Detecting Emotions through Electrodermal Activity in Learning Contexts: A Systematic Review
Abstract
:1. Introduction
1.1. Theoretical Background of Emotion and Learning
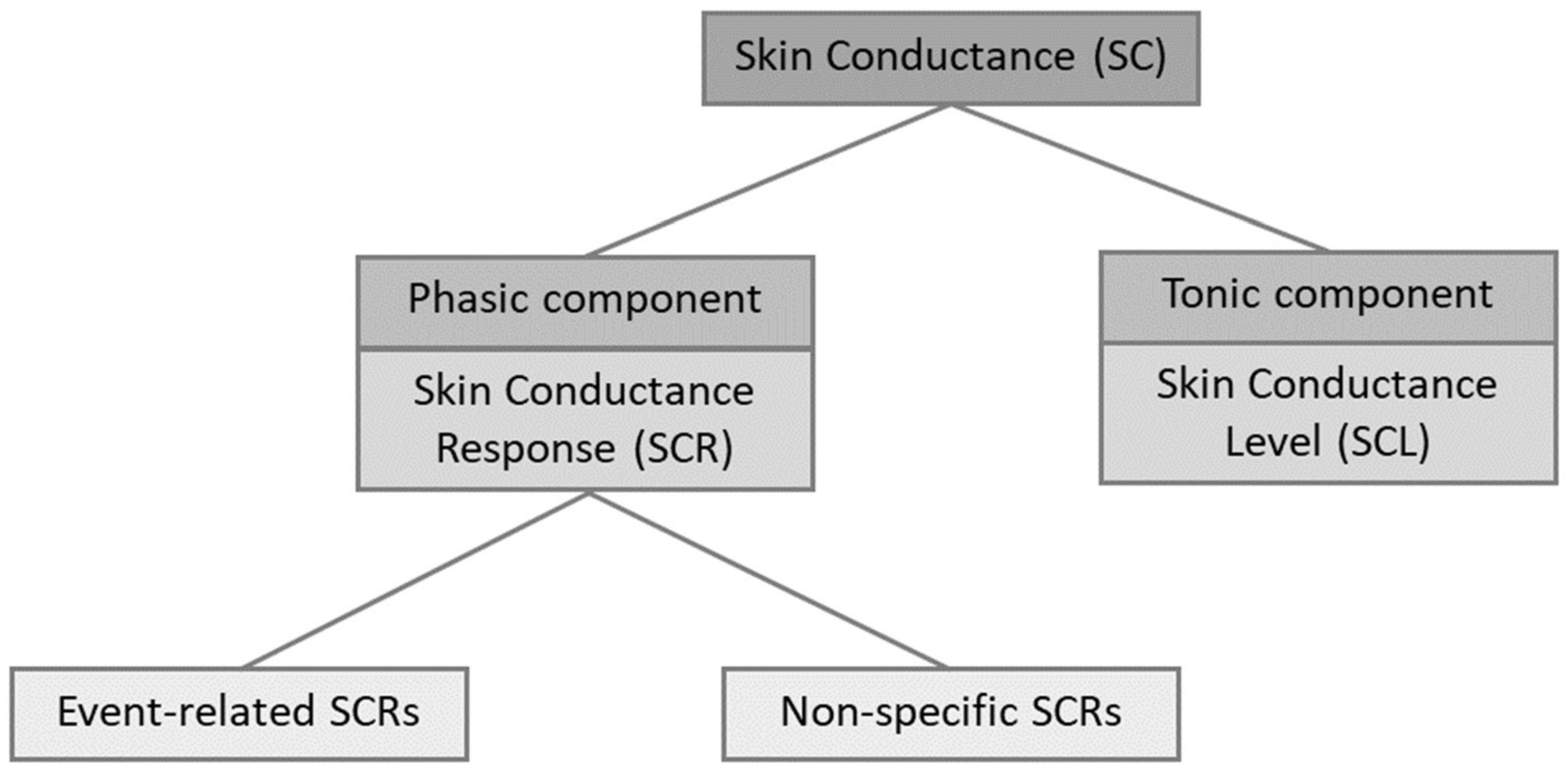
1.2. Electrodermal Activity
1.3. EDA: The Methodological Objective
1.4. Physiological Arousal and Learning: The Empirical Objectives
1.5. This Study
- (1)
- Methodological objective: Provide an overview of methodological aspects of EDA and investigate implicit guidelines and standards for EDA processing in educational research.
- (2)
- Empirical objectives:
- Examine existing empirical evidence of the interaction between physiological arousal as measured by EDA and learning outcomes
- Examine existing empirical evidence of physiological arousal as measured by EDA during the learning process
- (a)
- Examine how physiological arousal as measured by EDA varies during the learning process (unimodal)
- (b)
- Examine combinations of EDA with multimodal data streams to understand learning processes (multimodal)
2. Research Method
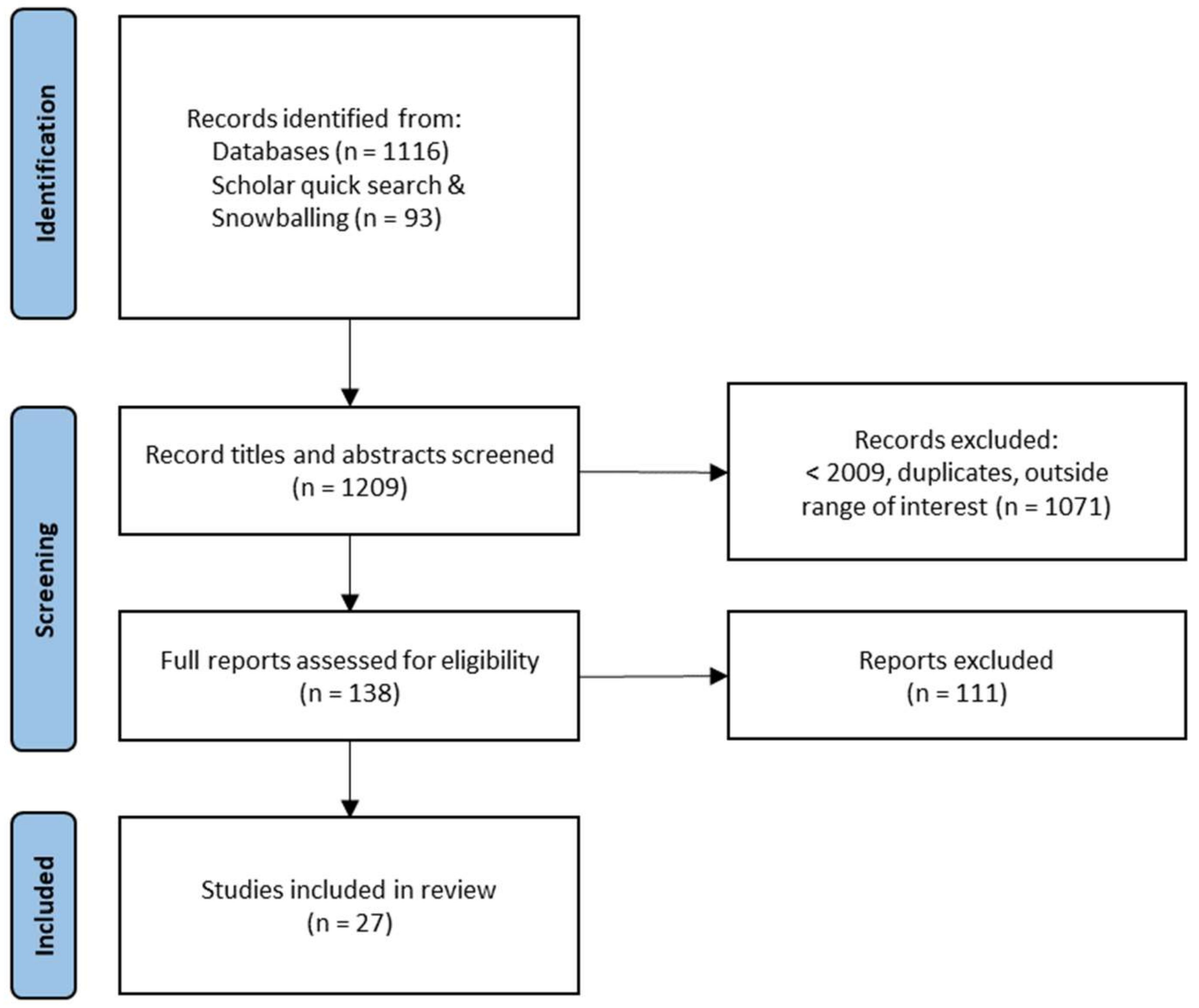
2.1. Search and Inclusion of Studies
2.2. Study Feature Coding
3. Results
3.1. Methodological Aspects of EDA
3.1.1. Devices to Measure EDA
3.1.2. Processing EDA
3.1.3. Signal Processing: Filtering, Cleaning, and Normalization
3.1.4. Baseline Measurement
3.1.5. Features of EDA
3.1.6. Feature Extraction Methods
3.2. Empirical Results
3.2.1. Learning Outcomes
3.2.2. Unimodal Approaches to Studying Learning Processes
3.2.3. Multimodal Approaches to Studying Learning Processes
4. Discussion
- Use devices capable of measuring EDA through electrodes placed on the fingers of the nondominant hand, in authentic settings, and with a sufficient sampling rate
- Justify choices for using tonic or phasic components
- Report data cleaning and filtering procedures clearly
- Look for good practices regarding baselines in other scholarly fields
- Provide argumentation for choice of features
- Define an appropriate response window
- Need for guidelines and standards for EDA processing
- Potential in investigating EDA changes at critical moments during the learning process
- More research needed into experiential measures regarding valence
- Facial expression detection seems promising to connect EDA with behavioural measures
- Analyze the relation between EDA and other physiological measures (EEG, ECG, EMG, heart rate, and skin temperature)
- Potential in investigating combinations of EDA and experiential, behavioural, and other physiological measures at critical moments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cacioppo, J.T.; Tassinary, L.G.; Berntson, G. (Eds.) Handbook of Psychophysiology; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Ley, M.; Hanke, S. Emotion Recognition from Physiological Signal Analysis: A Review. Electron. Notes Theor. Comput. Sci. 2019, 343, 35–55. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal Activity, 2nd ed.; Springer Science + Business Media: Berlin, Germany, 2012. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Chon, K.H. Innovations in Electrodermal Activity Data Collection and Signal Processing: A Systematic Review. Sensors 2020, 20, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, C.; Liu, Z.; Li, Q.; Liu, S. Sensor Data-Driven Emotion Perception in Physical Learning Spaces—A Review and Prospect. In Proceedings of the 2018 Seventh International Conference of Educational Innovation through Technology (EITT), Auckland, New Zealand, 12–14 December 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Pekrun, R. The Control-Value Theory of Achievement Emotions: Assumptions, Corollaries, and Implications for Educational Research and Practice. Educ. Psychol. Rev. 2006, 18, 315–341. [Google Scholar] [CrossRef]
- D’Mello, S.K. Emotional Learning Analytics. In Handbook of Learning Analytics; Society for Learning Analytics Research: Beaumont, Alberta, 2017; pp. 115–127. [Google Scholar] [CrossRef] [Green Version]
- Eliot, J.A.R.; Hirumi, A. Emotion theory in education research practice: An interdisciplinary critical literature review. Educ. Technol. Res. Dev. 2019, 67, 1065–1084. [Google Scholar] [CrossRef] [Green Version]
- Pijeira-Díaz, H.J. Electrodermal Activity and Sympathetic Arousal during Collaborative Learning. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2019. [Google Scholar]
- Harley, J.M. Measuring emotions: A survey of cutting edge methodologies used in computer-based learning environ-ment research. In Emotions, Technology, Design and Learning; Tettegah, S., Gartmeier, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 89–114. [Google Scholar]
- Schneider, B.; Dich, Y.; Radu, I. Unpacking the relationship between existing and new measures of physiological synchrony and collaborative learning: A mixed methods study. Int. J. Comput. Collab. Learn. 2020, 15, 89–113. [Google Scholar] [CrossRef]
- Dawson, M.E.; Schell, A.M.; Filion, D.L. The Electrodermal System. In Handbook of Psychophysiology, 4th ed.; Cacioppo, J.T., Tassinary, L.G., Berntson, G.G., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 217–243. [Google Scholar] [CrossRef]
- Eteläpelto, A.; Kykyri, V.-L.; Penttonen, M.; Hökkä, P.; Paloniemi, S.; Vähäsantanen, K.; Eteläpelto, T.; Lappalainen, V. A multi-componential methodology for exploring emotions in learning. Front. Learn. Res. 2018, 6, 6–36. [Google Scholar] [CrossRef] [Green Version]
- Lehman, B.; D’Mello, S.; Graesser, A. Confusion and complex learning during interactions with computer learning environments. Internet High. Educ. 2012, 15, 184–194. [Google Scholar] [CrossRef]
- Loderer, K.; Pekrun, R.; Lester, J.C. Beyond cold technology: A systematic review and meta-analysis on emotions in technology-based learning environments. Learn. Instr. 2020, 70, 101162. [Google Scholar] [CrossRef]
- Boekaerts, M.; Pekrun, R. Emotions and emotion regulation in academic settings. In Handbook of Educational Psychology; Corno, L., Anderman, E.M., Eds.; Routledge: England, UK, 2016; pp. 76–90. [Google Scholar]
- D’Mello, S.; Graesser, A. The half-life of cognitive-affective states during complex learning. Cogn. Emot. 2011, 25, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Götz, T.; Hall, N.C. Emotion and achievement in the classroom. In International Guide to Student Achievement; Hattie, J., Ed.; Routledge: England, UK, 2013; pp. 192–195. [Google Scholar]
- Ekman, P. Basic emotions. In Handbook of cognition and emotion; Dalgleish, T., Power, M.J., Eds.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 1999; pp. 45–60. [Google Scholar]
- Broekens, J.; Brinkman, W.-P. AffectButton: A method for reliable and valid affective self-report. Int. J. Hum.-Comput. Stud. 2013, 71, 641–667. [Google Scholar] [CrossRef]
- Graesser, A.; Mcdaniel, B.; Chipman, P.; Witherspoon, A.; D’mello, S.; Gholson, B. Detection of emotions during learning with AutoTutor. In Proceedings of the Annual Meeting of the Cognitive Science Society, Vancouver, BC, Canada, 26–29 July 2006; Volume 28, pp. 285–290. [Google Scholar]
- Kort, B.; Reilly, R.; Picard, R. An Affective Model of Interplay Between Emotions and Learning: Reengineering Educational Pedagogy-Building a Learning Companion. In Proceedings of the IEEE International Conference on Advanced Learning Technologies, Madison, WI, USA, 6–8 August 2001; pp. 43–46. [Google Scholar] [CrossRef]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Harley, J.M.; Bouchet, F.; Azevedo, R. Measuring Learners’ Co-Occurring Emotional Responses during Their Interaction with a Pedagogical Agent in MetaTutor. In Computer Vision; Springer: Singapore, 2012; Volume 7315, pp. 40–45. [Google Scholar] [CrossRef]
- Pekrun, R. Emotion and Achievement During Adolescence. Child Dev. Perspect. 2017, 11, 215–221. [Google Scholar] [CrossRef]
- Calvo, R.; D’Mello, S. Affect Detection: An Interdisciplinary Review of Models, Methods, and Their Applications. IEEE Trans. Affect. Comput. 2010, 1, 18–37. [Google Scholar] [CrossRef]
- Malmberg, J.; Haataja, E.; Seppänen, T.; Järvelä, S. Are we together or not? The temporal interplay of monitoring, physiological arousal and physiological synchrony during a collaborative exam. Int. J. Comput. Collab. Learn. 2019, 14, 467–490. [Google Scholar] [CrossRef] [Green Version]
- Wampfler, R.; Klingler, S.; Solenthaler, B.; Schinazi, V.; Gross, M. Affective state prediction in a mobile setting using wearable biometric sensors and stylus. In Proceedings of the 12th International Conference on Educational Data Mining, Montreal, QC, Canada, 2–5 July 2019; pp. 198–207. [Google Scholar] [CrossRef]
- Fritz, T.; Begel, A.; Müller, S.C.; Yigit-Elliott, S.; Züger, M. Using psycho-physiological measures to assess task difficulty in software development. In Proceedings of the 36th International Conference on Software Engineering, Hyderabad, India, 31 May 2014; pp. 402–413. [Google Scholar] [CrossRef] [Green Version]
- Thammasan, N.; Stuldreher, I.V.; Schreuders, E.; Giletta, M.; Brouwer, A.-M. A Usability Study of Physiological Measurement in School Using Wearable Sensors. Sensors 2020, 20, 5380. [Google Scholar] [CrossRef]
- Di Mitri, D.; Schneider, J.; Specht, M.; Drachsler, H. From signals to knowledge: A conceptual model for multimodal learning analytics. J. Comput. Assist. Learn. 2018, 34, 338–349. [Google Scholar] [CrossRef] [Green Version]
- McCraty, R.; Atkinson, M.; Tiller, W.A.; Rein, G.; Watkins, A.D. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am. J. Cardiol. 1995, 76, 1089–1093. [Google Scholar] [CrossRef]
- Pijeira-Díaz, H.; Drachsler, H.; Kirschner, P.; Järvelä, S. Profiling sympathetic arousal in a physics course: How active are students? J. Comput. Assist. Learn. 2018, 34, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures. Publication recommendations for electrodermal measurements. Psychophysiol 2012, 49, 1017–1034. [Google Scholar] [CrossRef]
- Braithwaite, J.J.; Watson, D.G.; Jones, R.; Rowe, M. A guide for analysing electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments. Psychophysiology 2015, 49, 1017–1034. Available online: https://www.biopac.com/wp-content/uploads/EDA-SCR-Analysis.pdf (accessed on 30 July 2021).
- Khan, T.H.; Villanueva, I.; Vicioso, P.; Husman, J. Exploring relationships between electrodermal activity, skin temperature, and performance during. In Proceedings of the 2019 IEEE Frontiers in Education Conference (FIE), Covington, KY, USA, 16–19 October 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Geršak, G. Electrodermal activity—A beginner’s guide. Electrotech. Rev. 2021, 87, 175–182. [Google Scholar]
- Schmidt, S.; Walach, H. Electrodermal activity (EDA)—State-of-the-art measurement and techniques for parapsychological purposes. J. Parapsychol. 2000, 64, 139–163. [Google Scholar]
- Carroll, A.; Gillies, R.M.; Cunnington, R.; McCarthy, M.; Sherwell, C.; Palghat, K.; Goh, F.; Baffour, B.; Bourgeois, A.; Rafter, M.; et al. Changes in science attitudes, beliefs, knowledge and physiological arousal after implementation of a multimodal, cooperative intervention in primary school science classes. Inf. Learn. Sci. 2019, 120, 409–425. [Google Scholar] [CrossRef]
- Hoogerheide, V.; Renkl, A.; Fiorella, L.; Paas, F.; van Gog, T. Enhancing example-based learning: Teaching on video increases arousal and improves problem-solving performance. J. Educ. Psychol. 2019, 111, 45–56. [Google Scholar] [CrossRef]
- Daley, S.G.; Willett, J.B.; Fischer, K.W. Emotional responses during reading: Physiological responses predict real-time reading comprehension. J. Educ. Psychol. 2014, 106, 132–143. [Google Scholar] [CrossRef]
- Dich, Y.; Reilly, J.; Schneider, B. Using physiological synchrony as an indicator of collaboration quality, task performance and learning. In Lecture Notes in Computer Science; Springer International Publishing: New York, NY, USA, 2018; Volume 10947, pp. 98–110. [Google Scholar] [CrossRef]
- Dindar, M.; Malmberg, J.; Järvelä, S.; Haataja, E.; Kirschner, P.A. Matching self-reports with electrodermal activity data: Investigating temporal changes in self-regulated learning. Educ. Inf. Technol. 2020, 25, 1785–1802. [Google Scholar] [CrossRef] [Green Version]
- Mu, S.; Cui, M.; Huang, X. Multimodal Data Fusion in Learning Analytics: A Systematic Review. Sensors 2020, 20, 6856. [Google Scholar] [CrossRef] [PubMed]
- Noroozi, O.; Pijeira-Díaz, H.J.; Sobocinski, M.; Dindar, M.; Järvelä, S.; Kirschner, P.A. Multimodal data indicators for capturing cognitive, motivational, and emotional learning processes: A systematic literature review. Educ. Inf. Technol. 2020, 25, 5499–5547. [Google Scholar] [CrossRef]
- Harley, J.M.; Bouchet, F.; Hussain, M.S.; Azevedo, R.; Calvo, R. A multi-componential analysis of emotions during complex learning with an intelligent multi-agent system. Comput. Hum. Behav. 2015, 48, 615–625. [Google Scholar] [CrossRef]
- Mauss, I.B.; Robinson, M.D. Measures of emotion: A review. Cogn. Emot. 2009, 23, 209–237. [Google Scholar] [CrossRef]
- Peixoto, F.; Mata, L.; Monteiro, V.; Sanches, C.; Pekrun, R. The Achievement Emotions Questionnaire: Validation for Pre-Adolescent Students. Eur. J. Dev. Psychol. 2015, 12, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.K. The Bridge: Linking Mood Induction, Self-Report, and Psychophysiology to Vocabulary Learning on a Paired-Associates Learning Task. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2017. Available online: https://www.proquest.com/docview/1896532066 (accessed on 4 August 2021).
- Li, T.; Lajoie, S. Predicting aviation training performance with multimodal affective inferences. Int. J. Train. Dev. 2021, 25, 301–315. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ahonen, L.; Cowley, B.U.; Hellas, A.; Puolamäki, K. Biosignals reflect pair-dynamics in collaborative work: EDA and ECG study of pair-programming in a classroom environment. Sci. Rep. 2018, 8, 3138. [Google Scholar] [CrossRef]
- Antoniou, P.E.; Arfaras, G.; Pandria, N.; Athanasiou, A.; Ntakakis, G.; Babatsikos, E.; Nigdelis, V.; Bamidis, P. Biosensor Real-Time Affective Analytics in Virtual and Mixed Reality Medical Education Serious Games: Cohort Study. JMIR Serious Games 2020, 8, e17823. [Google Scholar] [CrossRef]
- Apostolidis, H.; Papantoniou, G.; Tsiatsos, T. Deployment and Dynamics of a Biofeedback System for Anxiety Awareness during Online Examination Activities. Appl. Sci. 2021, 11, 756. [Google Scholar] [CrossRef]
- Blikstein, P.; Gomes, J.S.; Akiba, H.; Schneider, B. The Effect of Highly Scaffolded Versus General Instruction on Students’ Exploratory Behavior and Arousal. Technol. Knowl. Learn. 2017, 22, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.; Regenbrecht, H.; Langlotz, T.; Can, Y.S.; Ersoy, C.; Butson, R. Measuring Cognitive Load and Insight: A Methodology Exemplified in a Virtual Reality Learning Context. In Proceedings of the 2019 IEEE International Symposium on Mixed and Augmented Reality (ISMAR), Beijing, China, 14–18 October 2019; pp. 351–362. [Google Scholar] [CrossRef]
- Cooper, D.G.; Arroyo, I.; Woolf, B.P.; Muldner, K.; Burleson, W.; Christopherson, R. Sensors model student self concept in the classroom sensors model student self concept in the classroom. In Proceedings of the International Conference on User Modeling, Adaptation, and Personalization, Trento, Italy, 22–26 June 2009; Volume 5535, pp. 30–41. [Google Scholar] [CrossRef] [Green Version]
- Cowley, B.; Fantato, M.; Jennett, C.; Ruskov, M.; Ravaja, N. Learning when serious: Psychophysiological evaluation of a technology-enhanced learning game. Educ. Technol. Soc. 2014, 17, 3–16. [Google Scholar]
- Geršak, V.; Vitulić, H.S.; Prosen, S.; Starc, G.; Humar, I.; Geršak, G. Use of wearable devices to study activity of children in classroom; Case study—Learning geometry using movement. Comput. Commun. 2020, 150, 581–588. [Google Scholar] [CrossRef]
- Hardy, M.; Wiebe, E.N.; Grafsgaard, J.F.; Boyer, K.E.; Lester, J.C. Physiological Responses to Events during Training. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting; SAGE Publications: Thousand Oaks, CA, USA, 2013; Volume 57, pp. 2101–2105. [Google Scholar] [CrossRef] [Green Version]
- Harley, J.M.; Jarrell, A.; Lajoie, S.P. Emotion regulation tendencies, achievement emotions, and physiological arousal in a medical diagnostic reasoning simulation. Instr. Sci. 2019, 47, 151–180. [Google Scholar] [CrossRef]
- Hussain, M.S.; Alzoubi, O.; Calvo, R.A.; D’Mello, S.K. Affect detection from multichannel physiology during learning sessions with autotutor. In Lecture Notes in Computer Science; Biswas, G., Bull, S., Kay, J., Mitrovic, A., Eds.; Springer: Singapore, 2011; Volume 6738, pp. 131–138. [Google Scholar]
- Irfan, C.M.A.; Nomura, S.; Yamagishi, T.; Kurosawa, Y.; Yajima, K.; Nakahira, K.T.; Ogawa, N.; Fukumura, Y. Utilization of Bio-Signals to Understand the Physiological States of e-Learners and Improve the Learning Contents. IEICE Trans. Inf. Syst. 2011, E94-D, 1235–1242. [Google Scholar] [CrossRef] [Green Version]
- Malmberg, J.; Järvelä, S.; Holappa, J.; Haataja, E.; Huang, X.; Siipo, A. Going beyond what is visible: What multichannel data can reveal about interaction in the context of collaborative learning? Comput. Hum. Behav. 2019, 96, 235–245. [Google Scholar] [CrossRef]
- Mason, L.; Zaccoletti, S.; Scrimin, S.; Tornatora, M.C.; Florit, E.; Goetz, T. Reading with the eyes and under the skin: Comprehending conflicting digital texts. J. Comput. Assist. Learn. 2019, 36, 89–101. [Google Scholar] [CrossRef]
- Meer, Y.; Breznitz, Z.; Katzir, T. Calibration of Self-Reports of Anxiety and Physiological Measures of Anxiety While Reading in Adults with and Without Reading Disability. Dyslexia 2016, 22, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Papamitsiou, Z.; Olsen, J.K.; Giannakos, M. Predicting learners’ effortful behaviour in adaptive assessment using multimodal data. In Proceedings of the 10th International Learning Analytics and Knowledge (LAK), Vancouver, BC, Canada, 13–17 March 2017. [Google Scholar] [CrossRef] [Green Version]
- Strohmaier, A.R.; Schiepe-Tiska, A.; Reiss, K.M. A comparison of self-reports and electrodermal activity as indicators of mathematics state anxiety. Front. Learn. Res. 2020, 8, 16–32. [Google Scholar] [CrossRef]
- Van Bruinessen, I.R.; Ende, I.V.D.; Visser, L.; Van Dulmen, S. The impact of watching educational video clips on analogue patients’ physiological arousal and information recall. Patient Educ. Couns. 2016, 99, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, I.; Campbell, B.D.; Raikes, A.C.; Jones, S.H.; Putney, L.G. A Multimodal Exploration of Engineering Students’ Emotions and Electrodermal Activity in Design Activities. J. Eng. Educ. 2018, 107, 414–441. [Google Scholar] [CrossRef]
- Villanueva, I.; Goodridge, W.H.; Call, B.J. An initial exploration of engineering students’ emotive responses to spatial and engineering statics problems. In Proceedings of the 2018 ASEE Annual Conference & Exposition, Salt Lake, UT, USA, 23–27 July 2018. [Google Scholar] [CrossRef]
- Geršak, G.; Drnovšek, J. Electrodermal activity patient simulator. PLoS ONE 2020, 15, e0228949. [Google Scholar] [CrossRef]
- Benedek, M.; Kaernbach, C. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 2010, 190, 80–91. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, A.M.; Van Beurden, M.; Nijboer, L.; Derikx, L.; Binsch, O.; Gjaltema, C.; Noordzij, M. A comparison of different electrodermal variables in response to an acute social stressor. In Lecture Notes in Computer Science; Ham, J., Spagnolli, A., Blankertz, B., Gamberini, L., Jacucci, G., Eds.; Springer: Singapore, 2018; Volume 10727, pp. 7–17. [Google Scholar] [CrossRef]
- Asuero, A.G.; Sayago, A.; Gonzalez, A.G. The Correlation Coefficient: An Overview. Crit. Rev. Anal. Chem. 2006, 36, 41–59. [Google Scholar] [CrossRef]
- de Bragança Pereira, C.A.; Wechsler, S. On the concept of p-value. Braz. J. Probab. Stat. 1993, 7, 159–177. [Google Scholar]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Kasuya, E. On the use of r and r squared in correlation and regression. Ecol. Res. 2019, 34, 235–236. [Google Scholar] [CrossRef]
- Banerjee, A.; Chitnis, U.B.; Jadhav, S.L.; Bhawalkar, J.S.; Chaudhury, S. Hypothesis testing, type I and type II errors. Ind. Psychiatry J. 2009, 18, 127. [Google Scholar] [CrossRef]
- Schumacker, R.; Tomek, S. F-Test. In Understanding Statistics Using R; Springer: New York, NY, USA, 2013; pp. 197–207. [Google Scholar]
- Bring, J. How to standardize regression coefficients. Am. Stat. 1994, 48, 209–213. [Google Scholar]
- Pekrun, R.; Muis, K.R.; Frenzel, A.C.; Götz, T. Emotions at School; Routledge: London, UK, 2018. [Google Scholar]
- Hernando-Gallego, F.; Luengo, D.; Artes-Rodriguez, A. Feature Extraction of Galvanic Skin Responses by Nonnegative Sparse Deconvolution. IEEE J. Biomed. Health Inform. 2018, 22, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Posada-Quintero, H.; Florian, J.P.; Orjuela-Cañón, A.D.; Aljama-Corrales, T.; Charleston-Villalobos, S.; Chon, K.H. Power Spectral Density Analysis of Electrodermal Activity for Sympathetic Function Assessment. Ann. Biomed. Eng. 2016, 44, 3124–3135. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Dimitrov, T.; Moutran, A.; Park, S.; Chon, K.H. Analysis of Reproducibility of Noninvasive Measures of Sympathetic Autonomic Control Based on Electrodermal Activity and Heart Rate Variability. IEEE Access 2019, 7, 22523–22531. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Florian, J.P.; Orjuela-Cañón, Á.D.; Chon, K.H. Highly sensitive index of sympathetic activity based on time-frequency spectral analysis of electrodermal activity. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R582–R591. [Google Scholar] [CrossRef] [Green Version]
| Ref. | Participants 1 | Age 2 | n | Type of Task | Domain | Study Type 3 |
|---|---|---|---|---|---|---|
| [52] | University students | 23.00 | 38 | Collaborative Programming | Computer sciences | Case study |
| [53] | University students + adults | - | 11 | VR: Virtual patient scenario | Medicine | Experiment |
| [54] | University students | 26.04 (2.30) | 15 | Educational game virtual patient | Medicine | Experiment |
| [55] | High school students | - | 21 | Building a bridge and a tower | Physics | Experiment |
| [39] | Primary school students | 11.60 (0.54) | 214 | Inquiry-based learning lessons | Sciences | Case study |
| [56] | University students + adults | 18–45 | 24 | VR: problem-solving task | Problem- solving | Case study |
| [57] | High school students | - | 35 | ITS: geometry tasks | Geometry | Experiment |
| [58] | Adults | 25.87 (3.85) | 15 | Educational game: stakeholder management | Project management | Experiment |
| [49] | University students | 23.50 (6.57) | 70 | Vocabulary training | Language | Experiment |
| [59] | Primary school students | 7.50 (0.47) | 104 | Geometry tasks & physical learning | Geometry | Experiment |
| [60] | Students | - | 38 | Programming tasks | Computer sciences | Case study |
| [46] | University students | 21.00 (1.90) | 67 | ITS: human circulatory system tasks | Biology | Case study |
| [61] | University students | 24.30 (3.50) | 37 | Diagnostic reasoning tasks | Medicine | Experiment |
| [40] | University students | 20.63 (2.13) | 61 | Electrical circuits troubleshooting | Physics | Experiment |
| [62] | University students | 18–30 | 20 | ITS: physics, computer literacy, critical thinking tasks | Physics | Experiment |
| [63] | University students | 19–20 | 18 | E-learning: mathematics & electric circuit tasks | Mathematics & physics | Experiment |
| [36] | University students | - | 76 | Exam | Engineering | Experiment |
| [50] | University students | 24.37 (5.81) | 19 | Aviation training | Aviation | Experiment |
| [64] | High school students | 17.4 (0.67) | 48 | CSCL: design a healthy breakfast | Biology | Case study |
| [65] | Primary school students | 12.37 (0.55) | 48 | Reading comprehension task | Language | Experiment |
| [66] | Adults | 21–34 | 39 | Reading task | Language | Experiment |
| [33] | High school students | 16–17 | 24 | Online exam | Physics | Case study |
| [67] | University students | 19.24 (0.83) | 32 | Programming questions | Computer sciences | Case study |
| [68] | University students | 23.20 (4.07) | 95 | Test | Mathematics | Experiment |
| [69] | Adults | 33.10 (13.40) | 75 | Educational video’s | Medicine | Experiment |
| [70] | University students | 18–20 | 18 | Workshop design | Design | Experiment |
| [71] | University students | - | 7 | Engineering problems | Engineering | Experiment |
| Ref. | Device | Processing | Baseline | |||
|---|---|---|---|---|---|---|
| Filtering | Cleaning | Activity | Length | Usage | ||
| [52] | Shimmer3 GSR+ | - | Interpolation Normalization | Video | 7 min | - |
| [53] | Empatica E4 | - | - | Learning session | - | Average in plots |
| [54] | Self-assembled | Low-pass filter | - | - | - | - |
| [55] | ProComp Infiniti | - | Manual and visual | Different tasks & video | 22 min | Calculate difference score |
| [39] | Empatica E3 | High and low-pass filter | - | - | - | - |
| [56] | Empatica E4 | - | Machine learning | - | - | - |
| [57] | MIT sensor | - | - | - | - | - |
| [58] | Electrodes Ag/AgCl filled | Low-pass filter & down-sampling | - | No specific activities | 5 min | Mean baseline as covariate |
| [49] | BioSemi Active 2 | Down-sampling | - | Resting time & practice video’s | 5 min | Segmenting signal |
| [59] | BodyMedia Core | Non-specified | Accelerometer | - | - | - |
| [60] | Not specified | - | - | - | - | - |
| [46] | Q-Sensor 2.0 | - | - | No specific activities | 10–15 min | Correction for normalization |
| [61] | Q-Sensor 2.0; Biopac | - | - | No specific activities | 2–5 min | Correction for normalization |
| [40] | Empatica E4 | - | - | Learning session | - | Used in analysis |
| [62] | Biopac | - | - | - | - | - |
| [63] | Biopac | High-pass | Normalization | - | - | - |
| [36] | Empatica E4 | - | Accelerometer L2 norm calculation | - | - | - |
| [50] | BioNomadix | Non-specified | Non-specified | - | - | - |
| [64] | Empatica E3 | Adaptive Gaussian filter | Manual, visual Normalization | - | - | - |
| [65] | ProComp Infiniti | - | Normalization | Watching video & learning session | 4 min | Calculate difference score |
| [66] | Biosemi Active 2 | Down-sampling | - | No specific activities | - | Analysis |
| [33] | Empatica E4 | No processing | No processing | - | - | - |
| [67] | Empatica E4 | - | - | - | - | - |
| [68] | Empatica E4 | - | - | Breathing exercise | 5 min | Analysis |
| [69] | Biopac | Low-pass filter | - | Watching video | 30 s | Comparing to baseline |
| [70] | Empatica E3 | - | Normalization | No specific activities | - | - |
| [71] | Empatica E3 | - | Accelerometer Normalization | - | - | - |
| Ref. | Features | Extraction Features in Segments/Whole Session (Time) 1 | Feature Extraction Methods |
|---|---|---|---|
| [52] | Standardized SCR & SCL score | Time segment around event (20 s) | Ledalab |
| [53] | Mean | Task segment (varying) | - |
| [54] | Mean | Task segment (varying) | Manual |
| [55] | Standardized SCL score | Time segment (2 min) | Biograph Infiniti |
| [39] | Mean | Whole learning session (45–60 min) | Manual |
| [56] | Mean, SD, min, max, percentiles | Time segment (1 min) | cvxEDA-tool |
| [57] | Mean, SD, min, max | Time segment around event (90 s) | - |
| [58] | Mean | Time segment (1 min) | Ledalab |
| [49] | Mean | Task segment (40 s) | Ledalab |
| [59] | Mean | Whole learning session (2 h) | Manual |
| [60] | Standardized SCL score | Time segment around event (5 s) | Ledalab |
| [46] | Mean, range | Time segment around event (10 s) | Augsburg toolbox |
| [61] | Number of SCR peaks, Standardized SCL score | Whole learning session (2.5 h) | - |
| [40] | Mean | Task segment (varying) | - |
| [62] | - | Time segment (10 s) | Augsburg toolbox |
| [63] | Mean | Time segment (1 min) | - |
| [36] | Mean | Whole learning session (-) | Ledalab |
| [50] | Mean | Task segment (-) | Neurokit |
| [64] | Number of SCR peaks, Frequency of SCR peaks | Time segment (1 min) | Ledalab |
| [65] | Mean | Task segment (4 min) | - |
| [66] | Amplitude sum of SCR peaks, Latency of SCR peaks | Whole learning session (1 h) | Ledalab |
| [33] | Number of SCR peaks, Onset of SCR peaks | Time segment (1 min) | Ledalab |
| [67] | Mean | Task segment (varying) | - |
| [68] | Frequency of SCR peaks | Time segment (1 min) | Ledalab |
| [69] | Mean, Number of SCR peaks | Task segment (59–79 s) | Acqknowledge |
| [70] | Mean | Whole learning session (75 min) | Manual |
| [71] | Mean | Whole learning session (-) | - |
| Ref. | Interaction EDA— Learning Outcomes | Unimodal | Multimodal | |||
|---|---|---|---|---|---|---|
| Interaction EDA— Learning Process | Experiential | Behavioral | Other | Multimodal Results | ||
| [52] | Differences before and after pass and fail events | Multimodal | - | - | Heart rate | Correlation between heart rate and SCR |
| [53] | - | Increasing EDA during learning | - | - | Heart rate, EEG | No results |
| [54] | - | Variations in EDA during segments of learning | Self-report anxiety | - | EEG | Correlation between EDA and self-report (no results EDA—EEG) |
| [55] | - | U-shaped EDA during learning | x | x | x | x |
| [39] | Positive correlation between science knowledge and changes in EDA | Increasing EDA during learning | x | x | x | x |
| [56] | Classifier with EDA to indicate Aha! Moment (83.66%) | - | - | - | Heart rate | No results |
| [57] | - | Multimodal | Self-report emotion | Facial expression detection | Mouse & chair pressure | Predicting emotions during learning |
| [58] | Tonic EDA predicts learning gain | - | - | - | EMG & ECG | No results |
| [49] | Change in tonic EDA over time predicts performance | Self-report emotion | - | Heart rate, HRV, ECG | No significant relations | |
| [59] | - | Higher EDA in physical learning | Self-report valence | - | Skin temperature | No results |
| [60] | Bigger learning gains when SCR after specific event | - | Self-report engagement | - | - | No results |
| [46] | - | Multimodal | Self-report emotion | Facial expression detection | - | Relations between modalities |
| [61] | Phasic EDA can predict learning | Multimodal | Self-report emotion | - | - | SCL positively predicts anxiety and shame |
| [40] | No association EDA and performance | No difference baseline EDA and EDA during task | Self-report worry | - | - | No results |
| [62] | - | Multimodal | Self-report emotion | - | EMG & ECG | Predicting self-report with EDA |
| [63] | - | Decreasing EDA during learning (SCL) | - | - | ECG | No results |
| [36] | Positive correlation EDA and performance | Multimodal | - | - | Skin temperature | Positive correlation skin temperature and EDA |
| [50] | Phasic EDA can predict performance | - | Self-report | Facial expression detection | - | - |
| [64] | - | Multimodal | - | Facial expression detection | - | Negative (40%), neutral (33%), positive facial expressions (22%)—physiological synchrony |
| [65] | High arousal relates to low performance | - | Self-report emotional problems | Eye-tracking | - | No significant relations |
| [66] | - | EDA oral reading > silent reading (skilled readers) | Self-report anxiety | - | Heart rate | Positive correlation self-report anxiety and EDA (no results heart rate) |
| [33] | Frequency of arousal periods correlates with performance | Mean 60% low arousal, 24% medium, 17% high | x | x | x | |
| [67] | - | Multimodal | - | Facial expression detection, eye-tracking | Heart rate, EEG, skin temperature | High EDA correlates with high emotion, high heart rate, low mental workload, and memory load |
| [68] | No association EDA and performance | Multimodal | Self-report Anxiety | - | - | No significant relations |
| [69] | - | Increasing SCL during learning compared to baseline (not for SCR) | Self-report arousal | - | Heart rate | No significant relations |
| [70] | - | Increase in EDA during learning (more when active learning) | Self-report emotion | - | - | Correlation between EDA and negative emotions and positive emotions |
| [71] | No significant relation EDA and performance on tasks | Decrease EDA in two of three tasks | Self-report emotion | - | - | Correlation between EDA and self-reported emotion before the task |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvers, A.; Tombeng, N.; Bosse, T.; Lazonder, A.W.; Molenaar, I. Detecting Emotions through Electrodermal Activity in Learning Contexts: A Systematic Review. Sensors 2021, 21, 7869. https://doi.org/10.3390/s21237869
Horvers A, Tombeng N, Bosse T, Lazonder AW, Molenaar I. Detecting Emotions through Electrodermal Activity in Learning Contexts: A Systematic Review. Sensors. 2021; 21(23):7869. https://doi.org/10.3390/s21237869
Chicago/Turabian StyleHorvers, Anne, Natasha Tombeng, Tibor Bosse, Ard W. Lazonder, and Inge Molenaar. 2021. "Detecting Emotions through Electrodermal Activity in Learning Contexts: A Systematic Review" Sensors 21, no. 23: 7869. https://doi.org/10.3390/s21237869
APA StyleHorvers, A., Tombeng, N., Bosse, T., Lazonder, A. W., & Molenaar, I. (2021). Detecting Emotions through Electrodermal Activity in Learning Contexts: A Systematic Review. Sensors, 21(23), 7869. https://doi.org/10.3390/s21237869





