Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors
Abstract
1. Introduction
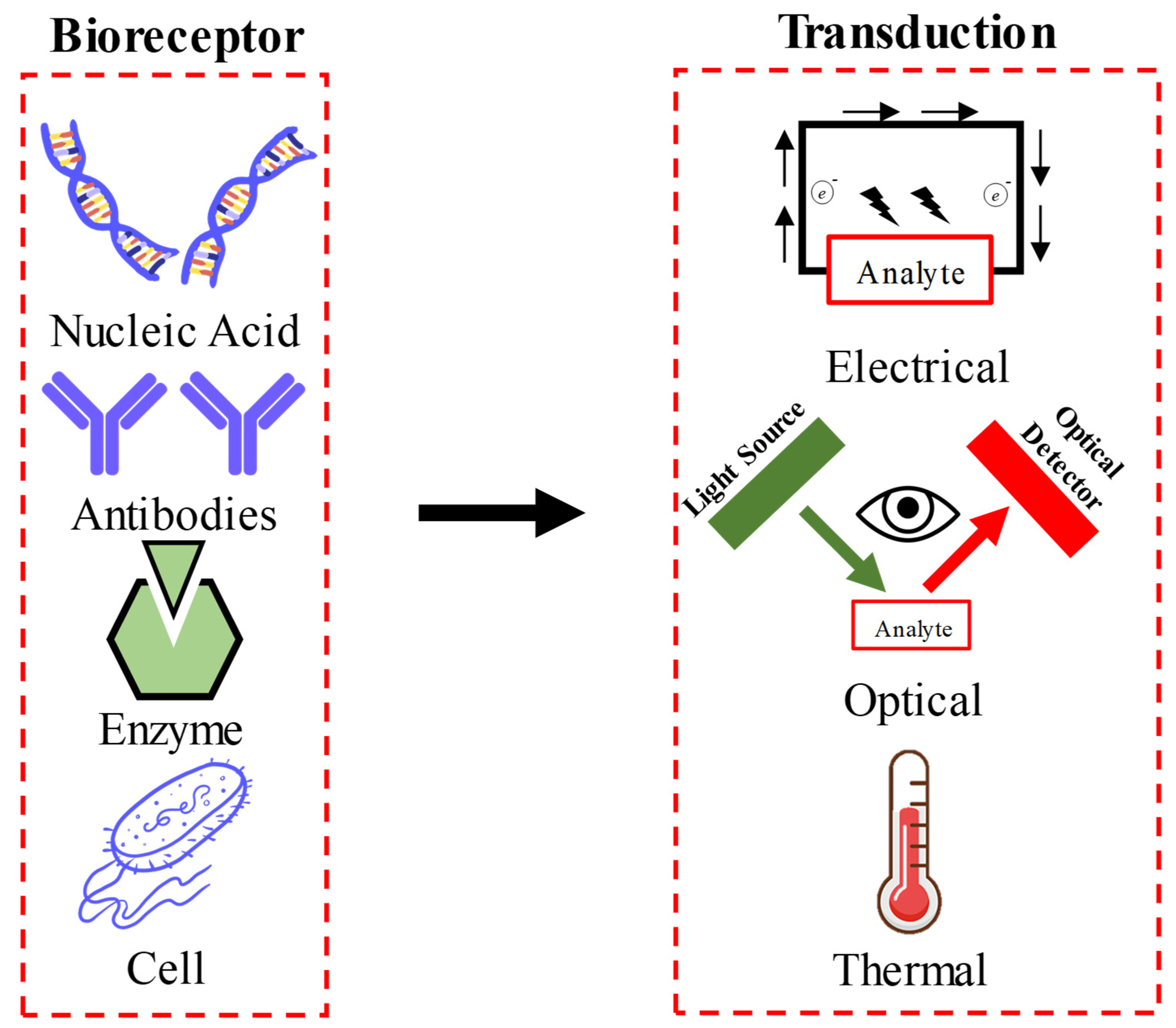
2. Nano-Biosensors: An Overview
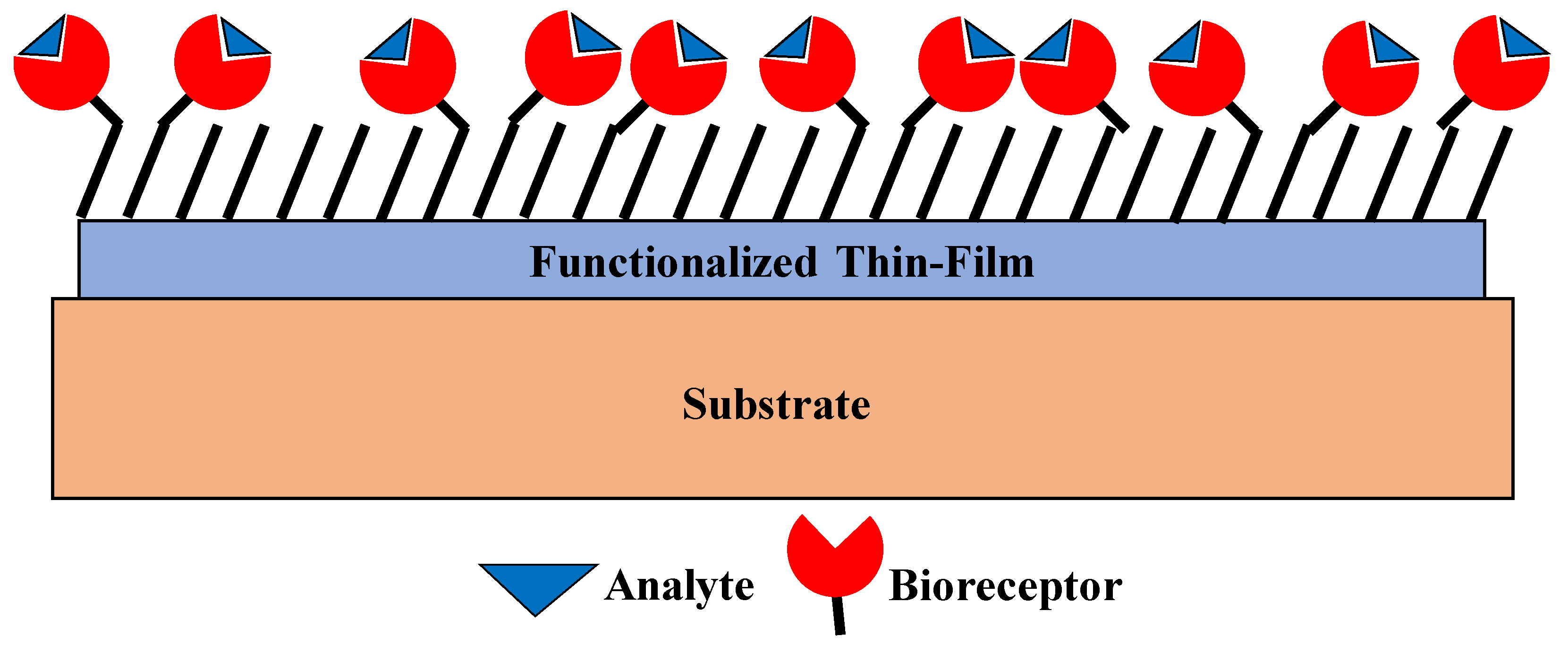
3. Thin-Film-Based Biosensors
4. Thin-Film Biosensors
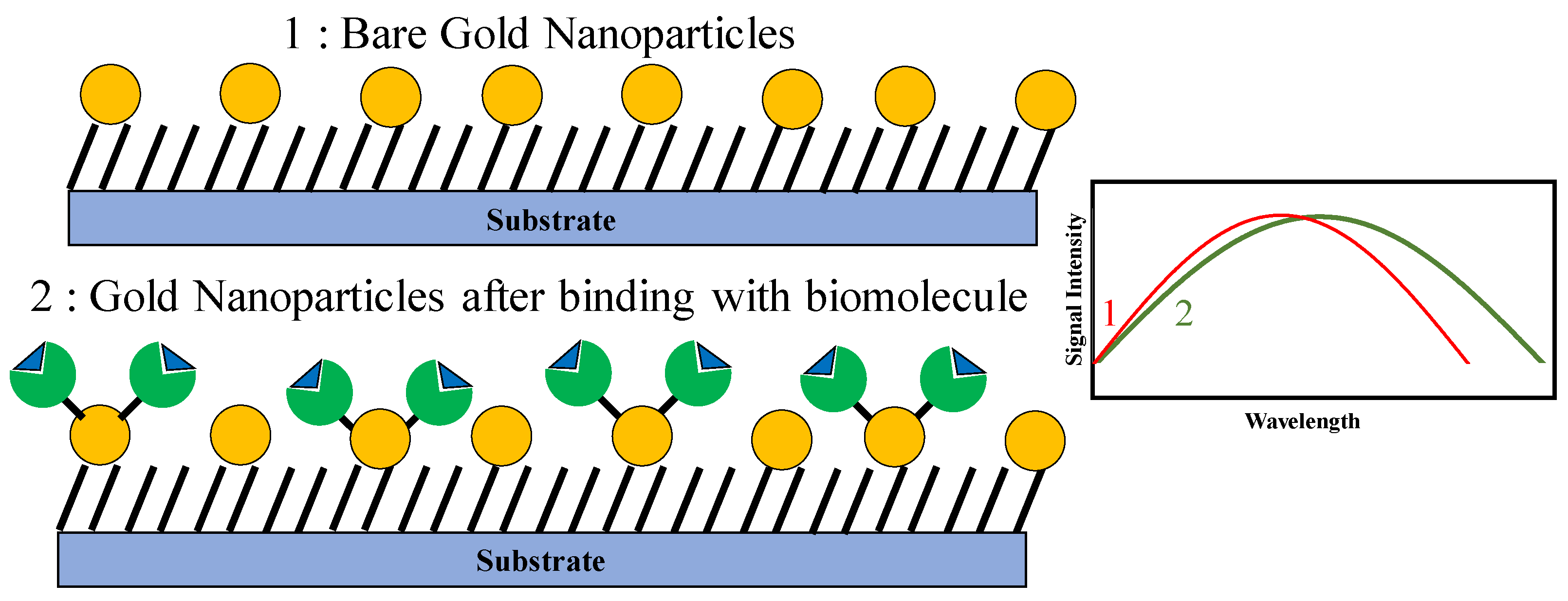
4.1. Gold Nanoparticles
4.2. Carbon Nanotubes
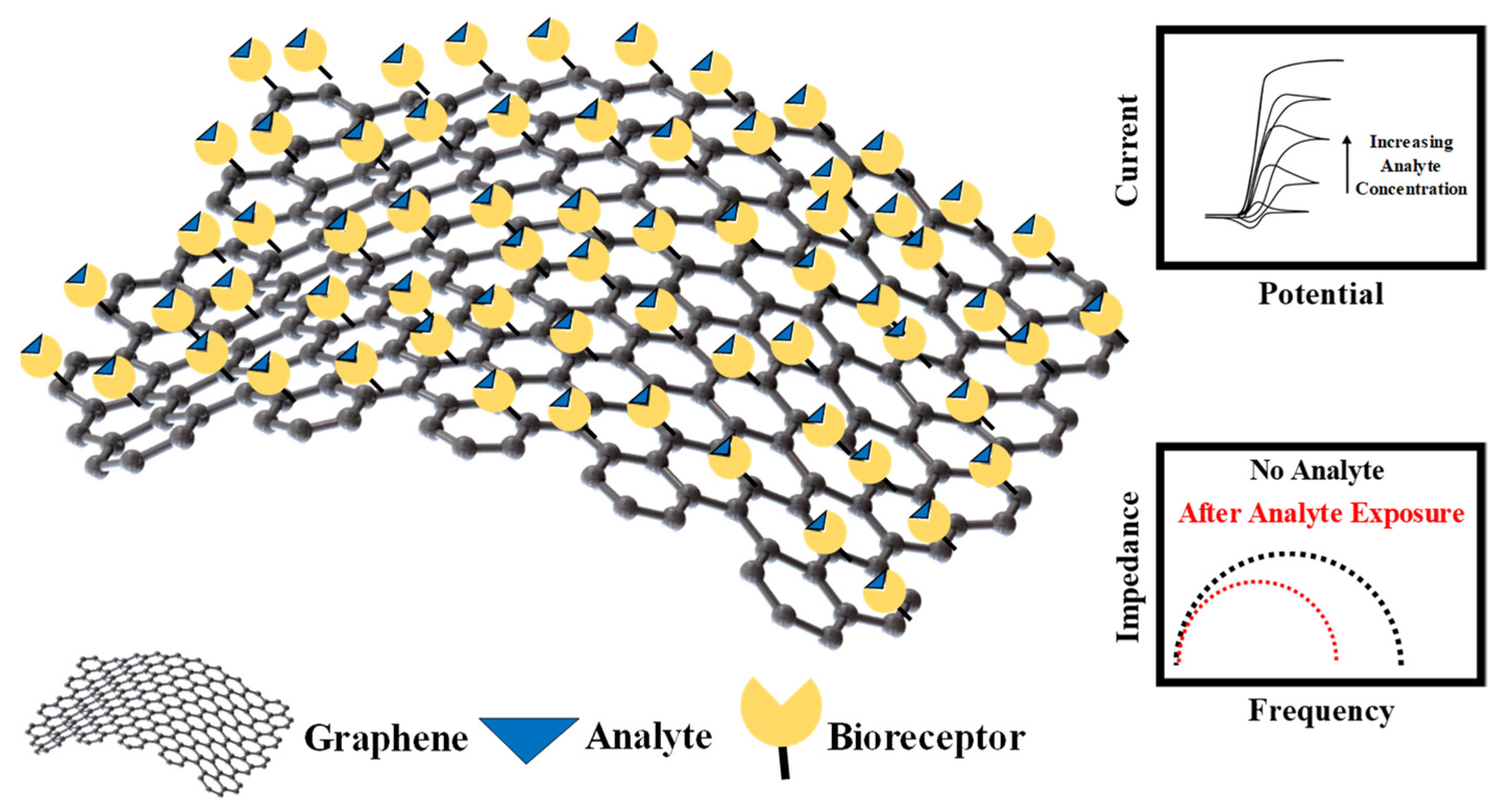
4.3. Graphene
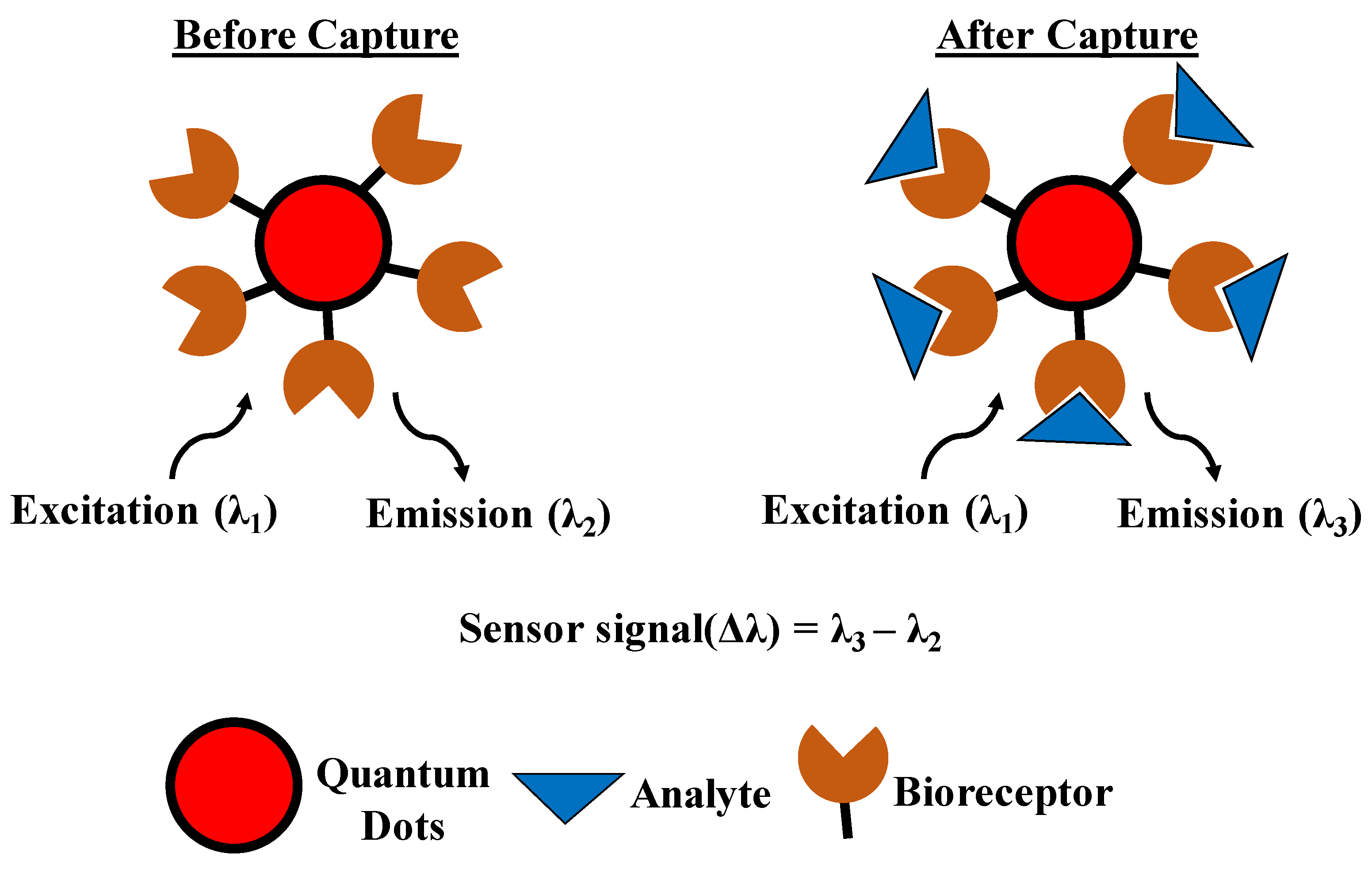
4.4. Quantum Dots
5. Nano-Biosensors for Cancer Detection and Future Prospective Including the Internet of Things and the Role of Machine Learning in Smart Biosensing
5.1. Cancer Diagnosis
5.2. Low-Power Sensors for Internet of Things
5.3. Machine Learning for Nano-Biosensors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pickel, D.; Manucy, G.P.; Walker, D.B.; Hall, S.B.; Walker, J.C. Evidence for Canine Olfactory Detection of Melanoma. Appl. Anim. Behav. Sci. 2004, 89, 107–116. [Google Scholar] [CrossRef]
- Fields, R.D. The Shark’s Electric Sense. Sci. Am. 2007, 297, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Telefoncu, A. Telefoncu Biochemistry Graduate Summer School -Biosensors; Ege University Science Faculty Press: Izmir, Turkey, 1999. [Google Scholar]
- Farré, M.; Kantiani, L.; Barceló, D. Microfluidic Devices: Biosensors; Academic Press: Cambridge, CA, USA, 2012; ISBN 9780123848628. Available online: https://www.sciencedirect.com/science/article/pii/B9780128132661000061 (accessed on 1 December 2020).
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Montalvo, J.G. Urea-Specific Enzyme Electrode. J. Am. Chem. Soc. 1969, 91, 2164–2165. [Google Scholar] [CrossRef] [PubMed]
- Scheller, F.W.; Schubert, F.; Neumann, B.; Pfeiffer, D.; Hintsche, R.; Dransfeld, I.; Wollenberger, U.; Renneberg, R.; Warsinke, A.; Johansson, G.; et al. Second Generation Biosensors. Biosens. Bioelectron. 1991, 6, 245–253. [Google Scholar] [CrossRef]
- Clark, M.F.; Lister, R.M.; Bar-Joseph, M. ELISA Techniques. 1986, pp. 742–766. Available online: https://www.abcam.com/kits/elisa-principle (accessed on 1 December 2020).
- Homola, J.; Piliarik, M. Surface Plasmon Resonance (SPR) Sensors; Springer: Berlin/Heidelberg, Germany, 2006; pp. 45–67. [Google Scholar]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, P.A.; Sandhyarani, N. Electrochemical DNA Sensors Based on the Use of Gold Nanoparticles: A Review on Recent Developments. Microchim. Acta 2017, 184, 981–1000. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef]
- Aldewachi, H.; Chalati, T.; Woodroofe, M.N.; Bricklebank, N.; Sharrack, B.; Gardiner, P. Gold Nanoparticle-Based Colorimetric Biosensors. Nanoscale 2018, 10, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O.; Ganta, D. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent Advances in Graphene-Based Biosensor Technology with Applications in Life Sciences. J. Nanobiotechnol. 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Coroş, M.; Pruneanu, S.; Stefan-van Staden, R.-I. Review—Recent Progress in the Graphene-Based Electrochemical Sensors and Biosensors. J. Electrochem. Soc. 2020, 167, 037528. [Google Scholar] [CrossRef]
- Merkoçi, A. Graphene-Based Biosensors. 2D Mater. 2020, 7, 040401. [Google Scholar] [CrossRef]
- Sánchez, A.; Villalonga, A.; Martínez-García, G.; Parrado, C.; Villalonga, R. Dendrimers as Soft Nanomaterials for Electrochemical Immunosensors. Nanomaterials 2019, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Kesici, E.; Yarali, E. Dendrimers Integrated Biosensors for Healthcare Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128138854. [Google Scholar]
- Chandra, S.; Mayer, M.; Baeumner, A.J. PAMAM Dendrimers: A Multifunctional Nanomaterial for ECL Biosensors. Talanta 2017, 168, 126–129. [Google Scholar] [CrossRef]
- Wang, N.; Hang, T.; Ling, H.; Hu, A.; Li, M. High-Performance Si-Based 3D Cu Nanostructured Electrode Assembly for Rechargeable Lithium Batteries. J. Mater. Chem. A 2015, 3, 11912–11919. [Google Scholar] [CrossRef]
- Weber, J.; Jeedigunta, S.; Kumar, A. Fabrication and Characterization of ZnO Nanowire Arrays with an Investigation into Electrochemical Sensing Capabilities. J. Nanomater. 2008, 2008, 1–5. [Google Scholar] [CrossRef]
- Parikh, R.Y.; Singh, S.; Prasad, B.L.V.; Patole, M.S.; Sastry, M.; Shouche, Y.S. Extracellular Synthesis of Crystalline Silver Nanoparticles and Molecular Evidence of Silver Resistance from Morganella Sp.: Towards Understanding Biochemical Synthesis Mechanism. ChemBioChem 2008, 9, 1415–1422. [Google Scholar] [CrossRef]
- Singh, S. Emerging Trends in Nanotechnology: Nanozymes, Imaging Probes and Biosensors and Nanocarriers. Curr. Drug Metab. 2019, 20, 414–415. [Google Scholar] [CrossRef]
- Banerjee, A.; Khan, S.H.; Broadbent, S.; Bulbul, A.; Kim, K.H.; Looper, R.; Mastrangelo, C.H.; KIM, H. Molecular Bridge Mediated Ultra-Low-Power Gas Sensing. arXiv 2019, arXiv:1911.05965v1. [Google Scholar]
- Sokolov, A.N.; Tee, B.C.-K.; Bettinger, C.J.; Tok, J.B.-H.; Bao, Z. Chemical and Engineering Approaches To Enable Organic Field-Effect Transistors for Electronic Skin Applications. Acc. Chem. Res. 2012, 45, 361–371. [Google Scholar] [CrossRef]
- Xiao, Y. “Plugging into Enzymes”: Nanowiring of Redox Enzymes by a Gold Nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef]
- Schierhorn, M.; Lee, S.J.; Boettcher, S.W.; Stucky, G.D.; Moskovits, M. Metal–Silica Hybrid Nanostructures for Surface-Enhanced Raman Spectroscopy. Adv. Mater. 2006, 18, 2829–2832. [Google Scholar] [CrossRef]
- Cai, H.; Xu, Y.; Zhu, N.; He, P.; Fang, Y. An Electrochemical DNA Hybridization Detection Assay Based on a Silver Nanoparticle Label. Analyst 2002, 127, 803–808. [Google Scholar] [CrossRef]
- Luo, X.-L.; Xu, J.-J.; Zhao, W.; Chen, H.-Y. A Novel Glucose ENFET Based on the Special Reactivity of MnO2 Nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Polsky, R.; Merkoçi, A. Electrochemical Stripping Detection of DNA Hybridization Based on Cadmium Sulfide Nanoparticle Tags. Electrochem. Commun. 2002, 4, 722–726. [Google Scholar] [CrossRef]
- Huang, Y.-Y.; Tian, Y.; Liu, X.-Q.; Niu, Z.; Yang, Q.-Z.; Ramamurthy, V.; Tung, C.-H.; Chen, Y.-Z.; Wu, L.-Z. Luminescent Supramolecular Polymer Nanoparticles for Ratiometric Hypoxia Sensing, Imaging and Therapy. Mater. Chem. Front. 2018, 2, 1893–1899. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Maity, S.; Yazhini, D.; Ghosh, A. Surface-Directed Disparity in Self-Assembled Structures of Small-Peptide l-Glutathione on Gold and Silver Nanoparticles. Langmuir 2020, 36, 11255–11261. [Google Scholar] [CrossRef] [PubMed]
- Englebienne, P. Use of Colloidal Gold Surface Plasmon Resonance Peak Shift to Infer Affinity Constants from the Interactions between Protein Antigens and Antibodies Specific for Single or Multiple Epitopes. Analyst 1998, 123, 1599–1603. [Google Scholar] [CrossRef]
- Lin, T.-J.; Huang, K.-T.; Liu, C.-Y. Determination of Organophosphorous Pesticides by a Novel Biosensor Based on Localized Surface Plasmon Resonance. Biosens. Bioelectron. 2006, 22, 513–518. [Google Scholar] [CrossRef]
- He, L.; Musick, M.D.; Nicewarner, S.R.; Salinas, F.G.; Benkovic, S.J.; Natan, M.J.; Keating, C.D. Colloidal Au-Enhanced Surface Plasmon Resonance for Ultrasensitive Detection of DNA Hybridization. J. Am. Chem. Soc. 2000, 122, 9071–9077. [Google Scholar] [CrossRef]
- Li, Y.; Wark, A.W.; Lee, H.J.; Corn, R.M. Single-Nucleotide Polymorphism Genotyping by Nanoparticle-Enhanced Surface Plasmon Resonance Imaging Measurements of Surface Ligation Reactions. Anal. Chem. 2006, 78, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamaguchi, I.; Kobayashi, T. Local Plasmon Sensor with Gold Colloid Monolayers Deposited upon Glass Substrates. Opt. Lett. 2000, 25, 372. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR Sensor Chip for Detection of Small Molecules Using Molecularly Imprinted Polymer with Embedded Gold Nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Honma, I.; Zhou, H. Humidity Sensor Based on Localized Surface Plasmon Resonance of Multilayer Thin Films of Gold Nanoparticles Linked with Myoglobin. Opt. Lett. 2006, 31, 1854. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-T.; Chuang, Y.-J.; Wu, Y.-C.; Yang, C.-S.; Wang, M.-C.; Tseng, F.-G. A Gold-Nanoparticle-Enhanced Immune Sensor Based on Fiber Optic Interferometry. Nanotechnology 2008, 19, 345501. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I.; Wang, J. Electroanalytical and Bioelectroanalytical Systems Based on Metal and Semiconductor Nanoparticles. Electroanalysis 2004, 16, 19–44. [Google Scholar] [CrossRef]
- Brown, K.R.; Fox, A.P.; Natan, M.J. Morphology-Dependent Electrochemistry of Cytochrome c at Au Colloid-Modified SnO2 Electrodes. J. Am. Chem. Soc. 1996, 118, 1154–1157. [Google Scholar] [CrossRef]
- Xu, Q.; Mao, C.; Liu, N.-N.; Zhu, J.-J.; Sheng, J. Direct Electrochemistry of Horseradish Peroxidase Based on Biocompatible Carboxymethyl Chitosan–Gold Nanoparticle Nanocomposite. Biosens. Bioelectron. 2006, 22, 768–773. [Google Scholar] [CrossRef]
- Wu, J.; Yan, F.; Zhang, X.; Yan, Y.; Tang, J.; Ju, H. Disposable Reagentless Electrochemical Immunosensor Array Based on a Biopolymer/Sol-Gel Membrane for Simultaneous Measurement of Several Tumor Markers. Clin. Chem. 2008, 54, 1481–1488. [Google Scholar] [CrossRef]
- Andreescu, S.; Luck, L.A. Studies of the Binding and Signaling of Surface-Immobilized Periplasmic Glucose Receptors on Gold Nanoparticles: A Glucose Biosensor Application. Anal. Biochem. 2008, 375, 282–290. [Google Scholar] [CrossRef]
- Kang, J.; Li, X.; Wu, G.; Wang, Z.; Lu, X. A New Scheme of Hybridization Based on the Aunano–DNA Modified Glassy Carbon Electrode. Anal. Biochem. 2007, 364, 165–170. [Google Scholar] [CrossRef]
- Cai, H.; Xu, C.; He, P.; Fang, Y. Colloid Au-Enhanced DNA Immobilization for the Electrochemical Detection of Sequence-Specific DNA. J. Electroanal. Chem. 2001, 510, 78–85. [Google Scholar] [CrossRef]
- Cui, R.; Huang, H.; Yin, Z.; Gao, D.; Zhu, J.-J. Horseradish Peroxidase-Functionalized Gold Nanoparticle Label for Amplified Immunoanalysis Based on Gold Nanoparticles/Carbon Nanotubes Hybrids Modified Biosensor. Biosens. Bioelectron. 2008, 23, 1666–1673. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.H.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.G.; Lambert, R.M. Selective Oxidation with Dioxygen by Gold Nanoparticle Catalysts Derived from 55-Atom Clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef]
- Raj, C.R.; Jena, B.K. Efficient Electrocatalytic Oxidation of NADH at Gold Nanoparticles Self-Assembled on Three-Dimensional Sol-Gel Network. Chem. Commun. 2005, 2005. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.; Nogamia, M. A Glucose Biosensor Based on Electrodeposited Biocomposites of Gold Nanoparticles and Glucose Oxidase Enzyme. Analyst 2001. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, H.; Li, J.; Tang, J.; Duan, M.; Jiang, L. Study on Colloidal Au-Enhanced DNA Sensing by Quartz Crystal Microbalance. Biochem. Biophys. Res. Commun. 2000, 274, 817–820. [Google Scholar] [CrossRef]
- Zhou, X.C.; O’Shea, S.J.; Li, S.F.Y. Amplified Microgravimetric Gene Sensor Using Au Nanoparticle Modified Oligonucleotides. Chem. Commun. 2000, 953–954. [Google Scholar] [CrossRef]
- Pang, L.; Li, J.; Jiang, J.; Shen, G.; Yu, R. DNA Point Mutation Detection Based on DNA Ligase Reaction and Nano-Au Amplification: A Piezoelectric Approach. Anal. Biochem. 2006, 358, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Wu, V.C.H.; Chuang, Y.-C.; Lin, C.-S. Using Oligonucleotide-Functionalized Au Nanoparticles to Rapidly Detect Foodborne Pathogens on a Piezoelectric Biosensor. J. Microbiol. Methods 2008, 73, 7–17. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, Z.-L.; Shen, G.-L.; Yu, R.-Q. Quartz Crystal Microbalance Immunoassay with Dendritic Amplification Using Colloidal Gold Immunocomplex. Sens. Actuators B Chem. 2006, 114, 696–704. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, J.; Wang, H.; Shen, G.; Yu, R. A Piezoelectric Immunosensor for the Detection of α-Fetoprotein Using an Interface of Gold/Hydroxyapatite Hybrid Nanomaterial. Biomaterials 2007, 28, 2147–2154. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, G.; Zhao, L.; Lai, K.W.C. Carbon Nanomaterial-Based Biosensors: A Review of Design and Applications. IEEE Nanotechnol. Mag. 2019, 13, 4–14. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon Nanostructures in Biology and Medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Vamvakaki, V.; Chaniotakis, N. Carbon Nanostructures as Transducers in Biosensors. Sens. Actuators B Chem. 2007, 126, 193–197. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Carbon Nanostructures as Immobilization Platform for DNA: A Review on Current Progress in Electrochemical DNA Sensors. Biosens. Bioelectron. 2017, 97, 226–237. [Google Scholar] [CrossRef]
- Iijima, S.; Yudasaka, M.; Yamada, R.; Bandow, S.; Suenaga, K.; Kokai, F.; Takahashi, K. Nano-Aggregates of Single-Walled Graphitic Carbon Nano-Horns. Chem. Phys. Lett. 1999, 309, 165–170. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z. Carbon Nanomaterial-Based Electrochemical Biosensors: An Overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angew. Chemie Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef]
- Li, G.; Liao, J.M.; Hu, G.Q.; Ma, N.Z.; Wu, P.J. Study of Carbon Nanotube Modified Biosensor for Monitoring Total Cholesterol in Blood. Biosens. Bioelectron. 2005, 20, 2140–2144. [Google Scholar] [CrossRef]
- Santos, R.M.; Rodrigues, M.S.; Laranjinha, J.; Barbosa, R.M. Biomimetic Sensor Based on Hemin/Carbon Nanotubes/Chitosan Modified Microelectrode for Nitric Oxide Measurement in the Brain. Biosens. Bioelectron. 2013, 44, 152–159. [Google Scholar] [CrossRef]
- Prasad, B.B.; Prasad, A.; Tiwari, M.P.; Madhuri, R. Multiwalled Carbon Nanotubes Bearing ‘Terminal Monomeric Unit’ for the Fabrication of Epinephrine Imprinted Polymer-Based Electrochemical Sensor. Biosens. Bioelectron. 2013, 45, 114–122. [Google Scholar] [CrossRef]
- Kress, G.J.; Shu, H.-J.; Yu, A.; Taylor, A.; Benz, A.; Harmon, S.; Mennerick, S. Fast Phasic Release Properties of Dopamine Studied with a Channel Biosensor. J. Neurosci. 2014, 34, 11792–11802. [Google Scholar] [CrossRef] [PubMed]
- Zelada-Guillén, G.A.; Tweed-Kent, A.; Niemann, M.; Göringer, H.U.; Riu, J.; Rius, F.X. Ultrasensitive and Real-Time Detection of Proteins in Blood Using a Potentiometric Carbon-Nanotube Aptasensor. Biosens. Bioelectron. 2013, 41, 366–371. [Google Scholar] [CrossRef]
- Zhang, J.; Boghossian, A.A.; Barone, P.W.; Rwei, A.; Kim, J.-H.; Lin, D.; Heller, D.A.; Hilmer, A.J.; Nair, N.; Reuel, N.F.; et al. Single Molecule Detection of Nitric Oxide Enabled by d(AT) 15 DNA Adsorbed to Near Infrared Fluorescent Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2011, 133, 567–581. [Google Scholar] [CrossRef]
- Jin, H.; Heller, D.A.; Strano, M.S. Single-Particle Tracking of Endocytosis and Exocytosis of Single-Walled Carbon Nanotubes in NIH-3T3 Cells. Nano Lett. 2008, 8, 1577–1585. [Google Scholar] [CrossRef]
- Fei, S.; Chen, J.; Yao, S.; Deng, G.; He, D.; Kuang, Y. Electrochemical Behavior of L-Cysteine and Its Detection at Carbon Nanotube Electrode Modified with Platinum. Anal. Biochem. 2005, 339, 29–35. [Google Scholar] [CrossRef]
- Antiochia, R.; Lavagnini, I.; Magno, F. Amperometric Mediated Carbon Nanotube Paste Biosensor for Fructose Determination. Anal. Lett. 2004, 37, 1657–1669. [Google Scholar] [CrossRef]
- Lübbers, D.W.; Opitz, N. The PCO2-/PO2-Optode: A New Probe for Measurement of PCO2 or PO in Fluids and Gases (Authors Transl). Z. Naturforsch. C Biosci. 1975, 30, 532–533. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, J.-H.; Barone, P.W.; Jin, H.; Zhang, J.; Heller, D.A.; Strano, M.S. A Luciferase/Single-Walled Carbon Nanotube Conjugate for Near-Infrared Fluorescent Detection of Cellular ATP. Angew. Chem. Int. Ed. 2010, 49, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.A.; Pratt, G.W.; Zhang, J.; Nair, N.; Hansborough, A.J.; Boghossian, A.A.; Reuel, N.F.; Barone, P.W.; Strano, M.S. Peptide Secondary Structure Modulates Single-Walled Carbon Nanotube Fluorescence as a Chaperone Sensor for Nitroaromatics. Proc. Natl. Acad. Sci. USA 2011, 108, 8544–8549. [Google Scholar] [CrossRef]
- Yi, H.; Ghosh, D.; Ham, M.-H.; Qi, J.; Barone, P.W.; Strano, M.S.; Belcher, A.M. M13 Phage-Functionalized Single-Walled Carbon Nanotubes As Nanoprobes for Second Near-Infrared Window Fluorescence Imaging of Targeted Tumors. Nano Lett. 2012, 12, 1176–1183. [Google Scholar] [CrossRef]
- Yang, S.; Fernando, K.A.S.; Liu, J.; Wang, J.; Sun, H.; Liu, Y.; Chen, M.; Huang, Y.; Wang, X.; Wang, H.; et al. Covalently PEGylated Carbon Nanotubes with Stealth Character In Vivo. Small 2008, 4, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kruss, S.; Hilmer, A.J.; Shimizu, S.; Schmois, Z.; De La Cruz, F.; Barone, P.W.; Reuel, N.F.; Heller, D.A.; Strano, M.S. A Rapid, Direct, Quantitative, and Label-Free Detector of Cardiac Biomarker Troponin T Using Near-Infrared Fluorescent Single-Walled Carbon Nanotube Sensors. Adv. Healthc. Mater. 2014, 3, 412–423. [Google Scholar] [CrossRef]
- Zhang, J.; Landry, M.P.; Barone, P.W.; Kim, J.-H.; Lin, S.; Ulissi, Z.W.; Lin, D.; Mu, B.; Boghossian, A.A.; Hilmer, A.J.; et al. Molecular Recognition Using Corona Phase Complexes Made of Synthetic Polymers Adsorbed on Carbon Nanotubes. Nat. Nanotechnol. 2013, 8, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.R. The Band Theory of Graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, R.; Yu, X.-Y.; Wang, L.; Liu, J.-H.; Huang, X.-J. Stripping Voltammetry Study of Ultra-Trace Toxic Metal Ions on Highly Selectively Adsorptive Porous Magnesium Oxide Nanoflowers. Analyst 2012, 137, 2183. [Google Scholar] [CrossRef]
- Kong, N.; Liu, J.; Kong, Q.; Wang, R.; Barrow, C.J.; Yang, W. Graphene Modified Gold Electrode via π–π Stacking Interaction for Analysis of Cu2+ and Pb2+. Sens. Actuators B Chem. 2013, 178, 426–433. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, W.; Via, B.K. Facile Synthesis of Soluble Graphene Quantum Dots and Its Improved Property in Detecting Heavy Metal Ions. Colloids Surf. B Biointerfaces 2014, 118, 72–76. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Park, S.J.; Kwon, O.S.; Bae, J.; Jang, J. High-Performance Flexible Graphene Aptasensor for Mercury Detection in Mussels. ACS Nano 2013, 7, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, G.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Poly(Amidoamine) Modified Graphene Oxide as an Efficient Adsorbent for Heavy Metal Ions. Polym. Chem. 2013, 4, 2164. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Wu, D.; Ma, H.; Mao, K.; Fan, D.; Du, B.; Li, H.; Wei, Q. Electrochemical Bisphenol A Sensor Based on N-Doped Graphene Sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef]
- Qu, Y.; Ma, M.; Wang, Z.; Zhan, G.; Li, B.; Wanga, X.; Fang, H.; Zhang, H.; Li, C. Sensitive Amperometric Biosensor for Phenolic Compounds Based on Graphene–Silk Peptide/Tyrosinase Composite Nanointerface. Biosens. Bioelectron. 2013, 44, 85–88. [Google Scholar] [CrossRef]
- Zaijun, L.; Xiulan, S.; Qianfang, X.; Ruiyi, L.; Yinjun, F.; Shuping, Y.; Junkang, L. Green and Controllable Strategy to Fabricate Well-Dispersed Graphene–Gold Nanocomposite Film as Sensing Materials for the Detection of Hydroquinone and Resorcinol with Electrodeposition. Electrochim. Acta 2012, 85, 42–48. [Google Scholar] [CrossRef]
- Liu, F.; Piao, Y.; Choi, J.S.; Seo, T.S. Three-Dimensional Graphene Micropillar Based Electrochemical Sensor for Phenol Detection. Biosens. Bioelectron. 2013, 50, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Tian, D.; Liu, S.; Zheng, X.; Duan, S.; Zhou, C. β-Cyclodextrin Functionalized Graphene Material: A Novel Electrochemical Sensor for Simultaneous Determination of 2-Chlorophenol and 3-Chlorophenol. Sens. Actuators B Chem. 2014, 195, 452–458. [Google Scholar] [CrossRef]
- Andreescu, S.; Marty, J.-L. Twenty Years Research in Cholinesterase Biosensors: From Basic Research to Practical Applications. Biomol. Eng. 2006, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Choi, B.G.; Park, H.; Park, T.J.; Yang, M.H.; Kim, J.S.; Jang, S.-Y.; Heo, N.S.; Lee, S.Y.; Kong, J.; Hong, W.H. Solution Chemistry of Self-Assembled Graphene Nanohybrids for High-Performance Flexible Biosensors. ACS Nano 2010, 4, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Meng, Y.; Zhang, W.; Zhou, J.; Xie, J.; Diao, G. β-Cyclodextrin Polymer Functionalized Reduced-Graphene Oxide: Application for Electrochemical Determination Imidacloprid. Electrochim. Acta 2013, 108, 1–9. [Google Scholar] [CrossRef]
- Chai, Y.; Niu, X.; Chen, C.; Zhao, H.; Lan, M. Carbamate Insecticide Sensing Based on Acetylcholinesterase/Prussian Blue-Multi-Walled Carbon Nanotubes/Screen-Printed Electrodes. Anal. Lett. 2013, 46, 803–817. [Google Scholar] [CrossRef]
- Wang, M.; Huang, J.; Wang, M.; Zhang, D.; Chen, J. Electrochemical Nonenzymatic Sensor Based on CoO Decorated Reduced Graphene Oxide for the Simultaneous Determination of Carbofuran and Carbaryl in Fruits and Vegetables. Food Chem. 2014, 151, 191–197. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.; Jiang, H.-J.; Yang, H.; Chen, H.-Y. Direct Electrochemistry and Bioelectrocatalysis of Microperoxidase-11 Immobilized on Chitosan-Graphene Nanocomposite. Electroanalysis 2010, 22, 1323–1328. [Google Scholar] [CrossRef]
- Song, H.; Ni, Y.; Kokot, S. Investigations of an Electrochemical Platform Based on the Layered MoS2–Graphene and Horseradish Peroxidase Nanocomposite for Direct Electrochemistry and Electrocatalysis. Biosens. Bioelectron. 2014, 56, 137–143. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-Based Wireless Bacteria Detection on Tooth Enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Liu, Y.; Li, L.-J.; Chen, P. Graphene-Based Biosensors for Detection of Bacteria and Their Metabolic Activities. J. Mater. Chem. 2011, 21, 12358. [Google Scholar] [CrossRef]
- Jia, F.; Duan, N.; Wu, S.; Ma, X.; Xia, Y.; Wang, Z.; Wei, X. Impedimetric Aptasensor for Staphylococcus Aureus Based on Nanocomposite Prepared from Reduced Graphene Oxide and Gold Nanoparticles. Microchim. Acta 2014, 181, 967–974. [Google Scholar] [CrossRef]
- Bruchez, M., Jr. Semiconductor Nanocrystals as Fluorescent Biological Labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef]
- Chan, W.C. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Q.; Zhang, C. Liposome–Quantum Dot Complexes Enable Multiplexed Detection of Attomolar DNAs without Target Amplification. J. Am. Chem. Soc. 2013, 135, 2056–2059. [Google Scholar] [CrossRef]
- Shanehsaz, M.; Mohsenifar, A.; Hasannia, S.; Pirooznia, N.; Samaei, Y.; Shamsipur, M. Detection of Helicobacter Pylori with a Nanobiosensor Based on Fluorescence Resonance Energy Transfer Using CdTe Quantum Dots. Microchim. Acta 2013, 180, 195–202. [Google Scholar] [CrossRef]
- Noor, M.O.; Shahmuradyan, A.; Krull, U.J. Paper-Based Solid-Phase Nucleic Acid Hybridization Assay Using Immobilized Quantum Dots as Donors in Fluorescence Resonance Energy Transfer. Anal. Chem. 2013, 85, 1860–1867. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Zhang, Q.; Tang, B.; Zhang, C. Single Quantum Dot-Based Nanosensor for Sensitive Detection of 5-Methylcytosine at Both CpG and Non-CpG Sites. Chem. Sci. 2018, 9, 1330–1338. [Google Scholar] [CrossRef]
- Qiu, X.; Hildebrandt, N. Rapid and Multiplexed MicroRNA Diagnostic Assay Using Quantum Dot-Based Förster Resonance Energy Transfer. ACS Nano 2015, 9, 8449–8457. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, M.; Zhang, C. Integration of Isothermal Amplification with Quantum Dot-Based Fluorescence Resonance Energy Transfer for Simultaneous Detection of Multiple MicroRNAs. Chem. Sci. 2018, 9, 4258–4267. [Google Scholar] [CrossRef]
- Jou, A.F.; Lu, C.-H.; Ou, Y.-C.; Wang, S.-S.; Hsu, S.-L.; Willner, I.; Ho, J.A. Diagnosing the MiR-141 Prostate Cancer Biomarker Using Nucleic Acid-Functionalized CdSe/ZnS QDs and Telomerase. Chem. Sci. 2015, 6, 659–665. [Google Scholar] [CrossRef]
- Su, S.; Fan, J.; Xue, B.; Yuwen, L.; Liu, X.; Pan, D.; Fan, C.; Wang, L. DNA-Conjugated Quantum Dot Nanoprobe for High-Sensitivity Fluorescent Detection of DNA and Micro-RNA. ACS Appl. Mater. Interfaces 2014, 6, 1152–1157. [Google Scholar] [CrossRef]
- Sang, L.-J.; Wang, H.-F. Aminophenylboronic-Acid-Conjugated Polyacrylic Acid–Mn-Doped ZnS Quantum Dot for Highly Sensitive Discrimination of Glycoproteins. Anal. Chem. 2014, 86, 5706–5712. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Jin, Z.; Lindén, S.; Jennings, T.L.; Hildebrandt, N. Quantum-Dot-Based Förster Resonance Energy Transfer Immunoassay for Sensitive Clinical Diagnostics of Low-Volume Serum Samples. ACS Nano 2013, 7, 7411–7419. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Shu, J.; Tang, D. Bioresponsive Release System for Visual Fluorescence Detection of Carcinoembryonic Antigen from Mesoporous Silica Nanocontainers Mediated Optical Color on Quantum Dot-Enzyme-Impregnated Paper. Anal. Chem. 2017, 89, 5152–5160. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Y.; Tang, B.; Zhang, C. Multicolor Quantum Dot-Based Chemical Nose for Rapid and Array-Free Differentiation of Multiple Proteins. Anal. Chem. 2016, 88, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, Y.; Kim, S. Signal Amplification via Biological Self-Assembly of Surface-Engineered Quantum Dots for Multiplexed Subattomolar Immunoassays and Apoptosis Imaging. ACS Nano 2013, 7, 9416–9427. [Google Scholar] [CrossRef]
- Tyrakowski, C.M.; Snee, P.T. Ratiometric CdSe/ZnS Quantum Dot Protein Sensor. Anal. Chem. 2014, 86, 2380–2386. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Shi, X.; He, X.; Wei, W.; Ma, N.; Chen, H. Highly Sensitive Detection of Caspase-3 Activities via a Nonconjugated Gold Nanoparticle–Quantum Dot Pair Mediated by an Inner-Filter Effect. ACS Appl. Mater. Interfaces 2013, 5, 9798–9802. [Google Scholar] [CrossRef]
- Ma, F.; Liu, W.; Tang, B.; Zhang, C. A Single Quantum Dot-Based Nanosensor for the Signal-on Detection of DNA Methyltransferase. Chem. Commun. 2017, 53, 6868–6871. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R. Proteolytic Assays on Quantum-Dot-Modified Paper Substrates Using Simple Optical Readout Platforms. Anal. Chem. 2013, 85, 8817–8825. [Google Scholar] [CrossRef]
- Tedsana, W.; Tuntulani, T.; Ngeontae, W. A Highly Selective Turn-on ATP Fluorescence Sensor Based on Unmodified Cysteamine Capped CdS Quantum Dots. Anal. Chim. Acta 2013, 783, 65–73. [Google Scholar] [CrossRef]
- Yu, X.; Wen, K.; Wang, Z.; Zhang, X.; Li, C.; Zhang, S.; Shen, J. General Bioluminescence Resonance Energy Transfer Homogeneous Immunoassay for Small Molecules Based on Quantum Dots. Anal. Chem. 2016, 88, 3512–3520. [Google Scholar] [CrossRef]
- Tsuboi, S.; Jin, T. Bioluminescence Resonance Energy Transfer (BRET)-Coupled Annexin V-Functionalized Quantum Dots for Near-Infrared Optical Detection of Apoptotic Cells. ChemBioChem 2017, 18, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Lei, J.; Huang, Y.; Cheng, Y.; Ju, H. Electrochemiluminescent Quenching of Quantum Dots for Ultrasensitive Immunoassay through Oxygen Reduction Catalyzed by Nitrogen-Doped Graphene-Supported Hemin. Anal. Chem. 2013, 85, 5390–5396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhou, L.; Nie, Z.; Xu, X.; Li, W.; Huang, Y.; He, K.; Yao, S. Versatile Electrochemiluminescent Biosensor for Protein–Nucleic Acid Interaction Based on the Unique Quenching Effect of Deoxyguanosine-5′-Phosphate on Electrochemiluminescence of CdTe/ZnS Quantum Dots. Anal. Chem. 2013, 85, 6279–6286. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Yu, Y.; Zou, G. A Monochromatic Electrochemiluminescence Sensing Strategy for Dopamine with Dual-Stabilizers-Capped CdSe Quantum Dots as Emitters. Anal. Chem. 2014, 86, 2784–2788. [Google Scholar] [CrossRef]
- Wang, W.; Hao, Q.; Wang, W.; Bao, L.; Lei, J.; Wang, Q.; Ju, H. Quantum Dot-Functionalized Porous ZnO Nanosheets as a Visible Light Induced Photoelectrochemical Platform for DNA Detection. Nanoscale 2014, 6, 2710–2717. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.-C.; Han, L.; Zhang, J.-R.; Zhu, J.-J. Enhanced Photoelectrochemical Strategy for Ultrasensitive DNA Detection Based on Two Different Sizes of CdTe Quantum Dots Cosensitized TiO2/CdS:Mn Hybrid Structure. Anal. Chem. 2014, 86, 10877–10884. [Google Scholar] [CrossRef]
- Zeng, X.; Ma, S.; Bao, J.; Tu, W.; Dai, Z. Using Graphene-Based Plasmonic Nanocomposites to Quench Energy from Quantum Dots for Signal-On Photoelectrochemical Aptasensing. Anal. Chem. 2013, 85, 11720–11724. [Google Scholar] [CrossRef]
- Feng, Q.-M.; Pan, J.-B.; Zhang, H.-R.; Xu, J.-J.; Chen, H.-Y. Disposable Paper-Based Bipolar Electrode for Sensitive Electrochemiluminescence Detection of a Cancer Biomarker. Chem. Commun. 2014, 50, 10949. [Google Scholar] [CrossRef]
- Baj-Rossi, C.; De Micheli, G.; Carrara, S. Electrochemical Detection of Anti-Breast-Cancer Agents in Human Serum by Cytochrome P450-Coated Carbon Nanotubes. Sensors 2012, 12, 6520–6537. [Google Scholar] [CrossRef] [PubMed]
- Ovádeková, R.; Jantová, S.; Letašiová, S.; Štepánek, I.; Labuda, J. Nanostructured Electrochemical DNA Biosensors for Detection of the Effect of Berberine on DNA from Cancer Cells. Anal. Bioanal. Chem. 2006, 386, 2055–2062. [Google Scholar] [CrossRef]
- Liu, F.L.; Xiao, P.; Fang, H.L.; Dai, H.F.; Qiao, L.; Zhang, Y.H. Single-Walled Carbon Nanotube-Based Biosensors for the Detection of Volatile Organic Compounds of Lung Cancer. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 44, 367–372. [Google Scholar] [CrossRef]
- Park, Y.K.; Bold, B.; Lee, W.K.; Jeon, M.H.; An, K.H.; Jeong, S.Y.; Shim, Y.K. D-(+)-Galactose-Conjugated Single-Walled Carbon Nanotubes as New Chemical Probes for Electrochemical Biosensors for the Cancer Marker Galectin-3. Int. J. Mol. Sci. 2011, 12, 2946–2957. [Google Scholar] [CrossRef]
- Zheng, T.-T.; Zhang, R.; Zou, L.; Zhu, J.-J. A Label-Free Cytosensor for the Enhanced Electrochemical Detection of Cancer Cells Using Polydopamine-Coated Carbon Nanotubes. Analyst 2012, 137, 1316–1318. [Google Scholar] [CrossRef]
- Fayazfar, H.; Afshar, A.; Dolati, M.; Dolati, A. DNA Impedance Biosensor for Detection of Cancer, TP53 Gene Mutation, Based on Gold Nanoparticles/Aligned Carbon Nanotubes Modified Electrode. Anal. Chim. Acta 2014, 836, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Shobha, B.N.; Muniraj, N.J.R. Design, Modeling and Performance Analysis of Carbon Nanotube with DNA Strands as Biosensor for Prostate Cancer. Microsyst. Technol. 2015, 21, 791–800. [Google Scholar] [CrossRef]
- Lerner, M.B.; D’Souza, J.; Pazina, T.; Dailey, J.; Goldsmith, B.R.; Robinson, M.K.; Johnson, A.T.C. Hybrids of a Genetically Engineered Antibody and a Carbon Nanotube Transistor for Detection of Prostate Cancer Biomarkers. ACS Nano 2012, 6, 5143–5149. [Google Scholar] [CrossRef]
- Abdolahad, M.; Janmaleki, M.; Taghinejad, M.; Taghnejad, H.; Salehi, F.; Mohajerzadeh, S. Single-Cell Resolution Diagnosis of Cancer Cells by Carbon Nanotube Electrical Spectroscopy. Nanoscale 2013, 5, 3421. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, F.; Dan, W.; Fu, Y.; Liu, S. Construction of Carbon Nanotube Based Nanoarchitectures for Selective Impedimetric Detection of Cancer Cells in Whole Blood. Analyst 2014, 139, 5086–5092. [Google Scholar] [CrossRef]
- Veetil, J.V.; Ye, K. Development of Immunosensors Using Carbon Nanotubes. Biotechnol. Prog. 2008, 23, 517–531. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive Electrochemical Immunosensor for Oral Cancer Biomarker IL-6 Using Carbon Nanotube Forest Electrodes and Multilabel Amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef]
- Wan, Y.; Deng, W.; Su, Y.; Zhu, X.; Peng, C.; Hu, H.; Peng, H.; Song, S.; Fan, C. Carbon Nanotube-Based Ultrasensitive Multiplexing Electrochemical Immunosensor for Cancer Biomarkers. Biosens. Bioelectron. 2011, 30, 93–99. [Google Scholar] [CrossRef]
- Arkan, E.; Saber, R.; Karimi, Z.; Shamsipur, M. A Novel Antibody–Antigen Based Impedimetric Immunosensor for Low Level Detection of HER2 in Serum Samples of Breast Cancer Patients via Modification of a Gold Nanoparticles Decorated Multiwall Carbon Nanotube-Ionic Liquid Electrode. Anal. Chim. Acta 2015, 874, 66–74. [Google Scholar] [CrossRef]
- De la Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh Sensitivity Carbon Nanotube Agents for Photoacoustic Molecular Imaging in Living Mice. Nano Lett. 2010, 10, 2168–2172. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Qu, K.; Song, Y.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Ultrasensitive and Selective Detection of a Prognostic Indicator in Early-Stage Cancer Using Graphene Oxide and Carbon Nanotubes. Adv. Funct. Mater. 2010, 20, 3967–3971. [Google Scholar] [CrossRef]
- Wang, L.-J.; Luo, M.-L.; Zhang, Q.; Tang, B.; Zhang, C.-Y. Single Quantum Dot-Based Nanosensor for Rapid and Sensitive Detection of Terminal Deoxynucleotidyl Transferase. Chem. Commun. 2017, 53, 11016–11019. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Song, J.; Lee, W.; Ryu, Y.M.; Jung, Y.; Kim, S.-Y.; Kim, K.; Hong, S.C.; Myung, S.J.; Kim, S. Cancer-Microenvironment-Sensitive Activatable Quantum Dot Probe in the Second Near-Infrared Window. Nano Lett. 2017, 17, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Zhao, Y.; Niu, S. Amplified Electrochemiluminescence Detection of Cancer Cells Using a New Bifunctional Quantum Dot as Signal Probe. Biosens. Bioelectron. 2013, 50, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Uludag, Y.; Tothill, I.E. Cancer Biomarker Detection in Serum Samples Using Surface Plasmon Resonance and Quartz Crystal Microbalance Sensors with Nanoparticle Signal Amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Altintas, Z.; Uludag, Y.; Gurbuz, Y.; Tothill, I.E. Surface Plasmon Resonance Based Immunosensor for the Detection of the Cancer Biomarker Carcinoembryonic Antigen. Talanta 2011, 86, 377–383. [Google Scholar] [CrossRef]
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact Imprinting Based Surface Plasmon Resonance (SPR) Biosensor for Real-Time and Ultrasensitive Detection of Prostate Specific Antigen (PSA) from Clinical Samples. Sens. Actuators B Chem. 2016, 224, 823–832. [Google Scholar] [CrossRef]
- Homola, J. (Ed.) Surface Plasmon Resonance Based Sensors; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4, ISBN 978-3-540-33918-2. [Google Scholar]
- Yang, M.; Yi, X.; Wang, J.; Zhou, F. Electroanalytical and Surface Plasmon Resonance Sensors for Detection of Breast Cancer and Alzheimer’s Disease Biomarkers in Cells and Body Fluids. Analyst 2014, 139, 1814. [Google Scholar] [CrossRef]
- Chen Yong Kah, J.; Wei Kho, K.; Guat Leng Lee, C.; James Richard Sheppard, C.; Xiang Shen, Z.; Chee Soo, K.; Carolene Olivo, M. Early Diagnosis of Oral Cancer Based on the Surface Plasmon Resonance of Gold Nanoparticles. Int. J. Nanomed. 2007, 2, 785–798. [Google Scholar]
- Law, W.-C.; Yong, K.-T.; Baev, A.; Prasad, P.N. Sensitivity Improved Surface Plasmon Resonance Biosensor for Cancer Biomarker Detection Based on Plasmonic Enhancement. ACS Nano 2011, 5, 4858–4864. [Google Scholar] [CrossRef]
- Li, Y.; Lee, H.J.; Corn, R.M. Detection of Protein Biomarkers Using RNA Aptamer Microarrays and Enzymatically Amplified Surface Plasmon Resonance Imaging. Anal. Chem. 2007, 79, 1082–1088. [Google Scholar] [CrossRef]
- Jang, H.S.; Park, K.N.; Kang, C.D.; Kim, J.P.; Sim, S.J.; Lee, K.S. Optical Fiber SPR Biosensor with Sandwich Assay for the Detection of Prostate Specific Antigen. Opt. Commun. 2009, 282, 2827–2830. [Google Scholar] [CrossRef]
- Krishnan, S.; Mani, V.; Wasalathanthri, D.; Kumar, C.V.; Rusling, J.F. Attomolar Detection of a Cancer Biomarker Protein in Serum by Surface Plasmon Resonance Using Superparamagnetic Particle Labels. Angew. Chemie Int. Ed. 2011, 50, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Treviño, J.; Calle, A.; Rodríguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Surface Plasmon Resonance Immunoassay Analysis of Pituitary Hormones in Urine and Serum Samples. Clin. Chim. Acta 2009, 403, 56–62. [Google Scholar] [CrossRef]
- Uludağ, Y.; Tothill, I.E. Development of a Sensitive Detection Method of Cancer Biomarkers in Human Serum (75%) Using a Quartz Crystal Microbalance Sensor and Nanoparticles Amplification System. Talanta 2010, 82, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Formisano, N.; Jolly, P.; Bhalla, N.; Cromhout, M.; Flanagan, S.P.; Fogel, R.; Limson, J.L.; Estrela, P. Optimisation of an Electrochemical Impedance Spectroscopy Aptasensor by Exploiting Quartz Crystal Microbalance with Dissipation Signals. Sens. Actuators B Chem. 2015, 220, 369–375. [Google Scholar] [CrossRef]
- Wang, D.; Tang, W.; Wu, X.; Wang, X.; Chen, G.; Chen, Q.; Li, N.; Liu, F. Highly Selective Detection of Single-Nucleotide Polymorphisms Using a Quartz Crystal Microbalance Biosensor Based on the Toehold-Mediated Strand Displacement Reaction. Anal. Chem. 2012, 84, 7008–7014. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhang, J.; Zhang, Y.; Han, J.; Jiang, L. Quantitative Investigation of the Influence of Gold Nanoparticles on the Dynamics of DNA Hybridization Using a Programmed Multi-Channel Quartz Crystal Microbalance System. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 158–162. [Google Scholar] [CrossRef]
- Atay, S.; Pişkin, K.; Yılmaz, F.; Çakır, C.; Yavuz, H.; Denizli, A. Quartz Crystal Microbalance Based Biosensors for Detecting Highly Metastatic Breast Cancer Cells via Their Transferrin Receptors. Anal. Methods 2016, 8, 153–161. [Google Scholar] [CrossRef]
- Kumar, M.; Yigit, M.; Dai, G.; Moore, A.; Medarova, Z. Image-Guided Breast Tumor Therapy Using a Small Interfering RNA Nanodrug. Cancer Res. 2010, 70, 7553–7561. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Fryxell, G.E.; Zhang, M. A Bifunctional Poly(Ethylene Glycol) Silane Immobilized on Metallic Oxide-Based Nanoparticles for Conjugation with Cell Targeting Agents. J. Am. Chem. Soc. 2004, 126, 7206–7211. [Google Scholar] [CrossRef]
- Chen, F.-H.; Zhang, L.-M.; Chen, Q.-T.; Zhang, Y.; Zhang, Z.-J. Synthesis of a Novel Magnetic Drug Delivery System Composed of Doxorubicin-Conjugated Fe3O4 Nanoparticle Cores and a PEG-Functionalized Porous Silica Shell. Chem. Commun. 2010, 46, 8633. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Huh, Y.-M.; Yang, J.; Lee, K.; Suh, J.-S.; Haam, S. PH-Triggered Drug-Releasing Magnetic Nanoparticles for Cancer Therapy Guided by Molecular Imaging by MRI. Adv. Mater. 2011, 23, 2436–2442. [Google Scholar] [CrossRef] [PubMed]
- Bakandritsos, A.; Mattheolabakis, G.; Chatzikyriakos, G.; Szabo, T.; Tzitzios, V.; Kouzoudis, D.; Couris, S.; Avgoustakis, K. Doxorubicin Nanocarriers Based on Magnetic Colloids with a Bio-Polyelectrolyte Corona and High Non-Linear Optical Response: Synthesis, Characterization, and Properties. Adv. Funct. Mater. 2011, 21, 1465–1475. [Google Scholar] [CrossRef]
- Santra, S.; Kaittanis, C.; Grimm, J.; Perez, J.M. Drug/Dye-Loaded, Multifunctional Iron Oxide Nanoparticles for Combined Targeted Cancer Therapy and Dual Optical/Magnetic Resonance Imaging. Small 2009, 5, 1862–1868. [Google Scholar] [CrossRef]
- Hwu, J.R.; Lin, Y.S.; Josephrajan, T.; Hsu, M.-H.; Cheng, F.-Y.; Yeh, C.-S.; Su, W.-C.; Shieh, D.-B. Targeted Paclitaxel by Conjugation to Iron Oxide and Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 66–68. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.-G.; Noh, H.-J.; Park, J.-H.; Hyeon, T. Large-Scale Synthesis of Uniform and Crystalline Magnetite Nanoparticles Using Reverse Micelles as Nanoreactors under Reflux Conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H. Bin Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Carta, D.; Casula, M.F.; Floris, P.; Falqui, A.; Mountjoy, G.; Boni, A.; Sangregorio, C.; Corrias, A. Synthesis and Microstructure of Manganese Ferrite Colloidal Nanocrystals. Phys. Chem. Chem. Phys. 2010, 12, 5074. [Google Scholar] [CrossRef] [PubMed]
- Chikkadi, K.; Muoth, M.; Maiwald, V.; Roman, C.; Hierold, C. Ultra-Low Power Operation of Self-Heated, Suspended Carbon Nanotube Gas Sensors. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Nguyen, H.; Hung, C.M.; Trung, N.N.; Van Duy, N. H2S Sensing Characteristics of Self-Heated Ag-Coated SnO2 Nanowires H2S Sensing Characteristics of Self-Heated Ag-Coated SnO2 Nanowires. In Proceedings of the The 12th Asian Conference on Chemical Sensors (ACCS2017), Hanoi, Vietnam, 12–15 November 2017; pp. 1–5. [Google Scholar]
- Wang, B.; Zhu, L.F.; Yang, Y.H.; Xu, N.S.; Yang, G.W. Fabrication of a SnO2 Nanowire Gas Sensor and Sensor Performance for Hydrogen. J. Phys. Chem. C 2008, 112, 6643–6647. [Google Scholar] [CrossRef]
- Liu, D.; Lin, L.; Chen, Q.; Zhou, H.; Wu, J. Low Power Consumption Gas Sensor Created from Silicon Nanowires/TiO2 Core-Shell Heterojunctions. ACS Sens. 2017, 2, 1491–1497. [Google Scholar] [CrossRef]
- Han, J.W.; Rim, T.; Baek, C.K.; Meyyappan, M. Chemical Gated Field Effect Transistor by Hybrid Integration of One-Dimensional Silicon Nanowire and Two-Dimensional Tin Oxide Thin Film for Low Power Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 21263–21269. [Google Scholar] [CrossRef]
- Cho, M.; Yun, J.; Kwon, D.; Kim, K.; Park, I. High-Sensitivity and Low-Power Flexible Schottky Hydrogen Sensor Based on Silicon Nanomembrane. ACS Appl. Mater. Interfaces 2018, 10, 12870–12877. [Google Scholar] [CrossRef]
- Alreshaid, A.T.; Hester, J.G.; Su, W.; Fang, Y.; Tentzeris, M.M. Review—Ink-Jet Printed Wireless Liquid and Gas Sensors for IoT, SmartAg and Smart City Applications. J. Electrochem. Soc. 2018, 165, B407–B413. [Google Scholar] [CrossRef]
- Stetter, J.R.; Carter, M.T. High Volume Zero Power Low Cost PPB Level Printed Nano-Sensors for IoT. ECS Trans. 2017, 77, 1825–1832. [Google Scholar] [CrossRef]
- Al Mamun, M.A.; Yuce, M.R. Sensors and Systems for Wearable Environmental Monitoring Toward IoT-Enabled Applications: A Review. IEEE Sens. J. 2019, 19, 7771–7788. [Google Scholar] [CrossRef]
- Long, H.; Turner, S.; Yan, A.; Xu, H.; Jang, M.; Carraro, C.; Maboudian, R.; Zettl, A. Plasma Assisted Formation of 3D Highly Porous Nanostructured Metal Oxide Network on Microheater Platform for Low Power Gas Sensing. Sens. Actuators B Chem. 2019, 301, 127067. [Google Scholar] [CrossRef]
- Eyal Weiss, R.A. Low-Power and High-Sensitivity Magnetic Sensors and Systems; Artech House: London, UK, 2018; ISBN 9781630812430. [Google Scholar]
- Villani, C.; Balsamo, D.; Brunelli, D.; Benini, L. Ultra-Low Power Sensor for Autonomous Non-Invasive Voltage Measurement in IoT Solutions for Energy Efficiency. Smart Sens. Actuators MEMS VII Cyber Phys. Syst. 2015, 9517, 95172I. [Google Scholar] [CrossRef]
- Kassal, P.; Steinberg, I.M.; Steinberg, M.D. Wireless Smart Tag with Potentiometric Input for Ultra Low-Power Chemical Sensing. Sens. Actuators B Chem. 2013, 184, 254–259. [Google Scholar] [CrossRef]
- Laubhan, K.; Talaat, K.; Riehl, S.; Aman, M.S.; Abdelgawad, A.; Yelamarthi, K. A Low-Power IoT Framework: From Sensors to the Cloud. In Proceedings of the 2016 IEEE International Conference on Electro Information Technology (EIT), Grand Forks, ND, USA, 19–21 May 2016; pp. 648–652. [Google Scholar] [CrossRef]
- Kuo, Y.W.; Li, C.L. Design of Long Range Low Power Sensor Node for the Last Mile of IoT. In Proceedings of the 2016 IEEE International Conference on Consumer Electronics-Taiwan (ICCE-TW), Nantou, Taiwan, 27–29 May 2016; pp. 15–16. [Google Scholar] [CrossRef]
- Akella Kamakshi, D.; Shrivastava, A.; Calhoun, B.H. A 0.2 V, 23 NW CMOS Temperature Sensor for Ultra-Low-Power IoT Applications. J. Low Power Electron. Appl. 2016, 6, 10. [Google Scholar] [CrossRef]
- Garulli, N.; Boni, A.; Caselli, M.; Magnanini, A.; Tonelli, M. A Low Power Temperature Sensor for IOT Applications in CMOS 65nm Technology. In Proceedings of the 2017 IEEE 7th International Conference on Consumer Electronics—Berlin (ICCE-Berlin), Berlin, Germany, 3–6 September 2017; pp. 92–96. [Google Scholar] [CrossRef]
- Banerjee, A.; Ghosh, T.; Likhite, R.; Hasan, N.; Kim, H.; Mastrangelo, C.H. Electrical Detection of Proteins Using Batch-Fabricated Vertical Metal Nanogap Break-Junctions. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2016, Dublin, Ireland, 9–13 October 2016; pp. 1230–1231. [Google Scholar]
- Banerjee, A.; Farhoudi, N.; Ghosh, C.; Mastrangelo, C.H.; Kim, H.; Broadbent, S.J.; Looper, R. Picowatt Gas Sensing and Resistance Switching in Tunneling Nano-Gap Electrodes. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar] [CrossRef]
- Ghosh, C.; Khan, S.H.; Broadbent, S.J.; Hsieh, H.C.; Noh, S.; Banerjee, A.; Farhoudi, N.; Mastrangelo, C.H.; Looper, R.; Kim, H. Nano-Gap Vapor Sensor. In Proceedings of the 2017 IEEE SENSORS, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Khan, S.H.; Banerjee, A.; Jung, Y.J.; Hsieh, H.C.; Wu, T.; Mastrangelo, C.H.; Kim, H. Ultra-Low-Power Chemical Sensor Node. In Proceedings of the GOMACTech 2018, Miami, FL, USA, 9 January 2018; pp. 6–9. [Google Scholar]
- Khan, S.H.; Banerjee, A.; Broadbent, S.; Kairy, P.D.; Kim, K.H.; Mastrangelo, C.H.; Looper, R.; Kim, H. Molecular Length Based Target Identification Using a Nano-Gap Sensor. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 27–31 January 2019; pp. 460–463. [Google Scholar]
- Khan, S.H.; Banerjee, A.; Broadbent, S.; Bulbul, A.; Simmons, M.C.; Kim, K.H.; Mastrangelo, C.H.; Looper, R.; Kim, H. Statistics-Based Gas Sensor. In Proceedings of the 2019 IEEE 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 27–31 January 2019; pp. 137–140. [Google Scholar]
- Banerjee, A.; Khan, S.-U.H.; Broadbent, S.; Likhite, R.; Looper, R.; Kim, H.; Mastrangelo, C.H. Batch-Fabricated α-Si Assisted Nanogap Tunneling Junctions. Nanomaterials 2019, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Banerjee, A.; Kim, K.H.; Salvant, J.; Looper, R.; Mastrangelo, C.H.; Kim, H. Threshold Point Modulation of a Wake-Up Nano-Gap Gas Sensor. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 733–736. [Google Scholar]
- Truong, S.K.M.; Kim, K.H.; Khan, S.H.; Salvant, J.; Banerjee, A.; Looper, R.; Mastrangelo, C.H.; Kim, H. Demonstration of $155.1\\mu\mathrm{W}$ Wake-Up Gas Sensor Node Toward 8 Month Lifetime. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 622–625. [Google Scholar]
- Pandey, S.S.; Banerjee, N.; Banerjee, A.; Hasan, N.; Kim, H.; Mastrangelo, C.H. High-Sensitivity Parametrically Amplified Chemo-Mechanical Vapor Sensors. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; pp. 1–4. [Google Scholar]
- Likhite, R.; Pandey, S.S.; Banerjee, A.; Kim, H.; Mastrangelo, C.H. Amplified Chemomechanical Comb Gas Sensor. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar] [CrossRef]
- Likhite, R.; Banerjee, A.; Kim, H.; Mastrangelo, C.H. Self-Leveling Micromechanical Gas Sensors. In Proceedings of the 2018 IEEE SENSORS, New Delhi, India, 28–31 October 2018; pp. 1–4. [Google Scholar]
- Likhite, R.; Banerjee, A.; Majumder, A.; Karkhanis, M.; Kim, H.; Mastrangelo, C.H. Parametrically Amplified Low-Power MEMS Capacitive Humidity Sensor. Sensors 2019, 19, 3954. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Likhite, R.; Kim, H.; Mastrangelo, C. An Ultra-Low Power Highly-Sensitive Vapor Sensor Based on Quantum Tunneling. In Proceedings of the The 23rd International Conference onMiniaturized Systems for Chemistry and Life Sciences MicroTAS 2019, Basel, Switzerland, 27–31 October 2019; pp. 1330–1331. [Google Scholar]
- Banerjee, A.; Likhite, R.; Kim, H.; Mastrangelo, C.H. Quantum Tunneling Hygrometer with Temperature Stabilized Nanometer Gap. Sci. Rep. 2020, 10, 4440. [Google Scholar] [CrossRef]
- Likhite, R.; Banerjee, A.; Majumder, A.; Karkhanis, M.; Kim, H.; Mastrangelo, C.H. VOC Sensing Using Batch-Fabricated Temperature Compensated Self-Leveling Microstructures. Sens. Actuators B Chem. 2020, 311, 127817. [Google Scholar] [CrossRef]
- Beach, C.; Krachunov, S.; Pope, J.; Fafoutis, X.; Piechocki, R.J.; Craddock, I.; Casson, A.J. An Ultra Low Power Personalizable Wrist Worn ECG Monitor Integrated with IoT Infrastructure. IEEE Access 2018, 6, 44010–44021. [Google Scholar] [CrossRef]
- Chen, X.; Rhee, W.; Wang, Z. Low Power Sensor Design for IoT and Mobile Healthcare Applications. China Commun. 2015, 12, 42–54. [Google Scholar] [CrossRef]
- Gatouillat, A.; Massot, B.; Badr, Y.; Sejdic, E.; Gehin, C. Evaluation of a Real-Time Low-Power Cardiorespiratory Sensor for the IoT. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 5382–5385. [Google Scholar] [CrossRef]
- Medu, H. Available online: http://www.ecnmag.com/article/2018/11/low-power-memory-iot-wearables-and-portable-medical-devices (accessed on 1 January 2021).
- Hayashikoshi, M.; Noda, H.; Kawai, H.; Murai, Y.; Otani, S.; Nii, K.; Matsuda, Y.; Kondo, H. Low-Power Multi-Sensor System with Power Management and Nonvolatile Memory Access Control for IoT Applications. IEEE Trans. Multi Scale Comput. Syst. 2018, 4, 784–792. [Google Scholar] [CrossRef]
- Banerjee, N.; Banerjee, A.; Hasan, N.; Pandey, S.S.; Gogoi, B.P.; Mastrangelo, C.H. A Monolithically Integrated Multisensor Platform. IEEE Sens. J. 2016, 16. [Google Scholar] [CrossRef]
- Tresanchez, M.; Pujol, A.; Pallejà, T.; Martínez, D.; Clotet, E.; Palacín, J. A Proposal of Low-Cost and Low-Power Embedded Wireless Image Sensor Node for IoT Applications. Procedia Comput. Sci. 2018, 134, 99–106. [Google Scholar] [CrossRef]
- Fayyazi, A.; Ansari, M.; Kamal, M.; Afzali-Kusha, A.; Pedram, M. An Ultra Low-Power Memristive Neuromorphic Circuit for Internet of Things Smart Sensors. IEEE Internet Things J. 2018, 5, 1011–1022. [Google Scholar] [CrossRef]
- Ma, S.; Liu, Q.; Sheu, P.C.Y. Foglight: Visible Light-Enabled Indoor Localization System for Low-Power IoT Devices. IEEE Internet Things J. 2018, 5, 175–185. [Google Scholar] [CrossRef]
- Apple Is the Last Major Smart Home Provider to Join Thread Group. Available online: https://www.fiercewireless.com/tech/apple-last-major-smart-home-provider-to-join-thread-group (accessed on 1 December 2020).
- Google Details Its Vision for the IoT. Available online: https://www.networkworld.com/article/3021820/google-ubiquity-summit-iot.html (accessed on 1 December 2020).
- Amazon Intros “Sidewalk” Protocol for Low-Power IoT Networks. Available online: https://www.lightreading.com/iot/m2m-platforms/amazon-intros-sidewalk-protocol-for-low-power-iot-networks/d/d-id/754392 (accessed on 1 December 2020).
- IoT Market Research Reports: Technology, Application, M2M & Semiconductor Wireless Sensor Internet of Things 2014 Analysis and 2020 Forecasts. Available online: https://go-gale-com.ezproxy.lib.utah.edu/ps/i.do?id=GALE%7CA369414466&v=2.1&u=marriottlibrary&it=r&p=AONE&sw=w (accessed on 1 December 2020).
- Semtech LoRa Technology Used by Trimble for IoT Water Monitoring Sensor Series. GlobeNewswire 2016: GlobeNewswire. 8 December 2016. Available online: https://www.globenewswire.com/news-release/2016/12/08/896112/0/en/Semtech-LoRa-Technology-Used-by-Trimble-for-IoT-Water-Monitoring-Sensor-Series.html. (accessed on 1 December 2020).
- New BluetoothA® Low Energy and Energy Harvesting Sensor Shields Further Extend the Capabilities of ON Semiconductor’s IoT Development Kit. Ultra-Low Power Connectivity and Battery-Free Sensing Solutions Enable Development of Novel IoT Use. M2 Pressw. Available online: https://www.electronicsmedia.info/2017/11/30/new-bluetooth-low-energy-energy-harvesting-sensor-shields-extend-capabilities-semiconductors-iot-development-kit/ (accessed on 1 December 2020).
- Reducing Power failures with netl’s low-cost electrical asset monitoring sensor technology. States News Service 2018: States News Service. 22 January 2018. Available online: https://www.engineering.pitt.edu/News/2018/NETL-Chen-Sensor-Tech/ (accessed on 1 December 2020).
- Industry Veteran Jeremy Jaech Lands $1.5 Million from Madrona Venture Group and Radar Partners for Innovative Low Power Sensor Technology; Founds SNUPI Technologies. PR Newswire 2012: PR Newswire. 11 December 2012. Available online: https://www.prnewswire.com/news-releases/industry-veteran-jeremy-jaech-lands-15-million-from-madrona-venture-group-and-radar-partners-for-innovative-low-power-sensor-technology-founds-snupi-technologies-183008391.html (accessed on 1 December 2020).
- Future Electronics Features RSL10 Solar Cell Multi-Sensor Platform from ON Semiconductor. PR.Com (Press Releases) 2019: PR.Com (Press Releases). 5 October 2019. Available online: https://www.freepressreleasedb.com/pr/Future-Electronics-Features-RSL10-Solar-Cell-Multi-Sensor-Platform-from-ON-Semiconductor-PR126273/ (accessed on 1 December 2020).
- Socionext Unveils New, Next-Generation Radar Sensors for IoT, Smart Home, and Other Applications. PR Newswire 2019: PR Newswire. 3 October 2019. Available online: https://www.rfglobalnet.com/doc/socionext-unveils-next-generation-radar-sensors-for-iot-smart-home-and-other-applications-0001 (accessed on 1 December 2020).
- Sales 13 IoT Statistics Defining the Future of Internet of Things. Available online: https://www.newgenapps.com/blog/iot-statistics-internet-of-things-future-research-data (accessed on 1 December 2020).
- Goswami, P. Advanced Materials and Techniques for Biosensors and Bioanalytical Applications, 1st ed.; Goswami, P., Ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9781003083856. [Google Scholar]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef]
- Guselnikova, O.; Trelin, A.; Skvortsova, A.; Ulbrich, P.; Postnikov, P.; Pershina, A.; Sykora, D.; Svorcik, V.; Lyutakov, O. Label-Free Surface-Enhanced Raman Spectroscopy with Artificial Neural Network Technique for Recognition Photoinduced DNA Damage. Biosens. Bioelectron. 2019, 145, 111718. [Google Scholar] [CrossRef] [PubMed]
- Erzina, M.; Trelin, A.; Guselnikova, O.; Dvorankova, B.; Strnadova, K.; Perminova, A.; Ulbrich, P.; Mares, D.; Jerabek, V.; Elashnikov, R.; et al. Precise Cancer Detection via the Combination of Functionalized SERS Surfaces and Convolutional Neural Network with Independent Inputs. Sens. Actuators B Chem. 2020, 308, 127660. [Google Scholar] [CrossRef]
- Thrift, W.J.; Ragan, R. Quantification of Analyte Concentration in the Single Molecule Regime Using Convolutional Neural Networks. Anal. Chem. 2019, 91, 13337–13342. [Google Scholar] [CrossRef]
- Lu, W.; Chen, X.; Wang, L.; Li, H.; Fu, Y.V. Combination of an Artificial Intelligence Approach and Laser Tweezers Raman Spectroscopy for Microbial Identification. Anal. Chem. 2020, 92, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Banerjee, T.; Srivastava, I.; Nie, S.; Pan, D. Machine Learning-Assisted Array-Based Biomolecular Sensing Using Surface-Functionalized Carbon Dots. ACS Sens. 2019, 4, 2730–2737. [Google Scholar] [CrossRef]
- Solmaz, M.E.; Mutlu, A.Y.; Alankus, G.; Kılıç, V.; Bayram, A.; Horzum, N. Quantifying Colorimetric Tests Using a Smartphone App Based on Machine Learning Classifiers. Sens. Actuators B Chem. 2018, 255, 1967–1973. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Gautam, S.H.; Mitra, S.K. Editors’ Choice—Artificial Intelligence Based Mobile Application for Water Quality Monitoring. J. Electrochem. Soc. 2019, 166, B3031–B3035. [Google Scholar] [CrossRef]
- Kim, H.; Awofeso, O.; Choi, S.; Jung, Y.; Bae, E. Colorimetric Analysis of Saliva–Alcohol Test Strips by Smartphone-Based Instruments Using Machine-Learning Algorithms. Appl. Opt. 2017, 56, 84. [Google Scholar] [CrossRef]
- Ballard, Z.S.; Joung, H.-A.; Goncharov, A.; Liang, J.; Nugroho, K.; Di Carlo, D.; Garner, O.B.; Ozcan, A. Deep Learning-Enabled Point-of-Care Sensing Using Multiplexed Paper-Based Sensors. NPJ Digit. Med. 2020, 3, 66. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Ma, G.; Bi, S.; Duan, Q.; Chen, J.; Feng, Y.; Liu, F.; Lee, J. Machine Learning for Total Organic Carbon Analysis of Environmental Water Samples Using High-Throughput Colorimetric Sensors. Analyst 2020, 145, 2197–2203. [Google Scholar] [CrossRef]
- Gonzalez-Navarro, F.; Stilianova-Stoytcheva, M.; Renteria-Gutierrez, L.; Belanche-Muñoz, L.; Flores-Rios, B.; Ibarra-Esquer, J. Glucose Oxidase Biosensor Modeling and Predictors Optimization by Machine Learning Methods. Sensors 2016, 16, 1483. [Google Scholar] [CrossRef]
- Ali, S.; Hassan, A.; Hassan, G.; Eun, C.-H.; Bae, J.; Lee, C.H.; Kim, I.-J. Disposable All-Printed Electronic Biosensor for Instantaneous Detection and Classification of Pathogens. Sci. Rep. 2018, 8, 5920. [Google Scholar] [CrossRef]
- Albrecht, T.; Slabaugh, G.; Alonso, E.; Al-Arif, S.M.R. Deep Learning for Single-Molecule Science. Nanotechnology 2017, 28, 423001. [Google Scholar] [CrossRef]
- Tsutsui, M.; Yoshida, T.; Yokota, K.; Yasaki, H.; Yasui, T.; Arima, A.; Tonomura, W.; Nagashima, K.; Yanagida, T.; Kaji, N.; et al. Discriminating Single-Bacterial Shape Using Low-Aspect-Ratio Pores. Sci. Rep. 2017, 7, 17371. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, Z.; Leng, K.; Han, W.; Niu, H.; Yu, Y.; Ling, Q.; Liu, J.; Wu, Z.; Zang, J. Nonintrusive Monitoring of Mental Fatigue Status Using Epidermal Electronic Systems and Machine-Learning Algorithms. ACS Sens. 2020, 5, 1305–1313. [Google Scholar] [CrossRef]
- Jeong, H.; Rogers, J.A.; Xu, S. Continuous On-Body Sensing for the COVID-19 Pandemic: Gaps and Opportunities. Sci. Adv. 2020, 6, eabd4794. [Google Scholar] [CrossRef] [PubMed]
- Tatarko, M.; Muckley, E.S.; Subjakova, V.; Goswami, M.; Sumpter, B.G.; Hianik, T.; Ivanov, I.N. Machine Learning Enabled Acoustic Detection of Sub-Nanomolar Concentration of Trypsin and Plasmin in Solution. Sens. Actuators B Chem. 2018, 272, 282–288. [Google Scholar] [CrossRef]
- Adak, M.F.; Lieberzeit, P.; Jarujamrus, P.; Yumusak, N. Classification of Alcohols Obtained by QCM Sensors with Different Characteristics Using ABC Based Neural Network. Eng. Sci. Technol. Int. J. 2020, 23, 463–469. [Google Scholar] [CrossRef]
- Yan, W.; Wang, K.; Xu, H.; Huo, X.; Jin, Q.; Cui, D. Machine Learning Approach to Enhance the Performance of MNP-Labeled Lateral Flow Immunoassay. Nano-Micro Lett. 2019, 11, 7. [Google Scholar] [CrossRef]


| Nanostructures | Transduction Mechanism | Analyte | Limit of Detection | Ref. |
|---|---|---|---|---|
| AuNPs | Electrochemical | Tumor markers | 0.1 μg/L | [47] |
| AuNPs | Electrochemical | DNA hybridization | 1.52 × 10−10 mol/L | [49] |
| AuNPs | Electrochemical | Glucose | 0.18 μM | [48] |
| AuNPs | Electrochemical | Horseradish peroxidase | 4.01 × 10−7 M | [46] |
| AuNPs | Electrochemical | |||
| AuNPs | Optical | Antibody/Antigen interaction | 25 ng/mL | [36] |
| AuNPs | Optical | Enzymatic ligation reactions | 1 pM | [39] |
| AuNPs | Optical | Organophosphorus pesticides | 0.234 ppb | [37] |
| AuNPs | Optical | DNA hybridization | 10 pM | [38] |
| AuNPs | Piezoelectric | Gene sensing | 3.2 × 10−11 M | [56] |
| AuNPs | Piezoelectric | DNA sensing | 10 μg/mL | [55] |
| AuNPs | Piezoelectric | DNA mutation detection | 2.6 × 10−9 mol/L | [57] |
| AuNPs | Piezoelectric | α-fetoprotein | 15.3 ng/mL | [60] |
| AuNPs | Piezoelectric | rabbit/goat anti-human IgG | 10.9 μg/mL | [59] |
| CNT | Electrochemical | Fructose | 1.0 × 10−6 mol/L | [76] |
| CNT | Electrochemical | Glucose oxidase | 20 mM | [67] |
| CNT | Electrochemical | L-cysteine | 0.3 μM | [75] |
| CNT | Electrochemical | Cholesterol | 100 mg/dL | [68] |
| CNT | Electrochemical | Surface glycoprotein | ~aM | [72] |
| CNT | Electrochemical | Nitric oxide | 25 nM | [69] |
| CNT | Electrochemical | Epinephrine | 0.02 ng/mL | [70] |
| CNT | Electrochemical | Nitric oxide | 300 nM | [73] |
| CNT | Optical | Troponin T | 100 ng/mL | [82] |
| CNT | Optical | Cellular ATP | 240 nM | [78] |
| CNT | Optical | Tumor cells | 2 μg/mL | [80] |
| CNT | Optical | riboflavin, L-thyroxine, oestradiol. | 100 μM | [83,84] |
| CNT | Optical | nitroaromatics | 9 μM | [79] |
| Graphene | Electrochemical | Carbamate Insecticide | 5.32 × 10−8 g/L | [100] |
| Graphene | Electrochemical | Pb(II) and Cd(II) | 2.1 pM and 81 pM | [86] |
| Graphene | Electrochemical | Cu2+, Zn2+, Fe3+, Pb2+ and Cr3+ | ||
| Graphene | Electrochemical | bisphenol A | 5.0 × 10−9 mol/L | [91,92] |
| Graphene | Electrochemical | Cholinesterase | 0.3 ppb | [96] |
| Graphene | Electrochemical | Phenol | 50 nM | [94] |
| Graphene | Electrochemical | Pb2+ | 5.0 × 10−9M | [88] |
| Graphene | Electrochemical | Hydroquinone, resorcinol | 5.2 × 10−9 mol/L, 2.2 × 10−9 mol/L | [93] |
| Graphene | Electrochemical | imidacloprid | 2.2 × 10−8 mol/L | [99] |
| Graphene | Electrochemical | Cu2+ and Pb2+ | 1.5–20 nM and 0.4–20 nM | [87] |
| Graphene | Electrochemical | 2-chlorophenol and 3-chlorophenol | 0.2 and 0.09 μM, | [95] |
| Graphene | Electrochemical | organophosphate | 1.37 × 10−7 M | [98] |
| Graphene | Electrochemical | Mercury | 10 pM | [89] |
| Quantum Dots | Bioluminescent | enrofloxacin | 0.023 ng/mL | [126] |
| Quantum Dots | Chemiluminescent | Dopamine | 3.0 nM | [130] |
| Quantum Dots | Chemiluminescent | carcinoembryonic antigen | 24 fg/mL | [128] |
| Quantum Dots | Chemiluminescent | protein−DNA interactions | 0.1 nM | [129] |
| Quantum Dots | Fluorescent | Glycoproteins | 0.15 μM | [117] |
| Quantum Dots | Fluorescent | Nucleic acid hybridization | 300 fmol | [111] |
| Quantum Dots | Fluorescent | MicroRNA | <1 pM | [113] |
| Quantum Dots | Fluorescent | DNA and MicroRNA | 1 fM and 10 fM | [116] |
| Quantum Dots | Fluorescent | miR-141 prostate cancer biomarker | 1.00 × 10−12 M | [115] |
| Quantum Dots | Fluorescent | 5-methylcytosine | 1 aM | [112] |
| Quantum Dots | Fluorescent | MicroRNA | 1.6 × 10−17 M | [114] |
| Quantum Dots | Fluorescent | DNA | <1 aM | [109] |
| Quantum Dots | Fluorescent | prostate specific antigen | 1.6 ng/m | [118] |
| Quantum Dots | Fluorescent | Helicobacter pylori | 4.5 × 10−9 M | [110] |
| Quantum Dots | Photo-electrochemical | Carcino-embryonic antigen | 0.47 pg/mL | [133] |
| Quantum Dots | Photo-electrochemical | DNA | 27 aM | [132] |
| Quantum Dots | Photo-electrochemical | DNA | <1 fM | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors 2021, 21, 1253. https://doi.org/10.3390/s21041253
Banerjee A, Maity S, Mastrangelo CH. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors. 2021; 21(4):1253. https://doi.org/10.3390/s21041253
Chicago/Turabian StyleBanerjee, Aishwaryadev, Swagata Maity, and Carlos H. Mastrangelo. 2021. "Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors" Sensors 21, no. 4: 1253. https://doi.org/10.3390/s21041253
APA StyleBanerjee, A., Maity, S., & Mastrangelo, C. H. (2021). Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors, 21(4), 1253. https://doi.org/10.3390/s21041253





