Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care
Abstract
1. An Introduction to Lactate Metabolism
The Importance of Lactate Monitoring in Critical Care
2. Current Methodologies Used for Lactate Analysis in Critical Care
3. Current Non-Invasive Technology for Lactate Measurements Outside of Critical Care
3.1. Sweat Lactate Sensors
3.2. Alternative Fluid Lactate Sensors
4. Adaption and Application of Non-Invasive Technologies into the Clinical Setting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Jubouri, M.A. Pathology User Guide. 2019. Available online: http://www.sthk.nhs.uk/atoz/Documents/Pathology/Pathology%20User%20Guide%20%2021%20draft%20Jan%2019%20v10.pdf (accessed on 27 January 2021).
- Alberti, K.G. The biochemical consequences of hypoxia. J. Clin. Pathol. 1977, 11, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gerich, J.E.; Meyer, C.; Woerle, H.J.; Stumvoll, M. Renal gluconeogenesis: Its importance in human glucose homeostasis. Diabetes Care 2001, 24, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Jorfeldt, L. Metabolism of L(plus)-lactate in human skeletal muscle during exercise. Acta Physiol. Scand. Suppl. 1970, 338, 1–67. [Google Scholar] [PubMed]
- Richter, E.A.; Kiens, B.; Saltin, B.; Christensen, N.J.; Savard, G. Skeletal muscle glucose uptake during dynamic exercise in humans: Role of muscle mass. Am. J. Physiol. Metab. 1988, 254, E555–E561. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G. Lactate as a fuel for mitochondrial respiration. Acta Physiol. Scand. 2000, 168, 643–656. [Google Scholar] [CrossRef]
- Bai, Z.; Zhu, X.; Li, M.; Hua, J.; Zhu, L.; Pan, J.; Wang, J.; Li, Y. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr. 2014, 14, 83. [Google Scholar] [CrossRef]
- Kruse, O.; Grunnet, N.; Barfod, C. Blood lactate as a predictor for in-hospital mortality in patients admitted acutely to hospital: A systematic review. Scand. J. Trauma Resusc. Emerg. Med. 2011, 19, 74. [Google Scholar] [CrossRef]
- Martin, J.; Blobner, M.; Busch, R.; Moser, N.; Kochs, E.; Luppa, P.B. Point-of-care testing on admission to the intensive care unit: Lactate and glucose independently predict mortality. Clin. Chem. Lab. Med. 2013, 51, 405–412. [Google Scholar] [CrossRef]
- Nichol, A.; Egi, M.; Pettilä, V.; Bellomo, R.; French, C.; Hart, G.K.; Davies, A.; Stachowski, E.; Reade, M.C.; Bailey, M.; et al. Relative hyperlactatemia and hospital mortality in critically ill patients: A retrospective multi-centre study. Crit. Care 2010, 14, R25. [Google Scholar] [CrossRef]
- Rishu, A.H.; Khan, R.M.; Al-Dorzi, H.M.; Tamim, H.; Al-Qahtani, S.; Al-Ghamdi, G.; Arabi, Y.M. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit. Care 2013, 17, R197. [Google Scholar] [CrossRef]
- Nijsten, M.W.N.; Bakker, J. Lactate monitoring in the ICU. ICU Manag. Pract. 2015, 15, 62–66. [Google Scholar]
- Vincent, J.-L.; e Silva, A.Q.A.; Couto, L., Jr.; Taccone, F.S. The value of blood lactate kinetics in critically ill patients: A systematic review. Crit. Care 2016, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.C.; van Bommel, J.; Schoonderbeek, F.J.; Sleeswijk Visser, S.J.; van der Klooster, J.M.; Lima, A.P.; Willemsem, S.P.; Bakker, J.; LACTATE Study Group. Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized controlled trial. Am. J. Respir. Crit. Care Med. 2010, 182, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Tisherman, S.S.; Barie, P.; Bokhari, F.; Bonadies, J.; Daley, B.; Diebel, L.; Eachempati, S.R.; Kurek, S.; Luchette, F.; Puyana, J.C.; et al. Clinical practice guideline: Endpoints of resuscitation. J. Trauma Inj. Infect. Crit. Care 2004, 57, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Woods, H.F. Clinical and Biochemical Aspects of Lactic Acidosis; Blackwell Scientific Publications: Oxford, UK, 1976. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Gu, W.-J.; Zhang, Z.; Bakker, J. Early lactate clearance-guided therapy in patients with sepsis: A meta-analysis with trial sequential analysis of randomized controlled trials. Intensive Care Med. 2015, 41, 1862–1863. [Google Scholar] [CrossRef]
- Jones, A.E.; Shapiro, N.I.; Trzeciak, S.; Arnold, R.C.; Claremont, H.A.; Kline, J.A.; Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 2010, 303, 739–746. [Google Scholar] [CrossRef]
- Masyuk, M.; Wernly, B.; Lichtenauer, M.; Franz, M.; Kabisch, B.; Muessig, J.M.; Zimmermann, G.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med. 2019, 45, 55–61. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2007, 34, 17–60. [Google Scholar] [CrossRef]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The Surviving Sepsis Campaign bundle: 2018 update. Intensive Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef]
- Genga, K.R.; Russell, J.A. Update of Sepsis in the intensive care unit. J. Innate Immun. 2017, 9, 441–455. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Tridente, A.; Dempsey-Hibbert, N.C. Immature platelet indices alongside procalcitonin for sensitive and specific identification of bacteremia in the intensive care unit. Platelets 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Exline, M.C.; Crouser, E.D. Mitochondrial mechanisms of sepsis-induced organ failure. Front. Biosci. 2008, 13, 5030–5041. [Google Scholar] [PubMed]
- Kumar, S.; Gupta, E.; Kaushik, S.; Srivastava, V.K.; Mehta, S.K.; Jyoti, A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scand. J. Immunol. 2018, 87, e12653. [Google Scholar] [CrossRef]
- Vary, T.C.; Martin, L.F. Potentiation of decreased pyruvate dehydrogenase activity by inflammatory stimuli in sepsis. Circ. Shock 1993, 39, 299–305. [Google Scholar]
- Vary, T.C. Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: Effects on plasma lactate. Shock 1996, 6, 89–94. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Bakker, A.C.; Vanhaesebroeck, B.; Beyaert, R.; Jacob, W.A.; Fiers, W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 1992, 267, 5317–5323. [Google Scholar] [CrossRef]
- Revelly, J.-P.; Tappy, L.; Martinez, A.; Bollmann, M.; Cayeux, M.-C.; Berger, M.M.; Chioléro, R.L. Lactate and glucose metabolism in severe sepsis and cardiogenic shock. Crit. Care Med. 2005, 33, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Huang, Y.; Zhu, Y.; Xia, L.; Wang, R.; Xiao, K.; Wang, H.; Yan, P.; Wen, B.; Cao, L.; et al. Discrimination of sepsis stage metabolic profiles with an LC/MS-MS-based metabolomics approach. BMJ Open Respir. Res. 2014, 1, e000056. [Google Scholar] [CrossRef] [PubMed]
- Sacha, G.L.; Lam, S.W.; Duggal, A.; Torbic, H.; Bass, S.N.; Welch, S.C.; Butler, R.S.; Bauer, S.R. Predictors of response to fixed-dose vasopressin in adult patients with septic shock. Ann. Intensive Care 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Wutrich, Y.; Barraud, D.; Conrad, M.; Cravoisy-Popovic, A.; Nace, L.; Bollaert, P.-E.; Levy, B.; Gibot, S. Early increase in arterial lactate concentration under epinephrine infusion is associated with a better prognosis during shock. Shock 2010, 34, 4–9. [Google Scholar] [CrossRef]
- Levy, B. Bench-to-bedside review: Is there a place for epinephrine in septic shock? Crit. Care 2005, 9, 561–565. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ward, M.J.; Self, W.H.; Singer, A.; Lazar, D.; Pines, J.M. Cost-effectiveness analysis of early point-of-care lactate testing in the emergency department. J. Crit. Care 2016, 36, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tridente, A.; Bion, J.; Mills, G.H.; Gordon, A.C.; Clarke, G.M.; Walden, A.; Hutton, P.; Holloway, P.A.H.; Chiche, J.-D.; Stuber, F.; et al. Derivation and validation of a prognostic model for postoperative risk stratification of critically ill patients with faecal peritonitis. Ann. Intensive Care 2017, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Astles, T. Iatrogenic anaemia in the critically ill: A survey of the frequency of blood testing in a teaching hospital intensive care unit. J. Intensive Care Soc. 2009, 10, 279–281. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; De Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Burden, R.J.; Pedlar, C.; Lewis, N.A. Biomarkers in elite sport: Where innovations in technology and application combine. Exp. Physiol. 2019, 104, 275–277. [Google Scholar] [CrossRef]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; Berg, A.V.D. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef]
- Smutok, O.; Karkovska, M.; Serkiz, R.; Vus, B.; Cenas, N.; Gonchar, M.V. A novel mediatorless biosensor based on flavocytochrome b2 immobilized onto gold nanoclusters for non-invasive L-lactate analysis of human liquids. Sens. Actuators B Chem. 2017, 250, 469–475. [Google Scholar] [CrossRef]
- Alam, F.; Roychoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Claver, J.B.; Mirón, M.C.V.; Capitán-Vallvey, L.F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst 2009, 134, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-E.; Hiraka, K.; Matloff, D.; Johns, J.; Deng, A.; Sode, K.; Labelle, J.T. Development toward a novel integrated tear lactate sensor using Schirmer test strip and engineered lactate oxidase. Sens. Actuators B Chem. 2018, 270, 525–529. [Google Scholar] [CrossRef]
- Ito, N.; Matsumoto, T.; Fujiwara, H.; Kayashima, S.; Arai, T.; Kikuchi, M.; Karube, I.; Matsumoto, Y. Transcutaneous lactate monitoring based on a micro-planar amperometric biosensor. Anal. Chim. Acta 1995, 312, 323–328. [Google Scholar] [CrossRef]
- Faridnia, M.H.; Palleschi, G.; Lubrano, G.J.; Guilbault, G.G. Amperometric biosensor for determination of lactate in sweat. Anal. Chim. Acta 1993, 278, 35–40. [Google Scholar] [CrossRef]
- Petropoulos, K.; Piermarini, S.; Bernardini, S.; Palleschi, G.; Moscone, D. Development of a disposable biosensor for lactate monitoring in saliva. Sens. Actuators B Chem. 2016, 237, 8–15. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fu, C. Os-complex-based amperometric bienzyme biosensor for continuous determination of lactate in saliva. Anal. Methods 2015, 7, 6158–6164. [Google Scholar] [CrossRef]
- Modali, A.; Vanjari, S.R.K.; Dendukuri, D. Wearable woven electrochemical biosensor patch for non-invasive diagnostics. Electroanalysis 2016, 28, 1276–1282. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Chua, K.Y.; Wicaksono, D.H.B.; Córcoles, E.P. Cotton fabric-based electrochemical device for lactate measurement in saliva. Analyst 2014, 139, 3009–3016. [Google Scholar] [CrossRef]
- Payne, M.E.; Zamarayeva, A.; Pister, V.I.; Yamamoto, N.A.; Arias, A.C. Printed, flexible lactate sensors: Design considerations before performing on-body measurements. Sci. Rep. 2019, 9, 13720. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Bandodkar, A.J.; Valdes-Ramirez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Baysal, G.; Önder, S.; Göcek, I.; Trabzon, L.; Kızıl, H.; Kök, F.N.; Kayaoğlu, B.K. Microfluidic device on a nonwoven fabric: A potential biosensor for lactate detection. Text. Res. J. 2014, 84, 1729–1741. [Google Scholar] [CrossRef]
- Kuşbaz, A.; Göcek, I.; Baysal, G.; Kök, F.N.; Trabzon, L.; Kizil, H.; Kayaoğlu, B.K. Lactate detection by colorimetric measurement in real human sweat by microfluidic-based biosensor on flexible substrate. J. Text. Inst. 2019, 110, 1725–1732. [Google Scholar] [CrossRef]
- Nagamine, K.; Mano, T.; Nomura, A.; Ichimura, Y.; Izawa, R.; Furusawa, H.; Matsui, H.; Kumaki, D.; Tokito, S. Noninvasive sweat-lactate biosensor emplsoying a hydrogel-based touch pad. Sci. Rep. 2019, 9, 10102. [Google Scholar] [CrossRef]
- Luo, X.; Shi, W.; Yu, H.; Xie, Z.; Li, K.; Cui, Y. Wearable carbon nanotube-based biosensors on gloves for lactate. Sensors 2018, 18, 3398. [Google Scholar] [CrossRef]
- Lin, K.-C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Guan, H.; Zhong, T.; He, H.; Zhao, T.; Xing, L.; Zhang, Y.; Xue, X. A self-powered wearable sweat-evaporation-biosensing analyzer for building sports big data. Nano Energy 2019, 59, 754–761. [Google Scholar] [CrossRef]
- Mao, Y.; Yue, W.; Zhao, T.; Shen, M.; Liu, B.; Chen, S. A self-powered biosensor for monitoring maximal lactate steady state in sport training. Biosensors 2020, 10, 75. [Google Scholar] [CrossRef]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef]
- Abrar, A.; Dong, Y.; Lee, P.K.; Kim, W.S. Bendable electro-chemical lactate sensor printed with silver nano-particles. Sci. Rep. 2016, 6, 30565. [Google Scholar] [CrossRef] [PubMed]
- Calabria, D.; Caliceti, C.; Zangheri, M.; Mirasoli, M.; Simoni, P.; Roda, A. Smartphone–based enzymatic biosensor for oral fluid L-lactate detection in one minute using confined multilayer paper reflectometry. Biosens. Bioelectron. 2017, 94, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Curto, V.F.; Fraser, K.J.; Gurfinkel, M.; Byrne, R.; Diamond, D.; Malliaras, G.G.; Benito-Lopez, F.; Owens, R.M. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem. 2012, 22, 4440–4443. [Google Scholar] [CrossRef]
- Minami, T.; Sato, T.; Minamiki, T.; Fukuda, K.; Kumaki, D.; Tokito, S. A novel OFET-based biosensor for the selective and sensitive detection of lactate levels. Biosens. Bioelectron. 2015, 74, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Guardigli, M.; Calabria, D.; Calabretta, M.M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef]
- Zhang, Z.; Kwok, R.T.K.; Yu, Y.; Tang, B.Z.; Ng, K.M. Sensitive and specific detection of L-lactate using an AIE-active fluorophore. ACS Appl. Mater. Interfaces 2017, 9, 38153–38158. [Google Scholar] [CrossRef]
- Tur-García, E.L.; Davis, F.; Collyer, S.D.; Holmes, J.L.; Barr, H.; Higson, S.P.J. Novel flexible enzyme laminate-based sensor for analysis of lactate in sweat. Sens. Actuators B Chem. 2017, 242, 502–510. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martínez, M.J. Dual range lactate oxidase-based screen printed amperometric biosensor for analysis of lactate in diversified samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef]
- Shimomura, T.; Sumiya, T.; Ono, M.; Itoh, T.; Hanaoka, T.-A. An electrochemical biosensor for the determination of lactic acid in expiration. Procedia Chem. 2012, 6, 46–51. [Google Scholar] [CrossRef]
- Rosati, G.; Gherardi, G.; Grigoletto, D.; Marcolin, G.; Cancellara, P.; Mammucari, C.; Scaramuzza, M.; De Toni, A.; Reggiani, C.; Rizzuto, R.; et al. Lactate dehydrogenase and glutamate pyruvate transaminase biosensing strategies for lactate detection on screen-printed sensors. Catalysis efficiency and interference analysis in complex matrices: From cell cultures to sport medicine. Sens. Bio-Sens. Res. 2018, 21, 54–64. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Pang, Y.; Yang, Z.; Yang, Y.; Ren, T.L. Wearable electronics based on 2D materials for human physiological information detection. Small 2020, 16, 1901124. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Lim, S. Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef]
- Karpova, E.V.; Laptev, A.I.; Andreev, E.A.; Karyakina, E.E.; Karyakin, A.A. Relationship between sweat and blood lactate levels during exhaustive physical exercise. ChemElectroChem 2020, 7, 191–194. [Google Scholar] [CrossRef]
- Pilardeau, P.A.; Lavie, F.; Vaysse, J.; Garnier, M.; Harichaux, P.; Margo, J.N.; Chalumeau, M.T. Effect of different work-loads on sweat production and composition in man. J. Sports Med. Phys. Fit. 1988, 28, 247–252. [Google Scholar]
- Green, J.M.; Bishop, P.A.; Muir, I.H.; McLester, J.R., Jr.; Heath, H.E. Effects of high and low blood lactate concentrations on sweat lactate response. Int. J. Sports Med. 2000, 21, 556–560. [Google Scholar] [CrossRef]
- Ament, W.; Huizenga, J.R.; Mook, G.A.; Gips, C.H.; Verkerke, G.J. Lactate and ammonia concentration in blood and sweat during incremental cycle ergometer exercise. Int. J. Sports Med. 1997, 18, 35–39. [Google Scholar] [CrossRef]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33–37. [Google Scholar] [CrossRef]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Palleschi, G.; Faridnia, M.H.; Lubrano, G.J.; Guilbault, G.G. Determination of lactate in human saliva with an electrochemical enzyme probe. Anal. Chim. Acta 1991, 245, 151–157. [Google Scholar] [CrossRef]
- Ashley, B.K.; Brown, M.S.; Park, Y.; Kuan, S.; Koh, A. Skin-inspired, open mesh electrochemical sensors for lactate and oxygen monitoring. Biosens. Bioelectron. 2019, 132, 343–351. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, X.; Yang, F.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, eaax0649. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef]
- Zamarayeva, A.M.; Yamamoto, N.A.; Toor, A.; Payne, M.E.; Woods, C.; Pister, V.I.; Khan, Y.; Evans, J.W.; Arias, A.C. Optimization of printed sensors to monitor sodium, ammonium, and lactate in sweat. APL Mater. 2020, 8, 100905. [Google Scholar] [CrossRef]
- Luo, X.; Guo, L.; Liu, Y.; Shi, W.; Gai, W.; Cui, Y. Wearable tape-based smart biosensing systems for lactate and glucose. IEEE Sens. J. 2020, 20, 3757–3765. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, X.; Shi, W.; Cui, Y. Microplate-integrated biosensors for glucose and lactate. J. Electrochem. Soc. 2019, 166, B421–B425. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef] [PubMed]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Kim, J.; Kurniawan, J.F.; Sempionatto, J.R.; Moreto, J.R.; Tang, G.; Campbell, A.S.; Shin, A.; Lee, M.Y.; Liu, X.; et al. Epidermal microfluidic electrochemical detection system: Enhanced sweat sampling and metabolite detection. ACS Sens. 2017, 2, 1860–1868. [Google Scholar]
- Promphet, N.; Rattanawaleedirojn, P.; Siralertmukul, K.; Soatthiyanon, N.; Potiyaraj, P.; Thanawattano, C.; Hinestroza, J.P.; Rodthongkum, N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Nakagawa, T.; Pavinatto, A.; Mensah, S.T.; Imani, S.; Mercier, P.; Wang, J. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip 2017, 17, 1834–1842. [Google Scholar] [CrossRef]
- Han, W.; He, H.; Zhang, L.; Dong, C.; Zeng, H.; Dai, Y.; Xing, L.; Zhang, Y.; Xue, X. A self-powered wearable noninvasive electronic-skin for perspiration analysis based on piezo-biosensing unit matrix of enzyme/ZnO nanoarrays. ACS Appl. Mater. Interfaces 2017, 9, 29526–29537. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Xiao, G.; He, J.; Qiao, Y.; Wang, F.; Xia, Q.; Wang, X.; Yu, L.; Lu, Z.; Li, C.-M. Facile and low-cost fabrication of a thread/paper-based wearable system for simultaneous detection of lactate and pH in human sweat. Adv. Fiber Mater. 2020, 2, 265–278. [Google Scholar] [CrossRef]
- Zhang, Z.; Azizi, M.; Lee, M.; Davidowsky, P.; Lawrence, P.; Abbaspourrad, A. A versatile, cost-effective, and flexible wearable biosensor for in situ and ex situ sweat analysis, and personalized nutrition assessment. Lab Chip 2019, 19, 3448–3460. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Yokus, M.A.; Songkakul, T.; Pozdin, V.A.; Bozkurt, A.; Daniele, M.A. Wearable multiplexed biosensor system toward continuous monitoring of metabolites. Biosens. Bioelectron. 2020, 153, 112038. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.-H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.O.; Ulyanova, Y.V.; Figueroa-Teran, R.; Bhatt, K.H.; Singhal, S.; Atanassov, P. Wearable sensor system powered by a biofuel cell for detection of lactate levels in sweat. ECS J. Solid State Sci. Technol. 2016, 5, M3075–M3081. [Google Scholar] [CrossRef]
- Goh, J.H.; Mason, A.; Al-Shamma’a, A.I.; Field, M.; Shackcloth, M.; Browning, P. Non invasive microwave sensor for the detection of lactic acid in cerebrospinal fluid (CSF). J. Phys. Conf. Ser. 2011, 307, 012017. [Google Scholar] [CrossRef]
- Mason, A.; Korostynska, O.; Louis, J.; Cordova-Lopez, L.; Abdullah, B.; Greene, J.; Connell, R.; Hopkins, J. Noninvasive in-situ measurement of blood lactate using microwave sensors. IEEE Trans. Biomed. Eng. 2017, 65, 698–705. [Google Scholar] [CrossRef]
- Khan, R.R.; Oh, S.; Choi, G.; Lee, H.S. Highly sensitive, fast and wide dynamic range lactate sensor containing solvatochromic sensing membrane by combining the capacitance-to-phase conversion technique. Sens. Actuators B Chem. 2020, 309, 127783. [Google Scholar] [CrossRef]
- Kim, S.; Yang, W.S.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Seo, S.-J.; Park, Y.M. Highly sensitive non-enzymatic lactate biosensor driven by porous nanostructured nickel oxide. Ceram. Int. 2019, 45, 23370–23376. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Ni, Y.; Li, J.; Liao, K.; Zhao, G. Porous cuprous oxide microcubes for non-enzymatic amperometric hydrogen peroxide and glucose sensing. Electrochem. Commun. 2009, 11, 812–815. [Google Scholar] [CrossRef]
- Wang, G.; Gu, A.; Wang, G.; Huang, Y.; Ji, H.; Fang, B. Porous Cu–NiO modified glass carbon electrode enhanced nonenzymatic glucose electrochemical sensors. Analyst 2011, 136, 5175–5180. [Google Scholar] [CrossRef]
- Baba, D.; Nugraha, A.S.; Iqbal, M.; Bo, J.; Li, C.; Alshehri, A.A.; You, J.; Malgras, V.; Yamachi, Y.; Asahi, T. Nafion®-coated mesoporous Pd film toward remarkably enhanced detection of lactic acid. RSC Adv. 2018, 8, 10446–10449. [Google Scholar] [CrossRef]
- Kumar, G.S.; Soorya, V.; Kumar, R.S.; Sivasubramanian, R.; Bhattacharyya, A. Multi-layer patch with aligned poly (acrylonitrile-co-acrylic acid) nanofibers for lactate detection in human sweat. Mater. Lett. 2021, 283, 128829. [Google Scholar] [CrossRef]
- Onor, M.; Gufoni, S.; Lomonaco, T.; Ghimenti, S.; Salvo, P.; Sorrentino, F.; Bramanti, E. Potentiometric sensor for non invasive lactate determination in human sweat. Anal. Chim. Acta 2017, 989, 80–87. [Google Scholar] [CrossRef]
- Mengarda, P.; Dias, F.A.; Peixoto, J.V.; Osiecki, R.; Bergamini, M.; Marcolino-Junior, L. Determination of lactate levels in biological fluids using a disposable ion-selective potentiometric sensor based on polypyrrole films. Sens. Actuators B Chem. 2019, 296, 126663. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 2, 100018. [Google Scholar] [CrossRef]
- Haupt, K. Molecularly imprinted polymers in analytical chemistry. Analyst 2001, 126, 747–756. [Google Scholar] [CrossRef]
- Pereira, T.C.; Stradiotto, N.R. Electrochemical sensing of lactate by using an electrode modified with molecularly imprinted polymers, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 764. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Hudson, A.; Foster, C.W.; Eersels, K.; Van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent advances in electrosynthesized molecularly imprinted polymer sensing platforms for bioanalyte detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef]
- Krishna, U.; Joshi, S.P.; Modh, M. An evaluation of serial blood lactate measurement as an early predictor of shock and its outcome in patients of trauma or sepsis. Indian J. Crit. Care Med. 2009, 13, 66–73. [Google Scholar] [CrossRef]
- Puskarich, M.A.; Trzeciak, S.; Shapiro, N.I.; Albers, A.B.; Heffner, A.C.; Kline, J.A.; Jones, A.E. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013, 143, 1548–1553. [Google Scholar] [CrossRef]
- Studer, P.; Vaucher, A.; Candinas, D.; Schnüriger, B. The value of serial serum lactate measurements in predicting the extent of ischemic bowel and outcome of patients suffering acute mesenteric ischemia. J. Gastrointest. Surg. 2015, 19, 751–755. [Google Scholar] [CrossRef]
- Dübendorfer, C.; Billeter, A.; Seifert, B.; Keel, M.; Turina, M. Serial lactate and admission SOFA scores in trauma: An analysis of predictive value in 724 patients with and without traumatic brain injury. Eur. J. Trauma Emerg. Surg. 2012, 39, 25–34. [Google Scholar] [CrossRef]
- Bhide, A.; Cheeran, S.; Muthukumar, S.; Prasad, S. Enzymatic low volume passive sweat based assays for multi-biomarker detection. Biosensors 2019, 9, 13. [Google Scholar] [CrossRef]
- Joshi, S.; Becherer, M.; Lugli, P.; Bhatt, V.; Wu, H. Flexible lactate and glucose sensors using electrolyte-gated carbon nanotube field effect transistor for non-invasive real-time monitoring. IEEE Sens. J. 2017, 17, 4315–4321. [Google Scholar] [CrossRef]
- Shi, W.; Luo, X.; Cui, Y. A tube-integrated painted biosensor for glucose and lactate. Sensors 2018, 18, 1620. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive label-free detection of cortisol and lactate using graphene embedded screen-printed electrode. Nano-Micro Lett. 2018, 10, 41. [Google Scholar] [CrossRef]
- Yeknami, A.F.; Wang, X.; Jeerapan, I.; Imani, S.; Nikoofard, A.; Wang, J.; Mercier, P.P. A 0.3-V CMOS biofuel-cell-powered wireless glucose/lactate biosensing system. IEEE J. Solid-State Circuits 2018, 53, 3126–3139. [Google Scholar] [CrossRef]
- Van den Heuvel, I.; Vlasselaers, D.; Wouters, P.J.; Milants, I.; Ellger, B.; Vanhorebeek, I.; Van den Berghe, G. Serial lactate measurements using microdialysis of interstitial fluid do not correlate with plasma lactate in children after cardiac surgery. Pediatr. Crit. Care Med. 2009, 10, 66–70. [Google Scholar] [CrossRef]
- Ellmerer, M.; Haluzik, M.; Blaha, J.; Kremen, J.; Svacina, S.; Plasnik, A.; Ikeoka, D.; Bodenlenz, M.; Schaupp, L.; Plank, J.; et al. Clinical evaluation of subcutaneous lactate measurement in patients after major cardiac surgery. Int. J. Endocrinol. 2009, 2009, 390975. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef]
- Fellmann, N.; Grizard, G.; Coudert, J. Human frontal sweat rate and lactate concentration during heat exposure and exercise. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 54, 355–360. [Google Scholar] [CrossRef]

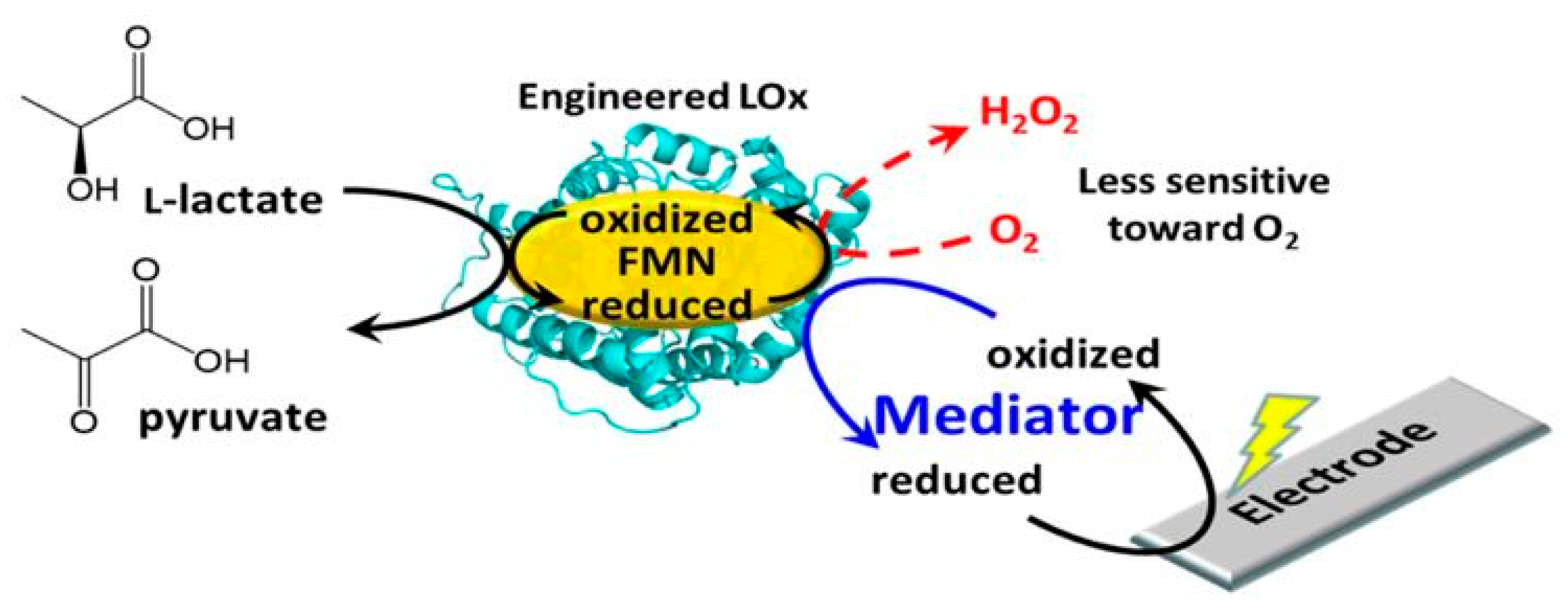
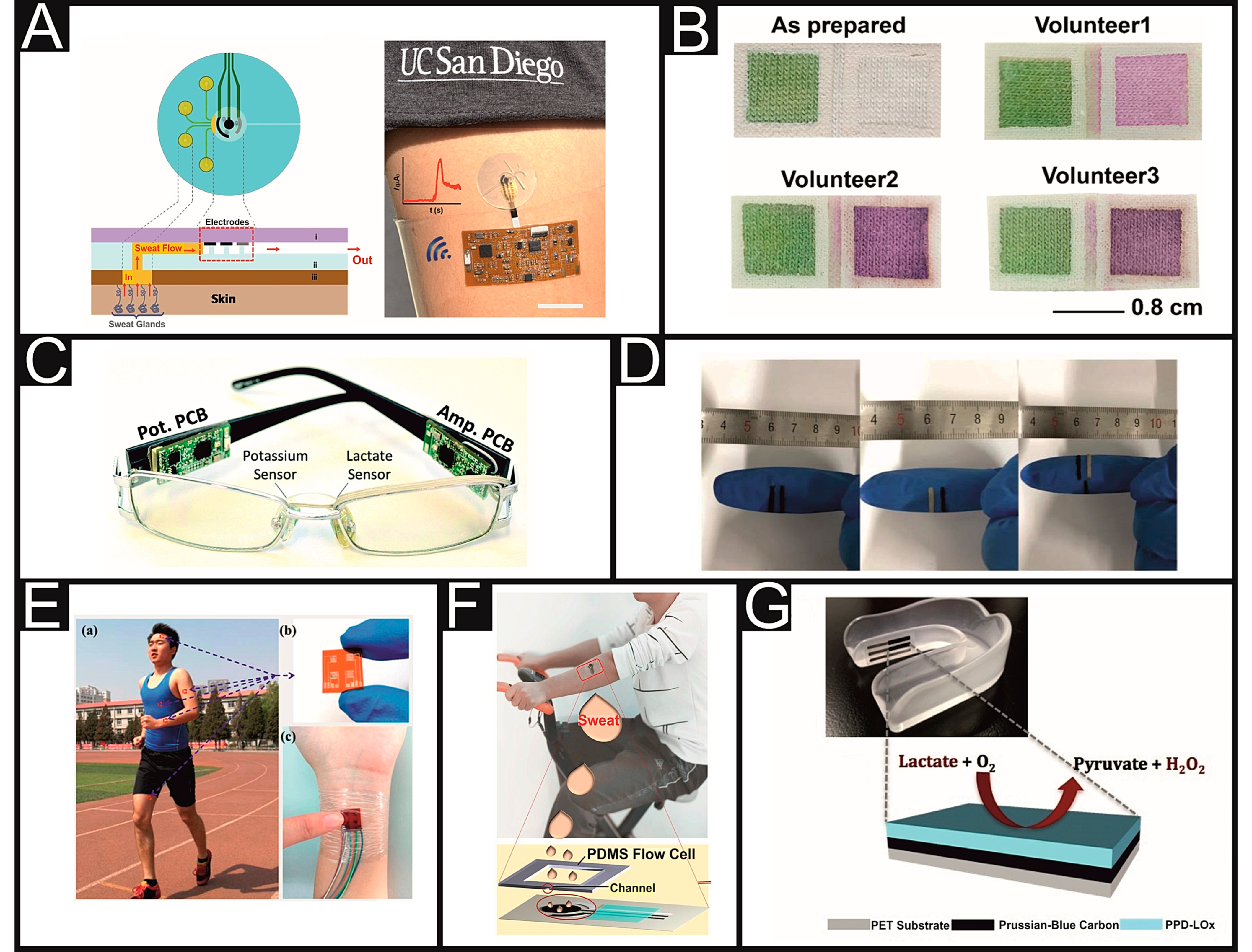
| Cohen & Woods’ Classification | Cause | Clinical Conditions in Which the Hyperlactaemia Is Observed |
|---|---|---|
| A | Global Hypoxia | Shock (Cardiogenic, obstructive, distributive, hypovolaemic), sepsis, profound hypotension, severe anaemia, cardiac arrest, trauma. |
| Regional hypoxia | Mesenteric ischaemia, limb ischaemia, localised trauma, microcirculatory dysfunction. | |
| Exertional hypoxia | Seizures, acute asthma or other increased work of breathing. | |
| B1 | Lactate elevation associated with underlying disease processes | Malignancy, sepsis, liver dysfunction, renal insufficiency, diabetic ketoacidosis, alcoholic ketoacidosis. |
| B2 | Lactate elevation caused by drugs or toxins | Metformin, acetaminophen, β2 adrenergic receptor agonists, sympathomimetics, nucleoside reverse-transcriptase inhibitors, alcohol, cyanide, carbon monoxide. |
| B3 | Lactate elevation caused by congenital errors of metabolism | Mitochondrial myopathy Pyruvate Dehydrogenase deficiency, glucose-6 phosphatase deficiency, congenital mitochondriopathies. |
| Enzyme | Sample Medium | Sensor Material | Method of Detection | Limit of Detection | Linear Range | Reference |
|---|---|---|---|---|---|---|
| LOx a | Saliva | SPE f/Methocel | Electrochemiluminescence | 5 µM | 10–500 µM | [45] |
| LOx a | Tears | SPE f | Amperometric | - | 0.39–16.60 mM | [46] |
| LOx a | Suction effusion fluid | Pt | Amperometric | - | 0.5–25 mM | [47] |
| LOx a | Sweat | Pt/PTFE g | Amperometric | - | 0–120 mg dL−1 | [48] |
| LOx a | Saliva | PB i-SPE f | Amperometric | 0.01 mM | 0.025–0.25 mM | [49] |
| LOx a | Saliva | Graphite/Os/PPhenol | Amperometric | 13 µM | 0.1–1 mM | [50] |
| LOx a | Aqueous | Nylon/carbon ink | Amperometric | - | 4–20 mM | [51] |
| LOx a | Saliva | PB i-SPE f | Amperometric | 0.3 mM | 0.1–5 mM | [52] |
| LOx a | Aqueous | Au/TTF p-CNT h | Amperometric | - | 0–24 mM | [53] |
| LOx a | Sweat | SPE f/CNT h | Amperometric | - | 1–20 mM | [54] |
| LOx a | Aqueous | Evolon fabric | Colourimetric | - | <5 mM> | [55] |
| LOx a | Sweat | SU-8 polymer | Colourimetric | - | 0–11 mM | [56] |
| LOx a | Sweat | PB i-SPE f | Potentiometric | - | 0–1 mM | [57] |
| LOx a | Sweat | CNT h | Amperometric | 6.0 µM | 0.047–1.52 mM | [58] |
| LOx a | Sweat | Pd/GO k | EIS u | 1 mM | 1–100 mM | [59] |
| LOx a | Sweat | Carbon film | Potentiometric | - | 0–21 mM | [60] |
| LOx a | Sweat | PVDF l/T-ZnO m | Piezoelectric | - | 0–8 mM | [61] |
| LOx a | Saliva | PB i-SPE f/PPDj | Amperometric | - | 0.1–1 mM | [62] |
| LOx a | Sweat | AgNP c/Nafion | Amperometric | - | 1–25 mM | [63] |
| LOx a | Saliva | PSS d/PAH e | Colourimetric | 0.1 mM | 0.6–10 mM | [64] |
| LOx a | Sweat | Prussian Blue | OECT t | - | <1 mM | [65] |
| LOx a | Sweat | Ionogel | OECT t | - | 1–100 mM | [66] |
| LOx a | Aqueous | Au/HP n-ORD o | OFET v | 66 nM | 0–1 µM | [67] |
| LOx a | Saliva/Sweat | Nitrocellulose | Chemiluminescence | 0.5/0.1 mM | 0–10 mM | [68] |
| LOx a | Sweat | Au/PB i | Amperometric | 0.137 mM | 0–5 mM | [69] |
| LOx a | Aqueous | TPE q | Fluorescence | 5.5 µM | 0–200 µM | [70] |
| LOx a | Sweat | Pt | Amperometric | - | 0–70 mM | [71] |
| LOx a | Saliva/Sweat | Cu-MOF r/CS s/Pt/SPE f | Amperometric | 0.75 µM | 0.00075–1 mM | [72] |
| LOx a | Breath | PB i-SPE f | Amperometric | - | 150 nM–1.1 mM | [73] |
| LDH b | Sweat | SPE f | Cyclic Voltammetry | 10 µM | 0–100 µM | [74] |
| Sensor Material | Sample Medium | Method of Detection | Limit of Detection | Linear Range | Reference |
|---|---|---|---|---|---|
| Cu/Nile Red | aqueous | Capacitance | - | 100 nM–1 M | [109] |
| NiO | aqueous | Amperometric | 27 µM | 0.01–7.75 mM | [110] |
| Pd film | sweat | Amperometric | 0.34 mM | 0.34–15 mM | [113] |
| Cu/PAN c/P(AN-co-AA) d | artificial sweat | Resistance | - | 27–270 ppm | [114] |
| SPCE a/Fe (III) | sweat | Potentiometric | 1 mM | 1–180 mM | [115] |
| Graphite/PPy b | tears, sweat, blood | Potentiometric | 81 µM | 0.1–10 mM | [116] |
| Recognition Method | Sample Medium | Additional Analytes | Sensor Material | Detection Methodology | Linear Range (mM) | Reference |
|---|---|---|---|---|---|---|
| LOx a | Aqueous | Oxygen | Au/SWCNT b/PB c | Amperometric | 0.05–0.85 | [87] |
| LOx a | Sweat | Glucose, ascorbic acid, uric acid, Na+, K+ | SilkNCT g | Amperometric | 5–35 | [88] |
| LOx a | Sweat | Na+, NH4+ | Au/TTF n/CNT f | Amperometric | 0–30 | [90] |
| LOx a | Aqueous | Glucose | SPE h/PB c | Amperometric | 0.48–2.59 | [91] |
| LOx a | Aqueous | Glucose | SPE h/PB c | Amperometric | 0.083–10.4 | [92] |
| LOx a | Sweat | pH, Na+ | SPEES d/PES e | Amperometric | 0–28 | [94] |
| LOx a | Sweat | Glucose | SPE h/PB c | Amperometric | 4–20 | [95] |
| LOx a | Sweat | pH | 4-AAP i/TOOS j | Colorimetric | 0–25 | [96] |
| LOx a | Sweat | Glucose | SPE h/PB c | Amperometric | 0–14 | [97] |
| LOx a | Sweat | Glucose, uric acid, urea | ZnO nanowires | Piezoelectric | 0–20 | [98] |
| LOx a | Sweat | pH | Assay Kit | Colorimetric | 0–25 | [100] |
| LOx a | Sweat | Glucose, pH | SPE h/o-PD o | Colorimetric | 0–0.025 | [101] |
| LOx a | Sweat | Glucose, pH, temperature | AuNP m/PB c | Amperometric | 1–40 | [104] |
| LOx a | Sweat | Glucose, pH | CNT f/Ti3C2Tx/PB c | Amperometric | 0–22 | [105] |
| LOx a | Artificial Sweat | Glucose, alcohol | ZnO film | Amperometric | 1–100 | [125] |
| LOx a | Aqueous | Glucose | CNT f | Field Effect Transistor | pM–mM | [126] |
| LOx a | Aqueous | Glucose | SPE h | Amperometric | 0.1–8 | [127] |
| Antibody | Artificial Sweat | Cortisol | SPE h/RGO k | Amperometric | 0.5–25 | [128] |
| LOx a | Aqueous | Glucose | CNT f/NQ l | Potentiometric | 2.5–15 | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crapnell, R.D.; Tridente, A.; Banks, C.E.; Dempsey-Hibbert, N.C. Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care. Sensors 2021, 21, 879. https://doi.org/10.3390/s21030879
Crapnell RD, Tridente A, Banks CE, Dempsey-Hibbert NC. Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care. Sensors. 2021; 21(3):879. https://doi.org/10.3390/s21030879
Chicago/Turabian StyleCrapnell, Robert D., Ascanio Tridente, Craig E. Banks, and Nina C. Dempsey-Hibbert. 2021. "Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care" Sensors 21, no. 3: 879. https://doi.org/10.3390/s21030879
APA StyleCrapnell, R. D., Tridente, A., Banks, C. E., & Dempsey-Hibbert, N. C. (2021). Evaluating the Possibility of Translating Technological Advances in Non-Invasive Continuous Lactate Monitoring into Critical Care. Sensors, 21(3), 879. https://doi.org/10.3390/s21030879







