Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines
Abstract
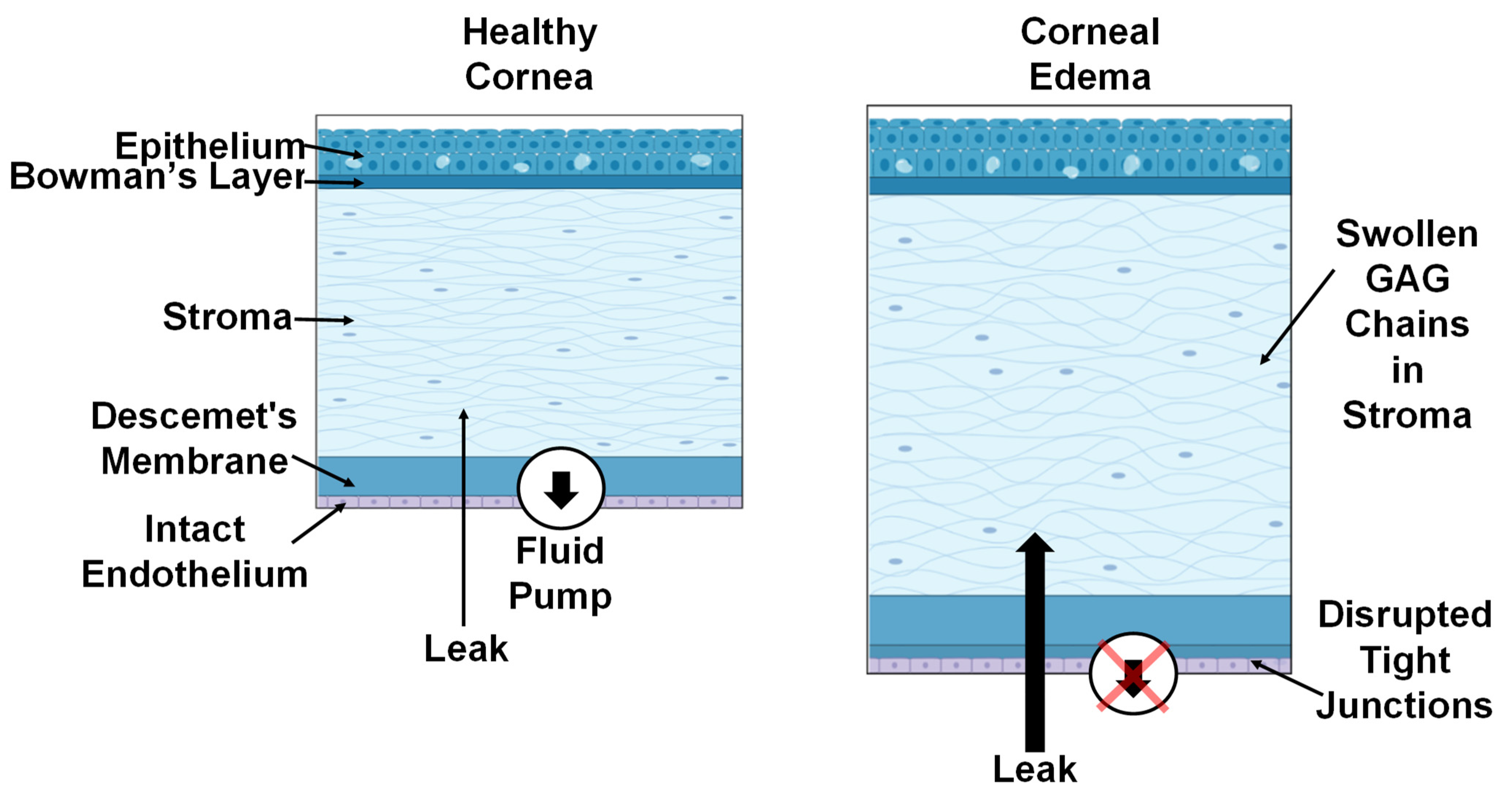
1. Introduction
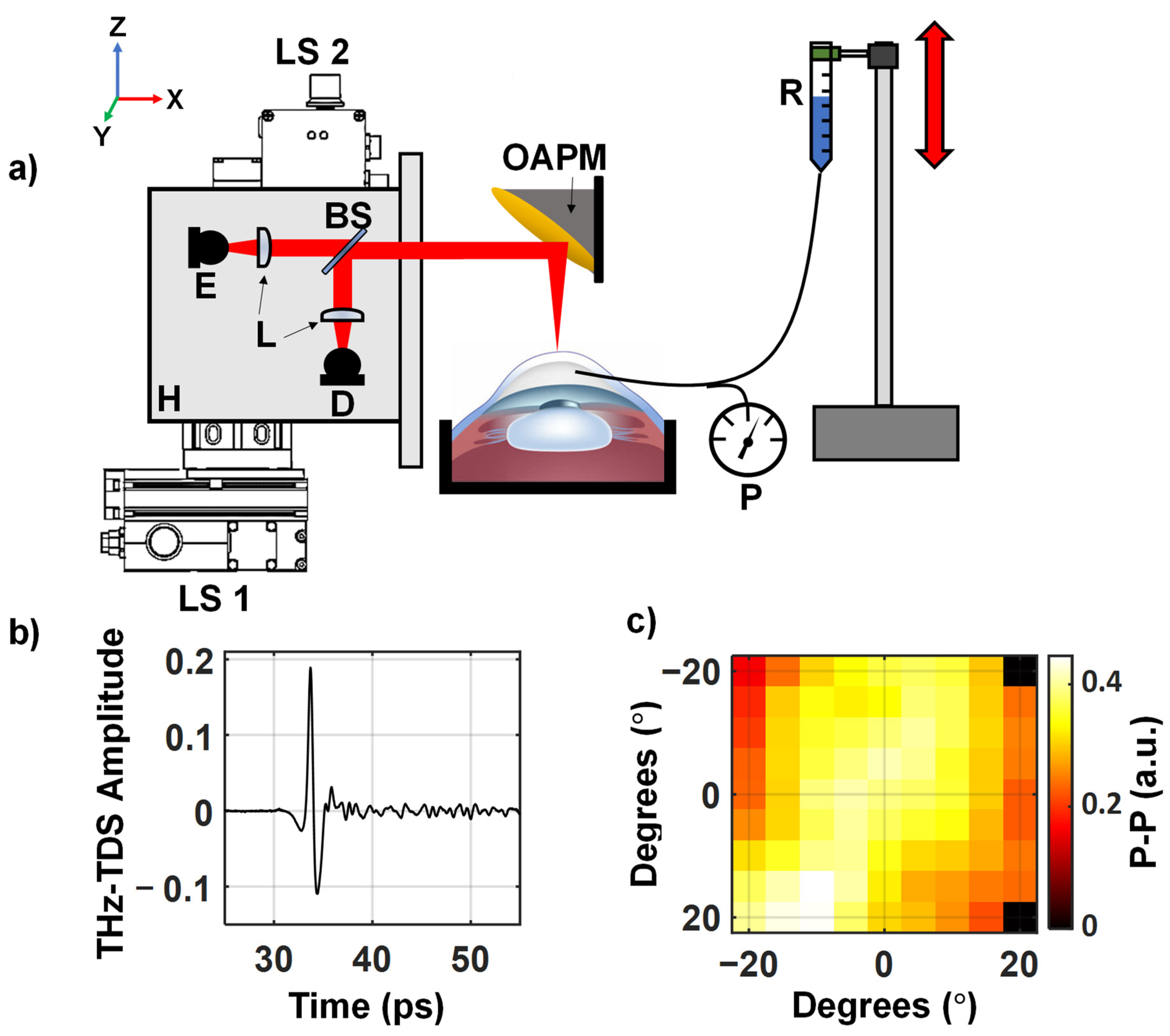
2. Materials and Methods
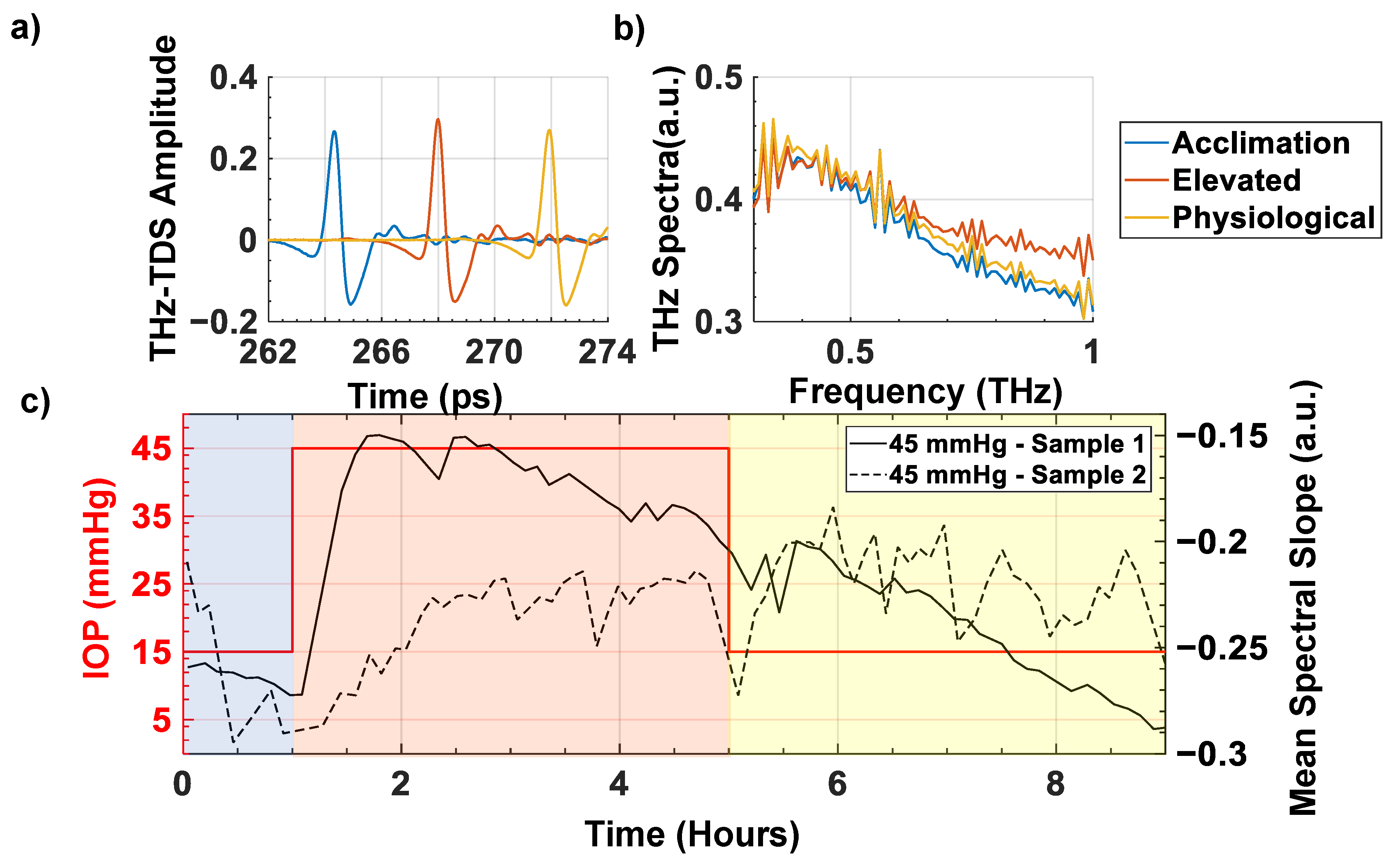
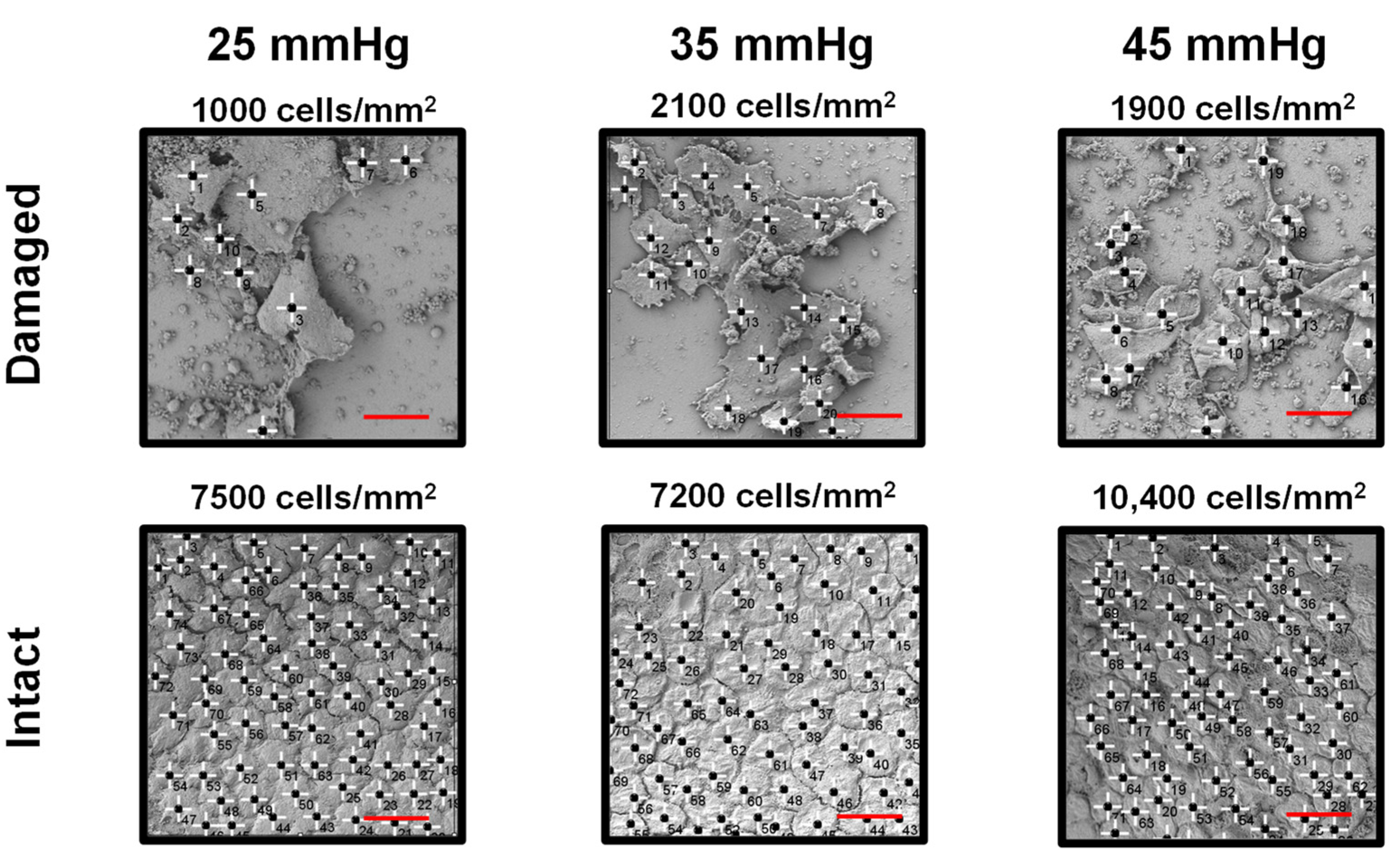
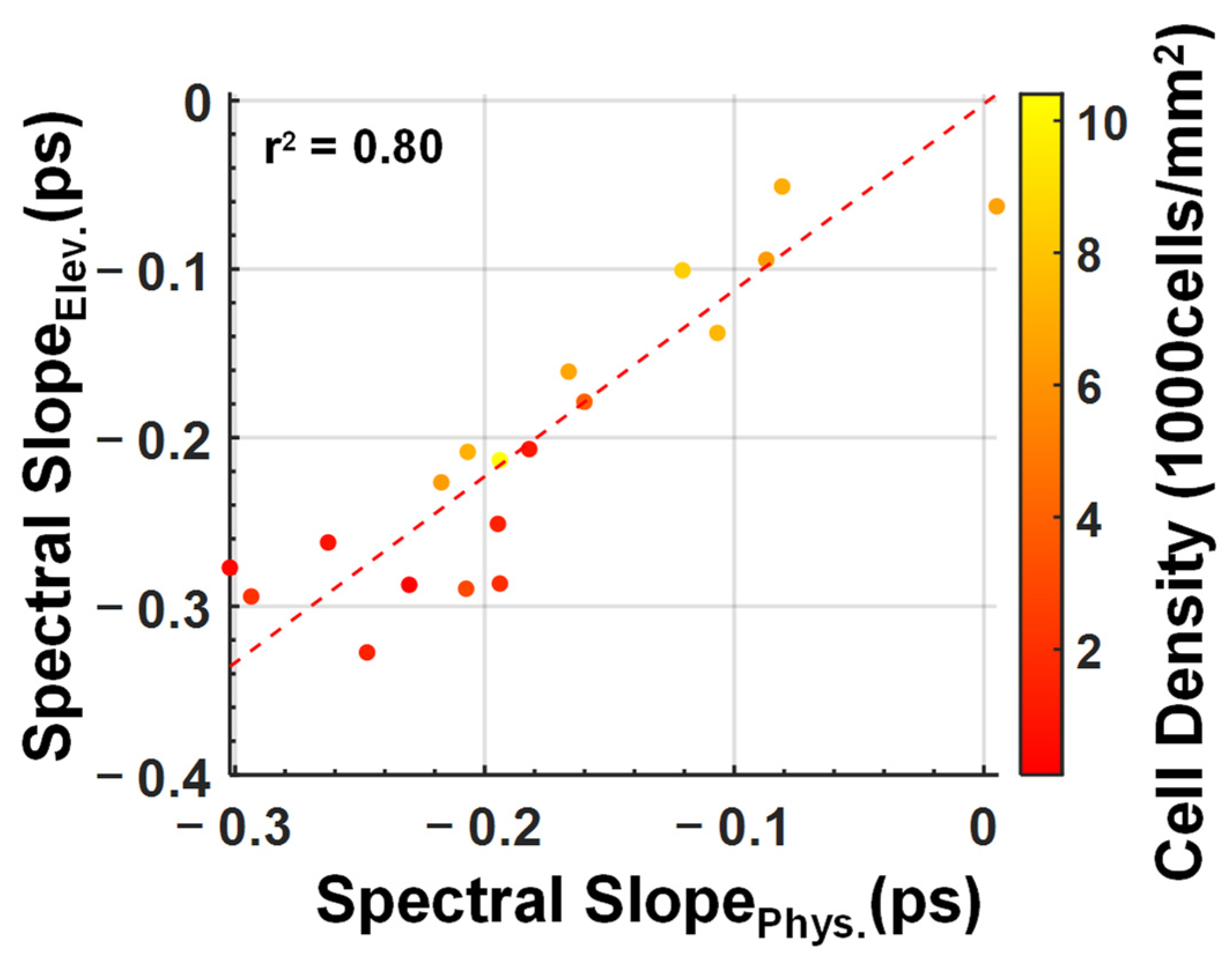
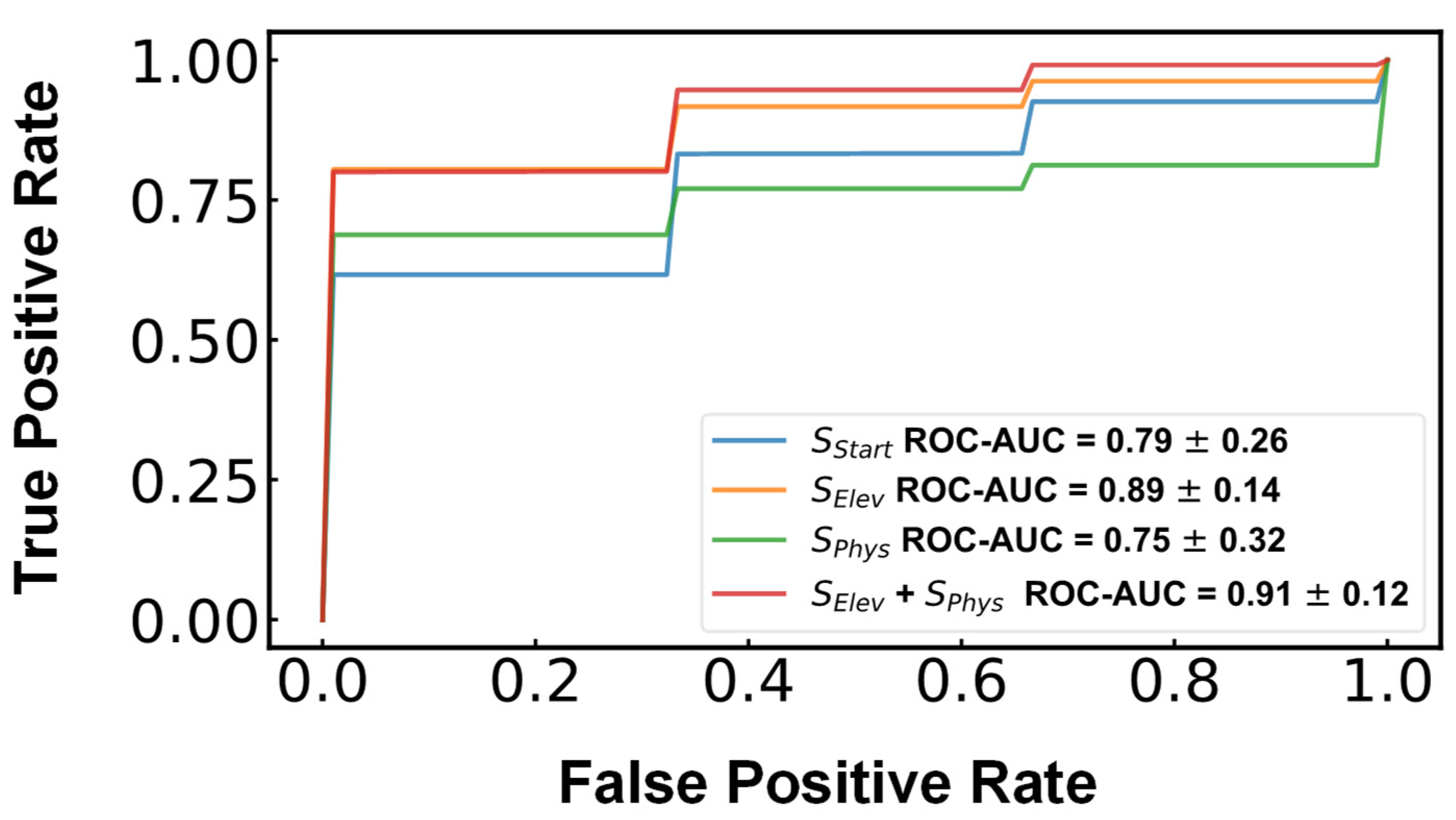
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ytteborg, J.; Dohlman, C.H. Corneal edema and intraocular pressure. II. Clinical results. Arch. Ophthalmol. 1965, 74, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zucker, B.B. Hydration and Transparency of Corneal Stroma. Arch. Ophthalmol. 1966, 75, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pinsky, P.M. Mechanisms of self-organization for the collagen fibril lattice in the human cornea. J. R. Soc. Interface 2013, 10, 20130512. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Petsche, S.J.; Pinsky, P.M. A structural model for the in vivo human cornea including collagen-swelling interaction. J. R. Soc. Interface 2015, 12, 20150241. [Google Scholar] [CrossRef]
- Srinivas, S. Cell Signaling in Regulation of the Barrier Integrity of the Corneal Endothelium. Exp. Eye Res. 2011, 95, 8–15. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kim, E.; Bonanno, J.A. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am. J. Physiol. Cell Physiol. 2016, 311, C116–C126. [Google Scholar] [CrossRef] [PubMed]
- Adamis, A.P.; Filatov, V.; Tripathi, B.J.; Tripathi, R.C. Fuchs’ endothelial dystrophy of the cornea. Surv. Ophthalmol. 1993, 38, 149–168. [Google Scholar] [CrossRef]
- Lefebvre, V.; Sowka, J.W.; Frauens, B.J. The clinical spectrum between posterior polymorphous dystrophy and iridocorneal endothelial syndromes. Optometry 2009, 80, 431–436. [Google Scholar] [CrossRef]
- Bigar, F.; Witmer, R. Corneal Endothelial Changes in Primary Acute Angle-closure Glaucoma. Ophthalmology 1982, 89, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.P.Y.; Broadway, D.C.; Khawaja, A.P.; Yip, J.L.Y.; Garway-Heath, D.F.; Burr, J.M.; Luben, R.; Hayat, S.; Dalzell, N.; Khaw, K.-T.; et al. Glaucoma and intraocular pressure in EPIC-Norfolk Eye Study: Cross sectional study. BMJ 2017, 358, j3889. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.M.; Boisjoly, H.M.; Brunette, I.; Charest, M.; Amyot, M. Corneal endothelial cell density in glaucoma. Cornea 1997, 16, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.A.; D'Oria, F.; Alio, J.L. Surgery for glaucoma in modern corneal graft procedures. Surv. Ophthalmol. 2021, 66, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Corneal Endothel. Health Dis. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sie, N.M.; Yam, G.H.-F.; Soh, Y.Q.; Lovatt, M.; Dhaliwal, D.; Kocaba, V.; Mehta, J.S. Regenerative capacity of the corneal transition zone for endothelial cell therapy. Stem Cell Res. Ther. 2020, 11, 523. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, D.K.; Bahn, C.F.; Lillie, J.H.; Meyer, R.F.; Martonyi, C.L. Evidence for corneal endothelial cell hypertrophy during postnatal growth of the cat cornea. Investig. Ophthalmol. Vis. Sci. 1983, 24, 247–250. [Google Scholar]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Taylor, Z.D.; Garritano, J.; Sung, S.; Bajwa, N.; Bennett, D.B.; Nowroozi, B.; Tewari, P.; Sayre, J.W.; Hubschman, J.-P.; Deng, S.X.; et al. THz and mm-Wave Sensing of Corneal Tissue Water Content: In Vivo Sensing and Imaging Results. IEEE Trans. Terahertz Sci. Technol. 2015, 5, 184–196. [Google Scholar] [CrossRef]
- Ammar, D.A.; Lei, T.C.; Kahook, M.Y.; Masihzadeh, O. Imaging the Intact Mouse Cornea Using Coherent Anti-Stokes Raman scattering (CARS). Investig. Ophthalmol. Vis. Sci. 2013, 54, 5258–5265. [Google Scholar] [CrossRef]
- Shao, P.; Seiler, T.G.; Eltony, A.M.; Ramier, A.; Kwok, S.J.J.; Scarcelli, G.; Ii, R.P.; Yun, S.-H. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3020–3027. [Google Scholar] [CrossRef]
- El-Shenawee, M.; Vohra, N.; Bowman, T.; Bailey, K. Cancer detection in excised breast tumors using terahertz imaging and spectroscopy. Biomed. Spectrosc. Imaging 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Patel, R.; Neel, V.; Giles, R.H.; Yaroslavsky, A.N. Multimodal Optical and Terahertz Biopsy of Nonmelanoma Cancers Skin. In Proceedings of the Biophotonics Congress: Biomedical Optics Congress 2018 (Microscopy/Translational/Brain/OTS), Hollywood, FL, USA, 3–6 April 2018; p. MF4A.4. [Google Scholar]
- George, D.K.; Chen, J.Y.; He, Y.; Knab, J.R.; Markelz, A.G. Functional-State Dependence of Picosecond Protein Dynamics. J. Phys. Chem. B 2021, 125, 11134–11140. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cardoso, G.G.; Amador-Medina, L.F.; Gutierrez-Torres, G.; Reyes-Reyes, E.S.; Benavides Martínez, C.A.; Cardona Espinoza, C.; Arce Cruz, J.; Salas-Gutierrez, I.; Murillo-Ortíz, B.O.; Castro-Camus, E. Terahertz imaging demonstrates its diagnostic potential and reveals a relationship between cutaneous dehydration and neuropathy for diabetic foot syndrome patients. Sci. Rep. 2022, 12, 3110. [Google Scholar] [CrossRef] [PubMed]
- Harris, Z.B.; Arbab, M.H. Terahertz PHASR Scanner with 2 kHz, 100 picosecond Time-Domain Trace Acquisition Rate and an Extended Field-of-View Based on a Heliostat Design. IEEE Trans. Terahertz Sci. Technol. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Harris, Z.B.; Khani, M.E.; Arbab, M.H. Terahertz Portable Handheld Spectral Reflection (PHASR) Scanner. IEEE Access 2020, 8, 228024–228031. [Google Scholar] [CrossRef]
- Harris, Z.B.; Katletz, S.; Khani, M.E.; Virk, A.; Arbab, M.H. Design and characterization of telecentric f-θ scanning lenses for broadband terahertz frequency systems. AIP Adv. 2020, 10, 125313. [Google Scholar] [CrossRef]
- Stantchev, R.I.; Li, K.; Pickwell-MacPherson, E. Rapid Imaging of Pulsed Terahertz Radiation with Spatial Light Modulators and Neural Networks. ACS Photonics 2021, 8, 3150–3155. [Google Scholar] [CrossRef]
- Arbab, M.H.; Winebrenner, D.P.; Dickey, T.C.; Klein, M.B.; Chen, A.; Mourad, P.D. A Noninvasive Terahertz Assessment of 2nd and 3rd Degree Burn Wounds. In Proceedings of the Conference on Lasers and Electro-Optics 2012, San Jose, CA, USA, 6–11 May 2012; p. CTu3B.3. [Google Scholar]
- Osman, O.B.; Jack Tan, T.; Henry, S.; Warsen, A.; Farr, N.; McClintic, A.M.; Wang, Y.-N.; Arbabi, S.; Arbab, M.H. Differentiation of burn wounds in an in vivo porcine model using terahertz spectroscopy. Biomed. Opt. Express 2020, 11, 6528–6535. [Google Scholar] [CrossRef]
- Khani, M.E.; Osman, O.B.; Harris, Z.B.; Chen, A.; Zhou, J.W.; Singer, A.J.; Arbab, M.H. Accurate and early prediction of the wound healing outcome of burn injuries using the wavelet Shannon entropy of terahertz time-domain waveforms. J. Biomed. Opt. 2022, 27, 116001. [Google Scholar] [CrossRef]
- Osman, O.B.; Harris, Z.B.; Zhou, J.W.; Khani, M.E.; Singer, A.J.; Arbab, M.H. In Vivo Assessment and Monitoring of Burn Wounds Using a Handheld Terahertz Hyperspectral Scanner. Adv. Photonics Res. 2022, 3, 2100095. [Google Scholar] [CrossRef]
- Khani, M.E.; Harris, Z.B.; Osman, O.B.; Zhou, J.W.; Chen, A.; Singer, A.J.; Arbab, M.H. Supervised machine learning for automatic classification of in vivo scald and contact burn injuries using the terahertz Portable Handheld Spectral Reflection (PHASR) Scanner. Sci. Rep. 2022, 12, 5096. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.E.; Winebrenner, D.P.; Arbab, M.H. Phase Function Effects on Identification of Terahertz Spectral Signatures Using the Discrete Wavelet Transform. IEEE Trans. Terahertz Sci. Technol. 2020, 10, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.E.; Arbab, M.H. Chemical Identification in the Specular and Off-Specular Rough-Surface Scattered Terahertz Spectra Using Wavelet Shrinkage. IEEE Access 2021, 9, 29746–29754. [Google Scholar] [CrossRef]
- Osman, O.B.; Arbab, M.H. Mitigating the effects of granular scattering using cepstrum analysis in terahertz time-domain spectral imaging. PLoS ONE 2019, 14, e0216952. [Google Scholar] [CrossRef]
- Khani, M.E.; Osman, O.B.; Arbab, M.H. Diffuse terahertz spectroscopy in turbid media using a wavelet-based bimodality spectral analysis. Sci. Rep. 2021, 11, 22804. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Bayati, E.; Oguchi, K.; Watanabe, S.; Winebrenner, D.P.; Hassan Arbab, M. Terahertz time-domain polarimetry (THz-TDP) based on the spinning E-O sampling technique: Determination of precision and calibration. Opt. Express 2020, 28, 13482–13496. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Osman, O.B.; Harris, Z.B.; Abazri, A.; Honkanen, R.; Arbab, M.H. Investigation of water diffusion dynamics in corneal phantoms using terahertz time-domain spectroscopy. Biomed. Opt. Express 2020, 11, 1284–1297. [Google Scholar] [CrossRef]
- Chen, A.; Virk, A.; Harris, Z.; Abazari, A.; Honkanen, R.; Arbab, M.H. Non-contact terahertz spectroscopic measurement of the intraocular pressure through corneal hydration mapping. Biomed. Opt. Express 2021, 12, 3438–3449. [Google Scholar] [CrossRef]
- Zhou, B.; Sit, A.J.; Zhang, X. Noninvasive measurement of wave speed of porcine cornea in ex vivo porcine eyes for various intraocular pressures. Ultrasonics 2017, 81, 86–92. [Google Scholar] [CrossRef]
- Stockslager, M.; Samuels, B.; Allingham, R.R.; Klesmith, Z.; Schwaner, S.; Forest, C.; Ethier, C. System for Rapid, Precise Modulation of Intraocular Pressure, toward Minimally-Invasive In Vivo Measurement of Intracranial Pressure. PLoS ONE 2016, 11, e0147020. [Google Scholar] [CrossRef]
- Ruiz-Ederra, J.; García, M.; Hernandez, M.; Urcola, H.; Hernández-Barbáchano, E.; Araiz, J.; Vecino, E. The pig eye as a novel model of glaucoma. Exp. Eye Res. 2005, 81, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Jones, J.; Tyrer, J.R.; Marshall, J. An interferometric ex vivo study of corneal biomechanics under physiologically representative loading, highlighting the role of the limbus in pressure compensation. Eye Vis. 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Virk, A.S.; Harris, Z.B.; Arbab, M.H. Development of a terahertz time-domain scanner for topographic imaging of spherical targets. Opt. Lett. 2021, 46, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Selvin, S.; Bajwa, N.; Chantra, S.; Nowroozi, B.; Garritano, J.; Goell, J.; Li, A.; Deng, S.X.; Brown, E.; et al. THz imaging system for in vivo human cornea. IEEE Trans. Terahertz. Sci. Technol. 2018, 8, 27–37. [Google Scholar] [CrossRef]
- Sung, S.; Dabironezare, S.; Llombart, N.; Selvin, S.; Bajwa, N.; Chantra, S.; Nowroozi, B.; Garritano, J.; Goell, J.; Li, A.; et al. Optical System Design for Noncontact, Normal Incidence, THz Imaging of in vivo Human Cornea. IEEE Trans. Terahertz Sci. Technol. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Sung, S.; Garritano, J.; Bajwa, N.; Deng, S.; Hubschman, J.-P.; Grundfest, W.; Taylor, Z. Preliminary results of non-contact THz imaging of cornea. In Proceedings of the Conference on Terahertz, RF, Millimeter, and Submillimeter-Wave Technology and Applications VIII, San Francisco, CA, USA, 10–12 February 2015; Volume 9362. [Google Scholar] [CrossRef]
- Jonuscheit, S.; Doughty, M.J.; Ramaesh, K. In vivo confocal microscopy of the corneal endothelium: Comparison of three morphometry methods after corneal transplantation. Eye (Lond.) 2011, 25, 1130–1137. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef]
- Jiang, W.; Simon, R. A comparison of bootstrap methods and an adjusted bootstrap approach for estimating the prediction error in microarray classification. Stat. Med. 2007, 26, 5320–5334. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Ye, L.; Meng, J.; Zhao, Z.; Liu, Z.; Hu, J. Acute ocular hypertension disrupts barrier integrity and pump function in rat corneal endothelial cells. Sci. Rep. 2017, 7, 6951. [Google Scholar] [CrossRef]
- Acar, B.T.; Vural, E.T.; Acar, S. Changes in endothelial cell density following penetrating keratoplasty and deep anterior lamellar keratoplasty. Int. J. Ophthalmol. 2011, 4, 644–647. [Google Scholar] [CrossRef]
- Lee, S.E.; Mehra, R.; Fujita, M.; Roh, D.; Long, C.; Lee, W.; Funderburgh, J.; Ayares, D.; Cooper, D.; Hara, H. Characterization of Porcine Corneal Endothelium for Xenotransplantation. Semin. Ophthalmol. 2013, 29, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.F.; McGhee, C.N.J.; Lee, W.R. A Scanning Electron Microscope Study of Porcine Corneal Endothelium Stored in Chondroitin Sulphate. Cornea 1992, 11, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Nikara, S.; Ahmadi, E.; Nia, A.A. Effects of different preparation techniques on the microstructural features of biological materials for scanning electron microscopy. J. Agric. Food Res. 2020, 2, 100036. [Google Scholar] [CrossRef]
- Müller, A.; Craig, J.P.; Grupcheva, C.N.; McGhee, C.N. The effects of corneal parameters on the assessment of endothelial cell density in the elderly eye. Br. J. Ophthalmol. 2004, 88, 325–330. [Google Scholar] [CrossRef][Green Version]
- Yamashita, K.; Hatou, S.; Inagaki, E.; Higa, K.; Tsubota, K.; Shimmura, S. A Rabbit Corneal Endothelial Dysfunction Model Using Endothelial-Mesenchymal Transformed Cells. Sci. Rep. 2018, 8, 16868. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Harris, Z.B.; Virk, A.; Abazari, A.; Varadaraj, K.; Honkanen, R.; Arbab, M.H. Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors 2022, 22, 9071. https://doi.org/10.3390/s22239071
Chen A, Harris ZB, Virk A, Abazari A, Varadaraj K, Honkanen R, Arbab MH. Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors. 2022; 22(23):9071. https://doi.org/10.3390/s22239071
Chicago/Turabian StyleChen, Andrew, Zachery B. Harris, Arjun Virk, Azin Abazari, Kulandaiappan Varadaraj, Robert Honkanen, and Mohammad Hassan Arbab. 2022. "Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines" Sensors 22, no. 23: 9071. https://doi.org/10.3390/s22239071
APA StyleChen, A., Harris, Z. B., Virk, A., Abazari, A., Varadaraj, K., Honkanen, R., & Arbab, M. H. (2022). Assessing Corneal Endothelial Damage Using Terahertz Time-Domain Spectroscopy and Support Vector Machines. Sensors, 22(23), 9071. https://doi.org/10.3390/s22239071









