqueous extracts from O. aquatica (OA-A) and O. silaifolia (OS-A) on FaDu and HeLa cells, with both cell lines being equally sensitive, as can be concluded from similar dose-response curves (Figure 1) and CC50 values (Table 5). The highest anticancer activity and selectivity was found for OA-A on FaDu and HeLa cells with CC50 values of 57.36 (SI > 17.43) and 47.16 µg/mL (SI > 21.2), respectively.
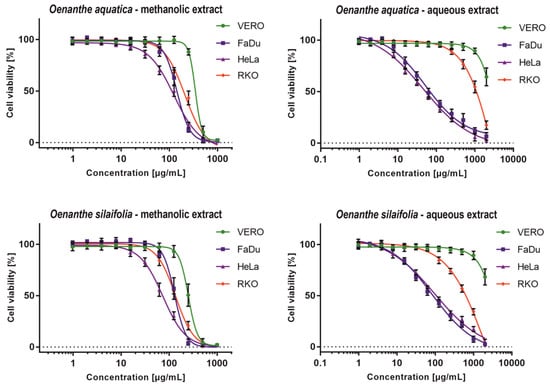
Figure 1.
Dose-response influence of O. aquatica and O. silaifolia extracts on cell lines.
The statistical significance of anticancer activity was analyzed using GraphPad Prism software (two-way ANOVA followed by Dunnett’s multiple comparisons test, analyzed with 95% confidence interval). We have compared the CC50 values calculated for cancer cell lines with the ones observed for the VERO cells. In case of both methanolic extracts, the differences of CC50 values between cancer and normal cells were statistically highly significant (p < 0.001). However, because our study design did not allow for accurate measurement of aqueous extracts CC50 on VERO cells, we could not subject them to statistical evaluation. Nevertheless, the SI values indicated that aqueous extracts of both species exert anticancer potential, especially towards FaDu and HeLa cells.
To evaluate the cytotoxicity of plant extracts, a classification based on the values of CC50 (μg/mL), has been proposed []. Taken into account this criterion, all tested extracts can be classified as moderately cytotoxic to FaDu and HeLa cells, whereas, in the case of RKO cells, only O. silaifolia methanolic extract can be classified as such. Aqueous extracts showed no cytotoxic activity towards the VERO cells, and methanolic extracts were weakly cytotoxic.
2.5. Antiviral Activitiy
The antiviral activity of O. aquatica and O. silaifolia extracts was tested on HSV-1 infected VERO cells. Based on the dose-response curves presented in Figure 1, we have selected the highest non-toxic concentrations of OS-M, OA-M to be 150 and 200 µg/mL, and for both aqueous extracts to be 1000 µg/mL. The microscopic observations of the cytopathic effect formation in extract treated HSV-1 infected cells in comparison with untreated infected cells (virus control) showed that in case of both aqueous extracts, the CPE was inhibited, suggesting a potential antiviral effect. The inhibition of CPE formation by OS-A at 1000 µg/mL was presented on Figure 2. Also, O. aquatica methanolic extract showed noticeable inhibition of CPE, but not as potent as aqueous extract. Acyclovir was used as a standard antiviral reference substance and completely inhibited CPE formation at 60 µg/mL (Figure 2), whereas a lower dose of 30 µg/mL only partially inhibited the development of CPE.
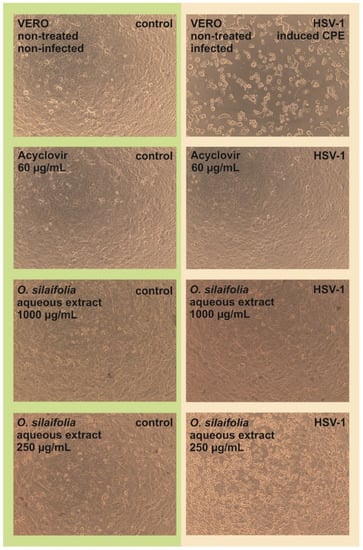
Figure 2.
The influence of O. silaifolia aqueous extracts on human herpesvirus-1 CPE in VERO cells.
To evaluate the infectious titer of HSV-1 in collected samples, the end-point titration assay was used and the results are presented in Table 6. The O. aquatica methanolic extract showed dose-response antiviral activity (Figure 3C) with the highest decrease of HSV-1 titer (Δlog) of 2.29 at 200 µg/mL (Table 6). Figure 4 shows the end-point HSV-1 titration assay of sample collected from infected VERO cells treated with O. aquatica methanolic extract (200 µg/mL) in comparison with the corresponding virus control, confirming the reduction of the infectious titer of HSV-1. In case of O. silaifolia, the methanolic extract showed only minor reduction of the virus titer. The acyclovir at 60 µg/mL completely abolished the infectivity of HSV-1 (Figure 3D), whereas the concentration of 30 µg/mL reduced the viral titer by 2.05 log (logCCID50/mL) (Table 6). Both aqueous extracts exerted potent reduction of HSV-1 titer at 1000 µg/mL and despite the fact that the accurate amount of viral titer was not calculated (Figure 3A,B), the decrease of the virus titer was no less than 3 log (logCCID50/mL). Since a Δlog of at least 3 is required to regard a substance to possess significant antiviral activity, it can be concluded that both aqueous extracts can be classified as such. Herein, the authors would like to point out that the reported antiviral activity of O. aquatica and O. silaifolia aqueous extracts was observed at relatively high concentration which might be deemed too high for any practical application. However, if future studies allow for separation of bioactive sub-fractions with higher antiviral activity or isolation of compounds responsible for the observed activity, it would greatly benefit the knowledge of natural product derived antivirals. It is still worth mentioning, that this work is the first one to describe anti-HSV activity of O. aquatica and O. silaifolia.

Table 6.
Decrease of HSV-1 titer by the O. aquatica and O. silaifolia extracts.
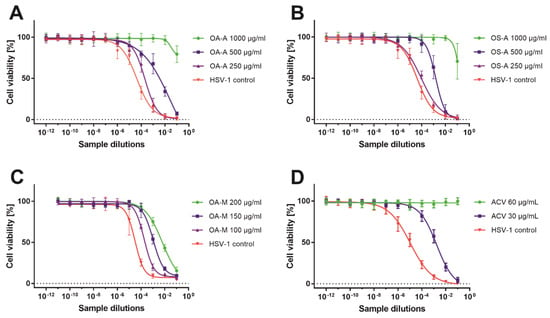
Figure 3.
The HSV-1 titration assay of selected samples.
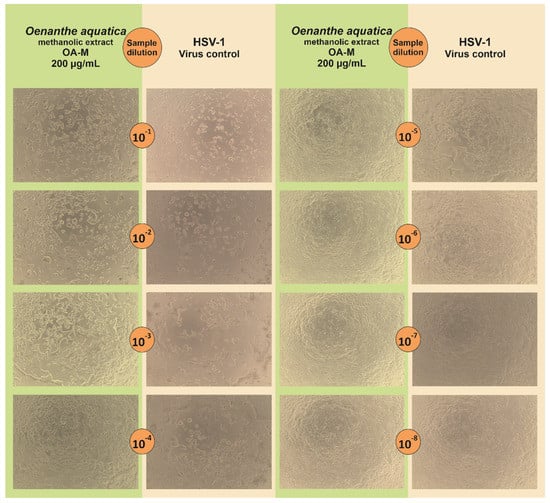
Figure 4.
Oenanthe aquatica methanolic extract (200 µg/mL) end-point HSV-1 titration assay (the numbers in circles refer to tenfold dilutions of samples collected from antiviral assays).
Organic acids identified in extracts of O. aquatica and O. silaifolia, namely citric acid (OA-M, OS-A) and malic acid (OA-M, OA-A, OS-A), were shown to exert virucidal activity towards avian influenza virus (AIV, H9N2) [] and multiple rhinovirus serotypes []. However, our study design was focused on the assessment of potential antiviral activity, which is mostly based on the influence of tested substances on intracellular steps of the viral replication cycle, and not on the assessment of virucidal activity.
Caffeic acid and its derivatives identified in O. aquatica and O. silaifolia, like caffeic acid glucoside, chlorogenic acid, cryptochlorogenic acid, and neochlorogenic acid, were reported to possess antiviral activities towards influenza viruses [,,], human herpesviruses (type-1, type-2), adenoviruses (type -3, -8, and -11) [], and hepatitis B virus (HBV) []. Chlorogenic acid was found to inhibit influenza viruses, strains A/PuertoRico/8/1934(H1N1) (EC50 44.87 μM, EC50–50% effective concentration), A/Beijing/32/92(H3N2) (EC50 62.33 μM), as well as strains resistant to oseltamivir; the inhibition occurs during the late stages of the influenza virus infectious cycle, probably due to the inhibition of viral neuraminidase and blocking the release of viral progeny from infected cells []. The chlorogenic acid was found to possess antiherpetic (HSV-1, HSV-2), and anti-adenovirus (ADV type-3, -8, and -11) activity, whereas the caffeic acid inhibited both HSV-1 and HSV-2 but only one type of adenovirus (ADV-3) []. Interestingly, neochlorogenic acid was reported as a potential anti-SARS-CoV-2 drug, inhibiting the RdRp (RNA-dependent RNA polymerase) of this virus. In silico, neochlorogenic acid interacted with key residues (Arg349, Tyr346 and Phe396) of RdRp []. It was also found that the anti-HSV-1 effect of caffeic acid is not because of either the direct HSV-1 inactivation (virucidal effect) or the cytotoxicity of caffeic acid to cells used for virus propagation, but is probably because of the ability to bind to enzymes involved in virus replication cycle []. The caffeic acid was found in all tested extracts from both O. aquatica and O. silaifolia and all extracts showed the ability to decrease the infectious titer of HSV-1; however, only aqueous extracts showed significant antiviral properties. Interestingly, only aqueous extracts showed the presence of neochlorogenic acid and cryptochlorogenic acid (Table 2), and thus we can theorize that those compounds may be responsible for the anti-HSV-1 properties; this requires further studies on isolated compounds. Of course, it is also possible that the synergism of different phenolic acids present in aqueous extracts may contribute to antiviral activity. The methanolic extracts showed higher cytotoxicity to VERO cells which resulted in lower concentrations used in antiviral assays and possibly also contributed to lower antiviral effects.
3. Materials and Methods
3.1. Plant Material and Extraction Procedure
Oenanthe species were collected in July 2020 from Turkey (O. silaifolia M. Bieb.: Tunceli, Mazgirt, Yukarı Oyumca village, 1400 m; O. aquatica (L.) Poir.: Edirne; Enez, Gala Lake, 150 m). Voucher specimens were deposited in Munzur University (voucher numbers: Paksoy 1397 and Paksoy 1187, respectively). The aerial parts were dried in a well-ventilated place for ten days and then ground using one laboratory mill.
For each species, methanol and water were selected as solvents. In the preparation of methanol extracts, the plant materials (5 g) were extracted at room temperature with 100 mL of methanol for 24 h. Then, the extracts were filtered, and the solvents were removed by using a rotary-evaporator. As for the water extracts, the plant samples (5 g) were extracted with 100 mL of boiled water for 15 min. Then, the mixtures were filtered and lyophilized. The obtained extracts were kept at 4 °C before analysis. The extraction yields were given in Table 1.
3.2. Total Content of Phenolics and Flavonoids
Total phenolic content (TPC) and total flavonoid content (TFC) were assessed according to previously described methods [,]. TPC was expressed as mg gallic acid equivalents (GAE)/g dry extract, whereas TFC was calculated as mg rutin equivalents (RE)/g dry extract. All experimental details of the assays are given in Supplemental Material.
3.3. Antioxidant Properties and Enzyme Inhibition
In the current work, the antioxidant effects of the tested extracts were detected by different assays []. The assays were [1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline) 6-sulfonic acid (ABTS) radical scavenging, cupric ion reducing antioxidant capacity (CUPRAC), ferric ion reducing antioxidant power (FRAP), metal chelating ability (MCA) and phosphomolybdenum assay (PDA)]. For DPPH, ABTS, CUPRAC and FRAP assay data were expressed as mg Trolox equivalents (TE)/g extract, whereas in MCA and PDA, mg EDTA equivalents (EDTAE)/g extract and mmol TE/g extract, respectively, were used. The experimental details for acetylcholinesterase, butyrylcholinesterase, tyrosinase, amylase and glucosidase assays were previously provided. Galathamine was used as a positive control in cholinesterase assays and data were evaluated as mg galanthamine equivalents (GALAE)/g extract. Kojic acid was used as a standard inhibitor in tyrosinase inhibitory assay and the results were expressed as mg kojic acid equivalents (KAE)/g extract [,]. Acarbose was selected as inhibitor for amylase and glucosidase inhibitory assays and the results are given as mmol acarbose equivalents (ACAE)/g extract. All experimental details of the assays are given in supplemental material. The assays were performed in triplicate and the differences in the extracts were evaluated by ANOVA assays (Tukey’s test).
3.4. LC-ESI-QTOF-MS/MS Analysis
The constituents of the studied extracts were separated on the on Gemini® column (3 μm i.d. C18 with TMS endcapping, 110 Å, 100 × 2 mm) guarded with pre-column (Phenomenex Inc, Torrance, CA, USA) following chromatographic conditions previously described []. The separation was performed on Agilent 1200 Infinity HPLC (Agilent Technologies, Santa Clara, CA, USA), whereas detection was obtained on Agilent 6530B QTOF (Agilent Technologies, Santa Clara, CA, USA). 2 mass spectra/s were registered in a scan range 100–1700 m/z for MS and MS/MS, applying collision energy of 10 and 40 eV. The drying gas temperature and flow were set at 300 °C and 12 L/min, whereas the sheath gas temperature and flow were set at 325 °C and 12 L/min, respectively. The ion source operated in negative mode with 40 psig, capillary V (+): 4000 V, skimmer 65 V. Freely available mass spectra databases (MassBank, PubChem, HMDB, Metlin, MoNA) were used for tentative identification of compounds, which was also supported by fragmentation patterns published in scientific literature.
3.5. Cell Assays
Media used for in vitro culturing included Dulbecco Modified Eagle Medium (DMEM, Corning, Tewksbury, MA, USA) used for VERO cells, and Modified Eagle Medium (MEM, Corning) used for other cell lines. Cell media used in the experiments were supplemented with antibiotics and fetal bovine serum. Incubation was carried out in a CO2 incubator (5% CO2, 37 °C). Detailed description of cell line maintenance can be found in the Supplementary Materials.
O. aquatica and O. silaifolia methanolic extracts were dissolved in DMSO, and aqueous extracts in PBS. Stock solutions (50 mg/mL) were membrane filtered (0.2 µm) and stored frozen (−20 °C) until used.
3.6. Cytotoxicity Assessment
Cytotoxicity was tested using MTT based assay following a previously described protocol []. Briefly, the monolayers of the appropriate cell lines in 96-well plates were incubated for 72 h with serial dilutions of extract stock solutions (methanolic extracts: 1000–0.98 µg/mL, aqueous extracts: 2000–0.98 µg/mL). Afterwards, the MTT assay was used for the evaluation of cellular viability and the absorbance was measured using a microplate reader. Collected data was analyzed with GraphPad Prism software and the values of CC50 (the 50% cytotoxic concentration–concentration resulting in 50% reduction of cell viability) were calculated from dose-response curves (non-linear regression). Moreover, the selectivity towards cancer cells was assessed by calculating the selectivity indexes (VERO CC50/cancer cell line CC50). In additional, CC10 (10% cytotoxic concentration–concentration resulting in 10% reduction of cell viability) values of tested extracts towards VERO cells were calculated for use in antiviral studies. Detailed descriptions of cytotoxicity testing can be found in the Supplementary Materials.
3.7. Antiherpetic Assay and the Evaluation of HSV-1 Titer
Antiviral activity was evaluated towards human herpesvirus-1 (HSV-1) replicating in VERO cells, as previously described []. The infectious titer of HSV-1 used in this study was 5.5 ± 0.25 logCCID50/mL (CCID50–50% cell culture infectious dose). This assay is based on the evaluation of the effect of tested extract on the formation of the cytopathic effect in virus infected cell line (after 1 h preincubation with 100-fold CCID50 of HSV-1). Extracts showing antiviral activity should inhibit the CPE.
Samples collected from antiviral assays were further subjected to end-point dilution assay to evaluate HSV-1 titers. Briefly, the VERO cells (monolayer) in 96-well plates were incubated with ten-fold dilutions of samples (3 replicates) in cell media for 72 h. Daily observation was conducted to monitor the development of CPE during titration. Subsequently, the MTT test was used to evaluate CCID50 and the values obtained for tested extracts were compared with the titer of non-treated infected cells. The detailed methodology can be found in the Supplementary Materials. In order to report that an extract significantly inhibits viral replication it should reduce the CCID50 by ≥3 log.
3.8. Data Analysis
The GraphPad Prism software was used for the evaluation of statistical significance of obtained data (two-way ANOVA with Dunnett’s multiple comparisons test).
4. Conclusions
The investigation of O. aquatica and O. silaifolia bio components described in this study adds to our understanding of the phytochemistry of these plants and enhances our understanding of their antioxidant, enzyme inhibitory properties, cytotoxicity, and antiviral activities. Aqueous extract of both plants showed the most potent antioxidant properties, with most of tests indicating correlation with their significant TPC. The methanolic extract of O. aquatica depressed the inhibitory activities of most enzymes including AChE, tyrosinase, and α-amylase. All tested extracts exerted noticeable anticancer activity towards hypopharyngeal squamous cell carcinoma (FaDu) and cervical adenocarcinoma (HeLa), whereas colon carcinoma derived cells (RKO) proved to be more resistant. The data presented herein demonstrate that neochlorogenic acid and cryptochlorogenic acid present in only aqueous extracts may be responsible for the anti-HSV-1 properties. However, further study, including clinical in vivo investigations, is needed to examine these aforementioned characteristics in order to incorporate these traditional plants as possible pharmaceutical components.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph15010050/s1, Figure S1: The BPC chromatogram of O. aquatica-MeOH; numbers correspond to Table 2.
Author Contributions
Conceptualization, Ł.Ś., E.S. and G.Z.; methodology, Ł.Ś., E.S., K.K.W., B.R., M.P.-D. and M.Y.P. software, M.F.M., N.B.S. and G.Z.; validation, Ł.Ś., E.S. and G.Z.; formal analysis, G.Z.; investigation, G.Z.; resources, M.Y.P. and G.Z.; data curation, G.Z.; writing—original draft preparation, Ł.Ś., E.S., M.F.M. and N.B.S.; writing—review and editing, G.Z.; visualization, Ł.Ś., E.S.; supervision, G.Z.; project administration, G.Z.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akgul, A.; Akgul, A.; Senol, S.G.; Yildirim, H.; Secmen, O.; Dogan, Y. An ethnobotanical study in Midyat (Turkey), a city on the silk road where cultures meet. J. Ethnobiol. Ethnomed. 2018, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Selseleh, M.; Nejad Ebrahimi, S.; Aliahmadi, A.; Sonboli, A.; Mirjalili, M.H. Metabolic profiling, antioxidant, and antibacterial activity of some Iranian Verbascum L. species. Ind. Crops Prod. 2020, 153, 112609. [Google Scholar] [CrossRef]
- Hroudová, Z.; Zákravský, P.; Hrouda, L.; Ostrý, I. Oenanthe aquatica (L.) Poir.: Seed reproduction, population structure, habitat conditions and distribution in Czechoslovakia. Folia Geobot. Phytotaxon. 1992, 27, 301–335. [Google Scholar] [CrossRef]
- Hançer, Ç.K.; Sevgi, E.; Altinbaşak, B.; Çakir, E.A.; Akkaya, M. Traditional knowledge of wild edible plants of Biga (Çanakkale), Turkey. Acta Soc. Bot. Pol. 2020, 89, 8914. [Google Scholar]
- Jodrugs. Available online: http://www.jodrugs.com/toxicologies/3636-plants-oenanthe.aspx (accessed on 12 August 2021).
- Vincieri, F.F.; Coran, S.A.; Bambagiotti, M. Composition of the Oenanthe aquatica essential oil. Planta Med. 1976, 29, 101–112. [Google Scholar] [CrossRef]
- Yang, X.B.; Huang, Z.M.; Cao, W.B.; Zheng, M.; Chen, H.Y.; Zhang, J.Z. Antidiabetic effect of Oenanthe javanica flavone. Acta Pharmacol. Sin. 2000, 21, 239–242. [Google Scholar] [PubMed]
- Kwon, D.; Yoon, S.; Carter, O.; Bailey, G.S.; Dashwood, R.H. Antioxidant and antigenotoxic activities of Angelica keiskei, Oenanthe javanica and Brassica oleracea in the Salmonella mutagenicity assay and in HCT116 human colon cancer cells. BioFactors 2006, 26, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-R.; Hwang, I.-G.; Kim, H.-Y.; Kang, T.-S.; Kim, Y.-B.; Joo, S.-S.; Lee, J.-S.; Jeong, H.-S. Antioxidant component and activity of dropwort (Oenanthe javanica) ethanol extracts. J. Korean Soc. Food Sci. Nutr. 2011, 40, 316–320. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Huang, Z.-M.; Yang, X.-B.; Liu, H.-Z.; Wu, G.-X. In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. J. Ethnopharmacol. 2008, 118, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.H.; Baek, H.H. Identification of Characteristic Aroma-Active Compounds from Water Dropwort (Oenanthe javanica DC.). J. Agric. Food Chem. 2005, 53, 6766–6770. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Yu, Y.-B.; Lee, J.-H. Isolation of Steroids and Flavonoids from the Herb of Oenanthe javanica Dc. Korean J. Pharmacogn. 1993, 24, 244–246. [Google Scholar]
- Lu, C.-L.; Li, X.-F. A review of Oenanthe javanica (Blume) DC. as traditional medicinal plant and its therapeutic potential. Evid. Based Complement. Alternat. Med. 2019, 2019, 6495819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-J.; Kong, K.-M.; Qi, W.-L.; Ye, W.-L.; Song, P.-S. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol. Sin. 2005, 26, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.G.; Wei, R.R.; Sang, Z.P. Biphenyl Derivatives from the Aerial Parts of Oenanthe javanica and Their COX-2 Inhibitory Activities. Chem. Biodivers. 2019, 16, e1800480. [Google Scholar] [CrossRef] [Green Version]
- Her, Y.; Shin, B.-N.; Lee, Y.L.; Park, J.H.; Kim, D.W.; Kim, K.S.; Kim, H.; Song, M.; Kim, J.-D.; Won, M.-H. Oenanthe javanica extract protects mouse skin from UVB radiation via attenuating collagen disruption and inflammation. Int. J. Mol. Sci. 2019, 20, 1435. [Google Scholar] [CrossRef] [Green Version]
- Bicchi, C.; Rubiolo, P.; Ballero, M.; Sanna, C.; Matteodo, M.; Esposito, F.; Zinzula, L.; Tramontano, E. HIV-1-inhibiting activity of the essential oil of Ridolfia segetum and Oenanthe crocata. Planta Med. 2009, 75, 1331–1335. [Google Scholar] [CrossRef]
- Schep, L.J.; Slaughter, R.J.; Becket, G.; Beasley, D.M.G. Poisoning due to water hemlock. Clin. Toxicol. 2009, 47, 270–278. [Google Scholar] [CrossRef]
- Authority, E.F.S. Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012, 10, 2663. [Google Scholar] [CrossRef] [Green Version]
- Bakhouche, I.; Aliat, T.; Boubellouta, T.; Gali, L.; Şen, A.; Bellik, Y. Phenolic contents and in vitro antioxidant, anti-tyrosinase, and anti-inflammatory effects of leaves and roots extracts of the halophyte Limonium delicatulum. S. Afr. J. Bot. 2021, 139, 42–49. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Fettach, S.; Mrabti, H.N.; Sayah, K.; Bouyahya, A.; Salhi, N.; Cherrah, Y.; El Abbes, F.M. Phenolic content, acute toxicity of Ajuga iva extracts and assessment of their antioxidant and carbohydrate digestive enzyme inhibitory effects. S. Afr. J. Bot. 2019, 125, 381–385. [Google Scholar] [CrossRef]
- Herrera-Pool, E.; Ramos-Díaz, A.L.; Lizardi-Jiménez, M.A.; Pech-Cohuo, S.; Ayora-Talavera, T.; Cuevas-Bernardino, J.C.; García-Cruz, U.; Pacheco, N. Effect of solvent polarity on the Ultrasound Assisted extraction and antioxidant activity of phenolic compounds from habanero pepper leaves (Capsicum chinense) and its identification by UPLC-PDA-ESI-MS/MS. Ultrason. Sonochem. 2021, 76, 105658. [Google Scholar] [CrossRef] [PubMed]
- Bhaigyabati, T.; Devi, P.G.; Devi, N.R.; Bag, G.C. Antioxidant activity, total phenolic and total flavonoid content of Oenanthe javanica Blume (DC) collected from Imphal West District. Int. Reseach J. Pharm 2017, 8, 63–68. [Google Scholar] [CrossRef]
- He, S.D.; Tang, M.M.; Zhang, Z.Y.; Liu, H.Y.; Luo, M.F.; Sun, H.J. Hypoglycemic effects of phenolic compound-rich aqueous extract from water dropwort (Oenanthe javanica DC.) on streptozotocin-induced diabetic mice. N. J. Chem. 2020, 44, 5190–5200. [Google Scholar] [CrossRef]
- Lee, K.; Padzil, A.; Syahida, A.; Abdullah, N.; Zuhainis, S.; Maziah, M.; Sulaiman, M.; Israf, D.; Shaari, K.; Lajis, N. Evaluation of anti-inflammatory, antioxidant and anti-nociceptive activities of six Malaysian medicinal plants. J. Med. Plants Res. 2011, 5, 5555–5563. [Google Scholar]
- Rafat, A.; Philip, K.; Muniandy, S. Antioxidant potential and phenolic content of ethanolic extract of selected Malaysian plants. Res. J. Biotechnol. 2010, 5, 16–19. [Google Scholar]
- Souilah, N.; Bendif, H.; Ullah, Z.; Miara, M.D.; Laib, M.; Akkal, S.; Medjroubi, K.; Mustafa, A.M. LC-MS/MS Profiling of 37 Fingerprint Phytochemicals in Oenanthe fistulosa L. and its Biological Activities. Nat. Prod. J. 2021, 11, 63–73. [Google Scholar] [CrossRef]
- Murata, T.; Katagiri, T.; Ishikawa, Y.; Abe, M.; Takahashi, E.; Iwahana, R.; Sakamoto, Y.; Sasaki, K. Inhibitory effects of phenylpropanoid derivatives from Oenanthe javanica on antigen-stimulated degranulation in RBL-2H3 cells. J. Nat. Prod. 2019, 82, 1518–1526. [Google Scholar] [CrossRef]
- Fujita, T.; Kadoya, Y.; Aota, H.; Nakayama, M. A new phenylpropanoid glucoside and other constituents of Oenanthe javanica. Biosci. Biotechnol. Biochem. 1995, 59, 526–528. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Sinan, K.I.; Ferrarese, I.; Sut, S.; Bene, K.; Mahomoodally, M.F.; Bibi Sadeer, N.; Ak, G.; Zengin, G. Chromatographic Separation of Breynia retusa (Dennst.) Alston Bark, Fruit and Leaf Constituents from Bioactive Extracts. Molecules 2020, 25, 5537. [Google Scholar] [CrossRef]
- Sinan, K.I.; Mahomoodally, M.F.; Eyupoglu, O.E.; Etienne, O.K.; Sadeer, N.B.; Ak, G.; Behl, T.; Zengin, G. HPLC-FRAP methodology and biological activities of different stem bark extracts of Cajanus cajan (L.) Millsp. J. Pharm. Biomed. Anal. 2021, 192, 113678. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Bibi Sadeer, N.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamočlija, J.; Soković, M. Identification of Chemical Profiles and Biological Properties of Rhizophora racemosa G. Mey. Extracts Obtained by Different Methods and Solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; Rengasamy, K.R.; Mahomoodally, M.F. Assessment of the Pharmacological Properties and Phytochemical Profile of Bruguiera gymnorhiza (L.) Lam Using In Vitro Studies, In Silico Docking, and Multivariate Analysis. Biomolecules 2020, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Segura Campos, M.R.; Ruiz Ruiz, J.; Chel-Guerrero, L.; Betancur Ancona, D. Coccoloba uvifera (L.) (Polygonaceae) Fruit: Phytochemical Screening and Potential Antioxidant Activity. J. Chem. 2015, 2015, 534954. [Google Scholar] [CrossRef] [Green Version]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef] [Green Version]
- Ricciutelli, M.; Bartolucci, G.; Campana, R.; Salucci, S.; Benedetti, S.; Caprioli, G.; Maggi, F.; Sagratini, G.; Vittori, S.; Lucarini, S. Quantification of 2- and 3-isopropylmalic acids in forty Italian wines by UHPLC-MS/MS triple quadrupole and evaluation of their antimicrobial, antioxidant activities and biocompatibility. Food Chem. 2020, 321, 126726. [Google Scholar] [CrossRef] [PubMed]
- Lauberte, L.; Fabre, G.; Ponomarenko, J.; Dizhbite, T.; Evtuguin, D.V.; Telysheva, G.; Trouillas, P. Lignin Modification Supported by DFT-Based Theoretical Study as a Way to Produce Competitive Natural Antioxidants. Molecules 2019, 24, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velika, B.; Kron, I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant properties of flavonol glycosides from green beans. Redox Rep. 1999, 4, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 34993. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.C.; Ramesh, N.; Sreevatsan, S.; Joseph, B.; Alle, P.; Belani, K.G.; Osterholm, M.T. Knowledge, attitudes, and poultry-handling practices of poultry workers in relation to avian influenza in India. Indian J. Occup. Environ. Med. 2013, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and biological activities of Polemonium caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Chae, W.-S.; Cha, C.-N.; Yoo, C.-Y.; Kim, S.; Lee, H.-J. Virucidal efficacy of a disinfectant solution composed of citric acid, malic acid and phosphoric acid against avian influenza virus. J. Prev. Vet. Med. 2018, 42, 16–21. [Google Scholar] [CrossRef]
- Hayden, G.F.; Gwaltney, J.M., Jr.; Thacker, D.F.; Hendley, J.O. Rhinovirus inactivation by nasal tissues treated with virucide. Antivir. Res. 1985, 5, 103–109. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, Z.; Cao, L.; Ding, G.; Wang, Z.; Xiao, W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017, 7, 45723. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.; Elhassan Taha, M.M.; Makeen, H.A.; Alhazmi, H.A.; Al Bratty, M.; Sultana, S.; Ahsan, W.; Najmi, A.; Khalid, A. Bioactive natural antivirals: An updated review of the available plants and isolated molecules. Molecules 2020, 25, 4878. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, A.; Shan, J.; Wang, S.; Cai, B.; Di, L. Study on the rationality for antiviral activity of Flos Lonicerae Japonicae-Fructus Forsythiae herb couple preparations improved by chito-oligosaccharide via integral pharmacokinetics. Molecules 2017, 22, 654. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.; Chiang, W.; Chang, M.; Ng, L.; Lin, C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Mahrosh, H.S.; Mustafa, G. An in silico approach to target RNA-dependent RNA polymerase of COVID-19 with naturally occurring phytochemicals. Environ. Dev. Sustain. 2021, 23, 16674–16687. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar] [PubMed] [Green Version]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef]
- Zengin, G.; Sieniawska, E.; Senkardes, I.; Picot-Allain, M.C.N.; Ibrahime Sinan, K.; Fawzi Mahomoodally, M. Antioxidant abilities, key enzyme inhibitory potential and phytochemical profile of Tanacetum poteriifolium Grierson. Ind. Crops Prod. 2019, 140, 111629. [Google Scholar] [CrossRef]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).