A Point-Line-Area Paradigm: 3D Printing for Next-Generation Health Monitoring Sensors
Abstract
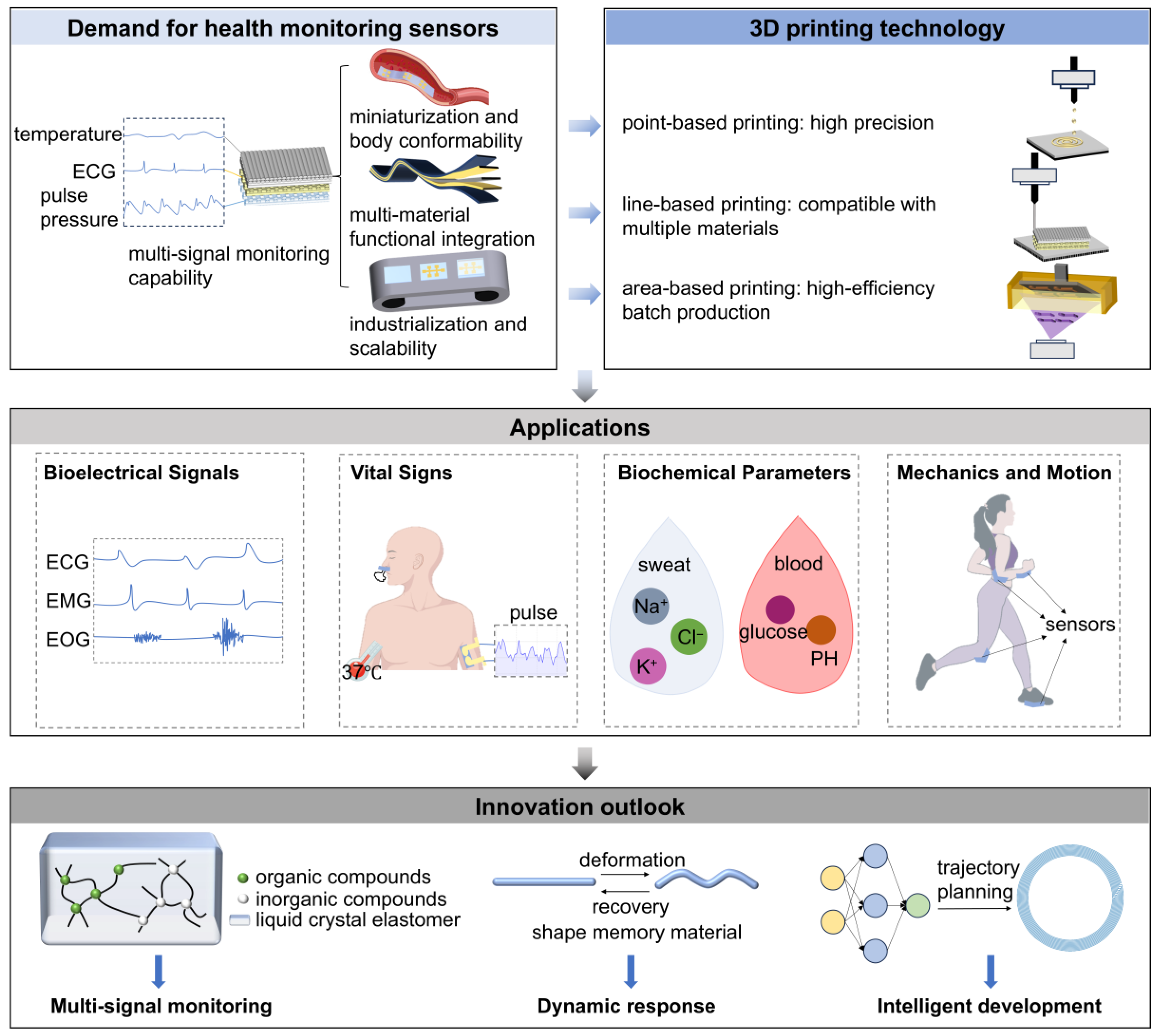
1. Introduction
2. Requirements Analysis for Health Monitoring Sensors
3. Introduction to Printing Modalities: A Unit-Dimension-Based Classification
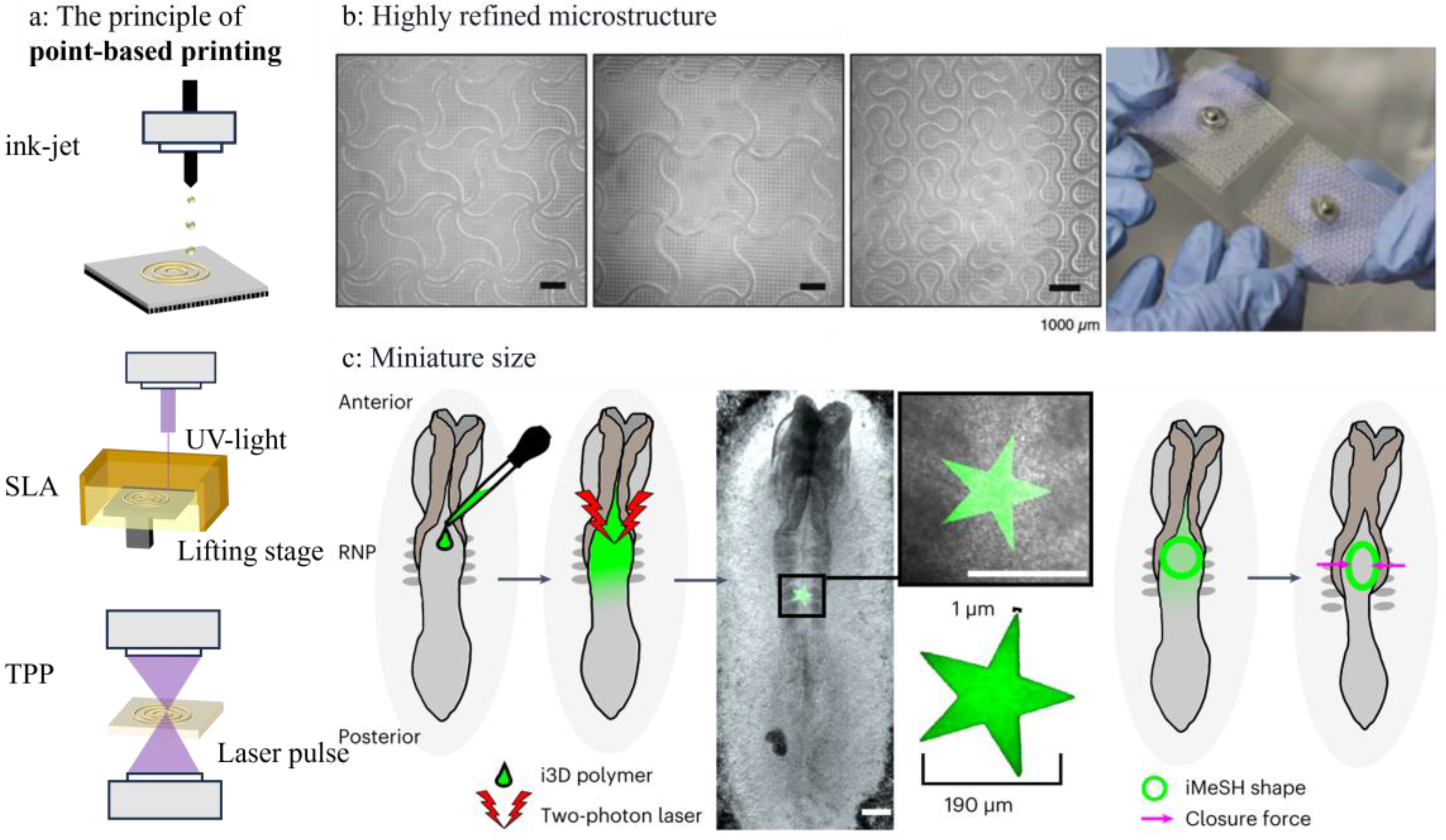
3.1. Point-Based Printing
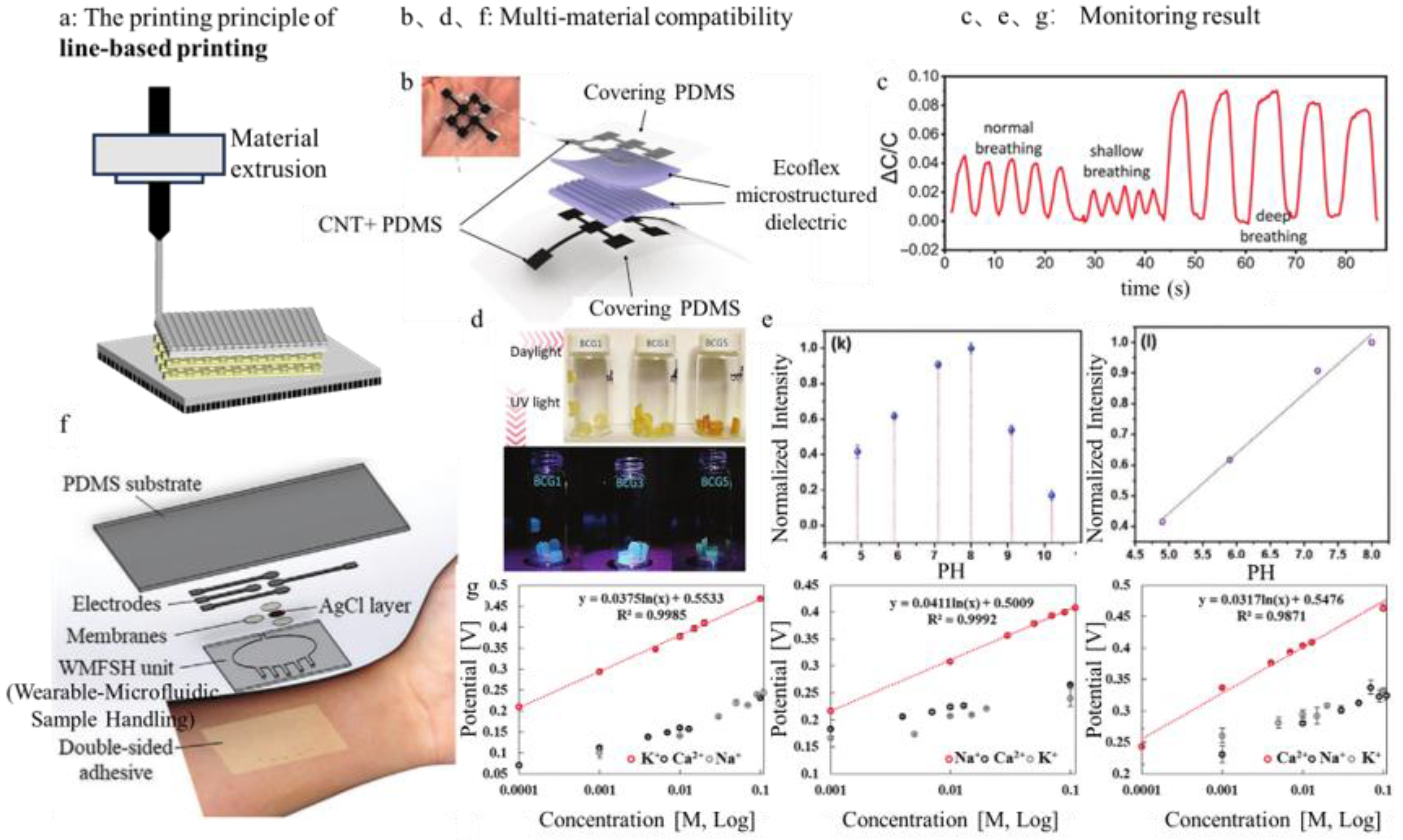
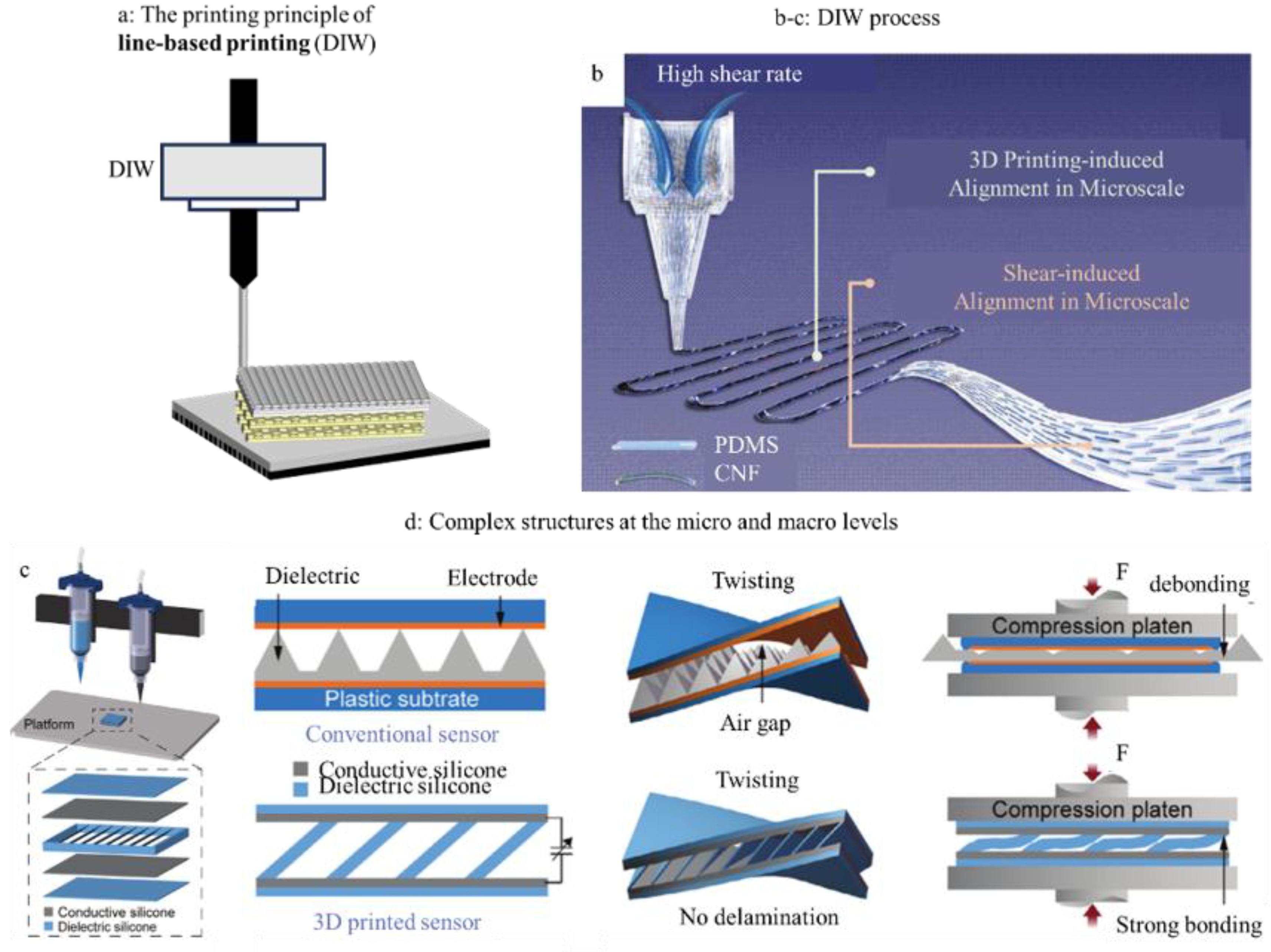
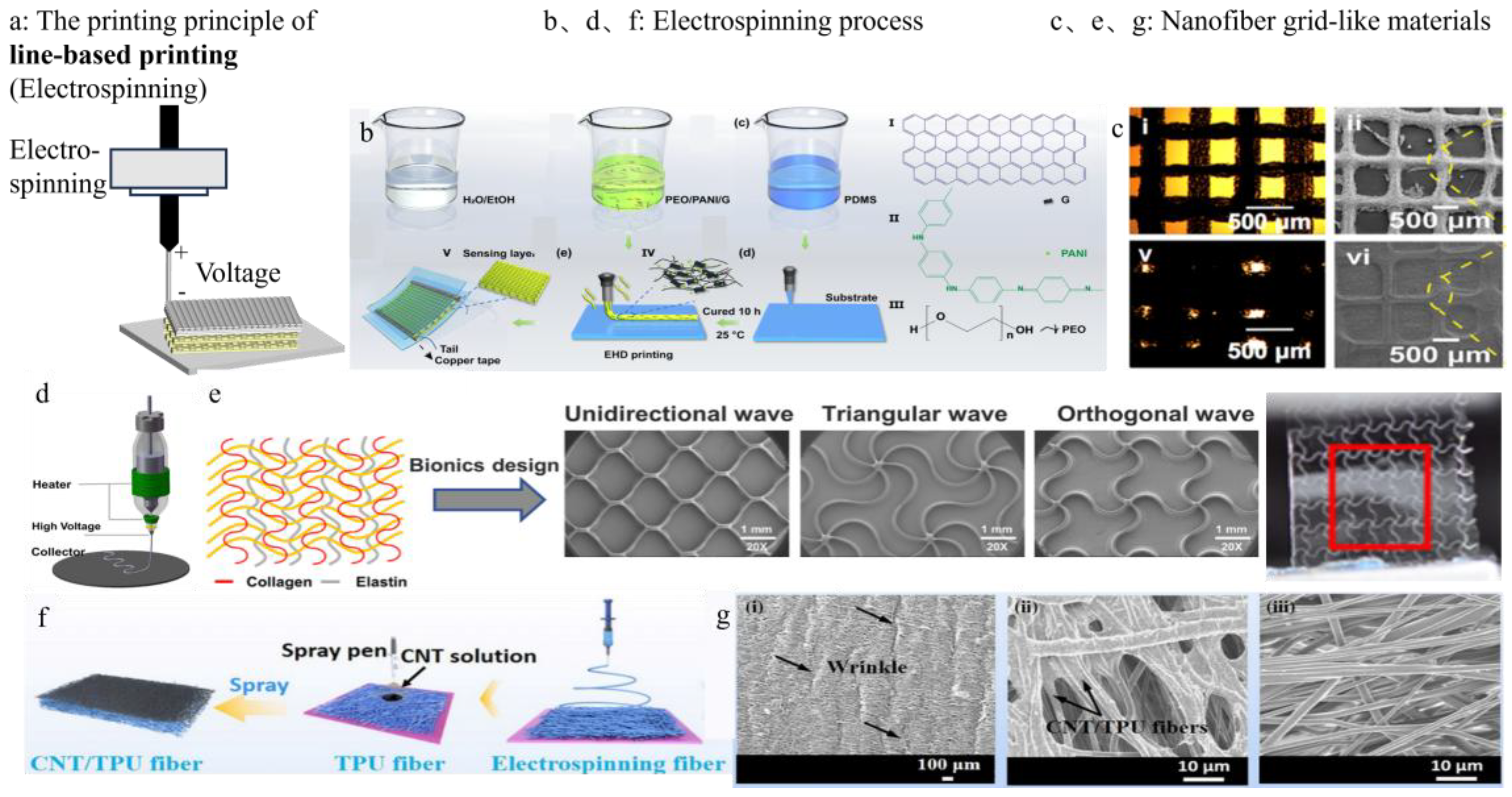
3.2. Line-Based Printing
| Term | Explanation |
|---|---|
| Shear-thinning | A property where the material’s viscosity decreases under shear stress (e.g., during extrusion), allowing it to flow easily through the nozzle. Once the stress is removed, it thickens again. |
| Storage Modulus (G′) | Measures the solid-like behavior of a material; higher G′ indicates stronger structural rigidity and shape-holding ability. |
| Loss Modulus (G″) | Reflects the liquid-like behavior; when G″ > G′, the material flows. |
3.3. Area-Based Printing
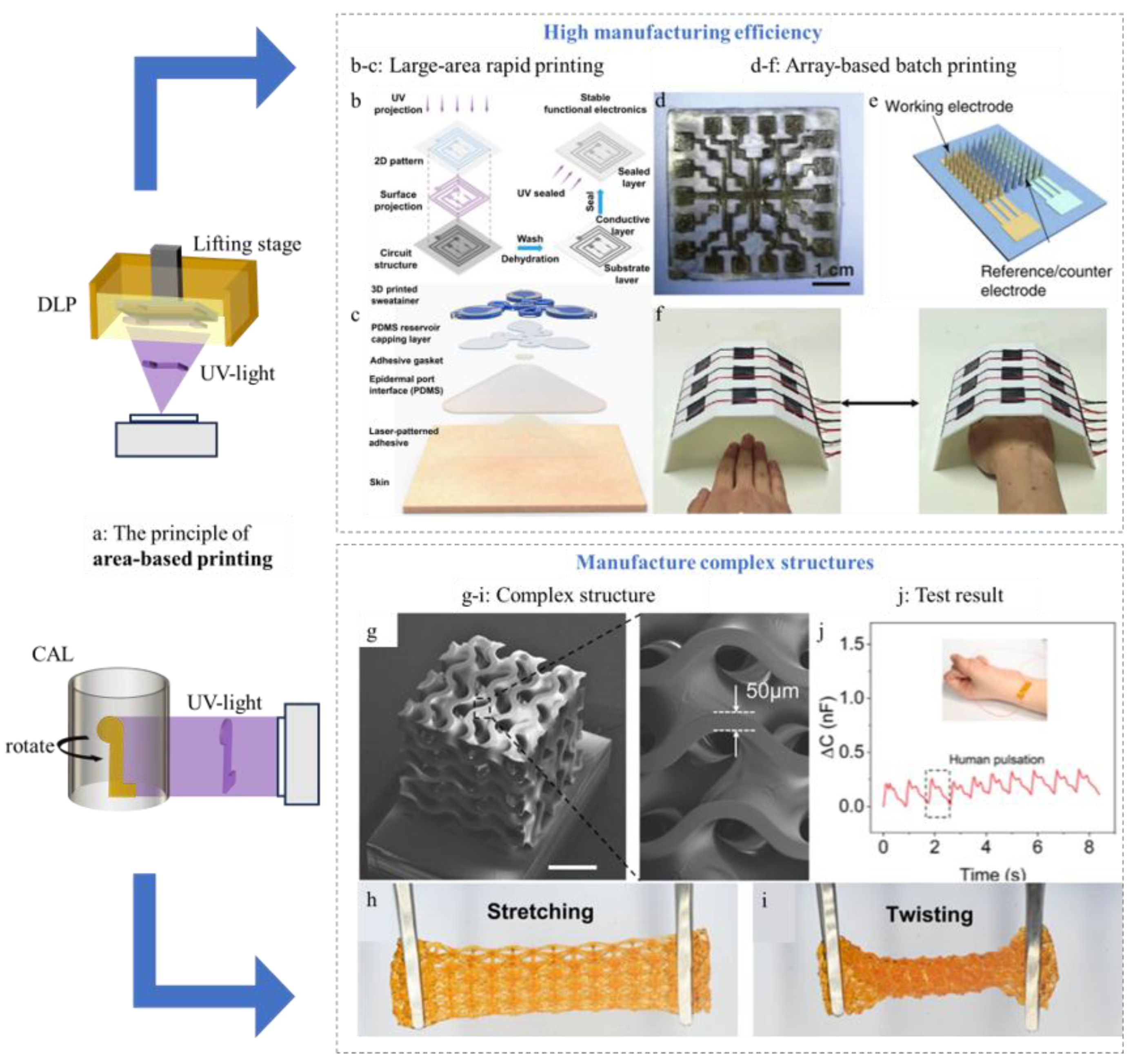
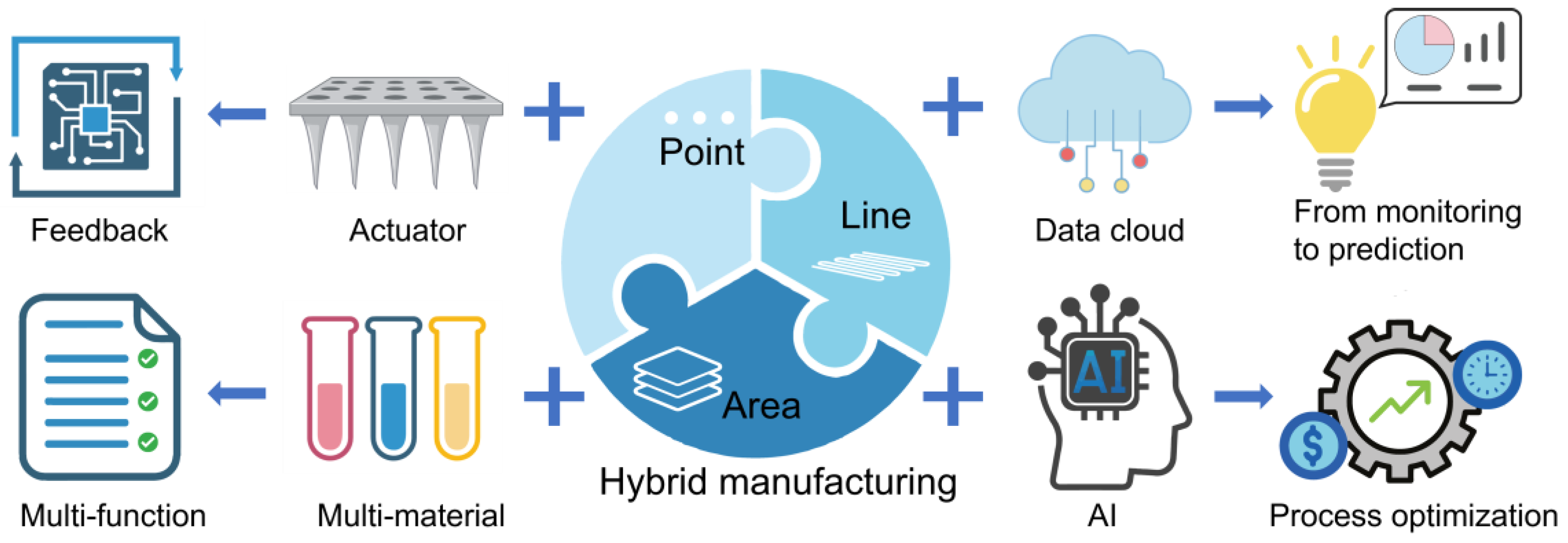
4. Current Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Report on Ageing and Health. 2015. Available online: https://www.who.int/publications/i/item/9789241565042 (accessed on 29 September 2015).
- Ren, Z. China Aging Report. Dev. Res. 2023, 40, 22–30. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; IDF: Brussels, Belgium, 2021; Available online: https://diabetesatlas.org/ (accessed on 14 September 2025).
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Barbagallo, C.M. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Li, H.; Wu, J.; Wan, Q.; Chen, T.; Luo, Y. Smart hydrogel sensors for health monitoring and early warning. Adv. Sens. Res. 2024, 3, 2400003. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Li, S.; Luo, Y.; Deng, S.; Yang, B.; Shao, J. On-Skin Epidermal Electronics for Next-Generation Health Management. Nano Micro Lett. 2026, 18, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Lu, H.; Zhu, M.; Liang, H.; Wu, X.; Zhang, Y. Carbon-based flexible devices for comprehensive health monitoring. Small Methods 2023, 7, 2201340. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Jiang, J.; An, F.; Ma, X.; Wu, J. Research progress of 3D printing technology in functional food, powering the future of food. Trends Food Sci. Technol. 2024, 149, 104545. [Google Scholar] [CrossRef]
- Xin, Y.; Zhou, X.; Bark, H.; Lee, P.S. The role of 3D printing technologies in soft grippers. Adv. Mater. 2024, 36, 2307963. [Google Scholar] [CrossRef]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Ren, J. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025, D1, D1670–D1676. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible sensing electronics for wearable/attachable health monitoring. Small 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Alam, F.; Ashfaq Ahmed, M.; Jalal, A.H.; Siddiquee, I.; Adury, R.Z.; Hossain, G.M.; Pala, N. Recent progress and challenges of implantable biodegradable biosensors. Micromachines 2024, 15, 475. [Google Scholar] [CrossRef]
- Truby, R.; Lewis, J. Printing soft matter in three dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, B.; Ananthavel, S.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Perry, J.W. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Prabhakar, M.M.; Saravanan, A.K.; Lenin, A.H.; Mayandi, K.; Ramalingam, P.S. A short review on 3D printing methods, process parameters and materials. Mater. Today Proc. 2021, 45, 6108–6114. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Jin, H.; Yasmin, S.A.-R.; Hossam, H. Advanced materials for health monitoring with skin-based wearable devices. Adv. Healthc. Mater. 2017, 6, 1700024. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Hossam, H. Autonomous flexible sensors for health monitoring. Adv. Mater. 2018, 30, 1802337. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, L.; Yeo, J.C.; Lim, C.T. Flexible hybrid sensors for health monitoring: Materials and mechanisms to render wearability. Adv. Mater. 2020, 32, 1902133. [Google Scholar] [CrossRef]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K.K. 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing–A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Yue, W.; Guo, Y.; Lee, J.C.; Ganbold, E.; Wu, J.K.; Li, Y.; Kim, N.Y. Advancements in passive wireless sensing systems in monitoring harsh environment and healthcare applications. Nano Micro Lett. 2025, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-B.; Shang, X.; Sun, M.; Bo, X.; Bai, J.; Du, Y.; Zhou, M. Emerging Multifunctional Wearable Sensors: Integrating Multimodal Sweat Analysis and Advanced Material Technologies for Next-Generation Health Monitoring. ACS Sens. 2025, 10, 2388–2408. [Google Scholar] [PubMed]
- Macdonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, aaf2093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, J.; He, Y. A review of 3D printing technologies for soft polymer materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Feng, J.; Fu, J.; Lin, Z.; Shang, C.; Li, B. A review of the design methods of complex topology structures for 3D printing. Vis. Comput. Ind. Biomed. Art 2018, 1, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Chai, J.; Zhan, Y.; Cui, D.; Wang, X.; Gao, L. Design, Fabrication, and Application of Large-Area Flexible Pressure and Strain Sensor Arrays: A Review. Micromachines 2025, 16, 330. [Google Scholar] [CrossRef]
- Qin, X.; Zhong, B.; Xu, H.; Jackman, J.A.; Xu, K.; Cho, N.J.; Wang, L. Manufacturing high-performance flexible sensors via advanced patterning techniques. Int. J. Extrem. Manuf. 2025, 7, 032003. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Dong, B.; Lee, J.; Ware, H.O.T.; Zhang, H.F.; Sun, C. High-speed 3D printing of millimeter-size customized aspheric imaging lenses with sub 7 nm surface roughness. Adv. Mater. 2018, 30, 1705683. [Google Scholar] [CrossRef]
- Kuang, X.; Zhao, Z.; Chen, K.; Fang, D.; Kang, G.; Qi, H.J. High-speed 3D printing of high-performance thermosetting polymers via two-stage curing. Macromol. Rapid Commun. 2018, 39, 1700809. [Google Scholar] [CrossRef]
- Gholami, F.; Yue, L.; Li, M.; Jain, A.; Mahmood, A.; Fratarcangeli, M.; Qi, H.J. Fast and Efficient Fabrication of Functional Electronic Devices through Grayscale Digital Light Processing 3D Printing. Adv. Mater. 2024, 36, 2408774. [Google Scholar] [CrossRef] [PubMed]
- Zastrow, M. The new 3D printing. Nature 2020, 578, 20–23. [Google Scholar] [CrossRef]
- Subedi, S.; Liu, S.; Wang, W.; Naser Shovon, S.A.; Chen, X.; Ware, H.O.T. Multi-material vat photopolymerization 3D printing: A review of mechanisms and applications. npj Adv. Manuf. 2024, 1, 9. [Google Scholar] [CrossRef]
- Olawumi, M.A.; Oladapo, B.I.; Olugbade, T.O. Evaluating the impact of recycling on polymer of 3D printing for energy and material sustainability. Resour. Conserv. Recycl. 2024, 209, 107769. [Google Scholar] [CrossRef]
- Zhang, Y.; Rui, K.; Huang, A.; Ding, Y.; Hu, K.; Shi, W.; Cao, X.; Lin, H.; Zhu, J.; Huang, W. Stereoassembled V2O5@ FeOOH hollow architectures with lithiation volumetric strain self-reconstruction for lithium-ion storage. Research 2020, 2360796. [Google Scholar]
- Zhang, C.; Li, H.; Huang, A.; Zhang, Q.; Rui, K.; Lin, H.; Sun, G.; Zhu, J.; Peng, H.; Huang, W. Rational design of a flexible CNTs@ PDMS film patterned by bio-inspired templates as a strain sensor and supercapacitor. Small 2019, 15, 1805493. [Google Scholar] [CrossRef] [PubMed]
- ISO/ASTM 52900:2021; Additive Manufacturing—General Principles—Terminology. International Organization for Standardization: Geneva, Switzerland; ASTM International: West Conshohocken, PA, USA, 2021.
- Jayasinghe, S.N.; Townsend-Nicholson, A. Bio-electrosprays: The next generation of electrified jets. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 1018–1022. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Yu, K.; Dong, G. Innovative polymer-based composite materials in additive manufacturing: A review of methods, materials, and applications. Polym. Compos. 2024, 45, 15389–15420. [Google Scholar] [CrossRef]
- Rocha, V.G.; Saiz, E.; Tirichenko, I.S.; García-Tuñón, E. Direct ink writing advances in multi-material structures for a sustainable future. J. Mater. Chem. A 2020, 8, 15646–15657. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, S.; Ravichandran, D.; Ramanathan, A.; Sobczak, M.T.; Sacco, A.F.; Song, K. 3D-Printed Polymeric Biomaterials for Health Applications. Adv. Healthc. Mater. 2024, 14, 2402571. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Jan, F.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Gu, Z. Photoresist development for 3D printing of conductive microstructures via two-photon polymerization. Adv. Mater. 2024, 36, 2409326. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Huang, S.; Huang, X.; Liu, Z.; Yao, C.; Xie, X. Multichannel microneedle dry electrode patches for minimally invasive transdermal recording of electrophysiological signals. Microsyst. Nanoeng. 2024, 10, 72. [Google Scholar] [CrossRef]
- Liu, D.; Tian, X.; Bai, J.; Wang, S.; Dai, S.; Wang, Y.; Zhang, S. A wearable in-sensor computing platform based on stretchable organic electrochemical transistors. Nat. Electron. 2024, 7, 1176–1185. [Google Scholar] [CrossRef]
- Alsharif, A.A.; Syed, A.M.; Li, X.; Alsharif, N.A.; Lubineau, G.; El-Atab, N. Hybrid 3D Printing of a Nature-Inspired Flexible Self-Adhesive Biopatch for Multi-Biosignal Sensing. Adv. Funct. Mater. 2024, 34, 2406341. [Google Scholar] [CrossRef]
- Herbert, R.; Lim, H.R.; Rigo, B.; Yeo, W.H. Fully implantable wireless batteryless vascular electronics with printed soft sensors for multiplex sensing of hemodynamics. Sci. Adv. 2022, 8, eabm1175. [Google Scholar] [CrossRef] [PubMed]
- Maniou, E.; Todros, S.; Urciuolo, A.; Moulding, D.A.; Magnussen, M.; Ampartzidis, I.; Elvassore, N. Quantifying mechanical forces during vertebrate morphogenesis. Nat. Mater. 2024, 23, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Kanatzidis, E.E.; Avila, R.; Zhou, M.; Bai, Y.; Chen, S.; Rogers, J.A. 3D-printed epidermal sweat microfluidic systems with integrated microcuvettes for precise spectroscopic and fluorometric biochemical assays. Mater. Horiz. 2023, 10, 4992–5003. [Google Scholar] [CrossRef]
- Parrilla, M.; Vanhooydonck, A.; Johns, M.; Watts, R.; De Wael, K. 3D-printed microneedle-based potentiometric sensor for pH monitoring in skin interstitial fluid. Sens. Actuators B Chem. 2023, 378, 133159. [Google Scholar] [CrossRef]
- Prabhu, A.; Baliga, V.; Shenoy, R.; Dessai, A.D.; Nayak, U.Y. 3D printed microneedles: Revamping transdermal drug delivery systems. Drug Deliv. Transl. Res. 2025, 15, 436–454. [Google Scholar] [CrossRef]
- Jiang, Y.; Islam, M.N.; He, R.; Huang, X.; Cao, P.F.; Advincula, R.C.; Choi, W. Recent Advances in 3D Printed Sensors: Materials, Design, and Manufacturing. Adv. Mater. Technol. 2022, 8, 2200492. [Google Scholar] [CrossRef]
- Schiavone, N.; Vincent, V.; Haroutioun, A. Effect of 3D printing temperature profile on polymer materials behavior. 3D Print. Addit. Manuf. 2020, 7, 311–325. [Google Scholar] [CrossRef]
- Sehhat, M.H.; Ali, M.; Farzad, Y. Impact of temperature and material variation on mechanical properties of parts fabricated with fused deposition modeling (FDM) additive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 4791–4801. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Han, W.; Lin, H.; Li, R.; Zhu, J.; Huang, W. 3D Printed Flexible Strain Sensors: From Printing to Devices and Signals. Adv. Mater. 2021, 33, 2004782. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Haghniaz, R.; Rashidi, A.; Ahadian, S.; Sheikhi, A.; Toyserkani, E. 3D-printed ultra-robust surface-doped porous silicone sensors for wearable biomonitoring. ACS Nano 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Rahman, M.M. Direct ink writing: A 3D printing technology for diverse materials. Adv. Mater. 2022, 34, 2108855. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Meng, J.; Bao, X.; Huang, Y.; Yan, X.P.; Qian, H.L.; Liu, T. Direct-ink-write 3D printing of programmable micro-supercapacitors from MXene-regulating conducting polymer inks. Adv. Energy Mater. 2023, 13, 2203683. [Google Scholar] [CrossRef]
- Hui, Y.; Yao, Y.; Qian, Q.; Luo, J.; Chen, H.; Qiao, Z.; Zhou, N. Three-dimensional printing of soft hydrogel electronics. Nat. Electron. 2022, 5, 893–903. [Google Scholar] [CrossRef]
- Chung, K.Y.; Tan, D.; He, Z.; Li, X.; Lu, J.; Yang, Q.; Xu, B. Cottonseed-Derived Reusable Bio-Carbon Gel Ink for DIW Printing Soft Electronic Textiles. Adv. Mater. 2025, 2415702. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulos, N.D.; Angeliki, B. Direct Ink writing for electrochemical device fabrication: A review of 3D-printed electrodes and Ink rheology. Catalysts 2024, 14, 110. [Google Scholar] [CrossRef]
- Tofangchi, A.; Han, P.; Izquierdo, J.; Iyengar, A.; Hsu, K. Effect of ultrasonic vibration on interlayer adhesion in fused filament fabrication 3D printed ABS. Polymers 2019, 11, 315. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Zhang, X. Developments of advanced electrospinning techniques: A critical review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Ji, D.; Lin, Y.; Guo, X.; Ramasubramanian, B.; Wang, R.; Radacsi, N.; Ramakrishna, S. Electrospinning of nanofibres. Nat. Rev. Methods Primers 2024, 4, 1. [Google Scholar] [CrossRef]
- Nazemi, M.M.; Khodabandeh, A.; Hadjizadeh, A. Near-field electrospinning: Crucial parameters, challenges, and applications. ACS Appl. Bio Mater. 2022, 5, 394–412. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Sun, J.; Zhang, R.; Liang, X.; Long, J.; Lu, R. Near-field direct writing based on piezoelectric micromotion for the programmable manufacturing of serpentine structures. Micromachines 2024, 15, 1478. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Najafikhoshnoo, S.; Das, P.; Noh, S.; Hoang, E.; Kim, T.; Esfandyarpour, R. All-3D-printed, flexible, and hybrid wearable bioelectronic tactile sensors using biocompatible nanocomposites for health monitoring. Adv. Mater. Technol. 2022, 7, 2101034. [Google Scholar] [CrossRef]
- Zhu, Z.; Park, H.S.; Mcalpine, M.C. 3D printed deformable sensors. Sci. Adv. 2020, 6, eaba5575. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Malkoc, A.; La Belle, J.T. The development of a glucose dehydrogenase 3D-printed glucose sensor: A proof-of-concept study. J. Diabetes Sci. Technol. 2018, 12, 176–182. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Huang, H.; Li, J.; Liu, H.; Guo, Z.; Lei, Y. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater. Sci. Eng. C 2020, 117, 111299. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Marvi, P.K.; Sherazee, M.; Tang, X.; Srinivasan, S.; Rajabzadeh, A.R. Carbon Dots Infused 3D Printed Cephalopod Mimetic Bactericidal and Antioxidant Hydrogel for Uniaxial Mechano-Fluorescent Tactile Sensor. Adv. Mater. 2024, 36, 2409819. [Google Scholar]
- Kim, T.; Yi, Q.; Hoang, E.; Esfandyarpour, R. A 3D printed wearable bioelectronic patch for multi-sensing and in situ sweat electrolyte monitoring. Adv. Mater. Technol. 2021, 6, 2001021. [Google Scholar]
- Liu, Y.; Wang, Z.; Song, X.; Shen, X.; Wei, Y.; Hua, C.; Liu, Y. 3D Printing-Induced Hierarchically Aligned Nanocomposites with Exceptional Multidirectional Strain Sensing Performance. Small 2024, 20, 2404810. [Google Scholar] [CrossRef]
- Xiao, F.; Wei, Z.; Xu, Z.; Wang, H.; Li, J.; Zhu, J. Fully 3D-Printed Soft Capacitive Sensor of High Toughness and Large Measurement Range. Adv. Sci. 2025, 12, 2410284. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Fu, J.; Xu, Y.; He, Y. Liquid metal microgels for three-dimensional printing of smart electronic clothes. ACS Appl. Mater. Interfaces 2022, 14, 13458–13467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ji, J.; Zhang, Y.; Liu, J.; Yu, R.; Yang, X.; Zhao, W. Ultra-elastic conductive silicone rubber composite foams for durable piezoresistive sensors via direct ink writing three-dimensional printing. Chem. Eng. J. 2025, 504, 158733. [Google Scholar] [CrossRef]
- Song, Y.; Dong, H.; Liu, W.; Fu, X.; Fu, Z.; Li, P.; Chang, M.W. Electrostatic jet engineering of flexible composite pressure sensors for physical applications. ACS Appl. Polym. Mater. 2022, 4, 868–878. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Hu, Z.; Lu, W.; Li, Z.; Gao, N.; He, Y. Gelatin-based metamaterial hydrogel films with high conformality for ultra-soft tissue monitoring. Nano Micro Lett. 2024, 16, 34. [Google Scholar] [CrossRef]
- Wang, P.; Sun, G.; Hua, S.; Yu, W.; Meng, C.; Han, Q.; Li, Y. Multifunctional all-nanofiber cloth integrating personal health monitoring and thermal regulation capabilities. InfoMat 2024, 7, e12629. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Dai, C. Design of DLP 3D printer control system based on Arduino. In Proceedings of the 2020 3rd World Conference on Mechanical Engineering and Intelligent Manufacturing (WCMEIM), Shanghai, China, 4–6 December 2020; pp. 497–500. [Google Scholar]
- Li, Y.; Mao, Q.; Yin, J.; Wang, Y.; Fu, J.; Huang, Y. Theoretical prediction and experimental validation of the digital light processing (DLP) working curve for photocurable materials. Addit. Manuf. 2021, 37, 101716. [Google Scholar] [CrossRef]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Li, H.; Dai, J.; Wang, Z.; Zheng, H.; Li, W.; Wang, M.; Cheng, F. Digital light processing (DLP)-based (bio) printing strategies for tissue modeling and regeneration. Aggregate 2023, 4, e270. [Google Scholar] [CrossRef]
- Kim, G.T.; Go, H.B.; Yu, J.H.; Yang, S.Y.; Kim, K.M.; Choi, S.H.; Kwon, J.S. Cytotoxicity, colour stability and dimensional accuracy of 3D printing resin with three different photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef]
- Mani, G.; Thepperumal, S.K. Recent Trends on Additive Manufacturing Biomaterial Composites in Tissue Regeneration Future Perspectives, Challenges, and Road Maps to Clinics for Biomedical Applications—A Review. 3D Print. Addit. Manuf. 2024. [Google Scholar] [CrossRef]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef]
- Loterie, D.; Delrot, P.; Moser, C. High-resolution tomographic volumetric additive manufacturing. Nat. Commun. 2020, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Darkes-Burkey, C.; Shepherd, R.F. Volumetric 3D printing of endoskeletal soft robots. Adv. Mater. 2024, 36, 2402217. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Castaño, M.I.; Madsen, A.G.; Madrid-Wolff, J.; Sgarminato, V.; Boniface, A.; Glückstad, J.; Moser, C. Holographic tomographic volumetric additive manufacturing. Nat. Commun. 2025, 16, 1551. [Google Scholar] [CrossRef]
- Alparslan, C.; Bayraktar, Ş. Advances in digital light processing (DLP) bioprinting: A review of biomaterials and its applications, innovations, challenges, and future perspectives. Polymers 2025, 17, 1287. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, J.; Feng, S.; Cui, J.; Guo, Y.; Liang, C.; Zhang, B. Projection Stereolithography 3D Printing High-Conductive Hydrogel for Flexible Passive Wireless Sensing. Adv. Mater. 2024, 36, 2400103. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ma, H.J.H.; Baessler, P.; Balanay, R.K.; Ray, T.R. Skin-interfaced microfluidic systems with spatially engineered 3D fluidics for sweat capture and analysis. Sci. Adv. 2023, 9, eadg4272. [Google Scholar] [CrossRef]
- Xiao, T.; Qian, C.; Yin, R.; Wang, K.; Gao, Y.; Xuan, F. 3D printing of flexible strain sensor array based on UV-curable multiwalled carbon nanotube/elastomer composite. Adv. Mater. Technol. 2021, 6, 2000745. [Google Scholar] [CrossRef]
- Tang, J.; Gou, K.; Wang, C.; Wei, M.; Tan, Q.; Weng, G. Self-Powered and 3D Printable Soft Sensor for Human Health Monitoring, Object Recognition, and Contactless Hand Gesture Recognition. Adv. Funct. Mater. 2024, 34, 2411172. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Q.; Luo, X.; Yang, L.; Cui, Y. Continuous monitoring of diabetes with an integrated microneedle biosensing device through 3D printing. Microsyst. Nanoeng. 2021, 7, 75. [Google Scholar] [CrossRef]
- Tang, H.; Yang, Y.; Liu, Z.; Li, W.; Zhang, Y.; Huang, Y.; Zang, J. Injectable ultrasonic sensor for wireless monitoring of intracranial signals. Nature 2024, 630, 84–90. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, B.; Liu, Q.; Chen, H.; Cheng, J.; Jian, B.; Ge, Q. Highly conductive and stretchable nanostructured ionogels for 3D printing capacitive sensors with superior performance. Nat. Commun. 2024, 15, 6431. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Chen, G.; Luo, X.; Zhang, X.; Li, D.; Xu, Y.; Liu, Y. Volumetric 3D printing of ionic conductive elastomers for multifunctional flexible electronics. Addit. Manuf. 2024, 95, 104536. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.; Wan, X.; Ma, J.; Li, N.; Li, J.; Yin, Q. Ultrafast, high-resolution and large-size three-dimensional structure manufacturing through high-efficiency two-photon polymerization initiators. Addit. Manuf. 2021, 47, 102358. [Google Scholar] [CrossRef]
- Zhao, C.F.; Wang, J.; Zhang, Z.Q.; Chi, C. Research progress on the design of structural color materials based on 3D printing. Adv. Mater. Technol. 2023, 8, 2200257. [Google Scholar] [CrossRef]
- Ali, N.B.; Khlif, M.; Hammami, D.; Bradai, C. Mechanical and morphological characterization of spherical cell porous structures manufactured using FDM process. Eng. Fract. Mech. 2019, 216, 106527. [Google Scholar] [CrossRef]
- Ge, L.; Dong, L.; Wang, D.; Ge, Q.; Gu, G. A digital light processing 3D printer for fast and high-precision fabrication of soft pneumatic actuators. Sens. Actuators A Phys. 2018, 273, 285–292. [Google Scholar] [CrossRef]
- Rau, D.A.; Michael, J.B.; Christopher, B.W. A rheology roadmap for evaluating the printability of material extrusion inks. Addit. Manuf. 2023, 75, 103745. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; de Leon, A.S.; Franchini, M.C.; Molina, S.I. Sustainable approaches in vat photopolymerization: Advancements, limitations, and future opportunities. Green Chem. 2025, 27, 8710–8754. [Google Scholar] [CrossRef]
- Chen, J.V.; Dang, A.B.C.; Dang, A. Comparing cost and print time estimates for six commercially-available 3D printers obtained through slicing software for clinically relevant anatomical models. 3D Print. Med. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Correia, J.P.M.; Liu, Y.; Ahzi, S. Investigating gradient temperature control for enhanced interfacial bonding behavior in material extrusion 3D printing continuous fiber reinforced polymer composites. Eur. J. Mech. A Solids 2024, 107, 105349. [Google Scholar] [CrossRef]
- Distler, T.; Sulistio, A.; Schneidereit, D.; Friedrich, O.; Boccaccini, A.R. 3D printed oxidized alginate-gelatin bioink provides guidance for C2C12 muscle precursor cell orientation and differentiation via shear stress during bioprinting. Biofabrication 2020, 12, 045005. [Google Scholar] [CrossRef]
- Teng, K.; An, Q.; Chen, Y.; Zhang, Y.; Zhao, Y. Recent development of alginate-based materials and their versatile functions in biomedicine, flexible electronics, and environmental uses. ACS Biomater. Sci. Eng. 2021, 7, 1302–1337. [Google Scholar] [CrossRef]
- Browning, M.B.; Cereceres, S.N.; Luong, P.T.; Cosgriff-Hernandez, E.M. Determination of the in vivo degradation mechanism of PEGDA hydrogels. J. Biomed. Mater. Res. Part A 2014, 102, 4244–4251. [Google Scholar]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet printing for materials and devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Sabyrov, N.; Abilgaziyev, A.; Ali, M.H. Enhancing interlayer bonding strength of FDM 3D printing technology by diode laser-assisted system. Int. J. Adv. Manuf. Technol. 2020, 108, 603–611. [Google Scholar] [CrossRef]
- Yin, J.; Lu, C.; Fu, J.; Huang, Y.; Zheng, Y. Interfacial bonding during multi-material fused deposition modeling (FDM) process due to inter-molecular diffusion. Mater. Des. 2018, 150, 104–112. [Google Scholar] [CrossRef]
- Perez, D.B.; Celik, E.; Karkkainen, R.L. Investigation of interlayer interface strength and print morphology effects in fused deposition modeling 3D-printed PLA. 3D Print. Addit. Manuf. 2021, 8, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Borrello, J.; Nasser, P.; Iatridis, J.C.; Costa, K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit. Manuf. 2018, 23, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Y.; Yang, H.; Wei, A.; Zhang, Y.; Mensah, A.; Wei, Q. Scalable functionalized liquid crystal elastomer fiber soft actuators with multi-stimulus responses and photoelectric conversion. Mater. Horiz. 2023, 10, 2587–2598. [Google Scholar] [CrossRef]
- Kashi, P.A.; Bachlechner, C.; Huc-Mathis, D.; Jäger, H.; Shahbazi, M. A 3D porous biofilm-inspired alginate/gellan hydrogel: Investigating printability and rheological properties affected by dual-wavelength UV crosslink 3D printing. Carbohydr. Polym. 2025, 370, 124246. [Google Scholar] [CrossRef]
- Kibrete, F.; Trzepieciński, T.; Gebremedhen, H.S.; Woldemichael, D.E. Artificial intelligence in predicting mechanical properties of composite materials. J. Compos. Sci. 2023, 7, 364. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Zhang, C. Applications of artificial intelligence/machine learning to high-performance composites. Compos. Part B Eng. 2024, 285, 111740. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B.; Dai, J.G.; Kazmi, S.M.S.; Munir, M.J. Evolutionary artificial intelligence approach for performance prediction of bio-composites. Constr. Build. Mater. 2021, 290, 123254. [Google Scholar] [CrossRef]
- Elbadawi, M.; Castro, B.M.; Gavins, F.K.H.; Ong, J.J.; Gaisford, S.; Pérez, G.; Goyanes, A. M3DISEEN: A novel machine learning approach for predicting the 3D printability of medicines. Int. J. Pharm. 2020, 590, 119837. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-photon polymerization for biological applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Van der Linden, P.J.E.M.; Popov, A.M.; Pontoni, D. Accurate and rapid 3D printing of microfluidic devices using wavelength selection on a DLP printer. Lab Chip 2020, 20, 4128–4140. [Google Scholar]
- Montgomery, S.M.; Demoly, F.; Zhou, K.; Qi, H.J. Pixel-level grayscale manipulation to improve accuracy in digital light processing 3D printing. Adv. Funct. Mater. 2023, 33, 2213252. [Google Scholar]
- Peng, X.; Kuang, X.; Roach, D.J.; Wang, Y.; Hamel, C.M.; Lu, C.; Qi, H.J. Integrating digital light processing with direct ink writing for hybrid 3D printing of functional structures and devices. Addit. Manuf. 2021, 40, 101911. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Chen, W.; Yue, X.; Zhao, Z.; Yin, Z. Intelligent path planning algorithm system for printed display manufacturing using graph convolutional neural network and reinforcement learning. J. Manuf. Syst. 2025, 79, 73–85. [Google Scholar] [CrossRef]
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2021, 6, 27–47. [Google Scholar] [CrossRef]
- Yadav, D.; Chhabra, D.; Garg, R.K.; Ahlawat, A.; Phogat, A. Optimization of FDM 3D printing process parameters for multi-material using artificial neural network. Mater. Today Proc. 2020, 21, 1583–1591. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, P.; Mishra, A.; You, K.; Zong, Y.; Lu, W.F.; Sriram, G. 3D Bioprinting and Artificial Intelligence-Assisted Biofabrication of Personalized Oral Soft Tissue Constructs. Adv. Healthc. Mater. 2025, 14, 2402727. [Google Scholar] [CrossRef]
- Bo, R.; Xu, S.; Yang, Y.; Zhang, Y. Mechanically-guided 3D assembly for architected flexible electronics. Chem. Rev. 2023, 123, 11137–11189. [Google Scholar] [CrossRef]
- Xue, Z.; Jin, T.; Xu, S.; Bai, K.; He, Q.; Zhang, F.; Zhang, Y. Assembly of complex 3D structures and electronics on curved surfaces. Sci. Adv. 2022, 8, eabm6922. [Google Scholar] [CrossRef]
- Gao, T.; Jia, S.; Wang, J.; Cai, Y.; Zhang, H.; Jiang, H.; Qu, J.P. Advanced adjustable sensor for multi-signal analysis via construction of co-continuous dual-sensing networks. Compos. Sci. Technol. 2022, 230, 109733. [Google Scholar] [CrossRef]
- Le, T.T.T.; Moh, S. Interference mitigation schemes for wireless body area sensor networks: A comparative survey. Sensors 2015, 15, 13805–13838. [Google Scholar] [CrossRef]
- Zolfagharian, A.; Kaynak, A.; Bodaghi, M.; Kouzani, A.Z.; Gharaie, S.; Nahavandi, S. Control-based 4D printing: Adaptive 4D-printed systems. Appl. Sci. 2020, 10, 3020. [Google Scholar] [CrossRef]
- Kuang, X.; Roach, D.J.; Wu, J.; Hamel, C.M.; Ding, Z.; Wang, T.; Qi, H.J. Advances in 4D printing: Materials and applications. Adv. Funct. Mater. 2019, 29, 1805290. [Google Scholar] [CrossRef]
- Leist, S.K.; Zhou, J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials. Virtual Phys. Prototyp. 2016, 11, 249–262. [Google Scholar] [CrossRef]
- Roppolo, I.; Caprioli, M.; Pirri, C.F.; Magdassi, S. 3D Printing of Self-Healing Materials. Adv. Mater. 2024, 36, 2305537. [Google Scholar] [CrossRef]
- Sun, T.; Feng, B.; Huo, J.; Xiao, Y.; Wang, W.; Peng, J.; Liu, L. Artificial intelligence meets flexible sensors: Emerging smart flexible sensing systems driven by machine learning and artificial synapses. Nano Micro Lett. 2024, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Benfradj, A.; Thaljaoui, A.; Moulahi, T.; Khan, R.U.; Alabdulatif, A.; Lorenz, P. Integration of artificial intelligence (AI) with sensor networks: Trends, challenges, and future directions. J. King Saud Univ. Comput. Inf. Sci. 2024, 36, 101892. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Jia, X.; Wei, H.; Hu, Z.; Li, W.; Wang, L. Recent progress on artificial intelligence-enhanced multimodal sensors integrated devices and systems. J. Semicond. 2025, 46, 011610. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Lee, C. Soft modular glove with multimodal sensing and augmented haptic feedback enabled by materials’ multifunctionalities. ACS Nano 2022, 16, 14097–14110. [Google Scholar] [CrossRef]
- Maibohm, C.; Silvestre, O.F.; Borme, J.; Sinou, M.; Heggarty, K.; Nieder, J.B. Multi-beam two-photon polymerization for fast large area 3D periodic structure fabrication for bioapplications. Sci. Rep. 2020, 10, 8740. [Google Scholar] [CrossRef]
- Lee, S.G.; Yu, K.J.; Won, S.M.; Yoo, J.Y. Advanced approaches to decoupled sensory signal monitoring in human interface systems. Int. J. Extrem. Manuf. 2025, 7, 042003. [Google Scholar] [CrossRef]
- Dabbagh, S.R.; Ozcan, O.; Tasoglu, S. Machine learning-enabled optimization of extrusion-based 3D printing. Methods 2022, 206, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, M.; Goushchi, S.J.; Keshtiban, P.M. Optimization of 3D printing process parameters to minimize surface roughness with hybrid artificial neural network model and particle swarm algorithm. Prog. Addit. Manuf. 2021, 6, 199–215. [Google Scholar] [CrossRef]
- Rojek, I.; Mikołajewski, D.; Dostatni, E.; Macko, M. AI-optimized technological aspects of the material used in 3D printing processes for selected medical applications. Materials 2020, 13, 5437. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Wiener, J.; Roth, M.; Moser, A.; Gmeiner, R.; Edler, M.; Griesser, T. Tough and degradable photopolymers derived from alkyne monomers for 3D printing of biomedical materials. Polym. Chem. 2016, 7, 5169–5180. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.J. The use of renewable feedstock in UV-curable materials–A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Teh, D.; Khan, T.; Corbitt, B.; Ong, C.E. Sustainability strategy and blockchain-enabled life cycle assessment: A focus on materials industry. Environ. Syst. Decis. 2020, 40, 605–622. [Google Scholar] [CrossRef]
- Zhang, A.; Zhong, R.Y.; Farooque, M.; Kang, K.; Venkatesh, V.G. Blockchain-based life cycle assessment: An implementation framework and system architecture. Resour. Conserv. Recycl. 2020, 152, 104512. [Google Scholar] [CrossRef]
- 3D Systems Advances Regenerative Medical Solutions with First-of-Its-Kind Peripheral Nerve Repair. Available online: https://www.globenewswire.com/news-release/2025/06/26/3105841/8852/en/index.html (accessed on 26 June 2025).
| Point-Based | Line-Based | Area-Based | References | |
|---|---|---|---|---|
| Resolution | Nano to micrometer scale | Micrometer to millimeter scale | Micrometer scale | [42] |
| Printing Speed | Slow, point-by-point scanning (e.g., 1.5 mm3 takes ~1 h). | Moderate, suitable for centimeter-scale Structures. | Fast, entire layer cured simultaneously (seconds per layer). | [101,102,103,104] |
| Material Compatibility | Mainly photosensitive resins or hydrogels. | Capable of printing thermoplastics, hydrogels, composite pastes, bio-inks, etc. | Dependent on photopolymerizable materials; limited selection of biocompatible materials. | [105] |
| Biocompatibility | Requires specially developed biocompatible photosensitive ink. | Customizable bio-inks with natural/synthetic polymers, cells, or functional fillers. | Some photopolymerizable materials may have toxicity or produce harmful degradation byproducts. | [106] |
| Cost | Inkjet printing is cheaper. The printing technology involving special light sources is relatively expensive. | Material extrusion is the cheapest. | The printing technology involving special light sources is relatively expensive. | [107] |
| Scalability | Not suitable for large-scale devices. | Weaker interfacial bonding in multi-material printing. | Suitable for batch fabrication. | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, M.; Yin, X.; Luo, Y.; Zhang, B.; Xue, Q. A Point-Line-Area Paradigm: 3D Printing for Next-Generation Health Monitoring Sensors. Sensors 2025, 25, 5777. https://doi.org/10.3390/s25185777
Ming M, Yin X, Luo Y, Zhang B, Xue Q. A Point-Line-Area Paradigm: 3D Printing for Next-Generation Health Monitoring Sensors. Sensors. 2025; 25(18):5777. https://doi.org/10.3390/s25185777
Chicago/Turabian StyleMing, Mei, Xiaohong Yin, Yinchen Luo, Bin Zhang, and Qian Xue. 2025. "A Point-Line-Area Paradigm: 3D Printing for Next-Generation Health Monitoring Sensors" Sensors 25, no. 18: 5777. https://doi.org/10.3390/s25185777
APA StyleMing, M., Yin, X., Luo, Y., Zhang, B., & Xue, Q. (2025). A Point-Line-Area Paradigm: 3D Printing for Next-Generation Health Monitoring Sensors. Sensors, 25(18), 5777. https://doi.org/10.3390/s25185777






