Highlights
What are the main findings?
- Deep learning models applied to lumbar IMU data predict postoperative mobility (CHARMI) and activities of daily living (Barthel Index) in older surgical patients, with R2 values of 0.65 and 0.70, respectively.
- Recommended discharge destinations were predicted with 82% accuracy using lumbar IMU data and deep learning.
What is the implication of the main finding?
- IMU-based assessment and deep learning offer the potential for automated, objective, and continuous monitoring of functional recovery.
Abstract
The growing proportion of older adults in the population necessitates improved methods for assessing functional recovery. Objective, continuous monitoring using wearable sensors offers a promising alternative to traditional, often subjective assessments. This study aimed to investigate the utility of inertial measurement unit (IMU)-based data, combined with deep learning, to predict postoperative mobility, activities of daily living, and discharge destination in older adults following surgery. Data from the SURGE-Ahead project was analyzed, involving 39 patients (mean age 79.05 years) wearing lumbar IMU sensors for up to five postoperative days. Deep learning models (TabPFN) were applied and validated using leave-one-out cross-validation to predict the Charité Mobility Index (CHARMI), the Barthel Index, and discharge destination. The TabPFN model achieved R2 values of 0.65 and 0.70 for predicting CHARMI and Barthel Index scores, respectively, with moderate to strong agreement with human assessments (weighted kappa ≥ 0.80). Discharge destination was predicted with an accuracy of 82%. The z-channel IMU data and parameters related to walking bouts were most predictive of outcomes. IMU-based data, combined with deep learning, demonstrates potential for automated functional assessment and discharge decision support in older adults following surgery.
1. Introduction
The growing proportion of older adults in the population presents significant challenges to healthcare systems worldwide, characterized by a rising incidence of multimorbidity, functional decline, and subsequent healthcare needs [1,2]. Following surgery, particularly in the geriatric population, accurate assessment of recovery trajectories is crucial for optimizing patient management, resource allocation, and ultimately, achieving successful discharge [3,4]. Traditionally, assessing these outcomes relies on clinical observation and periodic manual assessments, which can be subjective, time-consuming, and fail to capture the oscillations of real-world functional performance.
Recent advancements in wearable sensor technology, specifically inertial measurement units (IMUs), offer a promising avenue for objective and continuous monitoring of patient mobility and activity levels [5,6,7]. IMUs provide a plethora of data regarding movement patterns, enabling the quantification of gait parameters, activity engagement, and functional performance with increasing precision [8,9]. Furthermore, the potential to leverage this data for predictive modelling is gaining momentum, with applications ranging from fall risk assessment to potentially predicting rehabilitation outcomes [10,11,12].
The application of machine learning and deep learning techniques to IMU-derived data has demonstrated remarkable success in various healthcare domains. Recent studies have shown the capability to accurately predict gait parameters and assess frailty [11,13]. However, the translation to the specific context of orthogeriatric patients requires careful consideration. In addition, leveraging IMU-derived functional parameters to inform clinical decision-making requires further investigation.
This study aims to investigate the utility of IMU-based data, combined with artificial intelligence (AI), to predict key outcomes following orthogeriatric surgery, including mobility, activities of daily living (ADL), and discharge destination. By leveraging the continuous, objective data provided by IMUs and the predictive power of deep learning, we seek to provide a proof-of-concept that explores the potential for automated clinical decision-making, optimizing rehabilitation strategies, and ultimately improving patient outcomes in this vulnerable population.
2. Materials and Methods
2.1. Study Population
This study constitutes a secondary analysis of data collected during the Supporting Surgery with Geriatric Co-Management and AI Observational and AI Development (SURGE-Ahead) study [14], conducted at three surgical departments of Ulm University Medical Center between February 2023 and March 2024. Included patients were aged 70 years and older, undergoing surgery, and had an Identification of Seniors at Risk (ISAR) score of 2 or higher. Baseline characteristics were assessed using several standardized tools. Frailty and multimorbidity were evaluated using the Clinical Frailty Scale (CFS), American Society of Anesthesiologists (ASA) score, Identification of Seniors at Risk (ISAR) score, and number of medications. Cognitive function was assessed with the Montreal Cognitive Assessment 5-min (MoCA), and dementia status was recorded. Nutritional status was assessed using the Nutritional Risk Screening (NRS) score. Psychological distress was assessed with the Patient Health Questionnaire-4 (PHQ-4). Functional status was measured using the Barthel Index [15], and mobility was further assessed using the Charité Mobility Index (CHARMI; [16]) and the New Mobility Score (NMS). Additional data collected included age, sex, type of surgery, emergency surgery status, body mass index (BMI), care level, and history of falls (for details, see study protocol [14]). Of 178 patients enrolled in the study, 169 completed the study protocol until discharge (n = 3 drop-outs; n = 6 deaths). A subset of 39 participants wore an IMU (Axivity AX6; axivity Ltd., Newcastle upon Tyne, UK) in the lumbar region for at least 24 h, with 22 concurrently completing a movement diary. Sensors were attached to the participants’ lumbar spine using an adhesive patch on postoperative day 1, with recording starting at 00:00 the following day and detached on postoperative day 5 or earlier if the patient was discharged. The X-axis of the sensor was oriented vertically, the Y-axis horizontally, and the Z-axis pointed towards the patient’s abdomen, as illustrated in the Supplementary Figure S2. The sensor operated at a sampling rate of 100 Hz, with a resolution of 16 bits, and a battery lasting approximately 7 days. The Axivity AX6 contains a tri-axial accelerometer and gyroscope; however, in the current study, only the accelerometer data were analyzed. All participants underwent a comprehensive geriatric assessment. On postoperative day 3, assessments included the CHARMI and Barthel Index for mobility and ADL, respectively. In addition, discharge recommendations (back home, acute geriatric care unit, post-acute rehabilitation facility, or nursing home) were independently provided by two geriatricians, considering clinical assessment, patient preferences, and rehabilitation potential, and reconciled through joint review with 3-month follow-up data. For a STROBE chart, see the Supplementary Figure S1.
2.2. IMU Feature Extraction
IMU sensor data was preprocessed using the scikit-digital-health 0.17.2 library [17] for Python 3.12.2 (Python Software Foundation). The DETACH algorithm [18] was used to detect IMU-wearing episodes in each participant, and data was sliced accordingly. For each IMU channel (x, y, z), a comprehensive set of features was extracted across the entirety of the wearing episode, using the features class from the scikit-digital-health library. Signal distribution was characterized by calculating the mean, standard deviation, skewness, kurtosis, range, interquartile range, range count percentage, and the ratio beyond r sigma. Time domain analysis included the mean cross rate, autocorrelation, signal entropy, jerk metric, root mean square value, and power. Frequency domain characteristics were assessed using dominant frequency, power spectral sum, power range sum, spectral flatness, and spectral arc length. Furthermore, we calculated the vector magnitude for the root mean square value, power, and power spectral sum. All basic signal features were extracted using the scikit-digital-health library’s default parameters.
Beyond basic signal features, we utilized the Sit-2-Stand algorithm [19] and the Mobilise-D pipeline [8,9], implemented within the scikit-digital-health 0.17.2 and mobgap 0.10.0 libraries [20] for Python (Python Software Foundation), respectively. The Sit-2-Stand algorithm analyzes transitions from a sitting to a standing position, yielding features including episode count, duration, maximum and minimum acceleration, spectral arc length, and displacement. The Mobilise-D pipeline identifies walking bouts and calculates their duration, cadence, stride duration, and walking speed, along with their average, variance, and maximum values (also see Supplementary Table S2). For more information on the Mobilise-D project, please refer to the project page (https://mobilise-d.eu/, accessed on 28 June 2025) and associated publications [8,9].
2.3. Gait Detection
Gait detection performance of the Mobilise-D pipeline was assessed using movement diaries from 22 participants. Documented ambulation events defined ‘true positive’ walking bouts; instances of pipeline detection outside these events were ‘false positives,’ and missed events were ‘false negatives.’ Precision, recall, and F1 scores (a balanced measure that considers both false positives and false negatives, particularly useful with imbalanced datasets). To determine the relationship between detection performance and patient mobility, F1 scores were correlated with the number of detected walking bouts, CHARMI scores, and Barthel Index scores, by calculating Spearman’s Rho.
2.4. Deep Learning
This study followed the TRIPOD + AI reporting guidelines [21] (see Supplementary Figure S2) to ensure transparency and reproducibility. To predict postoperative outcomes, we employed TabPFN [22], a prior-fitted transformer designed for small tabular datasets, requiring no preprocessing or hyperparameter tuning, and capable of handling missing data. As a generative foundation model for tabular data, it was trained across millions of synthetic datasets, enabling it to learn robust representations and perform well with limited and complex data often encountered in geriatric populations. TabPFN’s prior-fitting approach reduces the risk of overfitting and allows for meaningful analysis even with relatively small sample sizes, a significant advantage in the context of geriatric research where data acquisition can be challenging. This contrasts with traditional deep learning methods that require extensive retraining and large datasets to achieve reliable performance. The model was utilized to predict CHARMI and Barthel Index scores at postoperative day 3 (using regression) and to classify optimal discharge destination (a multiclass task). Prior to model application, a feature selection pipeline utilizing Spearman correlation (for regression) and the Kruskal–Wallis test (for classification) identified significant features (p < 0.05) from the initial set of 94 extracted features. Model performance was evaluated using leave-one-out cross-validation, with R2, mean error, and weighted kappa (an inter-rater metric) for regression, and accuracy and receiver operating characteristic area under the curve (ROC AUC) micro average for classification. Finally, 95% confidence intervals (CI) were estimated via bootstrapping with 1000 iterations.
3. Results
3.1. Study Population
The study cohort comprised 39 patients (mean age 79.05 years ±6.4, 43.6% male) undergoing surgery at Ulm University Medical Center. The majority (74.4%) had undergone trauma surgery. The average BMI was 26.52 kg/m2 (±4.69), and the mean number of medications taken was 8.87 (±3.5), highlighting the significant multimorbidity present in the population. All demographic and clinical characteristics are displayed in Table 1. IMU sensors were worn for an average of 3.10 days (±1.41) following surgery, starting on postoperative day 1. At postoperative day 3, the mean CHARMI score was 4.91 (±2.84) and the mean Barthel Index score 59.46 (±24.32). Discharge recommendations favored home discharge for 22 patients; 16 were recommended for acute geriatric care, one for rehabilitation, and none for a nursing home.

Table 1.
Study Population Characteristics. BMI = body mass index, NRS = nutritional risk screening, MoCA = Montreal Cognitive Assessment, PHQ4 = Patient Health Questionnaire-4, ISAR = identification of seniors at risk, CFS = Clinical Frailty Scale, ASA = American Society of Anesthesiologists score, CHARMI = Charité Mobility Index, NMS = New Mobility Score, pre-OP = preoperative, post-OP = postoperative.
3.2. Gait Detection
The Mobilise-D pipeline [8,9] detected walking bouts with a precision of 0.51, a recall of 0.87, and an F1 score of 0.64 across all events. Notably, the F1 score showed a highly significant correlation with several mobility-related parameters, including the number of walking bouts, CHARMI scores, and Barthel Index scores (p < 0.001; see Figure 1).
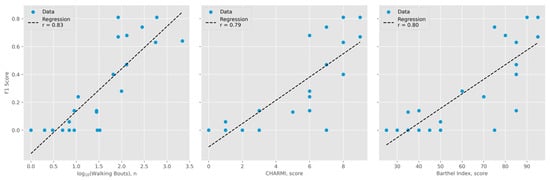
Figure 1.
Gait detection performance compared to activity level. Shared Y-axis represents F1 scores. (Left): number of walking bouts detected by the Mobilise-D algorithm (logarithmic scale, base 10; r = 0.83). (Middle): Charité Mobility Index (CHARMI) scores (r = 0.79). (Right): Barthel Index scores (r = 0.80).
3.3. Deep Learning
The feature selection revealed significant associations between IMU features and both CHARMI and Barthel Index scores, as well as with recommended discharge destinations. The strongest associations were observed in the IMU z-channel, which records acceleration during forward/backward motion and, importantly, gravitational pull when the patient is lying on their back (supine position). Furthermore, parameters related to walking bouts also demonstrated significant associations with all outcomes. Detailed results of the exploratory analysis are available in the Supplementary Table S2.
TabPFN demonstrated accurate prediction of both CHARMI and Barthel Index scores at postoperative day 3. For CHARMI scores, the model achieved an R2 of 0.65 (95% CI: 0.34–0.80), a mean error of 1.32 (95% CI: 1.01–1.66), and a weighted kappa of 0.80 (95% CI: 0.64–0.89). For the Barthel Index, the corresponding values were an R2 of 0.70 (95% CI: 0.53–0.82), a mean error of 10.87 (95% CI: 8.71–13.23), and a weighted kappa of 0.81 (95% CI: 0.69–0.88). A visual comparison of estimated and assessed scores is presented in Figure 2. TabPFN also yielded an accuracy of 0.82 (95% CI: 0.69–0.92) and an ROC AUC micro-average of 0.86 (95% CI: 0.74–0.96) for predicting the recommended discharge destination.
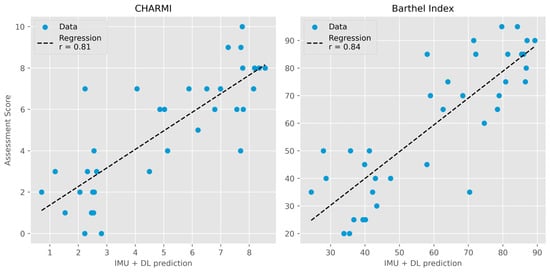
Figure 2.
Deep learning prediction of assessment scores compared to actual scores. (Left): Charité Mobility Index (CHARMI; r = 0.81). (Right): Barthel Index (r = 0.84). IMU = inertial measurement unit. DL = deep learning (TabPFN).
4. Discussion
This study presents a proof-of-concept for automated functional assessments and discharge decision support in older adults following surgery, utilizing IMU sensors and deep learning.
Good predictions of both mobility (CHARMI; R2 = 0.65) and ADL (Barthel Index; R2 = 0.70) were achieved using a single lumbar sensor. Furthermore, an inter-rater analysis demonstrated moderate to strong agreement (weighted kappa ≥ 0.80) between predicted and actual assessment scores [23], highlighting the potential for automated functional assessment in geriatric inpatients post-surgery, although it should be noted that functional assessments provide detailed clinical information beyond a composite score, informing individualized care planning. A single lumbar IMU sensor and deep learning also accurately predicted the recommended discharge destination in 82% of cases, representing a novel application of IMU data analysis. These results are comparable to those achieved by models relying primarily on electronic health record data [24] and are particularly encouraging given the challenges of optimal discharge planning in older adults and the frequent occurrence of undertreatment. Observational data from the SURGE-Ahead project indicated a standard-of-care accuracy of 0.73 compared to geriatric expert recommendations [14], suggesting a potential for improvement through the application of artificial intelligence.
Notably, the Z-channel data from the IMU sensor showed the most significant association with mobility, ADL scores, and the recommended discharge destination. In contrast, analyses of the X- and Y-axis did not reveal comparable associations with discharge destinations (see Supplementary Table S3). When correctly positioned on the lumbar region, the Z-channel exhibits heightened activity during periods of patient recumbency, potentially indicating immobility and a bed-ridden state. Conversely, data derived from the walking bout analysis—reflecting a state of mobility—were similarly predictive in our exploratory analyses (see Supplementary Table S2).
Analysis of movement diaries from 22 patients demonstrated reliable identification of walking bouts using the Mobilise-D pipeline, achieving an F1 score of 0.64. This is lower than the F1 score of 0.77 found by Kirk and colleagues (2024) [9], likely attributable to the higher proportion of immobilized patients within our study cohort: Correlation analyses indicated a reduction in walking bout detection F1 scores among patients exhibiting few detected walking bouts, limited mobility, and impaired self-care capabilities (see Figure 1). We also performed an additional analysis excluding algorithmic walking bout detection, utilizing only raw sensor data (see Supplementary Table S4). Notably, this approach yielded inferior predictive performance (mobility R2 0.52, ADL R2 0.39, discharge destination accuracy 0.79), suggesting that, while imperfect, the walking bout detection algorithm contributes valuable information to the overall model. Analysis of IMU wearing time revealed no significant correlation with discharge destination or outcome scores, suggesting that the algorithm’s predictive performance is not primarily driven by the length of sensor use, but rather by the kinematic data acquired during that period (see Supplementary Table S2).
During the observational and AI development study [14], approximately one-quarter (n = 46) agreed to wear an IMU sensor, others declined participation due to postoperative immobilization and pain that hindered sensor application. During the sensor application process, several participants (n = 7) expressed discomfort with lumbar sensor placement. While all continued their participation in the study, these seven patients opted to wear the IMU sensor on their right thigh instead. Data collected from the thigh-mounted sensors could not be included in this analysis, as the Mobilise-D algorithm is not designed to process data from alternate anatomical locations. Furthermore, the time required for sensor attachment and data retrieval (typically 10–20 min) represents a potential limitation and must be taken into account when evaluating the overall efficiency gains afforded by automated assessment and discharge decision support. Occasional instances of accidental sensor removal by patients, who mistook the device for a wound dressing, also presented a logistical challenge. Consequently, the SURGE-Ahead study group is now exploring the use of wrist-worn sensors in a follow-up study, an approach supported by emerging evidence. Recent research by Kluge and colleagues (2024) supports the validity of using wrist-worn sensors to detect gait sequence in a variety of patient populations [6], including those recovering from fractures. While performance was generally lower than with sensors placed at the lower back, the study demonstrated acceptable sensitivity (0.55–0.81) and specificity (0.95–0.98) compared to a multi-sensor reference system.
The ultimate goal of the research project is to develop a tool to augment clinical decision-making. Defining acceptable performance thresholds requires careful consideration of the prediction’s risk and the level of human oversight. Fully automated assessments, such as ADL evaluation, would necessitate high accuracy, approaching inter-rater reliability between clinicians (weighted kappa ≥ 0.9). However, even a model with moderate predictive power can be valuable if it aids clinician insights or outperforms current standards of care, as demonstrated by our discharge destination predictions. Future research should focus on refining accuracy, integrating explainability methods for human supervision, and validating performance across diverse clinical applications.
Limitations
This study represents a proof-of-concept investigation and should be interpreted considering several limitations. First, the relatively small sample size (n = 39) limits the generalizability of our findings. Consequently, the reported R2 and AUC values should be interpreted with consideration of the wide confidence intervals, which accurately reflect the uncertainty associated with these estimates. Second, the limited availability of IMU data (approximately 20% of the cohort) introduces potential bias and further constrains the statistical power of our analysis. Third, a significant limitation is the substantial class imbalance observed in discharge destinations, with no patients recommended for nursing home care. This imbalance poses challenges for model training and requires cautious interpretation of accuracy scores, as a majority class prediction can yield deceptively high overall accuracy. While calibration techniques can mitigate some effects, accurate prediction of minority class cases remains a concern. Fourth, the reliance on patient-reported events, which are subject to bias and potential inaccuracies, is a potentially limiting factor; the absence of objective, autonomous event verification techniques, such as image-based analysis, may have impacted the accuracy of the results. Fifth, while weighted kappa values demonstrated moderate to strong agreement with human assessments, they were lower than the 0.90–0.96 range typically observed between different human Barthel Index assessors [25]. Sixth, the observational nature of data collection led to variability in sensor wearing time periods, introducing potential bias. This will be addressed in future studies through the implementation of a standardized protocol with fixed sensor wearing durations. Seventh, this analysis was largely exploratory, and while deep learning techniques demonstrated promising predictive capabilities, further research is required to identify the optimal modeling approach. Eighth, some patients may have been bed-ridden, resulting in no walking bout episodes and a corresponding lack of related feature extraction. However, the TabPFN model automatically handles missing data, and the absence of walking bout data itself provides information regarding patient mobility. Finally, explainability of the model’s predictions was not explored and remains an important area for future research. Providing clinicians with a satisfactory understanding of the model’s reasoning as well as possible reasons for inaccurate predictions will be crucial for establishing trust and facilitating clinical adoption. While attribution methods such as SHAP may identify important features [26], these often correspond to low-level sensor signals that lack inherent clinical meaning and are not readily interpretable by clinicians.
5. Conclusions
This study demonstrates the potential of leveraging IMU sensor data and deep learning techniques for objective, automated assessment of functional recovery and discharge planning in older adults following surgery. Our findings support further investigation of IMU-based technologies as a valuable tool to optimize rehabilitation strategies and improve patient outcomes in this vulnerable population, ultimately paving the way for more personalized and efficient geriatric care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/s25165021/s1, Figure S1: STROBE chart; Figure S2: TRIPOD + AI checklist; Table S1: Movement diaries; Table S2: IMU statistics; Table S3: IMU axes stratified; Table S4: Raw IMU performance; Table S5: TRIPOD + AI checklist.
Author Contributions
Conceptualization, T.D.K.; methodology, T.D.K.; software, T.D.K. and J.K.; validation, U.L.R.; formal analysis, T.D.K. and U.L.R.; investigation, T.D.K., S.B. and C.L.; resources, M.D. and H.K.; data curation, T.D.K.; writing—original draft preparation, T.D.K.; writing—review and editing, S.B., U.L.R., C.L., M.D. and D.D.; visualization, T.D.K.; supervision, M.D., H.K. and J.K.; project administration, M.D.; funding acquisition, M.D. All authors have read and agreed to the published version of the manuscript.
Funding
T.D.K.: U.L.R., C.L. and S.B. were or currently are employed in the SURGE-Ahead project, for which funding was granted by the German Federal Ministry of Education and Research (Grant number: 01GY2101). The funding body had no influence on the study design, data collection and analysis, the decision to publish or the preparation of the manuscript.
Institutional Review Board Statement
The observational and AI development study of the SURGE-Ahead project was conducted following ethical guidelines set by the Declaration of Helsinki. It was approved by the University of Ulm’s Ethical Committee, with reference number # 310/22-Sta.
Informed Consent Statement
All participants provided their written informed consent.
Data Availability Statement
Data used in this specific analysis can be accessed on the project’s GitHub page: https://github.com/IfGF-UUlm/SA_Sensor-lumbar/, accessed on 28 June 2025. Raw IMU sensor data is not available on the project’s GitHub page due to file size limitations but is available upon request. Further information, data and results from the SURGE-Ahead observational and AI development study can be accessed on the project’s GitHub page: https://github.com/IfGF-UUlm/SA_OKIE/, accessed on 28 June 2025.
Acknowledgments
The SURGE-Ahead Study Group comprises Karen Andersen-Ranberg, Sophia Andres, Sibylle Beck, Christian Bolenz, Simone Brefka, Raffael Cintean, Karen Clauss, Dhayana Dallmeier, Genia Decker, Miriam Deniz, Michael Denkinger, Vanessa Disque, Anna Lena Flagmeier, Marina Fotteler, Florian Gebhard, Julia Geiger, Janina Hahn, David Hansen, Thomas Hoffmann, Wolfgang Janni, Hans Kestler, Reinhold Kilian, Jochen Klenk, Thomas Derya Kocar, Aleyna Koese, Johann Kraus, Christoph Leinert, Elena Leinert, Fabia Mangold, Judith Mantz, Annabel Müller-Stierlin, Gabi Müller, Nadir Nasir, Graziano Onder, Desmond O’Neill, Marcin Orzechowski, Carlos Pankratz, Nina Parchmann, Nuh Rahbari, Utz Lovis Rieger, Johannes Schobel, Fabienne Schochter, Konrad Schütze, Tobias Skuban-Eiseler, Vytautas Stasiunaitis, Florian Steger, Walter Swoboda, Adriane Uihlein, Sebastian Voigt-Radloff, Martin Wehling, Pascal Wendel, Felix Wezel, Jana Willems, Jessica Wolf, Philip Wolf, Friedemann Zengerling, and Marco Zeptner. Generative AI was used to improve the quality of writing. It assisted with proof-reading and enhancing the proficiency in professional English language. The following model was utilized: gemma3-27b-it published by Google under a permissive license [27]. The model was run locally on an RTX 6000 Ada GPU using 8-bit quantization. We would like to thank google for their contribution to large language models.
Conflicts of Interest
The authors declare no conflicts of interest for this study.
Abbreviations
The following abbreviations are used in this manuscript:
| IMU | Inertial Measurement Unit |
| AI | Artificial Intelligence |
| ADL | Activities of Daily Living |
| SURGE-Ahead | Supporting SURgery with GEriatric co-management and AI |
| ISAR | Identification of seniors at risk |
| CHARMI | Charité Mobility Index |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
| CI | Confidence interval |
| BMI | Body mass index |
| NRS | Nutritional Risk Screening |
| MoCA | Montreal Cognitive Assessment |
| PHQ-4 | Patient Health Questionnaire-4 |
| CFS | Clinical Frailty Scale |
| ASA | American Society of Anesthesiologists score |
| NMS | New Mobility Score |
| pre-OP | Preoperative |
| post-OP | Postoperative |
References
- United Nations Department for Economic and Social Affairs. World Population Prospects 2024: Summary of Results; United Nations: New York, NY, USA, 2025; ISBN 978-92-1-003169-1.
- Karthika, M.; Abraham, J.; Kodali, P.B.; Mathews, E. Emerging Trends of Chronic Diseases and Their Care Among Older Persons Globally. In Handbook of Aging, Health and Public Policy; Springer Nature: Singapore, 2023; pp. 1–24. ISBN 978-981-16-1914-4. [Google Scholar]
- Dhanani, H.; Tabata-Kelly, M.; Jarman, M.; Cooper, Z. A Scoping Review of Hospital-Based Geriatric-Centered Interventions on Trauma Surgery Services. J. Am. Geriatr. Soc. 2025, 73, 1250–1266. [Google Scholar] [CrossRef]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef]
- Chan, L.L.Y.; Choi, T.C.M.; Lord, S.R.; Brodie, M.A. Development and Large-Scale Validation of the Watch Walk Wrist-Worn Digital Gait Biomarkers. Sci. Rep. 2022, 12, 16211. [Google Scholar] [CrossRef] [PubMed]
- Kluge, F.; Brand, Y.E.; Micó-Amigo, M.E.; Bertuletti, S.; D’Ascanio, I.; Gazit, E.; Bonci, T.; Kirk, C.; Küderle, A.; Palmerini, L.; et al. Real-World Gait Detection Using a Wrist-Worn Inertial Sensor: Validation Study. JMIR Form. Res. 2024, 8, e50035. [Google Scholar] [CrossRef]
- MacLean, M.K.; Rehman, R.Z.U.; Kerse, N.; Taylor, L.; Rochester, L.; Del Din, S. Walking Bout Detection for People Living in Long Residential Care: A Computationally Efficient Algorithm for a 3-Axis Accelerometer on the Lower Back. Sensors 2023, 23, 8973. [Google Scholar] [CrossRef]
- Micó-Amigo, M.E.; Bonci, T.; Paraschiv-Ionescu, A.; Ullrich, M.; Kirk, C.; Soltani, A.; Küderle, A.; Gazit, E.; Salis, F.; Alcock, L.; et al. Assessing Real-World Gait with Digital Technology? Validation, Insights and Recommendations from the Mobilise-D Consortium. J. Neuroeng. Rehabil. 2023, 20, 78. [Google Scholar] [CrossRef]
- Kirk, C.; Küderle, A.; Micó-Amigo, M.E.; Bonci, T.; Paraschiv-Ionescu, A.; Ullrich, M.; Soltani, A.; Gazit, E.; Salis, F.; Alcock, L.; et al. Mobilise-D Insights to Estimate Real-World Walking Speed in Multiple Conditions with a Wearable Device. Sci. Rep. 2024, 14, 1754. [Google Scholar] [CrossRef]
- Friedrich, B.; Lau, S.; Elgert, L.; Bauer, J.M.; Hein, A. A Deep Learning Approach for TUG and SPPB Score Prediction of (Pre-) Frail Older Adults on Real-Life IMU Data. Healthcare 2021, 9, 149. [Google Scholar] [CrossRef]
- Arshad, M.Z.; Jung, D.; Park, M.; Shin, H.; Kim, J.; Mun, K.-R. Gait-Based Frailty Assessment Using Image Representation of IMU Signals and Deep CNN. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual Event, 1–5 November 2021; pp. 1874–1879. [Google Scholar]
- Sczuka, K.S.; Schneider, M.; Bourke, A.K.; Mellone, S.; Kerse, N.; Helbostad, J.L.; Becker, C.; Klenk, J. Template-Based Recognition of Human Locomotion in IMU Sensor Data Using Dynamic Time Warping. Sensors 2021, 21, 2601. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Renani, M.; Myers, C.A.; Zandie, R.; Mahoor, M.H.; Davidson, B.S.; Clary, C.W. Deep Learning in Gait Parameter Prediction for OA and TKA Patients Wearing IMU Sensors. Sensors 2020, 20, 5553. [Google Scholar] [CrossRef] [PubMed]
- Leinert, C.; Fotteler, M.; Kocar, T.D.; Dallmeier, D.; Kestler, H.A.; Wolf, D.; Gebhard, F.; Uihlein, A.; Steger, F.; Kilian, R.; et al. Supporting SURgery with GEriatric Co-Management and AI (SURGE-Ahead): A Study Protocol for the Development of a Digital Geriatrician. PLoS ONE 2023, 18, e0287230. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Liebl, M.E.; Elmer, N.; Schroeder, I.; Schwedtke, C.; Baack, A.; Reisshauer, A. Introduction of the Charité Mobility Index (CHARMI)—A Novel Clinical Mobility Assessment for Acute Care Rehabilitation. PLoS ONE 2016, 11, e0169010. [Google Scholar] [CrossRef]
- Adamowicz, L.; Christakis, Y.; Czech, M.D.; Adamusiak, T. SciKit Digital Health: Python Package for Streamlined Wearable Inertial Sensor Data Processing. JMIR mHealth uHealth 2022, 10, e36762. [Google Scholar] [CrossRef] [PubMed]
- Vert, A.; Weber, K.S.; Thai, V.; Turner, E.; Beyer, K.B.; Cornish, B.F.; Godkin, F.E.; Wong, C.; McIlroy, W.E.; Van Ooteghem, K. Detecting Accelerometer Non-Wear Periods Using Change in Acceleration Combined with Rate-of-Change in Temperature. BMC Med. Res. Methodol. 2022, 22, 147. [Google Scholar] [CrossRef]
- Adamowicz, L.; Karahanoglu, F.I.; Cicalo, C.; Zhang, H.; Demanuele, C.; Santamaria, M.; Cai, X.; Patel, S. Assessment of Sit-to-Stand Transfers during Daily Life Using an Accelerometer on the Lower Back. Sensors 2020, 20, 6618. [Google Scholar] [CrossRef]
- Küderle, A.; Tasca, P.; Bicer, M.; Kirk, C.; Megaritis, D.; Hinchliffe, C.; Stihi, A.; Muecke, A.; Babar, Z.; Kluge, F.; et al. MobGap 2025. Available online: https://github.com/mobilise-d/mobgap/ (accessed on 28 June 2025).
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; Van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, e078378. [Google Scholar] [CrossRef]
- Hollmann, N.; Müller, S.; Purucker, L.; Krishnakumar, A.; Körfer, M.; Hoo, S.B.; Schirrmeister, R.T.; Hutter, F. Accurate Predictions on Small Data with a Tabular Foundation Model. Nature 2025, 637, 319–326. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Pahlevani, M.; Taghavi, M.; Vanberkel, P. A Systematic Literature Review of Predicting Patient Discharges Using Statistical Methods and Machine Learning. Health Care Manag. Sci. 2024, 27, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Duffy, L.; Gajree, S.; Langhorne, P.; Stott, D.J.; Quinn, T.J. Reliability (Inter-Rater Agreement) of the Barthel Index for Assessment of Stroke Survivors: Systematic Review and Meta-Analysis. Stroke 2013, 44, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. arXiv 2017, arXiv:1705.07874. [Google Scholar] [CrossRef]
- Kamath, A.; Pathak, S.; Vieillard, N.; Merhej, R.; Perrin, S.; Matejovicova, T.; Ramé, A.; Rivière, M.; Rouillard, L.; Mesnard, T.; et al. Gemma 3 Technical Report 2025. arXiv 2025, arXiv:2503.19786. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).