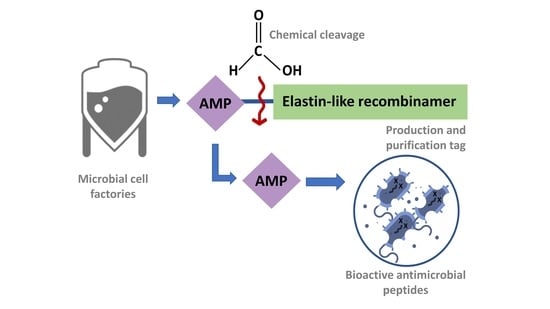
Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag
Abstract
:1. Introduction
2. Results
2.1. 3D Structure Analysis of CM4 and Synoeca
2.2. Production and Purification of CM4DP-A200 and SynDP-A200
2.3. Isolation and Purification of Free AMPs
2.4. Antibacterial Activity of Free AMPs
3. Discussion
4. Materials and Methods
4.1. Biological Materials
4.2. 3D Structure Analysis
4.3. Gene Construction and Protein Production
4.4. Cleavage and AMP Isolation
4.5. MALDI-TOF Mass Spectrometry
4.6. Minimum Inhibitory Concentration (MIC)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thabit, A.K.; Crandon, J.L.; Nicolau, D.P. Antimicrobial resistance: Impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin. Pharmacother. 2015, 16, 159–177. [Google Scholar] [CrossRef]
- Maragakis, L.L.; Perencevich, E.N.; Cosgrove, S.E. Clinical and economic burden of antimicrobial resistance. Expert Rev. Anti-Infect. Ther. 2008, 6, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Raman, G.; Sulham, K.; Gavaghan, M.; Menon, V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2014, 3, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Naylor, N.R.; Pouwels, K.B.; Hope, R.; Green, N.; Henderson, K.L.; Knight, G.M.; Atun, R.; Robotham, J.V.; Deeny, S.R. The health and cost burden of antibiotic resistant and susceptible Escherichia coli bacteraemia in the English hospital setting: A national retrospective cohort study. PLoS ONE 2019, 14, e0221944. [Google Scholar] [CrossRef]
- Graffunder, E.M.; Venezia, R.A. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J. Antimicrob. Chemother. 2002, 49, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Rolo, J.; De Lencastre, H.; Miragaia, M. Strategies of adaptation of Staphylococcus epidermidis to hospital and community: Amplification and diversification of SCCmec. J. Antimicrob. Chemother. 2012, 67, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.N.; Clegg, S. Klebsiella pneumoniae and type 3 fimbriae: Nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012, 7, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, C.P.; Dos Santos, S.C.; Madeira, A.; Mira, N.P.; Moreira, A.S.; Sá-Correia, I. Long-term colonization of the cystic fibrosis lung by Burkholderia cepacia complex bacteria: Epidemiology, clonal variation, and genome-wide expression alterations. Front. Cell. Infect. Microbiol. 2011, 1, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.; Hamill, P.; Hancock, R.E.W. Peptide Antimicrobial Agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [Green Version]
- Malmsten, M. Interactions of Antimicrobial Peptides with Bacterial Membranes and Membrane Components. Curr. Top. Med. Chem. 2016, 16, 16–24. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Genet. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. [Google Scholar] [CrossRef] [Green Version]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Zhao, C.-X. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Appl. Microbiol. Biotechnol. 2019, 103, 659–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, T.; Ge, H.; He, H.; Liu, Y.; Zhai, C.; Feng, L.; Yi, L. The heterologous expression strategies of antimicrobial peptides in microbial systems. Protein Expr. Purif. 2017, 140, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Parachin, N.; Mulder, K.C.; Viana, A.A.B.; Dias, S.C.; Franco, O.L. Expression systems for heterologous production of antimicrobial peptides. Peptides 2012, 38, 446–456. [Google Scholar] [CrossRef]
- Li, Y. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Expr. Purif. 2011, 80, 260–267. [Google Scholar] [CrossRef]
- Pereira, A.M.; Gomes, D.; da Costa, A.; Dias, S.C.; Casal, M.; Machado, R. Protein-Engineered Polymers Functionalized with Antimicrobial Peptides for the Development of Active Surfaces. Appl. Sci. 2021, 11, 5352. [Google Scholar] [CrossRef]
- Girotti, A.; López, I.M.; Arias, F.J.; Fernández-Colino, A.; Rodríguez-Cabello, J.C. Elastin-like recombinamers: Biosynthetic strategies and biotechnological applications. Biotechnol. J. 2011, 6, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Cabello, J.C. Smart Elastin-like Polymers. Results Probl. Cell Differ. 2004, 553, 45–57. [Google Scholar] [CrossRef]
- Machado, R.; Ribeiro, A.J.; Padrão, J.; Silva, D.; Nobre, A.; Teixeira, J.A.; Arias, F.J.; Cunha, A.M.; Rodríguez-Cabello, J.C.; Casal, M. Exploiting the Sequence of Naturally Occurring Elastin: Construction, Production and Characterization of a Recombinant Thermoplastic Protein-Based Polymer. J. Nano Res. 2009, 6, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, A.; Pereira, A.M.; Gomes, A.C.; Rodriguez-Cabello, J.C.; Casal, M.; Machado, R. Production of bioactive hepcidin by recombinant DNA tagging with an elastin-like recombinamer. New Biotechnol. 2018, 46, 45–53. [Google Scholar] [CrossRef]
- Li, B.-C.; Zhang, S.-Q.; Dan, W.-B.; Chen, Y.-Q.; Cao, P. Expression in Escherichia coli and purification of bioactive antibacterial peptide ABP-CM4 from the Chinese silk worm, Bombyx mori. Biotechnol. Lett. 2007, 29, 1031–1036. [Google Scholar] [CrossRef]
- Freire, D.O.; Da Cunha, N.B.; Leite, M.L.; Kostopoulos, A.G.C.; Da Silva, S.N.B.; De Souza, A.C.B.; Nolasco, D.O.; Franco, O.L.; Mortari, M.R.; Dias, S.C. Wasp venom peptide, synoeca-MP, fromSynoeca surinamashows antimicrobial activity against human and animal pathogenic microorganisms. Pept. Sci. 2020, 112, e24141. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Zhang, S.-Q. Antifungal mechanism of antibacterial peptide, ABP-CM4, from Bombyx mori against Aspergillus niger. Biotechnol. Lett. 2008, 30, 2157–2163. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Min, C.; Sang, M.; Han, Y.Y.; Ma, X.; Xue, X.Q.; Zhang, S.Q. A cationic amphiphilic peptide ABP-CM4 exhibits selective cytotoxicity against leukemia cells. Peptides 2010, 31, 1504–1510. [Google Scholar] [CrossRef]
- Da Costa, A.; Machado, R.; Ribeiro, A.; Collins, T.; Thiagarajan, V.; Neves-Petersen, M.T.; Rodríguez-Cabello, J.C.; Gomes, A.C.; Casal, M. Development of Elastin-Like Recombinamer Films with Antimicrobial Activity. Biomacromolecules 2015, 16, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, A.; Pereira, A.M.; Gomes, A.C.; Rodriguez-Cabello, J.C.; Sencadas, V.; Casal, M.; Machado, R. Single step fabrication of antimicrobial fibre mats from a bioengineered protein-based polymer. Biomed. Mater. 2017, 12, 045011. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.F.; Freire, M.S.; Cantuária, A.P.C.; Martins, D.C.M.; Amorim, I.A.; Dantas, E.M.G.L.; Farias, J.O.; Castro, M.B.; Silva, J.S.; Barriviera, F.A.; et al. The use of host defense peptides in root canal therapy in rats. Clin. Oral Investig. 2021, 25, 3623–3632. [Google Scholar] [CrossRef]
- Crimmins, D.L.; Mische, S.M.; Denslow, N.D. Chemical Cleavage of Proteins in Solution. Curr. Protoc. Protein Sci. 2005, 41, 11.4.1–11.4.11. [Google Scholar] [CrossRef] [PubMed]
- Bessa, P.C.; Machado, R.; Nürnberger, S.; Dopler, D.; Banerjee, A.; Cunha, A.M.; Rodríguez-Cabello, J.C.; Redl, H.; van Griensven, M.; Reis, R.L.; et al. Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J. Control. Release 2010, 142, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.; Ye, Z.; Aparicio, C.; Alonso, M.; Rodríguez-Cabello, J.C. Dual Self-Assembled Nanostructures from Intrinsically Disordered Protein Polymers with LCST Behavior and Antimicrobial Peptides. Biomacromolecules 2020, 21, 4043–4052. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human β-Defensin-1 Is a Salt-Sensitive Antibiotic in Lung That Is Inactivated in Cystic Fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.; Wang, J.; Shang, L.; Akhtar, M.U.; Wang, Z.; Shi, B.; Feng, X.; Shan, A. Short, symmetric-helical peptides have narrow-spectrum activity with low resistance potential and high selectivity. Biomater. Sci. 2019, 7, 2394–2409. [Google Scholar] [CrossRef]
- Li, A.; Sowder, R.C.; Henderson, L.E.; Moore, S.P.; Garfinkel, D.J.; Fisher, R.J. Chemical Cleavage at Aspartyl Residues for Protein Identification. Anal. Chem. 2001, 73, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.; Bessa, P.C.; Reis, R.L.; Rodriguez-Cabello, J.C.; Casal, M. Elastin-based nanoparticles for delivery of bone morphogenetic proteins. In Nanoparticles in Biology and Medicine: Methods and Protocols; Soloviev, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 353–363. [Google Scholar] [CrossRef]
- Machado, R.; da Costa, A.; Pereira, A.M.; Rodriguez-Cabello, J.C.; Casal, M. Elastins-Based Antimicrobial Particles for Delivery of Bioactive Compounds. Methods Mol. Biol. 2020, 2118, 29–43. [Google Scholar] [CrossRef]
- Shen, Y.; Ai, H.-X.; Song, R.; Liang, Z.-N.; Li, J.-F.; Zhang, S.-Q. Expression and purification of moricin CM4 and human β-defensins 4 in Escherichia coli using a new technology. Microbiol. Res. 2010, 165, 713–718. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef] [Green Version]
- CLSI—Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. In Sixteenth Informational Supplement; CLSI document M100-S16CLSI; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa Biofilms in Disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents—How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, B.S.; Zhang, W.; Harrison, J.J.; Quach, T.P.; Song, J.L.; Penterman, J.; Singh, P.K.; Chopp, D.L.; Packman, A.I.; Parsek, M.R. The extracellular matrix protectsPseudomonas aeruginosabiofilms by limiting the penetration of tobramycin. Environ. Microbiol. 2013, 15, 2865–2878. [Google Scholar] [CrossRef] [Green Version]
- Leitão, J.H.; Sousa, S.A.; Cunha, M.V.; Salgado, M.J.; Melo-Cristino, J.; Barreto, M.C.; Sá-Correia, I. Variation of the antimicrobial susceptibility profiles of Burkholderia cepacia complex clonal isolates obtained from chronically infected cystic fibrosis patients: A five-year survey in the major Portuguese treatment center. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 1101. [Google Scholar] [CrossRef]
- Madeira, A.; Santos, P.M.; Coutinho, C.P.; Pinto-De-Oliveira, A.; Sá-Correia, I. Quantitative proteomics (2-D DIGE) reveals molecular strategies employed by Burkholderia cenocepacia to adapt to the airways of cystic fibrosis patients under antimicrobial therapy. Proteomics 2011, 11, 1313–1328. [Google Scholar] [CrossRef]
- Mira, N.P.; Madeira, A.; Moreira, A.S.; Coutinho, C.P.; Sá-Correia, I. Genomic Expression Analysis Reveals Strategies of Burkholderia cenocepacia to Adapt to Cystic Fibrosis Patients’ Airways and Antimicrobial Therapy. PLoS ONE 2011, 6, e28831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, M.V.; Leitão, J.H.; Mahenthiralingam, E.; Vandamme, P.; Lito, L.; Barreto, C.; Salgado, M.J.; Sá-Correia, I. Molecular Analysis of Burkholderia cepacia Complex Isolates from a Portuguese Cystic Fibrosis Center: A 7-Year Study. J. Clin. Microbiol. 2003, 41, 4113–4120. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kucukural, A.; Zhang, Y. I-TASSER: A unified platform for automated protein structure and function prediction. Nat. Protoc. 2010, 5, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Huehne, R.; Suehnel, J. The Jena Library of Biological Macromolecules—JenaLib. Nat. Preced. 2009. [Google Scholar] [CrossRef]
- Machado, R.; Azevedo-Silva, J.; Correia, C.; Collins, T.; Arias, F.J.; Rodríguez-Cabello, J.C.; Casal, M. High level expression and facile purification of recombinant silk-elastin-like polymers in auto induction shake flask cultures. AMB Express 2013, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, F.; Castro, T.; Nogueira, E.; Pires, R.; Silva, C.; Ribeiro, A.; Cavaco-Paulo, A. OBP fused with cell-penetrating peptides promotes liposomal transduction. Colloids Surf. B Biointerfaces 2018, 161, 645–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]





| Antimicrobial Peptide | Theoretical | m/z (MALDI-TOF) |
|---|---|---|
| Synoeca | 1843.30 | 1872.39 |
| CM4 | 4008.78 | 4020.90 |
| Bacterial Species | MIC (mg/L) | |
|---|---|---|
| Synoeca | CM4 | |
| Escherichia coli | 25.00 | 50.00 |
| Staphylococcus aureus | 1.56 | 3.13 |
| Klebsiella pneumoniae | >100.00 | 50.00 |
| Pseudomonas aeruginosa | >100.00 | >100.00 |
| Burkholderia cenocepacia IST439 | 12.50 | ~200.00 |
| Burkholderia cenocepacia IST4113 | 12.50 | 200.00 |
| Staphylococcus epidermidis | 6.25 | 12.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.M.; Costa, A.d.; Dias, S.C.; Casal, M.; Machado, R. Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals 2021, 14, 956. https://doi.org/10.3390/ph14100956
Pereira AM, Costa Ad, Dias SC, Casal M, Machado R. Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals. 2021; 14(10):956. https://doi.org/10.3390/ph14100956
Chicago/Turabian StylePereira, Ana Margarida, André da Costa, Simoni Campos Dias, Margarida Casal, and Raul Machado. 2021. "Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag" Pharmaceuticals 14, no. 10: 956. https://doi.org/10.3390/ph14100956
APA StylePereira, A. M., Costa, A. d., Dias, S. C., Casal, M., & Machado, R. (2021). Production and Purification of Two Bioactive Antimicrobial Peptides Using a Two-Step Approach Involving an Elastin-Like Fusion Tag. Pharmaceuticals, 14(10), 956. https://doi.org/10.3390/ph14100956