Abstract
The progressive nature of dementia necessitates early detection strategies capable of identifying preclinical cognitive decline. Gait disturbances, mediated by higher-order cognitive functions, have emerged as potential digital biomarkers in this context. This bibliometric review systematically maps the scientific output from 2010 to 2025 on the application of wearable sensor technologies and gait analysis in the early diagnosis of dementia. A targeted search of the Scopus database yielded 126 peer-reviewed studies, which were analyzed using VOSviewer for performance metrics, co-authorship networks, bibliographic coupling, co-citation, and keyword co-occurrence. The findings delineate a multidisciplinary research landscape, with major contributions spanning neurology, geriatrics, biomedical engineering, and computational sciences. Four principal thematic clusters were identified: (1) Cognitive and Clinical Aspects of Dementia, (2) Physical Activity and Mobility in Older Adults, (3) Technological and Analytical Approaches to Gait and Frailty and (4) Aging, Cognitive Decline, and Emerging Technologies. Despite the proliferation of research, significant gaps persist in longitudinal validation, methodological standardization, and integration into clinical workflows. This review emphasizes the potential of sensor-derived gait metrics to augment early diagnostic protocols and advocates for interdisciplinary collaboration to advance scalable, non-invasive diagnostic solutions for neurodegenerative diseases.
1. Introduction
Dementia, with Alzheimer’s disease (AD) as its most prevalent form, represents one of the most pressing public health challenges of the 21st century. Currently, over 55 million people globally are living with dementia—a number projected to soar to 139 million by 2050 due to aging populations, particularly in low- and middle-income countries where the sharpest increases are anticipated [1]. AD alone accounts for 50–70% of dementia cases and imposes a profound and multifaceted burden on individuals, families, health systems, and economies worldwide [2]. The global cost of dementia care was estimated at $1.3 trillion in 2019 and is expected to escalate to $1.7 trillion by 2030, with nearly half of this cost attributed to informal care provided by unpaid family members, most of whom are women [1]. Despite advances in understanding the disease, there is still no curative treatment, making prevention, early diagnosis, and integrated care essential policy priorities [3]. The World Health Organization (WHO) and major scientific bodies have recognized dementia as a global public health priority, emphasizing the urgent need for coordinated international strategies to reduce risk factors, support caregivers, and alleviate health disparities [4]. Around 40% of global dementia cases may be preventable through modification of key risk factors such as hypertension, smoking, obesity, physical inactivity, and social isolation—factors that disproportionately affect disadvantaged populations [3]. However, national policy responses remain inadequate, with only a quarter of countries having implemented dedicated dementia plans, many of which lack adequate funding or sustainability [1]. As the prevalence of dementia continues to rise, inaction risks overwhelming healthcare systems, exacerbating social inequities, and undermining the well-being of older adults globally. The growing burden of dementia calls for immediate public health action grounded in scientific evidence, social justice, and economic foresight.
Early diagnosis of dementia is a critical public health imperative with significant implications for individual well-being, health care systems, and societal burden. Despite increasing awareness, it is estimated that up to 60% of dementia cases remain undiagnosed in primary care settings, thereby delaying access to vital resources and interventions [5]. As dementia progresses gradually, early identification—especially at the stage of mild cognitive impairment (MCI)—enables clinicians to initiate timely interventions that may slow cognitive decline and optimize the patient’s remaining functional years [6,7,8]. Furthermore, early diagnosis empowers patients and caregivers to plan for future care needs, make informed decisions about legal and financial matters, and access support services that improve quality of life [6,9]. From a systemic perspective, delaying the onset or progression of dementia through early detection could result in substantial cost savings and a reduced strain on long-term care services [10]. Despite these benefits, multiple barriers, such as stigma, limited training in primary care, and disparities in access among rural and minority populations, continue to hinder widespread implementation of early detection strategies. Therefore, enhancing clinical protocols, policy support, and public awareness around early diagnosis is essential to reduce the burden of dementia and improve outcomes for affected individuals and their families.
The integration of gait analysis and wearable sensor technologies is increasingly recognized as a promising avenue for the early detection and monitoring of cognitive impairment, including dementia. Gait, a complex motor function governed by cognitive processes such as attention, executive functioning, and memory, often deteriorates subtly during the early stages of cognitive decline, making it a sensitive behavioral biomarker [11,12,13]. Recent studies have demonstrated that individuals with amnestic mild cognitive impairment (aMCI), a precursor to Alzheimer’s disease, exhibit significant abnormalities in gait parameters, including reduced walking velocity, shorter stride length, and increased stride time variability compared to cognitively healthy controls [14]. These abnormalities correlate with specific domains of cognitive decline, particularly memory and executive function, supporting the view that motor and cognitive deficits share underlying neural pathways. Traditional diagnostic tools such as neuroimaging and cerebrospinal fluid biomarkers, although effective, are expensive, invasive, and often inaccessible in primary care or low-resource settings. In contrast, gait analysis using wearable sensors or depth cameras like Kinect v.2 offers a cost-effective, non-invasive, and scalable solution [12]. Wearable devices enable continuous, real-world monitoring of spatiotemporal gait parameters, which may better reflect functional impairments in daily life compared to laboratory-based assessments [14]. Furthermore, machine learning algorithms applied to gait data have shown high accuracy in distinguishing individuals with MCI from healthy controls, particularly when analyzing more cognitively demanding walking tasks like oval-path walking [12]. This convergence of digital health technologies, sensor data, and artificial intelligence not only enhances diagnostic precision but also holds the potential to transform routine clinical assessments by enabling earlier, more personalized interventions for individuals at risk of dementia.
Despite the growing body of evidence supporting gait analysis and wearable sensors as valuable tools in the detection of cognitive decline, the field still lacks systematic bibliometric mapping that could consolidate existing knowledge and guide future research directions. While numerous studies have independently highlighted the diagnostic potential of gait-related biomarkers, there is a notable absence of comprehensive, data-driven analyses that chart trends, influential publications, collaborative networks, and emerging hotspots in this interdisciplinary field. As recently noted by Zhong et al. [15], the research landscape on gait analysis in older adults with MCI remains fragmented and underexplored from a bibliometric perspective, hindering the development of a unified scientific framework. The lack of such systematic reviews and knowledge maps limits researchers’ ability to avoid redundancy, build on existing evidence, and strategically prioritize innovative approaches that could accelerate early diagnosis of dementia. Therefore, bibliometric mapping should be considered a critical component of advancing the field, promoting interdisciplinarity, and enhancing both the scientific and clinical impact of gait-based cognitive assessments.
This study aims to provide a focused bibliometric analysis of peer-reviewed journal articles published between 2010 and 2025 that examine the role of gait analysis and wearable sensor technologies in the early diagnosis or detection of dementia and Alzheimer’s disease. Despite increasing interest in this interdisciplinary field, no prior study has systematically mapped the scientific output using this combination of clinical, biomechanical, and technological criteria. The goal is to offer a structured overview of the field and to support future interdisciplinary research aimed at improving early detection and intervention strategies through non-invasive, sensor-based gait assessment.
2. Materials and Methods
2.1. Search Strategy
A targeted literature search was conducted in Scopus database to identify peer-reviewed articles related to the use of gait analysis, motion capture, and biomechanics in the early detection and diagnosis of dementia, with particular emphasis on Alzheimer’s disease. The search aimed to capture studies that explore the application of wearable technologies or sensors for assessing movement-related biomarkers in human subjects.
The following search query was used:
TITLE-ABS-KEY ((“dementia” OR “Alzheimer” OR “mild cognitive impairment” OR “cognitive decline”) AND (“early diagnosis” OR “early detection” OR “screening” OR “biomarker” OR “predict” OR “prognos” OR “monitoring” OR “assessment”) AND (“gait” OR “gait analysis” OR “walking” OR “locomotion” OR “mobility” OR “motion capture” OR “movement analysis” OR “biomechanics” OR “motor function”) AND (“wearable sensor” OR “inertial measurement unit” OR “IMU” OR “acceleromet” OR “gyroscope” OR “wearable device” OR “sensor” OR “portable device”)) AND PUBYEAR > 2009 AND PUBYEAR < 2026 AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (EXACTKEYWORD, “Humans”)).
The search was limited to articles published in English between 2010 and 2025 and included only original research involving human participants. Studies that met the criteria were drawn from reputable scientific databases, ensuring relevance to both clinical and technological aspects of dementia research. The goal was to highlight recent advances in wearable-assisted movement analysis as a tool for detecting cognitive decline at early stages.
2.2. Selection Criteria
Studies were selected according to predefined inclusion and exclusion criteria, with the aim of identifying research that aligns with the study objective: the use of gait-related or biomechanical markers, obtained through wearable technologies, for the early detection or diagnosis of dementia in human populations. The inclusion criteria required that studies: (i) be original research articles published in peer-reviewed journals, (ii) involve human participants, and (iii) focus on the application of gait analysis, motion capture, or biomechanical methods in the context of early-stage dementia or Alzheimer’s disease, utilizing wearable devices or sensors.
The exclusion criteria were applied to ensure the relevance and scientific rigor of the included literature. Studies were excluded if they: (i) involved non-human subjects, particularly animal models such as rats; (ii) were case reports, which often lack generalizability and statistical validity; or (iii) were protocol papers, which describe intended methodologies but do not report empirical findings. These selection criteria were applied during the screening and eligibility assessment phases of the review to ensure that only studies with direct applicability to human-centered, technology-driven approaches for early dementia detection were retained for analysis.
2.3. Data Extraction
Bibliographic data for all articles meeting the inclusion criteria were retrieved directly from the Scopus database. The export was conducted in CSV format, which included detailed metadata for each publication. Specifically, the dataset comprised information on article title, list of authors, author affiliations, journal of publication, year of publication, abstract, number of citations, and assigned keywords. This structured format allowed for a comprehensive overview of the publication characteristics relevant to the scope of this study. It should be noted that funding information was not included in the export, as this field is not available by default in the Scopus CSV output.
Following data export, the file was imported into VOSviewer (version 1.6.20.0), a widely used open-source tool for constructing and visualizing bibliometric networks [16,17]. The software was employed to perform bibliometric mapping and co-occurrence analysis of author keywords and terms extracted from abstracts. These analyses facilitated the identification of thematic clusters, emerging trends, and interrelationships among key concepts within the literature. This methodological step supported both the descriptive characterization of the research landscape and the quantitative exploration of conceptual linkages in the field of early dementia detection using gait analysis and wearable technologies.
2.4. Bibliometric Analysis
The bibliometric analysis in this study combined performance analysis and science mapping techniques, following established methodological standards in bibliometric research [18,19,20]. All analyses were conducted using VOSviewer (version 1.6.20.0), a specialized software tool for constructing and visualizing bibliometric networks. Prior to analysis, preprocessing procedures were implemented to enhance data consistency, including the use of custom thesaurus files to correct for variations and spelling inconsistencies in author names, journal titles, and keywords.
2.4.1. Performance Analysis
The performance analysis aimed to quantify research productivity and influence across both individual and source-level contributions. In the author productivity analysis, only authors with a minimum of four publications were included. The size of each node in the visualizations represented the total number of documents contributed by each author. Similarly, the source analysis evaluated journal-level productivity, applying a threshold of three documents per source, with node weights again determined by document count. Summary tables and network diagrams were generated to highlight the most prolific authors and publication venues within the dataset.
2.4.2. Science Mapping Analysis
Science mapping techniques were employed to uncover structural, thematic, and collaborative patterns within the literature. Four distinct approaches were used:
- Co-authorship Analysis: This analysis focused on institutional affiliations, treating organizations as the unit of analysis. Institutions with at least two published documents were included. The resulting network visualized collaboration patterns between institutions, with node sizes reflecting the number of documents associated with each organization.
- Bibliographic Coupling: Journals were used as the unit of analysis to assess thematic similarity based on shared references. A minimum of two documents per source was required for inclusion. The resulting overlay visualization identified clusters of journals with overlapping citation profiles.
- Co-citation Analysis: This technique was used to identify the intellectual structure and key scholarly influences within the field. It focuses on detecting sources (i.e., journals) that are frequently cited together across different publications, which may indicate shared conceptual backgrounds or thematic alignment. Τhe unit of analysis was the source (journal), and a minimum threshold of 10 co-citations was applied to ensure relevance and statistical robustness. This mapping approach enables the identification of influential publications and intellectual schools of thought [8,16].
- Keyword Co-occurrence Analysis: Author keywords served as the unit of analysis to explore recurring themes and emerging trends. Keywords that appeared at least three times across the corpus were included. Co-occurrence patterns were visualized as a network, with node size weighted by keyword frequency. Clustering was automatically performed by VOSviewer based on link strength and proximity, revealing major thematic groupings in the field.
A detailed summary of the methodological settings applied in each science mapping technique is provided in Table 1.

Table 1.
Overview of Science Mapping Techniques.
3. Results
3.1. Included Studies
A total of 184 records were initially retrieved from the Scopus database following the application of the search strategy described in Section 2.1. These records were screened based on predefined inclusion and exclusion criteria (see Section 2.2). After removing studies that did not meet the eligibility requirements, 126 articles were included (Table S1).
3.2. Bibliometric Performance Analysis
The performance analysis focused on identifying the most prolific contributors in the field by examining author productivity, using the number of published documents as the primary metric. Authors who had contributed to at least three publications within the final set of included studies were retained for analysis. A total of 25 authors met this threshold. Table 2 summarizes the bibliometric performance of the most productive and influential authors within the analyzed research domain. The data include the number of publications and corresponding citation counts, reflecting both research productivity and scholarly impact. Among the listed authors, Kaye, Jeffrey A. exhibits the highest citation record (563 citations from 6 documents), indicating a strong academic influence. Other authors with notable citation performance include Mattek, Nora C. (426 citations), Hayes, Tamara L. (407 citations), and Najafi, Bijan (289 citations). The overall distribution reveals a concentrated contribution pattern, wherein a limited number of authors account for a substantial proportion of citations, underscoring their pivotal roles in shaping the intellectual structure of the field.

Table 2.
Most Productive Authors by Number of Documents and Citations.
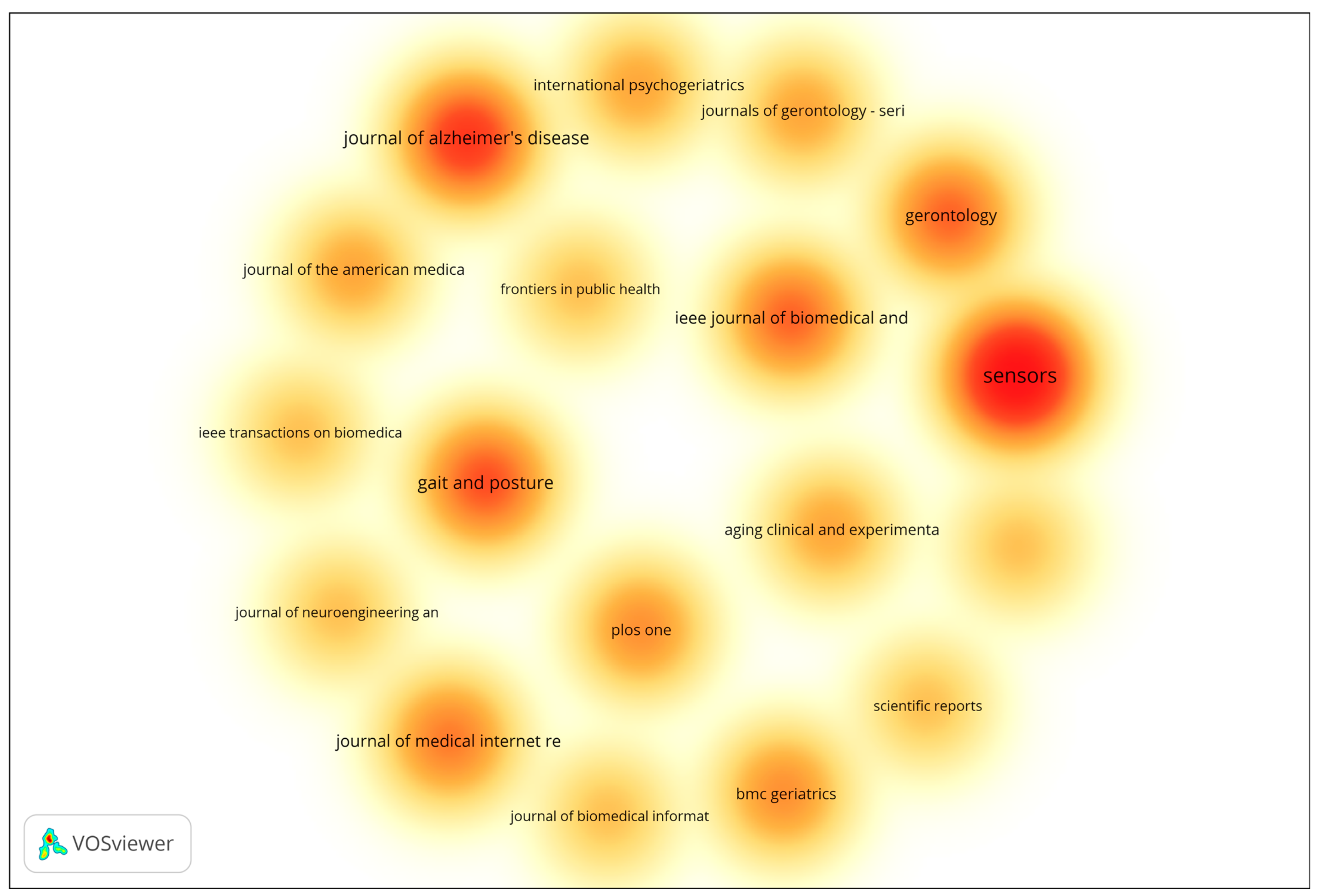
In addition to author-level productivity, source-level performance was also analyzed to identify the most prominent journals publishing research on gait analysis and wearable technologies for early dementia detection. The analysis included all sources with a minimum of two documents, using the number of documents as the weighting metric. The resulting density visualization (Figure 1) highlights clusters of influential publication venues based on document frequency. The visualization highlights the density and frequency of publications across various scientific outlets. Journals represented by warmer colors (red and orange) correspond to higher publication activity, indicating their prominence within the field. The results reveal that Sensors, Journal of Alzheimer’s Disease, and Gait and Posture are among the most influential publication venues, characterized by their high concentration of documents and strong citation performance. Other journals such as IEEE Journal of Biomedical and Health Informatics, Gerontology, and Journal of Medical Internet Research also demonstrate considerable research activity, underscoring the multidisciplinary nature of the field, which spans biomedical engineering, gerontology, and digital health. Overall, the overlay visualization underscores the dominance of a select group of specialized journals that serve as key dissemination platforms for research on aging, mobility, and sensor-based health monitoring. These outlets play a pivotal role in advancing interdisciplinary collaboration and the translation of technological innovations into clinical and public health applications.
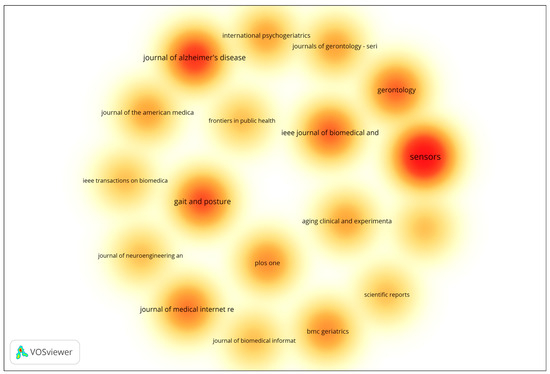
Figure 1.
Density Visualization of Most Productive Journals. The density map uses a color gradient to represent the concentration of publications across journals. Warmer colors (yellow → orange → red) indicate higher publication density and greater research activity, with red areas representing the most productive journals. Cooler and lighter colors indicate lower publication density and fewer contributions.
3.3. Science Mapping
3.3.1. Co-Authorship Analysis by Country
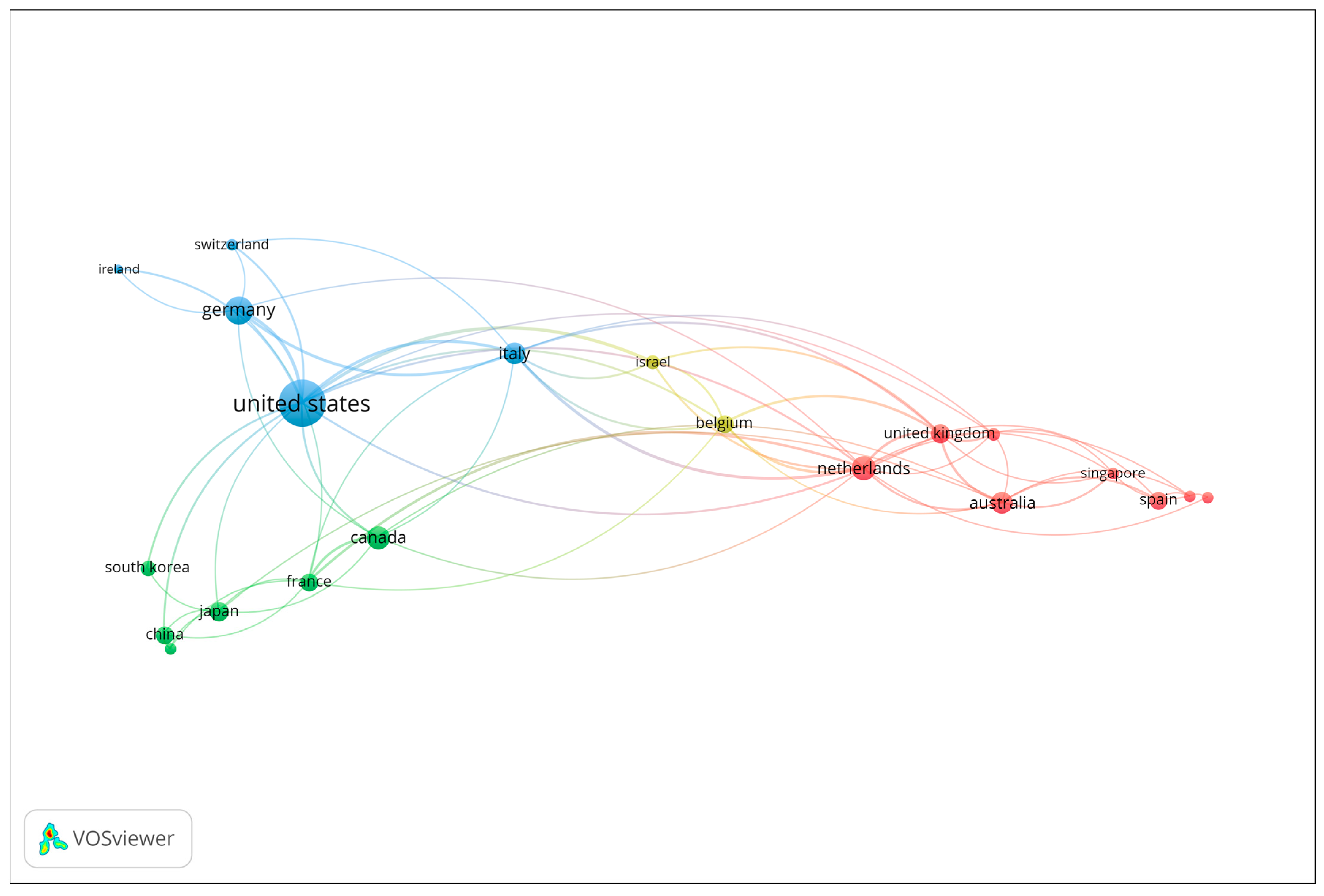
Co-authorship analysis at the country level was performed to explore patterns of international collaboration in the field of gait analysis and wearable technologies for early dementia detection. Countries contributing to at least two publications were included in the analysis. The number of documents served as the weighting metric, and the resulting network visualization is presented in Figure 2. Each node represents a country, with node size corresponding to the number of publications, and the connecting lines (links) indicating collaborative relationships based on co-authored documents. The color coding reflects distinct collaboration clusters, which highlight regional and transnational research partnerships. The visualization reveals that the United States occupies a central and dominant position within the network, demonstrating extensive collaborative links with several European and Asian countries. Strong research connections are observed between the United States, the United Kingdom, Italy, Germany, and the Netherlands, forming a core collaborative cluster that drives much of the scholarly output in the field. Other notable contributors, including Canada, Australia, and Japan, also exhibit active engagement within this network, though to a lesser extent. Overall, the co-authorship network underscores the highly collaborative and international nature of research in this domain, with a concentration of productivity and influence in a few key countries that serve as major hubs of scientific exchange and interdisciplinary innovation.
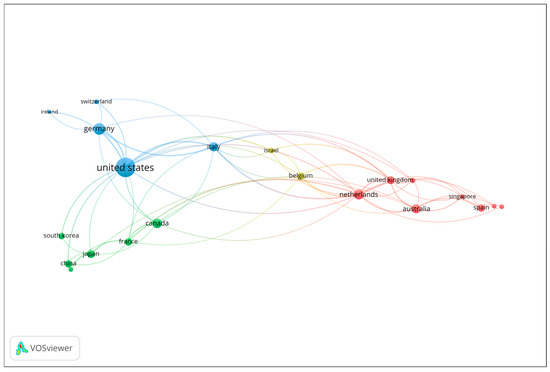
Figure 2.
Co-authorship network of contributing countries (Minimum 2 publications; Node size = number of documents).
3.3.2. Bibliographic Coupling Analysis of Organizations
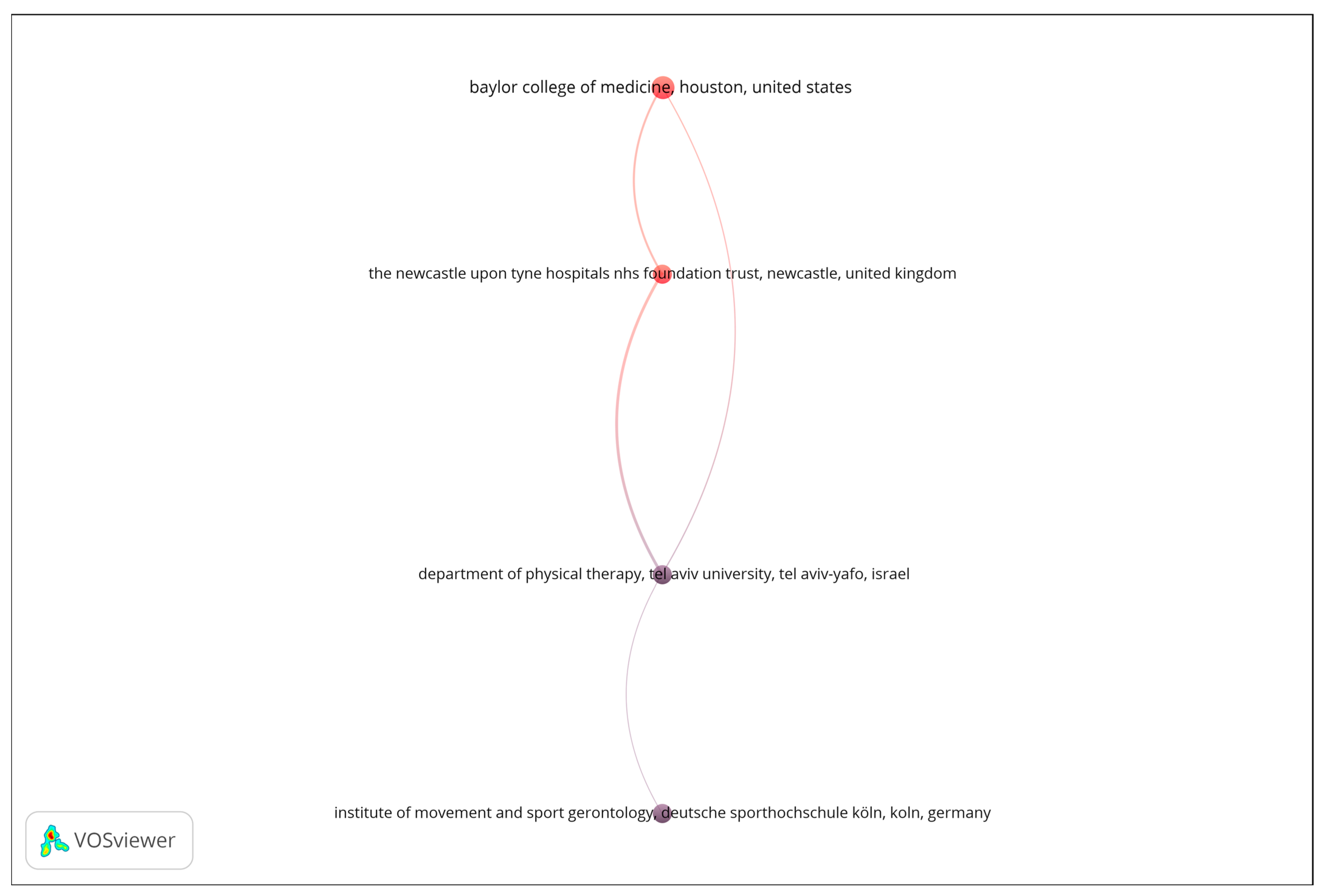
A bibliographic coupling analysis was performed to explore thematic similarities among publication venues based on shared reference patterns. The analysis was restricted to organizations with a minimum of four documents, and the number of documents was used as the weighting metric. As shown in Figure 3, the resulting network reveals a limited number of interconnected institutions, suggesting focused but relatively narrow collaboration patterns in the analyzed research domain. The visualization displays four nodes linked through bibliographic coupling relationships, forming a single, vertically oriented structure that represents distinct yet thematically aligned research entities. The strength of the coupling among these institutions indicates shared research interests and overlapping citation behavior, reflecting a convergence around specific topics such as biomedical engineering, sensor technologies, and aging-related health monitoring. Overall, the bibliographic coupling map underscores the thematic coherence of the leading organizations contributing to this field, while also highlighting the opportunity for broader institutional collaboration to enhance knowledge exchange and interdisciplinary integration.
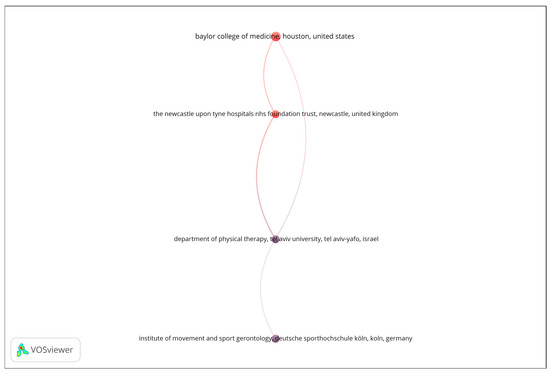
Figure 3.
Bibliographic coupling network of organizations.
3.3.3. Co-Citation Analysis
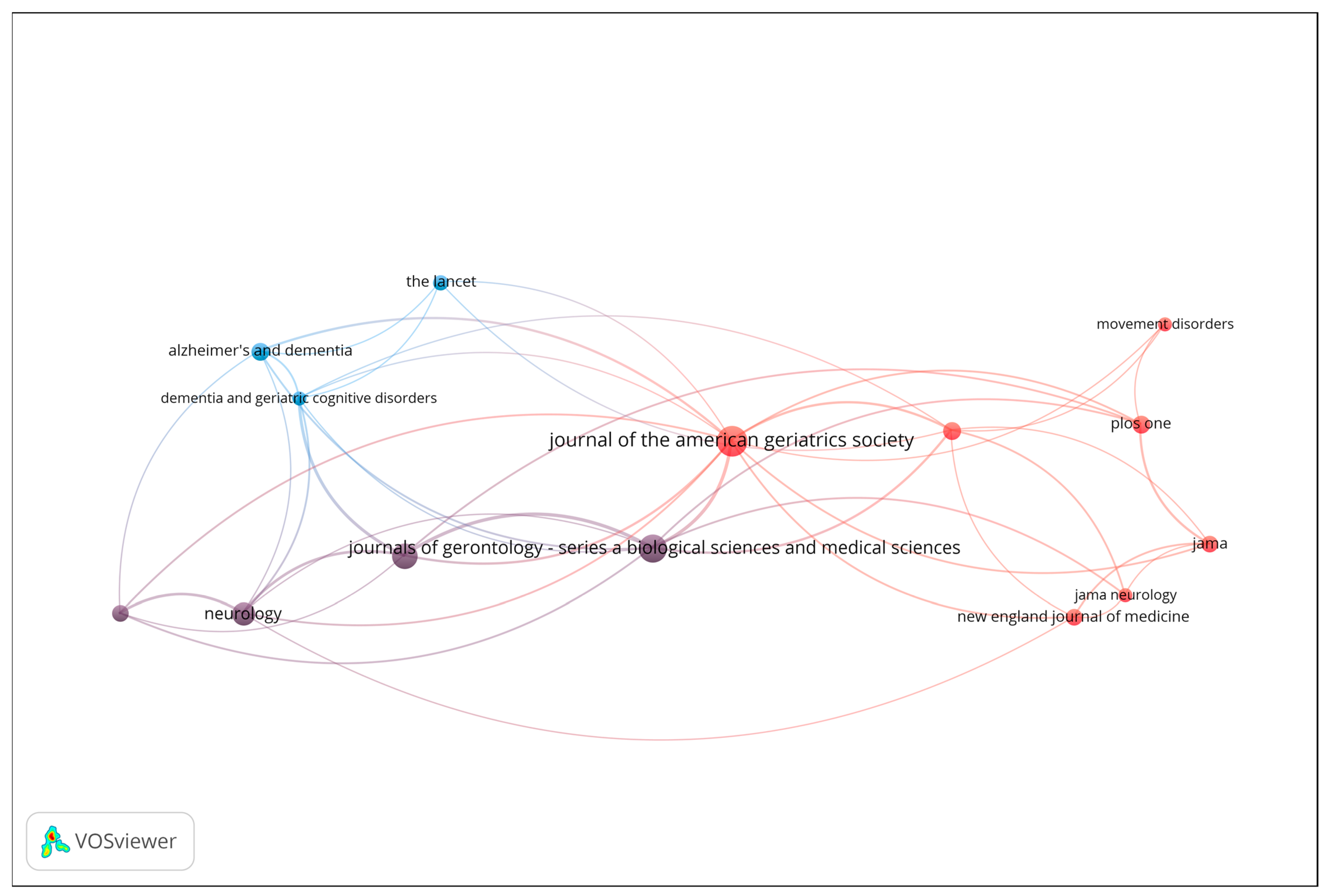
Co-citation analysis was conducted to uncover the intellectual structure and influential sources within the field of gait analysis and wearable sensor technologies for early dementia detection. This technique identifies journals and articles that are frequently cited together, reflecting shared conceptual foundations or complementary scientific contributions. Figure 4 presents the resulting co-citation network, including only sources with a minimum of 6 citations. The size of each node represents the total number of co-citations, while the proximity and thickness of connecting lines indicate the strength of co-citation relationships.
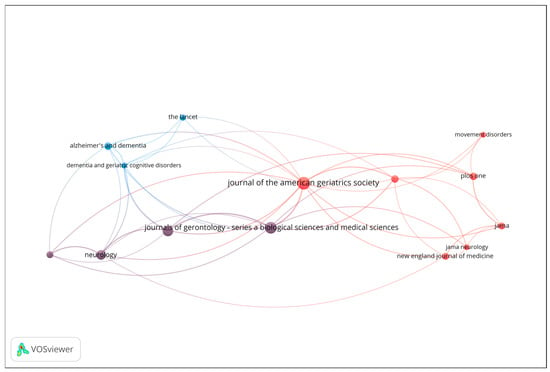
Figure 4.
Co-citation network of influential sources (Minimum 6 citations; Node size = number of co-citations).
The red cluster, located at the center-right of the map, represents the clinical and neurology-oriented core of the field. It includes highly influential journals such as Journal of the American Geriatrics Society (JAGS), JAMA Neurology, The New England Journal of Medicine (NEJM), JAMA, PLOS ONE, and Movement Disorders. This cluster signifies a strong concentration of general medical and neurological research, highlighting journals that serve as hubs for interdisciplinary clinical studies. The blue cluster, positioned toward the left of the visualization, is associated with dementia and geriatrics research. It comprises journals such as The Lancet, Alzheimer’s & Dementia, and Dementia and Geriatric Cognitive Disorders. These titles form a cohesive subfield focused on neurodegenerative diseases and aging-related health, while maintaining strong citation links to broader clinical outlets, particularly those in the red cluster. The purple cluster, situated between the blue and red regions, acts as a bridge between neurology and gerontology. It includes Neurology and the Journal of Gerontology: Series A (Biological Sciences & Medical Sciences). This cluster connects basic research on aging and neurobiology with clinical and population-level studies, thereby linking specialized and general medical discourses.
Overall, the co-citation analysis reveals a clear intellectual gradient extending from dementia and geriatrics (blue) through neurology and aging sciences (purple) to the general and clinical medicine core (red). This structure underscores the multidisciplinary nature of the field, where clinical geriatrics, neurological disorders, and aging research converge through shared citation patterns and overlapping thematic concerns.
3.3.4. Co-Occurrence Analysis of Author Keywords
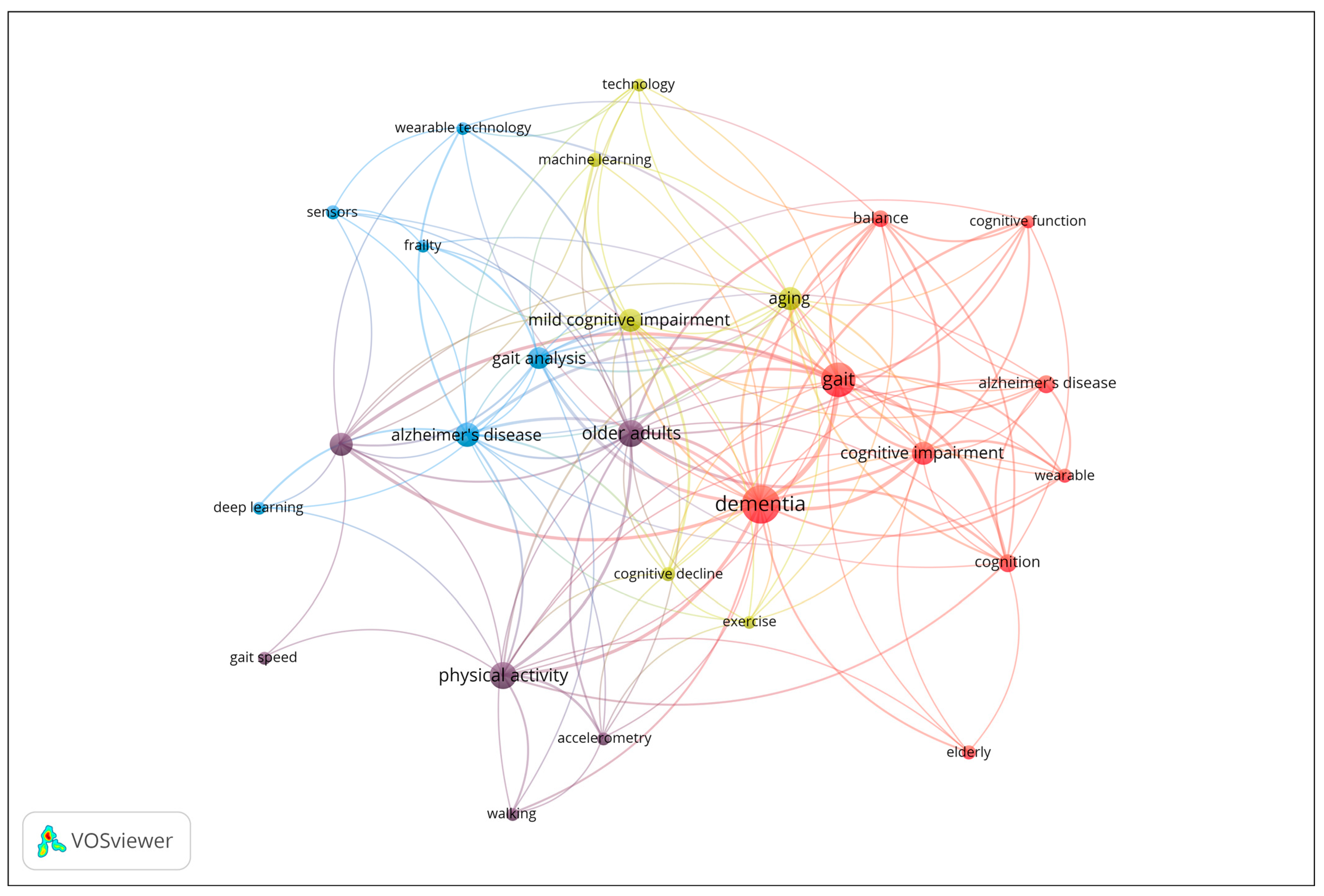
To investigate the thematic structure of the research field, a co-occurrence analysis of author keywords was performed. Keywords with a minimum occurrence of four were included in the analysis, and occurrence frequency was used as the weight metric. The results are displayed in the network visualization in Figure 5, where node size corresponds to the frequency of keyword use, and clustering is determined by co-occurrence strength. The network reveals four main clusters representing distinct but interrelated research themes. The map reveals four distinct thematic clusters. Cluster 1 (red) focuses on cognitive and clinical aspects of dementia, including keywords such as Alzheimer’s disease, cognition, dementia, and gait. Cluster 2 (purple) centers on physical activity and mobility in older adults, with terms like accelerometer, gait speed, and walking. Cluster 3 (blue) represents technological and analytical approaches, including deep learning, sensors, and wearable technology. Finally, Cluster 4 (yellow) highlights aging, cognitive decline, and emerging technologies, with keywords such as machine learning, exercise, and mild cognitive impairment. The network illustrates the interdisciplinary nature of the field, linking clinical, cognitive, and technological research domains in aging and dementia studies.
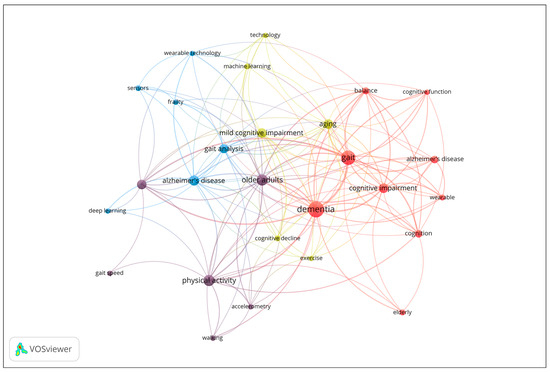
Figure 5.
Co-occurrence network of author keywords (Minimum 4 occurrences; Node size = frequency of occurrence).
From the co-occurrence analysis of author keywords in the included publications, three main thematic clusters emerged, reflecting the interdisciplinary nature of research on dementia and gait analysis:
- Cluster 1. Cognitive and Clinical Aspects of Dementia (9 items): Alzheimer’s disease, Balance, Cognition, Cognitive function, Cognitive impairment, Dementia, Elderly, Gait, Wearable [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
- Cluster 2. Physical Activity and Mobility in Older Adults (6 items): Accelerometer, Accelerometry, Gait speed, Older adults, Physical activity, Walking [65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].
- Cluster 3. Technological and Analytical Approaches to Gait and Frailty (6 items): Alzheimer’s disease, Deep learning, Frailty, Gait analysis, Sensors [80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111]
- Cluster 4. Aging, Cognitive Decline, and Emerging Technologies (6 items): Aging, Cognitive decline, Exercise, Machine learning, Mild cognitive impairment, Technology [112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]
4. Discussion
4.1. Interpretation of Thematic Cluster 1
The first thematic cluster highlights the growing convergence between cognitive, motor, and clinical dimensions of dementia. Across the included studies, dementia emerges not as a purely cognitive disorder but as a multisystem condition, detectable through alterations in movement, rhythm, environmental exposure, and affective responses.
A recurring theme involves the integration of cognitive–motor assessments as sensitive biomarkers of early cognitive decline. Dual-task paradigms that couple cognitive and motor performance outperform traditional screening tools. Schiavo et al. [22] validated the Performance Index (P-Index) as a quantitative measure combining gait and cognitive accuracy during the instrumented Timed Up and Go test, strongly predicting MMSE outcomes. Similarly, Greene et al. [31] introduced the Dual Task Ball Balancing Test, a safe digital assessment linked with both MCI and AD related dementia, emphasizing the shift toward ecologically valid, sensor-based cognitive testing.
Motor parameters such as gait variability and balance serve as additional indicators of neurodegeneration. Labott et al. [23] showed higher minimum toe clearance variability in MCI, while Schmidt et al. [29] and Chiba et al. [32] found poorer balance and gait patterns predicting falls and wheelchair dependence. These findings, supported by Aznielle-Rodríguez et al. [33], confirm that spatiotemporal gait features reflect global cognitive status and can function as digital proxies for brain health. Beyond motor correlates, neurobiological and environmental determinants are increasingly recognized. Xiong et al. [21] linked individual exposure to particulate matter and volatile organic compounds with cerebrospinal tau and amyloid biomarkers, revealing potential environmental pathways in dementia pathogenesis. Likewise, Elasfar et al. [27] differentiated AD from dementia with Lewy bodies (DLB) through gait analysis, suggesting that movement alterations precede amyloid accumulation.
Advances in wearable and digital monitoring expand clinical observation into real-world settings. Chan et al. [24] demonstrated that wrist-worn sensor data on walking speed, running duration, and bedtime predicted incident dementia with accuracy comparable to risk scores, while Hammink et al. [26] used biometric sensors in nursing homes to associate emotional responses with daily activities, offering insights into lived experience and under-stimulation in dementia care. Finally, studies like Slusarenko et al. [25] illuminate the embodied and rhythmic aspects of cognition. They showed that rhythmic proficiency and musical engagement enhance motor performance in healthy adults but not in MCI, implying that cognitive impairment disrupts rhythm-based coordination—yet such therapies retain rehabilitative potential.
In synthesis, this cluster portrays dementia as a complex interaction of cognitive, motor, emotional, and environmental factors. The convergence of dual-task testing, digital gait metrics, and ecological monitoring supports a personalized, sensor-informed approach to diagnosis and care. Individualized assessment whether through environmental exposure, mobility behavior, or affective response constitutes the foundation for next-generation dementia management.
4.2. Interpretation of Thematic Cluster 2
This cluster emphasizes how physical activity and mobility serve as core indicators of healthy aging, linking movement patterns with physical, cognitive, and functional outcomes. The integration of wearable technologies and objective accelerometry has transformed our understanding of mobility from a descriptive behavior to a quantifiable biomarker of aging health.
Evidence consistently shows that quality, not just quantity, of movement matters most. Continuous walking in longer bouts and higher cadence patterns have been associated with reduced care service use and lower risk of cognitive impairment, even after adjusting for age and comorbidities [66,67]. These findings suggest that mobility rhythm and endurance provide early insight into overall resilience and independence. Physiological analyses further support this link: moderate-to-vigorous activity enhances skeletal muscle energetics [70], while light daily activity improves short-term memory and inhibition among older adults with chronic illness [74].
The technological evolution of wearables enables continuous, non-invasive monitoring of health behaviors. Reviews and bibliometric analyses reveal rapid growth in smart wearable applications for fall detection, disease prevention, and mobility tracking [65,68]. When combined with machine learning, sensor data can predict functional decline and physical impairment with high precision [69]. Importantly, studies stress the need for user-centered design and long-term validation to ensure reliability in older populations. Mobility monitoring also plays a preventive role in fall and dependency prediction. Gait quality and walking speed derived from wrist-worn devices predict injurious falls [72], while sensor-based activity patterns outperform traditional tests in identifying fall risk among people with dementia [79]. Even in the oldest-old, higher activity intensity relates to better nutrition and motor performance, whereas sedentary behavior in institutional care remains a persistent challenge [71,76,78].
Overall, the cluster reveals a paradigm shift toward data-driven mobility assessment. Physical activity emerges as a multidimensional biomarker connecting musculoskeletal, cognitive, and psychosocial domains. Sustained, achievable levels of activity supported by wearable monitoring may thus represent one of the most practical strategies for maintaining autonomy and health in older adulthood.
4.3. Interpretation of Thematic Cluster 3
This cluster illustrates the rapid convergence of sensor technologies and artificial intelligence in analyzing gait and frailty among older and neurologically impaired adults. Collectively, these studies show how digital and analytical innovations are transforming movement assessment from clinical observation to continuous, data-driven monitoring.
A primary theme is the digitalization of gait assessment. Automated tools such as the electronic Short Physical Performance Battery (eSPPB) kiosk have demonstrated reliable, scalable frailty screening in community settings [80]. Similarly, ear-worn and depth-camera systems provide accurate gait speed and step length estimates, extending precision analysis beyond laboratories [87,91]. These advances make remote, objective mobility evaluation feasible in daily environments.
A second trend involves machine learning–based analytics capable of recognizing complex gait signatures. Deep learning models, such as LSTM and Enhanced Band-Dependent Learning, have achieved accurate classification of gait cycles and the detection of disease-related abnormalities, including Alzheimer’s-linked movement changes [81,85]. Such models move gait research toward predictive and diagnostic applications, offering real-time interpretation of sensor data.
At the same time, digital biomarkers derived from wearable devices are gaining clinical utility. Studies using explainable AI and quantile regression [88] improve the precision of gait feature extraction, while multicenter trials confirm their ability to distinguish neurological subtypes such as Alzheimer’s and Lewy body dementia [82]. Platforms like FACET further demonstrate that integrated home-based monitoring can reduce frailty progression [86].
Across this research, the fusion of multimodal sensing and analytical intelligence defines a shift toward precision gerontechnology. Inertial, radar, and vision-based systems now operate synergistically, offering multidimensional and longitudinal perspectives of human movement. These innovations position gait and frailty as dynamic, quantifiable, and modifiable health indicators, central to early detection and personalized intervention.
4.4. Interpretation of Thematic Cluster 4
This cluster highlights the rapid integration of digital innovation into aging and dementia research, where wearable sensors, smart-home systems, and artificial intelligence provide new ways to detect, monitor, and manage cognitive decline. The studies collectively show that behavioral and physiological data gathered unobtrusively can serve as early digital biomarkers of neurodegenerative change.
A dominant theme concerns AI-assisted diagnostics. Machine learning models using actigraphy and motor data have achieved high accuracy in distinguishing Alzheimer’s disease from other dementias and in detecting mild cognitive impairment [112,116,129]. Movement complexity, gait variability, and circadian rhythm metrics emerge as sensitive predictors of preclinical decline. These methods illustrate how everyday activity can act as a continuous reflection of cognitive health.
Equally important is the use of connected technologies for prevention and intervention. Telehealth and mHealth systems combine behavioral feedback with real-time monitoring. Tai Chi programs supported by wearables improved cognitive performance and sleep in diabetic older adults [114], while passive sensing interventions like Sense4Safety proved feasible for fall prevention in older adults with mild cognitive impairment [113]. Such models mark a shift toward personalized, technology-supported aging care.
The cluster also includes smart-home and ambient sensing applications capable of tracking behavioral and psychological symptoms of dementia [115,118]. These unobtrusive systems detect deviations in daily routines, while digital biomarkers have been correlated with Alzheimer’s pathology postmortem [119], underscoring their biological relevance. Moreover, longitudinal data link poor sleep and low physical activity with cognitive decline [120], and web-based multidomain programs demonstrate the feasibility of online cognitive and lifestyle interventions [128].
Overall, this cluster reveals a paradigm shift toward continuous, data-driven aging research, where cognition, mobility, and lifestyle are monitored dynamically. Emerging technologies not only enhance early detection but also support individualized prevention and care, transforming the traditional model of aging assessment into a proactive and personalized digital health framework.
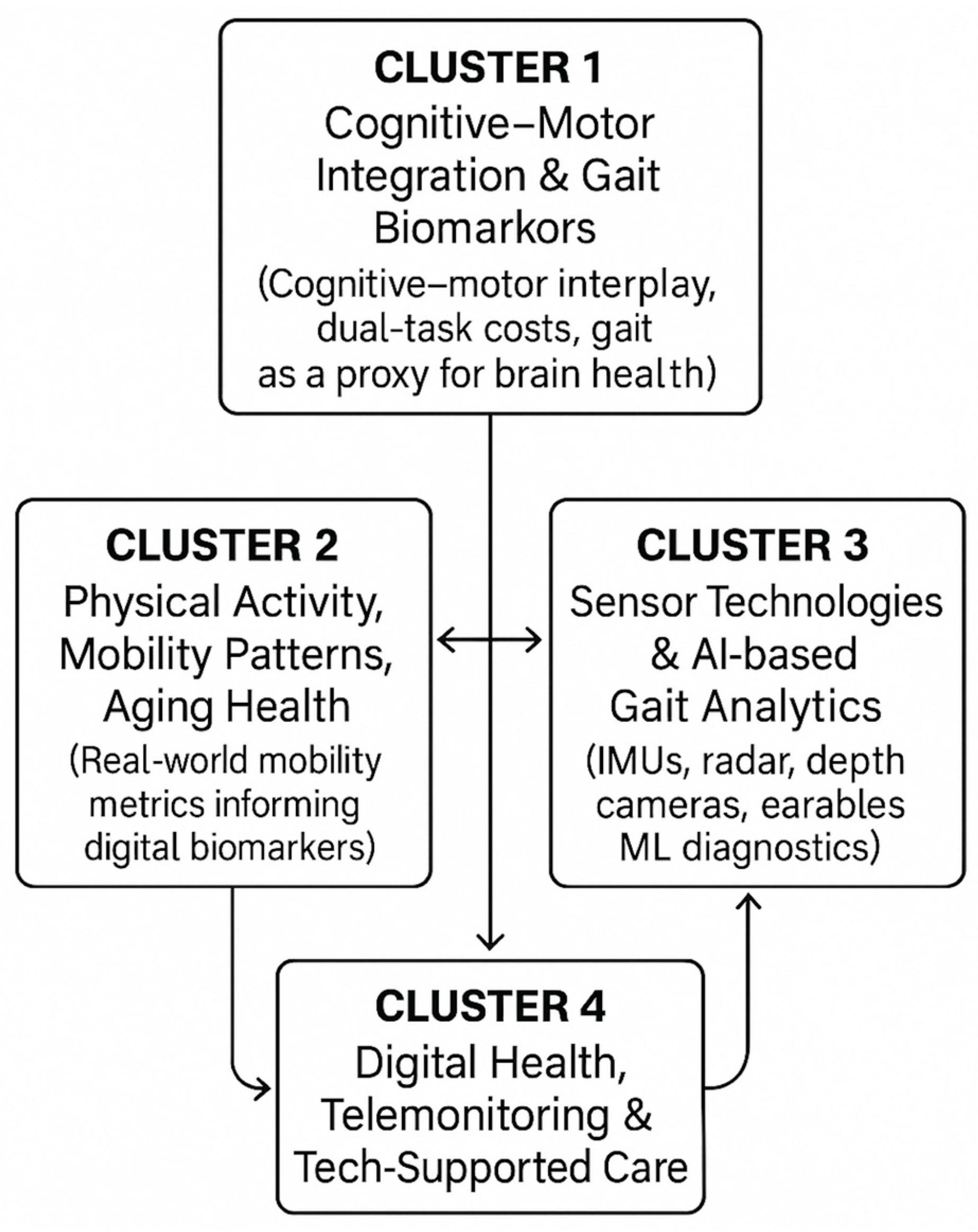
Figure 6 visually depicts the interactions among the four thematic clusters (cognitive–motor integration, physical activity and mobility patterns, sensor/AI-driven gait analytics, and digital-health interventions). This figure highlights how the clusters connect conceptually (e.g., cognitive–motor biomarkers linking to gait features; sensor innovations linking to real-world mobility monitoring) and clarifies the overarching narrative of technological and clinical convergence in the field.
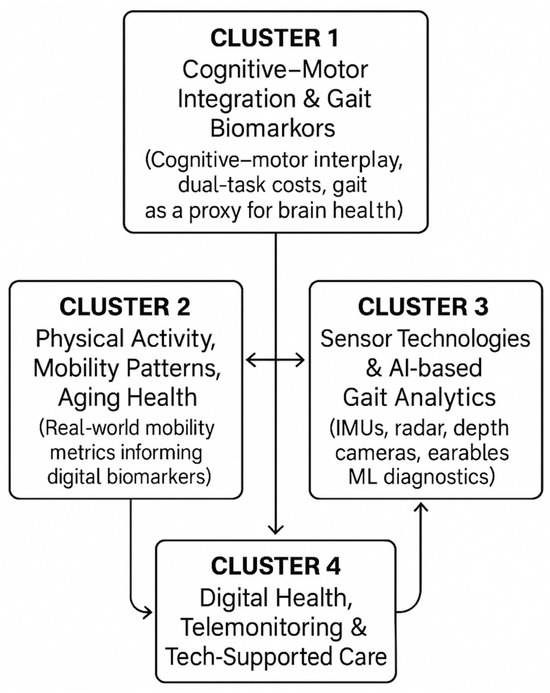
Figure 6.
A Cluster Relationship Map.
4.5. Causal Relationships and Neural Mechanisms Linking AD Pathology to Gait Abnormalities
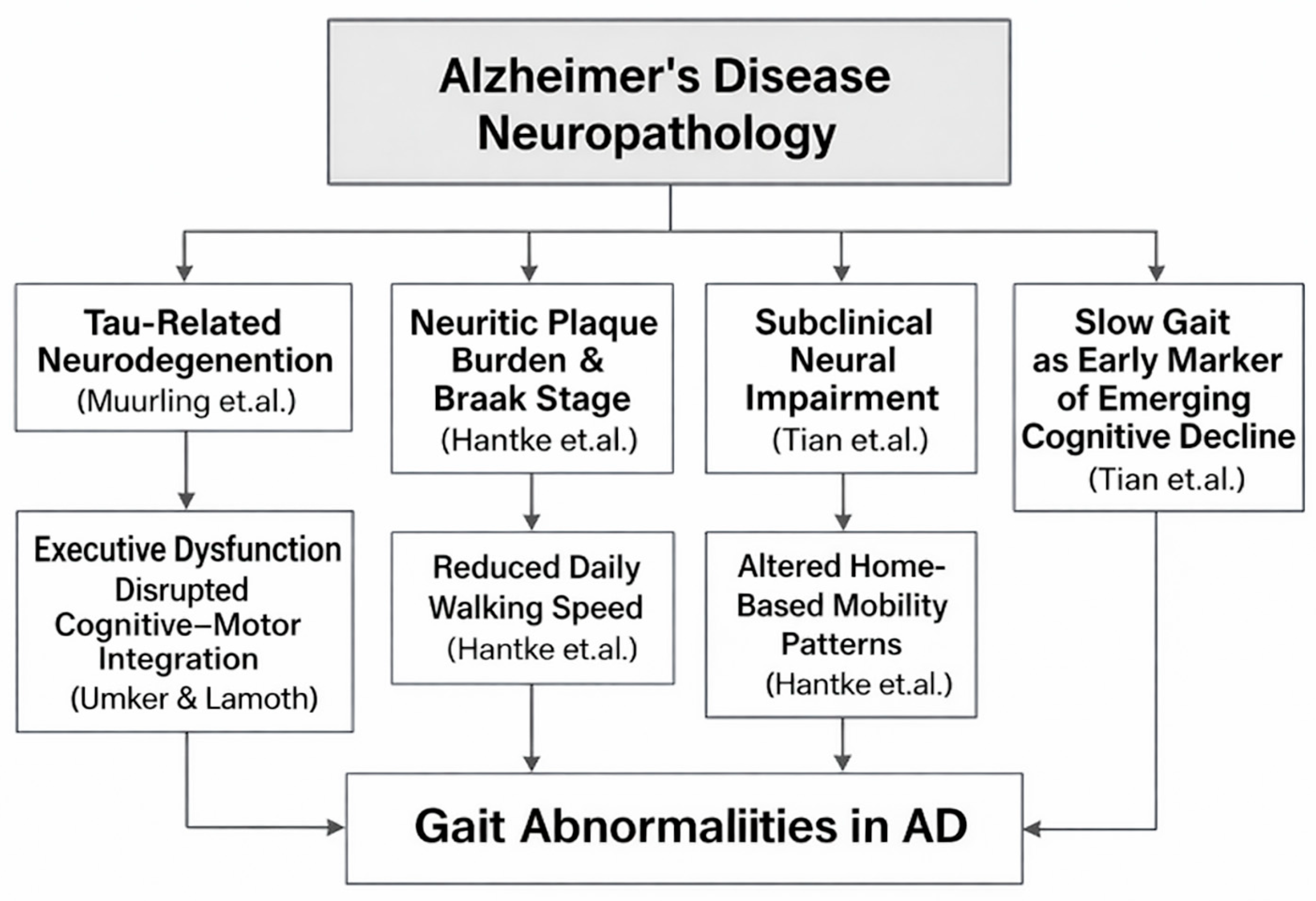
Growing evidence suggests that gait impairments in AD reflect underlying neurodegenerative processes rather than merely age-related motor slowing. Within the provided studies, several findings connect biomarkers of AD pathology, including tau-related neurodegeneration and neuritic plaque severity, to altered gait domains, such as pace, rhythm, or walking speed (Figure 7). These relationships support the hypothesis that gait serves as an externally measurable expression of central nervous system decline. Among the studies linking biological markers to gait, Muurling et al. [52] demonstrate that specific gait domains, particularly rhythm, correlate with cerebrospinal fluid (CSF) tau levels, especially under dual-task conditions. This relationship suggests a mechanistic pathway wherein tau-associated neuronal loss, especially in fronto-subcortical and motor-integration networks, disrupts the temporal regulation of gait. Because dual-task walking imposes cognitive-motor demands, the finding that rhythm disturbances magnify in this condition aligns with known impairments in executive control and attentional resource allocation in tau-driven AD pathology.
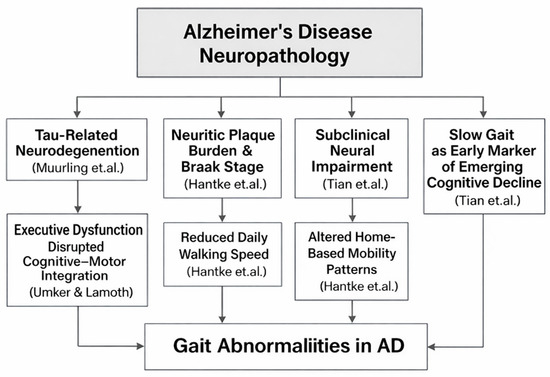
Figure 7.
Mechanistic Pathways Linking AD Neuropathology to Alterations in Gait Parameters. The figure summarizes findings from studies on tau-related neurodegeneration (Muurling et al. [52]), neuritic plaque burden and Braak stage (Hantke et al. [119]), subclinical neural impairment and slow gait as an early marker of cognitive decline (Tian et al. [45]), and executive dysfunction affecting cognitive–motor integration (IJmker & Lamoth [64]).
In contrast to tau effects, Elasfar et al. [27] report that regional amyloid-β (Aβ) deposition shows no significant association with gait outcomes. This supports the broader view that amyloid burden alone does not directly modulate motor coordination or locomotor timing. Instead, gait abnormalities appear to emerge more strongly from downstream neurodegenerative changes, including tau accumulation, synaptic dysfunction, and cortical/subcortical atrophy, rather than from amyloid deposition itself. Adding further support, Hantke et al. [119] demonstrate that postmortem neuropathological markers, including neuritic plaque (NP) severity and Braak stage, correlate with reduced walking speed and other digital mobility indicators captured through long-term in-home monitoring. These findings underscore a direct physiological coupling between progressive AD neuropathology and declines in everyday motor behavior, strengthening the mechanistic argument that gait slowing and disruption serve as measurable proxies of accumulating plaques and tangles.
The longitudinal study by Tian et al. [45] further supports the mechanistic link between neural degeneration and gait by showing that slower gait speed in older adults predicts later development of MCI/AD, particularly when slow gait is not accompanied by compensatory activity fragmentation. The interpretation offered is that persistent slow gait in the absence of behavioral adaptation may reflect subclinical neurological impairmen, likely including early tau-driven degeneration in cortical and subcortical regions that regulate motor function. Further mechanistic insight is provided by IJmker & Lamoth [64], who show that dementia patients exhibit irregular trunk acceleration patterns and instability, linked closely to deficits in executive function. Given that executive impairments in AD are strongly associated with frontal and parietal tau pathology, the study supports a causal chain: Tau-driven executive dysfunction → impaired motor planning and stability → increased gait irregularity.
4.6. Diagnostic Thresholds and Quantitative Gait Indicators for Early Cognitive Decline
To strengthen the quantitative dimension of gait-related findings, we additionally synthesized numerical differences in key gait parameters across the included studies. Older adults with MCI consistently demonstrated slower maximal walking speed, with reductions of approximately 0.25–0.35 m/s compared to healthy controls, and this decrease was strongly predictive of incident dementia in long-term follow-ups (32% reduced hazard per SD increase in speed). Gait variability measures showed robust discriminatory power: minimum toe clearance (MTC) variability was significantly higher in MCI (p = 0.016, d = 0.53), while increased step-time and swing-time variability differentiated dementia subtypes and reflected underlying motor-cognitive impairment. Dual-task paradigms further amplified these differences, with dual-task walking producing notable decreases in gait speed, stride length, mid-swing elevation, and increased double-limb support among individuals with cognitive impairment. Balance-related quantitative differences were also evident, with cognitively impaired older adults showing significantly higher sway velocity and path length (ηp2 = 0.144–0.190) during standing tasks (Table 3).

Table 3.
Quantitative Differences in Key Gait Parameters Between MCI and Healthy Controls Across Included Studies.
4.7. Emerging Trends
Recent research across the analyzed literature reveals a clear evolution toward continuous, data-driven, and personalized monitoring of aging, mobility, and cognitive decline. Advances in multimodal sensing, ranging from inertial [147] and radar-based systems to ear-worn and depth-camera devices, are enabling unobtrusive, real-world gait analysis that extends beyond traditional laboratory settings [78,85,89]. These innovations, supported by the growing use of artificial intelligence and deep learning frameworks, have demonstrated high precision in identifying disease-related gait abnormalities and early motor-cognitive changes associated with Alzheimer’s disease and mild cognitive impairment [148]. At the same time, novel analytical approaches such as explainable AI and quantile regression models enhance the interpretability and reliability of digital biomarkers derived from wearable sensors [86]. Collectively, these developments signify a paradigm shift from static, episodic clinical assessments to continuous digital phenotyping, where movement, behavior, and physiological signals act as dynamic indicators of health and disease progression.
Parallel to diagnostic advances, technology-supported interventions are increasingly emerging as tools for prevention and rehabilitation [149,150,151,152,153]. Integrated mobile and telehealth systems demonstrate the potential to reduce frailty progression and improve functional outcomes through personalized feedback loops [84,111]. Digital therapeutics such as wearable-assisted Tai Chi programs show measurable benefits in cognition and sleep quality among older adults [112], while passive sensing systems like Sense4Safety provide real-time fall prevention and behavioral monitoring for individuals with mild cognitive impairment [111]. Furthermore, smart-home and ambient sensing technologies allow continuous detection of behavioral and psychological symptoms of dementia, correlating digital biomarkers with neuropathological findings [113,117]. These approaches collectively reflect a broader transformation of geriatric and dementia care from reactive diagnosis to proactive, sensor-informed management. As emphasized by recent studies, ensuring user accessibility, privacy, and ethical design remains essential for the equitable deployment of such technologies [116,124,125].
4.8. Interdisciplinary Nature of the Field
The bibliometric and thematic analysis underscores the profoundly interdisciplinary character of research on gait analysis, wearable sensors, and early dementia detection. The field brings together diverse domains (clinical neuroscience, geriatrics, biomechanics, computer science, and data analytics) to create an integrated understanding of how motor and cognitive functions interact across the aging process [20,63,78,113]. This convergence reflects a shift from discipline-specific approaches to a system-oriented framework, where cognition, mobility, physiology, and environment are analyzed as interdependent components of brain health. Studies combining clinical gait metrics with advanced sensor technologies and artificial intelligence exemplify this integration, demonstrating how digital methods can bridge the gap between traditional neurology, behavioral science, and engineering [79,83,86].
The interdisciplinary nature of the field is also evident in its collaborative research networks. International co-authorship patterns reveal strong partnerships between clinicians, engineers, and data scientists across the United States, the United Kingdom, Germany, and the Netherlands, countries that serve as central hubs for innovation in digital aging research [Figure 2]. Such collaborations have enabled the development of shared methodologies, open-access datasets, and translational frameworks that accelerate progress from laboratory research to clinical application. Importantly, this cross-sector cooperation supports the creation of ecologically valid digital biomarkers capable of detecting early cognitive decline in real-world environments [84,111,112].
Ultimately, the interdisciplinarity of this field is not merely structural but conceptual. It redefines dementia research through the integration of clinical insight, technological precision, and behavioral context. The joint efforts of clinicians, computer scientists, and public health experts are paving the way for precision gerontechnology, an emerging paradigm in which artificial intelligence and sensor-based analytics inform early diagnosis, prevention, and personalized care. Sustaining this momentum will require continued cross-disciplinary collaboration, standardized data frameworks, and ethical design principles to ensure that innovation in digital health remains inclusive, transparent, and clinically meaningful [116,124,125].
4.9. Gaps in the Literature and Practical Implications for Clinical and Technological Research
Despite the growing body of evidence supporting the use of digital technologies in the early detection of cognitive decline, the literature still presents significant limitations that hinder both generalizability and real-world implementation. One major gap is the lack of longitudinal studies that evaluate the long-term validity and diagnostic precision of tools such as wearable devices and machine learning models in naturalistic settings. Most current studies rely on short-term data collection or controlled environments, which do not adequately reflect the complex and evolving nature of cognitive trajectories in aging populations. In addition, the literature reveals a conceptual and methodological fragmentation. While various studies successfully highlight individual digital biomarkers, such as gait variability, speech patterns, or sleep disturbances, there is a noticeable absence of integrative frameworks that combine these modalities into comprehensive diagnostic models. This fragmentation reduces the potential for multimodal analysis, which could enhance sensitivity to subtle and preclinical changes in cognitive function.
Another challenge lies in the limited diversity of study populations. Many datasets are drawn from homogeneous, high-income cohorts, thereby constraining the applicability of findings to broader, more diverse aging communities. Furthermore, ethical and privacy concerns related to the passive and continuous collection of behavioral data are often insufficiently addressed, despite the vulnerable status of the target population. These limitations carry important implications for both clinical practice and future technological development. Clinically, there is a growing need for tools that not only detect early signs of decline but also support individualized screening and intervention strategies. This presupposes close collaboration between clinicians, engineers, and data scientists to ensure that new technologies are both technically robust and aligned with user needs and clinical realities. Technologically, future tools must be designed with usability and accessibility in mind. Considering the sensory, motor, and cognitive challenges faced by older adults, systems must adopt human-centered design principles that facilitate long-term adherence and minimize user burden. Finally, broader access to open, multimodal datasets and standardized evaluation protocols would accelerate the development of clinically meaningful AI applications and foster replicable, interdisciplinary research.
4.10. Limitations of the Study
While this review offers a comprehensive exploration of the literature at the intersection of wearable technology, gait analysis, and early dementia detection, certain limitations must be acknowledged. First and foremost, the study was based solely on articles indexed in the Scopus database, which, although extensive, may not encompass relevant works from other platforms. This could have led to the exclusion of key contributions, particularly from technical or clinical subfields that use alternative publication channels. Another important constraint is the included research is based on short-term or cross-sectional designs conducted in controlled laboratory environments. These settings, while methodologically sound, do not always capture the complexity of real-world behavior or the long-term progression of cognitive decline in diverse, aging populations. The interpretability of results is also affected by variability in how gait features, sensor technologies, and cognitive assessments are measured across studies. This lack of standardization hampers direct comparison and synthesis. Moreover, although the analysis reveals rich thematic clustering, the reliance on automated bibliometric tools may oversimplify the nuanced overlap between different domains, particularly in such an interdisciplinary field. These limitations underscore the importance of ongoing interdisciplinary collaboration, methodological refinement, and inclusivity in future research efforts.
5. Conclusions
This bibliometric and thematic analysis provides a comprehensive overview of the evolving landscape of research on wearable sensor-based gait and behavioral analysis for the early detection of dementia and mild cognitive impairment. The findings highlight a dynamic and rapidly expanding field, characterized by increasing interdisciplinary collaboration across clinical neuroscience, biomedical engineering, data science, and gerontology. Importantly, the study underscores both the promise and complexity of using gait as a digital biomarker. On one hand, wearable sensors enable continuous, non-invasive monitoring of motor and behavioral indicators associated with cognitive decline. On the other, methodological heterogeneity and a lack of standardization pose challenges to comparability, reproducibility, and clinical adoption. As the global burden of dementia continues to rise, there is an urgent need for accessible, cost-effective, and ecologically valid tools to support early diagnosis and monitoring. This review advocates for future research that emphasizes longitudinal study designs, cross-cultural validation, ethical data governance, and the integration of sensor-based methods into routine care pathways. Strengthening collaboration between technologists, clinicians, and policy-makers will be essential to harness the full potential of wearable systems in addressing the pressing challenges of cognitive aging.
Supplementary Materials
The following supporting information can be downloaded at https://doi.org/10.5281/zenodo.17439813. Table S1: Overview of included studies.
Author Contributions
Conceptualization, A.T. and S.P.; methodology, A.T., S.P. and G.G.; software, S.P. and G.G.; validation, G.T., F.C. and G.K.; formal analysis, A.T., S.P. and D.T.; investigation, V.G. and G.M.; resources, G.T., V.G. and G.M.; data curation, P.V.; writing—original draft preparation, A.T., F.C. and G.K.; writing—review and editing, A.T. and G.T.; visualization, S.P. and G.G.; supervision, N.A. and K.V.; project administration, A.T. and P.V.; funding acquisition, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGpt 4.0. for English editing. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Health, T.L.P. Reinvigorating the public health response to dementia. Lancet Public Health 2021, 6, e696. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Comas-Herrera, A.; Knapp, M.; Guerchet, M.; Karagiannidou, M. World Alzheimer Report 2016. Improving Healthcare for People Living with Dementia: Coverage, Quality and Costs Now and in the Future; Alzheimer’s Disease International: London, UK, 2016. [Google Scholar]
- Aliberti, M.J.R.; Avelino-Silva, T.J.; Suemoto, C.K. Maximizing early dementia detection through medicare annual wellness visits. JAMA Netw. Open 2024, 7, e2437162. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s disease—Why we need early diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Bakirtzis, C.; Plakias, S.; Vlotinou, P.; Vadikolias, K.; Terzoudi, A.; Christidi, F. Predictive models for the transition from mild neurocognitive disorder to major neurocognitive disorder: Insights from clinical, demographic, and neuropsychological data. Biomedicines 2024, 12, 1232. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Kokkotis, C.; Vlotinou, P.; Kyriazidou, S.; Giarmatzis, G.; Kallivoulos, S.; Terzoudi, A.; Tsiptsios, D.; Merai, S. Instrumental Activities of Daily Living in Neurocognitive Disorders: Determinants and Clinical Implications for Health Promotion. Brain Sci. 2025, 15, 417. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Vadikolias, K.; Tripsianis, G.; Vlotinou, P.; Serdari, A.; Terzoudi, A.; Heliopoulos, I. Influence of social and demographic factors on the Montreal Cognitive Assessment (MoCA) Test in rural population of north-eastern Greece. Geriatrics 2021, 6, 43. [Google Scholar] [CrossRef]
- Vinze, S.; Chodosh, J.; Lee, M.; Wright, J.; Borson, S. The national public health response to Alzheimer’s disease and related dementias: Origins, evolution, and recommendations to improve early detection. Alzheimer’s Dement. 2023, 19, 4276–4286. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Karakitsiou, G.; Nikova, A.; Christidi, F.; Kokkotis, C.; Giarmatzis, G.; Tsakni, G.; Katsouri, I.-G.; Dimitrios, S. Mapping the Landscape of Biomechanics Research in Stroke Neurorehabilitation: A Bibliometric Perspective. Biomechanics 2024, 4, 664–684. [Google Scholar] [CrossRef]
- Seifallahi, M.; Galvin, J.E.; Ghoraani, B. Detection of mild cognitive impairment using various types of gait tests and machine learning. Front. Neurol. 2024, 15, 1354092. [Google Scholar] [CrossRef] [PubMed]
- Tsiakiri, A.; Plakias, S.; Giarmatzis, G.; Tsakni, G.; Christidi, F.; Papadopoulou, M.; Bakalidou, D.; Vadikolias, K.; Aggelousis, N.; Vlotinou, P. Gait Analysis in Multiple Sclerosis: A Scoping Review of Advanced Technologies for Adaptive Rehabilitation and Health Promotion. Biomechanics 2025, 5, 65. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Y.; Tao, S.; Huang, S.; Zhang, C.; Lv, Z. Wearable sensor-based daily life walking assessment of gait for distinguishing individuals with amnestic mild cognitive impairment. Front. Aging Neurosci. 2019, 11, 285. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, S.; Zou, M.; Chen, Y.; Shen, P.; He, Y.; Li, Y.; Liu, C.; Chen, Z. Gait analysis in older adults with mild cognitive impairment: A bibliometric analysis of global trends, hotspots, and emerging frontiers. Front. Aging 2025, 6, 1592464. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Moral-Muñoz, J.A.; Herrera-Viedma, E.; Santisteban-Espejo, A.; Cobo, M.J. Software tools for conducting bibliometric analysis in science: An up-to-date review. Prof. Inf. 2020, 29. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Ellegaard, O. The application of bibliometric analysis: Disciplinary and user aspects. Scientometrics 2018, 116, 181–202. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact: Methods and Practice; Springer: Cham, Switzerland, 2014; pp. 285–320. [Google Scholar]
- Xiong, C.; Lu, R.; Bui, Q.; Popp, B.; Schindler, S.E.; Shriver, L.P.; Cruchaga, C.; Hassenstab, J.; Benzinger, T.L.S.; Agboola, F.; et al. Person-specific digital measurements of air pollutant exposure and biomarkers of Alzheimer’s disease: Findings from a pilot study. J. Alzheimer’s Dis. 2025, 107, 778–788. [Google Scholar] [CrossRef]
- Schiavo, A.; Tiecker, A.P.; Dos Santos Oliveira, M.; Ross, N.R.; de Oliveira Brauner, F.; Balbinot, G.; Gemerasca Mestriner, R.G. Age-stratified validation of the performance index (P-Index) as a metric for dual-task cost in functional mobility across the adult lifespan. Gait Posture 2025, 121, 273–280. [Google Scholar] [CrossRef]
- Labott, B.K.; Herold, F.; Langhans, C.; Halfpaap, N.; Grässler, B.; Hoekelmann, A.; Müller, N.G.; Hamacher, D. Minimum toe clearance variability in older adults with mild cognitive impairment: Differences to healthy controls and effects of a dance intervention. Gait Posture 2025, 121, 101–107. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Espinoza-Cerda, M.T.; Brodie, M.A.; Lord, S.R.; Taylor, M.E. Daily-life walking speed, running duration and bedtime from wrist-worn sensors predict incident dementia: A watch walk—UK biobank study. Int. Psychogeriatr. 2025, 37, 100031. [Google Scholar] [CrossRef] [PubMed]
- Slusarenko, A.; Rosenberg, M.C.; Kazanski, M.E.; McKay, J.L.; Emmery, L.; Kesar, T.M.; Hackney, M.E. Associations between music and dance relationships, rhythmic proficiency, and spatiotemporal movement modulation ability in adults with and without mild cognitive impairment. J. Alzheimer’s Dis. 2025, 105, 1165–1182. [Google Scholar] [CrossRef] [PubMed]
- Hammink, J.H.W.; van Buuren, L.P.G.; Moor, J.A.; Derks, D.A.J.A.; Mohammadi, M. Evolving dementia care: An explorative study on the lived experience of older adults living with dementia in nursing homes using observational and biometric sensor data. Dementia 2025, 24, 456–479. [Google Scholar] [CrossRef] [PubMed]
- Elasfar, S.; Hameed, H.; Boeve, B.F.; Fields, J.A.; Jack, C.R.; Kantarci, K.; St Louis, E.K.; Lowe, V.J.; Petersen, R.C.; Ali, F.; et al. Identifying gait differences between Alzheimer’s disease and dementia with Lewy bodies and their associations with regional amyloid deposition. Alzheimer’s Dement. 2025, 21, e14351. [Google Scholar] [CrossRef]
- Resnick, B.; Boltz, M.; Galik, E.; Kuzmik, A.; McPherson, R.; Drazich, B.; Kim, N.; Zhu, S.; Wells, C.L. Measurement of Physical Activity Among Hospitalized Older Adults Living With Dementia. Rehabil. Nurs. 2024, 49, 115–124. [Google Scholar] [CrossRef]
- Schmidt, L.; Zieschang, T.; Koschate, J.; Stuckenschneider, T. Impaired Standing Balance in Older Adults with Cognitive Impairment after a Severe Fall. Gerontology 2024, 70, 755–763. [Google Scholar] [CrossRef]
- Jehu, D.A.; Langston, R.; Sams, R.; Young, L.; Hamrick, M.; Zhu, H.; Dong, Y. The Impact of Dual-Tasks and Disease Severity on Posture, Gait, and Functional Mobility among People Living with Dementia in Residential Care Facilities: A Pilot Study. Sensors 2024, 24, 2691. [Google Scholar] [CrossRef]
- Greene, B.; Tobyne, S.; Jannati, A.; McManus, K.; Osman, J.G.; Banks, R.; Kher, R.; Showalter, J.; Bates, D.; Pascual- Leone, A. The Dual Task Ball Balancing Test and Its Association with Cognitive Function: Algorithm Development and Validation. J. Med. Internet Res. 2024, 26, e49794. [Google Scholar] [CrossRef]
- Chiba, Y.; Kumamoto, A.; Noguchi, N.; Yoshimi, A.; Suda, A.; Hishimoto, A.; Kase, A. Wheelchair dependence in patients with dementia: Focus on kinematic gait analysis using simple wearable accelerometers and gyroscopes. Assist. Technol. 2024, 36, 398–404. [Google Scholar] [CrossRef]
- Aznielle-Rodríguez, T.; Galán-García, L.; Ontivero-Ortega, M.; Aguilar-Mateu, K.; Castro-Laguardia, A.M.; Fernández-Nin, A.; Garcia-Agustin, D.; Valdés-Sosa, M. Relationship between gait parameters and cognitive indexes in adult aging. PLoS ONE 2023, 18, e0291963. [Google Scholar] [CrossRef] [PubMed]
- Kostic, E.; Kwak, K.; Kim, D. Assessing the Global Cognition of Community-Dwelling Older Adults Using Motor and Sensory Factors: A Cross-Sectional Feasibility Study. Sensors 2023, 23, 7384. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, K.; Javed, B.; Toosizadeh, N. Association between dual-task function and neuropsychological testing in older adults with cognitive impairment. Exp. Gerontol. 2023, 178, 112223. [Google Scholar] [CrossRef] [PubMed]
- Trumpf, R.; Häussermann, P.; Zijlstra, W.; Fleiner, T. Circadian aspects of mobility-related behavior in patients with dementia: An exploratory analysis in acute geriatric psychiatry. Int. J. Geriatr. Psychiatry 2023, 38, e5957. [Google Scholar] [CrossRef]
- Wang, C.; Zahiri, M.; Vaziri, A.; Najafi, B. Dual-Task Upper Extremity Motor Performance Measured by Video Processing as Cognitive-Motor Markers for Older Adults. Gerontology 2023, 69, 650–656. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ha, S.W.; Jeong, D.E.; Lee, J.; Kim, D.; Min, J.Y.; Min, K.B. Association Between the Loss of Gait Harmony and Cognitive Impairment: Cross-Sectional Study. JMIR Public Health Surveill. 2023, 9, e46264. [Google Scholar] [CrossRef]
- Poole, V.N.; Dawe, R.J.; Lamar, M.; Esterman, M.; Barnes, L.; Leurgans, S.E.; Bennett, D.A.; Hausdorff, J.M.; Buchman, A.S. Dividing attention during the Timed Up and Go enhances associations of several subtask performances with MCI and cognition. PLoS ONE 2022, 17, e0269398. [Google Scholar] [CrossRef]
- Ma, R.; Zhào, H.; Wei, W.; Liu, Y.; Huang, Y. Gait characteristics under single-/dual-task walking conditions in elderly patients with cerebral small vessel disease: Analysis of gait variability, gait asymmetry and bilateral coordination of gait. Gait Posture 2022, 92, 65–70. [Google Scholar] [CrossRef]
- Zhou, H.; Park, C.; Shahbazi, M.; York, M.K.; Kunik, M.E.; Naik, A.D.; Najafi, B. Digital Biomarkers of Cognitive Frailty: The Value of Detailed Gait Assessment beyond Gait Speed. Gerontology 2022, 68, 224–233. [Google Scholar] [CrossRef]
- Taraldsen, K.; Helbostad, J.L.; Follestad, T.; Bergh, S.; Selbæk, G.; Saltvedt, I. Gait, physical function, and physical activity in three groups of home-dwelling older adults with different severity of cognitive impairment—A cross-sectional study. BMC Geriatr. 2021, 21, 670. [Google Scholar] [CrossRef]
- Wouters, H.; van Campen, J.P.; Kuitert, M.J.; Kikkert, L.; Hilmer, S.N.; Taxis, K.; Van Der Meer, H.G.; Lamoth, C.J.C. Anticholinergic and Sedative Medications and Dynamic Gait Parameters in Older Patients. Drugs Aging 2021, 38, 1087–1096. [Google Scholar] [CrossRef]
- Hao, W.; Zhao, W.; Kimura, T.; Ukawa, S.; Kadoya, K.; Kondo, K.; Tamakoshi, A. Association of gait with global cognitive function and cognitive domains detected by MoCA-J among community-dwelling older adults: A cross-sectional study. BMC Geriatr. 2021, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Studenski, S.A.; An, Y.; Kuo, P.L.; Schrack, J.A.; Wanigatunga, A.A.; Simonsick, E.M.; Resnick, S.M.; Ferrucci, L. Association of Combined Slow Gait and Low Activity Fragmentation with Later Onset of Cognitive Impairment. JAMA Netw. Open 2021, 4, e2135168. [Google Scholar] [CrossRef] [PubMed]
- Mulas, I.; Putzu, V.; Asoni, G.; Viale, D.; Mameli, I.; Pau, M. Clinical assessment of gait and functional mobility in Italian healthy and cognitively impaired older persons using wearable inertial sensors. Aging Clin. Exp. Res. 2021, 33, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Del Din, S.; Donaghy, P.; Galna, B.; Thomas, A.J.; Rochester, L. The impact of environment on gait assessment: Considerations from real-world gait analysis in dementia subtypes. Sensors 2021, 21, 813. [Google Scholar] [CrossRef]
- Gabriel, A.; Lehner, C.T.; Höhler, C.; Schneider, T.; Pfeiffer, T.P.T.; Diehl-Schmid, J.; Hermsdörfer, J. Anticipatory and reactive grip force control in patients with alzheimer’s disease: A pilot study. J. Alzheimer’s Dis. 2021, 82, 1651–1665. [Google Scholar] [CrossRef]
- Trumpf, R.; Morat, T.; Zijlstra, W.; Häussermann, P.; Fleiner, T. Assessment of Functional Performance in Acute Geriatric Psychiatry—Time for New Strategies? J. Geriatr. Psychiatry Neurol. 2020, 33, 316–323. [Google Scholar] [CrossRef]
- Rantalainen, T.; Teo, W.P.; Ridgers, N.D.; Nuzum, N.D.; Valente, L.; MacPherson, H. Laboratory-Based Gait Variability and Habitual Gait Entropy Do Not Differentiate Community-Dwelling Older Adults from Those with Subjective Memory Complaints. Gait Posture 2020, 80, 20–25. [Google Scholar] [CrossRef]
- Pau, M.; Mulas, I.; Putzu, V.; Asoni, G.; Viale, D.; Mameli, I.; Leban, B.; Allali, G. Smoothness of gait in healthy and cognitively impaired individuals: A study on Italian elderly using wearable inertial sensor. Sensors 2020, 20, 3577. [Google Scholar] [CrossRef]
- Muurling, M.; Rhodius- Meester, H.F.M.; Pärkkä, J.; van Gils, M.; Frederiksen, K.S.; Bruun, M.; Hasselbalch, S.G.; Soininen, H.; Herukka, S.K.; Hallikainen, M.; et al. Gait Disturbances are Associated with Increased Cognitive Impairment and Cerebrospinal Fluid Tau Levels in a Memory Clinic Cohort. J. Alzheimer’s Dis. 2020, 76, 1061–1070. [Google Scholar] [CrossRef]
- Koohsari, M.J.; Nakaya, T.; McCormack, G.R.; Shibata, A.; Ishii, K.; Yasunaga, A.; Koichiro, K. Cognitive Function of Elderly Persons in Japanese Neighborhoods: The Role of Street Layout. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Brodie, M.A.; Van Schooten, K.S.; Delbaere, K.; Close, J.C.T.; Payne, N.; Webster, L.; Chow, J.; McInerney, G.; Kurrle, S.E.; et al. Older People with Dementia Have Reduced Daily-Life Activity and Impaired Daily-Life Gait When Compared to Age-Sex Matched Controls. J. Alzheimer’s Dis. 2019, 71, S125–S135. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, B.; Hobbelen, J.S.M.; Cagnie, B.; van Eetvelde, B.; van den Noortgate, N.; Cambier, D. Reproducible Measurements of Muscle Characteristics Using the MyotonPRO Device: Comparison between Individuals With and Without Paratonia. J. Geriatr. Phys. Ther. 2018, 41, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, L.; Vuillerme, N.; van Campen, J.P.; Appels, B.A.; Hortobágyi, T.; Lamoth, C.J.C. The relationship between gait dynamics and future cognitive decline: A prospective pilot study in geriatric patients. Int. Psychogeriatr. 2018, 30, 1301–1309. [Google Scholar] [CrossRef]
- Byun, S.; Han, J.W.; Kim, T.H.; Kim, K.; Kim, T.H.; Park, J.Y.; Suh, S.W.; Seo, J.Y.; So, Y.; Lee, K.H.; et al. Gait Variability Can Predict the Risk of Cognitive Decline in Cognitively Normal Older People. Dement. Geriatr. Cogn. Disord. 2018, 45, 251–261. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Hillel, I.; Shustak, S.; Del Din, S.; Bekkers, E.M.J.; Pelosin, E.; Nieuwhof, F.; Rochester, L.; Mirelman, A. Everyday stepping quantity and quality among older adult fallers with and without mild cognitive impairment: Initial evidence for new motor markers of cognitive defcits? J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 1078–1082. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, H.; Lee, J.; Schwenk, M.; Najafi, B. Motor planning error: Toward measuring cognitive frailty in older adults using wearables. Sensors 2018, 18, 926. [Google Scholar] [CrossRef]
- König, A.; Klaming, L.; Pijl, M.; Demeurraux, A.; David, R.; Robert, P. Objective measurement of gait parameters in healthy and cognitively impaired elderly using the dual-task paradigm. Aging Clin. Exp. Res. 2017, 29, 1181–1189. [Google Scholar] [CrossRef]
- Zhou, H.; Sabbagh, M.; Wyman, R.; Liebsack, C.; Kunik, M.E.; Najafi, B. Instrumented Trail-Making Task to Differentiate Persons with No Cognitive Impairment, Amnestic Mild Cognitive Impairment, and Alzheimer Disease: A Proof of Concept Study. Gerontology 2017, 63, 189–200. [Google Scholar] [CrossRef]
- Werner, C.; Wiloth, S.; Lemke, N.C.; Kronbach, F.; Jansen, C.P.; Oster, P.; Bauer, J.M.; Hauer, K. People with dementia can learn compensatory movement maneuvers for the sit-to-stand task: A randomized controlled trial. J. Alzheimer’s Dis. 2017, 60, 107–120. [Google Scholar] [CrossRef]
- Gillain, S.; Dramé, M.; Lekeu, F.; Wojtasik, V.; Ricour, C.; Croisier, J.L.; Salmon, E.; Pétermans, J. Gait speed or gait variability, which one to use as a marker of risk to develop Alzheimer disease? A pilot study. Aging Clin. Exp. Res. 2016, 28, 249–255. [Google Scholar] [CrossRef]
- Ijmker, T.; Lamoth, C.J.C. Gait and cognition: The relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture 2012, 35, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Liu, Z.; Hu, Z.; Yang, T.; Gong, C. Research progress and visual analysis of smart wearable devices for the elderly in the context of ageing 4.0. Technol. Health Care 2025, 33, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Ustad, A.; Edwin, T.H.; Melsæter, K.N.; Sverdrup, K.; Tangen, G.G.; Døhl, Ø.; Thingstad, P.; Vereijken, B.; Maroni, N. Daily physical activity and trajectories of care service use among older adults: The HUNT4 Trondheim 70+ study. Front. Public Health 2025, 13, 1539179. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Brown, S.; Paquette, D.R.; Orwig, T.A.; Spartano, N.L.; Lin, H. Passive Measures of Physical Activity and Cadence as Early Indicators of Cognitive Impairment: Observational Study. J. Med. Internet Res. 2025, 27, e72946. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, J.; Ji, M.; Li, X. Wearable Technologies for Health Promotion and Disease Prevention in Older Adults: Systematic Scoping Review and Evidence Map. J. Med. Internet Res. 2025, 27, e69077. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.; Hu, Y.; Zhang, J.; Wang, X.; Wang, F.; Wu, M.; Lin, T. Predicting physical functioning status in older adults: Insights from wrist accelerometer sensors and derived digital biomarkers of physical activity. J. Am. Med. Inform. Assoc. 2024, 31, 2571–2582. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Blackwell, T.L.; Cawthon, P.M.; Coen, P.M.; Cummings, S.R.; Distefano, G.; Farsijani, S.; Forman, D.E.; Goodpaster, B.H.; Kritchevsky, S.B.; et al. Associations of accelerometry-measured and self-reported physical activity and sedentary behavior with skeletal muscle energetics: The Study of Muscle, Mobility and Aging (SOMMA). J. Sport Health Sci. 2024, 13, 621–630. [Google Scholar] [CrossRef]
- Muurling, M.; Badissi, M.; de Boer, C.; Legdeur, N.; Barkhof, F.; van Berckel, B.N.M.; Maier, A.B.; Pijnappels, M.; Visser, P.J. Physical activity levels in cognitively normal and cognitively impaired oldest-old and the association with dementia risk factors: A pilot study. BMC Geriatr. 2023, 23, 129. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Brodie, M.A.; Lord, S.R. Prediction of Incident Depression in Middle-Aged and Older Adults using Digital Gait Biomarkers Extracted from Large-Scale Wrist Sensor Data. J. Am. Med. Dir. Assoc. 2023, 24, 1106–1113.e11. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Arbona, C.H.; Brodie, M.A.; Lord, S.R. Prediction of injurious falls in older adults using digital gait biomarkers extracted from large-scale wrist sensor data. Age Ageing 2023, 52, afad179. [Google Scholar] [CrossRef] [PubMed]
- Volders, E.; de Groot, R.H.M.; Bolman, C.A.W.; Lechner, L. The longitudinal associations between change in physical activity and cognitive functioning in older adults with chronic illness (es). BMC Geriatr. 2021, 21, 478. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Dawe, R.J.; Leurgans, S.E.; Curran, T.A.; Truty, T.; Yu, L.; Barnes, L.; Hausdorff, J.M.; Bennett, D.A. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Parry, S.; Chow, M.; Batchelor, F.; Fary, R.E. Physical activity and sedentary behaviour in a residential aged care facility. Australas. J. Ageing 2019, 38, E12–E18. [Google Scholar] [CrossRef]
- Lu, Z.; Harris, T.B.; Shiroma, E.J.; Leung, J.; Kwok, T. Patterns of Physical Activity and Sedentary Behavior for Older Adults with Alzheimer’s Disease, Mild Cognitive Impairment, and Cognitively Normal in Hong Kong. J. Alzheimer’s Dis. 2018, 66, 1453–1462. [Google Scholar] [CrossRef]
- Moyle, W.; Jones, C.; Murfield, J.; Draper, B.; Beattie, E.; Shum, D.; Thalib, L.; O’Dwyer, S.; Mervin, C.M. Levels of physical activity and sleep patterns among older people with dementia living in long-term care facilities: A 24-h snapshot. Maturitas 2017, 102, 62–68. [Google Scholar] [CrossRef]
- Schwenk, M.; Hauer, K.; Zieschang, T.; Englert, S.; Mohler, J.; Najafi, B. Sensor-derived physical activity parameters can predict future falls in people with dementia. Gerontology 2014, 60, 483–492. [Google Scholar] [CrossRef]
- Yang, D.Z.; Rodrigues, E.E.; Hernandez, H.H.C.; Ong, E.H.; Heyzer, L.; Tan, C.N.; Kua, J.; Ismail, N.H.; Lim, W.S. Validation of the revised multi-sensor-based electronic Short Physical Performance Battery (eSPPB) kiosk in community-dwelling older adults. Eur. Geriatr. Med. 2025, 16, 975–983. [Google Scholar] [CrossRef]
- McCullough, A.K. Use of posterior probabilities from a long short-term memory network for characterizing dance behavior with multiple accelerometers. J. Alzheimer’s Dis. 2025, 105, 1069–1084. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Ryan, L.J.; Ur Rehman, R.Z.U.; Dignan, E.; Thompson, A.; Del Din, S.; Galna, B.; Thomas, A.J.; Rochester, L.; Alcock, L. Validation of an algorithm for detecting turning in people with cognitive impairment, considering dementia disease subtype. Gait Posture 2025, 118, 141–147. [Google Scholar] [CrossRef]
- Beichert, L.; Seemann, J.; Kessler, C.; Traschütz, A.; Müller, D.; Dillmann-Jehn, K.; Ricca, I.; Satolli, S.; Basak, N.A.; Coarelli, G.; et al. Patient-Relevant Digital-Motor Outcomes for Clinical Trials in Hereditary Spastic Paraplegia Type 7: A Multicenter PROSPAX Study. Neurology 2024, 103, e209887. [Google Scholar] [CrossRef] [PubMed]
- Cornish, B.F.; van Ooteghem, K.; Wong, M.; Weber, K.S.; Pieruccini-Faria, F.; Montero-Odasso, M.; McIlroy, W.E. Evaluation of a finite state machine algorithm to measure stepping with ankle accelerometry: Performance across a range of gait speeds, tasks, and individual walking ability. Med. Eng. Phys. 2024, 133, 104251. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Wu, T.; Lure, F.Y.M. E-BDL: Enhanced Band-Dependent Learning Framework for Augmented Radar Sensing. Sensors 2024, 24, 4620. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Aragonés, M.; Pérez-Rodríguez, R.; Carnicero Martínez, J.A.; Moreno-Sánchez, P.A.; Oviedo-Briones, M.; Villalba-Mora, E.; Abizanda-Soler, P.; Rodriguez-Mañas, L. Effects of Monitoring Frailty Through a Mobile/Web-Based Application and a Sensor Kit to Prevent Functional Decline in Frail and Prefrail Older Adults: FACET (Frailty Care and Well Function) Pilot Randomized Controlled Trial. J. Med. Internet Res. 2024, 26, e58312. [Google Scholar] [CrossRef]
- Seifer, A.K.; Kuderle, A.; Dorschky, E.; Moradi, H.; Hannemann, R.; Eskofier, B.M. Step Length and Gait Speed Estimation Using a Hearing Aid Integrated Accelerometer: A Comparison of Different Algorithms. IEEE J. Biomed. Health Inform. 2024, 28, 6619–6628. [Google Scholar] [CrossRef]
- Ghosal, R.; Varma, V.R.; Volfson, D.; Hillel, I.; Urbanek, J.; Hausdorff, J.M.; Watts, A.; Zipunnikov, V. Distributional data analysis via quantile functions and its application to modeling digital biomarkers of gait in Alzheimer’s Disease. Biostatistics 2023, 24, 539–561. [Google Scholar] [CrossRef]
- Haghi, M.; Ershadi, A.; Deserno, T.M. Recognizing Human Activity of Daily Living Using a Flexible Wearable for 3D Spine Pose Tracking. Sensors 2023, 23, 2066. [Google Scholar] [CrossRef]
- Peimankar, A.; Winther, T.S.; Ebrahimi, A.; Wiil, U.K. A Machine Learning Approach for Walking Classification in Elderly People with Gait Disorders. Sensors 2023, 23, 679. [Google Scholar] [CrossRef]
- Guess, T.M.; Bliss, R.; Hall, J.B.; Kiselica, A. Comparison of Azure Kinect overground gait spatiotemporal parameters to marker based optical motion capture. Gait Posture 2022, 96, 130–136. [Google Scholar] [CrossRef]
- Cicirelli, G.; Impedovo, D.; Dentamaro, V.; Marani, R.; Pirlo, G.; D’Orazio, T.R. Human Gait Analysis in Neurodegenerative Diseases: A Review. IEEE J. Biomed. Health Inform. 2022, 26, 229–242. [Google Scholar] [CrossRef]
- Guimarães, V.; Sousa, I.; Correia, M.V. A deep learning approach for foot trajectory estimation in gait analysis using inertial sensors. Sensors 2021, 21, 7517. [Google Scholar] [CrossRef]
- Sprint, G.; Cook, D.J.; Fritz, R. Behavioral differences between subject groups identified using smart homes and change point detection. IEEE J. Biomed. Health Inform. 2021, 25, 559–567. [Google Scholar] [CrossRef]
- Bringas, S.; Salomón, S.; Duque, R.; Lage-Martínez, C.; Montaña Arnáiz, J.L. Alzheimer’s Disease stage identification using deep learning models. J. Biomed. Inform. 2020, 109, 103514. [Google Scholar] [CrossRef]
- Sabo, A.; Mehdizadeh, S.; Ng, K.D.; Iaboni, A.; Taati, B. Assessment of Parkinsonian gait in older adults with dementia via human pose tracking in video data. J. Neuroeng. Rehabil. 2020, 17, 97. [Google Scholar] [CrossRef]
- Razjouyan, J.; Najafi, B.; Horstman, M.; Sharafkhaneh, A.; Amirmazaheri, M.; Zhou, H.; Kunik, M.E.; Naik, A.D. Toward using wearables to remotely monitor cognitive frailty in community-living older adults: An observational study. Sensors 2020, 20, 2218. [Google Scholar] [CrossRef]
- Schaat, S.; Koldrack, P.; Yordanova, K.; Kirste, T.; Teipel, S. Real-Time Detection of Spatial Disorientation in Persons with Mild Cognitive Impairment and Dementia. Gerontology 2020, 66, 85–94. [Google Scholar] [CrossRef]
- Dolatabadi, E.; Zhi, Y.X.; Flint, A.J.; Mansfield, A.; Iaboni, A.; Taati, B. The feasibility of a vision-based sensor for longitudinal monitoring of mobility in older adults with dementia. Arch. Gerontol. Geriatr. 2019, 82, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Fleiner, T.; Giannouli, E.; Jaekel, U.; Mellone, S.; Häussermann, P.; Zijlstra, W. Statistical learning of mobility patterns from long-term monitoring of locomotor behaviour with body-worn sensors. Sci. Rep. 2018, 8, 7079. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, M.; Cereatti, A.; Trojaniello, D.; Avanzino, L.; Pelosin, E.; Del Din, S.; Rochester, L.; Ginis, P.; Bekkers, E.M.J.; Mirelman, A.; et al. Estimation of spatio-temporal parameters of gait from magneto-inertial measurement units: Multicenter validation among Parkinson, mildly cognitively impaired and healthy older adults. Biomed. Eng. Online 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Morris, R.; Hickey, A.; Del Din, S.; Koychev, I.; Gunn, R.N.; Lawson, J.; Zamboni, G.; Ridha, B.; Sahakian, B.J.; et al. Gait in Mild Alzheimer’s Disease: Feasibility of Multi-Center Measurement in the Clinic and Home with Body-Worn Sensors: A Pilot Study. J. Alzheimer’s Dis. 2018, 63, 331–341. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Yorozu, A.; Adachi, D.; Takahashi, M.; Aoyama, T. Association between mild cognitive impairment and trajectory-based spatial parameters during timed up and go test using a laser range sensor. J. Neuroeng. Rehabil. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, T.; Häussermann, P.; Mellone, S.; Zijlstra, W. Sensor-based assessment of mobility-related behavior in dementia: Feasibility and relevance in a hospital context. Int. Psychogeriatr. 2016, 28, 1687–1694. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, A.; Martinikorena, I.; Lecumberri, P.; Gómez, M.; Millor, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Izquierdo, M. Dual Task Gait Performance in Frail Individuals with and without Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2016, 42, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Mitoma, H.; Sanjo, N.; Higuma, M.; Terashi, H.; Yokota, T. Ambulatory Gait Behavior in Patients with Dementia: A Comparison with Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 817–826. [Google Scholar] [CrossRef]
- González, I.; Lopez-Nava, I.H.; Fontecha, J.; Muñoz-Meléndez, A.; Pérez-SanPablo, A.I.; Quiñones-Urióstegui, I. Comparison between passive vision-based system and a wearable inertial-based system for estimating temporal gait parameters related to the GAITRite electronic walkway. J. Biomed. Inform. 2016, 62, 210–223. [Google Scholar] [CrossRef]
- Raknim, P.; Lan, K.C. Gait Monitoring for Early Neurological Disorder Detection Using Sensors in a Smartphone: Validation and a Case Study of Parkinsonism. Telemed. E-Health 2016, 22, 75–81. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chung, P.C.; Wang, W.H.; Pai, M.C.; Wang, C.Y.; Lin, C.W.; Wu, H.L.; Wang, J.S. Gait and balance analysis for patients with Alzheimer’s disease using an inertial-sensor-based wearable instrument. IEEE J. Biomed. Health Inform. 2014, 18, 1822–1830. [Google Scholar] [CrossRef]
- Gietzelt, M.; Feldwieser, F.; Gövercin, M.; Steinhagen-Thiessen, E.; Marschollek, M. A prospective field study for sensor-based identification of fall risk in older people with dementia. Inform. Health Soc. Care 2014, 39, 249–261. [Google Scholar] [CrossRef]
- Gillain, S.; Pétermans, J. Contribution of new techniques to study the gait in old populations. Ann. Phys. Rehabil. Med. 2013, 56, 384–395. [Google Scholar] [CrossRef][Green Version]
- Gramkow, M.H.; Brink-Kjaer, A.; Clemmensen, F.K.; Sjaelland, N.S.; Waldemar, G.; Jennum, P.; Hasselbalch, S.G.; Frederiksen, K.S. Diagnostic performance of actigraphy in Alzheimer’s disease using a machine learning classifier—A cross-sectional memory clinic study. Alzheimer’s Res. Ther. 2025, 17, 111. [Google Scholar] [CrossRef]
- Demiris, G.; Harrison, S.; Sefcik, J.; Skubic, M.; Richmond, T.S.; Hodgson, N.A. Feasibility and Acceptability of a Technology-Mediated Fall Risk Prevention Intervention for Older Adults with Mild Cognitive Impairment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2025, 80, glaf043. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.S.; Liu, H.Z.; Fang, J.; Wang, S.J.; Han, Y.X.; Meng, J.; Han, Y.; Zou, H.M.; Gu, Q.; Hu, X.; et al. Effects of Tai Chi on Cognitive Function in Older Adults with Type 2 Diabetes Mellitus: Randomized Controlled Trial Using Wearable Devices in a Mobile Health Model. J. Med. Internet Res. 2025, 27, e77014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yang, T.; Xie, Q.; Lledos, G.; Chou, W.H.; Yu, W. Modeling and mobile home monitoring of behavioral and psychological symptoms of dementia (BPSD). BMC Psychiatry 2024, 24, 197. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.B.; Akter, S.; Rao, P.; Kiselica, A.; Ranum, R.; Thomas, J.M.; Guess, T.M. Feasibility of Using a Novel, Multimodal Motor Function Assessment Platform with Machine Learning to Identify Individuals with Mild Cognitive Impairment. Alzheimer Dis. Assoc. Disord. 2024, 38, 344–350. [Google Scholar] [CrossRef]
- Snene, M.; Graf, C.; Vayne-Bossert, P.; Pautex, S. Pain Assessment for Patients with Dementia and Communication Impairment: Feasibility Study of the Usage of Artificial Intelligence-Enabled Wearables. Sensors 2024, 24, 6298. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Kristoffersson, A.; Folke, M.; Lindén, M.; Riboni, D. Unobtrusive Cognitive Assessment in Smart-Homes: Leveraging Visual Encoding and Synthetic Movement Traces Data Mining. Sensors 2024, 24, 1381. [Google Scholar] [CrossRef]
- Hantke, N.C.; Kaye, J.; Mattek, N.; Wu, C.Y.; Dodge, H.H.; Beattie, Z.; Woltjer, R. Correlating continuously captured home-based digital biomarkers of daily function with postmortem neurodegenerative neuropathology. PLoS ONE 2023, 18, e0286812. [Google Scholar] [CrossRef]
- Kimura, N.; Sasaki, Y.; Masuda, T.; Ataka, T.; Eguchi, A.; Kakuma, T.; Matsubara, E. Lifestyle factors that affect cognitive function–a longitudinal objective analysis. Front. Public Health 2023, 11, 1215419. [Google Scholar] [CrossRef]
- Freytag, J.; Mishra, R.K.; Street, R.L.; Catic, A.; Dindo, L.; Kiefer, L.; Najafi, B.; Naik, A.D. Using Wearable Sensors to Measure Goal Achievement in Older Veterans with Dementia. Sensors 2022, 22, 9923. [Google Scholar] [CrossRef]
- Ramazi, R.; Bowen, M.E.L.; Flynn, A.J.; Beheshti, R. Developing Acute Event Risk Profiles for Older Adults with Dementia in Long-Term Care Using Motor Behavior Clusters Derived from Deep Learning. J. Am. Med. Dir. Assoc. 2022, 23, 1977–1983.e1971. [Google Scholar] [CrossRef]
- Cullen, A.; Mazhar, M.K.A.; Smith, M.D.; Lithander, F.E.; Ó Breasail, M.Ó.; Henderson, E.J. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors 2022, 22, 3336. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Go, T.H.; Hong, S.H.; Kim, S.H.; Han, J.H.; Kang, Y.; Kang, D.R. Digital Biomarkers in Living Labs for Vulnerable and Susceptible Individuals: An Integrative Literature Review. Yonsei Med. J. 2022, 63, S43–S55. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.; Yoon, H.; Youm, C.; Kim, S.; Lee, M.; Park, H.; Kim, B.; Choi, H.; Noh, Y. Prediction of decline in global cognitive function using machine learning with feature ranking of gait and physical fitness outcomes in older adults. Int. J. Environ. Res. Public Health 2021, 18, 11347. [Google Scholar] [CrossRef] [PubMed]
- Mendez Colmenares, A.; Voss, M.W.; Fanning, J.; Salerno, E.A.; Gothe, N.P.; Thomas, M.L.; McAuley, E.; Kramer, A.F.; Burzynska, A.Z. White matter plasticity in healthy older adults: The effects of aerobic exercise. NeuroImage 2021, 239, 118305. [Google Scholar] [CrossRef]
- Dillon-Rossiter, K.; Prapavessis, H. REducing SEDENTary behavior among mild to moderate cognitively impaired assisted living residents: A pilot randomized controlled trial (RESEDENT study). J. Aging Phys. Act. 2021, 29, 27–35. [Google Scholar] [CrossRef]
- De Souto-Barreto, P.; Pothier, K.; Soriano, G.; Lussier, M.; Bherer, L.; Guyonnet, S.; Piau, A.; Ousset, P.J.; Vellas, B. A Web-Based Multidomain Lifestyle Intervention for Older Adults: The eMIND Randomized Controlled Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 142–150. [Google Scholar] [CrossRef]
- Khan, T.; Jacobs, P.G. Prediction of Mild Cognitive Impairment Using Movement Complexity. IEEE J. Biomed. Health Inform. 2021, 25, 227–236. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Ehsani, H.; Wendel, C.; Zamrini, E.; Connor, K.O.; Mohler, J. Screening older adults for amnestic mild cognitive impairment and early-stage Alzheimer’s disease using upper-extremity dual-tasking. Sci. Rep. 2019, 9, 10911. [Google Scholar] [CrossRef]
- Lussier, M.; Lavoie, M.; Giroux, S.; Consel, C.; Guay, M.; Macoir, J.; Hudon, C.; Lorrain, D.; Talbot, L.; Langlois, F.; et al. Early Detection of Mild Cognitive Impairment with In-Home Monitoring Sensor Technologies Using Functional Measures: A Systematic Review. IEEE J. Biomed. Health Inform. 2019, 23, 838–847. [Google Scholar] [CrossRef]
- Ehsani, H.; Mohler, J.; O’Connor, K.; Zamrini, E.; Tirambulo, C.; Toosizadeh, N. The association between cognition and dual-tasking among older adults: The effect of motor function type and cognition task difficulty. Clin. Interv. Aging 2019, 14, 659–669. [Google Scholar] [CrossRef]
- Kaye, J.; Reynolds, C.; Bowman, M.; Sharma, N.; Riley, T.; Golonka, O.; Lee, J.; Quinn, C.; Beattie, Z.; Austin, J.; et al. Methodology for establishing a community-wide life laboratory for capturing unobtrusive and continuous remote activity and health data. J. Vis. Exp. 2018, 2018, 56942. [Google Scholar] [CrossRef]
- Byrom, B.; Mc Carthy, M.; Schueler, P.; Muehlhausen, W. Brain Monitoring Devices in Neuroscience Clinical Research: The Potential of Remote Monitoring Using Sensors, Wearables, and Mobile Devices. Clin. Pharmacol. Ther. 2018, 104, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients with Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Hervas, R.; Fontecha, J.; González, I. m-Health: Lessons Learned by m-Experiences. Sensors 2018, 18, 1569. [Google Scholar] [CrossRef]
- Artusi, C.A.; Mishra, M.; Latimer, P.; Vizcarra, J.A.; Lopiano, L.; Maetzler, W.; Merola, A.; Espay, A.J. Integration of technology-based outcome measures in clinical trials of Parkinson and other neurodegenerative diseases. Park. Relat. Disord. 2018, 46, S53–S56. [Google Scholar] [CrossRef]
- Riboni, D.; Bettini, C.; Civitarese, G.; Janjua, Z.H.; Helaoui, R. SmartFABER: Recognizing fine-grained abnormal behaviors for early detection of mild cognitive impairment. Artif. Intell. Med. 2016, 67, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Sabbagh, M.; Lin, I.; Morgan, P.; Grewal, G.S.; Mohler, J.; Coon, D.W.; Najafi, B. Sensor-based balance training with motion feedback in people with mild cognitive impairment. J. Rehabil. Res. Dev. 2016, 53, 945–958. [Google Scholar] [CrossRef]
- Costa, L.; Gago, M.F.; Yelshyna, D.; Ferreira, J.; Silva, H.D.; Rocha, L.; Sousa, N.; Bicho, E. Application of Machine Learning in Postural Control Kinematics for the Diagnosis of Alzheimer’s Disease. Comput. Intell. Neurosci. 2016, 2016, 3891253. [Google Scholar] [CrossRef]
- Petersen, J.; Austin, D.; Mattek, N.; Kaye, J. Time out-of-home and cognitive, physical, and emotional wellbeing of older adults: A longitudinal mixed effects model. PLoS ONE 2015, 10, e0139643. [Google Scholar] [CrossRef]
- Akl, A.; Taati, B.; Mihailidis, A. Autonomous unobtrusive detection of mild cognitive impairment in older adults. IEEE Trans. Biomed. Eng. 2015, 62, 1383–1394. [Google Scholar] [CrossRef]
- Thielke, S.M.; Mattek, N.; Hayes, T.L.; Dodge, H.H.; Quiñones, A.R.; Austin, D.; Petersen, J.; Kaye, J. Associations between observed in-home behaviors and self-reported low mood in community-dwelling older adults. J. Am. Geriatr. Soc. 2014, 62, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Maxwell, S.A.; Mattek, N.; Hayes, T.L.; Dodge, H.H.; Pavel, M.; Jimison, H.B.; Wild, K.; Boise, L.; Zitzelberger, T.A. Intelligent Systems For Assessing Aging Changes: Home-based, unobtrusive, and continuous assessment of aging. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011, 66, i180–i190. [Google Scholar] [CrossRef] [PubMed]
- Kearns, W.D.; Nams, V.O.; Fozard, J.L. Tortuosity in movement paths is related to cognitive impairment wireless fractal Estimation in assisted living facility residents. Methods Inf. Med. 2010, 49, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hagler, S.; Austin, D.; Hayes, T.L.; Kaye, J.; Pavel, M. Unobtrusive and ubiquitous in-home monitoring: A methodology for continuous assessment of gait velocity in elders. IEEE Trans. Biomed. Eng. 2010, 57, 813–820. [Google Scholar] [CrossRef]
- Vlotinou, P.; Tsiakiri, A.; Frantzidis, C.A.; Katsouri, I.-G.; Aggelousis, N. The effect of an interventional movement program on the mechanical gait characteristics of a patient with dementia. Eng. Proc. 2023, 50, 4. [Google Scholar]
- Tsiara, A.A.; Plakias, S.; Kokkotis, C.; Veneri, A.; Mina, M.A.; Tsiakiri, A.; Kitmeridou, S.; Christidi, F.; Gourgoulis, E.; Doskas, T. Artificial Intelligence in the Diagnosis of Neurological Diseases Using Biomechanical and Gait Analysis Data: A Scopus-Based Bibliometric Analysis. Neurol. Int. 2025, 17, 45. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Vlotinou, P.; Kedraka, K.; Tsiptsios, D.; Karatzetzou, S.; Mitsou, L.; Tsamakis, K.; Kitmeridou, S.; Nikova, A.; Aggelousis, N. Remote learning for adults with mild cognitive impairment in the new landscape of COVID-19 restrictions. Adv. Soc. Sci. Res. J. 2022, 9, 382–394. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Plakias, S.; Vlotinou, P.; Athanasouli, P.; Terzoudi, A.; Kyriazidou, S.; Serdari, A.; Karakitsiou, G.; Megari, K.; Aggelousis, N. Ιnnovative health promotion strategies: A 6-month longitudinal study on computerized cognitive training for older adults with minor neurocognitive disorders. Eur. J. Investig. Health Psychol. Educ. 2025, 15, 34. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Koutzmpi, V.; Megagianni, S.; Toumaian, M.; Geronikola, N.; Despoti, A.; Kanellopoulou, S.; Arampatzi, X.; Margioti, E.; Davila, A. Remote neuropsychological evaluation of older adults. Appl. Neuropsychol. Adult 2024, 31, 796–803. [Google Scholar] [CrossRef]
- Tsiakiri, A.; Ioannidis, P.; Vlotinou, P.; Kokkotis, C.; Megagianni, S.; Toumaian, M.; Terzoudi, K.; Koutzmpi, V.; Despoti, A.; Megari, K. The role of a computerized cognitive intervention program on the neuropsychiatric symptoms in mild cognitive impairment. Aging Med. Healthc. 2024, 15, 122–128. [Google Scholar] [CrossRef]
- Despoti, A.; Megari, K.; Tsiakiri, A.; Toumaian, M.; Koutzmpi, V.; Liozidou, A.; Tsapanou, A. Effectiveness of remote neuropsychological interventions: A systematic review. Appl. Neuropsychol. Adult 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).