Continuous Movement Monitoring at Home Through Wearable Devices: A Systematic Review
Abstract
Highlights
- Wearable sensors—especially IMUs, accelerometers, and gyroscopes—are widely used for continuous home-based motor monitoring, particularly in neurological conditions like Parkinson’s disease.
- Most studies report high feasibility and patient compliance (≥70%), but only 5.6% were randomized trials, limiting the strength of clinical recommendations.
- Wearable devices are reliable tools for the real-world assessment of motor symptoms, potentially complementing traditional in-clinic evaluations.
- Broader clinical adoption will require overcoming challenges such as clinician awareness, standardization, data privacy, and equitable access to technology.
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Protocol
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Appraisal
3. Results
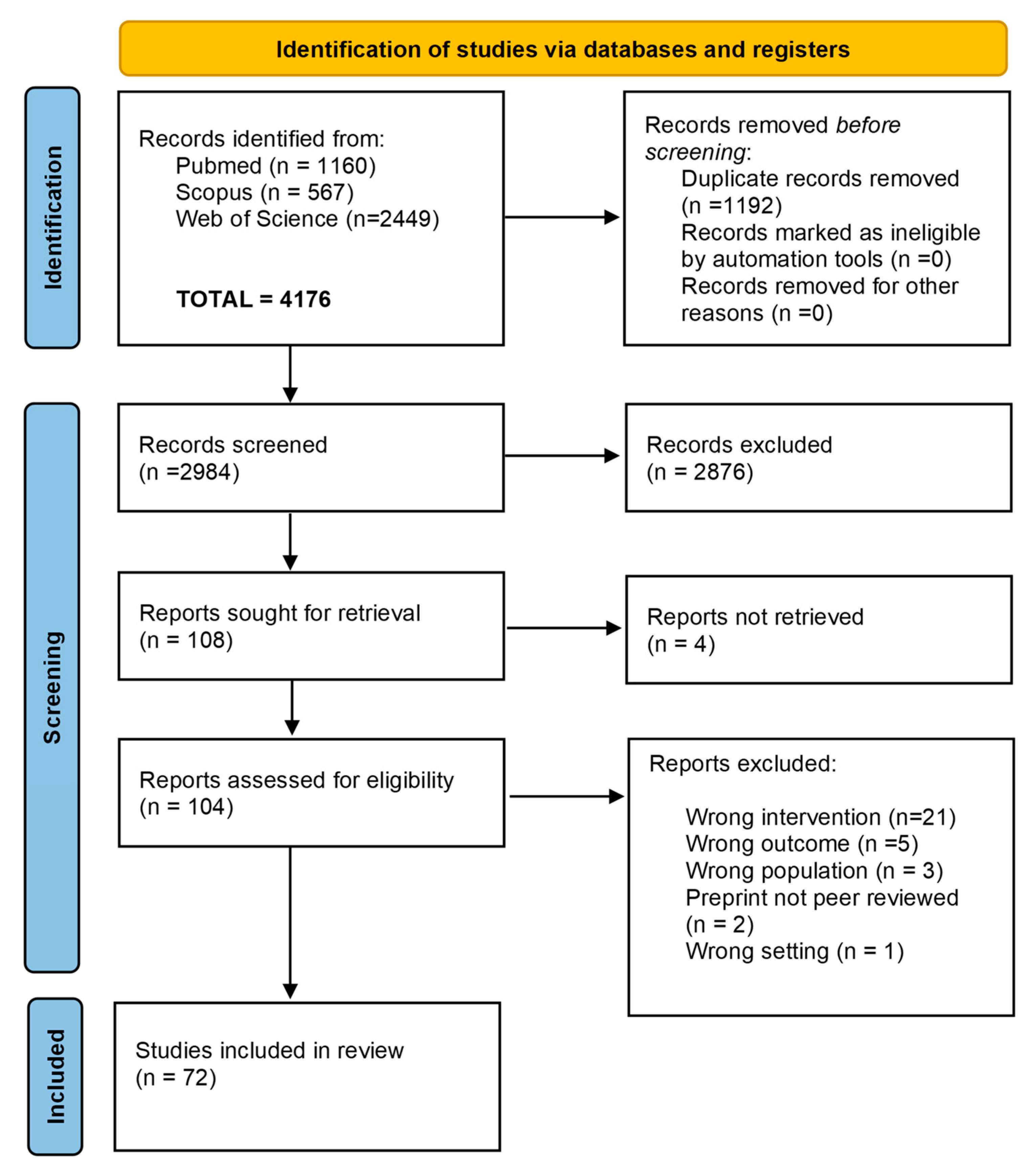
3.1. Study Selection and Quality Appraisal
3.2. Geographic Distribution
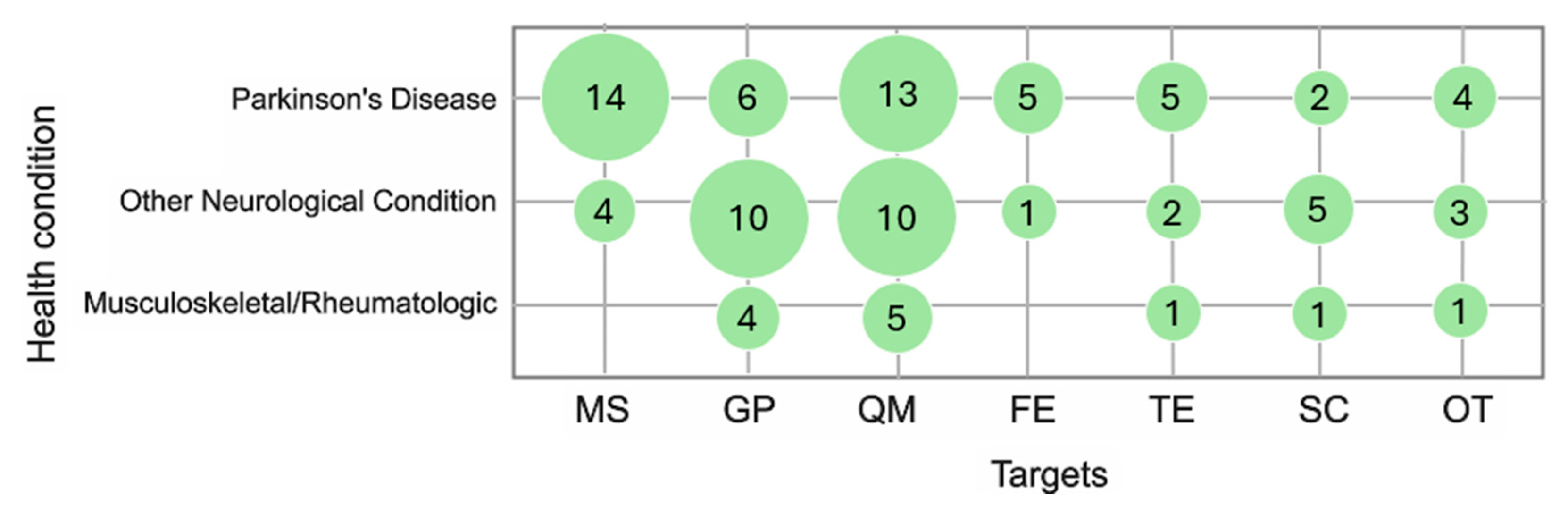
3.3. Health Conditions
3.4. Sensor Types
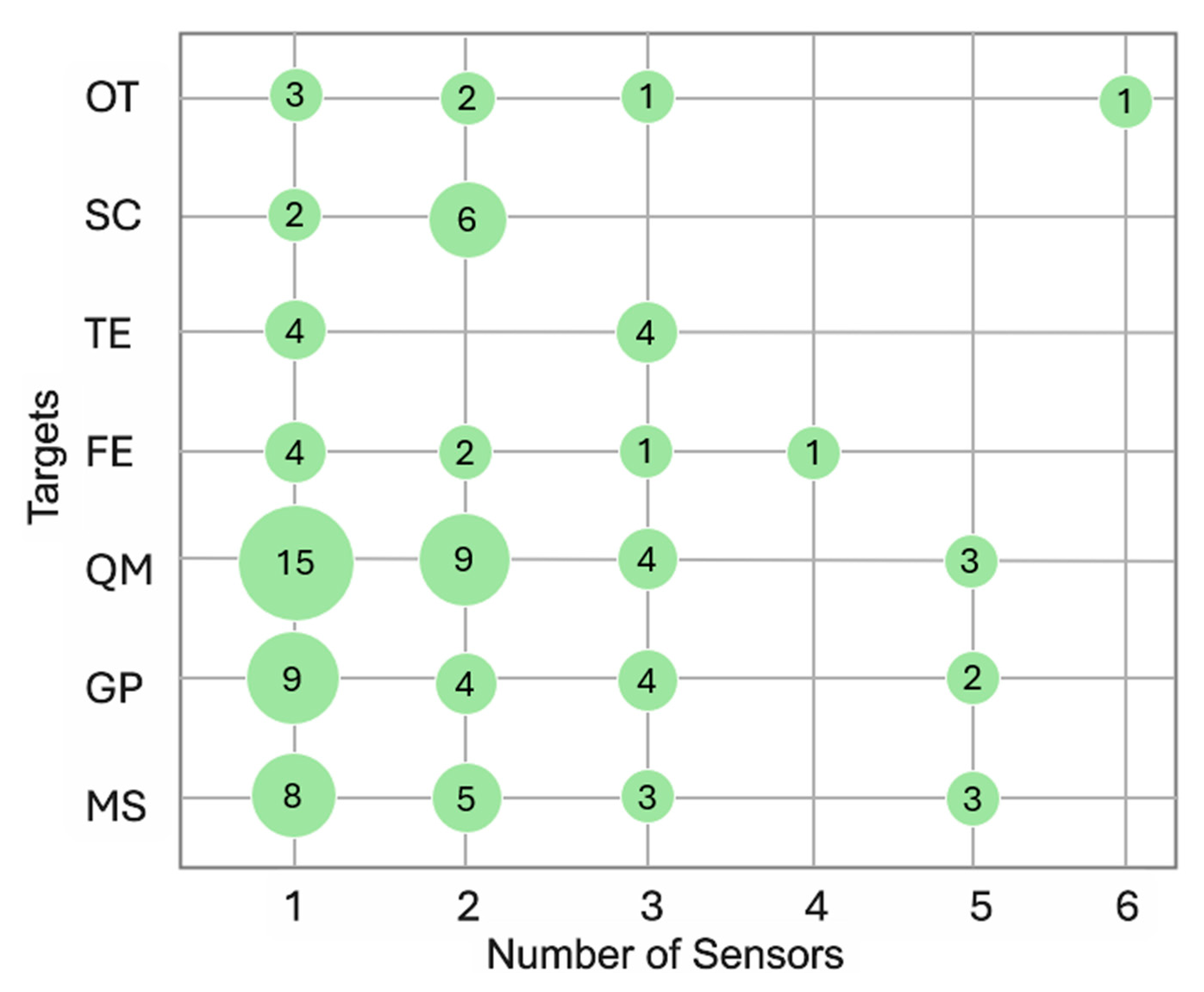
3.5. Monitoring: Timelines and Targets
3.6. Monitoring System Feasibility
4. Discussion
Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NCDs | Non-Communicable Diseases |
| WHO | World Health Organization |
| ECG | Electrocardiogram |
| IMUs | Inertial Measurement Units |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| OCEBM | Oxford Centre for Evidence-Based Medicine |
| STARD | Standards for Reporting Diagnostic Accuracy Studies |
| ICC | Intraclass Correlation Coefficient |
| OT | Other |
| SC | Self-reported Scales/Indexes |
| MSs | Motor Symptoms |
| GPs | Gait Parameters |
| TEs | Turning Events |
| FE | Fall Event/Risk |
| QM | Quantity of Movement |
| PD | Parkinson’s Disease |
| WEIRD | Western, Educated, Industrialized, Rich, and Democratic |
References
- The Lancet. Non-communicable diseases: What now? Lancet 2022, 399, 1201. [Google Scholar] [CrossRef] [PubMed]
- WHO [Online]. Non Communicable Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 27 June 2025).
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef] [PubMed]
- Barr, V.J.; Robinson, S.; Marin-Link, B.; Underhill, L.; Dotts, A.; Ravensdale, D.; Salivaras, S. The expanded Chronic Care Model: An integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp. Q. 2003, 7, 73–82. [Google Scholar] [CrossRef]
- Liu, L.; Stroulia, E.; Nikolaidis, I.; Miguel-Cruz, A.; Rios Rincon, A. Smart homes and home health monitoring technologies for older adults: A systematic review. Int. J. Med. Inform. 2016, 91, 44–59. [Google Scholar] [CrossRef]
- Capecci, M.; Ceravolo, M.G.; Ferracuti, F.; Grugnetti, M.; Iarlori, S.; Longhi, S.; Romeo, L.; Verdini, F. An instrumental approach for monitoring physical exercises in a visual markerless scenario: A proof of concept. J. Biomech. 2018, 69, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, L.S.; Aminorroaya, A.; Oikonomou, E.K.; Nargesi, A.A.; Wilson, F.P.; Krumholz, H.M.; Khera, R. Use of Wearable Devices in Individuals with or at Risk for Cardiovascular Disease in the US, 2019 to 2020. JAMA Netw. Open 2023, 6, e2316634. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Faranesh, A.Z.; Selvaggi, C.; Atlas, S.J.; McManus, D.D.; Singer, D.E.; Pagoto, S.; McConnell, M.V.; Pantelopoulos, A.; Foulkes, A.S. Detection of Atrial Fibrillation in a Large Population Using Wearable Devices: The Fitbit Heart Study. Circulation 2022, 146, 1415–1424. [Google Scholar] [CrossRef]
- Dagher, L.; Shi, H.; Zhao, Y.; Marrouche, N.F. Wearables in cardiology: Here to stay. Heart Rhythm. 2020, 17, 889–895. [Google Scholar] [CrossRef]
- Kamga, P.; Mostafa, R.; Zafar, S. The Use of Wearable ECG Devices in the Clinical Setting: A Review. Curr. Emerg. Hosp. Med. Rep. 2022, 10, 67–72. [Google Scholar] [CrossRef]
- Cappon, G.; Vettoretti, M.; Sparacino, G.; Facchinetti, A. Continuous Glucose Monitoring Sensors for Diabetes Management: A Review of Technologies and Applications. Diabetes Metab. J. 2019, 43, 383–397. [Google Scholar] [CrossRef]
- Wu, R.; Liaqat, D.; de Lara, E.; Son, T.; Rudzicz, F.; Alshaer, H.; Abed-Esfahani, P.; Gershon, A.S. Feasibility of Using a Smartwatch to Intensively Monitor Patients with Chronic Obstructive Pulmonary Disease: Prospective Cohort Study. JMIR Mhealth Uhealth 2018, 6, e10046. [Google Scholar] [CrossRef]
- Colantonio, S.; Govoni, L.; Dellacà, R.L.; Martinelli, M.; Salvetti, O.; Vitacca, M. Decision Making Concepts for the Remote, Personalized Evaluation of COPD Patients’ Health Status. Methods Inf. Med. 2015, 54, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; Stieglitz, E.J. Wearables for Neurologic Conditions: Considerations for Our Patients and Research Limitations. Neurol. Clin. Pract. 2021, 11, e537–e543. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, S.N.; Behrens, A.; Berner, J.; Sanmartin Berglund, J.; Anderberg, P. Objective sleep monitoring at home in older adults: A scoping review. J. Sleep Res. 2025, 34, e14436. [Google Scholar] [CrossRef] [PubMed]
- Ancona, S.; Faraci, F.D.; Khatab, E.; Fiorillo, L.; Gnarra, O.; Nef, T.; Bassetti, C.L.A.; Bargiotas, P. Wearables in the home-based assessment of abnormal movements in Parkinson’s disease: A systematic review of the literature. J. Neurol. 2021, 269, 100–110. [Google Scholar] [CrossRef]
- World Health Organization [Online]. International Classification of Functioning, Disability and Health: ICF. 2001. Available online: https://iris.who.int/handle/10665/42407 (accessed on 27 June 2025).
- Jayakody, O.; Breslin, M.; Srikanth, V.; Callisaya, M. Gait Characteristics and Cognitive Decline: A Longitudinal Population-Based Study. J. Alzheimer’s Disease. 2019, 71, S5–S14. [Google Scholar] [CrossRef]
- Patel, M.; Pavic, A.; Goodwin, V.A. Wearable inertial sensors to measure gait and posture characteristic differences in older adult fallers and non-fallers: A scoping review. Gait Posture 2019, 76, 110–121. [Google Scholar] [CrossRef]
- World Health Organization [Online]. WHO Guidelines on Physical Activity and Sedentary Behaviour. 2020. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 27 June 2025).
- Picerno, P.; Iosa, M.; D’Souza, C.; Benedetti, M.G.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis: A five-year update. Expert Rev. Med. Devices. 2021, 18 (Suppl. S1), 79–94. [Google Scholar] [CrossRef]
- Iosa, M.; Picerno, P.; Paolucci, S.; Morone, G. Wearable inertial sensors for human movement analysis. Expert. Rev. Med. Devices 2016, 13, 641–659. [Google Scholar] [CrossRef]
- Lee, S.; Chu, Y.; Ryu, J.; Park, Y.J.; Yang, S.; Koh, S.B. Artificial Intelligence for Detection of Cardiovascular-Related Diseases from Wearable Devices: A Systematic Review and Meta-Analysis. Yonsei Med. J. 2022, 63, S93–S106. [Google Scholar] [CrossRef]
- Nahavandi, D.; Alizadehsani, R.; Khosravi, A.; Acharya, U.R. Application of artificial intelligence in wearable devices: Opportunities and challenges. Comput. Methods Programs Biomed. 2022, 213, 106541. [Google Scholar] [CrossRef]
- Warmerdam, E.; Hausdorff, J.M.; Atrsaei, A.; Zhou, Y.; Mirelman, A.; Aminian, K.; Espay, A.J.; Hansen, C.; Evers, L.J.W.; Keller, A.; et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020, 19, 462–470. [Google Scholar] [CrossRef]
- Burton, E.; Hill, K.D.; Lautenschlager, N.T.; Thøgersen-Ntoumani, C.; Lewin, G.; Boyle, E.; Howie, E. Reliability and validity of two fitness tracker devices in the laboratory and home environment for older community-dwelling people. BMC Geriatr. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Fay-Karmon, T.; Galor, N.; Heimler, B.; Zilka, A.; Bartsch, R.P.; Plotnik, M.; Hassin-Baer, S. Home-based monitoring of persons with advanced Parkinson’s disease using smartwatch-smartphone technology. Sci. Rep. 2024, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Silva de Lima, A.L.; Smits, T.; Darweesh, S.K.L.; Valenti, G.; Milosevic, M.; Pijl, M.; Baldus, H.; de Vries, N.M.; Meinders, M.J.; Bloem, B.R. Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov. Disord. 2020, 35, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.G.; Paolucci, T.; Saggino, A.; Pezzi, L.; Bramanti, A.; Cimino, V.; Tommasi, M.; Saggini, R. The WeReha Project for an Innovative Home-Based Exercise Training in Chronic Stroke Patients: A Clinical Study. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520979866. [Google Scholar] [CrossRef]
- Ivorra, A.; Daniels, C.; Rubinsky, B. Minimally obtrusive wearable device for continuous interactive cognitive and neurological assessment. Physiol. Meas. 2008, 29, 543–554. [Google Scholar] [CrossRef]
- Muurling, M.; de Boer, C.; Hinds, C.; Atreya, A.; Doherty, A.; Alepopoulos, V.; Curcic, J.; Brem, A.-K.; Conde, P.; Kuruppu, S.; et al. Feasibility and usability of remote monitoring in Alzheimer’s disease. Digit. Health 2024, 10, 20552076241238133. [Google Scholar] [CrossRef]
- Capecci, M.; Gandolfi, M.; Straudi, S.; Calabrò, R.S.; Baldini, N.; Pepa, L.; Andrenelli, E.; Smania, N.; Ceravolo, M.G.; Morone, G.; et al. Shaping the future: An Italian survey unveils the unmet need to empower physical medicine and rehabilitation professionals with technological skills. Eur. J. Phys. Rehabil. Med. 2024, 60, 540–543. [Google Scholar] [CrossRef]
- Mertens, J.C.; Brzostowski, J.T.; Vamos, A.; Allyn, K.J.; Hafner, B.J.; Friedly, J.L.; DeGrasse, N.S.; Ballesteros, D.; Krout, A.; Larsen, B.G.; et al. A novel portable sensor to monitor bodily positions and activities in transtibial prosthesis users. Clin. Biomech. 2022, 99, 105741. [Google Scholar] [CrossRef]
- Ravichandran, V.; Sadhu, S.; Convey, D.; Guerrier, S.; Chomal, S.; Dupre, A.-M.; Akbar, U.; Solanki, D.; Mankodiya, K. iTex Gloves: Design and In-Home Evaluation of an E-Textile Glove System for Tele-Assessment of Parkinson’s Disease. Sensors 2023, 23, 2877. [Google Scholar] [CrossRef]
- OCEBM (Oxford Centre for Evidence-Based Medicine) Levels of Evidence Working Group [Online]. The Oxford Levels of Evidence 2. 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 27 June 2025).
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Burq, M.; Rainaldi, E.; Ho, K.C.; Chen, C.; Bloem, B.R.; Evers, L.J.W.; Helmich, R.C.; Myers, L.; Marks, W.J.; Kapur, R. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. NPJ Digit. Med. 2022, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Silva de Lima, A.L.; Hahn, T.; Evers, L.J.W.; de Vries, N.M.; Cohen, E.; Afek, M.; Bataille, L.; Daeschler, M.; Claes, K.; Boroojerdi, B.; et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS ONE 2017, 12, e0189161. [Google Scholar] [CrossRef] [PubMed]
- Silva de Lima, A.L.; Evers, L.J.W.; Hahn, T.; de Vries, N.M.; Daeschler, M.; Boroojerdi, B.; Terricabras, D.; Little, M.A.; Bloem, B.R.; Faber, M.J. Impact of motor fluctuations on real-life gait in Parkinson’s patients. Gait Posture 2018, 62, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Weiss, A.; Herman, T.; Hausdorff, J.M. Turn Around Freezing: Community-Living Turning Behavior in People with Parkinson’s Disease. Front. Neurol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Adams, J.L.; Kangarloo, T.; Tracey, B.; O’Donnell, P.; Volfson, D.; Latzman, R.D.; Zach, N.; Alexander, R.; Bergethon, P.; Cosman, J.; et al. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. NPJ Park. Dis. 2023, 9, 64. [Google Scholar] [CrossRef]
- Lipsmeier, F.; Simillion, C.; Bamdadian, A.; Tortelli, R.; Byrne, L.M.; Zhang, Y.-P.; Wolf, D.; Smith, A.V.; Czech, C.; Gossens, C.; et al. A Remote Digital Monitoring Platform to Assess Cognitive and Motor Symptoms in Huntington Disease: Cross-sectional Validation Study. J. Med. Internet Res. 2022, 24, e32997. [Google Scholar] [CrossRef]
- Block, V.J.; Lizée, A.; Crabtree-Hartman, E.; Bevan, C.J.; Graves, J.S.; Bove, R.; Green, A.J.; Nourbakhsh, B.; Tremblay, M.; Gourraud, P.-A.; et al. Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J. Neurol. 2017, 264, 316–326. [Google Scholar] [CrossRef]
- Pepa, L.; Capecci, M.; Andrenelli, E.; Ciabattoni, L.; Spalazzi, L.; Ceravolo, M.G. A Fuzzy Logic System for the Home Assessment of Freezing of Gait in Subjects with Parkinson’s Disease. Expert. Syst. Appl. 2020, 139, 112832. [Google Scholar] [CrossRef]
- Daneault, J.-F.; Vergara-Diaz, G.; Parisi, F.; Admati, C.; Alfonso, C.; Bertoli, M.; Bonizzoni, E.; Carvalho, G.F.; Costante, G.; Fabara, E.E.; et al. Accelerometer data collected with a minimum set of wearable sensors from subjects with Parkinson’s disease. Sci. Data 2021, 8, 48. [Google Scholar] [CrossRef]
- Iluz, T.; Gazit, E.; Herman, T.; Sprecher, E.; Brozgol, M.; Giladi, N.; Mirelman, A.; Hausdorff, J.M. Automated detection of missteps during community ambulation in patients with Parkinson’s disease: A new approach for quantifying fall risk in the community setting. J. Neuroeng. Rehabil. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Mucke, A.; Kuderle, A.; Roth, N.; Gladow, T.; Gabner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M.; Kluge, F. Detection of Unsupervised Standardized Gait Tests from Real-World Inertial Sensor Data in Parkinson’s Disease. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Diaz, G.; Daneault, J.-F.; Parisi, F.; Admati, C.; Alfonso, C.; Bertoli, M.; Bonizzoni, E.; Carvalho, G.F.; Costante, G.; Fabara, E.E.; et al. Limb and trunk accelerometer data collected with wearable sensors from subjects with Parkinson’s disease. Sci. Data 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, M.; Habets, J.G.V.; Herff, C.; Aarts, J.; Stevens, A.; Kuijf, M.L.; Kubben, P.L. Monitoring Parkinson’s disease symptoms during daily life: A feasibility study. NPJ Park. Dis. 2019, 5, 21. [Google Scholar] [CrossRef]
- Caballol, N.; Bay’es, A.; Prats, A.; Mart’ın-Baranera, M.; Quispe, P. Feasibility of a wearable inertial sensor to assess motor complications and treatment in Parkinson’s disease. PLoS ONE 2023, 18, e0279910. [Google Scholar] [CrossRef]
- Mancini, M.; Shah, V.V.; Stuart, S.; Curtze, C.; Horak, F.B.; Safarpour, D.; Nutt, J.G. Measuring freezing of gait during daily-life: An open-source, wearable sensors approach. J. Neuroeng. Rehabil. 2021, 18, 1. [Google Scholar] [CrossRef]
- Ullrich, M.; Roth, N.; Kuderle, A.; Richer, R.; Gladow, T.; Gasner, H.; Marxreiter, F.; Klucken, J.; Eskofier, B.M.; Kluge, F. Fall Risk Prediction in Parkinson’s Disease Using Real-World Inertial Sensor Gait Data. IEEE J. Biomed. Health Inform. 2023, 27, 319–328. [Google Scholar] [CrossRef]
- Bayés, À.; Samá, A.; Prats, A.; Pérez-López, C.; Crespo-Maraver, M.; Moreno, J.M.; Alcaine, S.; Rodriguez-Molinero, A.; Mestre, B.; Quispe, P.; et al. A “HOLTER” for Parkinson’s disease: Validation of the ability to detect on-off states using the REMPARK system. Gait Posture 2018, 59, 1–6. [Google Scholar] [CrossRef]
- Mazilu, S.; Blanke, U.; Dorfman, M.; Gazit, E.; Mirelman, A.; Hausdorff, J.; Troester, G. A Wearable Assistant for Gait Training for Parkinson’s Disease with Freezing of Gait in Out-of-the-Lab Environments. ACM Trans. Interact. Intell. Syst. 2015, 5, 1–31. [Google Scholar] [CrossRef]
- Stack, E.; King, R.; Janko, B.; Burnett, M.; Hammersley, N.; Agarwal, V.; Hannuna, S.; Burrows, A.; Ashburn, A. Could In-Home Sensors Surpass Human Observation of People with Parkinson’s at High Risk of Falling? An Ethnographic Study. BioMed Res. Int. 2016, 2016, 3703745. [Google Scholar] [CrossRef]
- Denk, D.; Herman, T.; Zoetewei, D.; Ginis, P.; Brozgol, M.; Cornejo Thumm, P.; Decaluwe, E.; Ganz, N.; Palmerini, L.; Giladi, N.; et al. Daily-Living Freezing of Gait as Quantified Using Wearables in People with Parkinson Disease: Comparison with Self-Report and Provocation Tests. Phys. Ther. 2022, 102, pzac129. [Google Scholar] [CrossRef] [PubMed]
- San-Segundo, R.; Zhang, A.; Cebulla, A.; Panev, S.; Tabor, G.; Stebbins, K.; Massa, R.E.; Whitford, A.; de la Torre, F.; Hodgins, J. Parkinson’s Disease Tremor Detection in the Wild Using Wearable Accelerometers. Sensors 2020, 20, 5817. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, D.; Dale, M.L.; Shah, V.V.; Talman, L.; Carlson-Kuhta, P.; Horak, F.B.; Mancini, M. Surrogates for Rigidity and PIGD MDS-UPDRS Subscores Using Wearable Sensors. Gait Posture 2022, 91, 186–191. [Google Scholar] [CrossRef]
- Chomiak, T.; Watts, A.; Meyer, N.; Pereira, F.V.; Hu, B. A Training Approach to Improve Stepping Automaticity While Dual-Tasking in Parkinson’s Disease: A Prospective Pilot Study. Medicine 2017, 96, e5934. [Google Scholar] [CrossRef] [PubMed]
- Heldman, D.A.; Harris, D.A.; Felong, T.; Andrzejewski, K.L.; Dorsey, E.R.; Giuffrida, J.P.; Goldberg, B.; Burack, M.A. Telehealth Management of Parkinson’s Disease Using Wearable Sensors: An Exploratory Study. Digit. Biomark. 2017, 1, 43–51. [Google Scholar] [CrossRef]
- Morgan, C.; Tonkin, E.L.; Craddock, I.; Whone, A.L. Acceptability of an In-home Multimodal Sensor Platform for Parkinson Disease: Nonrandomized Qualitative Study. JMIR Hum. Factors 2022, 9, e36370. [Google Scholar] [CrossRef]
- Rissanen, S.M.; Koivu, M.; Hartikainen, P.; Pekkonen, E. Ambulatory surface electromyography with accelerometry for evaluating daily motor fluctuations in Parkinson’s disease. Clin. Neurophysiol. 2021, 132, 469–479. [Google Scholar] [CrossRef]
- Mancini, M.; El-Gohary, M.; Pearson, S.; McNames, J.; Schlueter, H.; Nutt, J.G.; King, L.A.; Horak, F.B. Continuous Monitoring of Turning in Parkinson’s Disease: Rehabilitation Potential. NeuroRehabilitation 2015, 37, 3–10. [Google Scholar] [CrossRef]
- Atrsaei, A.; Hansen, C.; Elshehabi, M.; Solbrig, S.; Berg, D.; Liepelt-Scarfone, I.; Maetzler, W.; Aminian, K. Effect of Fear of Falling on Mobility Measured During Lab and Daily Activity Assessments in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 722830. [Google Scholar] [CrossRef]
- Haertner, L.; Elshehabi, M.; Zaunbrecher, L.; Pham, M.H.; Maetzler, C.; van Uem, J.M.T.; Hobert, M.A.; Hucker, S.; Nussbaum, S.; Berg, D.; et al. Effect of Fear of Falling on Turning Performance in Parkinson’s Disease in the Lab and at Home. Front. Aging Neurosci. 2018, 10, 78. [Google Scholar] [CrossRef]
- Shah, V.V.; McNames, J.; Carlson-Kuhta, P.; Nutt, J.G.; El-Gohary, M.; Sowalsky, K.; Mancini, M.; Horak, F.B. Effect of Levodopa and Environmental Setting on Gait and Turning Digital Markers Related to Falls in People with Parkinson’s Disease. Mov. Disord. Clin. Pract. 2022, 10, 223–230. [Google Scholar] [CrossRef]
- Cerff, B.; Maetzler, W.; Sulzer, P.; Kampmeyer, M.; Prinzen, J.; Hobert, M.A.; Blum, D.; van Lummel, R.; Del Din, S.; Gräber, S.; et al. Home-Based Physical Behavior in Late Stage Parkinson Disease Dementia: Differences between Cognitive Subtypes. Neurodegener. Dis. 2017, 17, 135–144. [Google Scholar] [CrossRef] [PubMed]
- van Wamelen, D.J.; Urso, D.; Ray Chaudhuri, K. How Time Rules: Diurnal Motor Patterns in de novo Parkinson’s Disease. J. Park. Dis. 2021, 11, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Marano, M.; Motolese, F.; Rossi, M.; Magliozzi, A.; Yekutieli, Z.; Di Lazzaro, V. Remote smartphone gait monitoring and fall prediction in Parkinson’s disease during the COVID-19 lockdown. Neurol. Sci. 2021, 42, 3089–3092. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.L.; Dinesh, K.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Sheth, N.; Aranyosi, A.J.; Zhu, W.; Goldenthal, S.; Biglan, K.M.; et al. Multiple Wearable Sensors in Parkinson and Huntington Disease Individuals: A Pilot Study in Clinic and at Home. Digit. Biomark. 2017, 1, 52–63. [Google Scholar] [CrossRef]
- Katzan, I.; Schuster, A.; Kinzy, T. Physical Activity Monitoring Using a Fitbit Device in Ischemic Stroke Patients: Prospective Cohort Feasibility Study. JMIR Mhealth Uhealth 2021, 9, e14494. [Google Scholar] [CrossRef]
- David, A.; ReethaJanetSureka, S.; Gayathri, S.; Annamalai, S.J.; Samuelkamleshkumar, S.; Kuruvilla, A.; Magimairaj, H.P.; Varadhan, S.; Balasubramanian, S. Quantification of the relative arm use in patients with hemiparesis using inertial measurement units. J. Rehabil. Assist. Technol. Eng. 2021, 8, 205566832110317. [Google Scholar] [CrossRef]
- Fang, Q.; Mahmoud, S.S.; Kumar, A.; Gu, X.; Fu, J. A Longitudinal Investigation of the Efficacy of Supported In-Home Post-Stroke Rehabilitation. IEEE Access 2020, 8, 138690–138700. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J. Predicting daily use of the affected upper extremity 1 year after stroke. J. Stroke Cerebrovasc. Dis. 2015, 24, 274–283. [Google Scholar] [CrossRef]
- Ezeugwu, V.E.; Manns, P.J. The Feasibility and Longitudinal Effects of a Home-Based Sedentary Behavior Change Intervention After Stroke. Arch. Phys. Med. Rehabil. 2018, 99, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.F.M.; Gonzalez, P.C.; Fong, K.N.K. Usability of a wearable device for home-based upper limb telerehabilitation in persons with stroke: A mixed-methods study. Digit. Health 2023, 9, 20552076231153737. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.M.; Tulipani, L.J.; Gurchiek, R.D.; Allen, D.A.; Solomon, A.J.; Cheney, N.; McGinnis, R.S. Open-source dataset reveals relationship between walking bout duration and fall risk classification performance in persons with multiple sclerosis. PLOS Digit. Health 2022, 1, e0000120. [Google Scholar] [CrossRef] [PubMed]
- Supratak, A.; Datta, G.; Gafson, A.R.; Nicholas, R.; Guo, Y.; Matthews, P.M. Remote Monitoring in the Home Validates Clinical Gait Measures for Multiple Sclerosis. Front. Neurol. 2018, 9, 561. [Google Scholar] [CrossRef]
- Arpan, I.; Shah, V.V.; McNames, J.; Harker, G.; Carlson-Kuhta, P.; Spain, R.; El-Gohary, M.; Mancini, M.; Horak, F.B. Fall Prediction Based on Instrumented Measures of Gait and Turning in Daily Life in People with Multiple Sclerosis. Sensors 2022, 22, 5940. [Google Scholar] [CrossRef]
- Gordon, M.F.; Grachev, I.D.; Mazeh, I.; Dolan, Y.; Reilmann, R.; Loupe, P.S.; Fine, S.; Navon-Perry, L.; Gross, N.; Papapetropoulos, S.; et al. Quantification of Motor Function in Huntington Disease Patients Using Wearable Sensor Devices. Digit. Biomark. 2019, 3, 103–115. [Google Scholar] [CrossRef]
- Dinesh, K.; Snyder, C.W.; Xiong, M.; Tarolli, C.G.; Sharma, S.; Dorsey, E.R.; Sharma, G.; Adams, J.L. A Longitudinal Wearable Sensor Study in Huntington’s Disease. J. Huntington’s Dis. 2020, 9, 69–81. [Google Scholar] [CrossRef]
- Andrzejewski, K.L.; Dowling, A.V.; Stamler, D.; Felong, T.J.; Harris, D.A.; Wong, C.; Cai, H.; Reilmann, R.; Little, M.A.; Gwin, J.T.; et al. Wearable Sensors in Huntington Disease: A Pilot Study. J. Huntington’s Dis. 2016, 5, 199–206. [Google Scholar] [CrossRef]
- Handelzalts, S.; Alexander, N.B.; Mastruserio, N.; Nyquist, L.V.; Strasburg, D.M.; Ojed, L.V. Detection of Real-World Trips in At-Fall Risk Community Dwelling Older Adults Using Wearable Sensors. Front. Med. 2020, 7, 514. [Google Scholar] [CrossRef]
- Srisuwan, B.; Klute, G.K. Locomotor Activities of Individuals with Lower-Limb Amputation. Prosthet. Orthot. Int. 2021, 45, 191–197. [Google Scholar] [CrossRef]
- Albert, M.V.; Deeny, S.; McCarthy, C.; Valentin, J.; Jayaraman, A. Monitoring Daily Function in Persons with Transfemoral Amputations Using a Commercial Activity Monitor: A Feasibility Study. PMR 2014, 6, 1120–1127. [Google Scholar] [CrossRef]
- Hordacre, B.; Barr, C.; Crotty, M. Community activity and participation are reduced in transtibial amputee fallers: A wearable technology study. BMJ Innov. 2015, 1, 10–16. [Google Scholar] [CrossRef]
- Sherman, K.; Roberts, A.; Murray, K.; Deans, S.; Jarvis, H. Daily step count of British military males with bilateral lower limb amputations: A comparison of in-patient rehabilitation with the consecutive leave period between admissions. Prosthet. Orthot. Int. 2019, 43, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Colabianchi, N.; Wensman, J.; Gates, D.H. Wearable Sensors Quantify Mobility in People with Lower Limb Amputation During Daily Life. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Charlton, J.M.; Krowchuk, N.M.; Eng, J.J.; Li, L.C.; Hunt, M.A. Remotely delivered, individualized, and self-directed gait modification for knee osteoarthritis: A pilot trial. Clin. Biomech. 2023, 106, 105981. [Google Scholar] [CrossRef]
- Perraudin, C.G.M.; Illiano, V.P.; Calvo, F.; O’Hare, E.; Donnelly, S.C.; Mullan, R.H.; Sander, O.; Caulfield, B.; Dorn, J.F. Observational Study of a Wearable Sensor and Smartphone Application Supporting Unsupervised Exercises to Assess Pain and Stiffness. Digit. Biomark. 2018, 2, 106–125. [Google Scholar] [CrossRef]
- Dinesh, K.; White, N.; Baker, L.; Sowden, J.E.; Behrens-Spraggins, S.; Wood, E.; Charles, J.; Herrmann, D.N.; Sharma, G.; Eichinger, K. Disease-specific wearable sensor algorithms for profiling activity, gait, and balance in individuals with Charcot-Marie-Tooth disease type 1A. J. Peripher. Nerv. Syst. 2023, 28, 368–381. [Google Scholar] [CrossRef]
- Gatward, M.E.; Logue, R.N.; Yang, L.J.S.; Brown, S.H. Quantifying Real-World Upper Limb Activity Via Patient-Initiated Spontaneous Movement in Neonatal Brachial Plexus Palsy. PMR 2023, 15, 604–612. [Google Scholar] [CrossRef]
- Wiedmann, I.; Grassi, M.; Duran, I.; Lavrador, R.; Alberg, E.; Daumer, M.; Schoenau, E.; Rittweger, J. Accelerometric Gait Analysis Devices in Children—Will They Accept Them? Results From the AVAPed Study. Front. Pediatr. 2021, 8, 574443. [Google Scholar] [CrossRef]
- Mc Ardle, R.; Morris, R.; Hickey, A.; Del Din, S.; Koychev, I.; Gunn, R.N.; Lawson, J.; Zamboni, G.; Ridha, B.; Sahakian, B.J.; et al. Gait in Mild Alzheimer’s Disease: Feasibility of Multi-Center Measurement in the Clinic and Home with Body-Worn Sensors: A Pilot Study. J. Alzheimer’s Dis. 2018, 63, 331–341. [Google Scholar] [CrossRef]
- Khan, N.C.; Pandey, V.; Gajos, K.Z.; Gupta, A.S. Free-Living Motor Activity Monitoring in Ataxia-Telangiectasia. Cerebellum 2022, 21, 368–379. [Google Scholar] [CrossRef]
- Eklund, N.M.; Ouillon, J.; Pandey, V.; Stephen, C.D.; Schmahmann, J.D.; Edgerton, J.; Gajos, K.Z.; Gupta, A.S. Real-life ankle submovements and computer mouse use reflect patient-reported function in adult ataxias. Brain Commun. 2023, 5, fcad064. [Google Scholar] [CrossRef]
- Yen, T.C.; Mohler, J.; Dohm, M.; Laksari, K.; Najafi, B.; Toosizadeh, N. The Effect of Pain Relief on Daily Physical Activity: In-Home Objective Physical Activity Assessment in Chronic Low Back Pain Patients after Paravertebral Spinal Block. Sensors 2018, 18, 3048. [Google Scholar] [CrossRef]
- Tsvyakh, A.I.; Hospodarskyy, A.Y.; Marchenkova, N.O.; Kopytchak, I.R.; Kostjuk, V.P.; Lymar, Y.A.; Gdanskyi, S.M. Telerehabilitation of the Knee Joints of Patients with Polytrauma. Wiadomo’sci Lek. 2021, 74, 48–51. [Google Scholar] [CrossRef]
- Schwenk, M.; Hauer, K.; Zieschang, T.; Englert, S.; Mohler, J.; Najafi, B. Sensor-Derived Physical Activity Parameters Can Predict Future Falls in People with Dementia. Gerontology 2014, 60, 483–492. [Google Scholar] [CrossRef]
- Mueller, A.; Paterson, E.; McIntosh, A.; Praestgaard, J.; Bylo, M.; Hoefling, H.; Wells, M.; Lynch, D.R.; Rummey, C.; Krishnan, M.L.; et al. Digital Endpoints for Self-Administered Home-Based Functional Assessment in Pediatric Friedreich’s Ataxia. Ann. Clin. Transl. Neurol. 2021, 8, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, S.M.; Hyppönen, J.; Silvennoinen, K.; Säisänen, L.; Karjalainen, P.A.; Mervaala, E.; Kälviäinen, R. Wearable Monitoring of Positive and Negative Myoclonus in Progressive Myoclonic Epilepsy Type 1. Clin. Neurophysiol. 2021, 132, 2464–2472. [Google Scholar] [CrossRef]
- Kamil, R.J.; Bakar, D.; Ehrenburg, M.; Wei, E.X.; Pletnikova, A.; Xiao, G.; Oh, E.S.; Mancini, M.; Agrawal, Y. Detection of Wandering Behaviors Using a Body-Worn Inertial Sensor in Patients with Cognitive Impairment: A Feasibility Study. Front. Neurol. 2021, 12, 529661. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Popp, W.L.; Brogioli, M.; Albisser, U.; Demkó, L.; Debecker, I.; Velstra, I.-M.; Gassert, R.; Curt, A. Reliability of Wearable-Sensor-Derived Measures of Physical Activity in Wheelchair-Dependent Spinal Cord Injured Patients. Front. Neurol. 2018, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Gidaro, T.; Gasnier, E.; Annoussamy, M.; Vissing, J.; Attarian, S.; Mozaffar, T.; Iyadurai, S.; Wagner, K.R.; Vissière, D.; Walker, G.; et al. Home-based gait analysis as an exploratory endpoint during a multicenter phase 1 trial in limb girdle muscular dystrophy type R2 and facioscapulohumeral muscular dystrophy. Muscle Nerve 2022, 65, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bendig, J.; Wolf, A.-S.; Mark, T.; Frank, A.; Mathiebe, J.; Scheibe, M.; Müller, G.; Stahr, M.; Schmitt, J.; Reichmann, H.; et al. Feasibility of a Multimodal Telemedical Intervention for Patients with Parkinson’s Disease—A Pilot Study. J. Clin. Med. 2022, 11, 1074. [Google Scholar] [CrossRef]
- Reichmann, H.; Klingelhoefer, L.; Bendig, J. The use of wearables for the diagnosis and treatment of Parkinson’s disease. J. Neural Transm. 2023, 130, 783–791. [Google Scholar] [CrossRef]
- Moore, K.; O’Shea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamäki, A.; Condell, J.; Nordström, A.; et al. Older Adults’ Experiences with Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR Mhealth Uhealth 2021, 9, e23832. [Google Scholar] [CrossRef]
- Lanotte, F.; O’Brien, M.K.; Jayaraman, A. AI in Rehabilitation Medicine: Opportunities and Challenges. Front. Neurol. 2023, 47, 444–458. [Google Scholar] [CrossRef]
- Guo, C.C.; Chiesa, P.A.; de Moor, C.; Fazeli, M.S.; Schofield, T.; Hofer, K.; Belachew, S.; Scotland, A. Digital Devices for Assessing Motor Functions in Mobility-Impaired and Healthy Populations: Systematic Literature Review. J. Med. Internet Res. 2022, 24, e37683. [Google Scholar] [CrossRef] [PubMed]
- Davergne, T.; Pallot, A.; Dechartres, A.; Fautrel, B.; Gossec, L. Use of Wearable Activity Trackers to Improve Physical Activity Behavior in Patients with Rheumatic and Musculoskeletal Diseases: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2019, 71, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.; Mazhar, M.K.A.; Smith, M.D.; Lithander, F.E.; ÓBreasail, M.; Henderson, E.J. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors 2022, 22, 3336. [Google Scholar] [CrossRef] [PubMed]
- Sica, M.; Tedesco, S.; Crowe, C.; Kenny, L.; Moore, K.; Timmons, S.; Barton, J.; O’Flynn, B.; Komaris, D.S. Continuous home monitoring of Parkinson’s disease using inertial sensors: A systematic review. PLoS ONE 2021, 16, e0246528. [Google Scholar] [CrossRef]
- Morgan, C.; Rolinski, M.; McNaney, R.; Jones, B.; Rochester, L.; Maetzler, W.; Craddock, I.; Whone, A.L. Systematic Review Looking at the Use of Technology to Measure Free-Living Symptom and Activity Outcomes in Parkinson’s Disease in the Home or a Home-like Environment. J. Park. Dis. 2020, 10, 429–454. [Google Scholar] [CrossRef]
- Vavasour, G.; Giggins, O.M.; Doyle, J.; Kelly, D. How wearable sensors have been utilised to evaluate frailty in older adults: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 112. [Google Scholar] [CrossRef]
- Henrich, J.; Heine, S.J.; Norenzayan, A. Most people are not WEIRD. Nature 2010, 466, 29. [Google Scholar] [CrossRef]
- Cristia, A.; Farabolini, G.; Scaff, C.; Havron, N.; Stieglitz, J. Infant-directed input and literacy effects on phonological processing: Non-word repetition scores among the Tsimane’. PLoS ONE 2020, 15, e0237702. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, G.; Cao, S.; Wang, S.; Wang, F.; Xu, M. A bibliometric analysis of wearable device research in sleep science: Trends and hotspots. Medicine 2025, 104, e42853. [Google Scholar] [CrossRef]
- Rahman, N.; Thamotharampillai, T.; Rajaratnam, V. Ethics, guidelines, and policy for technology in healthcare. In Medical Equipment Engineering: Design, Manufacture and Applications; Institution of Engineering and Technology (IET): London, UK, 2023; Chapter 9; pp. 119–147. [Google Scholar]
- Joshua, R.G.; Imoize, A.L. Examining the complex interactions between cybersecurity and ethics in emerging healthcare systems. In Cybersecurity in Emerging Healthcare Systems; Routledge: Abingdon, UK, 2024; Chapter 13; pp. 401–426. [Google Scholar]
- Vayena, E.; Blasimme, A. Health Research with Big Data: Time for Systemic Oversight. J. Law Med. Ethics 2018, 46, 119–129. [Google Scholar] [CrossRef]
- Binns, R. Tracking on the Web, Mobile and the Internet-of-Things. 2022. Available online: https://arxiv.org/abs/2201.10831 (accessed on 27 June 2025).
- Kluge, E.H.W. Artificial intelligence in healthcare: Ethical considerations. Healthc. Manag. Forum 2020, 33, 47–49. [Google Scholar] [CrossRef] [PubMed]
| Health Condition | Studies (n°) | Subjects (n°) |
|---|---|---|
| Parkinson’s Disease | 35 | 4646 |
| Stroke | 6 | 107 |
| Multiple Sclerosis | 4 | 189 |
| Huntington Disease | 4 | 263 |
| Ataxia | 3 | 62 |
| Alzheimer’s Disease | 2 | 28 |
| Other | 7 | 227 |
| Total Neurologic Conditions | 61 | 5522 |
| Loss of Balance | 1 | 5 |
| Total Aging-related Conditions | 1 | 5 |
| Amputee | 6 | 94 |
| Polytrauma | 1 | 48 |
| Degenerative Facet Osteoarthropathy | 1 | 8 |
| Total Musculoskeletal Conditions | 8 | 150 |
| Osteoarthritis | 1 | 20 |
| Osteoarthritis–rheumatoid arthritis–psoriatic arthritis | 1 | 30 |
| Total Rheumatologic Conditions | 2 | 50 |
| Subjects with Pathological Condition | 5727 | |
| Healthy Subjects (Control Groups) | 2222 | |
| Total | 72 | 7949 |
| Hardware | Device’s Name | Additional App or Data Logger | References | Commercial Device | Medical Device | CE Certification |
|---|---|---|---|---|---|---|
| Tri-axial accelerometer | BiostampRC (MC10 Inc., Lexington, KY, USA) | Not needed | [70,77,81,91] | yes | yes | yes |
| Axivity AX-3 (Axivity Ltd., Newcastle upon Tyne, UK) | [57,78,94] | yes | no | no | ||
| ActiGraph GT3X o GT9X (ActiGraph LLC, Pensacola, FL, USA) | [88,90,92] | yes | no | no | ||
| GeneActiv (ActivInsights Ltd., Kimbolton, UK) | [45,95,96] | yes | yes | no | ||
| RehaGait (Hasomed GmbH, Magdeburg, Germany) | [64,65] | yes | yes | no | ||
| PAMSys (BioSensics, London, UK) | [82,97] | yes | no | no | ||
| ActivPal (PAL Technologies Ltd., Glasgow, UK) | [75,87] | yes | no | no | ||
| PKG (Parkinson KinetiGraph) (Global Kinetics/PKG Health, Melbourne, Australia) | [68] | no | yes | no | ||
| REMPARK (CETpD—Universitat Politècnica de Catalunya, Barcelona, Spain) | [53] | no | no | no | ||
| Shimmer (Shimmer Research, Dublin, Ireland) | [48] | yes | no | yes | ||
| Kinesia (Great Lakes NeuroTechnologies Inc., Cleveland, OH, USA) | [60] | yes | yes | no | ||
| Actibelt RCT2 (Trium Analysis GmbH, Munich, Germany) | [93] | yes | yes | no | ||
| Actical (Philips Respironics, Bend, OR, USA) | [74] | yes | no | no | ||
| ITEX gloves (University of Rhode Island—Wearable Biosensing Lab, Kingston, UK) | [34] | no | no | no | ||
| Not specified | [61,72,73,98] | |||||
| Tri-axial accelerometer–gyroscope | Dynaport Hybrid (McRoberts B.V., The Hague, The Netherlands) | Not needed | [40,46,67] | yes | yes | yes |
| Physilog (MindMaze Assessments, Lausanne, Switzerland) | [99,100] | yes | no | yes | ||
| Mobile GaitLab (Portabiles HealthCare Technologies GmbH, Erlangen, Germany) | [47,52] | yes | yes | yes | ||
| MOX5 (Maastricht Instruments/Instrument Development Engineering & Evaluation department, Maastricht, The Netherlands) | [49] | yes | no | yes | ||
| PD Monitor (PD Neurotechnology Ltd., London, UK) | [105] | yes | yes | yes | ||
| STAT-ON (Sense4Care, Barcelona, Spain) | [50] | no | yes | yes | ||
| Gait Tutor System (MHealth Technologies, Bologna, Italy) | [56] | yes | yes | no | ||
| Not specified | [55,76,84] | |||||
| Tri-axial accelerometer–barometer | PERS-phylips (Philips Lifeline, Cambridge, MA, USA) | Not needed | [28] | yes | yes | yes |
| Tri-axial accelerometer–surface electromyography | FarosEMG (Bittium Biosignals Ltd., Kuopio, Finland) | Not needed | [62,101] | n.a. | n.a. | n.a. |
| Tri-axial accelerometer–gyroscope–magnetometer–barometer | ReSense (Rehabilitation Engineering Lab, ETH Zurich, Zurich, Switzerland) | Not needed | [103] | no | no | no |
| Accelerometer–gyroscope–magnetometer | Opal APDM, Inc (APDM Wearable Technologies, Portland, OR, USA) | Not needed | [51,58,63,66,79,83,102] | yes | no | no |
| GaitAssist (Swiss Federal Institute of Technology Zurich, Zurich, Switzerland) | [54] | no | no | no | ||
| ActiMyo (Institute of Myology & Sysnav partnership, Paris, France) | [104] | no | yes | yes | ||
| Not specified | [89] | |||||
| Coil antenna | WAFER (Department of Bioengineering, University of Washington; Seattle, WA, USA) | ECHO | [33] | no | no | no |
| Smartphone sensors (accelerometer, gyroscope, GPS) | Verily study watch (Verily Life Sciences LLC, Dallas, TX, USA) | Not needed | [37] | yes | yes | yes |
| Samsung Galaxy J7 (Samsung Electronics Co., Suwon, Republic of Korea) | ROCHE HD | [42] | yes | no | yes | |
| iPod touch 4th generation (Apple Inc., Cupertino, CA, USA) | Gait Reminder | [59] | yes | no | yes | |
| iPhone 5 or 6 (Apple Inc., Cupertino, CA, USA) | Customized App | [44] | yes | no | yes | |
| iPhone 10 or 11 (Apple Inc., Cupertino, CA, USA) | Brain Baseline | [41] | yes | no | yes | |
| Android Phone (not specified) | Encephalog Home FOX wearable companion Customized app | [38,39,69,80] | ||||
| Smartwatch sensors (accelerometer, gyroscope, GPS) | Moto G360 (Motorola Mobility LLC, Chicago, IL, USA) | ROCHE HD | [42] | yes | no | yes |
| Stepwatch Activity Monitor (Modus Health LLC, Washington, DC, USA) | Not needed | [86] | n.a. | n.a. | n.a. | |
| Apple Watch 4 or 5 (Apple Inc., Cupertino, CA, USA) | Brain Baseline | [41] | yes | no | yes | |
| FitBit (Fitbit Inc., San Francisco, CA, USA) | Not needed | [43,71,85] | yes | no | yes | |
| Pebble Smartwatch (Pebble Technology Corp., Palo Alto, CA, USA) | Fox wearable companion | [38,39] | yes | no | Yes |
| Health Condition | % Adherence /Compliance | Number of Sensors Used | References |
|---|---|---|---|
| Parkinson’s disease | 71 | 5 | [105] |
| 68 | 2 | [38] | |
| 59 | 1 | [37] | |
| 94 | 3 | [49] | |
| 96 | 1 | [60] | |
| Stroke | 91 | 1 | [76] |
| Cerebral palsy | Not expressed | 1 | [93] |
| Osteoarthritis–rheumatoid arthritis–psoriatic arthritis | 56 | 2 | [90] |
| Validation Method | References | Sensitivity | Specificity | Accuracy | Precision | ICC |
|---|---|---|---|---|---|---|
| Gold standard | [43,44,52,84,93] | n.a. | n.a. | 82.5% | n.a. | 0.76 |
| Patient-reported outcome | [53,80,83,90,101] | 92.6% | 97.6% | 97.6% | 96.4% | 0.70 |
| Clinical assessment | [33,34,37,42,44,45,46,47,48,49,50,51,71,72,77,78,80,81,89,91,92,99,101,102,103] | 82.5% | 63.5% | 70.0% | n.a. | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farabolini, G.; Baldini, N.; Pagano, A.; Andrenelli, E.; Pepa, L.; Morone, G.; Ceravolo, M.G.; Capecci, M. Continuous Movement Monitoring at Home Through Wearable Devices: A Systematic Review. Sensors 2025, 25, 4889. https://doi.org/10.3390/s25164889
Farabolini G, Baldini N, Pagano A, Andrenelli E, Pepa L, Morone G, Ceravolo MG, Capecci M. Continuous Movement Monitoring at Home Through Wearable Devices: A Systematic Review. Sensors. 2025; 25(16):4889. https://doi.org/10.3390/s25164889
Chicago/Turabian StyleFarabolini, Gianmatteo, Nicolò Baldini, Alessandro Pagano, Elisa Andrenelli, Lucia Pepa, Giovanni Morone, Maria Gabriella Ceravolo, and Marianna Capecci. 2025. "Continuous Movement Monitoring at Home Through Wearable Devices: A Systematic Review" Sensors 25, no. 16: 4889. https://doi.org/10.3390/s25164889
APA StyleFarabolini, G., Baldini, N., Pagano, A., Andrenelli, E., Pepa, L., Morone, G., Ceravolo, M. G., & Capecci, M. (2025). Continuous Movement Monitoring at Home Through Wearable Devices: A Systematic Review. Sensors, 25(16), 4889. https://doi.org/10.3390/s25164889







