High-Performance Hydrogen Gas Sensor Based on Pd-Doped MoS2/Si Heterojunction
Abstract
1. Introduction
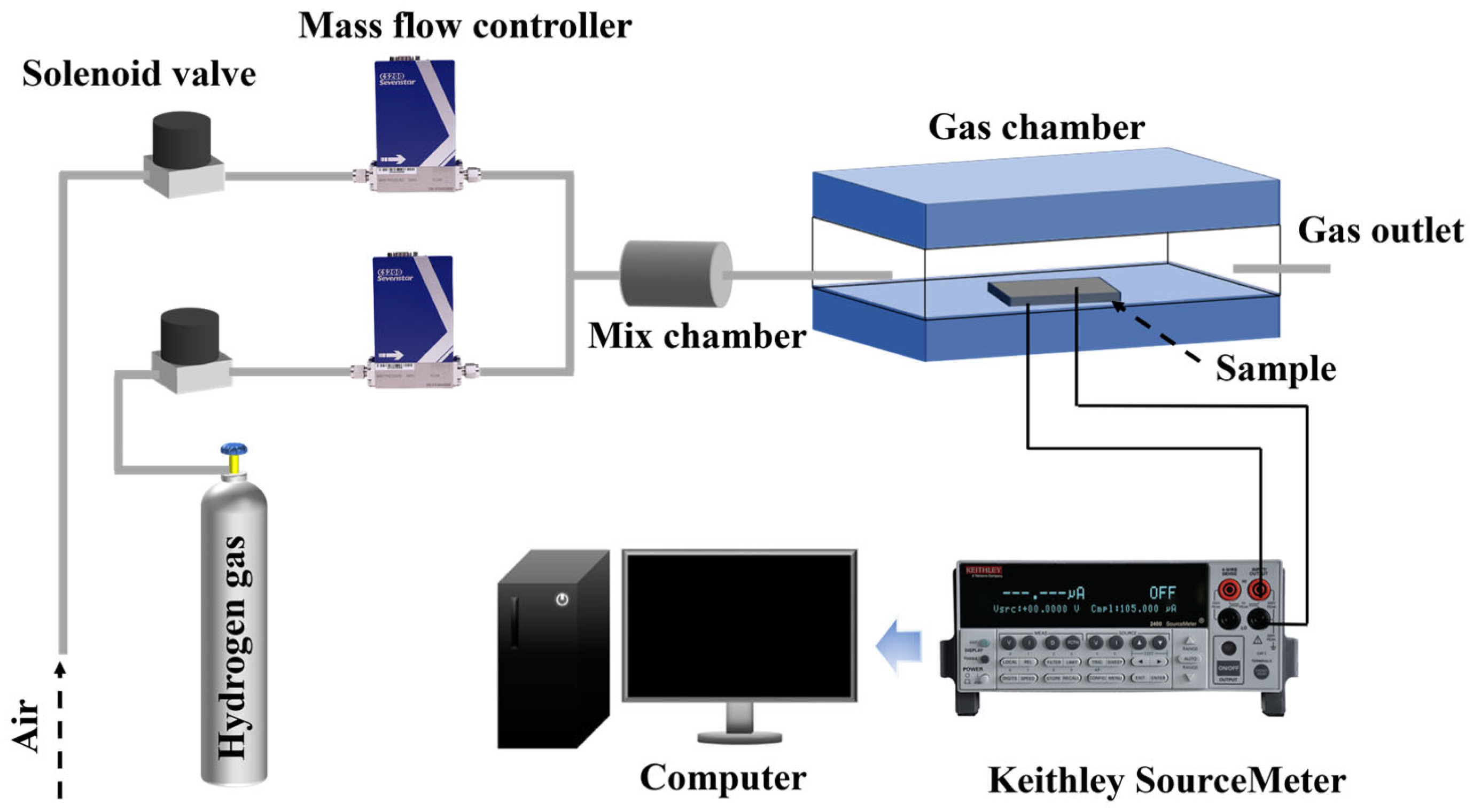
2. Materials and Methods
3. Results
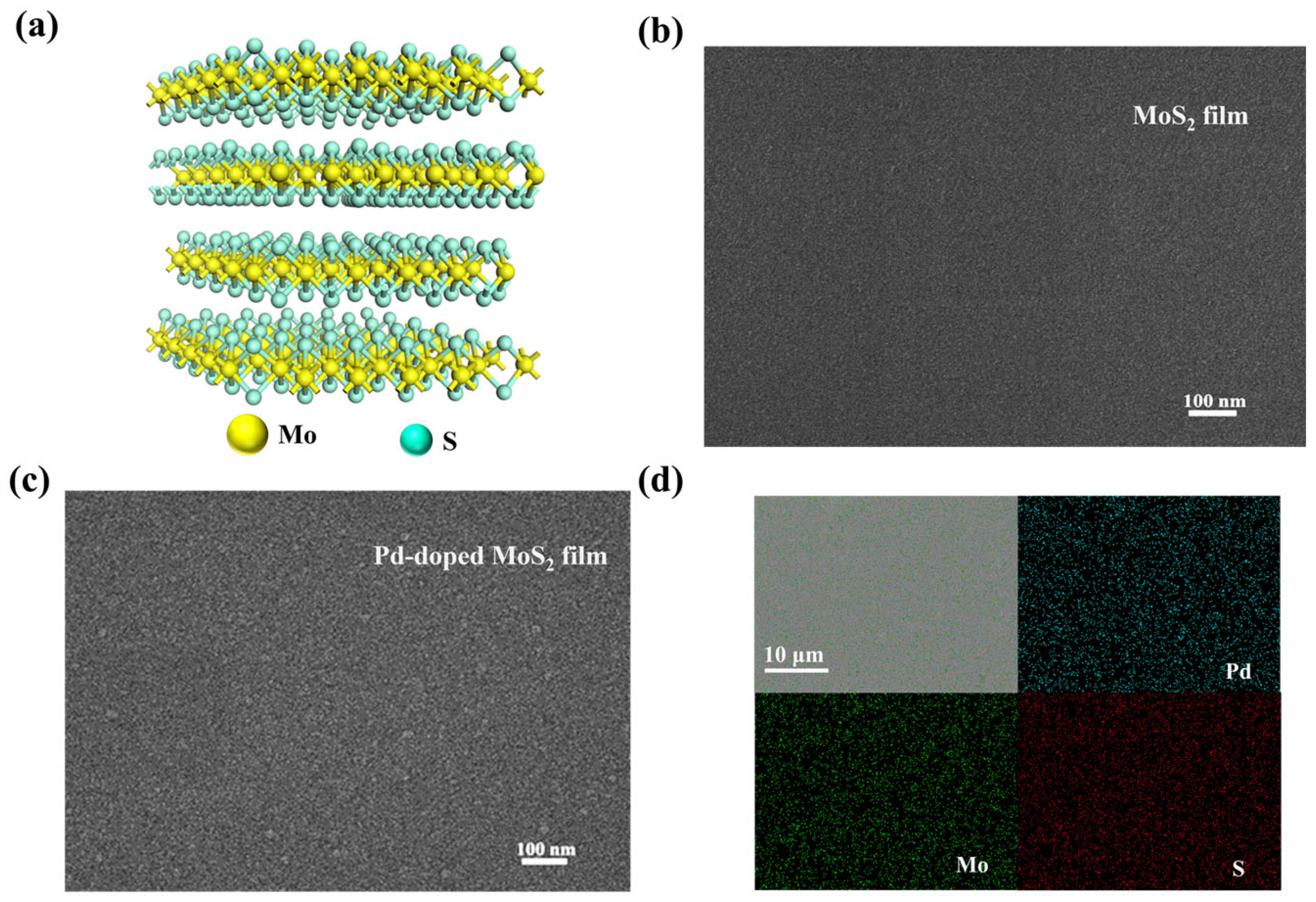
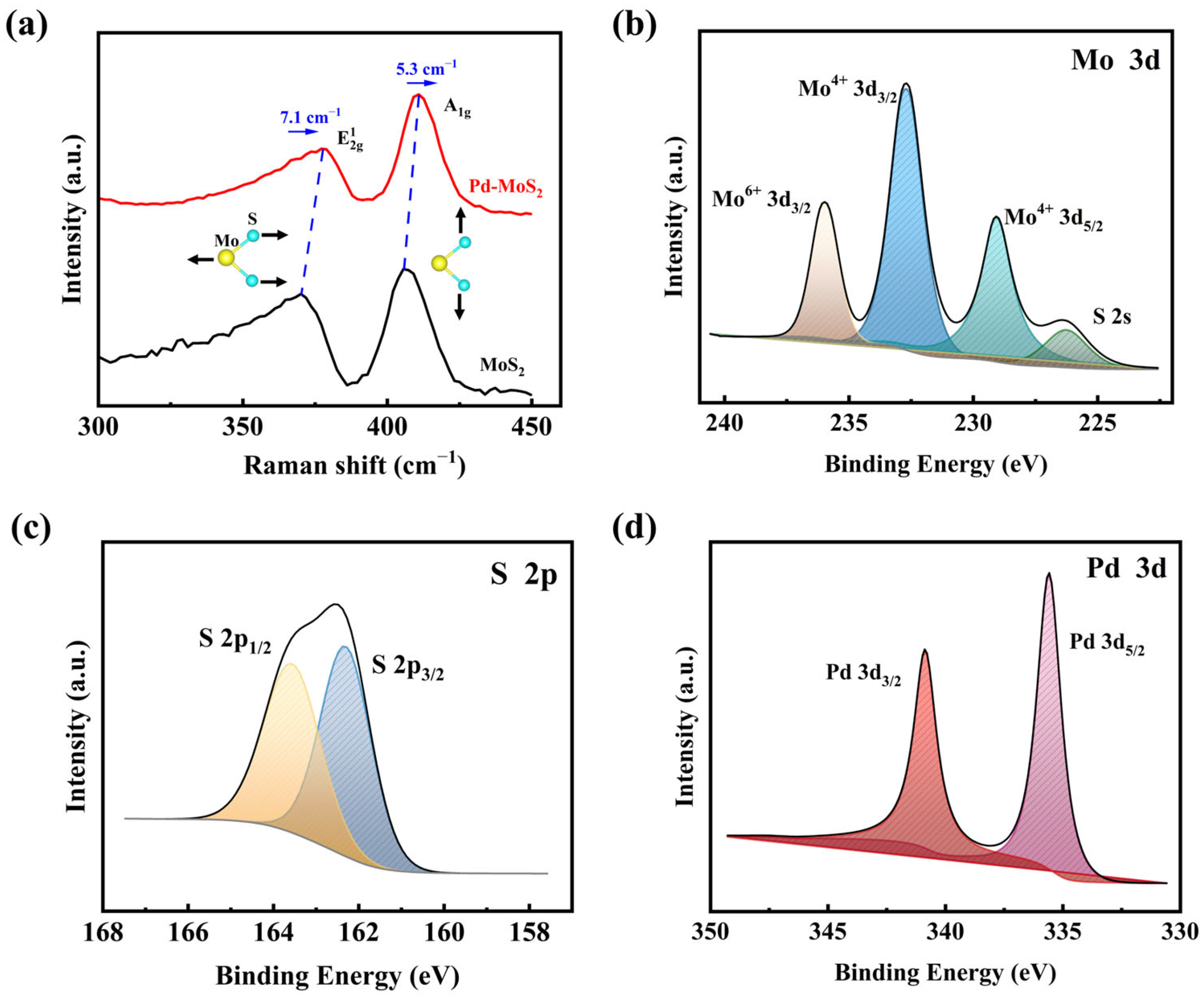
3.1. Thin Film’s Characterization
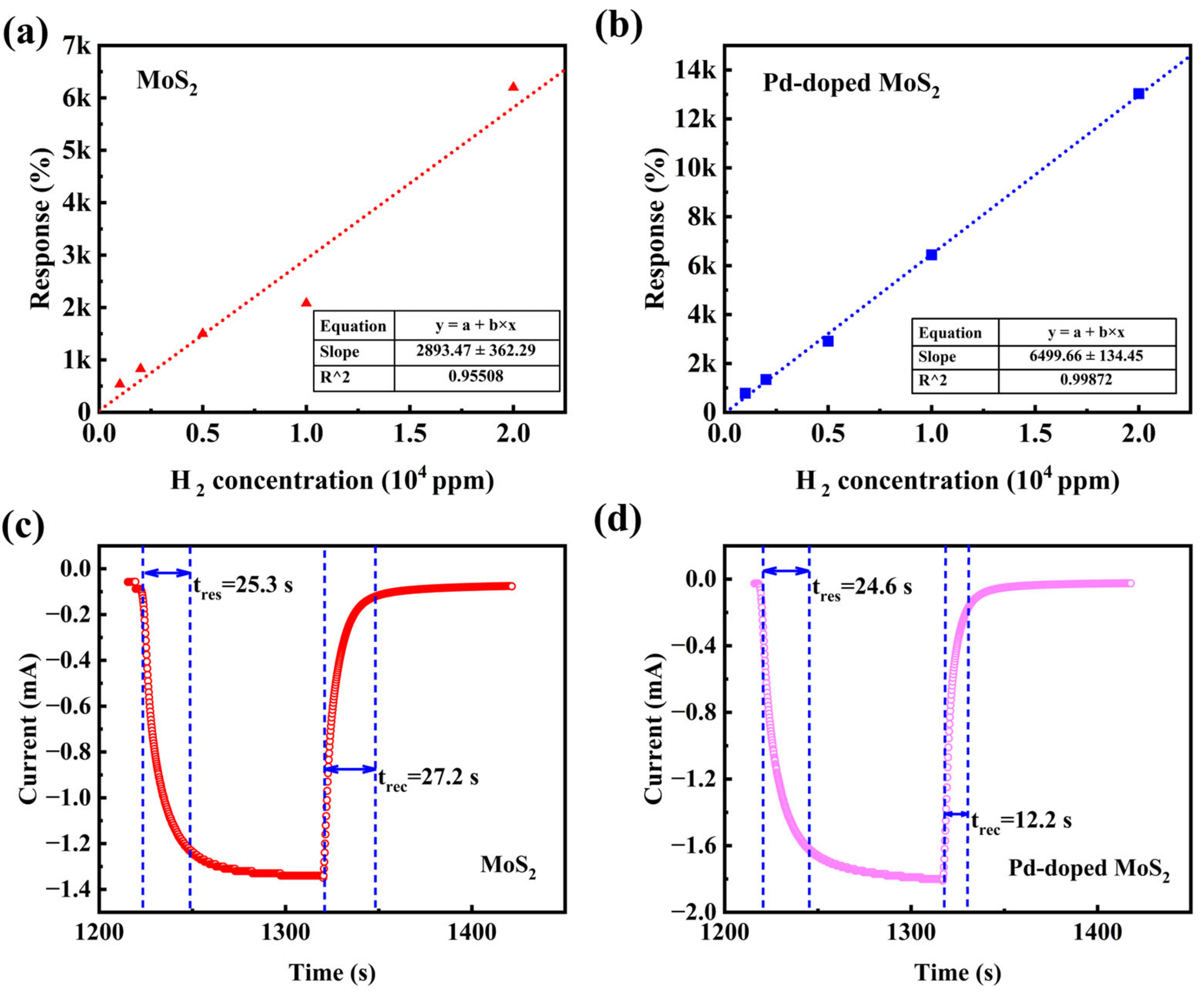
3.2. Gas Sensing Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A mini review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Kulagin, V.A.; Grushevenko, D.A. Will hydrogen be able to become the fuel of the future? Therm. Eng. 2020, 67, 189–201. [Google Scholar] [CrossRef]
- Calabrese, M.; Portarapillo, M.; Di Nardo, A.; Venezia, V.; Turco, M.; Luciani, G.; Di Benedetto, A. Hydrogen Safety Challenges: A Comprehensive Review on Production, Storage, Transport, Utilization, and CFD-Based Consequence and Risk Assessment. Energies 2024, 17, 1350. [Google Scholar] [CrossRef]
- Yang, F.; Wang, T.; Deng, X.; Dang, J.; Huang, Z.; Hu, S.; Li, Y.; Ouyang, M. Review on hydrogen safety issues: Incident statistics, hydrogen diffusion, and detonation process. Int. J. Hydrogen Energy 2021, 46, 31467–31488. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Jaiswal, J.; Chandra, R. A Fast Response/Recovery of Hydrophobic Pd/V2O5 Thin Films for Hydrogen Gas Sensing. Sens. Actuators B Chem. 2016, 236, 16–26. [Google Scholar] [CrossRef]
- Ab Kadir, R.; Li, Z.; Sadek, A.Z.; Abdul Rani, R.; Zoolfakar, A.S.; Field, M.R.; Ou, J.Z.; Chrimes, A.F.; Kalantar-zadeh, K. Electrospun Granular Hollow SnO2 Nanofibers Hydrogen Gas Sensors Operating at Low Temperatures. J. Phys. Chem. C 2014, 118, 3129–3139. [Google Scholar] [CrossRef]
- Mondal, B.; Gogoi, P.K. Nanoscale Heterostructured Materials Based on Metal Oxides for a Chemiresistive Gas Sensor. ACS Appl. Electron. Mater. 2022, 4, 59–86. [Google Scholar] [CrossRef]
- Tien, L.C.; Sadik, P.W.; Norton, D.P.; Voss, L.F.; Pearton, S.J.; Wang, H.T.; Kang, B.S.; Ren, F.; Jun, J.; Lin, J. Hydrogen Sensing at Room Temperature with Pt-Coated ZnO Thin Films and Nanorods. Appl. Phys. Lett. 2005, 87, 222106. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Cai, D.; Li, H.; Liu, Y.; Wang, D.; Wang, L.; Li, Q.; Wang, T. Room-Temperature Hydrogen Sensor Based on Grain-Boundary Controlled Pt Decorated In2O3 Nanocubes. Sens. Actuators B Chem. 2014, 201, 351–359. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Lai, P.T. Investigation of WO3/ZnO Thin-Film Heterojunction-Based Schottky Diodes for H2 Gas Sensing. Int. J. Hydrog. Energy 2014, 39, 10313–10319. [Google Scholar] [CrossRef]
- Sakalley, S.; Saravanan, A.; Kathiravan, D.; Tang, J.-C.; Cheng, W.-C.; Chen, S.-C.; Sun, H.; Huang, B.-R. Enhanced Hydrogen Gas Sensing through the Utilization of a Hybrid Nanostructure Combining ZnO Nanotubes and HiPIMS Cu3N Thin Film. Sens. Actuators B Chem. 2024, 402, 135107. [Google Scholar] [CrossRef]
- Khanikar, T.; Karki, D.; Su, Y.-D.; Young Hong, J.; Wang, Y.; Naeem, K.; Ohodnicki, P.R. Pd/PMMA Nanocomposite-Coated Optical Fiber Hydrogen Sensor Operating at Room Temperature with Humidity Tolerance. IEEE Sens. J. 2024, 24, 34498–34506. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotech. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Xu, T.; Pei, Y.; Liu, Y.; Wu, D.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. High-Response NO2 Resistive Gas Sensor Based on Bilayer MoS2 Grown by a New Two-Step Chemical Vapor Deposition Method. J. Alloys Compd. 2017, 725, 253–259. [Google Scholar] [CrossRef]
- Aysha Parveen, R.; Vinoth, E.; Hara, K.; Archana, J.; Ponnusamy, S.; Navaneethan, M. Graphene-Analogous Functional Materials: A Review and Perspective on the Synthesis, Properties, and Special Emphasis of Gas Sensors. Chem. Eng. J. 2025, 517, 163418. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-Enriched WS2 Nanosheets on Carbon Nanofibers Boosts NO2 Detection at Room Temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Cui, Z.; Wu, P.; Zhang, S.; Wang, L. Superior Carrier Mobility and Photogalvanic Effect of Si9C15 and WSSe Heterojunctions. Mater. Today Commun. 2025, 46, 112830. [Google Scholar] [CrossRef]
- Sultana, N.; Degg, A.; Upadhyaya, S.; Nilges, T.; Sarma, N.S. Synthesis, Modification, and Application of Black Phosphorus, Few-Layer Black Phosphorus (FLBP), and Phosphorene: A Detailed Review. Mater. Adv. 2022, 3, 5557–5574. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, H.; Li, M.; Chen, T.; Xiao, X.; Wang, Y. Recent Advances in MoS 2 -Based Nanomaterial Sensors for Room-Temperature Gas Detection: A Review. Sens. Diagn. 2023, 2, 361–381. [Google Scholar] [CrossRef]
- Yao, Y.; Tolentino, L.; Yang, Z.; Song, X.; Zhang, W.; Chen, Y.; Wong, C. High-Concentration Aqueous Dispersions of MoS2. Adv. Funct. Mater. 2013, 23, 3577–3583. [Google Scholar] [CrossRef]
- Taufik, A.; Asakura, Y.; Hasegawa, T.; Yin, S. MoS2–xSex Nanoparticles for NO Detection at Room Temperature. ACS Appl. Nano Mater. 2021, 4, 6861–6871. [Google Scholar] [CrossRef]
- Gu, Z.; He, Z.; Chen, F.; Meng, L.; Feng, J.; Zhou, R. Ionic Liquid Decelerates Single-Stranded DNA Transport through Molybdenum Disulfide Nanopores. ACS Appl. Mater. Interfaces 2022, 14, 32618–32624. [Google Scholar] [CrossRef]
- Aggarwal, R.; Saini, D.; Mitra, R.; Sonkar, S.K.; Sonker, A.K.; Westman, G. From Bulk Molybdenum Disulfide (MoS2) to Suspensions of Exfoliated MoS2 in an Aqueous Medium and Their Applications. Langmuir 2024, 40, 9855–9872. [Google Scholar] [CrossRef] [PubMed]
- Late, D.J.; Huang, Y.-K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhen, Y.; Zhang, J.; Li, Y.; Zhong, H.; Jia, Z.; Xiong, Y.; Xue, Q.; Yan, Y.; Alharbi, N.S. SnO2 Nanoparticles-Modified 3D-Multilayer MoS2 Nanosheets for Ammonia Gas Sensing at Room Temperature. Sens. Actuators B Chem. 2020, 321, 128471. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano-Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kumar, A.; Sarkar, R.; Mohanty, P.P.; Kumar, D.; Deswal, S.; Kumar, P.; Ahuja, R.; Chakraborty, S.; Kumar, M.; et al. Pt Nanoparticles on Vertically Aligned Large-Area MoS2 Flakes for Selective H2 Sensing at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 2527–2537. [Google Scholar] [CrossRef]
- Jaiswal, J.; Tiwari, P.; Singh, P.; Chandra, R. Fabrication of Highly Responsive Room Temperature H2 Sensor Based on Vertically Aligned Edge-Oriented MoS2 Nanostructured Thin Film Functionalized by Pd Nanoparticles. Sens. Actuators B Chem. 2020, 325, 128800. [Google Scholar] [CrossRef]
- Baek, D.-H.; Kim, J. MoS2 Gas Sensor Functionalized by Pd for the Detection of Hydrogen. Sens. Actuators B Chem. 2017, 250, 686–691. [Google Scholar] [CrossRef]
- Xu, H.; Hao, L.; Liu, H.; Dong, S.; Wu, Y.; Liu, Y.; Cao, B.; Wang, Z.; Ling, C.; Li, S.; et al. Flexible SnSe Photodetectors with Ultrabroad Spectral Response up to 10.6 Μm Enabled by Photobolometric Effect. ACS Appl. Mater. Interfaces 2020, 12, 35250–35258. [Google Scholar] [CrossRef]
- Hao, L.; Du, Y.; Wang, Z.; Wu, Y.; Xu, H.; Dong, S.; Liu, H.; Liu, Y.; Xue, Q.; Han, Z. Wafer-Size Growth of 2D Layered SnSe Films for UV-Visible-NIR Photodetector Arrays with High Responsitivity. Nanoscale 2020, 12, 7358–7365. [Google Scholar] [CrossRef]
- Wu, D.; Lou, Z.; Wang, Y.; Xu, T.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. Construction of MoS2/Si Nanowire Array Heterojunction for Ultrahigh-Sensitivity Gas Sensor. Nanotechnology 2017, 28, 435503. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Gao, W.; Wu, Z.; Lin, Y.; Li, G.; Guo, W.; Yu, L.; Zeng, H.; Zhu, J. Hydrogen Gas Sensing Properties of MoS2/Si Heterojunction. Sens. Actuators B Chem. 2015, 211, 537–543. [Google Scholar] [CrossRef]
- Qin, R.; Wang, P.; Lin, C.; Cao, F.; Zhang, J.; Chen, L.; Mu, S. Transition Metal Nitrides: Activity Origin, Synthesis and Electrocatalytic Applications. Acta Phys. Chim. Sin 2021, 37, 2009099. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv Funct Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Koh, H.-J.; Yoo, H.-W.; Kim, J.-S.; Jung, H.-T. Tunable Volatile-Organic-Compound Sensor by Using Au Nanoparticle Incorporation on MoS2. ACS Sens. 2017, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hao, J.; Zheng, S.; Sun, Q.; Wang, T.; Zhang, D.; Zhang, H.; Wang, Y.; Zhou, X. N Dopants Triggered New Active Sites and Fast Charge Transfer in MoS2 Nanosheets for Full Response-Recovery NO2 Detection at Room Temperature. Appl. Surf. Sci. 2022, 571, 151162. [Google Scholar] [CrossRef]
- Bruix, A.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Andersen, M.; Porsgaard, S.; Besenbacher, F.; Hammer, B.; Lauritsen, J.V. In Situ Detection of Active Edge Sites in Single-Layer MoS2 Catalysts. ACS Nano 2015, 9, 9322–9330. [Google Scholar] [CrossRef]
- Nazir, R.; Fageria, P.; Basu, M.; Gangopadhyay, S.; Pande, S. Decoration of Pd and Pt Nanoparticles on a Carbon Nitride (C3 N4) Surface for Nitro-Compounds Reduction and Hydrogen Evolution Reaction. New J. Chem. 2017, 41, 9658–9667. [Google Scholar] [CrossRef]
- Hau, H.H.; Duong, T.T.H.; Man, N.K.; Nga, T.T.V.; Xuan, C.T.; Le, D.T.T.; Van Toan, N.; Hung, C.M.; Van Duy, N.; Van Hieu, N. Enhanced NO2 Gas-Sensing Performance at Room Temperature Using Exfoliated MoS2 Nanosheets. Sens. Actuators A Phys. 2021, 332, 113137. [Google Scholar] [CrossRef]
- Dey, S.; Santra, S.; Sen, S.; Burman, D.; Ray, S.K.; Guha, P.K. Photon-Assisted Ultra-Selective Formaldehyde Sensing by Defect Induced NiO-Based Resistive Sensor. IEEE Sens. J. 2018, 18, 5656–5661. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Wu, Y.; Guo, F.; Zhang, M.; Zhu, X.; Xu, R.; Hao, L. High-Performance Flexible Photodetectors Based on CdTe/MoS2 Heterojunction. Nanoscale 2024, 16, 13932–13937. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Yuan, H.; Lin, G.; Xie, H.; Jiang, T. Enhanced H2 Sensing Performance of Pd Decorated MoS2: Experimental and DFT Insights. J. Alloys Compd. 2025, 1010, 178139. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Y.; Liu, H.; Dong, S.; Wu, Y.; Wang, Z.; Wang, Y.; Wu, M.; Han, Z.; Hao, L. Pd-Decorated 2D SnSe Ultrathin Film on SiO2/Si for Room-Temperature Hydrogen Detection with Ultrahigh Response. J. Alloys Compd. 2021, 851, 156844. [Google Scholar] [CrossRef]
- Yao, J.; Zheng, Z.; Yang, G. All-Layered 2D Optoelectronics: A High-Performance UV–Vis–NIR Broadband SnSe Photodetector with Bi2 Te3 Topological Insulator Electrodes. Adv Funct Mater. 2017, 27, 1701823. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, S.J.; Lee, Y.; Kim, J.-S.; Jung, W.-B.; Yoo, H.-W.; Kim, J.; Jung, H.-T. Highly Enhanced Gas Adsorption Properties in Vertically Aligned MoS2 Layers. ACS Nano 2015, 9, 9314–9321. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Zhu, Z.; Bao, J.; Kumar, M.; Kumar, M. Fast Detection and Low Power Hydrogen Sensor Using Edge-Oriented Vertically Aligned 3-D Network of MoS2 Flakes at Room Temperature. Appl. Phys. Lett. 2017, 111, 093102. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Jiang, C.; Zhang, Y. Room Temperature Hydrogen Gas Sensor Based on Palladium Decorated Tin Oxide/Molybdenum Disulfide Ternary Hybrid via Hydrothermal Route. Sens. Actuators B Chem. 2017, 242, 15–24. [Google Scholar] [CrossRef]
- Fan, L.; Xu, N.; Chen, H.; Zhou, J.; Deng, S. A Millisecond Response and Microwatt Power-Consumption Gas Sensor: Realization Based on Cross-Stacked Individual Pt-Coated WO3 Nanorods. Sens. Actuators B Chem. 2021, 346, 130545. [Google Scholar] [CrossRef]
- Hassan, K.; Chung, G.-S. Catalytically Activated Quantum-Size Pt/Pd Bimetallic Core–Shell Nanoparticles Decorated on ZnO Nanorod Clusters for Accelerated Hydrogen Gas Detection. Sens. Actuators B Chem. 2017, 239, 824–833. [Google Scholar] [CrossRef]
- Goel, N.; Kumar, R.; Jain, S.K.; Rajamani, S.; Roul, B.; Gupta, G.; Kumar, M.; Krupanidhi, S.B. A High-Performance Hydrogen Sensor Based on a Reverse-Biased MoS2/GaN Heterojunction. Nanotechnology 2019, 30, 314001. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, C. Pt-Activated TiO2-MoS2 Nanocomposites for H2 Detection at Low Temperature. J. Alloys Compd. 2018, 747, 550–557. [Google Scholar] [CrossRef]


| Materials | Fabrication Method | H2 Concentration (ppm) | Response (%) | Response/Recovery Times (s) | Temperature | Ref. |
|---|---|---|---|---|---|---|
| MoS2 flake | CVD | 10,000 | 1 | 14.3/137 | RT | [47] |
| Pd–SnO2/MoS2 | Hydrothermal method | 5000 | 18 | 30/19 | RT | [48] |
| Pt-WO3 | PVD | 5000 | 68 | ~ | 110 °C | [49] |
| Pt/Pd-ZnO | PLD | 10,000 | 58 | 5/76 | 100 °C | [50] |
| MoS2/GaN | Sputtering | 10,000 | 150 | ~ | 157 °C | [51] |
| Pt-MoS2 | Hydrothermal method | 2000 | 75 | 150/370 | 100 °C | [52] |
| Pd-doped MoS2/Si | Sputtering | 10,000 | 6400 | 24.6/12.2 | RT | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, E.; Xu, Z.; Sun, A.; Yang, S.; Jiang, J. High-Performance Hydrogen Gas Sensor Based on Pd-Doped MoS2/Si Heterojunction. Sensors 2025, 25, 4753. https://doi.org/10.3390/s25154753
Ma E, Xu Z, Sun A, Yang S, Jiang J. High-Performance Hydrogen Gas Sensor Based on Pd-Doped MoS2/Si Heterojunction. Sensors. 2025; 25(15):4753. https://doi.org/10.3390/s25154753
Chicago/Turabian StyleMa, Enyu, Zihao Xu, Ankai Sun, Shuo Yang, and Jianyu Jiang. 2025. "High-Performance Hydrogen Gas Sensor Based on Pd-Doped MoS2/Si Heterojunction" Sensors 25, no. 15: 4753. https://doi.org/10.3390/s25154753
APA StyleMa, E., Xu, Z., Sun, A., Yang, S., & Jiang, J. (2025). High-Performance Hydrogen Gas Sensor Based on Pd-Doped MoS2/Si Heterojunction. Sensors, 25(15), 4753. https://doi.org/10.3390/s25154753





