A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of Cobalt Oxide Nanoparticles
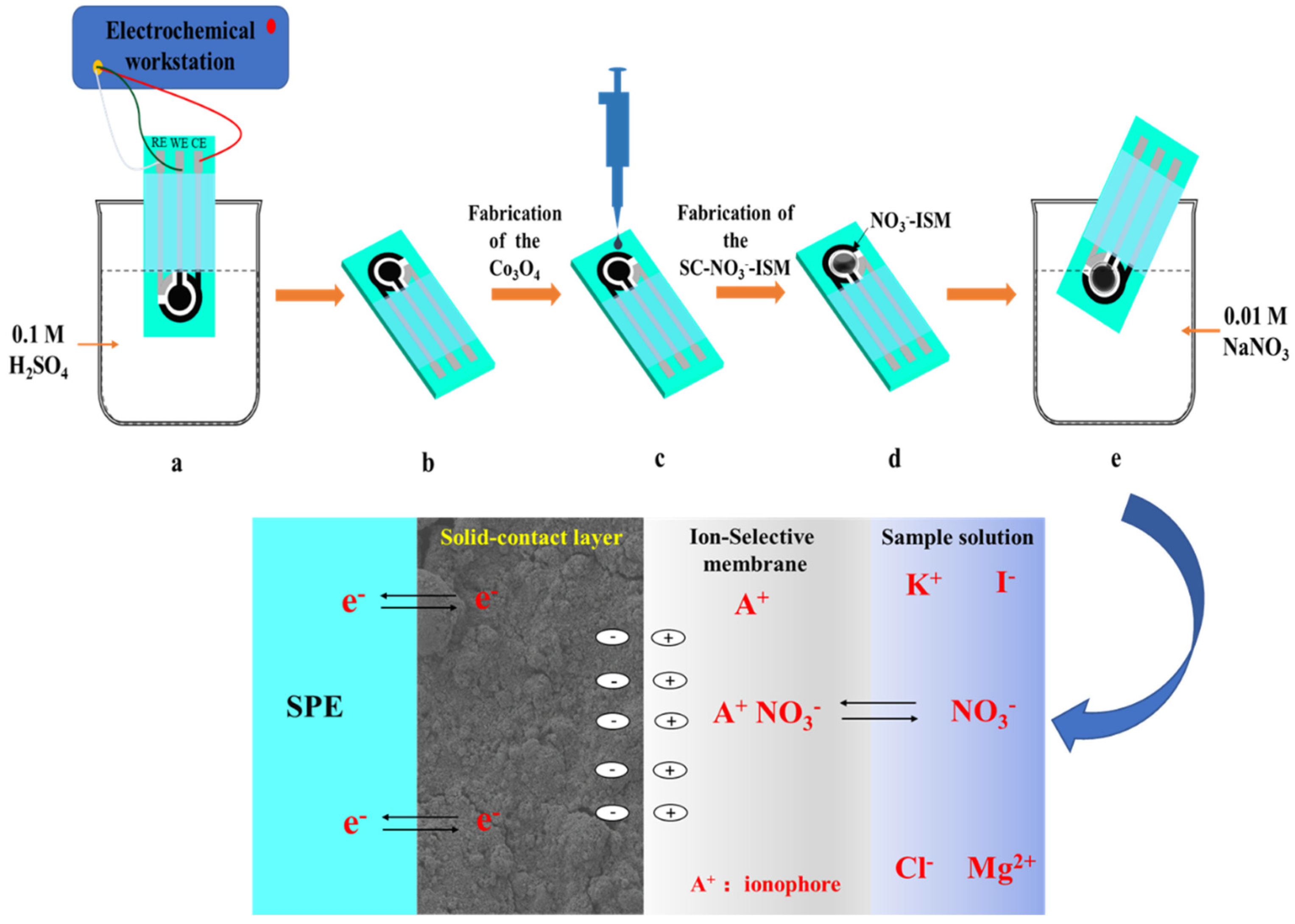
2.3. Preparation of Nitrate Ion-Selective Electrodes
3. Results and Discussions
3.1. Parameter Optimization for NO3−-ISM/Co3O4 NPs/SPE Fabrication
3.2. Physical Properties of the Synthesized Co3O4 Nanoparticles
3.3. Electrochemical Analysis of the Proposed Sensors
3.4. Chronopotentiometric Test
3.5. Potentiometric Water Layer Tests
3.6. Selectivity of the NO3−-ISM/SPE and NO3−-ISM/Co3O4 NPs/SPE
3.7. Effect of pH on the Response of the NO3−-ISM/Co3O4 NPs/SPE for NO3− Detection
3.8. Enhanced Sensing Principle of the NO3−-ISM/Co3O4 NPs/SPE
3.9. Potentiometric Characteristics
3.10. Lifetime Study for the NO3−-ISM/Co3O4 NPs/SPE
3.11. Analyses of Real Water Samples from an Aquaponic System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Li, W.; McEvan, M.; Li, D.; Lian, G.; Chen, T. Adaptive filtering-based soft sensor method for estimating total nitrogen in aquaponic systems. Comput. Electron. Agric. 2021, 186, 106175. [Google Scholar] [CrossRef]
- Colt, J.; Schuur, A.M. Comparison of nutrient costs from fish feeds and inorganic fertilizers for aquaponics systems. Aquac. Eng. 2021, 95, 102205. [Google Scholar] [CrossRef]
- Li, L.; Tan, L.; Yang, W.; Xu, X.; Shen, Y.; Li, J. Conjoint applications of meta-analysis and bioinformatic data toward understanding the effect of nitrate on fish. Sci. Total Environ. 2021, 794, 148645. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, S.; Jensen, F.B.; Huong, D.T.T.; Wang, T.; Phuong, N.T.; Bayley, M. Effect of nitrite exposure on functional haemoglobin levels, bimodal respiration, and swimming performance in the facultative air-breathing fish Pangasianodon hypophthalmus. Aquat. Toxicol. 2011, 104, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, Y.; Wang, Y.; Xu, S.; Zhou, L.; Li, J.; Li, X. Chronic nitrate exposure cause alteration of blood physiological parameters, redox status and apoptosis of juvenile turbot (Scophthalmus maximus). Environ. Pollut. 2021, 283, 117103. [Google Scholar] [CrossRef]
- Conlin, S.M.; Tudor, M.S.; Shim, J.; Gosse, J.A.; Neilson, A.; Hamlin, H.J. Elevated nitrate alters the metabolic activity of embryonic zebrafish. Environ. Pollut. 2018, 235, 180–185. [Google Scholar] [CrossRef]
- Li, G.; Yuan, H.; Mou, J.; Dai, E.; Zhang, H.; Li, Z.; Zhao, Y.; Dai, Y.; Zhang, X. Electrochemical detection of nitrate with carbon nanofibers and copper commodified carbon fiber electrodes. Compos. Comun. 2022, 29, 101043. [Google Scholar] [CrossRef]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical detection of nitrate, nitrite and ammonium for on-site water quality monitoring. Curr. Opin. Electrochem. 2022, 32, 100926. [Google Scholar] [CrossRef]
- Gapper, L.W.; Fong, B.Y.; Otter, D.E.; Indyk, H.E.; Woollard, D.C. Determination of nitrite and nitrate in dairy products by ion exchange LC with spectrophotometric detection. Int. Dairy J. 2004, 14, 881–887. [Google Scholar] [CrossRef]
- Kazemzadeh, A.; Ensafi, A.A. Simultaneous detemination of nitrite and nitrate in various samples using flow-injection spectrophotometric detection. Microchem. J. 2001, 69, 159–166. [Google Scholar] [CrossRef]
- Liu, F.; Reinmuth-Selzle, K.; Lai, S.; Weller, M.G.; Poschl, U.; Kampf, C.J. Simultaneous determination of nitrated and oligomerized proteins by size exclusion high-performance liquid chromatography coupled to photodiode array detection. J. Chromatogr. A 2017, 1495, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Tuma, P. The use of polarity switching for the sensitive determination of nitrate in human cerebrospinal fluid by capillary electrophoresis with contactless conductivity detection. J. Chromatogr. A 2016, 1447, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Anthis, A.H.C.; Afshar, M.G.; Pankratova, N.; Cuartero, M.; Crespo, G.A.; Bakker, E. All-Solid-State Potentiometric Sensors with Multi-Walled Carbon Nanotube Inner Transducing Layer for Anion Detection in Environmental Samples. Anal. Chem. 2015, 87, 8640–8645. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ion-selective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Zhang, M.; Zhang, F.; He, P. Home Detection Technique for Na+ and K+ in Urine Using a Self-Calibrated all-Solid-State Ion-Selective Electrode Array Based on Polystyrene-Au Ion-Sensing Nanocomposites. Anal. Chem. 2021, 93, 8318–8325. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Qin, W. A solid-contact Ca2+ ion-selective electrode based on an inorganic redox buffer of Ag@AgCl/1-tetradecyl-3-methylimidazolium chloride as ion-to-electron transducer. Talanta 2019, 209, 120570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, X.; Waterhouse, G.I.N.; Jiang, X.; Zhang, Z.; Yu, L. Potential stability improvement in Pb2+ ion selective electrodes by applying hydrophobic polyaniline as ion-to-electron transducer. Synth. Met. 2021, 281, 116898. [Google Scholar] [CrossRef]
- He, N.; Gyurcsanyi, R.E.; Lindfors, T. Electropolymerized hydrophobic polyazulene as solid-contact in potassium-selective electrodes. Analyst 2016, 141, 2990–2997. [Google Scholar] [CrossRef]
- Kojima, J.; Uchiyama, K.; Yoshida, Y. Influence of solid electrolyte upon the repeatability and reproducibility of all-solid-state io-selective electrodes with inorganic insertion material paste. Electrochim. Acta 2021, 373, 137896. [Google Scholar] [CrossRef]
- Vazquez, M.; Bobacka, J.; Ivaska, A.; Lewenstam, A. Influence of oxygen and carbon dioxide on the electrochemical stability of poly(3,4-ethylenedioxythiophene) used as ion-to-electron transducer in all-solid-state ion-selective electrodes. Sens. Actuators B 2002, 82, 7–13. [Google Scholar] [CrossRef]
- Pietrzak, K.; Krstulovic, N.; Blazeka, D.; Car, J.; Malinowski, S.; Wardak, C. Metal oxide nanoparticles as solid contact in ion-selective electrodes sensitive to potassium ions. Talanta 2022, 243, 123335. [Google Scholar] [CrossRef] [PubMed]
- Arjomandi, J.; Lee, J.Y.; Ahmadi, F.; Parvin, M.H.; Moghanni-Bavil-Olyaei, H. Spectroelectrochemistry and electrosynthesis of polypyrrole supercapacitor electrodes based on gamma aluminum oxide and gamma iron (III) oxide nanocomposites. Electrochim. Acta 2017, 251, 212–222. [Google Scholar] [CrossRef]
- Shen, H.; Gele, A. Facile synthesis of N-doped lignin-based carbon nanofibers decorated with iron oxides for flexible supercapacitor electrodes. Inorg. Chem. Commun. 2021, 128, 108607. [Google Scholar] [CrossRef]
- Liu, J.; Jin, R.; YinaQiao; Wu, Y.; Wang, X.; Wang, Y. Determination of Lead (II) Using Glassy Carbon Electrode Modified with Hexagonal Co3O4 Microparticles. Int. J. Electrochem. Sci. 2018, 13, 10415–10426. [Google Scholar] [CrossRef]
- Nallusamy, S.; Sujatha, K. Experimental analysis of nanoparticles with cobalt oxide synthesized by coprecipitation method on electrochemical biosensor using FTIR and TEM. Mater. Today Proc. 2021, 37, 728–732. [Google Scholar] [CrossRef]
- Perachiselvi, M.; Samraj, J.J.; Bagavathy, M.S.; Pushpalaksmi, E.; Annadura, G. Facile Synthesis of Cobalt Oxide Nanoparticle for Biological Studies. Appl. Ecol. Environ. Sci. 2020, 8, 269–272. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; van Le, Q.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloy. Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Buffon, E.; Stradiotto, N.R. A molecularly imprinted polymer on reduced graphene oxide-gold nanoparticles modified screen-printed electrode for selective determination of ferulic acid in orange peels. Microchem. J. 2021, 167, 106339. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Jang, H.; Shokouhimehr, M. A screen-printed electrode modified with Fe3O4@polypyrrole-Pt core-shell nanoparticles for electrochemical detection of 6-mercaptopurine and 6-thioguanine. Talanta 2021, 232, 122379. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mahmoudi-Moghaddam, H.; Tajik, S.; Jahani, S. A modified screen-printed electrode based on La3+-doped Co3O4 nanocubes for determination of sulfite in real samples. Microchem. J. 2019, 147, 590–597. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Costa-García, A.; de Muñiz, A. Electrochemical (Bio)Sensors for Pesticides Detection Using Screen-Printed Electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.; Lu, D.; Senecal, A.; Kurup, P. Voltammetric detection of arsenic (III) using gold nanoparticles modified carbon screen printed electrodes: Application for facile and rapid analysis in commercial apple juice. Food Chem. 2021, 352, 129327. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2015, 183, 503–517. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galata, G.; de Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Su, L.; Yang, C.; Ma, Y.; Zhang, H.; Chen, X. Colorimetric Detection of Sulfite in Foods by a TMB-O2-Co3O4 Nanoparticles Detection System. J. Agric. Food Chem. 2014, 62, 5827–5834. [Google Scholar] [CrossRef]
- Tang, W.; Ping, J.; Fan, K.; Wang, Y.; Luo, X.; Ying, Y.; Wu, J.; Zhou, Q. All-solid-state nitrate-selective electrode and its application in drinking water. Electrochim. Acta 2011, 81, 186–190. [Google Scholar] [CrossRef]
- Thangavelu, K.; Parameswari, K.; Kuppusamy, K.; Haldorai, Y. A simple and facile method to synthesize Co3O4 nanoparticles from metal benzoate dihydrazinate complex as a precursor. Mater. Lett. 2011, 65, 1482–1484. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Thatoi, D.N.; Sahoo, P.K.; Soam, A. Synthesis and characterization of cobalt oxide (Co3O4) nanoparticles. Mater. Today Proc. 2021, 41, 269–271. [Google Scholar] [CrossRef]
- Moro, F.; Tang, S.V.Y.; Tuna, F.; Lester, E. Magnetic properties of cobalt oxide nanoparticles synthesized by a continuous hydrothermal method. J. Magn. Magn. Mater. 2013, 348, 1–7. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, J.; Tour, J.M. Laser-induced graphene synthesis of Co3O4 in graphene for oxygen electrocatalysis and metal-air batteries. Carbon 2018, 139, 880–887. [Google Scholar] [CrossRef]
- Thuy, N.T.D.; Zhao, G.; Wang, X.; Awuah, E.; Zhang, L. Potassium ion-selective electrode with a sensitive ion-to-electron transducer composed of porous laser-induced graphene and MoS2 fabricated by one-step direct laser writing. Electroanalysis 2022. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Al Nafiey, A.; Addad, A.; Sieber, B.; Chastanet, G.; Barras, A.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide decorated with Co3O4 nanoparticles (rGO-Co3O4) nanocomposite: A reusable catalyst for highly efficient reduction of 4- nitrophenol, and Cr(VI) and dye removal from aqueous solutions. Chem. Eng. J. 2017, 322, 375–384. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef] [PubMed]
- Garland, N.T.; McLamore, E.S.; Cavallaro, N.D.; Mendivelso-Perez, D.; Smith, E.A.; Jing, D.; Claussen, J.C. Flexible Laser-Induced Graphene for Nitrogen Sensing in Soil. ACS Appl. Mater. Interfaces 2018, 10, 39124–39133. [Google Scholar] [CrossRef] [PubMed]
- Paczosa-Bator, B. Effects of type of nanosized carbon black on the performance of an all-solid-state potentiometric electrode for nitrate. Microchim. Acta 2014, 181, 1093–1099. [Google Scholar] [CrossRef]












| Interfering Ion | Concentration (mg L−1) | Selectivity Coefficient | |
|---|---|---|---|
| NO3−-ISM/SPE | NO3−-ISM/Co3O4 NPs/SPE | ||
| SO42− | 0.1 | −5.7 ± 0.2 | −5.4 ± 0.3 |
| Br− | 0.1 | −3.5 ± 0.2 | −3.8 ± 0.2 |
| Cl− | 0.1 | −3.0 ± 0.3 | −3.1 ± 0.1 |
| CO32− | 0.1 | −4.3 ± 0.2 | −4.8 ± 0.2 |
| I− | 0.1 | −3.1 ± 0.1 | −2.8 ± 0.1 |
| K+ | 0.1 | −5.8 ± 0.1 | −5.7 ± 0.2 |
| Na+ | 0.1 | −4.9 ± 0.2 | −4.5 ± 0.2 |
| Mg+ | 0.1 | −4.1 ± 0.3 | −3.6 ± 0.3 |
| Aquaculture Water Composition Parameters | Concentration | Value |
|---|---|---|
| NO3− | mg/L | 5.27 |
| NO2− | mg/L | 0.11 |
| NH4+ | mg/L | 21.2 |
| pH | 7.06 | |
| SS | mg/L | 16 |
| BOD | mg/L | 9.1 |
| DO | mg/L | 0.66 |
| Chlorophyll a | µg/L | 1.85 |
| Sample | NO3−-ISM/Co3O4 NPs/SPE (×10−5 M) | Spectrophotometer (×10−5 M) |
|---|---|---|
| Aquaculture water | 7.98 ± 0.06 | 8.50 ± 0.02 |
| Yangtze River water | 1.81 ± 0.04 | 1.69 ± 0.03 |
| Domestic water | 1.58 ± 0.02 | 1.62 ± 0.02 |
| Tap water | 2.02 ± 0.02 | 1.97 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuy, N.T.D.; Wang, X.; Zhao, G.; Liang, T.; Zou, Z. A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors 2022, 22, 9730. https://doi.org/10.3390/s22249730
Thuy NTD, Wang X, Zhao G, Liang T, Zou Z. A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors. 2022; 22(24):9730. https://doi.org/10.3390/s22249730
Chicago/Turabian StyleThuy, Nguyen Thi Dieu, Xiaochan Wang, Guo Zhao, Tingyu Liang, and Zaihan Zou. 2022. "A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems" Sensors 22, no. 24: 9730. https://doi.org/10.3390/s22249730
APA StyleThuy, N. T. D., Wang, X., Zhao, G., Liang, T., & Zou, Z. (2022). A Co3O4 Nanoparticle-Modified Screen-Printed Electrode Sensor for the Detection of Nitrate Ions in Aquaponic Systems. Sensors, 22(24), 9730. https://doi.org/10.3390/s22249730







