Abstract
We describe a new genus and species of the tribe Ichthyomyini (Rodentia: Sigmodontinae) based on three specimens collected in Machupicchu, Cusco, in the southern Peruvian Andes. Our study includes a comprehensive morphological analysis of 201 specimens representing all recognized species, employing multivariate statistics (principal component analysis) of external and cranial measurements, as well as phylogenetic methods. We used maximum parsimony for morphological data and concatenated molecular datasets (Cytochrome b [17 species], IRBP [15 species], and RAG1 [11 species]) analyzed via maximum likelihood and Bayesian inference. The new genus and species exhibit an allopatric distribution relative to other Ichthyomyini and are distinguished by the following combination of traits: dull slate-gray dorsal fur, lighter ventrally without contrast to the dorsum; incomplete philtrum; vestigial pinna concealed within head fur; long, broad hindfeet with a well-developed fringe of stiff hairs and brown soles; laterally compressed tail exceeding head-body length; type 1 carotid circulation pattern; absence of the orbicular apophysis of the malleus; presence of posteroloph and posterolophid in M1, M2, m1, and m2; unilocular hemiglandular stomach (non-reduced).
1. Introduction
Sigmodontine rodents represent the second most diverse group of mammals worldwide and exhibit a wide range of morphological adaptations that facilitate their utilization of different resources and habitats [1,2,3,4,5,6,7,8,9,10]. Within this taxon, the tribe Ichthyomyini comprises highly specialized semi-aquatic and carnivorous species [11,12,13,14,15] with two subtribes; six genera and another one not described [13]; and 23 species, four of them undescribed [13,16]. To date, five ichthyomyine rodent species have been documented in Peru: Neusticomys orcesi by Jenkins and Barnett, 1997, assigned to “Chibchanomys” and recognized as an undescribed genus and species [13]; Ichthyomys stolzmanni by Thomas, 1893; Daptomys peruviensis [17]; an undescribed Neusticomys [18]; and an undescribed Daptomys [16]. These records are based on a total of eleven specimens collected over a span of approximately 120 years [14,15,19,20].
Neusticomys orcesi in Peru (previously recorded as Chibchanomys) was primarily based on an immature specimen from the montane forests of the Cordillera Carpish, Department of Huánuco, in central Peru [14,15].
This specimen, initially identified as Anotomys leander Thomas, 1906 [21,22], was later assigned to Chibchanomys trichotis Voss, 1988 [14], who emphasized differences from the typical form of the genus Chibchanomys found in Colombia, suggesting that LSUMZ 14406 might represent a distinct biological entity. Subsequently, the Peruvian specimen was included in Chibchanomys orcesi by Jenkins and Barnett, 1997 [15,23]. Recently, based on molecular evidence, it has been proposed that C. orcesi belongs to the genus Neusticomys and Peruvian specimens would correspond to a new genus [13].
During a three-month fieldwork conducted in various localities within and surrounding the Santuario Histórico de Machupicchu in 2012, three adult specimens of an ichthyomyine rodent were collected. Previous morphological studies have shown uncertainty in the taxonomic classification of undescribed Peruvian ichthyomyine rodents. In this regard, they were considered synonymous: A. leander [21,22], C. trichotis [14], and C. orcesi [15,23], but recently, based on molecular evidence, they were identified as “a possible new genus and species from Peru” [13] (p. 2/71). In the present study, we conduct an in-depth review of both morphological and molecular data with the aim of defining and characterizing this new species and genus.
Consequently, the present study includes a comprehensive examination to provide further evidence for the existence of this new genus and species, utilizing detailed and extensive morphological comparisons, multivariate statistics, and morphological and molecular phylogenetic analyses.
2. Materials and Methods
2.1. Study Area and Specimen Collection
Three adult specimens (MUSA 13864, 18964, 18965) were collected in October 2012 near the Intipata Archeological Center (13°11′09′′ S and 72°32′34.5′′ W; 2825 m) and in April 2016 at two locations near the Wiñaywayna archeological complex (13°11′36.33′′ S and 72°32′10.41′′ W; 2628 m; and 13°11′34.31′′ S and 72°32′03.79′′ W; 2499 m) within the Santuario Histórico de Machupicchu, Province of Urubamba, Department of Cusco, Peru. Additionally, we obtained photographs of a specimen collected by A. L. Gardner in August 1968 (LSUMZ 14406) in the Cordillera Carpish (9°42.3′ S and 76°04.44′ W;) near the Área de Conservación Regional Bosque Montano de Carpish, Department of Huánuco, Peru (Figure 1).
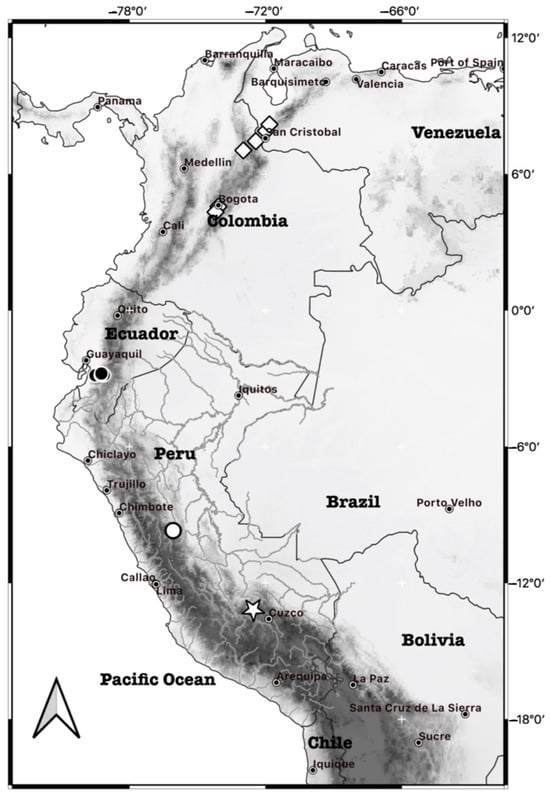
Figure 1.
Map of distribution records of new species and genus and species of Ichthyomyini from Machupicchu, type and paratypes from Wiñaywayna (star) and Carpish specimen (white circle); Chibchanomys trichotis (diamonds); and Neusticomys orcesi (black circles) also shown. Reference site at the latitude–longitude intersection (white cross).
The collection of animals adhered to the guidelines of the American Society of Mammalogists [24]. This study encompassed all recognized genera within the Ichthyomyine tribe [14,15].
Specimens—the new Peruvian species was compared with three specimens of C. trichotis, four of N. orcesi (including photos of two specimens from the British Museum Natural History (BMHN), namely the holotype (BM 1982.816) and paratype (BM 1982.815), detailed images of LSUMZ 14406 kindly provided by J. Salazar-Bravo, and C. trichotis specimen from Museo de Historia Natural de la Universidad Industrial de Santander, Colombia (MHN-UIS s/n), provided by Javier Colmenares. Additionally, holotypes of Neusticomys vossi (QCAZ 7830) and Ichthyomys pinei (MZUA-234) were included. In total, the dataset included 21 recognized species, comprising the newly described species and at least 3 undescribed forms with unclear taxonomic definitions [13,16], resulting in 201 specimens (Table S1), 9 of which were documented through high-resolution photographs (Table S1). These specimens are currently housed at the following institutions: Museo de Historia Natural de la Universidad Nacional de San Agustín (MUSA); Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos (MUSM); Museo de Zoología de la Pontificia Universidad Católica de Ecuador (QCAZ); Museo de la Escuela Politécnica Nacional de Ecuador (MEPN); Museo Ecuatoriano de Ciencias Naturales (MECN); Museo de Zoología de la Universidad de Azuay (MZUA); Museo de Historia Natural de la Universidad Nacional de Colombia (ICN); Museo de Historia Natural de la Universidad Industrial de Santander de Colombia (MHN-UIS); Museo de Historia Natural de la Universidad Industrial de Santander (MHN-UIS); Louisiana Natural History Museum (LSUMZ); American Museum of Natural History (AMNH); and National Museum of Natural History of the Smithsonian Institution (USNM). Additionally, only measurements from specimens [14] of C. trichotis housed at the Field Museum of Natural History, Chicago (FMNH), University of Michigan Museum of Zoology, Ann Arbor (UMMZ), and A. leander deposited at the Natural Historie Riksmuseet, Stockholm (NHRS).
2.2. Morphological Characters
To differentiate the genera of Ichthyomyini, Anthony [11] employed 5 characters, while Voss [14], in an extensive morphological review, utilized 18 characters to phylogenetically assess the species within this tribe. More recently, Salazar-Bravo et al. [13] expanded this framework by presenting 56 characters. In this study, we selected 39 characters (Table S2) to construct a matrix aimed at differentiating and phylogenetically evaluating the species of the tribe Ichthyomyini. Among these, four characters are newly introduced, while the remainder are primarily derived from the aforementioned authors. However, some characters have been structurally modified, and their states revised based on the examination of new material. Detailed descriptions of these characters are provided in the Supplementary Material, Table S2. A multivariate analysis was conducted on morphological measurements, encompassing the skull, mandible, and external body size (expressed in mm, as described later). A total of 39 diagnostic characters and their states (Table S2) were analyzed across all samples, which included 169 museum specimens representing 19 species plus 3 subspecies (Table S2). The descriptions of these characters were sourced from the relevant literature [11,14,17,23,25,26,27,28,29,30,31,32,33,34,35,36]. The age was determined using the dental wear classes TWC method (14), considering the state of basioccipital suture closure and hair characteristics. The nomenclature for external, cranial, dental, and stomach traits follow the guidelines provided by previous authors [14,23,37,38]. The fur color of museum specimens and digital images was described using Ridgway color standards [39] and color tables [40]. The following external measurements were recorded from specimen labels: total length (TL), length of the tail (LT), hindfoot length (HFL), and ear length (EL). Head and body length (HBL) was calculated by subtracting LT from TL. Cranial and dental measurements (following Voss, 1988) were taken using a digital caliper (0.01 mm) and included condylo-incisive length (CIL), length of diastema (LD), length of the maxillary molars (LM), length of the incisive foramina (LIF), breadth of the incisor tips (BIT), breadth of the incisive foramina (BIF), breadth of the palatal bridge (BPB), length of nasals (LN), breadth of nasals (BN), least interorbital breadth (LIB), zygomatic breadth (ZB), breadth of braincase (BB), breadth of the zygomatic plate (BZP), breadth of M1 (BM1), height of incisor (HI), depth of incisor (DI), and breadth of the occipital condyles (BOC). Additionally, measurements of mandible length (MAL), length of the mandibular molars (LMM), and bullae length (LBu) were included, which were previously not measured in similar studies. Throughout the text, measurements are presented as a range (minimum value–maximum value) or mean ± standard deviation (X ± SD). Ratios were calculated to quantify observed differences in nasal shape, condylar width, tympanic bullae, and relative teeth size, including nasal width divided by nasal length (NB/LN), length of the occipital condyle divided by braincase breadth (BOC/BB), length of bullae divided by condylo-incisive length (LBu/CIL), and molar length divided by condylo-incisive length (LM/CIL).
Of the 201 specimens reviewed, we obtained complete measurements of 144 especimens, which were measured directly by us, and were combined with those of 41 specimens taken from the literature. Only specimens with fully erupted dentition (TWC = 3–5) were included in the morphometric analyses. The specimens used in this study represented 21 nominal species and six subspecies: A. leander, 7; C. trichotis, 3; I. hydrobates hydrobates, 14; I. hydrobates soderstromi, 20; I. stolzmanni, 10; I. orientalis, 3; I. tweedii, 18; I. pittieri, 3; I. pinei, 2; N. monticolus, 33; N. vossi, 20; N. orcesi, 6 (two photographs); D. peruviensis, 10 (two photographs); D. venezuelae, 3; D. ferreirai, 2 (photographs); D. oyapocki, 1; D. mussoi, 1; undescribed Daptomys from Peru, 2; undescribed Daptomys from Bolivia, 1 (photograph). Rheomys mexicanus, 24; R. raptor raptor, 2; R. raptor hartmanni, 4; R. thomasi thomasi, 2; R. thomasi chiapensis, 1; R. underwoodi, 3; and the new Peruvian undescribed species, 4 (one photograph).
2.3. Morphometric Analyses
Two principal component analyses (PCA) were conducted to analyze the phenotypic variation in the new Peruvian form compared to other forms of the Ichthyomyini tribe. The PCAs were performed using R and RStudio 2023.12.1 software [41]. The first PCA involved log-transformed external and craniodental measurements of 22 nominal forms of ichthyomyine rodents, including 17 log-transformed linear craniodental measurements from 38 specimens with complete data. The second PCA included all available cranial measurements from 38 specimens: A. leander (AMNH 66201, 66202, 244605, 244606, 244607, NHRS 6100, BMNH 5.11.7.1); N. monticolus (ICN 12118, 16526, AMNH 62920, 63376, 64625–64629, 64632, 64633, 64639;); N. vossi (QCAZ 7830, 8956, AMNH 244609, 244608, MECN 4332); C. trichotis (FMNH 71226, ICN 10152, UMMZ 156532, 156376, MBUCV I-2795, NMNH 442606); N. orcesi (MEPN 12230, BMNH 82.816, 82.815, MZUA-MA 37); and the undescribed species, (LSUMZ 14406, MUSA 13864, 18964, 18965).
2.4. Phylogenetic Analysis
A morphological phylogenetic analysis was conducted using maximum parsimony based on 38 discrete characters (Table S1), with 20 recognized species of Ichthyomyini and three putative outgroup taxa (Sigmodon, Nectomys and Holochilus). Character definitions and criteria for state transitions were described in Supplementary Material, Table S2. The character scoring was based on the study of 169 museum specimens, photographs (indicated above), and literature description [11,14,23,27,37,42,43,44,45]. Moreover, the parsimony analysis was performed using Tree Analysis Using New Technology (TNT) software version 1.5 [46], employing a heuristic search with Tree Bisection Reconnection (TBR) branch-swapping and 1 million replicates. The strict consensus topology of the MP trees, tree length, length of branch, the consistency (CI) and retention (RI) indices were estimated. Additionally, Bootstrap resampling with ten thousand replicates and Bremer values were calculated using Aquickie.run implemented in TNT software, with 1, 3, 5, and 7 additional steps and suboptimal trees [47]. Genetic sequences from three genes of the Ichthyomyini species were obtained from GenBank, 19 sequences of mitochondrial Cytochrome b, 16 sequences of nuclear IRBP, and 11 sequences of nuclear RAG1. GenBank accession numbers (Table S3). We combined the mitochondrial and nuclear aligned sequences into a concatenated supermatrix. In the extended supermatrix, we included all ichthyomyine sequences, plus two Sigmodon sequences as outgroup (20 terminals, 3543 sites, with 21.9% of missing character states). This matrix was used to evaluate general patterns of generic and specific relationships among taxa with Bayesian inference and maximum likelihood analysis. The Bayesian analysis was conducted with MrBayes 3.1.2 [48] consisting of two independent runs, each with three heated and one cold Markov chains. They were allowed to proceed for 5 million generation and sampled every 1000 generations; these analyses were repeated two times. The model of molecular evolution used was GTR+G selected using the Bayesian information criterion (BIC) and the Akaike information criterion (corrected (AICc), both implemented in MEGA 11 [49]. The first 25% of the sampled trees were discarded as “burnin”, and the remaining trees were used to generate a 50% majority rule consensus tree and obtain posterior probability (PP) estimates for each clade, PP ≥ 0.95 were considered well supported. The maximum likelihood approach was conducted using the model of molecular evolution TIM2+G4 selected using the Bayesian information criterion (BIC), using Modeltest-NG v0.1.7. [50] implemented in RaxML GUI 2.0 [51,52,53] and bootstrap resampling [54] with 1000 bootstrap replicates was applied to assess support for individual nodes. Genetic distances (Table S4) and standard error between genera and within genus, were estimated using the Kimura two parameters evolution model [55], implemented in MEGA 11 [49], the analytical procedure encompassed 19 coding nucleotide sequences using 1st, 2nd, 3rd, and non-coding positions. The pairwise deletion option was applied to all ambiguous positions for each sequence pair resulting in a final dataset comprising 1134 positions.
3. Results
3.1. Multivariate Statistics
For the PCA based on the means of all nominal forms, the first two components accounted for 83.29% of the variance. The first component (63.35% of variance) primarily reflected size, as indicated by the heavy positive loadings of W, BZP, and BIT. The second component (19.94% of variance) was influenced by EL, LT, and LMM. This PCA revealed distinct separation of the genera of Ichthyomyini in morphospace, including (a) the split of Rheomys in at least two groups and separating Neusticomys from Daptomys, each; (b) the close phenotypic similarity of N. orcesi to N. monticolus and N. vossi; and (c) the close phenotypic convergence between the new species with C. trichotis and A. leander (Figure 2A).
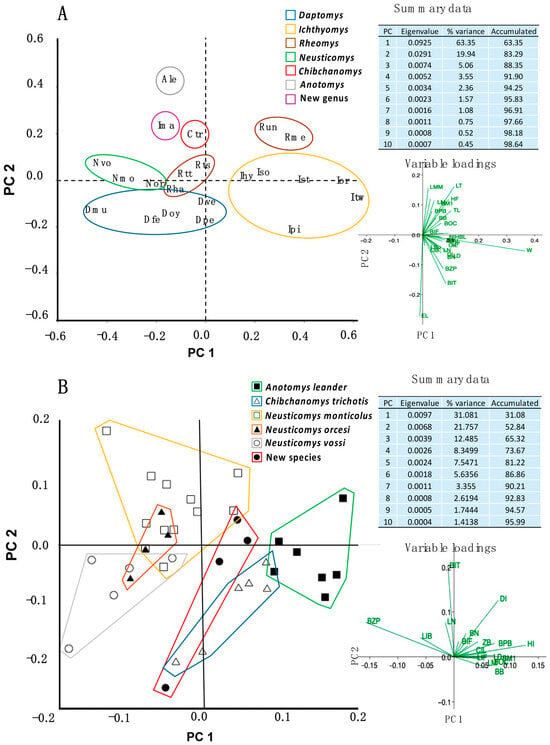
Figure 2.
Principal component analysis of Ichthyomyini rodents. (A) Above, scatter plot and scree plot developed with log-transformed arithmetic mean of external, weight, and cranial measurements of 22 taxa (20 species and 4 subspecies). Anotomys leander, Ale; C. trichotis, Ctr; D. ferreirai, Dfe; D. mussoi, Dmu; Daptomys oyapocki, Doy; D. peruviensis, Dpe; D. venezuelae, Dve; I. hydrobates hydrobates, Ihy; I. hydrobates soderstromi, Iso; I. orientalis, Ior; Ichthyomys pittieri, I. stolzmanni, Ist; Ipi; I. tweedii, Itw; New species, Ima (from Machupicchu and Carpish); Neusticomys monticolus, N. orcesi, Nor; Nmo; N. vossi, Nvo; R. raptor hartmanni, Rha; R. thomasi thomasi, Rtt; R. thomasi stirtoni, Rti; R. mexicanus, Rme; R. underwoodi, Run; and (B) Below, scree plot and scatter plot obtained from 38 log-transformed cranial measurements of six species with convergent morphology; in parenthesis the number of specimens: Anotomys leander (7), filled squares (green); Chibchanomys trichotis (6), open triangles (blue); Neusticomys orcesi (4), filled triangles (orange); New Peruvian undescribed species from Machupicchu (4), filled circles (red); Neusticomys vossi (5), open circles (gray); and Neusticomys monticolus (12), open squares (yellow). In both, in parenthesis of scree plot, Eigenvalue, and variance.
In the PCA based on cranial measurements of similar species (A. leander, C. trichotis, Neusticomys spp., and the new species), the first three components accounted for 65.32% of the variance. The first component (31.08% of variance) was influenced by BZP, DI, and HI; the second component (27.76% of variance) was influenced by BIT, BZP, DI, and Hi, and the third component (12.49% of variance) was influenced by BPB, LD, BZP, and LIF. The PCA revealed (a) remarkable separation of Neusticomys with respect to other genera; (b) close phenotypic similarity between Chibchanomys, Anotomys, and new species, divergent specimens below the group are immatures; (c); and (d) N. orcesi is depicted overlapping with other Neusticomys (Figure 2B).
3.2. Morphology
The evaluation and comparison of the diagnostic characteristics of the genus Chibchanomys (sensu Voss [14]) and the new Peruvian undescribed species from Machupicchu reveal significant morphological similarities and convergences. These shared features include reduced pinnae buried in the head fur (Figure 3a,h), longer tail than the combined HBL, five plantar pads on the hand, long and wide hindfoot, and with fringes of hair well-developed (Figure 3a,i,l), long nasal bones, supraorbital foramina open laterally, carotid circulation pattern 1, orbicular process of the malleus absent, stomach with glandular epithelium between the esophagus and pyloric sphincter. However, the new Peruvian undescribed species from Machupicchu can be distinguished from Chibchanomys by several morphological differences. The new species exhibits a more grayish dorsal coloration and darker ventral coloration compared to Chibchanomys (Figure 3b,c), and its hind feet are whitish with larger plantar pads and brown soles. The muzzle and chin of the new species are whitish (Figure 3e,f), and it possesses a partial or incomplete philtrum that is closed above and narrow distal (Figure 3e). The nasals of new species are longer (Pt = 0.001; Figure 4A,B) and extend to the interorbital region. The interorbital breadth is slightly narrower in new species (Pt = 0.005). The new species also exhibits a less inflated braincase (Pt = 0.005; Figure 4G,H,I) and less inflated bullae (Pt = 0.008) compared to Chibchanomys. The occipital condyles of new species are slightly broader (Pt = 0.005), and its breadth of the palatal bridge is narrower. Other distinguishing features include a more defined anteromedial flexid in m1, a non-reduced molar toothrow, the presence of an enterostyle in M1, the near absence of M3, a small entolophulid in m1, and a broad forward orientation of the incisive foramen (in contrast to the posterior broadness observed in Chibchanomys). The palatal process of the premaxillary bone in the new species is large and reaches the posterior edge of the incisive foramen, whereas in Chibchanomys, it occupies a third or half of the incisive foramen. When considering comparative indices, nasal bone in the new species is longer and less wide (NB/LN = 0.31–0.32) than in Chibchanomys (NB/LN = 0.36). The relative condylar breadth is narrower in Chibchanomys (BOC/BB = 0.55 in both species) compared to the new species (BOC/BB = 0.62–0.64). The bullae are inflated in Chibchanomys (LBu/CIL = 0.23) but not inflated in the new species (LBu/CIL = 0.17).
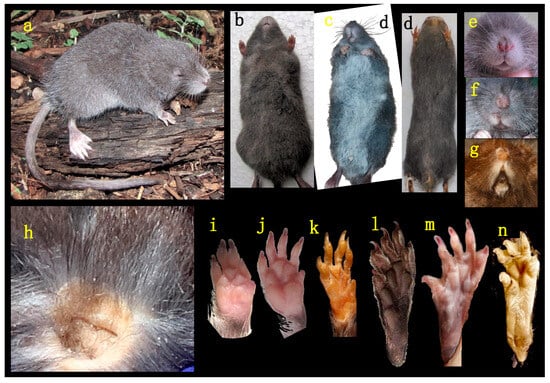
Figure 3.
Images of the external traits, (a) new species of Ichthyomyini from Machupicchu. (MUSA 13864). Ventral view of (b) new species, (c) Chibchanomys trichotis (Colombia MHN-UIS s/n) and (d) Neusticomys orcesi (BM 82.816, type). Frontal view of muzzle: (e) new species, (f) C. trichotis and (g) N. orcesi (MZUA-MA 37). (h) Pinna of new species. Palmar forefoot and plantar hindfoot: (i,l) new species, (j,m) C. trichotis, and (k,n) N. orcesi.
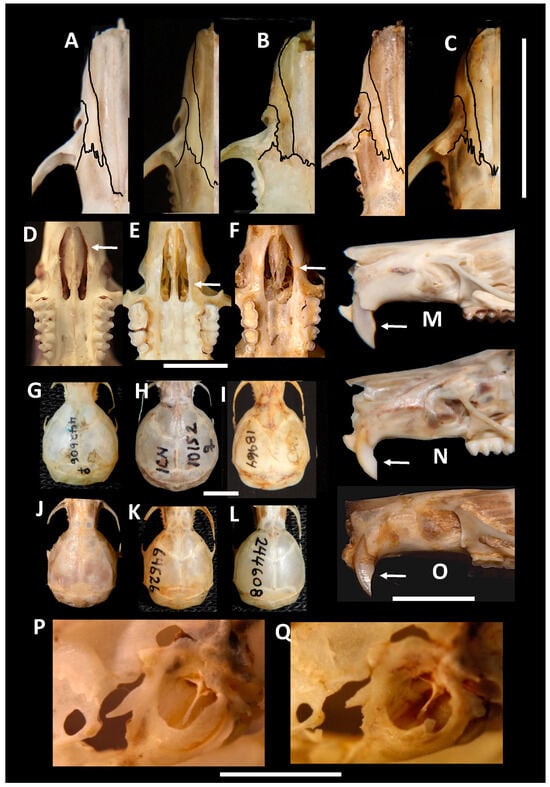
Figure 4.
Images of cranial structures of selected ichthyomyines. Partial view of upper part of rostrum: (A) new species (MUSA 13864, 18965), (B) C. trichotis (ICN 10152, NMNH 442606), and (C) N. orcesi (MZUA-MA 37) and N. monticolus (AMNH 64626); scale bar 10 mm. Palatal view, with detail on the form of the incisive foramen form, (D) new species (MUSA 18964), (E) C. trichotis (NMNH 442606), and (F) N. orcesi (MZUA-MA 37); scale bar 5 mm. Braincase form: (G,H) globular in C. trichotis (ICN 10152, NMNH 442606); (I) inflated in the new species (MUSA 18964); not inflated in (J) N. orcesi (MZUA-MA 37), (K) N. monticolus (ANMH 64626) and (L) N. vossi (AMNH 244608); scale bar 5 mm. Lateral view of rostrum: (M) opisthodont incisors in the new species (MUSA 13864) and (N) C. trichotis (ICN 10152), and near orthodont incisors in (O) N. orcesi (MZUA-MA 37); scale bar 5 mm. Lateral view of the bulla of the new species (P) MUSA 18964, (Q) MUSA 18965; scale bar 4 mm.
3.3. Morphologic Phylogeny
A maximum parsimony analysis resulted in 18 most parsimonious trees, each of them with 108 steps. The consensus tree (Figure 5) had a CI = 0.537; a homoplasy index = 0.427; RI = 0.795; and Bremer index = 7.0. The Ichthyomyini tribe was consistently resolved as a monophyletic group, in relation to the three outgroup genera. Within the tribe, Ichthyomys was identified as a sister to the remaining genera (Rheomys, Chibchanomys and Neusticomys), and each of these was also recovered as monophyletic. Two subclades were supported within Neusticomys where N. orcesi was associated with N. vossi and N. monticolus. The undescribed Peruvian form was consistently associated with C. trichotis in all the most parsimonious trees and the consensus tree, and both species were associated with A. leander (Figure 5).
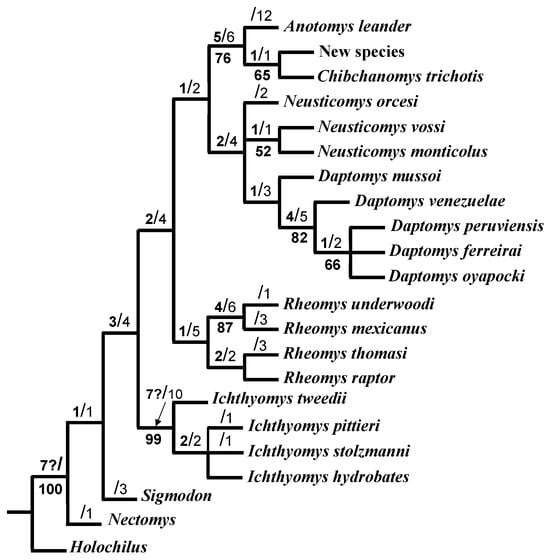
Figure 5.
Consensus of 18 most parsimonious trees resulting from maximum parsimony analysis of 38 morphological characters of Ichthyomyini rodents (tree lengths = 108; CI = 0.537; RI = 0.795); support values are indicated on each branch, standard bootstrap of 10000 replicates (below), Bremer support (above left), and branch length (above right). It is not present in all clades (?).
3.4. Molecular Phylogenies
The cladograms obtained by Bayesian inference and maximum likelihood methods are broadly coincident in their topology, generic and specific relationships, and high support (Figure 6) of recognized genera and species, with exception of Chibchanomys with a moderate support (Bayesian: 0.69, bootstrap: 70). Both methods consistently defined three clades, one clade consisted of A. leander, which was always basal to the entire radiation; the second clade included Ichthyomys and Rheomys; while the third clade comprised Chibchanomys, Daptomys, Neusticomys. Specimens of Machupicchu and Abiseo were recovered as monophyletic (Bayesian: 1.00, bootstrap: 100) and sister to Daptomys, and would be made up of two sister species (Bayesian: 1.00, bootstrap: 100), and with respect to Daptomys supported by high support (Bayesian: 0.97), and high support in relation to Neusticomys and Chibchanomys (Bayesian: 1.00, bootstrap: 92). Intrageneric genetic distances (K2P) based on the Cytochrome b gene (Table 1) reveal divergence values ≤ 3.73% in Daptomys, 6.99% in Neusticomys, 8.87% in Ichthyomys, and 6.91% in the new species (Supplementary Material, Table S4). In contrast, Rheomys exhibits a notably higher divergence (12.76%), suggesting that it may represent more than one genus. Intergeneric distances range from 13.82% to 23.54%, with the lowest values observed between Daptomys and Chibchanomys (13.82%) and between Ichthyomys and Rheomys (14.08%). The new species shows genetic distances of 14.99% to 22.40% relative to all other genera (Table 1). For the IRBP gene, intrageneric divergence ranges from 0.31% to 1.21% across all genera, while the new species differs from other genera by 0.81% to 2.61% (Supplementary Material, Table S4).
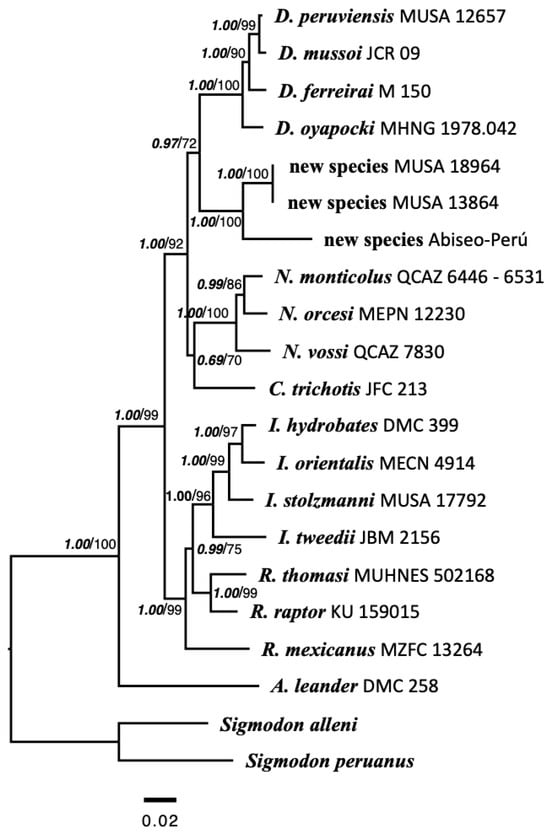
Figure 6.
Molecular phylogeny of Ichthyomyini rodents based on concatenated sequences of Cytochrome b, IRBP, and RAG1 genes. Nodal supports are indicated as follows: posterior probability values for Bayesian inference (left) and bootstrap for maximum likelihood (right).

Table 1.
Average evolutionary divergence within and between genera of the Ichthyomyini tribe, based on the Kimura 2-parameter model for Cytochrome b sequences. Standard error estimates (between genera) are shown above the diagonal (in blue).
3.5. Genus and Species Description
Considering the morphological and molecular comparisons that distinguish the undescribed Peruvian species from other genera and species within the Ichthyomyini tribe, the phylogenetic analyses support its classification as a new genus, indicating a distinct evolutionary lineage. The presence of a wide distributional gap and significant biogeographic barriers suggest the existence of reproductively isolated populations. Therefore, a new genus and species of ichthyomyine rodent is recognized and described as follows:
Family: Cricetidae Fischer, 1817
Subfamily: Sigmodontinae Wagner, 1843
Tribe: Ichthyomyini Vorontsov, 1959
Incanomys gen. nov. Zeballos
Incan water mouse, ratón acuático incaico
- Anotomys Gardner, 1971:1088. Not Anotomys leander Thomas, 1906.
- Chibchanomys Voss, 1988:321. Not Ichthyomys trichotis Thomas, 1897. Name combination
- Chibchanomys Jenkins & Barnett, 1997:126. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Name combination
- “Chibchanomys” sensu Salazar-Bravo et al. 2023. Not Chibchanomys Voss, 1988.
Type species. Incanomys mayopuma, new species.
Type series. PERU: Wiñaywayna, Aguas Calientes district, Urubamba province, Cusco department (MUSA 13864, 18964 and 18965).
Included species. The nominal form from Machupicchu and Carpish, and another undescribed from San Martín department, Peru.
Diagnosis. A medium-size Ichthyomyini (head and body length ranging from 102 to 118 mm, length of hindfoot with claw 28.9–31.1 mm; alveolar length of maxillary toothrow 4.2–4.4 mm, and weight 37–47 g). The new genus is defined by the following combination of characters: dull pelage, dorsal coloration slate gray with large white guard hairs; philtrum incomplete; vestigial ears, with a small conch buried in the fur; hindfoot broad with well-developed fringe of stiff hairs along the plantar and fingers margins; soles brownish; tail longer than the head and body length, slightly compressed; long nasal bones; orbicular apophysis of malleus absent; opisthodont incisors; enterostyle and mesoloph in M1; small anterolophs in M2 and M3, M3 sometimes missing; Posterolophids in m1 and m2. Karyotype: 2n: 92, FN: 98 or 98 or 100 [21].
Description. As the new genus description derives from the described species, its diagnostic features align with the original species account (provided below). A second species, recently discovered, is currently represented solely by cytochrome b sequence data.
Comparisons. The genus Incanomys can be easily distinguished from other non-aquatic sigmodontine species by its slate gray hair coat with fine and large, white guard hairs. The underparts are slightly pale-slate in color. Incanomys has large and broad hindfeet, with a hairy fringe along the metatarsal margins. The hypothenar pad is absent, and the pinnae are hairy and very reduced, forming a vestigial fold buried in the fur of the head. The philtrum is incomplete. The tail is unicolored and distally compressed.
Anotomys differs from Incanomys in having dorsal and ventral slate hair coat, with whitish underparts that do not exhibit countershading with the back. Anotomys also has superciliary vibrissae, while Incanomys does not. Anotomys has a pure white spot over the ears, four pads with the hypothenar and third interdigital fused, and a metatarsal configuration of IV > III > V > II > I. Its tail lacks a ventral fringe, and it has an orbicular apophysis of the malleus absent. The carotid circulation pattern in Anotomys is type 2. Anotomys also has anteromedial flexus and flexid on M1; tricuspidate M3, and a posteroloph present only in M1. The omohyoid muscle is absent in Anotomys, and its bacular cartilage is unidigitated. Additional comparative characteristics are presented in Table 2 and Table 3.

Table 2.
Morphological comparisons among genera of the tribe Ichthyomyini.

Table 3.
External and cranial measurements. Individual measurements in millimeters and weight in grams. Showing: range of measurements, number of specimens (n), mean (x), and standard error (SE) of Neusticomys orcesi, MEPN 12230, MZUA-MA 37 and others two [23]; Chibchanomys trichotis, USNM 442606, ICN 10152, MHN-UIS s/n and other two [15,33]; and Incanomys mayopuma, MUSA 13864, 18964, 18965 and LSU 14406 [14]. Significant difference based on t-student test of I. mayopuma, compared with C. trichotis (*) and N. orcesi (^).
Chibchanomys differs from Incanomys by having a more brownish dorsal color and paler ventral color. Chibchanomys also lacks a philtrum and has short nasals that do not reach the middle part of the interorbital. Its interorbital breadth is slightly broader, and it has a more inflated braincase and bullae. Chibchanomys also has broader occipital condyles and larger breadth of the palatal bridge. The species has a lightly defined anteromedial flexid in m1, lacks an enterostyle in M1, and has a broad posterior edge of the incisive foramen. The palatal process of the premaxillary bone occupies a third or a half of the incisive foramen in Chibchanomys.
Daptomys species are distinguished from Incanomys by their mouse-like appearance, characterized by a cylindrical tail and well-developed pinnae that protrude from the skin. The pinnae are normal and clearly visible above the unruffled fur of the head. Daptomys has a philtrum, and its metatarsal configuration is III ≥ IV > II >> V > I. The species has a weakly developed metatarsal fringe of hairs and narrow hindfeet. It also possesses a delicate ventral fringe on the tail. The skull of Daptomys is non-inflated, and it has an orbicular apophysis of the malleus. The molars of Daptomys are comparatively reduced in size, measuring between 3.3 and 4.4 mm, with the ratio of length to crown height (LM/CIL) varying between 0.13 and 0.17.
Neusticomys species are distinguished from Incanomys by the most mouse-like archetype, as well as their comparatively small adult size (less than 30 g). They have a coloration of slate gray and brownish gray, with white lips in the specimens of N. orcesi. The tail is cylindrical, and the pinnae are well-developed and protrude from the skin, like in Daptomys. Neusticomys also has a philtrum and a metatarsal configuration of III ≥ IV > II >> V > I. However, the metatarsal fringe of hairs is weakly developed, and the hindfeet are narrow. Unlike Daptomys, Neusticomys lacks a ventral fringe on the tail. The skull is rounded, and it has an orbicular apophysis of the malleus. The molars of Neusticomys are comparatively normal in size, with the LM/CIL ratio varying between 0.14 and 0.19. It is worth noting that southern populations of Neusticomys are geographically separated by a considerable distance of 835 km and significant biogeographical barriers.
Ichthyomys species differ from Incanomys by their glossy and grizzled or brownish pelage, which includes abundant guard hairs. The underparts are white or cream, exhibiting countershading with the back. Ichthyomys has a larger body size, weighing above 50 g, and a larger, conical tail that is bicolor or blackish. The pinnae are reduced and visible above the fur of the head, similar to Daptomys and Neusticomys. It also possesses a philtrum and supraorbital foramina in the dorsal position of the interorbital constriction. In dorsal view, the nasal bones are short and do not cancel the incisors or nasal orifice. The skull is non-inflated, and the occipital condyle is developed and visible in dorsal view. The orbicular apophysis of the malleus is present, and the carotid circulation pattern is type 3. Ichthyomys lacks an anteromedian flexid, bicuspidate M3, and posteroloph and posterolophid in M1, M2, m1 and m2. It lacks a gall bladder, and the glandular epithelium is restricted to the greater curvature of the stomach.
Rheomys species differ from Incanomys based on their size, with R. underwoodi and R. mexicanus weighing above 50 g, while R. thomasi weights 45 g, and R. raptor below 30 g. Rheomys has a glossy skin, except R. thomasi. The pelage is characterized by abundant guard hairs, and the tail is bicolor in R. underwoodi and R. mexicanus. The ears are plainly visible above the unruffled fur of the head, and a philtrum is present in R. thomasi and R. raptor. Rheomys has three palmar pads, and the tail is either cylindrical or conical. The interorbital constriction is short, and the carotid arterial supply pattern is type 3. Like other species discussed, Rheomys has an orbicular apophysis of the malleus. Furthermore, all species of Rheomys are found in Central America.
Etymology. name derived from the Incan Empire, whose inhabitants lived in the region, particularly in the citadel of Machupicchu located near the capture site (2.5 km S) of the described species.
Family: Cricetidae Fischer, 1817
Subfamily: Sigmodontinae Wagner, 1843
Tribe: Ichthyomyini Vorontsov, 1959
Incanomys mayopuma, new species Zeballos, Pari, Medina and Pino;
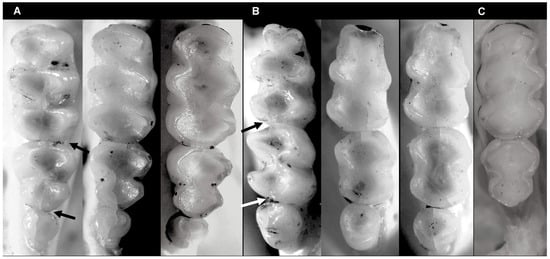
Figure 7.
View of molar tooth rows of Incanomys mayopuma new species showing molars series: (A) left upper molars (arrows indicate small anterolophs on M2 and M3); (B) lower right molars (arrows highlight posterolophids of m1 and m2). Specimens of A and B ordered left to right: MUSA 18964 (age 2), MUSA 18965 (age 3), and MUSA 13864 (age 4); (C) right upper molars without third molar MUSA 18965.
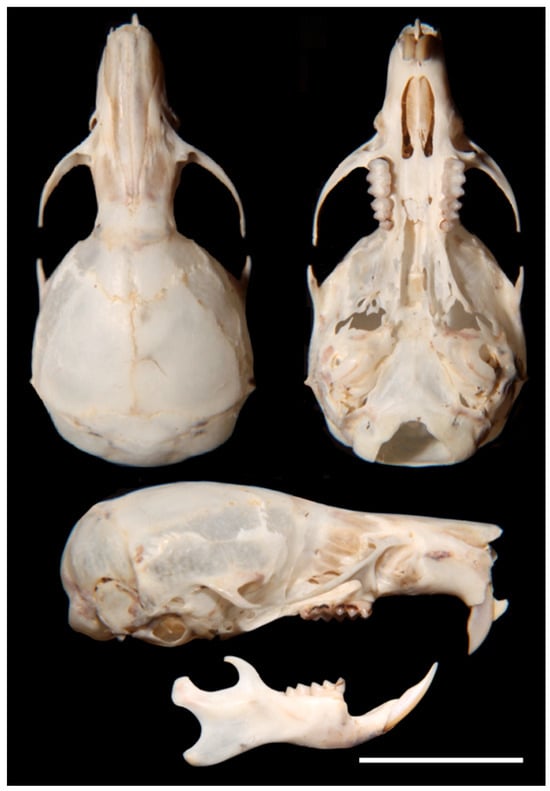
Figure 8.
Dorsal, ventral, lateral view of cranium and mandible of Incanomys mayopuma, new species (holotype MUSA 13864).
- Anotomys leander Gardner, 1971:1088. Not Anotomys leander Thomas, 1906
- Chibchanomys trichotis Voss, 1988:321. Not Ichthyomys trichotis Thomas, 1897. Name combination
- Chibchanomys orcesi Jenkins & Barnett, 1997:126. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Misidentification
- Chibchanomys orcesi Voss, 2015:282–283. Not Chibchanomys orcesi Jenkins & Barnett, 1997. Name combination
- Holotype. the holotype is an adult male collected by A. Pari and H. Zeballos (24 October 2012; field number APC 1755). It consists of a well-preserved skin and skull, with tissues preserved in ethanol. TWC 4, with basioccipital/basiesphenoid suture close and adult pelage, deposited in Museo de Historia Natural de la Universidad Nacional de San Agustín, Arequipa, Peru (MUSA 13864).
- Type locality. Peru, Department of Cusco, Province of Urubamba, Wiñaywayna, located 4 km southwest of the Aguas Calientes town (also known as Machupicchu Pueblo). The geographical coordinates are approximately 13°11′09′′ S and 72°32′34.5′′ W, at an elevation of 2825 m. The locality is within the Santuario Histórico de Machupicchu on the eastern slope of the Peruvian Andes (Figure 1).
- Paratypes. Two specimens collected by Sandra Arias (field number SAP 01, 02) from Wiñaywayna. The first is an immature female (MUSA 18964), which is a well-preserved specimen with the skin and skull in good condition, with TWC 2 and including basioccipital open suture and adult skin. The second is a male specimen (MUSA 18965) preserved in alcohol, with the skull removed, TWC 3, including basioccipital close suture and adult pelage.
- Etymology. The specific name mayopuma derives from Quechua, combining mayu (river) and puma (mountain lion). This name reflects the species’ semi-aquatic habits and carnivorous nature, akin to the neotropical otter. It emphasizes the animal’s adaptations to aquatic environments, predatory behavior, and ecological niche.
- Diagnosis. As for genus (see above)
- Description. A medium-size Ichthyomyini (Table 2). The dorsal skin is soft and dull. The coloration [38,39] is deep grayish olive to hair brown (Y90 M80 C80 to Y99 M70 C90). The dorsal guard hairs are deep grayish olive (Y90 M80 C80), while the bicolor hairs have a proximal part that is pale olive gray or pearl gray (N20 Y00 M00). The underparts are slightly paler than the back, lacking contrast (Figure 3b) and have bicolor hairs that are pale olive gray distally and olive gray or deep olive gray proximally. The muzzle is pale olive-buff (N10 Y40 M20; Figure 3a,e). The dorsal hairs measure 7.9 mm ± 0.29 and consists of long dorsal guard hairs that are deep grayish olive (Y90 M80 C80) and are more abundant on the sides. The color of the dorsal guard hairs is pale olive gray (N20 Y00 M00). The ventral fur is short (6.72 mm ± 0.62), while the underfur is long (7.90 ± 0.29) and fine. The large guard hairs measure 12.38 ± 0.04. The mystacial bristles are abundant and variable in size, and the supraorbital vibrissae are absent (Figure 3a,e). The tail is longer than combined length of head and body, measuring 121.30 mm ± 5.28. It is compressed laterally in the middle and distal part and has a terminal tuft of hairs measuring 6.3 mm. The tail is well-haired (Figure 3a), lacking visible epidermal scales. The dorsal hairs on the tail measure 3.03 mm ± 0.54, while the ventral hairs measure 6.83 mm ± 0.52. The color of the tail is unicolored Olive Brown (N90 Y80 M50, Figure 3a). The pinnae are significantly reduced (Figure 3a, h), measuring 1.83–3.11 mm from the border of the auditory meatus and are hidden among the fur. The philtrum absent or indistinct (Figure 3e). The manus has grayish upper parts and pale pinkish palms with whitish fingers. There are five pads on the manus (Figure 3i): three separate interdigital pads and two separate carpal pads. The metatarsal pattern is IV > III > II = V > I. The hind feet are very wide and large (Figure 3a,l); 30.41 mm ± 1.01, with brown soles that are naked (Figure 3l). The hypothenar pad of the pes is absent, and the thenar pad is developed. The hallux has a bearing nail, and the other four fingers have claws. The pes has a fringe of stiff hairs along the plantar margins and digits (Figure 3l), measuring 1.86 mm ± 0.07 on the internal margin and 2.23 mm ± 0.10 on the external margin. The dorsal parts of the pes have brown hairs, while the fingers are paler. The rostrum is narrow, and the nasolacrimal capsules are visible on dorsal view. The nasals are long (LN/CIL = 0.40–0.42; Figure 4A) and extend anteriorly over the incisors and nasal orifice, penetrating deeply into the skull to the middle part of the interorbital constriction. The zygomatic arch is complete (Figure 4H). The posterior border of the inferior zygomatic root is over the M1 anterocone. The interorbital is narrow, with a LIB/BB ratio of 0.34–0.36 in TWC 1–4 (Figure 4I and Figure 8). The interorbital is smooth without a postorbital ridge, and the supraorbital foramina are located on the border of the interorbital constriction. The braincase is flattened and globular, giving it a young appearance compared to other genera. The braincase (Figure 4H) is flattened and globular, giving it a young appearance compared to other genera. The incisive foramen broad anteriorly (Figure 4D). The palatal process of the premaxillary bone reaches the posterior edge of the incisive foramen. The occipital condyles are not visible dorsally (Figure 4H). The posterior edge of the incisive foramen is located at the level with the anterior border of M1, and it is shorter than or does not reach the posterior border of M3 (Figure 4D). The anterior palatal pits are conspicuous (Figure 4D), and the bullae are smaller and not inflated (Figure 8). The orbicular apophysis of the malleus is absent (Figure 4O,P). The carotid circulation pattern is type 1 (sensu Voss [14]). The dental formulae: I 1/1, C 0/0 PM 0/0 M 2–3/3 = 15–16 (MUSA 18965 with dM3 absent, Figure 7). The principal cusps of the molars are tall and sharp. M1 lacks an anteromedian flexus (Figure 7A), while M2 has a delicate anteromedian style. M1 has a small mesoloph, visible in young specimens (Figure 7A), while M2 lacks mesolophs. There are small posterolophs in M1 and M2. M3 lacks a conule behind the protocone/paracone. There is a small anteroloph in M2 and M3 (Figure 7A). M3 is small without a conulid behind the protoconid/metaconid. Both m1 and m2 have an anteromedian flexid (Figure 7B) and lack mesolophids (Figure 7B). They have small entolophulids, visible in young specimens. There are also small posterolophids in m1 and m2. The upper incisors are slightly opisthodonts, the angle at which incisors protrude from the jaw was 105 (Figure 4L and Figure 8) and have a very pale-yellow anterior face. The lower incisors are slender. An omohyoid muscle is present and a unilocular–hemiglandular stomach with glandular epithelium between the esophagus and pyloric sphincter, covering the antrum to the pyloric region and bordering fold insert on the small curvature between the esophagus and sphincter. It does not have contact with the incisura angularis, and it has a gall bladder. Karyotype 2N: 92, FN: 102 [21,22].
Measurements. holotype: LT, 253 mm; LC, 135 mm; LCC, 118 mm; LPP, 31.1 mm; LO, 6.2 mm; W, 47 g; CIL, 25.47 mm; LN, 10.16 mm; BN, 3.21 mm; LIB, 4.41 mm; BB, 12.82 mm; DI, 1.52 mm; HI, 4.05 mm; BIT, 1.53 mm; LIF, 4.57 mm; BIF, 1.78 mm; LD, 6.16 mm, LM, 4.42 mm; BM1, 1.45 mm; BPB, 3.04 mm; ZB, 12.88 mm; BZP, 0.98 mm; BOC, 7.93 mm; MAL, 16.22 mm; LMM, 4.99 mm; LBu, 4.27 mm; guard hair, 9 mm. Measurements of all specimens and comparison with C. trichotis and N. orcesi are presented in Table 3.
Comparisons. Incanomys mayopuma can be easily distinguished from other non-aquatic species of Sigmodontines by its medium-size, slate-colored skin with fine and large white guard hairs, slightly pale slate underparts, and a large and broad hindfoot with a hairy fringe along the metatarsal margins, the absence of a hypothenar pad, hairy and compressed distal tail of uniform color, greatly reduced pinnae hidden in the fur of the head, and indistinct or absent philtrum. Ichthyomys species differ from I. mayopuma in their glossy and grizzled or brownish pelage, abundant guard hairs, white- or cream-colored underparts with countershading on the back, larger body size (weight above 50 g), larger and conical tails, bicolor tails in I. stolzmanni, I. orientalis, and I. pinei, visible pinnae above the fur of the head, presence of philtrum, supraorbital foramina in dorsal position of interorbital constriction, short nasal bones that do not reach the incisors and nasal orifice in dorsal view, non-inflated braincase, developed occipital condyle visible on dorsal view, presence of orbicular apophysis of the malleus, carotid circulation pattern 3, absence of anteromedian flexid in M1, bicuspidate M3, absence of posteroloph in M1 and M2, absence of posterolophid in m1 and m2, and absence of gall bladder with glandular epithelium restricted to the greater curvature of the stomach. Anotomys leander differs from I. mayopuma in having slate-colored dorsal and ventral skin, whitish underparts without countershading on the back, presence of superciliary vibrissae, absence of pinnae, a pure white spot over the ears, four pads with fused hypothenar and third interdigital pads, metatarsal configuration IV > III > V > II > I, tail without ventral fringe, presence of orbicular apophysis of the malleus, carotid circulation pattern 2, presence of anteromedial flexus and flexid on M1, tricuspidate M3, presence of posteroloph in M1 only, absence of omohyoid muscle, and unidigitated bacular cartilage. Neusticomys species can be easily distinguished from I. mayopuma by their more mouse-like archetype, comparatively small adult size (less than 30 g), cylindrical tails, developed pinnae that protrude from the skin, presence of philtrum, metatarsal configuration III ≥ IV > II >> V > I, weakly developed metatarsal fringe of hairs, narrow hindfeet, absence of ventral tail fringe or delicacy, rounded skull, presence of orbicular apophysis of the malleus, and comparatively normal molars (LM/CIL varies between 0.18 and 0.23). Species of Rheomys differ from I. mayopuma by their size, with R. underwoodi and R. mexicanus weighing above 50 g, while R. thomasi and R. raptor weighing below 30 g, glossy skin (except R. thomasi), pelage with abundant guard hairs, bicolor tail in R. underwoodi and R. mexicanus, ears plainly visible above unruffled fur of head, presence of philtrum in R. thomasi and R. raptor, three palmar pads, cylindrical or conical tails, short interorbital constriction, carotid arterial supply pattern 3, presence of orbicular apophysis of the malleus and normal molars. In addition, all species of Rheomys lives in Central America. Incanomys mayopuma differs from C. trichotis in having a more grayish dorsal color and darker ventral color, whitish hindfeet with larger plantar pads and brown soles, whitish muzzle and chin (Figure 3e,f), indistinct or reduced philtrum that is closed above and narrow on the distal part, long nasals that reach the medial part of the interorbital (Pt = 0.001; Figure 4A,B), slightly narrower interorbital breadth, (Pt = 0.005), less inflated braincase (Pt = 0.005; Figure 4G,H), less inflated bullae (Pt = 0.008), slightly broader occipital condyles (Pt = 0.005), narrower breadth of the palatal bridge, more defined anteromedial flexid in m1, non-reduced molar toothrow, presence of enterostyle in M1, absence of M3 or barely present, small entolophulid in m1, broad incisive foramen positioned forward instead of backward as in C. trichotis, large palatal process of the premaxillary bone that reaches the posterior edge of the incisive foramen (in C. trichotis it occupies a third or half of the incisive foramen). Incanomys mayopuma is similar to C. trichotis in terms of body size (head and body length), hindfoot length, skull length, nasal breadth, metatarsal pattern, absence of orbicular apophysis of the malleus, absence of anteromedial flexus in M1, and presence of anteriomedial flexid in m1. Incanomys mayopuma is easily distinguished from N. orcesi by its larger body size, weight, tail length (Pt = 0.001), hindfoot size (Pt = 0.0005), slate gray coloration pattern (compared to brownish gray in N. orcesi), absence of white lips (Figure 3e,g), non-white tip of the tail, ear length, and toothrow length (Table 3), presence of a philtrum in N. orcesi and incomplete philtrum in I. mayopuma, different metatarsal pattern (III ≥ IV > II >> V in N. orcesi, and IV > III > II = V > I in I. mayopuma), narrower and larger nasals (Figure 4A,B and Figure 8, Pt = 0.031), broad forward incisive foramen (Figure 4D,F), larger palatal process of the premaxillary bone that reaches the posterior edge of the incisive foramen, smaller bullae (Pt = 0.013), absence of orbicular apophysis of the malleus (Figure 4O,P), and pale and opisthodont incisors (Figure 4L). Furthermore, there is a significant geographic distance and biogeographical barriers separating them; the distance between C. trichotis and Incanomys forms of northern Peru [56] is 1420 km, with respect of I. mayopuma from Cusco is about 2000 km; while N. orcesi is 520 km apart of Incanomys of northern Peru [56], 780 km from Carpish population, and 1330 km from Machupicchu population. N. orcesi has larger and plainly visible pinnae above unruffled fur of the head (9 mm or more) compared to I. mayopuma (less than 7 mm, pinnae less than 3.11 mm). In relation to comparative indices, nasal bone is longer and less wide in I. mayopuma (NB/LN = 0.31–0.32) than C. trichotis (NB/LN = 0.36) or N. orcesi (NB/LN = 0.34–0.35). The relative condylar breadth is narrower in C. trichotis and N. orcesi (BOC/BB = 0.55 in both) compared to I. mayopuma (BOC/BB = 0.62–0.64). The bullae are more inflated in C. trichotis (LBu/CIL = 0.23) and N. orcesi (LBu/CIL = 0.22) and non-inflated in I. mayopuma (LBu/CIL = 0.17).
Distribution. Incanomys mayopuma is found in the eastern slopes of the central Andes in Peru. Its range extends from Carpish in the Department of Huanuco to the southern region of Peru, including the vicinity of the Machupicchu archeological site in the Department of Cusco. The specimens have been collected at elevations ranging from 2400 to 2643 m. Currently, I. mayopuma is endemic to Peru and It is geographically separated from C. trichotis, with which it shares strong morphological similarity. It lies approximately 900 km from the Ecuadorian species N. orcesi, with which it was originally confused. This separation is primarily due to the presence of the Huancabamba depression (Marañon Valley), which acts as a significant biogeographic barrier in northern Peru, separating the Northern Andes from the Southern Andes (Figure 1).
Habitat and Ecology. the first specimen was captured over 300 m away from the nearest stream using a pitfall trap (20 L) after a storm. The second specimen was captured using a funnel trap (30 × 90 cm) within a stream, while the third specimen was found dead near a small waterfall. In the highlands of the collection region, there are abundant puddles during the rainy season and small streams, with the montane forest characterized by streams with steep slopes. The montane forest in the capture area can be classified as secondary forest, with trees reaching heights of up to 12 m and abundant vegetation. The stream is surrounded by gallery forest for most of its course. Some notable plant species in the study area include Escallonia pilgeriana, Myrica pubescens, and species from the genera Weinmannia, Clusia, Desmodium, Miconia, Lantana, and Piper. During the trapping period in April 2016, the water temperature at twelve points ranged between 11.5 and 13.5 °C, while the pH fluctuated between 7.34 and 7.88. The dissolved oxygen levels varied between 2.22 and 8.88 ppm, with the collection sites showing higher scores of dissolved oxygen (7.5 and 8.8 ppm). In the same habitat, other species of mammals were also captured, including five didelphids: Didelphis pernigra, Caluromys lanatus, Marmosa rapposa, Marmosops impavidus, and Monodelphis peruviana. Additionally, the cenolestid Lestoros inca and six rodents were captured: Akodon surdus, Microryzomys minutus, Oligoryzomys destructor, Thomasomys notatus, Cuscomys oblativus, and Dactylomys peruanus.
4. Discussion
The Peruvian specimens collected near the Machupicchu archeological site and the Carpish Mountains represent a new genus and species [13], described here as Incanomys mayopuma. While morphologically convergent with Chibchanomys trichotis and Anotomys leander in external and/or craniodental traits (see Figure 5, morphological tree), I. mayopuma exhibits significant genetic divergence. It also displays notable morphological and molecular distinctions from Neusticomys orcesi.
In the case of I. mayopuma, molecular phylogenies have revealed that the genus Chibchanomys is paraphyletic [13]. This paraphyly stems from the inclusion of both a juvenile Peruvian specimen from Carpish in central Peru [14] (p. 324) and N. orcesi [23] within this genus. To resolve this taxonomic issue, we propose a solution based on molecular, morphometric, and additional phylogenetic evidence [13,56]. Our results are consistent with the finding that Colombian and Venezuelan populations should be retained as C. trichotis (a monotypic species) [14], and the southern Ecuadorian form, previously referred to as Chibchanomys orcesi, should be transferred to the genus Neusticomys [13]; and the new species from southern Peru and San Martín Department to the new genus Incanomys, although the notable genetic distance (9.48% [56], and 12.10% in this study), branch length (Figure 6) and support (Bayesian: 1.00 and bootstrap: 100) makes a major revision necessary in the generic definition.
Incanomys is found in the Peruvian Yungas of the Central Andes, while C. trichotis inhabits the montane forests of the northern Andes in Colombia and Venezuela; N. orcesi has a range that extends from the Páramo and elfin forests of the northern Andes to southern Ecuador; and A. leander has a range that extends to the montane forest of northern Ecuador and south of Colombia. These species exhibit broad geographic separation (Figure 1), further reinforced by significant barriers such as the Huancabamba depression and Marañon valley [55], likely contributing to their allopatric evolution.
On the other hand, the morphological evidence from our analysis underscores the need for further studies, particularly molecular data, to clarify the phylogenetic relationships among certain ichthyomyine species and genera [13]. For instance, a small (HBL = 97 mm; W = 18 g), immature Bolivian assigned to Chibchanomys [57] may belong to different genus, given its comparatively short hindfeet (24 mm), large ears (11 mm), and short tail (81 mm). Also, the Central American genus Rheomys may consist of more than one genus. Another species that needs revision is I. tweedi, since the disjunct forms of Central America, northern Ecuador and the nominal form of southern Ecuador present morphological differences that would constitute different taxonomic entities. The same counts for I. hydrobates, which currently includes three variants.
Adaptations to a semiaquatic lifestyle vary significantly among ichthyomyines. Some species occupy both terrestrial and aquatic habitats, favoring calm waters in ponds and non-flood-prone streams. These species tend to be small- or medium-sized, with narrow feet, poorly developed fringing hairs, and short visible ears, traits exemplified by Neusticomys spp., Daptomys spp., and smaller Rheomys. In contrast, larger species with fusiform and hydrodynamic bodies, small ears, broad hindfeet, thick and strong tails, and depressed heads are specialized for fast-flowing (lotic) streams in Andean ecosystems [43,58], Ichthyomys and large Rheomys exemplify this adaptation, possessing webbed feet and fringe hairs that enhance propulsion, enabling them to navigate rapids and transport prey, sometimes as large as small trout (Nasario Huincho, pers. comm.). A third group includes medium-sized non-fusiform species (A. leander, C. trichotis, and I. mayopuma) with broad, and well-developed hindfeet featuring fringe hairs, absent or vestigial ears, and strong slightly compressed tails. These species are adapted to small or fast-flowing Andean streams, though their habitats are highly variable topography. They represent a convergent morphological archetype. The link between body design and aquatic habitat illustrates how environmental factors drive morphological, physiological, and behavioral adaptations for aquatic life [59,60,61]. Furthermore, adaptations to aquatic niches may have led to convergent traits with other aquatic mammals [14]. For example, the absence of an M3 in an adult I. mayopuma specimen could reflect molar reduction as an aquatic specialization, a pattern as observed in other ichthyomyines [36,60,62].
Despite these insights, the biology and distribution of these species remain poorly understood. The tribe’s initial comprehensive revision [14] recognized 14 species grouped in five genera. Thirty-four years later, new questions have arisen, and their diversity is now better documented, with 18 currently recognized species [5,15,28]. Today, ichthyomyine rodents comprising seven genera and at least 22 species [13,63] (and this study), represent a remarkable Sigmodontinae radiation with striking aquatic adaptations. Encouragingly, growing research attention has led to the description of new species and expanded known distributions [12,13,14,19,28,29,31,57,64,65,66,67,68,69,70,71] with at least two additional undescribed species reported in Bolivia and Peru [13,18,57].
The discovery of a new Ichthyomyini genus and species in the Peruvian Andes solidifies the neotropical region as the global epicenter of semi-aquatic mammal diversity. This finding is particularly striking given the remarkable morphological convergence observed among geographically isolated, paraphyletic lineages including A. leander (Ecuador–Colombia), C. trichotis (Colombia–Venezuela), R. underwoodi (Central America), and the newly described Peruvian taxon. Such exceptional diversity, driven by the complex topography of the Andes, highlights critical gaps in our understanding of the ecological and evolutionary mechanisms underlying these extraordinary adaptive radiations. At the same time, mounting threats to aquatic ecosystems—such as pollution, habitat degradation, and alterations to riparian zones—emphasize the urgent need to study and conserve these species, their ecological roles, and their fragile habitats.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17060406/s1 as Supplementary materials: Table S1: Specimens examined: the locality of collection (country, region/department/province, district, locality); Table S2: Qualitative morphological characters and character states selected for phylogenetic analysis are described below and the distribution of their states among ichthyomyine species; and matrix of morphological character states of species of Ichthyomyini tribe; Table S3: List of GenBank accession numbers for molecular data used in this study; Table S4: Genetic distances were estimated with the Kimura two parameters evolution model (Kimura 1980) of Ichthyomyini species, based on cytochrome b, genetic distance (below the diagonal) and standard error (upper diagonal).
Author Contributions
The individual contributions: H.Z. conceived and designed the experiments, performed the experiments, analyzed the data, taxonomy, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft. A.P. performed the field experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft. C.E.M. performed the field experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft. K.P. performed the field experiments analyzed the data, authored or reviewed drafts of the article, and approved the final draft. S.A. performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft. A.L.A. performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft. F.N.G. performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Internal Investigation Grants 2020 of Universidad Católica de Santa María (Resolution No. 27857-R-2021).
Data Availability Statement
The raw data and other results are available in the Supplementary Materials.
Acknowledgments
We would like to express our gratitude to the following individuals and institutions for allowing us to review the collections under your care. We are thankful to Evaristo López, the director of the Museo Historia Natural de la Universidad Nacional de San Agustín, Arequipa, Peru; Víctor Raúl Pacheco, the curator of the Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru; Santiago Burneo, the curator of the Museo de Zoología de la Pontificia Universidad Católica de Ecuador, Quito; Hugo López, the curator of the Museo de Historia Natural de la Universidad Nacional de Colombia, Bogotá; Robert S. Voss, the curator of mammals at the American Museum of Natural History, New York; the National Museum of Natural History; Darrin Lunde, the Collections Manager of the National Museum of Natural History, Washington D.C.; Jorge Brito, a researcher at Instituto Nacional de Biodiversidad de Ecuador, Quito, and Valentina Posse, for allowing us to examine specimens from the Museo de la Escuela Politécnica de Ecuador and the Museo Ecuatoriano de Ciencias Naturales; José González Maya for granting us access to a specimen from Costa Rica; Carlos Nivelo from the Museo de Zoología de la Universidad del Azuay; David Marín from the Instituto de Biología, Universidad de Antioquia for providing us with valuable information about specimens from Colombia; Javier Colmenares from Texas Tech University for the photos of a specimen from Colombia; to Gerardo Ceballos, Lourdes Martínez-Esteves, Andrés García, and Jesús Pacheco from the Instituto de Ecología of the Universidad Nacional Autónoma de México, who accompanied us during the Curso Latinoamericano de Biología de la Conservación, in the collection of the first specimen. To the park rangers of the Santuario Histórico Machupicchu; to L. Cruz from the Universidad Nacional San Antonio Abad del Cusco; to the Dirección Regional de Cultura—Cusco; and to S. Portillo from the Dirección Regional de Comercio Exterior y Turismo—DIRCETUR. Special thanks go to Jorge Salazar-Bravo of Texas Tech University, USA, for their thorough review and valuable comments on the manuscript. Enrique Lessa of the Universidad de la República, Uruguay deserves recognition for his assistance in improving the language and clarity of the manuscript. Finally, we would like to express our appreciation to the Dirección General de Flora y Fauna Silvestre (DGFFS), Servicio Nacional de Áreas Naturales Protegidas (SERNANP), Zona Reservada Sierra del Divisor and Santuario Histórico de Machupicchu for granting us study authorizations and providing field facilities (RD 503-2011-AG-DGFFS, RJ 003-2013-SERNANP-ZRSD y RJSHM-No. 054-2012-SERNANP-JEF). This study was funded by Universidad Católica de Santa María, Internal Investigation Grants 2020 (Resolution No. 27857-R-2021).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
In the case of Natural History Museums, they are described in the text. The following abbreviations are used in this manuscript:
| UCSM | Universidad Católica de Santa María |
| SERNANP | Servicio Naciona de Áreas Naturales Protegidas |
References
- Catzeflis, F.M.; Aguilar, J.P.; Jaeger, J.J. Muroid rodents: Phylogeny and evolution. Trends Ecol. Evol. 1992, 7, 122–126. [Google Scholar] [CrossRef]
- Hershkovitz, P. Evolution of Neotropical cricetine rodents (Muridae), with special reference to the phyllotine group. Fieldiana Zool. 1962, 46, 1–524. [Google Scholar]
- Musser, G.G.; Carleton, M.D. Superfamily Muroidea. In Mammal Species of the World, A Taxonomic and Geographic Reference; Wilson, D.E., Reeder, D.A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; Volume 2, pp. 894–1531. [Google Scholar]
- Pardiñas, U.F.J.; Shenbrot, G.; Patton, J.L.; Myers, P.; León-Paniagua, L.; Ordóñez Garza, N.; Cook, J.A.; Kryštufek, B.; Haslauer, R.; Bradley, R. Family Cricetidae (True Hamsters, Voles, Lemmings and New World Rats and Mice). In Handbook of the Mammals of the World, Rodents II; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2017; Volume 7, pp. 204–279. [Google Scholar]
- Pardiñas, U.F.J.; Ruelas, D.; Brito, J.; Bradley, L.C.; Bradley, R.D.; Ordóñez Garza, N.; Krystufek, B.; Cook, J.A.; Soto, E.C.; Salazar-Bravo, J.; et al. Species Accounts of Cricetidae. In Handbook of the Mammals of the World, Rodents II; Wilson, D.E., Lacher, T.E., Jr., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2017; Volume 7, pp. 280–535. [Google Scholar]
- Patton, J.; Pardiñas, U.F.J.; D’elía, G. (Eds.) Mammals of South America, Rodents; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 1–1336. [Google Scholar]
- Reig, O.A. Diversity pattern and differentiation of High Andean rodents. In High Altitude Tropical Biogeography; Vuillemier, F., Monasterio, M., Eds.; Oxford University Press: New York, NY, USA, 1986; pp. 404–439. [Google Scholar]
- Smith, M.F.; Patton, J.L. The diversification of South American murid rodents: Evidence from mitochondrial DNA sequence data for the Akodontine tribe. Biol. J. Linn. Soc. 1993, 50, 149–177. [Google Scholar] [CrossRef]
- Smith, M.F.; Patton, J.L. Phylogenetic relationships and the radiation of Sigmodontine rodents in South America: Evidence from Cytochrome b. J. Mamm. Evol. 1999, 6, 89–128. [Google Scholar] [CrossRef]
- Wilson, D.E.; Reader, D.A. (Eds.) Mammal Species of the World: A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. i–xvii+743–2142. [Google Scholar]
- Anthony, H.E.; Tate, G.H.H. Two new genera of rodents from South America. Am. Mus. Novit. 1929, 383, 6. Available online: http://hdl.handle.net/2246/4089 (accessed on 15 January 2023).
- Barnett, A.A. The ecology and natural history of a fishing mouse Chibchanomys spec. nov. (Ichthyomyini: Muridae) from the Andes of southern Ecuador. Mamm. Biol. 1997, 62, 43–52. [Google Scholar]
- Salazar-Bravo, J.; Tinoco, N.; Zeballos, H.; Brito, J.; Arenas-Viveros, D.; Marín-C, D.; Ramírez-Fernández, J.D.; Percequillo, A.R.; Lee, T.E., Jr.; Solari, S.; et al. Systematics and diversification of the Ichthyomyini (Cricetidae, Sigmodontinae) revisited: Evidence from molecular, morphological, and combined approaches. PeerJ 2023, 11, e14319. [Google Scholar] [CrossRef] [PubMed]
- Voss, R.S. Systematics and ecology of ichthyomyine rodents (Muroidea): Patterns of morphological evolution in a small adaptive radiation. Bull. Am. Mus. Nat. Hist. 1988, 188, 259–493. Available online: http://hdl.handle.net/2246/927 (accessed on 5 February 2006).
- Voss, R.S. Tribe Ichthyomyini Vorontsov, 1959. In Mammals of South America; Patton, J.L., Pardiñas, U.F.J., D’Elía, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; pp. 279–291. [Google Scholar]
- Zeballos, H.; Díaz, R.; Rodriguez, N.; Delgado, W.; Tuwits-Wajai, O.; Villoslaba, D.; Cahuaza, F.J.; Arce, A. Pequeños mamíferos no voladores del paisaje Alto Mayo, San Martín, Peru. In Capítulo 8, Evaluación Biológica Rápida del Paisaje Alto Mayo, San Martín Perú; Boletín RAP de Evaluación Biológica 73; Larsen, T.H., Palomino, W., Zeballos, H., Carrillo, P., Eds.; Conservation International: Arlington, VA, USA, 2024; pp. 307–326. [Google Scholar]
- Musser, G.G.; Gardner, A.L. A new species of the ichthyomyine Daptomys from Peru. Am. Mus. Novit. 1974, 2537, 1–23. Available online: http://hdl.handle.net/2246/2740 (accessed on 23 November 2006).
- SERNANP [Servicio Nacional de Áreas Naturales Protegidas]. Parque Nacional Tingo María 50 Años; SERNANP y Empresa de Generación Huallaga, S.A-Odebrecht: Lima, Peru, 2014; pp. 1–160. [Google Scholar]
- Pacheco, V.; Ugarte-Nuñez, J. New records of Stolzmann’s fish-eating rat Ichthyomys stolzmanni (Cricetidae. Sigmodontinae) in Peru: A rare species becoming a nuisance. Mamm. Biol. 2011, 76, 657–661. [Google Scholar] [CrossRef]
- Thomas, O. New mammals from Peru and Bolivia, with list of those recorded from Inambari River, Upper Madre de Dios. Ann. Mag. Nat. Hist. 1901, 7, 178–190. [Google Scholar] [CrossRef]
- Gardner, A.L. Karyotypes of two rodents from Peru, with a description of the highest diploid number recorded from a mammal. Experientia 1971, 27, 1088–1089. [Google Scholar] [CrossRef]
- Gardner, A.L.; Patton, J.L. Karyotypic variation in oryzomyine rodents (Cricetidae) with comments on Chromosomal evolution in the Neotropical Cricetine Complex. Occas. Pap. Mus. Nat. Sci. La. State Univ. 1976, 49, 1–48. [Google Scholar]
- Jenkins, P.D.; Barnett, A.A. A new species of water mouse of the genus Chibchanomys (Rodentia: Muridae: Simodontinae) from Ecuador. Bull. Nat. Hist. Mus. Lond. (Zool.) 1997, 63, 123–128. [Google Scholar]
- Sikes, R.S.; Gannon, W.L.; Animal Care and Use Committee of the ASM. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mamm. 2016, 97, 663–688. [Google Scholar] [CrossRef]
- Anthony, H.E. Preliminary report on Ecuadorean mammals, No. 1. Am. Mus. Novitates 1921, 20, 1–6. Available online: http://hdl.handle.net/2246/4636 (accessed on 1 April 2014).
- Anthony, H.E. 1923. Preliminary report on Ecuadorean mammals, No. 3. Am. Mus. Novitates 1923, 55, 1–13. Available online: http://hdl.handle.net/2246/4635 (accessed on 13 September 2013).
- Ellerman, J.R. The families and Genera of Living Rodents. In Family Muridae; Trustees British Mus (Nat Hist): London, UK, 1941; Volume 2, pp. xii+690. [Google Scholar]
- Hanson, J.D.; D’Elía, G.; Ayers, S.B.; Cox, S.B.; Burneo, S.F.; Lee, T.E. A new species of fish-eating rat, genus Neusticomys (Sigmodontinae), from Ecuador. Zool. Stud. 2015, 54, 49. [Google Scholar] [CrossRef]
- Ochoa, J.; Soriano, P. A new species of water rat, genus Neusticomys Anthony, from the Andes of Venezuela. J. Mamm. 1991, 72, 97–103. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Carmignotto, A.P.; de Silva, M.J. A new species of Neusticomys (Ichthyomyini. Sigmodontinae) from central Brazilian Amazonia. J. Mamm. 2005, 86, 873–880. [Google Scholar] [CrossRef]
- Percequillo, A.R.; Dalapicolla, J.; Abreu-Júnior, E.F.; Roth, P.R.O.; Ferraz, K.M.P.M.B.; Chiquito, E.A. How many species of mammals are there in Brazil? New records of rare rodents (Rodentia: Cricetidae: Sigmodontinae) from Amazonia raise the current known diversity. PeerJ 2017, 5, e4071. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O. On some mammals from Central Peru. Proc. Zool. Soc. Lond. 1893, XXIII, 333–340. [Google Scholar]
- Thomas, O. Descriptions of four new South American mammals. Ann. Mag. Nat. Hist. 1897, 20, 218–221. [Google Scholar] [CrossRef][Green Version]
- Thomas, O. A new aquatic genus of Muridae discovered by Consul, L. Soderström in Ecuador. Ann. Mag. Nat. Hist. 1906, 17, 86–88. [Google Scholar] [CrossRef]
- Thomas, O. A third genus of the Ichthyomys group. Ann. Mag. Nat. Hist. 1906, 17, 421–423. [Google Scholar] [CrossRef][Green Version]
- Voss, R.S.; Lunde, D.R.; Simmons, N.B. The Mammals of Paracou, French Guiana: A Neotropical Lowland Rainforest Fauna Part 2. Nonvolant Species. Bull. Am. Mus. Nat. Hist. 2001, 263, 1–236. Available online: http://hdl.handle.net/2246/386 (accessed on 18 August 2001). [CrossRef]
- Carleton, M.D. A Survey of Gross Stomach Morphology in New World Cricetinae (Rodentia, Muroidea), with Comments on Functional Interpretations; Miscellaneous Publications of the Museum of Zoology; University of Michigan Museum of Zoology: Ann Arbor, MI, USA, 1973; Volume 146, pp. 1–43. Available online: http://hdl.handle.net/2027.42/56390 (accessed on 13 May 2025).
- Reig, O.A. A proposed unified nomenclature for the enameled components of the molar teeth of Cricetidae (Rodentia). J. Zool. 1977, 181, 227–241. [Google Scholar] [CrossRef]
- Ridgway, R. Color Standards and Color Nomenclature; reprint by Elibron Clasics; Elibron Clasics: Washington, DC, USA, 1912; pp. i–v+1–43+LIII plates. [Google Scholar]
- Küppers, H. Atlas de Los Colores, 1st ed.; Blume Barcelona: Barcelona, España, 2002; pp. 1–165. [Google Scholar]
- RStudio Team. Integrated Development for R; RStudio PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 29 January 2024).
- Hooper, E.; Musser, G.G. The glans penis in Neotropical cricetines (family Muridae) with comments on classification of muroid rodents. Mis. Pub. Mus. Zool. Univ. Mich. 1964, 123, 1–57. [Google Scholar]
- Starrett, A.; Fisler, G.F. Aquatic adaptations of the water mouse, Rheomys underwoodi. Contrib. Sci. 1970, 182, 1–14. [Google Scholar] [CrossRef]
- Vorontzov, N.N. Evolution of the Alimentary System in Myomorph Rodents; Smithsonian Institution: Washington, DC, USA, 1979; pp. 1–346. [Google Scholar]
- Voss, R.S.; Linzey, A.V. Comparative gross morphology of male accessory glands among Neotropical Muridae (Mammalia: Rodentia) with comments on systematic implications. Misc. Pub Mus. Zool. Univ. Mich. 1981, 159, 1–41. [Google Scholar]
- Goloboff, P.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Goloboff, P.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Fluori, T.; Zozlov, A.; Morel, B.; Posada, D.; Stamatakis, A. Modeltest NG v0.1.7. Exilixis Lab. 2021. Available online: https://github.com/ddarriba/modeltest (accessed on 2 March 2025).
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2020, 12, 373–377. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Pavan, S.E.; Abreu, E.F.; Sánchez-Vendizú, P.Y.; Batista, R.; Murta-Fonseca, R.A.; Pradel, R.; Rengifo, E.M.; Leo, M.; Pacheco, V.; Aleixo, A.; et al. A hint on the unknown diversity of eastern Andes: High endemicity and new species of mammals revealed through DNA barcoding. Syst. Biodivers. 2024, 21, 2302196. [Google Scholar] [CrossRef]
- Anderson, S. Mammals of Bolivia, taxonomy and distribution. Bull. Am. Mus. Nat. Hist. 1997, 231, 1–652. Available online: http://hdl.handle.net/2246/1620 (accessed on 23 January 2006).
- Hooper, E.T. Habitats and food of amphibious mice of the genus Rheomys. J. Mamm. 1968, 49, 550–553. [Google Scholar] [CrossRef]
- Fish, F.E. Transitions from drag-based to lift-based propulsion in mammalian swimming. Am. Zool. 1996, 36, 628–641. Available online: https://www.jstor.org/stable/3884002 (accessed on 6 August 2018). [CrossRef]
- Fish, F.E. Secondary evolution of aquatic propulsion in higher vertebrates: Validation and prospect. Integr. Comp. Biol. 2016, 56, 1285–1297. [Google Scholar] [CrossRef]
- Kükenthal, W. XIX. On the adaptation of mammals to aquatic life. Ann. Mag. Nat. Hist. 1891, 38, 153–179. [Google Scholar] [CrossRef]
- Dubost, G.; Petter, F. Une espèce nouvelle de “rat-pechêur” de Guyane Francaise: Daptomys oyapocki sp. nov. (Rongeurs, Cricetidae). Mammalia 1978, 42, 435–439. [Google Scholar] [CrossRef]
- Handley, C.O.; Mondolfi, E. A new species of fish-eating rat, Ichthyomys, From Venezuela (Rodentia Cricetidae). Acta Biol. Venez. 1963, 3, 417–419. [Google Scholar]
- Fernández de Córdova, J.; Nivelo-Villavicencio, C.; Reyes-Puig, C.; Pardiñas, U.F.J.; Brito, J. A new species of crab-eating rat of the genus Ichthyomys, from Ecuador (Rodentia, Cricetidae, Sigmodontinae). Mammalia 2019, 84, 377–391. [Google Scholar] [CrossRef]
- Gonzales, F.N.; Arce-Merma, A.; Zeballos, H. Range extension of the Peruvian fish-eating rat Neusticomys peruviensis (Rodentia: Cricetidae) in Peru. Rev. Peru. Biol. 2017, 24, 413–416. [Google Scholar] [CrossRef]
- Leite, R.N.; Silva, M.N.F.; Gardner, T.A. New records of Neusticomys oyapocki (Rodentia, Sigmodontinae) from a human-dominated forest landscape in northeastern brazilian Amazonia. Mastozool. Neotrop. 2007, 14, 257–261. [Google Scholar]
- Medina, C.E.; Lopez, E.; Pino, K.; Pari, A.; Zeballos, H. Biodiversidad de la zona reservada Sierra del Divisor (Perú): Una visión desde los mamíferos pequeños. Rev. Peru. Biol. 2015, 22, 199–212. [Google Scholar] [CrossRef][Green Version]
- Miranda, C.L.; Rossi, V.; Semedo, T.B.F.; Flores, T.A. New records and geographic distribution extension of Neusticomys ferreirai and N. oyapocki (Rodentia, Sigmodontinae). Mammalia 2012, 76, 335–339. [Google Scholar] [CrossRef]
- Pacheco, V.; Sánchez-Vendizú, P.; Loaiza-Salazar, C.R.; Pino, K.; Medina, C.; Vivas-Ruiz, D. A revision of Neusticomys peruviensis (Rodentia: Cricetidae) with the description of a new subspecies. J. Mamm. 2020, 101, 858–871. [Google Scholar] [CrossRef]
- Rodríguez-Posada, M.E. Primer registro del ratón de agua del Táchira, Neusticomys mussoi (Rodentia, Cricetidae) en Colombia. Mastozool. Neotrop. 2014, 21, 367–372. [Google Scholar]
- Velandia-Perilla, J.H.; Saavedra-Rodríguez, C.A. Mammalia, Rodentia, Cricetidae, Neusticomys monticolus (Anthony, 1921): Noteworthy records of the Montane fish-eating rat in Colombia. Check List 2013, 9, 686–688. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).