Abstract
Carbon dioxide (CO2) is a predominant greenhouse gas significantly contributing to atmospheric heat retention, primarily driven by anthropogenic activities intensifying the greenhouse effect. This study aims to evaluate the diversity of plant species, above-ground biomass (AGB), and carbon stock within a dry dipterocarp forest, which is a vital local natural resource. This study presents a comprehensive evaluation of plant species diversity, AGB, and carbon stock capacity within a dry dipterocarp forest at the Nature Study Center, Mahasarakham University, located in the Kham Riang Subdistrict of Kantharawichai District, Maha Sarakham Province, spanning an area of 20.80 hectares. Ten sample plots, each measuring 40 × 40 m, were established and distributed across the study area. The diameter at breast height (DBH) and the height of the trees were meticulously recorded for all trees within these plots. Advanced statistical techniques were employed to calculate the relative dominance (RD), relative frequency (RF), and Importance Value Index (IVI), alongside a comprehensive assessment of plant species diversity. The AGB was assessed using precise allometric equations, with a focus on analyzing carbon storage within woody biomass. The findings revealed the presence of 52 tree species across 26 families within the forest. The total AGB was measured at 144.510 tons, with carbon stock reaching 67.920 tCO2. These results offer critical insights into enhancing land management strategies to optimize carbon stock, thereby playing a vital role in mitigating greenhouse gas emissions, a significant factor in climate change dynamics.
1. Introduction
Global warming has become an increasingly severe issue across all regions of the world [1]. The primary factor contributing to global warming is the rise in greenhouse gas concentrations in the atmosphere, which disrupts the natural balance. These greenhouse gases are primarily generated by human activities, such as industrial production, energy consumption, transportation, and agriculture. Furthermore, deforestation and land use changes significantly contribute to climate change on local, regional, and global scales [2,3]. Among the greenhouse gases released into the atmosphere, carbon dioxide (CO2) accounts for the largest share, representing 77% of all emissions. Methane (CH4) contributes 14%, and nitrous oxide (N2O) accounts for 8%, while other gases, such as hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6), collectively make up the remaining 1% [4,5,6]. Global carbon dioxide emissions from fossil fuels are projected to reach a record high of 37.4 billion tons in 2024, an increase of 0.8% compared to 2023, according to new data from the Global Carbon Project [7]. This trend pushes countries further away from achieving their climate targets to mitigate global warming. These findings were publicly released on November 13, 2024 during the COP29 summit held in Baku, Azerbaijan, leaders from around the world gathered to discuss strategies for raising trillions of dollars in funding to address the issue of rising global temperatures. Some experts have suggested that global greenhouse gas emissions could peak within this decade, as renewable energy technologies such as solar panels, wind turbines, and electric vehicles gain rapid popularity. However, to date, these technologies only meet a portion of global energy demands, leaving countries reliant on fossil fuels as their primary energy source [8,9,10].
The reduction in CO2 emissions in the atmosphere can be achieved through various methods, including the advancement of clean production technologies, the adoption of clean energy sources, and the implementation of carbon capture and sequestration techniques [11,12]. Among these methods, carbon capture and sequestration are recognized as effective strategies for preventing CO2 from entering the atmosphere. One of the most efficient, cost-effective, and natural approaches to sequestration involves utilizing trees and wooden products. Trees absorb CO2 from the atmosphere via photosynthesis, storing it as biomass in both above-ground components, such as stems, branches, and leaves, and below-ground components, primarily roots [13,14,15,16,17,18,19,20]. The carbon remains sequestered in the tree until it is harvested. Increased photosynthesis leads to greater CO2 absorption and accumulation [21,22,23]. The anatomical features of trees can be analyzed to estimate their biomass using allometric equations, which describe the relationship between biomass and tree structure [24,25]. Activities such as afforestation (the introduction of trees in areas that have never been forested), reforestation (the replanting of trees in areas that were once forested), the restoration of degraded forests, the transformation of agricultural lands into agroforestry systems, and the establishment of forest plantations are crucial for the expansion of forested regions. These initiatives promote carbon storage and mitigate CO2 emissions into the atmosphere, thus aiding in the balance of the carbon cycle and influencing global climate systems [26,27,28,29,30]. However, the quantity of carbon stored in forested areas is subject to variation based on multiple factors, including the type of forest, species diversity, tree density, topography, and environmental conditions [31,32,33]. Additionally, carbon stock is affected by the species of trees, their age, local environmental factors, and the silvicultural practices applied in forest management [34,35,36].
Dry dipterocarp forests, primarily found in the seasonal tropical regions of Thailand, Cambodia, Laos, and Vietnam, are characterized by their deciduous nature, with trees shedding their leaves during the dry season to conserve water. A dry dipterocarp forest is a type of deciduous plant community typically found in arid regions that experiences a dry season lasting 5 to 6 months each year, receiving an annual rainfall of 900 to 1200 mm. In the northern region, this type of forest often grows on rocky mountains with shallow, nutrient-poor soils, interspersed with gravel. Stunted and twisted trees are scattered throughout, while in relatively flat areas, sandy soil supports trees with straight trunks that grow quite well. The prominent species in this forest include four types of the family Dipterocapaceae: Shorea obtusa, S. siamensis, Dipterocarpus obtusifolius, and D. tuberculatus [37]. A dry dipterocarp forest is classified as a permanent forest community characterized by fire as a determining factor (fire climax community). In the absence of fire, this ecosystem would transition into a different type of forest. Fire plays a crucial role in shaping the structure, maintaining species diversity within the plant community, and facilitating the regeneration of tree species. Prolonged control of wildfires in dry dipterocarp forests can lead to a shift towards a more humid plant community, thereby diminishing the regeneration capacity of indicator species such as Shorea obtusa, S. siamensis, Dipterocarpus obtusifolius, and D. tuberculatus. This is primarily because the seeds of these trees fail to reach the ground. Seed germination occurs rapidly after falling, but the roots often dry out and die. Seedlings and medium-sized trees tend to weaken, especially when they receive insufficient light due to dense competition from other plants. The increased moisture in the soil may ultimately contribute to the decline in these indicator species, allowing other tree species that thrive in the new environmental conditions to replace the dry dipterocarp forest [38]. The premise of this research posits that dry dipterocarp forests harbor a considerable variety of plant species, which plays a crucial role in generating a significant quantity of above-ground biomass (AGB) and bolstering carbon stock capabilities. This research posits that the variety of plant species in dry dipterocarp forests is positively associated with the forests’ capacity for carbon stock. This suggests that greater species diversity results in increased AGB and improved carbon storage, thus aiding in the mitigation of greenhouse gas emissions. The objective of this study is to assess the diversity of plant species, AGB, and carbon stock in a dry dipterocarp forest, which serves as an essential local natural resource. The findings of this research may inform ecosystem restoration initiatives aimed at enhancing resilience to climate change and promoting sustainable management of forest resources.
2. Materials and Methods
2.1. Research Area
The study area for this research is a dry dipterocarp forest located within the Nature Study Center, Mahasarakham University, Kham Riang Subdistrict, Kantharawichai District, Maha Sarakham Province (Figure 1). The total forest area covers 20.80 hectares. Mahasarakham Province is situated in the central region of northeastern Thailand at an elevation of 153 m above sea level. The province’s topography primarily consists of high plains without mountains. The climate is divided into three distinct seasons: winter lasts from approximately mid-October to mid-February, summer from mid-February to mid-May, and the rainy season from mid-May to mid-October. According to the average monthly climate statistics of Maha Sarakham Province from 1981 to 2010, the annual average temperature was recorded at 27.4 °C, with the lowest average annual temperature being 22.4 °C and the highest average annual temperature reaching 33.7 °C. April is noted as the hottest month of the year. Furthermore, the average annual rainfall in Maha Sarakham Province ranges from 1000 to 1200 mm.
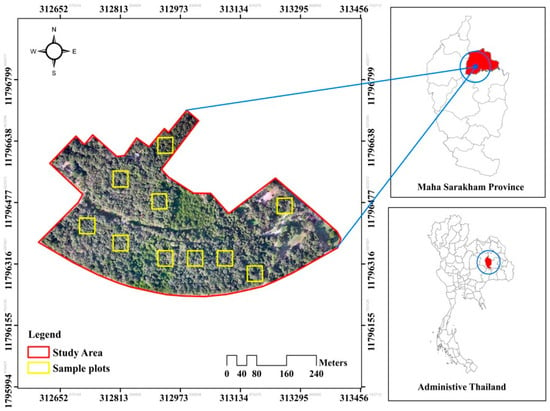
Figure 1.
Dry dipterocarp forest located within the nature study center.
2.2. Data Collection and Analysis
The assessment of carbon in ABG, particularly the carbon stored in trees, is relatively straightforward to measure and analyze. This is because the carbon content in trees can be calculated using established allometric equations, which require only the diameter at breast height (DBH) and tree height for the computations. In this study, a sample plot size of 40 × 40 m is commonly utilized in forestry research in Thailand, corresponding to an area of 0.16 hectares, a familiar unit of measurement for the Thai population. Furthermore, the sample plots were established using a simple random sampling method. From a total area of 20.80 hectares, ten sample plots measuring 40 × 40 m each were distributed throughout the study area to ensure comprehensive coverage [39]. In the designated sample plots, the data collection focused on measuring the diameter at breast height (DBH) and the overall height of the trees. DBH was measured at a height of 1.3 m above ground level for all trees with a DBH greater than 4.5 cm, using a diameter tape for precision. Tree heights were recorded using a Nikon Forest ProII device. Each tree within the specified plot was clearly marked and tagged to prevent any tree from being overlooked or counted multiple times, with measurements starting from the northern boundary and progressing inward. Additionally, species identification and classification were conducted for each tree.
For the analysis of plant communities, the Importance Value Index (IVI) was assessed, which comprises several components: density (D), frequency (F), dominance (Do), relative density (RD), relative frequency (RF), and relative dominance (RDo). The formulas for these calculations are detailed in Equations (1)–(7).
Relative density (RDi)
Density (Di)
Relative frequency (RFi)
Frequency (Fi)
Relative dominance (RDoi)
Dominance (Doi)
Furthermore, the species diversity was determined using the Shannon–Wiener diversity index, while the evenness index was evaluated through Pielou’s evenness index [40,41,42]. Biomass estimation can be performed both directly and indirectly. Direct biomass estimation might be achieved by cutting all the plants within the area of interest and weighing them. However, it is evident that this method is only suitable for estimating biomass in small study areas. In reality, when we need to estimate the biomass of forests, which are large ecosystems, direct measurement by cutting everything is difficult and would result in a massive destruction of resources. Therefore, indirect biomass estimation is considered a more practical method. One popular indirect method for estimating biomass is the allometry method. This approach is more convenient in practice as it avoids the need for destructive sampling and is suitable for larger areas. In this study, the calculations were conducted based on the formulas specified in Equations (8) and (9). The above-ground biomass of tree stems, branches, and leaves was determined using the allometric equation (Equation (10)) tailored for dry dipterocarp forests, as established by Ogawa et al. [43]. In the final stage, the carbon stock for individual species and the overall forest area was assessed by considering the carbon content in woody biomass, which is roughly 47% for dry dipterocarp forests [44]. The analysis of all data in this research was conducted using Microsoft Excel.
where H′ represents the Shannon diversity index, Pi denotes the proportion of the total population made up of species i, and S indicates the total number of species observed.
Ws = 0.0396 D2H0.9326
Wb = 0.003487 D2H1.0270
Wl = (28.0/wtc + 0.025)−1
Wt = Ws + Wb + Wl
Wb = 0.003487 D2H1.0270
Wl = (28.0/wtc + 0.025)−1
Wt = Ws + Wb + Wl
The variable D signifies the diameter taken at breast height, expressed in centimeters (cm), whereas H indicates the overall height of the tree, measured in meters (m). In the equation, wtc is defined as Ws + Wb. Furthermore, Ws, Wb, and Wl represent the dry weights of the tree’s stem, branches, and leaves, respectively, with measurements provided in kilograms (kg).
3. Results
3.1. Plant Species Diversity
In Appendix A, details of the tree species in the dry evergreen forest are presented, providing a comprehensive summary of different families, scientific names, the total number of trees, and the density of each species. The analysis of this data reveals several trends and anomalies, offering significant insights into the forest composition in terms of density and quantity of trees. The Fabaceae family stands out particularly (see Table 1 and Figure 2), especially the species Sindora siamensis Teijsm. & Miq, which has the highest density at 54.5, with 545 trees, indicating a prominent presence in the forest. This suggests that the environment is particularly conducive to this species. Similarly, Erythrophleum teysmannii (Kurz) Craib and Xylia xylocarpa (Roxb.) Taub. also exhibit relatively high densities, indicating their adaptability and proliferation within the forest ecosystem. Additionally, these three prominent species demonstrate significant potential for carbon stock.

Table 1.
The particularly notable Fabaceae species.
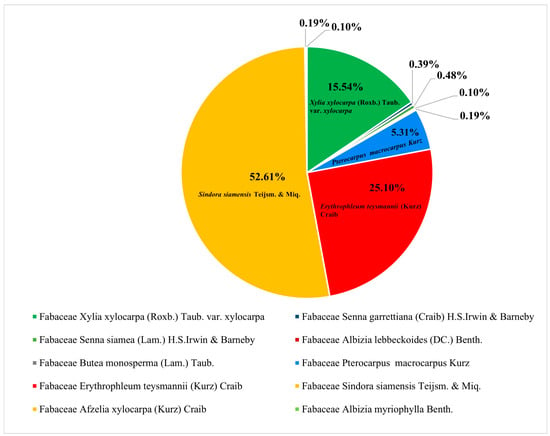
Figure 2.
Three notable species belonging to the family Fabaceae.
Conversely, there are numerous species with notably low densities and numbers, such as Diospyros rhodocalyx Kurz, Albizia lebbeckoides (DC.) Benth., and several others, with only one recorded tree and a density of 0.1. This could hint at various ecological factors, such as limited resources, competition, or recent environmental changes, affecting their population. The distribution of species across different families reveals a diverse ecosystem, albeit with unequal representation. While some families like Moraceae, Lauraceae, and Connaraceae have a limited number of species, others such as Fabaceae and Dipterocarpaceae display greater diversity and abundance. This variation suggests differing levels of ecological niches and adaptive strategies among families in this specific environment. Subjectively discussing these results, one might argue that the predominance of certain species like Sindora siamensis Teijsm. & Miq could shape the forest’s overall structure and function. The ecological role of such dominant species could include providing habitats for various fauna or influencing nutrient cycling. The presence of low-density species, while less impactful on a large scale, could contribute to the genetic diversity and resilience of the ecosystem, preventing monocultures that could be vulnerable to disease or climate change.
Nevertheless, although the data emphasizes a clear pattern of dominance and rarity of species, it does not imply a causal relationship. Further studies may investigate various factors such as soil composition, climate conditions, and human influences that could explain these observations. Additionally, temporal data could provide insights into trends over time, revealing whether these patterns are stable or indicative of ongoing ecological change. In conclusion, this analysis underscores the importance of both dominant and rare species in maintaining the ecological balance of the dry dipterocarp forest. While the subjective interpretation of their roles provides a narrative, an objective approach is essential for understanding the underlying factors driving these patterns. Further research could refine these findings, offering deeper insights into the forest’s dynamics and informing conservation efforts.
The results of the Important Value Index analysis are presented in Appendix B. The data analysis from Appendix B, which displays the Important Value Index (IVI) for various plant species, reveals several interesting patterns and differences. Initially, the species Sindora siamensis Teijsm. & Miq. stands out with an exceptionally high IVI of 82.507, indicating significant ecological importance in the study area. This may be due to its prominence in terms of density, frequency, and basal area. Similarly, Erythrophleum teysmannii (Kurz) Craib also shows a high IVI of 37.228, suggesting a significant role in the ecosystem. The top ten species with the highest IVI are illustrated in Table 2. In contrast, several species have a much lower IVI, indicating lesser ecological impact or presence in the area. For example, species such as Canthium coromandelicum and Pterospermum semisagittatum Ham. Ex Roxb exhibit very low IVIs of 0.116 and 0.137, respectively. These low values suggest that these species are either less abundant or have a lesser impact on the ecosystem in the studied environment.

Table 2.
Illustrates the top ten species with the highest IVI.
The data analysis suggests a significant variation in species importance within the ecosystem, with a few species contributing heavily to the structure and function of the plant community, while many others have minimal impact. This pattern reflects a common ecological principle, where a small number of species dominate the landscape, contributing to the majority of the ecological functions, while a larger number of species are present in smaller quantities. Additionally, the data highlights the importance of considering each component of the IVI—relative density (RD), relative dominance (RDo), and relative frequency (RF)—when analyzing ecological importance. For instance, Afzelia xylocarpa (Kurz) Craib has a high RFI of 30.2610, contributing significantly to its overall IVI, despite lower values in other metrics.
These results could lead to interpretations about biodiversity, conservation priorities, and the ecological roles of dominant versus less dominant species. A deeper analysis could investigate the ecological or environmental factors contributing to the observed dominance patterns, such as soil type, climate conditions, or human impact. Overall, the findings underscore the complexity of ecological systems and the importance of both dominant and minor species in maintaining biodiversity and ecological balance. Future studies could delve deeper into the interactions between these species and their environments to better understand the underlying causes of these patterns.
3.2. Results of Biomass and Carbon Stock
The study of biomass and carbon stock in the dry dipterocarp forest revealed a total biomass of 144.510 tons, distributed among stems (116.743 tons), branches (23.693 tons), and leaves (4.074 tons). The total carbon stock was 67.920 tCO2, with contributions from stems (54.869 tC), branches (11.136 tC), and leaves (1.915 tC) (Appendix C, Figure 3 and Figure 4). The study of biomass and carbon stock for individual tree species in the area revealed that the five species with the highest average biomass per unit area were Sindora siamensis Teijsm. & Miq. (52.240 ton), Erythrophleum teysmannii (Kurz) Craib (32.232 ton), Pterocarpus macrocarpus Kurz (11.022 ton), Xylia xylocarpa (Roxb.) Taub. (10.287 ton), and Shorea obtusa Will. ex Blume (8.989 ton). These species contributed to carbon stock values of 24.553, 15.196, 5.180, 4.835, and 4.225 tC, respectively. The proportions of the average carbon stock for these species were 36.150%, 22.374%, 7.627%, 7.119%, and 6.220%.
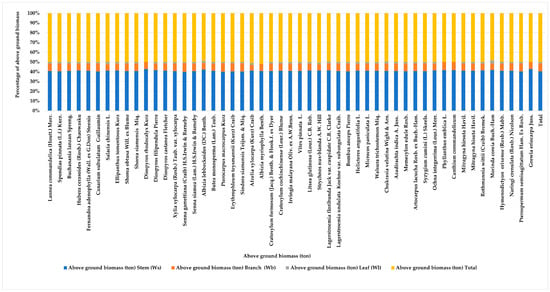
Figure 3.
The proportional contributions of stems, branches, and leaves to the total AGB in tree species found in the dry dipterocarp forest.
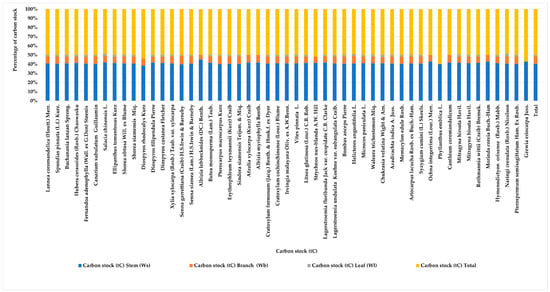
Figure 4.
The allocation of above-ground carbon stock among various components (stems, branches, leaves) across different tree species in the dry dipterocarp forest.
The information demonstrates the significant impact of the predominant tree species on the accumulation of biomass and the storage of carbon in the ecosystem. The prevalence of Sindora siamensis Teijsm. & Miq. and Erythrophleum teysmannii (Kurz) Craib in terms of biomass and carbon storage highlights their ecological significance and indicates their potential as species of interest for conservation efforts and carbon offset initiatives. Nevertheless, the research reveals that biomass and carbon stock are unevenly distributed across different species, with a select few making a significantly larger contribution to the overall amounts. This indicates that initiatives aimed at increasing carbon stock ought to take into account the encouragement of a wider variety of species to bolster the resilience and sustainability of ecosystems. The approach taken in this research offers an extensive evaluation of biomass and carbon storage; however, future investigations should consider examining below-ground biomass and the carbon present in soil to deliver a more complete understanding of carbon processes within these forests.
4. Discussion
This research focuses on a dry dipterocarp forest, an area that was previously classified as a degraded forest. Furthermore, the Royal Forest Department of Thailand defines a “degraded forest” as a forest area that has a limited number of valuable trees remaining, making it difficult for the ecosystem to naturally recover. This is characterized by the presence of saplings taller than 2 m, with a maximum density of 20 trees per 0.16 ha. Alternatively, it may contain trees with a diameter of 50–100 cm measured at a height of 1.30 m from the ground, with a maximum of eight trees per 0.16 ha. Additionally, if there are trees with a diameter exceeding 100 cm, their density should not exceed two trees per 0.16 ha. In summary, a forest area must meet all three criteria, with a total not exceeding 16 trees per 0.16 [45]. This area has been rehabilitated through collaborative efforts involving Mahasarakham University, local community leaders, and residents of Kham Riang Subdistrict. Agreements were reached to safeguard and conserve the forest, which included bans on deforestation, tree uprooting, forest fires, wildlife hunting, and littering. Villagers were permitted to gather non-timber forest resources, including wild fruits, edible forest plants, and red ant eggs. In addition, tree species that are native to the area were introduced to aid in the restoration of the forest. The synergy of these initiatives has resulted in the revitalization and expansion of a more comprehensive and flourishing forest ecosystem. Nevertheless, in this study, the discussion is divided into the following sections.
4.1. Biomass and Carbon Stock in Comparison to Other Forests, Along with the Impact of Fire Effects
The findings of this research align with the studies conducted by Wattayakorn and Diloksumpun [46], who reported that dry dipterocarp forests generally exhibit a total biomass ranging from 67 to 158 t ha−1 and carbon stock between 34 and 80 tC ha−1. Similarly, Kanhom et al. [47] studied the dry dipterocarp forest in Ban Nong Mek, Khok Sung District, Sa Kaeo Province, finding 39 species from 21 families, with a total biomass of 54.54 t ha−1 and carbon stock of approximately 25.64 tC ha−1. Further comparisons with the research of Wattanasuksakul et al. [37] reveal that dry dipterocarp forests affected by forest fires had 42 species from 22 families, while those without forest fires had 46 species from 25 families. The biomass in fire-affected areas was 106.6 t ha−1, with a carbon stock of 52.6 tC ha−1, whereas non-fire-affected areas showed a higher biomass of 128.3 t ha−1 and carbon stock of 63.4 tC ha−1. The study by Wattanasuksakul et al. [37] reveals that trees growing in areas unaffected by forest fires exhibit a higher biomass and carbon stock than those in frequently burned areas. This finding suggests that forest fires negatively impact tree growth, causing trees in fire-disturbed areas to be smaller than those in undisturbed areas. Implementing effective forest fire control measures in these areas could enhance the growth potential of trees, enabling them to accumulate biomass and sequester carbon more efficiently.
Additionally, proper forest fire management can help preserve biodiversity and sustain a healthy, balanced ecosystem over the long term. In contrast, the study by Kumros et al. [48] in the dry dipterocarp forest within the Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani Province, found that plots subjected to annual burning had a higher biomass and carbon stock in trees compared to plots that were unburned or under controlled burning. This was attributed to the greater diameter and height of trees in the annually burned plots, indicating that fire had minimal impact on mature trees. However, for saplings, unburned plots exhibited greater diameter and height, resulting in a higher biomass and carbon stock than burned plots, despite the higher density of saplings in the burned plots. These findings highlight the complexity of factors influencing biomass and carbon stock, such as post-fire tree growth, fire-resistant plant species capable of sprouting and growing after fire, and the physical and biological characteristics of each area following fires of varying frequency and intensity. Studying the multifaceted influences of these factors provides a deeper understanding of biomass and carbon stock in different plant communities, offering valuable insights for forest management and carbon storage strategies. Furthermore, implementing strict measures to regulate land use between conservation forests and forests designated for utilization, particularly through the prevention and protection of forest resources from encroachment and destruction, would encourage plant species to grow into larger trees. This would result in higher biomass and greater carbon stock potential in conservation forests compared to forests designated for utilization [49].
4.2. Role of Individual Species
This study highlights the roles of various plant species within diverse ecosystems, examining the number and density of each species in a given area. For instance, in the Fabaceae family, the species Sindora siamensis Teijsm. & Miq boasts a significant number of 545 trees and a density of 54.5, underscoring its pivotal role in establishing the forest’s structural foundation. Additionally, the species Erythrophleum teysmannii (Kurz) Craib also seems to play a substantial role, with 260 trees and a density of 26. Conversely, some species like Albizia lebbeckoides (DC.) Benth. and Strychnos nux-blanda A.W. Hill are represented by merely a single tree, indicating a lesser role in terms of tree count and density. However, despite their limited numbers, these species might have specific roles within the ecosystem, such as providing shade or serving as a unique food source for certain animals. Species in the Dipterocarpaceae family, such as Shorea obtusa Will. ex Blume and Shorea siamensis Miq., also play crucial roles, with tree counts of 154 and 36, respectively, contributing to forest diversity and providing habitats for other living organisms. The presence of diverse species in the forest, such as those from the Ebenaceae and Malvaceae families, further indicates the ecosystem’s richness. This diversity reflects interactions among various species, promoting biodiversity crucial for the ecosystem’s sustainability. In summary, each species listed in the table has a unique role within the ecosystem, whether in building the forest’s structural foundation, promoting biodiversity, or supporting other species. Collectively, these roles are vital in maintaining the natural balance and fostering sustainable development in forested areas.
4.3. Forest Management
The findings from this research bring forth crucial management implications for the conservation and enhancement of dry dipterocarp forests. To further substantiate these claims, let us delve deeper into each aspect and provide references from relevant research studies.
Collaborative Efforts in Forest Conservation: This study emphasizes the importance of collaboration among academic institutions, local communities, and government bodies. This notion is supported by Sayer et al. [50], who discuss the benefits of multi-stakeholder partnerships in forest landscape restoration. Their research highlights how such collaborations can lead to more effective policy-making that balances conservation with sustainable resource use.
Forest Fire Management: The critical role of forest fire management is underscored in this research. According to a study by Bowman et al. [51], implementing strict fire control measures is essential to prevent the detrimental effects of fires on forest ecosystems. Their findings suggest that controlled burning, when managed correctly, can help maintain the ecological balance and support biodiversity.
Land Use Demarcation and Regulation: Clear demarcation and regulation of land use between conservation and utilization zones are essential, as highlighted in this study. Chazdon et al. [52] argue that zoning can protect vital ecosystems from encroachment, allowing plant species to thrive and reach maturity. Their work demonstrates that such strategies can significantly increase biomass and carbon capture.
Climate Change Mitigation Strategies: Forest conservation plays a pivotal role in broader climate change mitigation strategies. Canadell and Raupach [53] emphasize the importance of forests in carbon storage and the reduction in atmospheric carbon levels. Their research supports the idea that adopting effective management practices can enhance the resilience of forests, contributing significantly to climate change mitigation.
By integrating these management practices, stakeholders can ensure the long-term health and resilience of dry dipterocarp forests. This approach not only contributes to ecological well-being but also supports the livelihoods of local communities. The insights from this research, backed by studies from [50,51,52,53], provide a comprehensive framework for sustainable forest management and conservation efforts.
5. Conclusions
This study on plant species diversity, ABG, and carbon stock in the dry dipterocarp forest within the Nature Study Center, Mahasarakham University, Kham Riang Subdistrict, Kantharawichai District, Mahasarakham Province, covering a total area of 20.80 hectares, revealed a total of 52 tree species across 26 families. The three most abundant families were Fabaceae (10 species), Rubiaceae (6 species), followed by Dipterocarpaceae and Lythraceae, each represented by two species. In terms of the biomass and carbon stock of individual tree species, the study found that the five species with the highest average biomass per unit area were Sindora siamensis Teijsm. & Miq. (52.240 ton), Erythrophleum teysmannii (Kurz) Craib (32.232 ton), Pterocarpus macrocarpus Kurz (11.022 ton), Xylia xylocarpa (Roxb.) Taub. (10.287 ton), and Shorea obtusa Will. ex Blume (8.989 ton). These species contributed to carbon stock values of 24.553, 15.196, 5.180, 4.835, and 4.225 tC, respectively. Their proportions of average biomass and carbon stock relative to the total were 36.150%, 22.374%, 7.627%, 7.119%, and 6.220%, respectively. However, the findings from this study pertain solely to the assessment of carbon storage value in trees, focusing exclusively on above-ground biomass. Therefore, it is essential to conduct further research on the carbon storage value of other biomass components, such as the understory, dead wood, and below-ground carbon, to enhance the accuracy and reliability of the data obtained.
Author Contributions
Conceptualization, C.P., T.L. and Y.U.; methodology, C.P., T.L., Y.U. and P.A.; validation, C.P., T.L., Y.U., P.A., T.R., J.I. and M.S.; formal analysis, C.P., T.L., Y.U., P.A., T.R., J.I. and M.S.; resources, T.L. and Y.U.; data curation, C.P., T.L., Y.U., P.A., T.R., J.I. and M.S.; funding acquisition, C.P. and T.L.; writing—review and editing, C.P., T.L. and Y.U.; project administration, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was financially supported by Mahasarakham University.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. Additionally, author Mehsa Singharath was employed by the company Nampapa Nakhoneluang. The remaining authors affirm that the research was conducted without any commercial or financial relationships that could be perceived as a potential conflict of interest.
Appendix A

Table A1.
Plant species in the dry dipterocarp forest.
Table A1.
Plant species in the dry dipterocarp forest.
| Family Name | Scientific Name | Number of Trees | Density |
|---|---|---|---|
| Anacardiaceae | Lannea coromandelica (Houtt.) Merr. | 45 | 4.5 |
| Spondias pinnata (L.f.) Kurz. | 10 | 1 | |
| Buchanania lanzan Spreng. | 14 | 1.4 | |
| Annonaceae | Hubera cerasoides (Roxb.) Chaowasku | 28 | 2.8 |
| Bignoniaceae | Fernandoa adenophylla (Wall. ex G.Don) Steenis | 2 | 0.2 |
| Burseraceae | Canarium subulatum Guillaumin | 24 | 2.4 |
| Celastraceae | Salacia chinensis L. | 4 | 0.4 |
| Connaraceae | Ellipanthus tomentosus Kurz | 10 | 1 |
| Dipterocarpaceae | Shorea obtusa Will. ex Blume | 154 | 15.4 |
| Shorea siamensis Miq. | 36 | 3.6 | |
| Ebenaceae | Diospyros rhodocalyx Kurz | 1 | 0.1 |
| Diospyros filipendula Pierre | 28 | 2.8 | |
| Diospyros castanea Fletcher | 78 | 7.8 | |
| Fabaceae | Xylia xylocarpa (Roxb.) Taub. var. xylocarpa | 161 | 16.1 |
| Senna garrettiana (Craib) H.S.Irwin & Barneby | 4 | 0.4 | |
| Senna siamea (Lam.) H.S.Irwin & Barneby | 5 | 0.5 | |
| Albizia lebbeckoides (DC.) Benth. | 1 | 0.1 | |
| Butea monosperma (Lam.) Taub. | 2 | 0.2 | |
| Pterocarpus macrocarpus Kurz | 55 | 5.5 | |
| Erythrophleum teysmannii (Kurz) Craib | 260 | 26 | |
| Sindora siamensis Teijsm. & Miq. | 545 | 54.5 | |
| Afzelia xylocarpa (Kurz) Craib | 2 | 0.2 | |
| Albizia myriophylla Benth. | 1 | 0.1 | |
| Hypericaceae | Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer | 18 | 1.8 |
| Cratoxylum cochinchinense (Lour.) Blume | 27 | 2.7 | |
| Irvingiaceae | Irvingia malayana Oliv. ex A.W.Benn. | 23 | 2.3 |
| Lamiaceae | Vitex pinnata L. | 6 | 0.6 |
| Lauraceae | Litsea glutinosa (Lour.) C.B. Rob. | 19 | 1.9 |
| Loganiaceae | Strychnos nux-blanda A.W. Hill | 1 | 0.1 |
| Lythraceae | Lagerstroemia floribunda Jack var. cuspidate C.B. Clarke | 3 | 0.3 |
| Lagerstroemia undulata Koehne var. subangulata Craib. | 5 | 0.5 | |
| Malvaceae | Bombax anceps Pierre | 20 | 2 |
| Helicteres angustifolia L. | 1 | 0.1 | |
| Microcos paniculata L. | 52 | 5.2 | |
| Meliaceae | Walsura trichostemon Miq. | 13 | 1.3 |
| Chukrasia velutina Wight & Arn. | 1 | 0.1 | |
| Azadirachta indica A. Juss. | 4 | 0.4 | |
| Memecylaceae | Memecylon edule Roxb. | 2 | 0.2 |
| Moraceae | Artocarpus lacucha Roxb. ex Buch.-Ham. | 3 | 0.3 |
| Myrtaceae | Syzygium cumini (L.) Skeels. | 15 | 1.5 |
| Ochnaceae | Ochna integerrima (Lour.) Merr. | 2 | 0.2 |
| Phyllanyhceae | Phyllanthus emblica L. | 1 | 0.1 |
| Rubiaceae | Canthium coromandelicum | 1 | 0.1 |
| Mitragyna hirsuta Havil. | 7 | 0.7 | |
| Mitragyna hisuta Havil. | 24 | 2.4 | |
| Rothmannia wittii (Craib) Bremek. | 75 | 7.5 | |
| Morinda coreia Buch.-Ham | 1 | 0.1 | |
| Hymenodictyon orixense (Roxb.) Mabb. | 4 | 0.4 | |
| Rutaceae | Naringi crenulata (Roxb.) Nicolson | 1 | 0.1 |
| Sterculiaceae | Pterospermum semisagittatum Ham. Ex Roxb. | 1 | 0.1 |
| Tiliaceae | Grewia eriocarpa Juss. | 1 | 0.1 |
| Total | 1801 | 180.1 |
Appendix B

Table A2.
The Importance Value Index.
Table A2.
The Importance Value Index.
| Scientific Name | Relative Density | Relative Dominance | Relative Frequency | Importance Value Index |
|---|---|---|---|---|
| Lannea coromandelica (Houtt.) Merr. | 2.498 | 2.278 | 2.498 | 7.276 |
| Spondias pinnata (L.f.) Kurz. | 0.555 | 0.778 | 0.555 | 1.889 |
| Buchanania lanzan Spreng. | 0.777 | 0.529 | 0.777 | 2.084 |
| Hubera cerasoides (Roxb.) Chaowasku | 1.554 | 0.585 | 1.554 | 3.694 |
| Fernandoa adenophylla (Wall. ex G.Don) Steenis | 0.111 | 0.006 | 0.055 | 0.173 |
| Canarium subulatum Guillaumin | 1.332 | 0.091 | 0.111 | 1.534 |
| Salacia chinensis L. | 0.222 | 2.749 | 1.332 | 4.303 |
| Ellipanthus tomentosus Kurz | 0.555 | 0.046 | 0.222 | 0.824 |
| Shorea obtusa Will. ex Blume | 8.550 | 0.195 | 0.555 | 9.301 |
| Shorea siamensis Miq. | 1.998 | 6.050 | 8.550 | 16.600 |
| Diospyros rhodocalyx Kurz | 0.055 | 2.074 | 1.998 | 4.129 |
| Diospyros filipendula Pierre | 1.554 | 0.014 | 0.055 | 1.625 |
| Diospyros castanea Fletcher | 4.330 | 0.292 | 1.554 | 6.177 |
| Xylia xylocarpa (Roxb.) Taub. var. xylocarpa | 8.939 | 2.041 | 4.330 | 15.311 |
| Senna garrettiana (Craib) H.S.Irwin & Barneby | 0.222 | 0.273 | 8.939 | 9.435 |
| Senna siamea (Lam.) H.S.Irwin & Barneby | 0.277 | 0.018 | 0.222 | 0.518 |
| Albizia lebbeckoides (DC.) Benth. | 0.055 | 7.038 | 0.277 | 7.372 |
| Butea monosperma (Lam.) Taub. | 0.111 | 0.070 | 0.055 | 0.236 |
| Pterocarpus macrocarpus Kurz | 3.053 | 6.249 | 0.111 | 9.414 |
| Erythrophleum teysmannii (Kurz) Craib | 14.436 | 19.738 | 3.053 | 37.228 |
| Sindora siamensis Teijsm. & Miq. | 30.261 | 37.809 | 14.436 | 82.507 |
| Afzelia xylocarpa (Kurz) Craib | 0.111 | 0.025 | 30.261 | 30.397 |
| Albizia myriophylla Benth. | 0.055 | 0.723 | 0.111 | 0.889 |
| Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer | 0.999 | 0.010 | 0.055 | 1.065 |
| Cratoxylum cochinchinense (Lour.) Blume | 1.499 | 0.892 | 0.999 | 3.390 |
| Irvingia malayana Oliv. ex A.W.Benn. | 1.277 | 0.784 | 1.499 | 3.560 |
| Vitex pinnata L. | 0.333 | 0.864 | 1.277 | 2.474 |
| Litsea glutinosa (Lour.) C.B. Rob. | 1.055 | 0.218 | 0.333 | 1.606 |
| Strychnos nux-blanda A.W. Hill | 0.055 | 0.345 | 1.055 | 1.456 |
| Lagerstroemia floribunda Jack var. cuspidate C.B. Clarke | 0.166 | 0.026 | 0.055 | 0.248 |
| Lagerstroemia undulata Koehne var. subangulata Craib. | 0.277 | 0.064 | 0.166 | 0.508 |
| Bombax anceps Pierre | 1.110 | 0.246 | 0.277 | 1.635 |
| Helicteres angustifolia L. | 0.055 | 1.450 | 1.110 | 2.616 |
| Microcos paniculata L. | 2.887 | 0.023 | 0.055 | 2.966 |
| Walsura trichostemon Miq. | 0.721 | 1.378 | 2.887 | 4.987 |
| Chukrasia velutina Wight & Arn. | 0.055 | 0.586 | 0.721 | 1.363 |
| Azadirachta indica A. Juss. | 0.222 | 0.039 | 0.055 | 0.316 |
| Memecylon edule Roxb. | 0.111 | 0.187 | 0.222 | 0.520 |
| Artocarpus lacucha Roxb. ex Buch.-Ham. | 0.166 | 0.080 | 0.111 | 0.358 |
| Syzygium cumini (L.) Skeels. | 0.832 | 0.152 | 0.166 | 1.152 |
| Ochna integerrima (Lour.) Merr. | 0.111 | 0.665 | 0.832 | 1.609 |
| Phyllanthus emblica L. | 0.055 | 0.033 | 0.111 | 0.199 |
| Canthium coromandelicum | 0.055 | 0.005 | 0.055 | 0.116 |
| Mitragyna hirsuta Havil. | 0.388 | 0.139 | 0.055 | 0.583 |
| Mitragyna hisuta Havil. | 1.332 | 0.755 | 0.388 | 2.476 |
| Rothmannia wittii (Craib) Bremek. | 4.164 | 0.010 | 1.332 | 5.507 |
| Morinda coreia Buch.-Ham | 0.055 | 1.0872 | 4.164 | 5.307 |
| Hymenodictyon orixense (Roxb.) Mabb. | 0.222 | 0.013 | 0.055 | 0.291 |
| Naringi crenulata (Roxb.) Nicolson | 0.055 | 0.102 | 0.222 | 0.379 |
| Pterospermum semisagittatum Ham. Ex Roxb. | 0.055 | 0.026 | 0.055 | 0.137 |
| Grewia eriocarpa Juss. | 0.055 | 0.126 | 0.055 | 0.237 |
| Total | 100.000 | 100.000 | 100.000 | 300.000 |
Appendix C

Table A3.
ABG and carbon stock.
Table A3.
ABG and carbon stock.
| Scientific Name | Above-Ground Biomass (ton) | Carbon Stock (tCO2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Stem (Ws) | Branch (Wb) | Leaf (Wl) | Total | Stem (Ws) | Branch (Wb) | Leaf (Wl) | Total | |
| Lannea coromandelica (Houtt.) Merr. | 2.342 | 0.437 | 0.092 | 2.871 | 1.101 | 0.205 | 0.043 | 1.349 |
| Spondias pinnata (L.f.) Kurz. | 0.808 | 0.160 | 0.030 | 0.998 | 0.380 | 0.075 | 0.014 | 0.469 |
| Buchanania lanzan Spreng. | 0.520 | 0.097 | 0.020 | 0.637 | 0.245 | 0.045 | 0.010 | 0.300 |
| Hubera cerasoides (Roxb.) Chaowasku | 0.621 | 0.107 | 0.025 | 0.753 | 0.292 | 0.050 | 0.012 | 0.354 |
| Fernandoa adenophylla (Wall. ex G.Don) Steenis | 0.082 | 0.015 | 0.003 | 0.100 | 0.038 | 0.007 | 0.002 | 0.047 |
| Canarium subulatum Guillaumin | 3.174 | 0.654 | 0.113 | 3.941 | 1.492 | 0.307 | 0.053 | 1.852 |
| Salacia chinensis L. | 0.037 | 0.006 | 0.002 | 0.045 | 0.018 | 0.003 | 0.001 | 0.021 |
| Ellipanthus tomentosus Kurz | 0.189 | 0.032 | 0.008 | 0.229 | 0.089 | 0.015 | 0.004 | 0.108 |
| Shorea obtusa Will. ex Blume | 7.326 | 1.380 | 0.283 | 8.989 | 3.443 | 0.649 | 0.133 | 4.225 |
| Shorea siamensis Miq. | 2.146 | 0.407 | 0.083 | 2.636 | 1.009 | 0.191 | 0.039 | 1.239 |
| Diospyros rhodocalyx Kurz | 0.012 | 0.002 | 0.000 | 0.014 | 0.005 | 0.001 | 0.000 | 0.007 |
| Diospyros filipendula Pierre | 0.285 | 0.045 | 0.012 | 0.342 | 0.134 | 0.021 | 0.005 | 0.161 |
| Diospyros castanea Fletcher | 1.825 | 0.321 | 0.073 | 2.219 | 0.858 | 0.151 | 0.034 | 1.043 |
| Xylia xylocarpa (Roxb.) Taub. var. xylocarpa | 8.369 | 1.599 | 0.320 | 10.287 | 3.934 | 0.751 | 0.150 | 4.835 |
| Senna garrettiana (Craib) H.S.Irwin & Barneby | 0.746 | 0.170 | 0.020 | 0.936 | 0.350 | 0.080 | 0.010 | 0.440 |
| Senna siamea (Lam.) H.S.Irwin & Barneby | 0.277 | 0.053 | 0.011 | 0.341 | 0.130 | 0.025 | 0.005 | 0.160 |
| Albizia lebbeckoides (DC.) Benth. | 0.019 | 0.003 | 0.001 | 0.022 | 0.009 | 0.001 | 0.000 | 0.010 |
| Butea monosperma (Lam.) Taub. | 0.072 | 0.013 | 0.003 | 0.088 | 0.034 | 0.006 | 0.001 | 0.041 |
| Pterocarpus macrocarpus Kurz | 8.828 | 1.910 | 0.284 | 11.022 | 4.149 | 0.897 | 0.133 | 5.180 |
| Erythrophleum teysmannii (Kurz) Craib | 26.076 | 5.332 | 0.925 | 32.332 | 12.256 | 2.506 | 0.435 | 15.196 |
| Sindora siamensis Teijsm. & Miq. | 41.976 | 8.926 | 1.337 | 52.240 | 19.729 | 4.195 | 0.629 | 24.553 |
| Afzelia xylocarpa (Kurz) Craib | 0.021 | 0.003 | 0.001 | 0.026 | 0.010 | 0.002 | 0.000 | 0.012 |
| Albizia myriophylla Benth. | 0.011 | 0.002 | 0.000 | 0.014 | 0.005 | 0.001 | 0.000 | 0.006 |
| Cratoxylum formosum (Jacq.) Benth. & Hook.f. ex Dyer | 1.021 | 0.194 | 0.039 | 1.254 | 0.480 | 0.091 | 0.019 | 0.590 |
| Cratoxylum cochinchinense (Lour.) Blume | 0.817 | 0.147 | 0.032 | 0.997 | 0.384 | 0.069 | 0.015 | 0.468 |
| Irvingia malayana Oliv. ex A.W.Benn. | 1.023 | 0.190 | 0.040 | 1.253 | 0.481 | 0.089 | 0.019 | 0.589 |
| Vitex pinnata L. | 0.254 | 0.047 | 0.010 | 0.311 | 0.120 | 0.022 | 0.005 | 0.146 |
| Litsea glutinosa (Lour.) C.B. Rob. | 0.364 | 0.062 | 0.015 | 0.441 | 0.171 | 0.029 | 0.007 | 0.207 |
| Strychnos nux-blanda A.W. Hill | 0.026 | 0.004 | 0.001 | 0.032 | 0.012 | 0.002 | 0.000 | 0.015 |
| Lagerstroemia floribunda Jack var. cuspidate C.B. Clarke | 0.074 | 0.013 | 0.003 | 0.089 | 0.035 | 0.006 | 0.001 | 0.042 |
| Lagerstroemia undulata Koehne var. subangulata Craib. | 0.247 | 0.047 | 0.009 | 0.303 | 0.116 | 0.022 | 0.004 | 0.143 |
| Bombax anceps Pierre | 1.746 | 0.348 | 0.065 | 2.158 | 0.820 | 0.164 | 0.030 | 1.014 |
| Helicteres angustifolia L. | 0.042 | 0.007 | 0.002 | 0.051 | 0.020 | 0.004 | 0.001 | 0.024 |
| Microcos paniculata L. | 1.321 | 0.235 | 0.053 | 1.608 | 0.621 | 0.110 | 0.025 | 0.756 |
| Walsura trichostemon Miq. | 0.520 | 0.094 | 0.021 | 0.635 | 0.244 | 0.044 | 0.010 | 0.298 |
| Chukrasia velutina Wight & Arn. | 0.038 | 0.007 | 0.002 | 0.046 | 0.018 | 0.003 | 0.001 | 0.022 |
| Azadirachta indica A. Juss. | 0.202 | 0.038 | 0.008 | 0.248 | 0.095 | 0.018 | 0.004 | 0.117 |
| Memecylon edule Roxb. | 0.098 | 0.019 | 0.004 | 0.121 | 0.046 | 0.009 | 0.002 | 0.057 |
| Artocarpus lacucha Roxb. ex Buch.-Ham. | 0.180 | 0.034 | 0.007 | 0.221 | 0.085 | 0.016 | 0.003 | 0.104 |
| Syzygium cumini (L.) Skeels. | 0.738 | 0.143 | 0.027 | 0.908 | 0.347 | 0.067 | 0.013 | 0.427 |
| Ochna integerrima (Lour.) Merr. | 0.024 | 0.004 | 0.001 | 0.029 | 0.012 | 0.002 | 0.000 | 0.014 |
| Phyllanthus emblica L. | 0.005 | 0.001 | 0.000 | 0.006 | 0.002 | 0.000 | 0.000 | 0.003 |
| Canthium coromandelicum | 0.010 | 0.002 | 0.000 | 0.012 | 0.005 | 0.001 | 0.000 | 0.006 |
| Mitragyna hirsuta Havil. | 0.150 | 0.026 | 0.006 | 0.182 | 0.071 | 0.012 | 0.003 | 0.085 |
| Mitragyna hisuta Havil. | 0.768 | 0.137 | 0.031 | 0.936 | 0.361 | 0.064 | 0.014 | 0.440 |
| Rothmannia wittii (Craib) Bremek. | 1.007 | 0.166 | 0.041 | 1.214 | 0.473 | 0.078 | 0.019 | 0.571 |
| Morinda coreia Buch.-Ham | 0.013 | 0.002 | 0.001 | 0.015 | 0.006 | 0.001 | 0.000 | 0.007 |
| Hymenodictyon orixense (Roxb.) Mabb. | 0.089 | 0.016 | 0.004 | 0.108 | 0.042 | 0.007 | 0.002 | 0.051 |
| Naringi crenulata (Roxb.) Nicolson | 0.032 | 0.006 | 0.001 | 0.039 | 0.015 | 0.003 | 0.001 | 0.018 |
| Pterospermum semisagittatum Ham. Ex Roxb. | 0.165 | 0.034 | 0.006 | 0.204 | 0.077 | 0.016 | 0.003 | 0.096 |
| Grewia eriocarpa Juss. | 0.006 | 0.001 | 0.000 | 0.007 | 0.003 | 0.000 | 0.000 | 0.004 |
| Total | 116.743 | 23.693 | 4.074 | 144.510 | 54.869 | 11.136 | 1.915 | 67.920 |
References
- Gabric, A.J. The Climate Change Crisis: A Review of Its Causes and Possible Responses. Atmosphere 2023, 14, 1081. [Google Scholar] [CrossRef]
- Prudil, J.; Pospíšilová, L.; Dryšlová, T.; Barančíková, G.; Smutný, V.; Sedlák, L.; Ryant, P.; Hlavinka, P.; Trnka, M.; Halas, J.; et al. Assessment of carbon stock as affected by different management practices using the RothC model. Plant Soil Environ. 2023, 69, 532–544. [Google Scholar] [CrossRef]
- Dagiliūtė, R.; Kazanavičiūtė, V. Impact of Land-Use Changes on Climate Change Mitigation Goals: The Case of Lithuania. Land 2024, 13, 131. [Google Scholar] [CrossRef]
- World Resources Institute: World GHG Emissions Flow Chart. Available online: http://pdf.wri.org/world_greenhouse_gas_emissions_flowchart.pdf (accessed on 2 February 2025).
- Tian, J.; Shan, Y.; Zheng, H.; Lin, X.; Liang, X.; Guan, D. Structural patterns of city-level CO2 emissions in Northwest China. J. Clean. Prod. 2019, 223, 553–593. [Google Scholar] [CrossRef]
- Sugsaisakon, S.; Kittipongvises, S. Citywide Energy-Related CO2 Emissions and Sustainability Assessment of the Development of Low-Carbon Policy in Chiang Mai, Thailand. Sustainability 2021, 13, 6789. [Google Scholar] [CrossRef]
- Policywatch: CO2 Emissions Around the World Challenge the Net Zero Target. Available online: https://policywatch.thaipbs.or.th/article/environment-97 (accessed on 3 February 2025).
- COP 29, Baku: In Solidarity for a Green World. Available online: https://cop29.az/en/home (accessed on 5 February 2025).
- Sdgmove: The Commencement of the ‘COP29’ Conference Focuses on the Critical Issue of Funding and the Urgent Need to Address Climate Change in Developing Countries. Available online: https://www.sdgmove.com/2024/11/12/start-cop29-key-points/ (accessed on 5 February 2025).
- Carlsen, L. The Baku Paradox: An Analysis of Selected Sustainable Development Goals. Sustainability 2025, 17, 2547. [Google Scholar] [CrossRef]
- Angkahad, T.; Laosuwan, T.; Sangpradid, S.; Prasertsri, N.; Uttaruk, Y.; Phoophathong, T.; Nuchthapho, J. An empirical analysis of above-ground biomass and carbon stock using UAV photogrammetry and machine learning techniques. IEEE Access 2024, 12, 86740. [Google Scholar] [CrossRef]
- Psistaki, K.; Tsantopoulos, G.; Paschalidou, A.K. An Overview of the Role of Forests in Climate Change Mitigation. Sustainability 2024, 16, 6089. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- Murphy, D.J. Carbon stock by Tropical Trees and Crops: A Case Study of Oil Palm. Agriculture 2024, 14, 1133. [Google Scholar] [CrossRef]
- Albrecht, A.; Kandji, S.T. Carbon stock in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Aide, T.M.; Almeyda Zambrano, A.M.; Balvanera, P.; Becknell, J.M.; Boukili, V.; Brancalion, P.H.S.; Broadbent, E.N.; Chazdon, R.L.; et al. Biomass Resilience of Neotropical Secondary Forests. Nature 2016, 530, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2: A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Csillik, O.; Asner, G.P. Aboveground Carbon Emissions from Gold Mining in the Peruvian Amazon. Environ. Res. Lett. 2020, 15, 014006. [Google Scholar] [CrossRef]
- Uttaruk, Y.; Khoa, P.V.; Laosuwan, T. A Guideline for Greenhouse Gas Emission Reduction and Carbon stock in Forest Sector Based on Thailand Voluntary Emission Reduction Programme. Sains Malaysiana 2024, 53, 477–486. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Sterling, A.; Trujillo-Briñez, A.; Suárez-Córdoba, Y.D.; Roa-Fuentes, L.L. Forest Attribute Dynamics in Secondary Forests: Insights for Advancing Ecological Restoration and Transformative Territorial Management in the Amazon. Diversity 2025, 17, 39. [Google Scholar] [CrossRef]
- Xiao, J.-L.; Zeng, F.; He, Q.-L.; Yao, Y.-X.; Han, X.; Shi, W.-Y. Responses of Forest Carbon Cycle to Drought and Elevated CO2. Atmosphere 2021, 12, 212. [Google Scholar] [CrossRef]
- Brisebois, A.; Major, J.E. Effects of CO2 Treatments on Functional Carbon Efficiencies and Growth of Forest Tree Seedlings: A Study of Four Early-Successional Deciduous Species. Forests 2024, 15, 193. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Zhu, Q.; Wang, W.; Peng, W.; Cai, Y. Estimating Carbon stock Potential of Forest and Its Influencing Factors at Fine Spatial-Scales: A Case Study of Lushan City in Southern China. Int. J. Environ. Res. Public Health 2022, 19, 9184. [Google Scholar] [CrossRef]
- Jundang, W.; Puangchit, L.; Diloksumpun, S. Carbon storage of dry dipterocarp forest and eucalypt plantation at Mancha Khiri Plantation, Khon Kaen Province. Thai J. For. 2010, 29, 36. [Google Scholar]
- Fujimoto, M.; Puangchit, L.; Sugawara, F.; Sripraram, D.; Jiamjeerakul, W.; Kato, H. Carbon stock Estimation of Urban Trees in Parks and Streets of Bangkok Metropolitan, Thailand. Thai J. For. 2016, 35, 30–41. [Google Scholar]
- Laosuwan, T.; Uttaruk, Y.; Sangpradid, S.; Butthep, C.; Leammanee, S. The Carbon stock Potential of Silky Oak (Grevillea robusta A.Cunn. ex R.Br.), a High-Value Economic Wood in Thailand. Forests 2023, 14, 1824. [Google Scholar] [CrossRef]
- Chavan, S.B.; Dhillon, R.S.; Sirohi, C.; Uthappa, A.R.; Jinger, D.; Jatav, H.S.; Chichaghare, A.R.; Kakade, V.; Paramesh, V.; Kumari, S.; et al. Carbon stock Potential of Commercial Agroforestry Systems in Indo-Gangetic Plains of India: Poplar and Eucalyptus-Based Agroforestry Systems. Forests 2023, 14, 559. [Google Scholar] [CrossRef]
- Newaj, R.; Dhyani, S.K.; Chavan, S.B.; Rizvi, R.H.; Prasad, R.; Ajit; Alam, B.; Handa, A.K. Methodologies for Assessing Biomass, Carbon Stock and Carbon Stock in Agroforestry Systems; National Research Centre for Agroforestry: Jhansi, India, 2014; p. 45. [Google Scholar]
- Boretti, A.; Florentine, S. Atmospheric CO2 Concentration and Other Limiting Factors in the Growth of C3 and C4 Plants. Plants 2019, 8, 92. [Google Scholar] [CrossRef]
- Dixon, R.K.; Solomon, A.M.; Brown, S.; Houghton, R.A.; Trexier, M.C.; Wisniewski, J. Carbon Pools and Flux of Global Forest Ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Van Con, T.; Thang, N.T.; Ha, D.T.T.; Khiem, C.C.; Quy, T.H.; Lam, V.T.; Van Do, T.; Sato, T. Relationship between above ground biomass and measures of structure and species diversity in tropical forests of Vietnam. For. Ecol. Manag. 2013, 310, 213–218. [Google Scholar] [CrossRef]
- Yin, L.; Bao, W.; Bongers, F.; Chen, B.; Chen, G.; Guo, K.; Jiang, M.; Lai, J.; Lin, D.; Liu, C.; et al. Drivers of tree carbon storage in subtropical forests. Sci. Total Environ. 2019, 654, 684–693. [Google Scholar]
- Spittlehouse, D.L.; Stewart, R.B. Adaptation to climate change in forest management. J. Ecosyst. Manag. 2003, 4, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Shao, H.; Sun, H.; Zhang, W.; Yan, Q. Optimizing Carbon stock in Forest Management Plans Using Advanced Algorithms: A Case Study of Greater Khingan Mountains. Forests 2023, 14, 1785. [Google Scholar] [CrossRef]
- Chen, Z.; Long, F.; Qi, H. Research on Forest Carbon Sink Potential and Economic Value in Zhejiang Province. J. Heilongjiang Bayi Agric. Univ. 2022, 34, 126–133. [Google Scholar]
- Wattanasuksakul, S.; Khamyong, S.; Sri-ngernyuang, K.; Anongrak, N. Plant Species Diversity and Carbon Stocks in Dry Dipterocarp Forest with and without Fire at Intakin Silvicultural Research Station, Chiang Mai Province. Thai J. For. 2012, 31, 1–14. [Google Scholar]
- Cooling, E.N.G. Fast Growing Timber Trees of the Lowland Tropics: Pinus merkusii; Commonwealth Forestry Institute, Oxford University: Oxford, UK, 1968. [Google Scholar]
- Royal Forest Department: Participatory Study of Carbon Stock and Biodiversity in Community Forests. Available online: https://www.forest.go.th/community-development/wp-content/uploads/sites/105/2020/03/%E0%B8%84%E0%B8%B9%E0%B9%88%E0%B8%A1%E0%B8%B7%E0%B8%AD%E0%B8%84%E0%B8%B2%E0%B8%A3%E0%B9%8C%E0%B8%9A%E0%B8%AD%E0%B8%99.pdf (accessed on 18 April 2025).
- Whittaker, R.H. Communities and Ecosystems; Macmillan, Collier Macmillan Ltd.: London, UK, 1970. [Google Scholar]
- Shannon, C.E.; Weaver, W.W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Pielou, E.C. Ecological Diversity; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Ogawa, H.; Yoda, K.; Ogino, K.; Kira, T. Comparative ecological studies on three main types of forest vegetation in Thailand II. Plant biomass. Nat. Life Southeast Asia 1965, 4, 49. [Google Scholar]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. (Eds.) IPCC Guidelines for National Greenhouse Gas Inventories; National Greenhouse Gas Inventories Programme; IGES: Kanagawa, Japan, 2006. [Google Scholar]
- Royal Forest Department: Royal Forest Department Regulations. Available online: https://forestinfo.forest.go.th/pfd/Files/FileEnactment/ET6.pdf (accessed on 20 April 2025).
- Wattayakorn, K.; Diloksumpun, S. Chapter 5 carbon cycle. In Thailand Climate Change Information Vol I; Thailand Research Fund’s Research and Development and Co-Ordination Center for Global Warming and Climate Change, The Thailand Research Fund: Bangkok, Thailand, 2011. [Google Scholar]
- Kanhom, B.; Moungsrimuangdee, B.; Waiboonya, P.; Yodsa-nga, P.; Larpkern, P. Plant diversity and biomass carbon stocks of Nong Mek Community Forest, Khok Sung District, Sa Kaeo Province. Thai J. For. 2019, 38, 41–55. [Google Scholar]
- Kumros, B.; Diloksumpun, S.; Wanthongchai, K.; Ninkhet, O. Carbon stocks in deciduous forest with different burning frequencies at Huai Kha Khaeng Wildlife Sanctuary, Uthai Thani province. Thai J. For. 2013, 32, 133–141. [Google Scholar]
- Ounkerd, K.; Sunthornhao, P.; Puangchit, L. Valuation of carbon stock in trees at Khao Wong community forest, Chaiyaphum province. Thai J. For. 2015, 34, 29–38. [Google Scholar]
- Sayer, J.; Sunderland, T.; Ghazoul, J.; Pfund, J.L.; Sheil, D.; Meijaard, E.; Venter, M.; Boedhihartono, A.K.; Day, M.; Garcia, C.; et al. Ten principles for a landscape approach to reconciling agriculture, conservation, and other competing land uses. Proc. Natl. Acad. Sci. USA 2013, 110, 8349–8356. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Balch, J.; Artaxo, P.; Bond, W.J.; Cochrane, M.A.; D’Antonio, C.M.; Defries, R.; Johnston, F.H.; Keeley, J.E.; Krawchuk, M.A.; et al. The Human Dimension of Fire Regimes on Earth. J. Biogeogr. 2011, 38, 2223–2236. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Harvey, C.A.; Komar, O.; Griffith, D.M.; Ferguson, B.G.; Ramos, M.M.; Morales, H.; Nigh, R.; Soto-Pinto, L.; van Breugel, M.; et al. Beyond reserves: A research agenda for conserving biodiversity in human-modified tropical landscapes. Biotropica 2009, 41, 142–153. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing Forests for Climate Change Mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).