Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation
Abstract
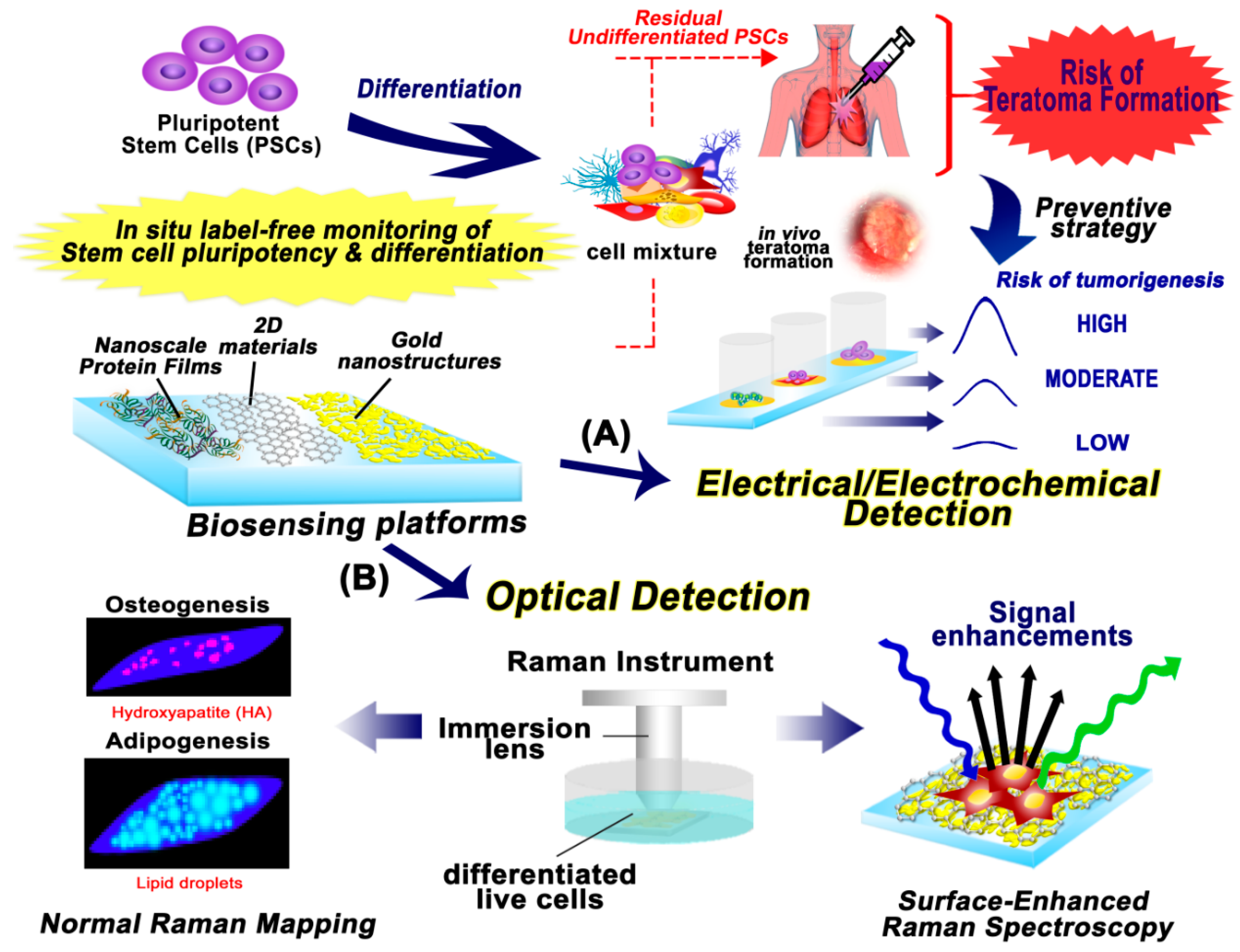
1. Introduction
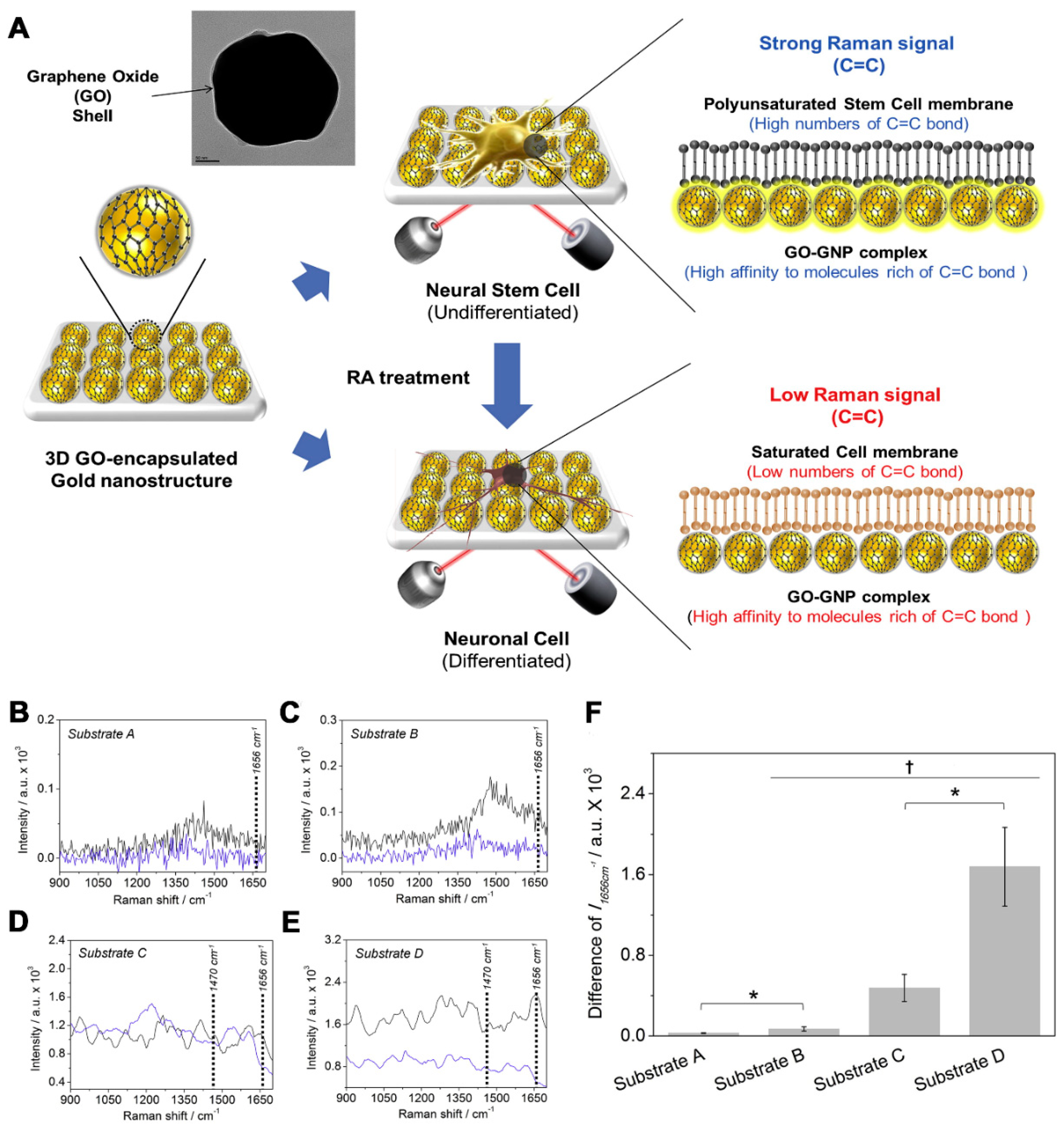
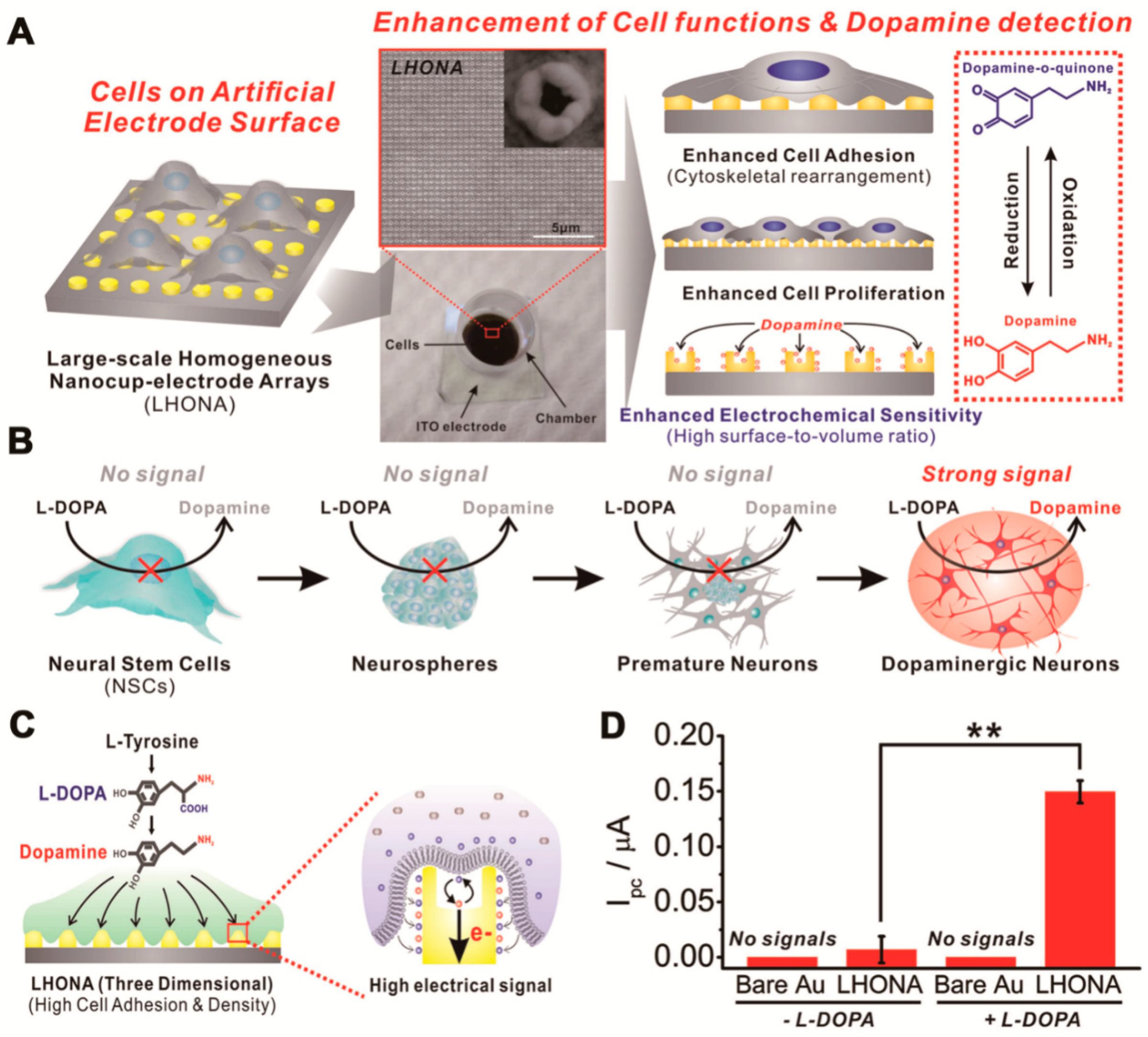
2. Monitoring NSC Multipotency and Differentiation
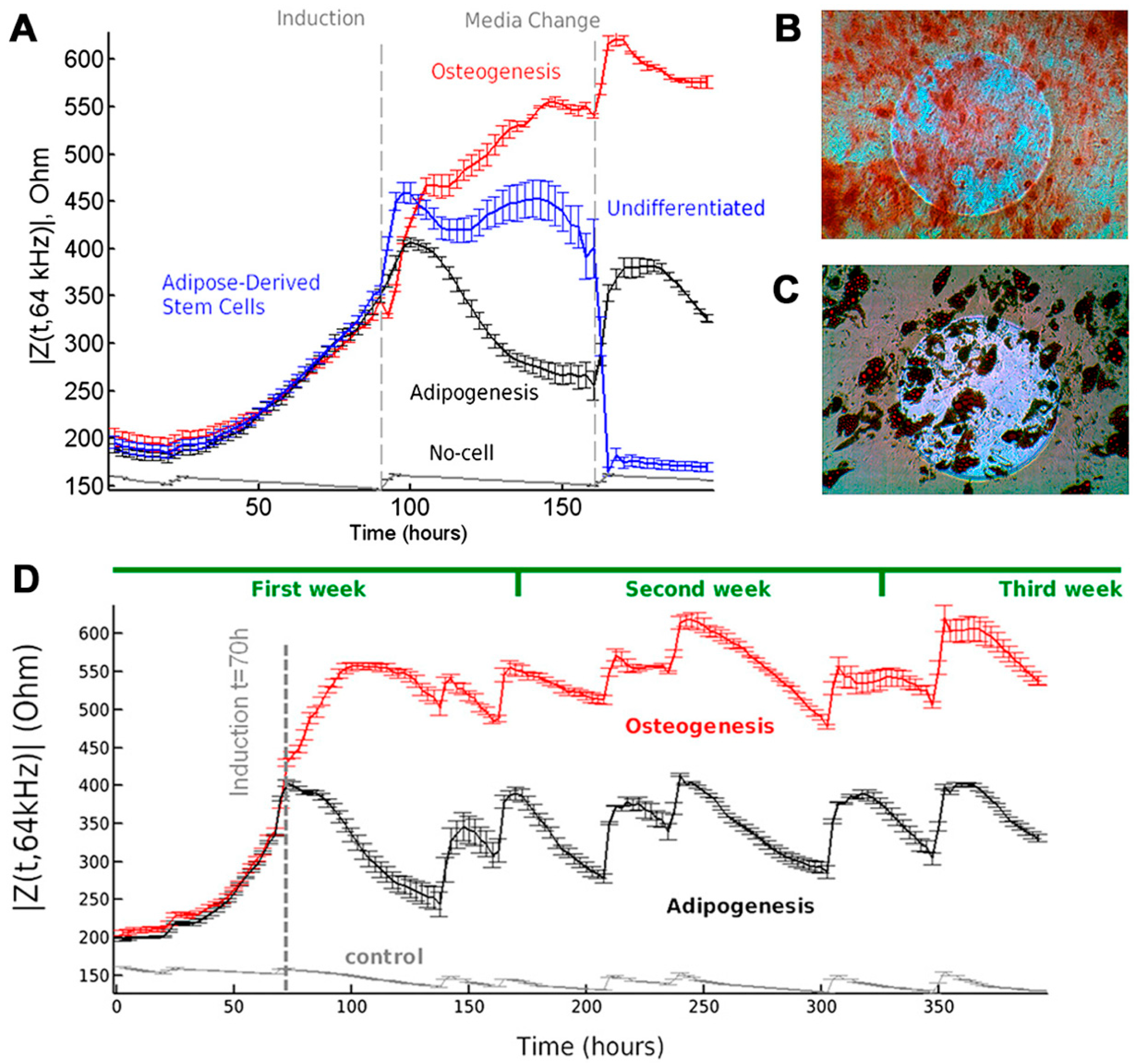
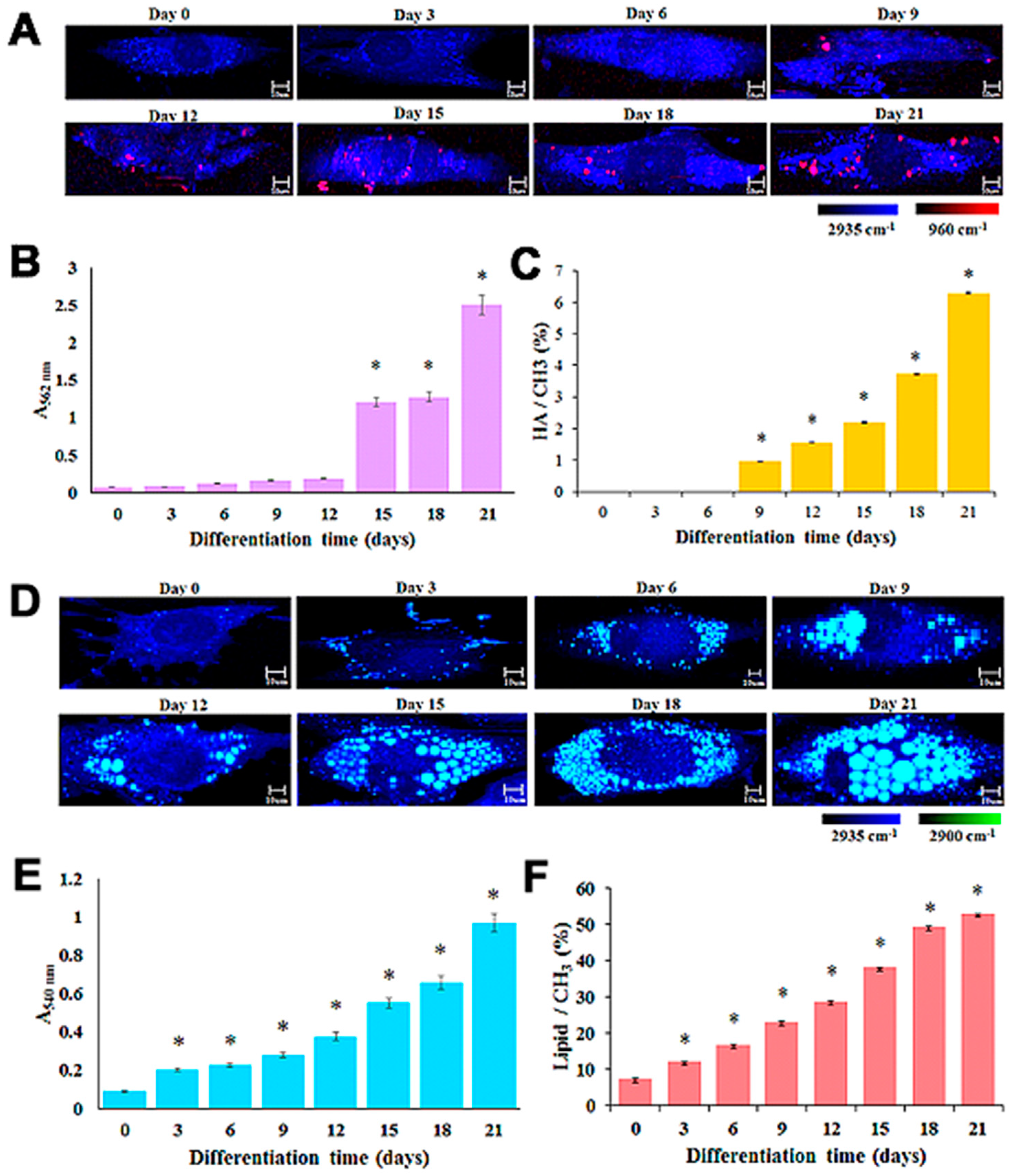
3. Monitoring of MSC Differentiation
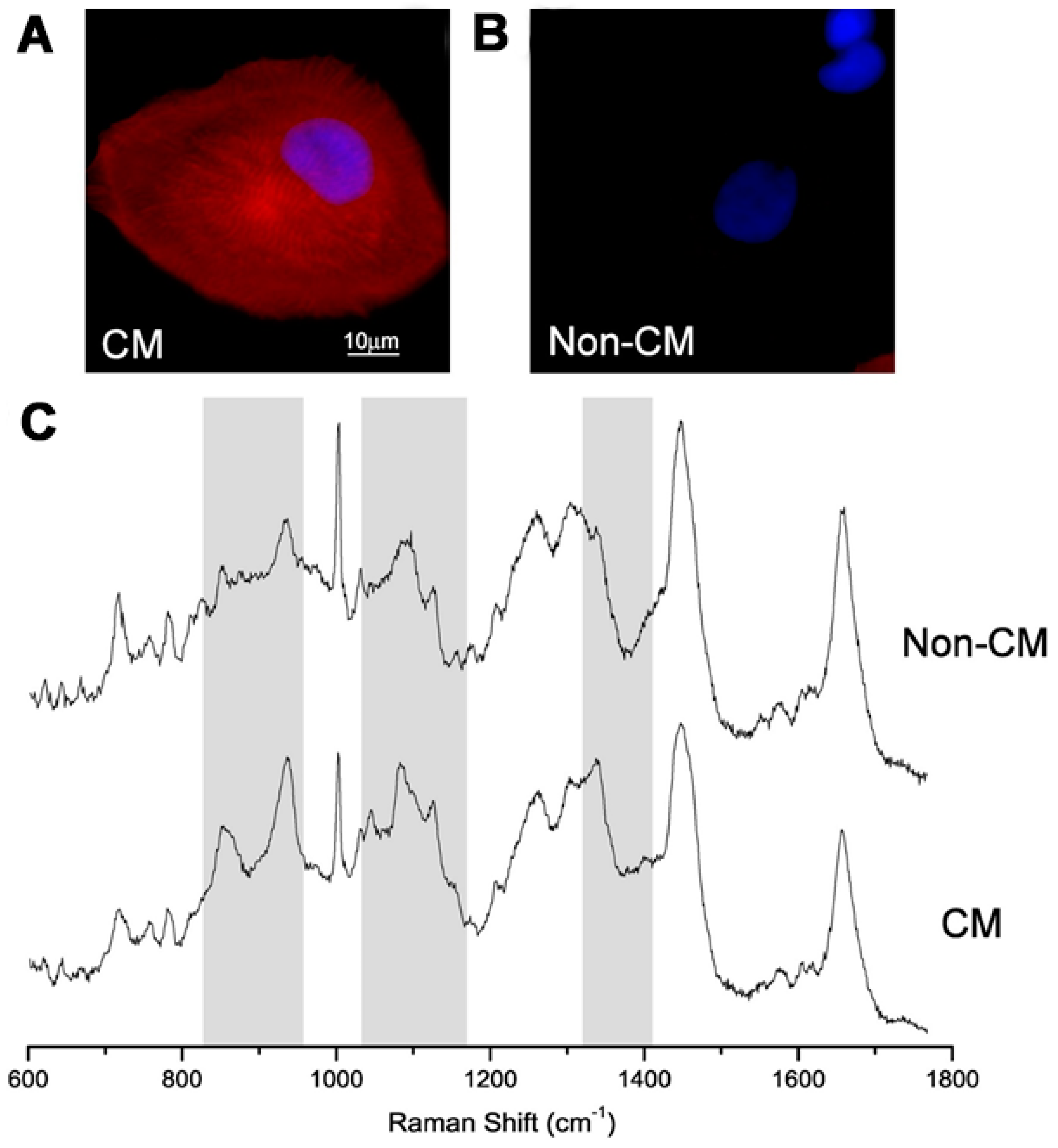
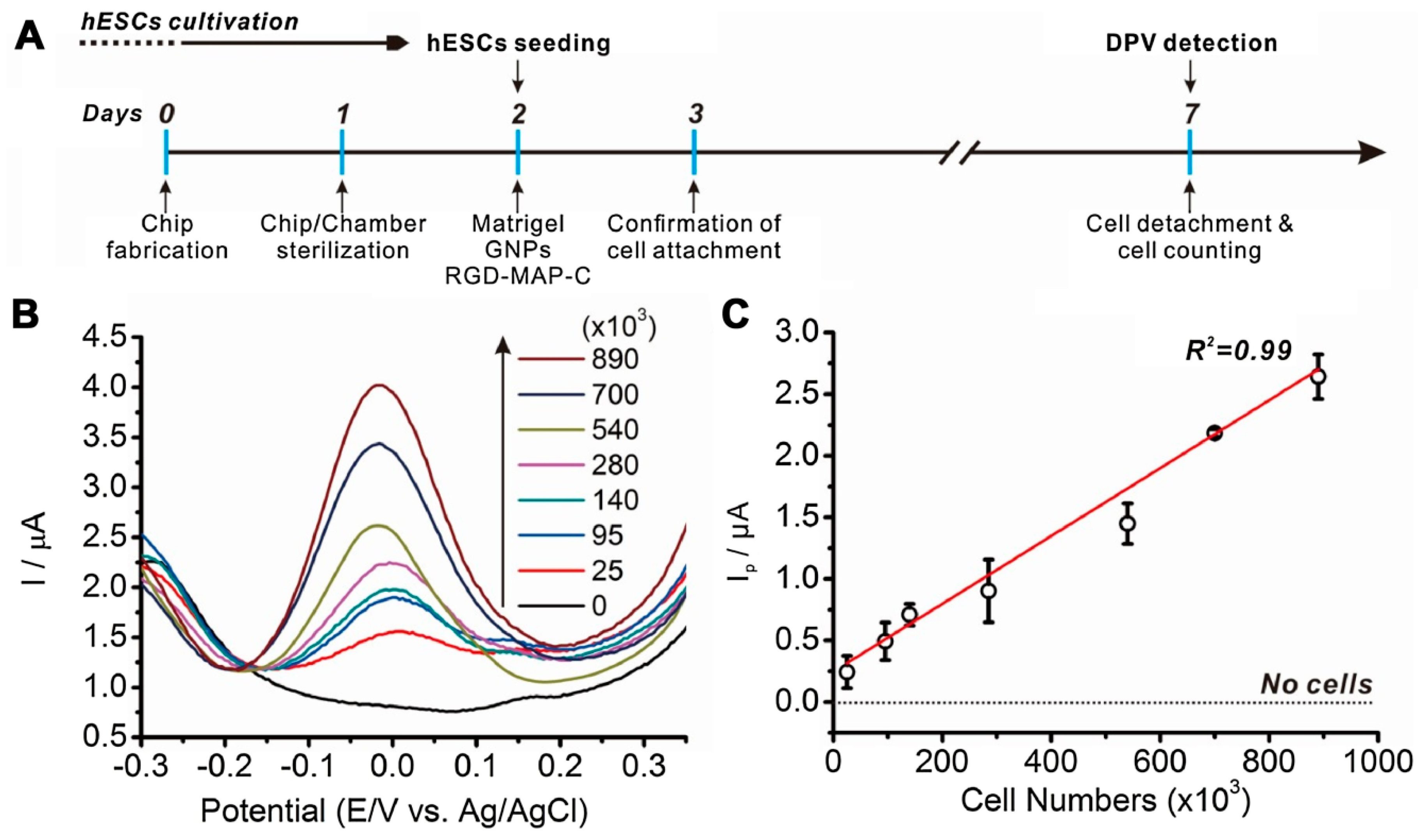
4. Monitoring of PSC Pluripotency and Differentiation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, X.; Zhang, Q.; Yang, X.; Tian, J. Development of New Technologies for Stem Cell Research. J. Biomed. Biotechnol. 2012, 2012, 741416. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Kahn, C.R. Transplantation of adipose tissue and stem cells: Role in Metabolism and Disease. Nat. Rev. Endocrinol. 2010, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S. Stem cells applications in regenerative medicine and disease therapeutics. Int. J. Cell Biol. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.; Krause, D.; Keating, A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, H.I. Introduction to neural stem cells. Stroke 2007, 38, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Kim, Y.J.; Son, Y.J.; Yoo, H.S.; Park, J.H. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017, 22, 561–568. [Google Scholar] [CrossRef]
- Shufaro, Y.; Reubinoff, B.E. Therapeutic applications of embryonic stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Kopper, O.; Benvenisty, N. Expanding the boundaries of embryonic stem cells. Cell Stem Cell 2012, 10, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Boddington, S.E.; Sutton, E.J.; Henning, T.D.; Nedopil, A.J.; Sennino, B.; Kim, A.; Daldrup-Link, H.E. Labeling Human Mesenchymal Stem Cells with Fluorescent Contrast Agents: The Biological Impact. Mol. Imaging Biol. 2011, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Choo, S.-S.; Kim, S.-J.; Song, I.; Kim, T.-H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Niu, C.; Willard, B.; Zhao, W.; Liu, L.; He, W.; Wu, T.; Yang, S.; Feng, S.; Mu, Y.; et al. Proteomic analysis of porcine mesenchymal stem cells derived from bone marrow and umbilical cord: Implication of the Proteins Involved in the Higher Migration Capability of Bone Marrow Mesenchymal Stem Cells. Stem Cell Res. Ther. 2015, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Viganò, M.; Rebulla, P.; Giordano, R.; Lazzari, L. What is beyond a qRT-PCR study on mesenchymal stem cell differentiation properties: How to Choose the Most Reliable Housekeeping Genes. J. Cell Mol. Med. 2013, 17, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Park, U.; Kim, K. Multiple growth factor delivery for skin tissue engineering applications. Biotechnol. Bioprocess Eng. 2018, 22, 659–670. [Google Scholar] [CrossRef]
- Bumbrah, G.S.; Sharma, R.M. Raman spectroscopy–Basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Kalantri, P.P.; Somani, R.R.; Makhija, D.T. Raman spectroscopy: APotential Technique in Analysis of Pharmaceuticals. Der Chem. Sin. 2010, 1, 1–12. [Google Scholar]
- Suhito, I.R.; Han, Y.; Min, J.; Son, H.; Kim, T.-H. In situ label-free monitoring of human adipose-derived mesenchymal stem cell differentiation into multiple lineages. Biomaterials 2018, 154, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Ramoji, A.; Galler, K.; Glaser, U.; Henkel, T.; Mayer, G.; Dellith, J.; Bauer, M.; Popp, J.; Neugebauer, U. Characterization of different substrates for Raman spectroscopic imaging of eukaryotic cells. J. Raman Spectrosc. 2016, 47, 773–786. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Albro, M.B.; Stevens, M.M. Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 2017, 140, 128–137. [Google Scholar] [CrossRef] [PubMed]
- McManus, L.L.; Bonnier, F.; Burke, G.A.; Meenan, B.J.; Boyd, A.R.; Byrne, H.J. Assessment of an osteoblast-like cell line as a model for human primary osteoblasts using Raman spectroscopy. Analyst 2012, 137, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- McManus, L.L.; Burke, G.A.; McCafferty, M.M.; O’Hare, P.; Modreanu, M.; Boyd, A.R.; Meenan, B.J. Raman spectroscopic monitoring of the osteogenic differentiation of human mesenchymal stem cells. Analyst 2011, 136, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-C.; Choo, S.-S.; Kim, K.-T.; Hong, K.-S.; Moon, S.-H.; Cha, H.-J.; Kim, T.-H. Conductive hybrid matrigel layer to enhance electrochemical signals of human embryonic stem cells. Sens. Actuators B Chem. 2017, 242, 224–230. [Google Scholar] [CrossRef]
- Yea, C.-H.; Jeong, H.-C.; Moon, S.-H.; Lee, M.-O.; Kim, K.-J.; Choi, J.-W.; Cha, H.-J. In situ label-free quantification of human pluripotent stem cells with electrochemical potential. Biomaterials 2016, 75, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, J.; Shin, W.; Choi, J.W.; Kim, H.J. Priming nanoparticle-guided diagnostics and therapeutics towards human organs-on-chips microphysiological system. Nano Converg. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.L.T.; Kim, E.J.; Chang, S.-K.; Park, T.J. Sensitive detection of lead ions using sodium thiosulfate and surfactant-capped gold nanoparticles. Biochip J. 2016, 10, 65–73. [Google Scholar] [CrossRef]
- Collinson, M.M. Nanoporous gold electrodes and their applications in analytical chemistry. ISRN Anal. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013, 2013, 49. [Google Scholar] [CrossRef]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of Particle Shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Kang, M.; Park, M.; Jeong, K.-H. Engineering hot spots on plasmonicnanopillar arrays for SERS: A review. Biochip J. 2016, 10, 297–309. [Google Scholar] [CrossRef]
- Alizadeh, R.; Hassanzadeh, G.; Joghataei, M.T.; Soleimani, M.; Moradi, F.; Mohammadpour, S.; Ghorbani, J.; Safavi, A.; Sarbishegi, M.; PirhajatiMahabadi, V.; et al. In vitro differentiation of neural stem cells derived from human olfactory bulb into dopaminergic-like neurons. Eur. J. Neurosci. 2017, 45, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Daadi, M.M.; Grueter, B.A.; Malenka, R.C.; Redmond, D.E., Jr.; Steinberg, G.K. Dopaminergic neurons from midbrain-specified human embryonic stem cell-derived neural stem cells engrafted in a monkey model of Parkinson’s disease. PLoS ONE 2012, 7, e41120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Garitaonandia, I.; Abramihina, T.; Wambua, G.K.; Ostrowska, A.; Brock, M.; Noskov, A.; Boscolo, F.S.; Craw, J.S.; Laurent, L.C.; et al. Deriving dopaminergic neurons for clinical use. A. practical approach. Sci. Rep. 2013, 3, 1463. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, Q.; Ma, Y.; Feng, Z.; Wang, T. Directional induction of dopaminergic neurons from neural stem cells using substantianigra homogenates and basic fibroblast growth factor. Neural Regen. Res. 2012, 7, 511–516. [Google Scholar] [PubMed]
- Furukawa, Y.; Shimada, A.; Kato, K.; Iwata, H.; Torimitsu, K. Monitoring neural stem cell differentiation using PEDOT-PSS based MEA. Biochim. Biophys. Acta 2013, 1830, 4329–4333. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kim, I.-S.; Han, N.; Yun, S.; Park, K.I.; Yoo, K.-H. Real-time discrimination between proliferation and neuronal and astroglial differentiation of human neural stem cells. Sci. Rep. 2014, 4, 6319. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Kim, S.U.; Choi, J.-W. Monitoring in vitro neural stem cell differentiation based on surface-enhanced Raman spectroscopy using a gold nanostar array. J. Mater. Chem. C 2015, 3, 3848–3859. [Google Scholar] [CrossRef]
- Hong, S.-G.; Kim, J.H.; Kim, R.E.; Kwon, S.-J.; Kim, D.W.; Jung, H.-T.; Dordick, J.S.; Kim, J. Immobilization of glucose oxidase on graphene oxide for highly sensitive biosensors. Biotechnol. Bioprocess Eng. 2016, 21, 573–579. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, K.B.; Choi, J.W. 3D graphene oxide-encapsulated gold nanoparticles to detect neural stem cell differentiation. Biomaterials 2013, 34, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Amato, L.; Heiskanen, A.; Caviglia, C.; Shah, F.; Zór, K.; Skolimowski, M.; Madou, M.; Gammelgaard, L.; Hansen, R.; Seiz, E.G.; et al. Pyrolysed 3D-Carbon Scaffolds Induce Spontaneous Differentiation of Human Neural Stem Cells and Facilitate Real-Time Dopamine Detection. Adv. Funct. Mater. 2014, 24, 7042–7052. [Google Scholar] [CrossRef]
- Kim, T.H.; Yea, C.H.; Chueng, S.T.; Yin, P.T.; Conley, B.; Dardir, K.; Pak, Y.; Jung, G.Y.; Choi, J.W.; Lee, K.B. Large-Scale Nanoelectrode Arrays to Monitor the Dopaminergic Differentiation of Human Neural Stem Cells. Adv. Mater. 2015, 27, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Yubo, M.; Yanyan, L.; Li, L.; Tao, S.; Bo, L.; Lin, C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: Ameta-analysis. PLoS ONE 2017, 12, e0175449. [Google Scholar] [CrossRef] [PubMed]
- Battiwalla, M.; Hematti, P. Mesenchymal Stem Cells in Hematopoietic Stem Cell Transplantation. Cytotherapy 2009, 11, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.A.; Haack-Sørensen, M.; Mathiasen, A.B.; Jørgensen, E.; Ekblond, A.; Kastrup, J. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): Study Design. Regen. Med. 2012, 7, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Vernardis, S.I.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Metabolomics analysis of the osteogenic differentiation of umbilical cord blood mesenchymal stem cells reveals differential sensitivity to osteogenic agents. Stem Cells Dev. 2017, 26, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Ghita, A.; Pascut, F.C.; Sottile, V.; Denning, C.; Notingher, I. Applications of Raman micro-spectroscopy to stem cell technology: Label-free molecular discrimination and monitoring cell differentiation. EPJ Tech. Instrum. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Ho, J.H.; Lee, O.K. Detection of hepatic maturation by Raman spectroscopy in mesenchymal stromal cells undergoing hepatic differentiation. Stem Cell Res. Ther. 2016, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sirivisoot, S.; Webster, T.J. Multiwalled carbon nanotubes enhance electrochemical properties of titanium to determine in situ bone formation. Nanotechnology 2008, 19, 295101. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Büth, H.; Cho, S.; Thielecke, H. Detection of the osteogenic differentiation of mesenchymal stem cells in 2D and 3D cultures by electrochemical impedance spectroscopy. J. Biotechnol. 2010, 148, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Duruksu, G.; Congur, G.; Karaoz, E. Genomagnetic assay for electrochemical detection of osteogenic differentiation in mesenchymal stem cells. Analyst 2013, 138, 5424–5430. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Kim, S.U.; Park, M.-K.; Choi, J.W. Electrochemical Detection of Human Mesenchymal Stem Cell Differentiation on Fabricated Gold Nano-Dot Cell Chips. J. Nanosci. Nanotechnol. 2015, 15, 7929–7934. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.B.; Son, S.J.; Min, J. Nanomaterials in label-free impedimetric biosensor: Current process and future perspectives. Biochip J. 2016, 10, 318–330. [Google Scholar] [CrossRef]
- Bogomolova, A.; Komarova, E.; Reber, K.; Gerasimov, T.; Yavuz, O.; Bhatt, S.; Aldissi, M. Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal. Chem. 2009, 81, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Fujita, K.; Smith, N.I.; Kobayashi, M.; Inouye, Y.; Kawata, S. Raman microscopy for dynamic molecular imaging of living cells. J. Biomed. Opt. 2008, 13, 044027. [Google Scholar] [CrossRef] [PubMed]
- Gomathy, S.; Stylianou, C.; Phang, I.; Cool, S.; Nurcombe, V.; Ample, F.; Lear, M.; Gorelik, S.; Hobley, J. Raman mapping glucose metabolism during adipogenesis from human mesenchymal stem cells. In Proceedings of the 2010 Photonics Global Conference, Singapore, 14–16 December 2010; pp. 1–5. [Google Scholar]
- Smith, R.; Wright, K.L.; Ashton, L. Raman spectroscopy: An Evolving Technique for Live Cell Studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Yamaguchi, Y.; Morimoto, C.; Fujita, K.; Takedachi, M.; Kawata, S.; Murakami, S.; Tamiya, E. Time-lapse Raman imaging of osteoblast differentiation. Sci. Rep. 2015, 5, 12529. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.; Fallon, J.; Pettingill, L.; De Silva, M.; Shepherd, R. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 2007, 313, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Downes, A.; Mouras, R.; Elfick, A. Optical spectroscopy for noninvasive monitoring of stem cell differentiation. BioMed Res. Int. 2010, 2010, 101864. [Google Scholar] [CrossRef] [PubMed]
- Kafi, M.A.; Cho, H.Y.; Choi, J.W. Engineered peptide-based nanobiomaterials for electrochemical cell chip. Nano Converg. 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.-H.; Min, J.; Choi, J.-W. The fabrication of cell chips for use as bio-sensors. Biochip J. 2007, 1, 219–227. [Google Scholar]
- Reubinoff, B.E.; Pera, M.F.; Fong, C.-Y.; Trounson, A.; Bongso, A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat. Biotechnol. 2000, 18, 399. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Takahashi, J.; Watanabe, K.; Hayashi, H.; Morizane, A.; Koyanagi, M.; Sasai, Y.; Hashimoto, N. Fluorescence-Activated Cell Sorting–Based Purification of Embryonic Stem Cell–Derived Neural Precursors Averts Tumor Formation after Transplantation. Stem Cells 2006, 24, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Pascut, F.C.; Goh, H.T.; Welch, N.; Buttery, L.D.; Denning, C.; Notingher, I. Noninvasive detection and imaging of molecular markers in live cardiomyocytes derived from human embryonic stem cells. Biophys. J. 2011, 100, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Pascut, F.C.; Kalra, S.; George, V.; Welch, N.; Denning, C.; Notingher, I. Non-invasive label-free monitoring the cardiac differentiation of human embryonic stem cells in-vitro by Raman spectroscopy. BBA-Gen. Subj. 2013, 1830, 3517–3524. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, W.; Zhu, M.-Q.; Han, J.J.; Hurst, J.K.; Li, A.D. Reversibly photoswitchable dual-color fluorescent nanoparticles as new tools for live-cell imaging. J. Am. Chem. Soc. 2007, 129, 3524–3526. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Oh, B.-K.; Koo, K.-K.; Jyoung, J.-Y.; Jeong, S.; Choi, J.-W. Biosensor arrays for environmental pollutants detection. Biochip J. 2008, 2, 223–234. [Google Scholar]
- Michalet, X.; Pinaud, F.; Bentolila, L.; Tsay, J.; Doose, S.; Li, J.; Sundaresan, G.; Wu, A.; Gambhir, S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.-H.; An, J.H.; Kim, J.; Choi, J.-W. In situ electrochemical detection of embryonic stem cell differentiation. J. Biotechnol. 2013, 166, 1–5. [Google Scholar] [CrossRef] [PubMed]
| Types of Stem Cells | Types of Differentiation | Substrate | Detection Method | Ref. |
|---|---|---|---|---|
| NSC | Neurogenesis | PEDOT-PSS modified MEA | Electrochemical impedance spectroscopy | [36] |
| NSC | Neurogenesis | Gold sensing electrode | Capacitance array sensor | [37] |
| NSC | Neurogenesis | Gold nanostar | Cyclic voltammetry and surface-enhanced Raman spectroscopy | [38] |
| NSC | Neurogenesis | 3D-GO encapsulated gold nanostructure | Raman spectroscopy | [40] |
| NSC | Neurogenesis | Pyrolyzed carbon 3D scaffolds | Amperometry | [41] |
| NSC | Neurogenesis | Large-scale homogeneous nanocup-electrode arrays | Cyclic voltammetry | [42] |
| MSC | Osteogenesis and adipogenesis | Quartz glass | Raman spectroscopy | [17] |
| MSC | Osteogenesis | Planar electrode-based chip | Electrochemical impedance spectroscopy | [51] |
| MSC | Adipogenesis and osteogenesis | Gold microelectrode arrays | Electrochemical impedance spectroscopy | [52] |
| MSC | Neurogenesis | Gold nano-dot surface | Cyclic voltammetry | [54] |
| MSC | Adipogenesis | Coverslip glass | Raman spectroscopy | [58] |
| MSC | Osteogenesis | Quartz dish | Raman spectroscopy | [60] |
| ESC | - | ITO/GNPs/RGD/Matrigel composites | Differential Pulse Voltammetry | [23] |
| ESC | - | Gold films | Cyclic voltammetry | [24] |
| ESC | Cardiogenesis | Tissue Culture flask and micro-bioreactors | Raman spectroscopy | [67,68] |
| ESC | - | Gold electrode | Cyclic voltammetry | [72] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhito, I.R.; Angeline, N.; Choo, S.-S.; Woo, H.Y.; Paik, T.; Lee, T.; Kim, T.-H. Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation. Sensors 2018, 18, 2755. https://doi.org/10.3390/s18092755
Suhito IR, Angeline N, Choo S-S, Woo HY, Paik T, Lee T, Kim T-H. Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation. Sensors. 2018; 18(9):2755. https://doi.org/10.3390/s18092755
Chicago/Turabian StyleSuhito, Intan Rosalina, Novi Angeline, Sung-Sik Choo, Ho Young Woo, Taejong Paik, Taek Lee, and Tae-Hyung Kim. 2018. "Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation" Sensors 18, no. 9: 2755. https://doi.org/10.3390/s18092755
APA StyleSuhito, I. R., Angeline, N., Choo, S.-S., Woo, H. Y., Paik, T., Lee, T., & Kim, T.-H. (2018). Nanobiosensing Platforms for Real-Time and Non-Invasive Monitoring of Stem Cell Pluripotency and Differentiation. Sensors, 18(9), 2755. https://doi.org/10.3390/s18092755






