Nuisance Growth of Cissus verticillata (Vitaceae) Negatively Affects the Structure of Mangroves in Marismas Nacionales Nayarit, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Forest Structure
2.3. Statistical Analysis
2.4. Natural Regeneration and Mortality
3. Results
3.1. Density of Mangrove Species
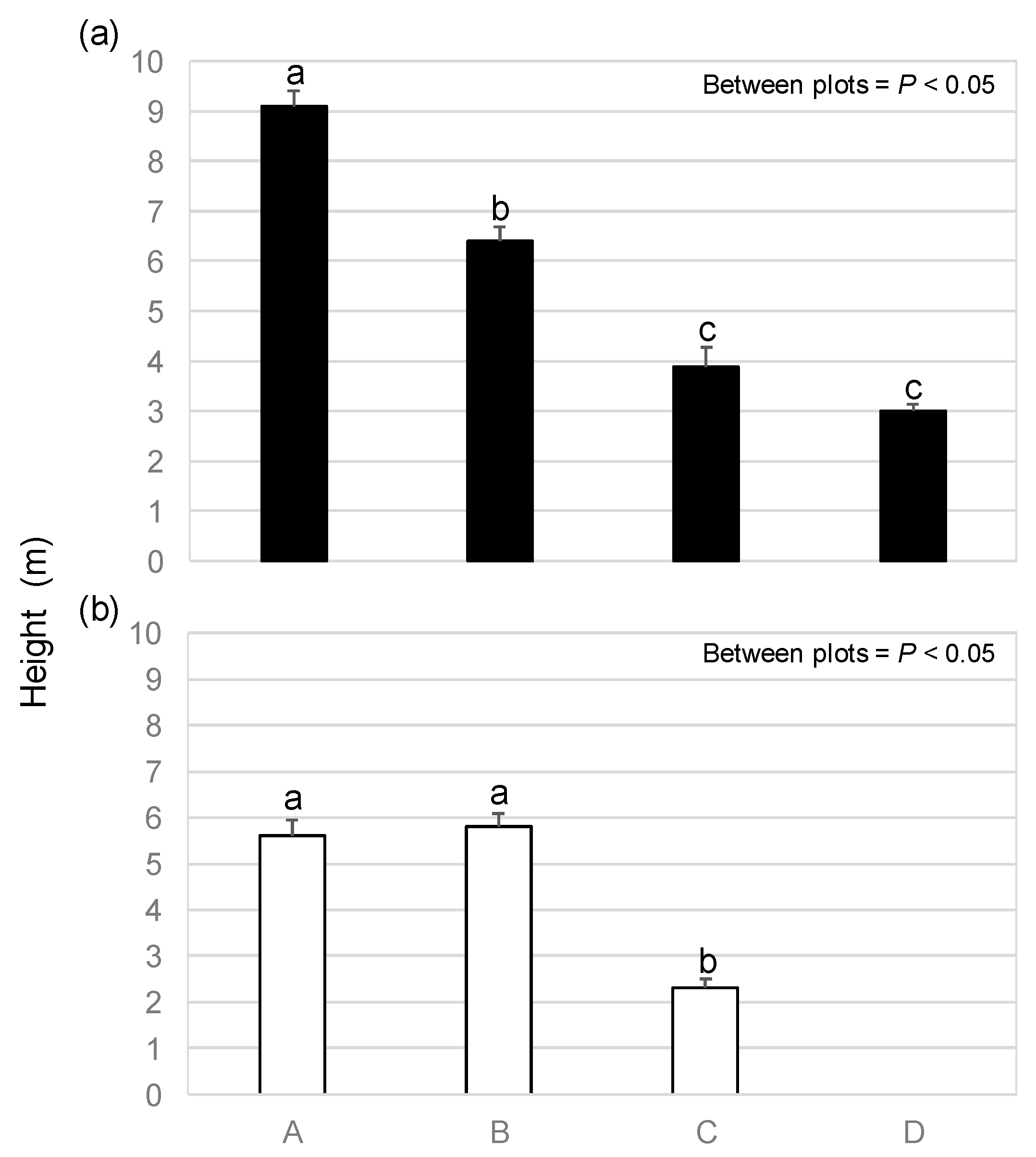
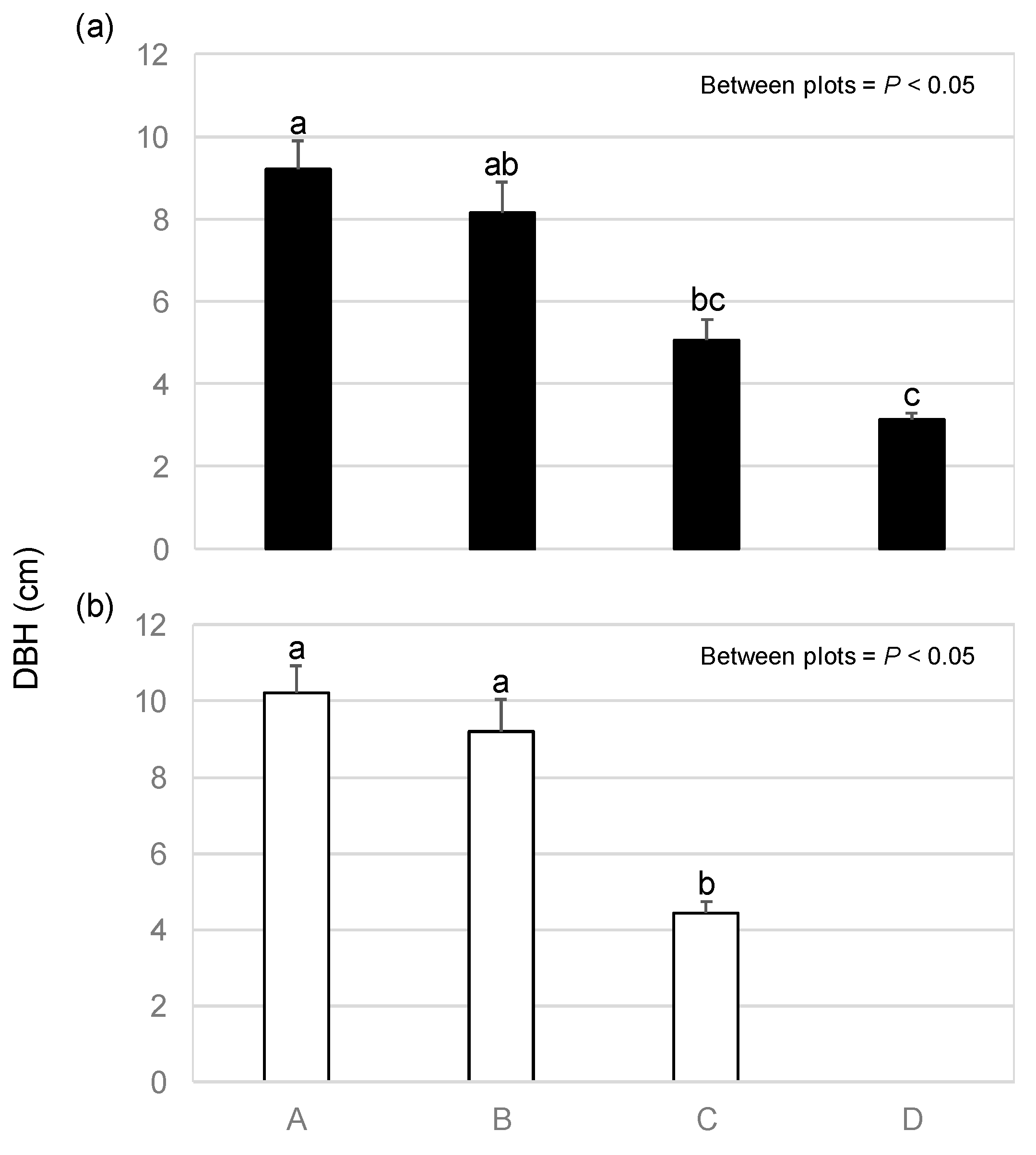
3.2. Height and DBH
3.3. Basal Area, Holdridge Complexity Index (HCI) and Importance Value Index (IVI)
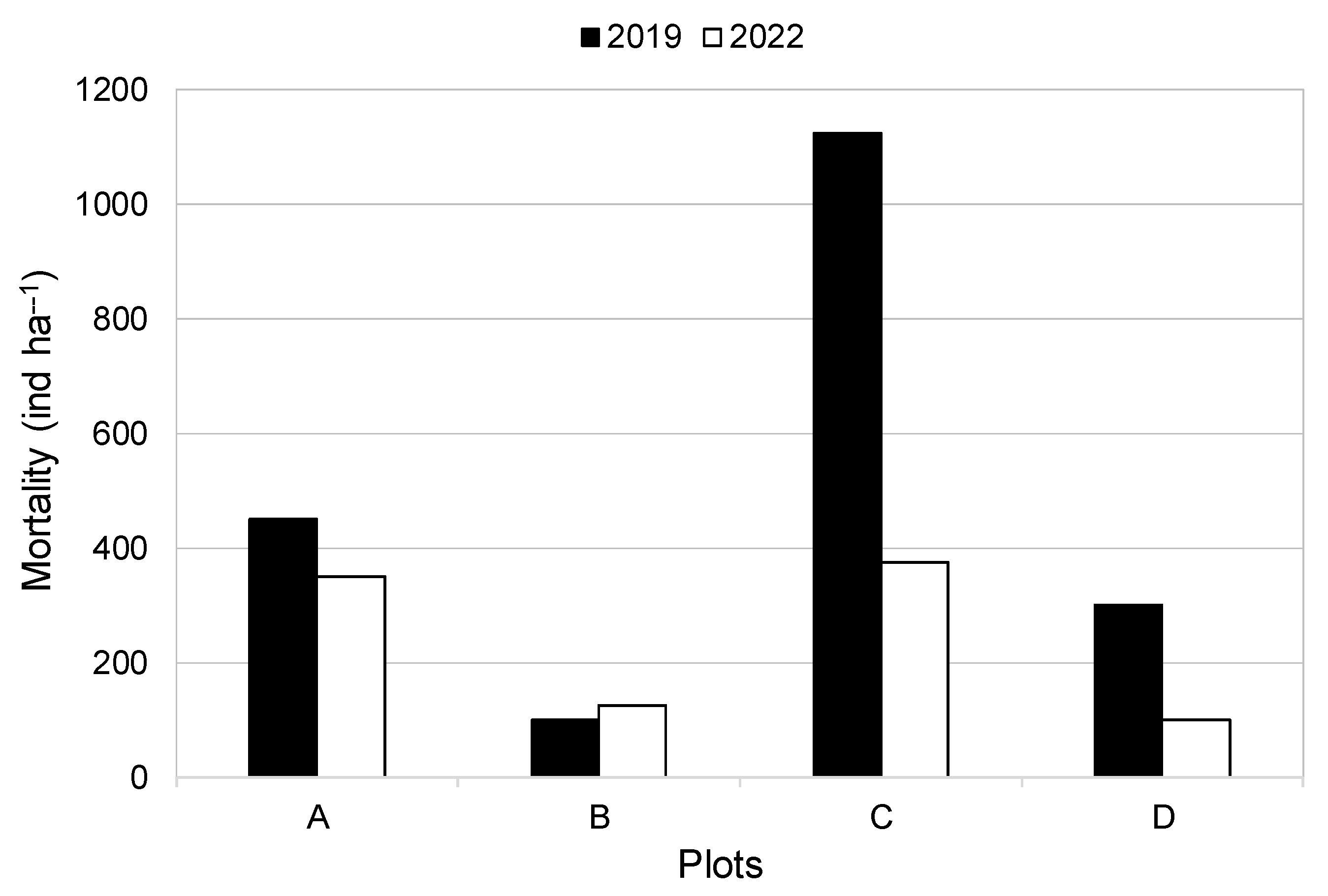
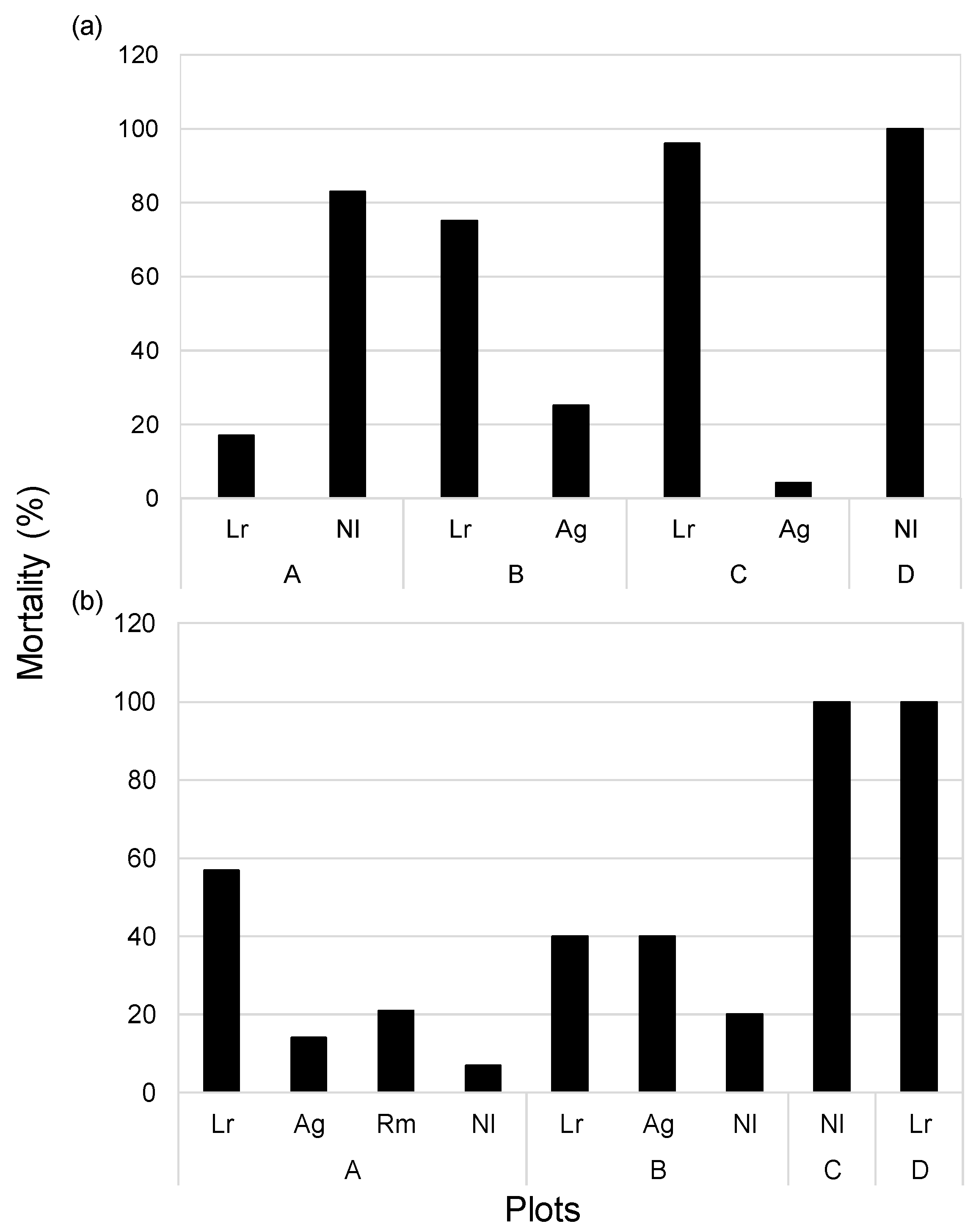
3.4. Mortality and Number of Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986; p. 419. [Google Scholar]
- Velázquez-Salazar, S.; Rodríguez-Zúñiga, M.T.; Alcántara-Maya, J.A.; Villeda-Chávez, E.; Valderrama-Landeros, L.; Troche-Souza, C.; Vázquez-Balderas, B.; Pérez-Espinosa, I.; Cruz-López, M.I.; Ressl, R.; et al. Manglares de México. Actualización y Análisis de los datos 2020; CONABIO: Coyoacan, México, 2021; p. 168. [Google Scholar]
- Sarma, V.V.; Hyde, K.D. A review on frequently occuring fungi in mangroves. Fung. Divers. 2001, 8, 34. [Google Scholar]
- Gómez-García, E.; Soto-Estrada, A.; Sol-Sánchez, A.; Pérez-Vázquez, A.; Sánchez-Soto, S.; Ruíz-Rosado, O. Daño foliar ocasionado por la herbivoría en árboles de mangle negro (Avicennia germinans L.) en Tabasco, México. Instituto de Investigaciones de Sanidad Vegetal, La Habana, Cuba. Fitosan 2015, 19, 213–219. [Google Scholar]
- Iannone, B.; Carnevale, S.; Main, M.; Hill, J.; McConnell, J.; Johnson, S.; Enloe, S.; Andreu, M.; Bell, E.; Cuda, J.; et al. Invasive Species Terminology: Standardizing for Stakeholder Education. J. Ext. 2020, 58, 27. [Google Scholar] [CrossRef]
- Ramírez-Carballo, H.; Ramírez-García, J.G. Estudio Para Identificar las vías de Introducción de la Enredadera Tripa de Zopilote (Cissus verticillata), Carrizo (Arundo donax) y Zacate Buffel (Cenchrus ciliaris) en la Reserva de la Biósfera Marismas Nacionales y su ÁREA de Influencia. Proyecto 00089333 “Aumentar las Capacidades de México Para Mejorar Especies Exóticas Invasoras a Través de la Implementación de la Estrategia Nacional de Especies Invasoras”; PNUD México (Programa de las Naciones Unidas para el Desarrollo): Mexico City, Mexico, 2017; p. 99, Anexo 1. [Google Scholar]
- French, J.V.; Lonard, R.I.; Everitt, J.H. Cissus sicyoides C. Linnaeus (Vitaceae), a Potential Exotic Pest in the Lower Rio Grande Valley, Texas. Sundrop Plant Sci. 2003, 55, 72–74. [Google Scholar]
- Rzendowsky, J.; Calderon, G. Vitaceae. In Flora del Bajio y de Regiones Adyacentes; Instituto de Ecología AC: Xalapa, Mexico, 2005; p. 131. [Google Scholar]
- PIER (Pacific Island Ecosystems at Risk). Cissus verticillata (L.) Nicolson & Jarvis, Vitaceae 2012. Available online: http://www.hear.org/pier/species/cissus_verticillata.htm (accessed on 6 May 2025).
- Rojas-Sandoval, J. Cissus verticillata (Possum Grape Vine); CABI Compendium: Wallingford, UK, 2022. [Google Scholar]
- Staples, G.W.; Herbst, D.R. A Tropical Garden Flora-Plants Cultivated in the Hawaiian Islands and Other Tropical Places; Bishop Museum Press: Honolulu, HI, USA, 2005; p. 908. [Google Scholar]
- Valderrama-Landeros, L.; Camacho-Cervantes, M.; Velázquez-Salazar, S.; Villeda-Chávez, E.; Flores-Verdugo, F.; Flores-de-Santiago, F. Detection of an invasive plant (Cissus verticillata) in the largest mangrove system on the eastern Pacific coast—A remote sensing approach. Wetl. Ecol. Manag. 2025, 33, 1–13. [Google Scholar] [CrossRef]
- Alongi, M.D. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Castillo, E.B.; Gervacio, J.H.; Bedolla, S.R. Estructura forestal de una zona de manglar en la laguna de Coyuca de Benítez, Guerrero. Rev. Mex. Cienc. For. 2017, 9, 45. [Google Scholar] [CrossRef][Green Version]
- DOF (Diario Oficial de la Federación). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres—Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio—Lista de Especies en Riesgo; DOF (Diario Oficial de la Federación): Mexico City, Mexico, 2010. [Google Scholar]
- CONANP (Comisión Nacional de Áreas Naturales Protegidas). Programa de Manejo Reserva de la Biósfera Marismas Nacionales Nayarit. Secretaría de Medio Ambiente y Recursos Naturales. 2013. p. 204. Available online: https://simec.conanp.gob.mx/pdf_libro_pm/77_libro_pm.pdf (accessed on 1 May 2025).
- Valdez-Hernández, J.I. Aprovechamiento Forestal de Manglares en el ESTADO de Nayarit, Costa Pacífica de México; Número Especial; Madera y Bosques: Veracruz, México, 2002; pp. 129–145. [Google Scholar]
- Brokaw, N.; Thompson, J. The H for the DBH. Forest Ecol. Manag. 2000, 129, 89–91. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Wiley & Sons: Hoboken, NJ, USA, 1974; p. 24. [Google Scholar]
- Shaeffer-Novelli, Y.; Cintrón, G. Guía Para Estudo de Áreas de Manguezal. Estrutura, função e Flora; Caribbean Ecological Research: São Paulo, Brazil, 1986. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An upland forest continuum in the prairie-forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San José, Costa Rica, 1967; p. 206. [Google Scholar]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Versión 8.0. 2007. Available online: www.statsoft.com (accessed on 10 April 2023).
- De Santana-Lopes, D.M.; Chaves, F.; Tognella, M. Mangrove Mortality: Analyses of Natural and Anthropic Causes and Their Effects on Forest Dynamics. J. Coast. Res. 2020, 95, 102–107. [Google Scholar] [CrossRef]
- De Sousa-Lino, C.; De Paiva-Sales, T.; Oliveira-Alexandre, F.S.; Ferreira, J.M.; Freire De Sousa, D.; Bezerra-Gomes, P.; Falcao do Amaral, J.; Maia, F.; Silveira, E.R.; Goreti Rodrigues de Queiroz, M.; et al. Antioxidant activity of a Cissus verticillata Fraction and Tyramine, its Bioactive Constituent, on Alloxan-Induced Diabetic Rats. Open Pharm. J. 2008, 2, 63–69. [Google Scholar] [CrossRef]
- Drobnik, J.; Barroncas de Oliveira, A. Cissus verticillata (L.) Nicolson and C.E. Jarvis (Vitaceae): Its Identification and usage in the sources from 16th to 19th century. J. Ethnopharm. 2015, 171, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Sáenz, D.; Lazcano, D.; Penilla-Juárez, A. Notes on the Herpetofauna of Western Mexico 10: Herpetofauna of the Protected Natural Area “Estero El Salado” and adjacent areas in Puerto Vallarta, Jalisco, México. Bullet Chic. Herpet. Soc. 2013, 48, 117–119. [Google Scholar]
- Sandoval-Parra, A.M. Diversidad y Cuantificación de Áreas Dañadas por Trepadoras del Ecosistema de Manglar, en el Ejido de los Morrillos, Nayarit. Bachelor’s Thesis, Universidad de Chapingo, Texcoco, Mexico, 2011; p. 74. [Google Scholar]
- Flores-Cárdenas, F. Caracterización Estructural de los Manglares Del Sistema Lagunar Huizache-Caimanero, Sinaloa. Bachelor’s Thesis, Universidad Autónoma de Sinaloa, Facultad de Ciencias del Mar, Mazatlán, Mexico, 2016; p. 82. [Google Scholar]
- Cintron, G.; Schaeffer-Novelli, Y. Methods for studying mangrove structure. In Mangr Ecos: Resear Meth; Snedaker, S.C., Snedaker, J.G., Eds.; UNESCO: Paris, France, 1984; pp. 91–113. [Google Scholar]
- Velázquez-Pérez, C.; Tovilla-Hernández, C.; Romero-Berny, E.I.; Navarrete, A.J. Estructura del manglar y su influencia en el almacén de carbono en la Reserva La Encrucijada, Chiapas, México. Madera Bosques 2019, 25, 14. [Google Scholar] [CrossRef]
- Flores-Cárdenas, F.; Hurtado-Oliva, M.A.; Doyle, T.W.; Nieves-Soto, M.; Díaz-Castro, S.; Manzano-Sarabia, M.M. Litterfall Production of Mangroves in Huizache-Caimanero Lagoon System, México. J. Coast. Res. 2017, 331, 118–124. [Google Scholar] [CrossRef]
- Torres, J.; Infante-Mata, D.; Sánchez, A.J.; Espinoza-Tenorio, A.; Barba, E. Atributos estructurales, productividad (hojarasca) y fenología del manglar en la Laguna Mecoacán, Golfo de México. Rev. Biol. Trop. 2017, 65, 18. [Google Scholar]
- Pool, D.J.; Snedaker, S.C.; Lugo, A.E. Structure of Mangrove Forests in Florida, Puerto Rico, Mexico, and Costa Rica. Biotropica 1977, 9, 195–212. [Google Scholar] [CrossRef]
- Peñaherrera-Colina, L. Situación actual sobre el uso del glifosato en Ecuador. In Viabilidad del Glifosato en Sistemas Productivos Sustentables; Ríos, A., Ed.; INIAP: Quito, Ecuador, 2013; p. 178. [Google Scholar]
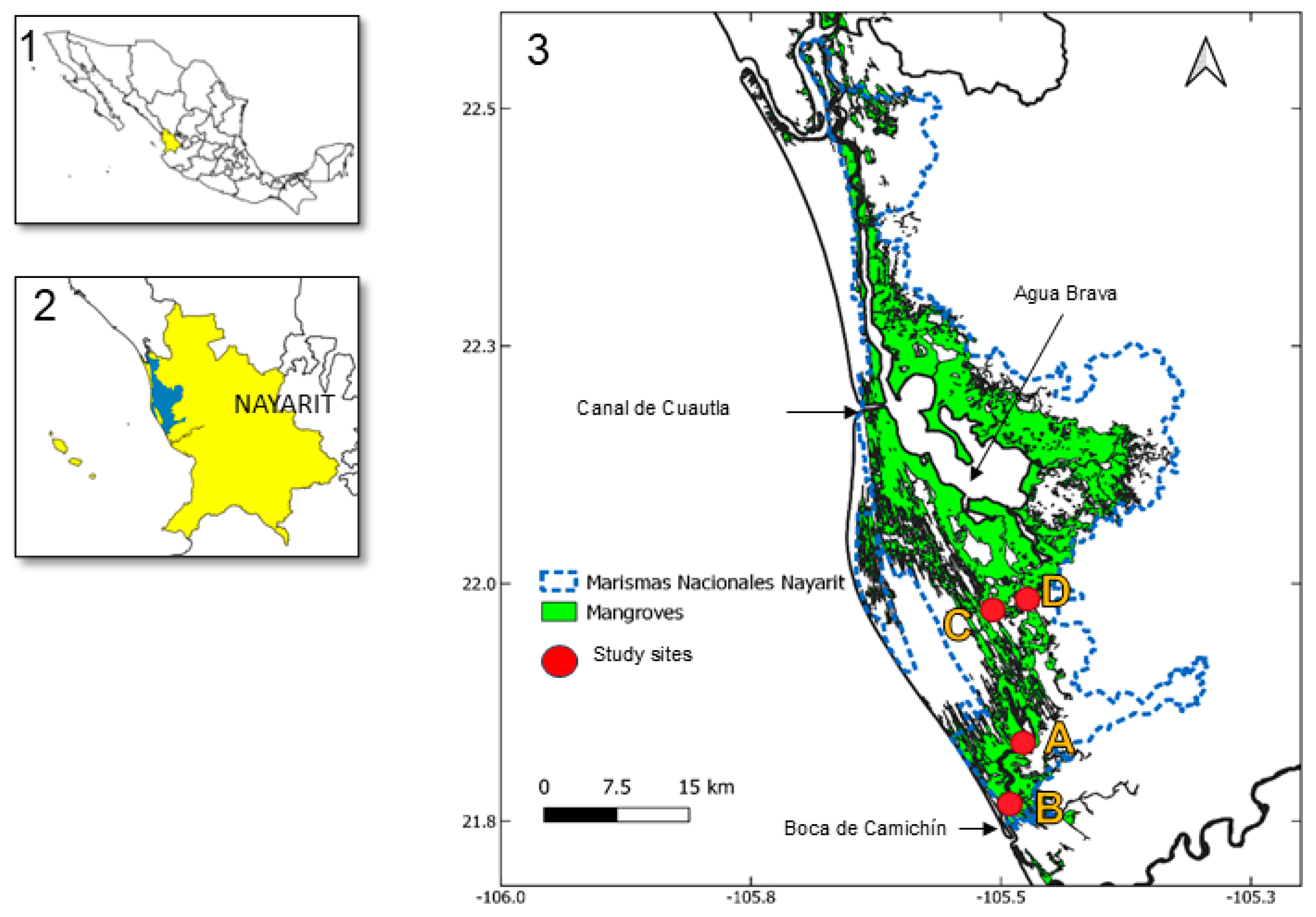

| Plot | Local Name of the Site | Latitude | Longitude | Sampling Dates |
|---|---|---|---|---|
| A | Boca de Camichín | 21.832292 | −105.477975 | 25 March 2019; 14 November 2022 |
| B | 21.768176 | −105.50823 | 26 March 2019; 14 November 2022 | |
| C | Unión de Corrientes | 21.972358 | −105.510564 | 27 March 2019; 18 November 2022 |
| D | 21.983529 | −105.474844 | 28 March 2019; 18 November 2022 |
| Plot | Density (Stems ha−1) | Relative density (%) | |||||||
| Lr | Ag | Rm | Lr | Ag | Rm | NI | |||
| 2019 | 2022 | 2019 | 2022 | ||||||
| A | 2075 | 1875 | 49 | 33 | 18 | 58 | 27 | 15 | 1 |
| B | 2050 | 1690 | 46 | 48 | 6 | 37 | 54 | 9 | 0 |
| C | 660 | 530 | 42 | 58 | 0 | 47 | 53 | 0 | 9 |
| D | 850 | Unrecorded | 65 | 35 | 0 | Unrecorded | |||
| Plot | Species | BA m2 0.1 ha−1 | IVI | BA by Plot m2 0.1 ha−1 | HCI | ||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2022 | 2019 | 2022 | 2019 | 2022 | 2019 | 2022 | ||
| A | Laguncularia racemosa | 0.84 | 1.07 | 140 | 167.5 | ||||
| Avicennia germinans | 1.07 | 0.93 | 118 | 98.4 | 2.02 | 2.09 | 11.39 | 6.48 | |
| Rhizophora mangle | 0.11 | 0.09 | 42 | 34.1 | |||||
| B | Laguncularia racemosa | 0.47 | 0.43 | 120 | 98.1 | 1.83 | 1.80 | 7.16 | 5.3 |
| Avicennia germinans | 1.34 | 1.32 | 167 | 183 | |||||
| Rhizophora mangle | 0.02 | 0.03 | 13 | 18.9 | |||||
| C | Laguncularia racemosa | 0.05 | 0.03 | 117 | 128 | 0.16 | 0.09 | 0.08 | 0.02 |
| Avicennia germinans | 0.11 | 0.06 | 183 | 172 | |||||
| D | Laguncularia racemosa | 0.04 | * | 181 | * | 0.07 | * | 0.04 | * |
| Avicennia germinans | 0.03 | 119 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán-Aguilar, O.; Ordóñez-Rosas, M.L.; Castillo-Cruz, I.; Rodríguez-Arredondo, L.; Ruiz-Domínguez, M.; Hurtado-Oliva, M.Á.; Manzano-Sarabia, M. Nuisance Growth of Cissus verticillata (Vitaceae) Negatively Affects the Structure of Mangroves in Marismas Nacionales Nayarit, Mexico. Diversity 2025, 17, 407. https://doi.org/10.3390/d17060407
Millán-Aguilar O, Ordóñez-Rosas ML, Castillo-Cruz I, Rodríguez-Arredondo L, Ruiz-Domínguez M, Hurtado-Oliva MÁ, Manzano-Sarabia M. Nuisance Growth of Cissus verticillata (Vitaceae) Negatively Affects the Structure of Mangroves in Marismas Nacionales Nayarit, Mexico. Diversity. 2025; 17(6):407. https://doi.org/10.3390/d17060407
Chicago/Turabian StyleMillán-Aguilar, Olivia, María Luisa Ordóñez-Rosas, Isaac Castillo-Cruz, Lidia Rodríguez-Arredondo, Marcelino Ruiz-Domínguez, Miguel Ángel Hurtado-Oliva, and Marlenne Manzano-Sarabia. 2025. "Nuisance Growth of Cissus verticillata (Vitaceae) Negatively Affects the Structure of Mangroves in Marismas Nacionales Nayarit, Mexico" Diversity 17, no. 6: 407. https://doi.org/10.3390/d17060407
APA StyleMillán-Aguilar, O., Ordóñez-Rosas, M. L., Castillo-Cruz, I., Rodríguez-Arredondo, L., Ruiz-Domínguez, M., Hurtado-Oliva, M. Á., & Manzano-Sarabia, M. (2025). Nuisance Growth of Cissus verticillata (Vitaceae) Negatively Affects the Structure of Mangroves in Marismas Nacionales Nayarit, Mexico. Diversity, 17(6), 407. https://doi.org/10.3390/d17060407







