The Development of a Measuring System for Intraoral SpO2
Abstract
1. Introduction
- Time delay in measurements: There is a time delay associated with measurements taken at the periphery of the body, such as the fingers, which makes it difficult to detect sudden changes in pathological conditions in real time [6].
- Dependence on blood flow: When blood flow decreases because of insufficient peripheral circulation, such as during hypothermia, it is difficult to obtain sufficiently strong signals [7].
- The influence of body movement: Body movement during sports or sleep causes the device to shift, which significantly affects the measurement accuracy [8].
- Location: Many conventional devices are worn on the surface of the body, which is difficult to accomplish in cases where the patient has burns or sensitive injuries [9].
- The influence of pressure: Accurate measurement is impossible without using the optimal contact pressure [10]. If the pressure of the sensor on the human body is insufficient, the amplitude of the AC signal is reduced, making it difficult to detect transmitted light. Conversely, when excessive pressure is applied, the waveform of the AC signal is distorted by the occluded artery, which also affects the accuracy [11].
- The influence of visible light: Interference from visible light in the surroundings can affect the accurate detection of the desired light signals [12].
2. Materials and Methods
2.1. Principles of Measurement
2.1.1. Pulse Oximeter
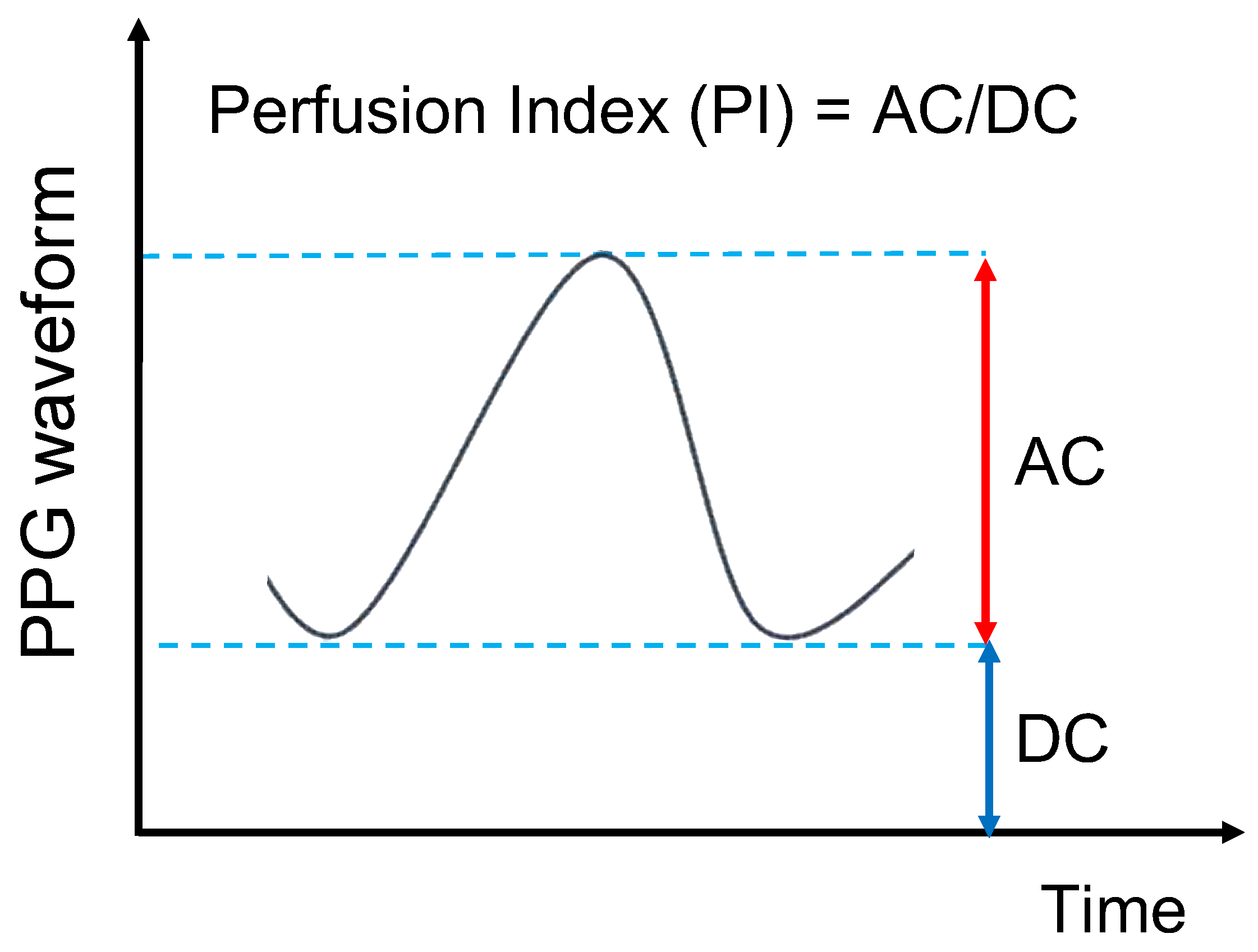
2.1.2. Perfusion Index (PI) Value
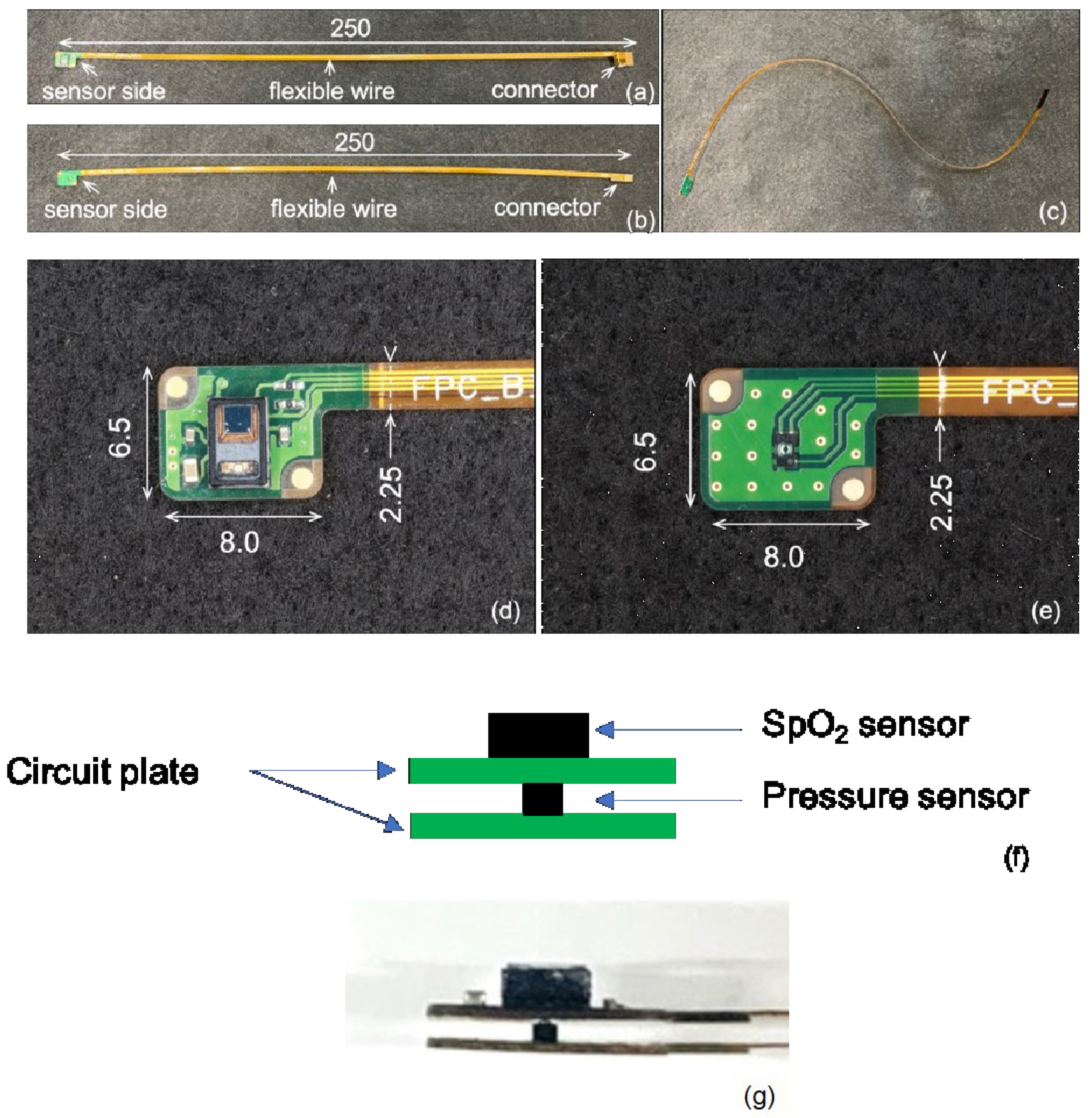
2.2. Intraoral Sensing Modules
2.2.1. Sensing Module and Measurement System
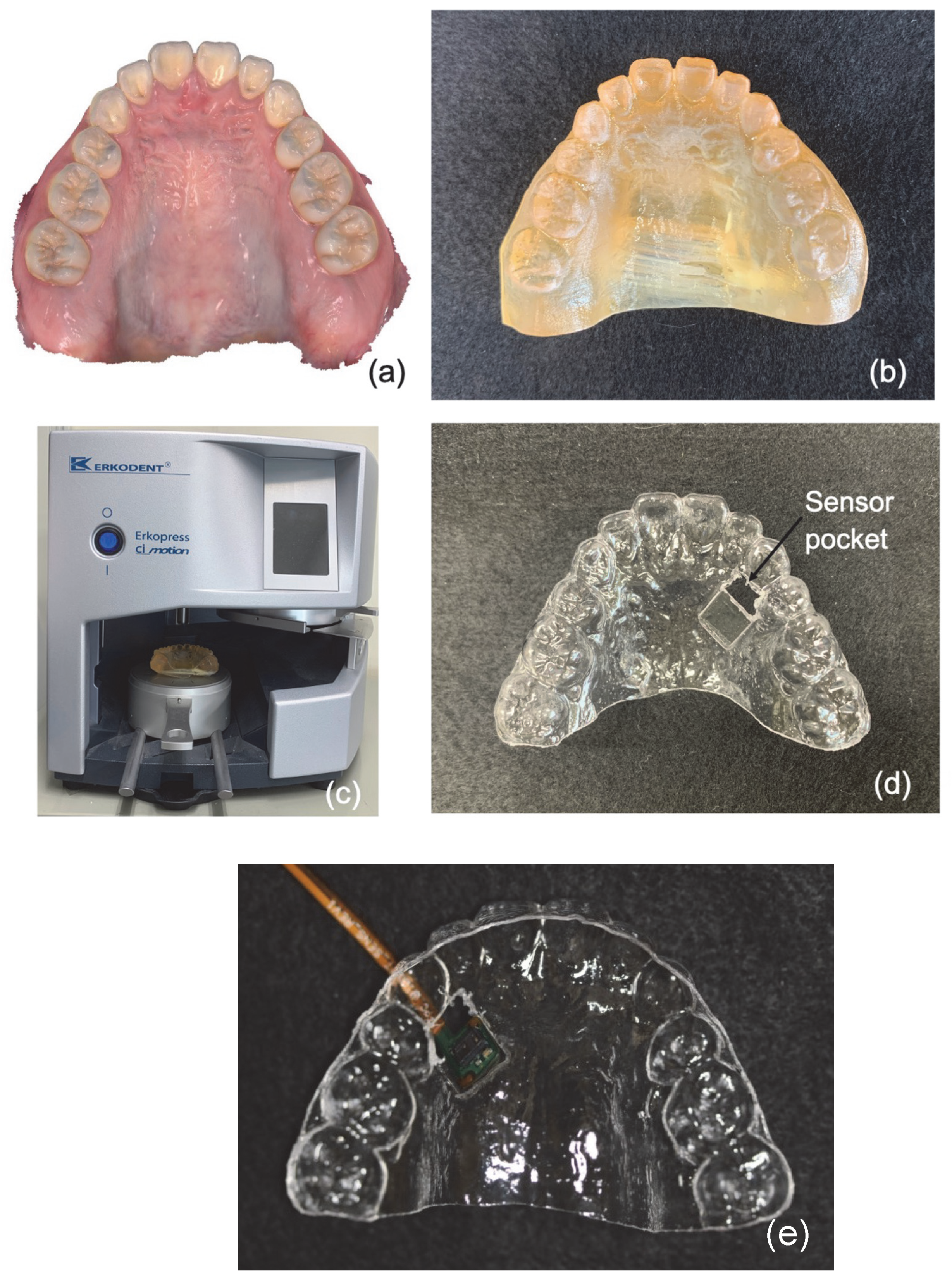
2.2.2. Method of Manufacturing the Mouthpiece Device
2.3. Measurement of SpO2
2.3.1. Verification of the Mouthpiece Device (Extraoral)
2.3.2. Accuracy Evaluation of the Mouthpiece Device (Intraoral and Extraoral)
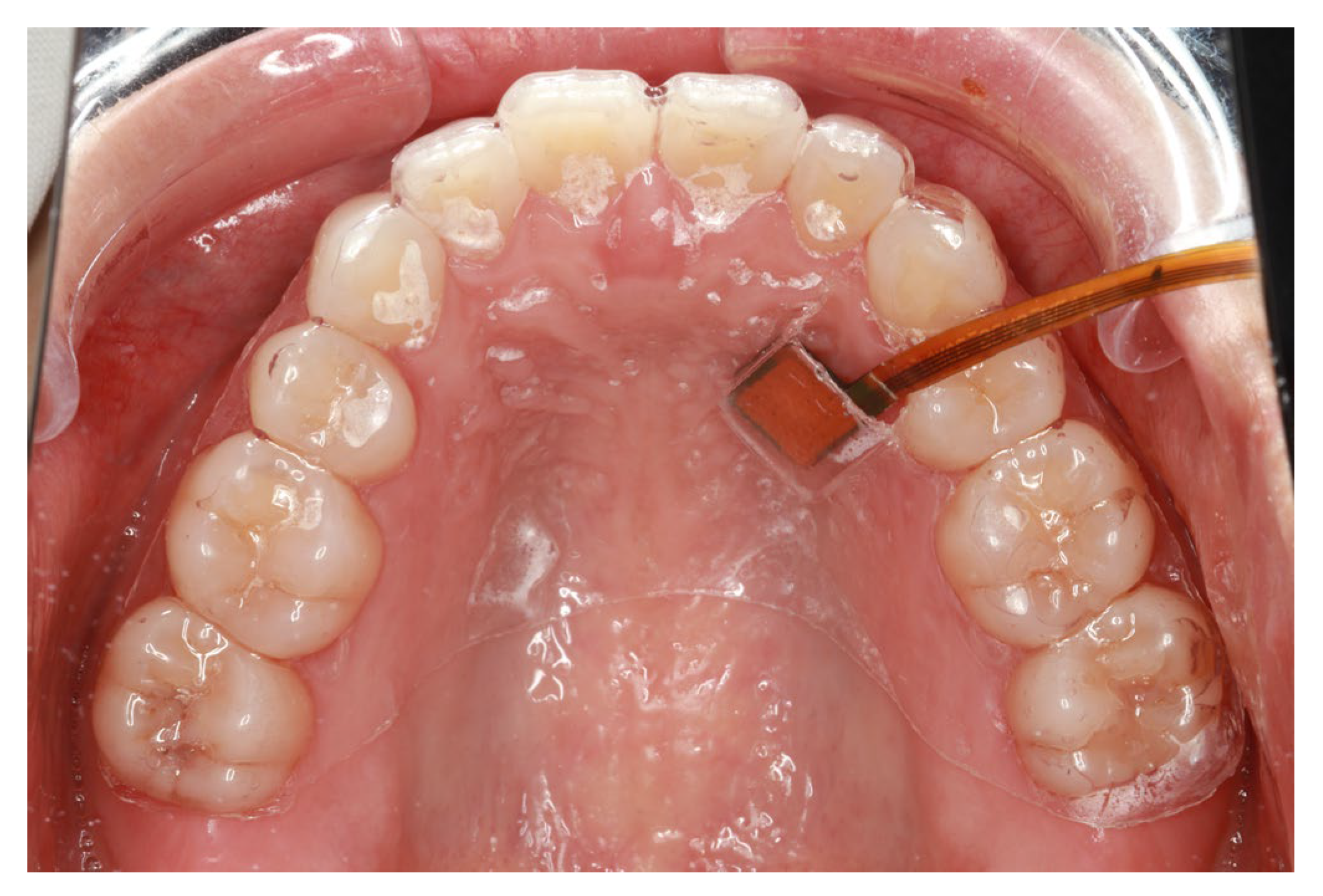
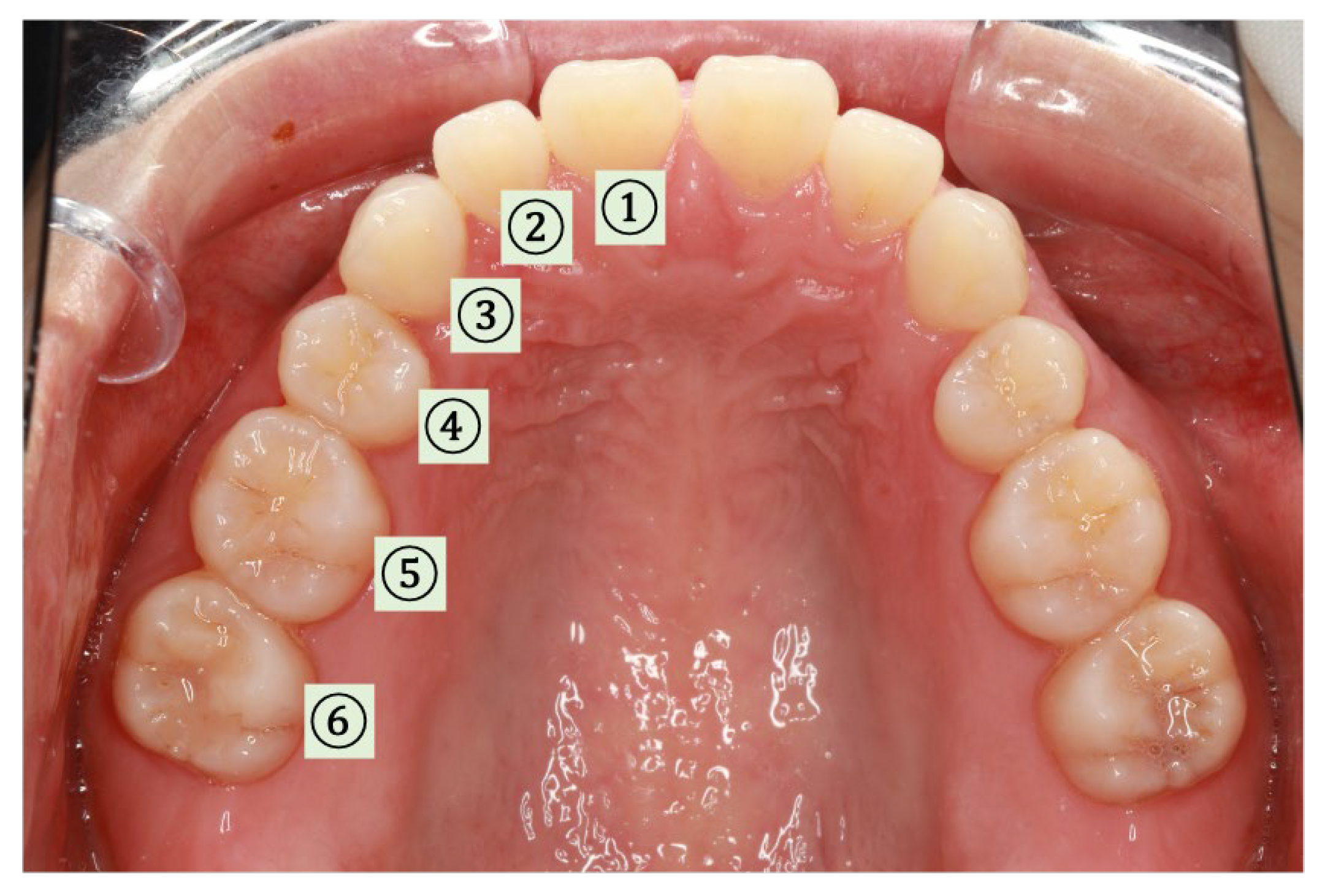
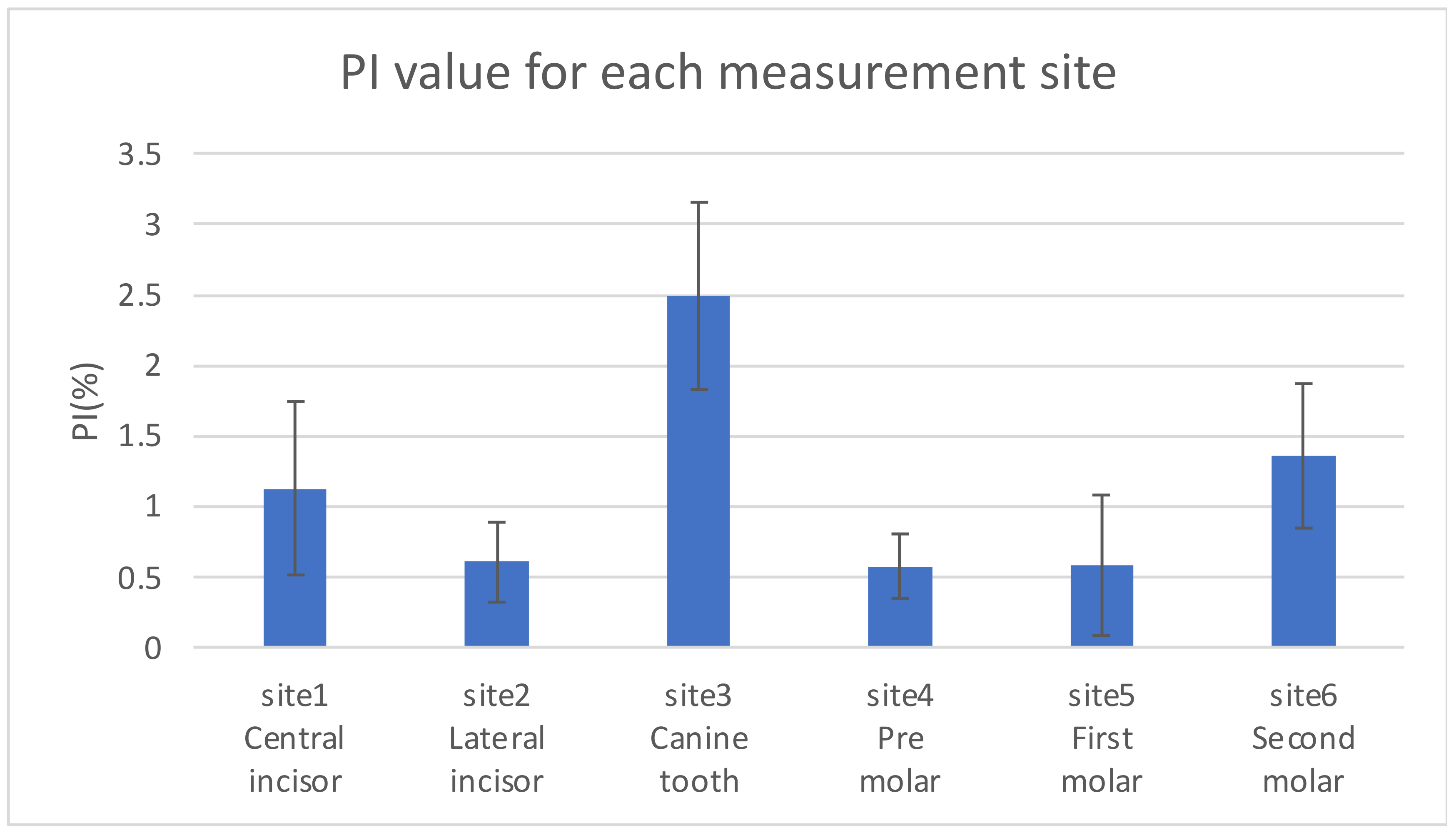
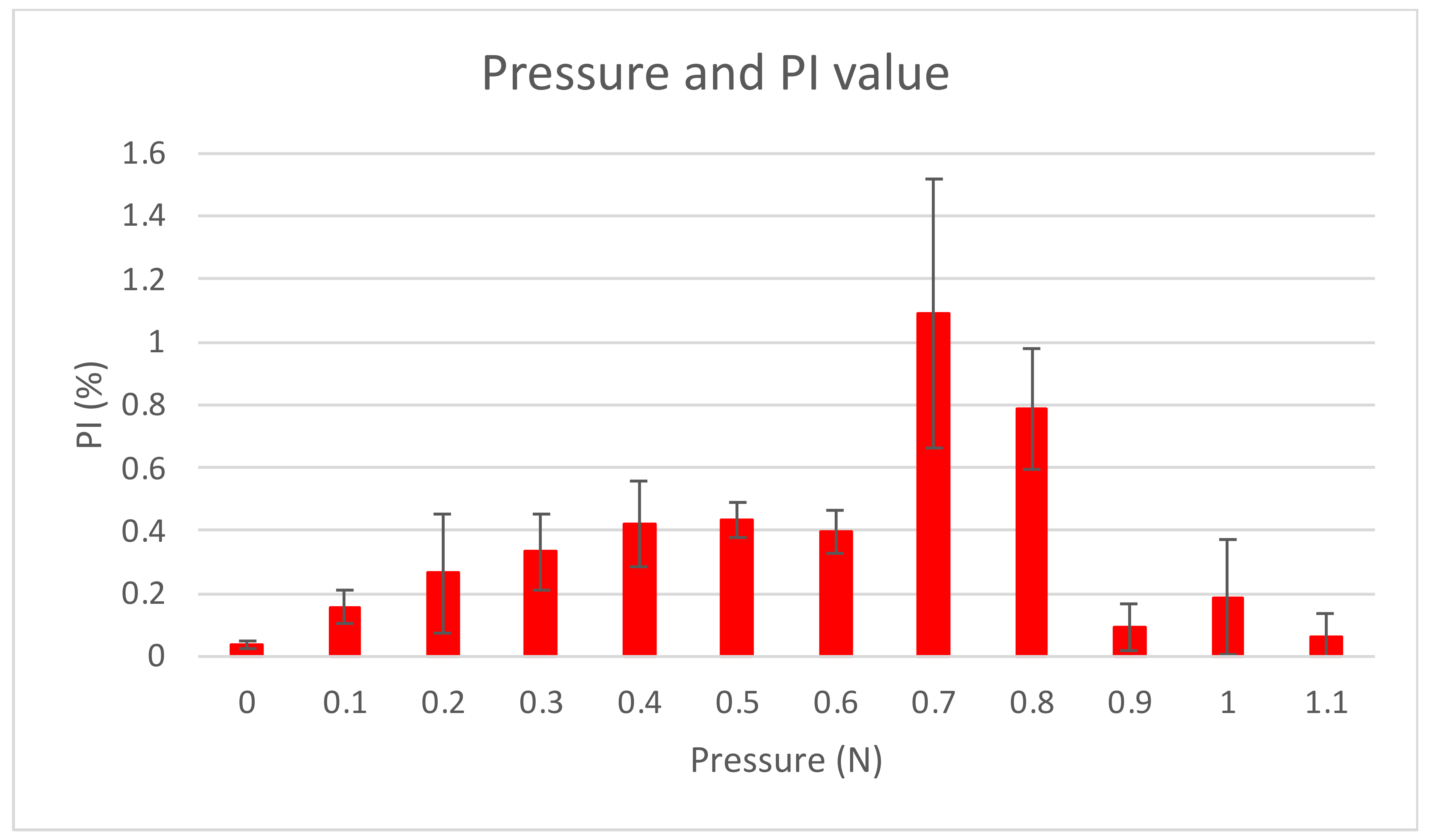
2.3.3. SpO2 and Pressure Measurements at Different Locations (Intraoral)
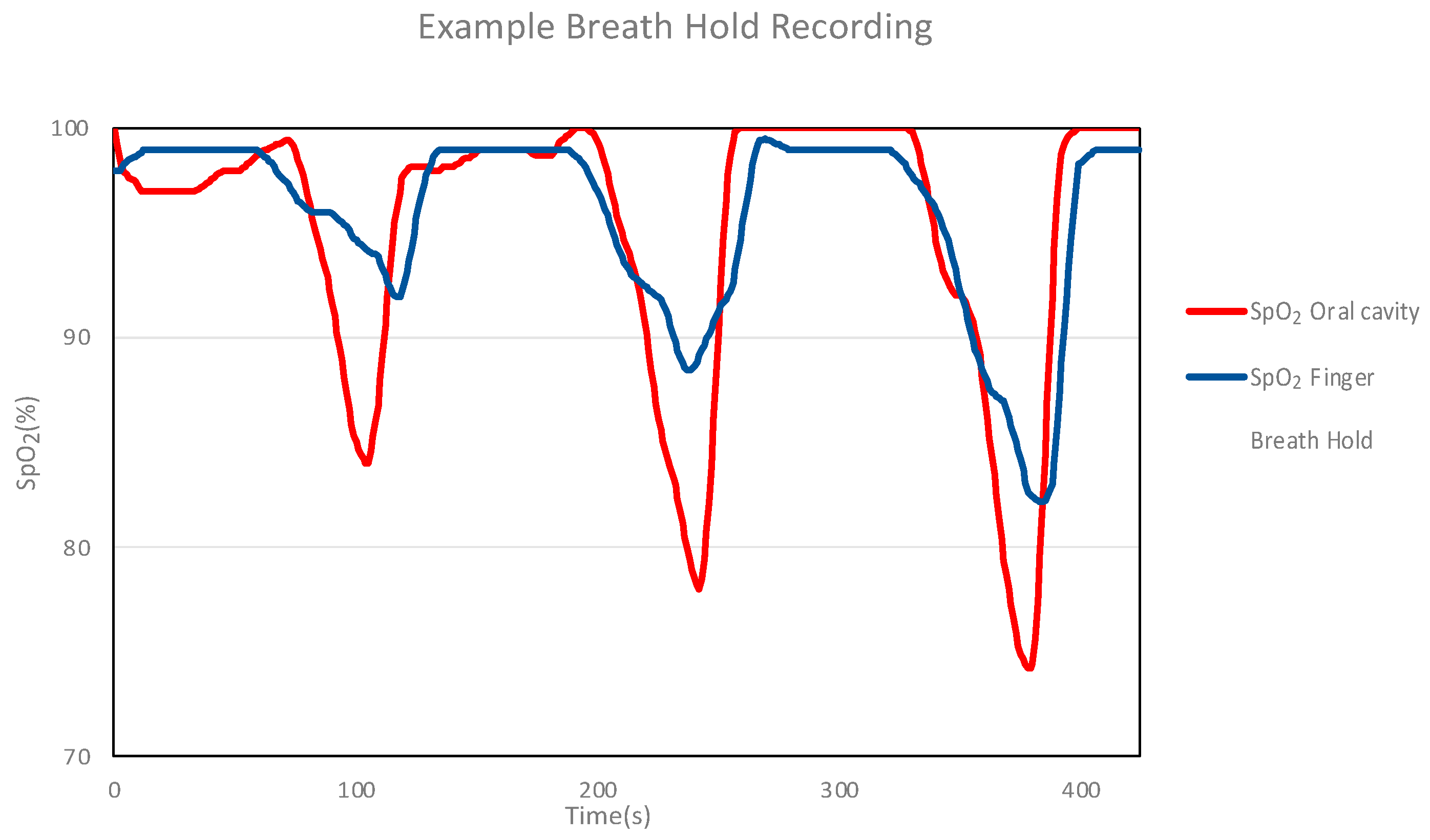
2.3.4. Breath-Holding Measurements Using the Mouthpiece Device (Intraoral and Extraoral)
3. Results
3.1. Accuracy of the Mouthpiece Device (Extraoral)
3.2. Accuracy of the Mouthpiece Device (Intraoral and Extraoral)
3.3. Evaluation of Measured Values Obtained Using the Mouthpiece Device
3.3.1. SpO2 and Pressure (Intraoral)
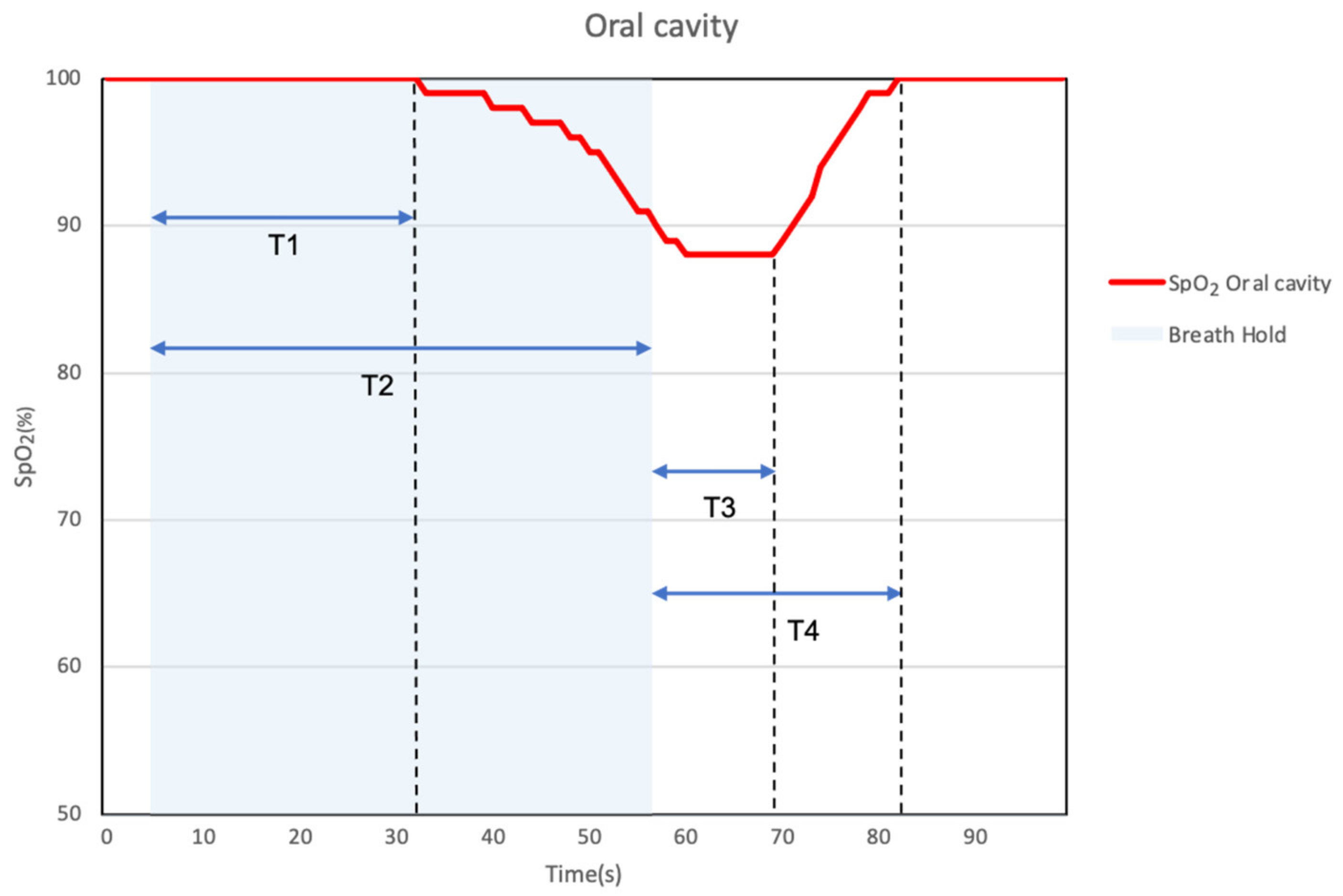
3.3.2. Breath-Holding and SpO2 Response (Intraoral and Extraoral)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hafen, B.B.; Sharma, S. Oxygen Saturation. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Koseeyaporn, J.; Wardkein, P.; Sinchai, A.; Kainan, P.; Tuwanut, P. Pulse Oximetry Based on Quadrature Multiplexing of the Amplitude Modulated Photoplethysmographic Signals. Sensors 2023, 23, 6106. [Google Scholar] [CrossRef]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Banik, P.P.; Hossain, S.; Kwon, T.-H.; Kim, H.; Kim, K.-D. Development of a wearable reflection-type pulse oximeter system to acquire clean PPG signals and measure pulse rate and SpO2 with and without finger motion. Electronics 2020, 9, 1905. [Google Scholar] [CrossRef]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef]
- MacLeod, D.B.; Cortinez, L.I.; Keifer, J.C.; Cameron, D.; Wright, D.R.; White, W.D.; Moretti, E.W.; Radulescu, L.R.; Somma, J. The desaturation response time of finger pulse oximeters during mild hypothermia. Anaesthesia 2005, 60, 65–71. [Google Scholar] [CrossRef]
- Budidha, K.; Kyriacou, P.A. In vivo investigation of ear canal pulse oximetry during hypothermia. J. Clin. Monit. Comput. 2018, 32, 97–107. [Google Scholar] [CrossRef]
- Masè, M.; Micarelli, A.; Strapazzon, G. Hearables: New Perspectives and Pitfalls of In-Ear Devices for Physiological Monitoring. A Scoping Review. Front. Physiol. 2020, 11, 568886. [Google Scholar] [CrossRef]
- Pal, S.K.; Kyriacou, P.A.; Kumaran, S.; Fadheel, S.; Emamdee, R.; Langford, R.M.; Jones, D.P. Evaluation of oesophageal reflectance pulse oximetry in major burns patients. Burns 2005, 31, 337–341. [Google Scholar] [CrossRef]
- Reisner, A.; Shaltis, P.A.; McCombie, D.; Asada, H.H. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology 2008, 108, 950–958. [Google Scholar] [CrossRef]
- Tamura, T. Current progress of photoplethysmography and SPO(2) for health monitoring. Biomed. Eng. Lett. 2019, 9, 21–36. [Google Scholar] [CrossRef]
- Torp, K.D.; Modi, P.; Simon, L.V. Pulse Oximetry. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023. [Google Scholar]
- Budidha, K.; Kyriacou, P.A. The human ear canal: Investigation of its suitability for monitoring photoplethysmographs and arterial oxygen saturation. Physiol. Meas. 2014, 35, 111–128. [Google Scholar] [CrossRef]
- Hickey, M.; Samuels, N.; Randive, N.; Langford, R.M.; Kyriacou, P.A. An in vivo investigation of photoplethysmographic signals and preliminary pulse oximetry estimation from the bowel using a new fiberoptic sensor. Anesth. Analg. 2011, 112, 1104–1109. [Google Scholar] [CrossRef]
- Kyriacou, P.A.; Jones, D.P.; Langford, R.M.; Petros, A.J. A pilot study of neonatal and pediatric esophageal pulse oximetry. Anesth. Analg. 2008, 107, 905–908. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, Y.; Salipante, P.; Kotula, A.P.; Lipshutz, S.; Graves, D.T.; Alimperti, S. Mechanical Regulation of Oral Epithelial Barrier Function. Bioengineering 2023, 10, 517. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Hamdoon, S.M.; AlSamak, S.; Ahmed, M.K.; Gasgoos, S. Evaluation of biofilm formation on different clear orthodontic retainer materials. J. Orthod. Sci. 2022, 11, 34. [Google Scholar]
- Shiotsu, R.; Yoshida, Y.; Haga, S.; Maki, K. Development of a new intraoral sensing system for measuring the wearing time of clear aligner. Jpn. J. Digit. Dent. 2022, 11, 118–126. [Google Scholar]
- Morikawa, K.; Isogai, R.; Nonaka, J.; Yoshida, Y.; Haga, S.; Maki, K. A New Intraoral Six-Degrees-of-Freedom Jaw Movement Tracking Method Using Magnetic Fingerprints. Sensors 2022, 22, 8923. [Google Scholar] [CrossRef]
- Corsini, I.; Cecchi, A.; Coviello, C.; Dani, C. Perfusion index and left ventricular output correlation in healthy term infants. Eur. J. Pediatr. 2017, 176, 1013–1018. [Google Scholar] [CrossRef]
- Tulay, C.M.; Gurmen, E.S. Dyspnea: Perfusion index and triage status. J. Clin. Monit. Comput. 2023, 37, 1103–1108. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Measurement in medicine: The analysis of method comparison studies. J. R. Stat. Soc. Ser. D Stat. 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef]
- Davies, H.J.; Williams, I.; Peters, N.S.; Mandic, D.P. In-Ear Measurement of Blood Oxygen Saturation: An Ambulatory Tool Needed To Detect The Delayed Life-Threatening Hypoxaemia in COVID-19. arXiv 2020, arXiv:2006.04231. [Google Scholar]
- Ueno, D.; Sekiguchi, R.; Morita, M.; Jayawardena, A.; Shinpo, S.; Sato, J.; Kobayashi, K. Palatal mucosal measurements in a Japanese population using cone-beam computed tomography. J. Esthet. Restor. Dent. 2014, 26, 48–58. [Google Scholar] [CrossRef]
- Sampietro-Martínez, R.; Pérez-Monreal, J.; Sánchez-Torres, A.; Bara-Casaus, J.; Gay-Escoda, C. Color doppler ultrasound for the assessment of palatal fibromucosa thickness and the trajectory of the greater palatine artery: A pilot study. J. Clin. Exp. Dent. 2022, 14, e528–e533. [Google Scholar] [CrossRef]
- Teng, X.; Zhang, Y.-T. The effect of contacting force on photoplethysmographic signals. Physiol. Meas. 2004, 25, 1323. [Google Scholar] [CrossRef]
- Choi, S.J.; Ahn, H.J.; Yang, M.K.; Kim, C.S.; Sim, W.S.; Kim, J.A.; Kang, J.G.; Kim, J.K.; Kang, J.Y. Comparison of desaturation and resaturation response times between transmission and reflectance pulse oximeters. Acta Anaesthesiol. Scand. 2010, 54, 212–217. [Google Scholar] [CrossRef]
- McCullough, L.; Arora, S. Diagnosis and treatment of hypothermia. Am. Fam. Physician 2004, 70, 2325–2332. [Google Scholar]
- Van Veelen, M.J.; Brodmann Maeder, M. Hypothermia in Trauma. Int. J. Environ. Res. Public Health 2021, 18, 8719. [Google Scholar] [CrossRef]
- Rauch, S.; Miller, C.; Bräuer, A.; Wallner, B.; Bock, M.; Paal, P. Perioperative Hypothermia—A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 8749. [Google Scholar] [CrossRef]
- Paal, P.; Pasquier, M.; Darocha, T.; Lechner, R.; Kosinski, S.; Wallner, B.; Zafren, K.; Brugger, H. Accidental Hypothermia: 2021 Update. Int. J. Environ. Res. Public Health 2022, 19, 501. [Google Scholar] [CrossRef]
- Kelly, K.L.; Carlson, A.R.; Allison, T.G.; Johnson, B.D. A comparison of finger and forehead pulse oximeters in heart failure patients during maximal exercise. Heart Lung 2020, 49, 259–264. [Google Scholar] [CrossRef]
- Wick, K.D.; Matthay, M.A.; Ware, L.B. Pulse oximetry for the diagnosis and management of acute respiratory distress syndrome. Lancet Respir. Med. 2022, 10, 1086–1098. [Google Scholar] [CrossRef]



| LED wavelength (nm) | 660 for red, 880 for IR |
| LED current (mA) | 7 for red and IR |
| LED radiation power (mW) | Approx. 3.2 for red, 2.7 for IR |
| Sampling rate (sps) | 50 |
| Averaging (pts) | 2 *1 |
| LED pulse width (μs) | 123.8 |
| LED settling time (μs) | 12 *2 |
| Photodiode (PD) spectral bandwidth (nm) | 420 to 1020 (860 nm at peak) |
| Evaluation of Time, T1–T4 | p-Value | ||
|---|---|---|---|
| Mean Value (Second) | SD | ||
| T1 | 7.4 | 2.8 | 0.01 * |
| T2 | 7.3 | 2.3 | 0.004 * |
| T3 | 7.4 | 1.6 | <0.0001 * |
| T4 | 6.5 | 1.9 | 0.002 * |
| Mean Value (%) | SD | p-Value | |
|---|---|---|---|
| Lowest point | 4.0 | 0.9 | 0.0002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashima, Y.; Onimaru, M.; Isogai, R.; Kawai, N.; Yoshida, Y.; Maki, K. The Development of a Measuring System for Intraoral SpO2. Sensors 2024, 24, 435. https://doi.org/10.3390/s24020435
Kashima Y, Onimaru M, Isogai R, Kawai N, Yoshida Y, Maki K. The Development of a Measuring System for Intraoral SpO2. Sensors. 2024; 24(2):435. https://doi.org/10.3390/s24020435
Chicago/Turabian StyleKashima, Yuki, Minako Onimaru, Ryosuke Isogai, Noboru Kawai, Yoshifumi Yoshida, and Koutaro Maki. 2024. "The Development of a Measuring System for Intraoral SpO2" Sensors 24, no. 2: 435. https://doi.org/10.3390/s24020435
APA StyleKashima, Y., Onimaru, M., Isogai, R., Kawai, N., Yoshida, Y., & Maki, K. (2024). The Development of a Measuring System for Intraoral SpO2. Sensors, 24(2), 435. https://doi.org/10.3390/s24020435






