Abstract
This study analyses for the first time the genetic diversity, connectivity, and evolutionary dynamics of the small red scorpionfish (Scorpaena notata) in the Balearic Islands, using two mitochondrial DNA markers: Cytochrome c oxidase subunit I (COI) and the Control Region (CR). Nucleotide diversity of the COI gene was found to be low compared to other commercial fish species, suggesting that fishing may be impacting the population despite being a by-catch species. In contrast, the CR showed higher genetic variability. Demographic history analyses suggest that S. notata underwent a population expansion during the Pleistocene, possibly driven by sea-level changes. Genetic structure analyses (Fst and AMOVA) indicated genetic homogeneity and high connectivity among the Balearic Islands’ population, likely facilitated by its passive dispersion via pelagic eggs and larvae and the oceanographic conditions of the region. Our results suggest that the entire Balearic Islands could be considered as a unique Management Unit, although its potential relation to other nearby areas, such as the Iberian Peninsula, along with the analysis of additional genetic markers, should be addressed in future studies.
1. Introduction
Marine functional connectivity refers to the various movements of marine organisms—both active and passive—that facilitate the transfer of organic matter, genes, and energy, thereby establishing functional links among habitat patches, separate regions, and ecosystems [1]. In this sense, patterns of intraspecific genetic diversity are key to understanding biodiversity, evolutionary and ecological processes, but knowledge of the distribution and drivers of genetic diversity remain limited [2]. The geographical distribution and population connectivity of many marine species are shaped by their dispersal potential and gene flow, which depend on both passive larval transport and active adult migration, e.g., [3,4,5]. Many marine fishes undergo a planktonic larval phase that takes place in the open water, separated from their adult habitat, which offers great potential for widespread dispersal [6,7].
Molecular DNA markers have been used to assess population connectivity and intraspecific genetic diversity of marine fishes, e.g., [8,9,10]. In particular, mitochondrial DNA (mtDNA) markers have been widely used as a robust indicator of population structure analysis, both for coding genes and the D-loop or Control Region (CR). The use of mitochondrial markers combined with a Bayesian approach [11] and coalescent theory [12,13] have also allowed us to infer the reconstruction of the historical demography over time of various marine species, e.g., [14], and thus provide insight into the effects of past climate changes [15]. These genetic approaches have provided insights into the dispersal patterns of marine fish larvae, the influence of oceanographic barriers, and the extent of connectivity among populations, e.g., [4,5]. Genetic findings have also improved fisheries management, primarily for the fisheries’ stock identification and delineation, which provide crucial information strategies by revealing whether populations are self-sustaining or reliant on external recruitment [16,17].
Scorpaenidae is among the most important fish families, in terms of biomass, in some littoral Mediterranean communities, such as in rocky areas and Posidonia oceanica beds [18], and are considered to play an important role in the rocky-reef ecosystems of tropical and temperate seas worldwide [19,20]. Given the prevalence of these habitats in numerous Mediterranean marine protected areas, rockfish populations could be considered good indicators of the condition of these marine reserves [21]. Our study species, the small red scorpionfish, Scorpaena notata, is a small-size species of the family Scorpaenidae present in the eastern Atlantic, from the Bay of Biscay to Senegal, Madeira, the Azores and the Canary Islands, and the Mediterranean and Black Sea [22]. In the Western Mediterranean, this species typically inhabits coastal waters, in caves, seagrass meadows and red algae bottoms, from the surface down to 150 m depth [18,23]. It is an oviparous species with external fertilization and its eggs are embedded in a gelatinous matrix [24].
In the Mediterranean, S. notata is a by-catch species caught with trammel nets and bottom trawling [25]. Within the Scorpaenidae family, S. notata is among the most abundant species in the infra- and circa-littoral communities, particularly in red algae sedimentary bottoms [18,23].
Although some biological studies have been performed on S. notata ([25] and references therein), nothing is known about its population genetics. Therefore, the main objective of the present study is to estimate the genetic diversity, connectivity and demographic history of the small red scorpionfish using two mitochondrial fragments, Control Region and Cytochrome Oxidase subunit I (COI), at a small spatial scale in the Balearic Islands (Western Mediterranean), an area with complex oceanographic conditions.
2. Materials and Methods
2.1. Study Area
The Balearic Islands are the most distant insular area in the Western Mediterranean, separated from the Iberian Peninsula by a minimum distance of 95 nautical miles and with depths ranging from 800 to almost 2000 m [26]. The Balearic Islands are a natural boundary between the Algerian sub-basin in the south, and the Balearic sub-basin in the north. These sub-basins are characterised by different oceanographic conditions [27] and are connected by a series of channels that play an important role in regional circulation, as passages for the exchange of water masses between them. The Algerian sub-basin receives warmer and fresher surface water from the Atlantic, whereas the Balearic sub-basin is characterised by colder and more saline waters that have remained for a longer period in the Mediterranean. The Balearic Islands are characterised by a high oligotrophy [28], which is somehow mitigated in the Balearic sub-basin by the influence of waters generated in the more productive area of the Gulf of Lions [29]. This contrasting oceanographic setting between the north and the south of the Balearic Islands has given rise to a complex oceanographic scenario, which has been reported to influence the population dynamics of both coastal and deep-water demersal resources in such key attributes as recruitment, spawning and body condition [30,31,32,33,34,35].
2.2. Sampling
The fin clip samples of Scorpaena notata were collected during the MEDITS (International bottom trawl survey in the Mediterranean) and CANAL (Menorca Channel) scientific surveys carried out throughout the Balearic Islands during the period 2021–2023 (Table S1). In these surveys, the experimental bottom trawl GOC-73 was used to sample demersal communities and resources of the exploited bottoms (for specific information, see [36]). A total of 90 samples were collected from 20 stations grouped in 6 location sites (or geographic regions) across the Balearic Islands (Figure 1 and Table S1), and these were preserved in 96% ethanol.
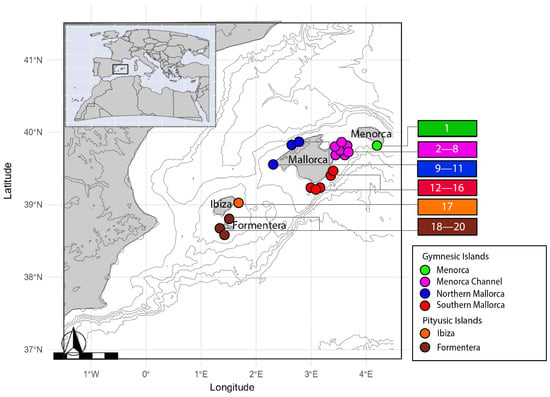
Figure 1.
Map of the sampling stations and location sites for Scorpaena notata in the Balearic Islands. Circles represent stations and colours the location sites used in this study. Colours are consistent in all figures. Sample sizes, coordinates and details of each sampling station are described in Table S1.
2.3. DNA Extraction, Amplification and Sequencing
Total genomic DNA was extracted with the DNeasy Blood and Tissue Extraction kit (Qiagen, West Sussex, UK) following the manufacturer’s instructions. Two mitochondrial fragments were amplified: Cytochrome c Oxidase subunit I gene using the primers FF2d/FR1d [37], and the Control Region with primers designed by Ostellari et al. [38]. However, this last set of primers did not amplify adequately due to the presence of double bands or a non-reproducible banding pattern (Figure S1). Because of this, we designed internal primers based on the Ostellari primers using the Primer3 InPut program (https://primer3.ut.ee/, accessed on 10 May 2023). Primers used in this study are included in Table S2.
PCR conditions consisted of 35 cycles after initial denaturalization at 94 °C for 2 min. The cycles comprised 30 s at 94 °C, 1 min at 51 °C (COI) or 56 °C (CR), and 1 min at 72 °C, followed by a final extension at 72 °C for ten min. The amplification products were purified using the MicroCLEAN kit (Microzone, UK) and sent to the external service provider MACROGEN (Madrid, Spain) for Sanger sequencing. To ensure accuracy, both forward and reverse strands were sequenced.
MEGA-X [39] was employed to edit and align the obtained sequences, using the ClustalW method, and checked for quality. Forward and reverse sequences were aligned and analysed to generate an individual consensus sequence for each sample. All sequences were deposited in GenBank (accession numbers are indicated in Table S1).
2.4. Data Analyses
Samples were grouped into six location sites from the Balearic Islands (BA): Menorca (MEN), Menorca Channel (MCH), Northern Mallorca (NML) and Southern Mallorca (SML), from the Gymnesic Islands (GI); and Ibiza (IBI) and Formentera (FOR) from the Pityusic Islands (PI) (Figure 1).
Genetic diversity parameters, such as the number of polymorphic sites (S), number of haplotypes (h), haplotype (Hd) and nucleotide (π) diversity and respective standard deviations, and mean nucleotide difference (k) were calculated for each location site and for all populations using the DnaSP v6 program [40] and Arlequin v3.5.2.2 [41].
Haplotype networks were constructed using the Median-Joining Network analysis implemented in PopArt 1.7 [42] for all the haplotypes detected for both mtDNA fragments and for the concatenated sequence. These networks aimed to obtain the genetic connectivity of S. notata in the regional area of the Balearic Islands.
To assess the genetic connectivity and the population structure, two analyses were carried out, an Analysis of Molecular Variance (AMOVA [43]) and the fixation index Fst [44], using Arlequin.
Previously to the analyses, the most adequate evolutionary models for the data were selected using IQ-tree2 [45]. A Bayesian coalescent approach implemented in MIGRATE version 2.0.6 [46] was used to assess migration rates between different regions within the Balearic Islands. The analysis was based on Bayesian inference, estimating population size and gene flow with an exponential distribution, assuming a constant mutation rate under the HKY evolutionary model, and utilizing a long Markov chain configuration. The analysis ran for 10 million generations and 0.1% of the initial generations were discarded due to burn-in.
To investigate the demographic history of the studied species in the Balearic Islands, neutrality tests, including Tajima’s D and Fu’s statistics and mismatch distribution, were analyzed with the DnaSP 6 program. In addition, a Multilocus Coalescent Extended Bayesian Skyline Plot (EBSP) implemented in BEAST v.2.7.6 [47] was performed for the entire population using the concatenated fragment of COI and CR sequences. To construct the posterior distribution of effective population size over time, a standard Markov Chain Monte Carlo (MCMC) sampling procedure was used assuming a single panmictic population. For this analysis, parameters and priors were configured in BEAUti 2 [48] and we specified a HKY substitution model with empirical frequencies and a strict molecular clock. Regarding the choice of the mutation rate, we considered the following: (1) the unavailability of substitution rate for COI and for CR for Scorpaenidae and (2) the differing mutation rates between mtDNA regions [49]. In view of these constraints, we chose distinct clock rates for each locus. For COI, a range of three molecular rates was used (2.3%, 4% and 6.6% per million years) and two for CR (5% and 10%), following the mutation rates described in other marine species ([50,51] respectively). The analyses were run for 20 million generations, sampling every 1000 and 10% burn-in of the samples. Tracer v 1.7.2 [52] was then used to analyse the runs and RStudio v2022.12 + 0.353 [53] to generate the skyline plots.
3. Results
3.1. Genetic Diversity
A concatenated fragment of 832 bp of mtDNA (COI: 554 bp; CR: 278 bp) was sequenced from 90 individuals of S. notata from the six location sites throughout the Balearic Islands (Figure 1). All sequences were deposited in GenBank database; accession numbers are given in Table S1 (PQ333157–PQ333216 plus PQ363180-PQ363249 for COI; and PQ468319–PQ468412 for CR).
A total of 101 variable sites were observed in the concatenated fragment. Among the 90 sequences, 85 haplotypes were identified. The mean haplotype diversity and nucleotide diversity for all the samples were 0.999 and 0.011, respectively. Similar diversity values were found in the Gymnesic and Pityusic Islands and also between the six locations. Diversity indices are summarized in Table 1.

Table 1.
Genetic diversity indices estimated from mtDNA data of Scorpaena notata for six location sites in the Balearic Islands.
Regarding the COI fragment, 26 haplotypes were recognized, with a mean haplotype and nucleotide diversity for all the samples of 0.732 and 0.002, respectively. The haplotype diversity of each location ranged from 0.511 (Menorca) to 0.857 (South of Mallorca). Nucleotide diversity of all the locations was low, ranging from 0.001 to 0.003. On the other hand, a much higher genetic diversity was observed for CR. In the 90 sequences, 78 haplotypes were observed, with a mean haplotype and nucleotide diversity of 0.997 and 0.028, respectively. Haplotype diversities in the locations ranged between 0.993 (Ibiza) and 1.0 (Menorca, Menorca Channel, Southern Mallorca and Formentera) and nucleotide diversity varied between 0.024 (Menorca Channel) and 0.032 (Formentera) (Table 1).
3.2. Genetic Differentiation and Connectivity
The obtained haplotype networks are included in Figure 2. The haplotype networks for the concatenated (Figure 2c) and CR (Figure 2b) fragment presented a high number of haplotypes, 85 and 78, respectively. Both networks revealed a hyper-diverse bush-like pattern, with a large number of singletons (84.6% and 94.1%, respectively). In contrast, the COI fragment network (Figure 2a) showed a star-like distribution, where a total of 26 haplotypes were observed, including 19 singletons and 7 shared haplotypes, one of which was very common, being shared by 45 (50%) of the specimens and all the location sites, suggesting a genetic homogeneity in the area studied. The network (Figure S2), which includes other Mediterranean S. notata samples, also exhibited no geographical pattern.
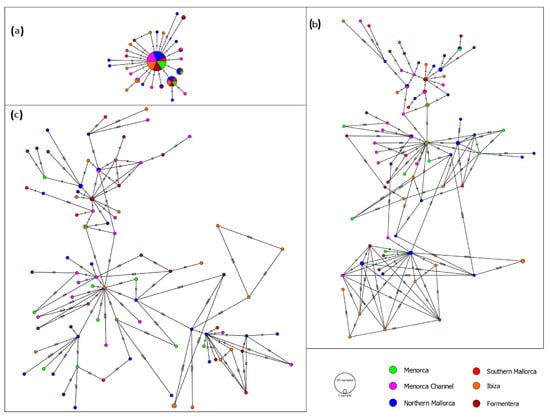
Figure 2.
Median joining networks of haplotypes constructed with mitochondrial sequences from (a) the COI (554 pb), (b) Control Region (278 bp) and (c) concatenated mtDNA sequences (832 pb), represented by location sites. Circle sizes are proportional to the number of sequences per haplotype. Distances are proportional to mutation numbers between haplotypes.
The overall coefficient of genetic differentiation was not significant (p = 0.41011 ± 0.03167) for the combined data set. All pair-wise Fst values were also low and non-significant (Table S3). The pair-wise population genetic differentiation in the concatenated fragment was similar to both the individual genes when analyzed separately. Therefore, these results also reveal genetic homogeneity among the S. notata location sites from the Balearic Islands.
A three-way AMOVA was performed, considering two groups, i.e., the Gymnesic and Pityusic Islands. This hierarchical analysis showed that the variation occurs primarily within location sites (99.78%), rather than between them or between groups of islands, with a low and non-significant Fst value (Fst = 0.00224, p = 0.21017) (Table 2), confirming the lack of genetic structure of S. notata individuals throughout the Balearic Islands. In agreement, the concatenated mtDNA data showed high connectivity, with elevated values of the number of migrant individuals per generation, between all location sites (Table S3). It is noteworthy that migrant events are slightly higher from the Pityusic Islands (Ibiza and Formentera), and that North of Mallorca and the Menorca Channel are the areas that receive more migrant individuals.

Table 2.
Analysis of molecular variance (AMOVA) among groups (Gymnesic and Pityusic Islands) and among and within location sites for the mitochondrial concatenated fragment of Scorpaena notata.
3.3. Population History
The mismatch distribution of concatenated sequences suggests a demographic expansion of the S. notata population (Figure 3a). This expansion was further supported by the significantly negative values of Fu’s Fs (−121.701; p < 0.001) and Tajima’s D (−1.963; p < 0.05) values (Table 3), which failed to reject the null hypothesis of constant population size. Additionally, the Bayesian Skyline Plot also showed evidence of demographic expansion. All distinct mutation rates suggest that the expansion dated to the Late Pleistocene (Figure S3). The mutation rates of 2.3% for COI and 5 % for CR (Figure 3b) suggest a scenario where the expansion may be attributed to an interglacial warmer climate (130,000–110,000 ya) and not with a glacial period, suggesting that these mutation rates might be more consistent with present data. Moreover, the BSP analysis showed that S. notata population had a stable growth rate, not showing significant population dynamics during the past 20,000 years (Figure S4).
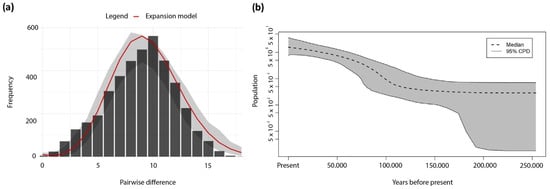
Figure 3.
Historical demographic analyses. (a) Mismatch distributions of concatenated mtDNA sequences (832 bp) of Scorpaena notata. The red line is the expected distribution calculated for the assumption of a demographically expanding population; grey area indicates up and low bound; and grey bars indicate the observed frequencies of pairwise differences of nucleotides among haplotypes. (b) Bayesian skyline plot reconstructions of changes in effective population size through time of S. notata populations around the Balearic Islands using the mitochondrial concatenated sequences. Mutation rates: 2.3% for COI and 5% for CR. Time on the x-axis is in years.

Table 3.
Neutrality tests (Tajima’s D and Fu’s statistics) for Scorpaena notata population (N = 90) in the Balearic Islands.
4. Discussion
In the present study, the genetic diversity, connectivity and demographic history of the small red scorpionfish Scorpaena notata were assessed for the first time in the Balearic Islands. This species is a significant by-catch species of the commercial trawlers working on the shallow continental shelf off the Balearic Islands and is the most common scorpaenid species at these depths [54]. It is worth noting that scorpionfishes play an important ecological role in coastal rocky-reef communities, beyond their relevance to fisheries [19,20]. Therefore, their populations should be closely monitored and properly managed—not only due to their importance to small-scale fisheries, but also because of their potential as indicators of the environmental health of coastal rocky-reef ecosystems [21].
In particular, the overall nucleotide diversity for COI fragment (π: 0.0021) was recorded, and it was found to be lower than that reported for commercially exploited fish species sampled and analysed in the Balearic Islands [55,56] (Table 4). Although the comparison should be made with caution, as the mutation rate may vary among genera, these results suggest that the population of S. notata in the Balearic Islands could be affected by the impact of fishing, despite being a by-catch species. Population declines might lead to a decrease in genetic diversity, which could also be influenced by environmental variables and life-history traits. It has been proposed that the loss of genetic diversity in mitochondrial genomes due to population declines correlates with the loss of genetic diversity in the nuclear genome, and thus can serve as indicator of the conservation status of fish species populations, e.g., [57,58]. Similarly, other by-catch fish species in the area were assessed as overexploited, some even at higher levels than that of M. surmuletus, which was one of the most targeted fish species in the Balearic Islands [59] before its recent recovery [60]. This recovery is probably the result of the implementation of the multiannual plan for fisheries exploiting demersal stocks in the Western Mediterranean Sea (Regulation EU 2019/1022 of the European Parliament and of the Council of 20 June 2019). In contrast, the overall nucleotide diversity of CR fragment (π: 0.0276) was slightly higher than that recorded in the littoral co-generic species S. maderensis from the Mediterranean Sea (π: 0.0258) [10], which is rarely present in the catches of Mediterranean fisheries.

Table 4.
Biological information, assessment and genetic diversity (based on COI fragment) of fish species distributed in the Balearic Islands.
A contrasting pattern with regard to genetic variability was found when comparing both mtDNA markers, COI and CR. While COI showed low values for genetic diversity, the CR recorded high values (Table 1). This genetic hypervariability in CR is comparable to results in S. maderensis [10] and, although documented in populations of other fish genera, e.g., [62,63], is uncommon in rocky coastal species [10]. Differences in variability between COI and CR can be due to the well-known evolutionary rate variations in different parts of the mtDNA molecular system [49] as well as evolutionary rate variation between lineages [64], life history traits (e.g., generation-time or self-recruitment [10,55,65]), or demographic or selective factors, (e.g., population size, bottlenecks or expansion events [55,66]).
Regarding genetic structure, genetic population analyses (Fst and AMOVA) suggest a genetic homogeneity and high connectivity between the location sites in the Balearic Islands. These results were supported by haplotype networks and MIGRATE analysis, which showed no evidence for genetic structure; therefore, the small red scorpionfish is not composed of discernible genetic groups within the Balearic Islands region. Other studies on the small scale connectivity of fish species found similar results. Schunter et al. [67] studied genetic connectivity and geographic fragmentation for the marine fish Serranus cabrilla, a serranid fish, following a fine sampling pattern along the Mediterranean. These authors did not find any population differentiation between the Balearic Islands (Formentera vs Menorca vs Mallorca), but they did find differences when comparing the Balearic Islands with the Central Mediterranean and the South Spanish coast. In the case of the dusky grouper Epinephelus marginatus, another serranid species, no genetic differentiation was observed between the Balearic Islands, nor between the majority of other Mediterranean populations studied [68]. In a genetic study of the black scorpionfish, Scorpaena porcus, in the Mediterranean and Black Seas, no small-scale population differences were observed, but large-scale differences were detected [69]. However, other studies in marine fish species suggest a complex population structure along the western Mediterranean e.g., [67,70], therefore, a possible population difference cannot be ruled out when comparing the Balearic Islands with other Mediterranean regions. Although COI sequences of S. notata indicate a shared genetic composition across the Mediterranean and even on the Atlantic coast of Portugal (Figure S2), further research is needed to confirm these findings.
The high connectivity observed in the present results suggests that S. notata is a species experiencing constant events of exchange of individuals in the continental shelf of the Balearic Islands. In marine species, these events generally occur in pelagic stages during the larval phase [71]. In the planktonic larval phase, the larvae are capable of broad dispersal, this being the main vector of genetic exchange [6,71]. Currently, there are no data on larval duration of S. notata, but for its co-generic species, S. porcus and S. guttata, it has been established as 29 and 30 days, respectively [72,73]. This period, if similar in S. notata, would be long enough to ensure passive dispersion for the exchange of individuals at the small spatial scale analyzed. Additionally, the Balearic Islands lie at the transition between the Algerian sub-basin in the south and the Balearic sub-basin in the north, with complex hydrodynamics interconnecting the two sub-basins through the channels between the islands [74] and references therein that can increase the exchange of individuals.
Traditionally, the degree of genetic connectivity has been related to both contemporary and historical factors that, when combined, shape the present-day observed patterns, e.g., [75,76]. The historical demographic analyses carried out here (mismatch distribution, neutrality tests and BSPs) suggest a demographic expansion in the Balearic S. notata population, which, according to BSPs analysis, could have occurred in the Late Pleistocene rather than in the Holocene, as is commonly observed in marine species [50,69,77,78,79]. The recent stable population dynamics support the hypothesis that this expansion probably took place before the Last Glacial Period, possibly near the last interglacial period (130–110 kya) [80,81]. According to Thunell [82], the Mediterranean showed large-scale temperature oscillations at a long time-series during the Pleistocene. This result contrasts with reported expansions for Scorpaena porcus in the Black and Adriatic Seas [69] in 17.5–20 kya and for other marine species in the Holocene epoch (17.5–20 Kya), during the warming period following the Last Glacial Maximum (LGM), e.g., [69,77,78,79]. Fossil evidence shows that Scorpaena notata has been present in the Mediterranean since the early and mid-Pliocene [83], surviving multiple glaciations. Distinct salinity and temperature conditions between Western and Eastern Mediterranean during the Last Glacial Maximum [84] could influence the differences between the expansion time described in S. notata and S. porcus. Moreover, calibration time might not give an accurate date due to the fact that the molecular clock has not been precisely determined for Scorpaeniformes or for marine fish in general [51,69] and, taking into account that mutation rates vary considerably among genes and taxa [85,86,87], the molecular clock selection in different studies can significantly affect results, particularly with highly variable regions like the CR. This may lead to anomalous or uncertain growth times [88] and obscure historical and demographic events further back in time.
5. Conclusions
In conclusion, here we studied for the first time the genetic diversity, connectivity, and evolutionary dynamics of the small red scorpionfish (Scorpaena notata). Our results suggest a genetic homogeneity and high connectivity between location sites in the Balearic Islands. This is most likely due to the pelagic larval phase and the complex oceanographic conditions in the study area. On the other hand, demographic analyses indicate the occurrence of past population expansion, likely influenced by Pleistocene sea-level changes. The results of this study suggest that the entire Balearic Islands could be considered as a unique Management Unit for S. notata.
However, it is important to keep in mind that mitochondrial DNA is maternally inherited and therefore reflects only maternal lineages and, although multiple fragments may be analyzed, it still represents a single, non-recombinant genetic locus, which limits its resolution. Therefore, to draw more robust conclusions, future research should incorporate biparentally inherited nuclear markers (e.g., microsatellites, SNPs) or genomic data (e.g., ddRAD-seq, GBS). Research using these markers should confirm the relationships with other Mediterranean areas, as suggested by COI, and evaluate the degree of panmixia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17060405/s1.
Author Contributions
Conceptualization, F.O., A.P. and S.R.-A.; methodology, J.R. and A.T.; data analysis, J.R. and S.R.-A.; writing—original draft preparation, A.P.; writing—review and editing, B.G., F.O., A.P. and S.R.-A.; funding acquisition, B.G., F.O. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the MARFISH project (PDR2020/69–ITS 2017-006), included in the Projectes de Recerca Científica i Tecnològica of the Direcció General de Política Universitària i Recerca, which are funded by the Comunitat Autònoma de les Illes Balears through the Direcció Conselleria de Fons Europeus, Universitat i Cultura, with resources from the Tourist Stay Tax Law. The MEDITS surveys are co-funded by the European Union through the European Maritime Fisheries and Aquaculture Fund (EMFAF) within the National Program of collection, management, and use of data in the fisheries sector and support for scientific advice regarding the Common Fisheries Policy. The CANAL surveys were part of the SosMed project (Improvement in scientific and technical knowledge for the sustainability of demersal fisheries in the western Mediterranean), funded by the European Union—Next Generation (Recovery, Transformation and Resilience Plan), with an agreement between the Ministry of Agriculture, Fisheries and Food and the Spanish National Research Council, through the Spanish Institute of Oceanography.
Institutional Review Board Statement
The sampling scheme followed a standardized protocol (MEDITS Handbook. MEDITS Working Group, ninth version; 2017; p. 106) approved by international authorities (EU/DG Mare, FAO/GFCM). If a live specimen of a rare species or a species subject to conservation measures were caught, it was quickly sampled (4–5 min) and returned to the sea unharmed, giving it a chance of survival, following recommendation GFCM/36/2012/3 (http://www.gfcmonline.org/decisions/, accessed on 8 August 2024) on fisheries management measures for conservation of sharks and rays in the GFCM area.
Data Availability Statement
All sequences were deposited in GenBank database; accession numbers are available in the Supplementary file.
Acknowledgments
The authors would like to thank all the participants in the MEDITS and CANAL surveys, as well as the crew of R/V Miguel Oliver, R/V Ángeles Alvariño and R/V Ramón Margalef.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMOVA | Analysis of Molecular Variance |
| BA | Balearic Islands |
| BSP | Bayesian Skyline Plot |
| CANAL | Menorca Channel scientific survey |
| COI | Cytochrome c oxidase subunit I |
| CRddRAD-seq | Control RegionDouble Digest Restriction-site Associated DNA Sequencing |
| EBSP | Extended Bayesian Skyline Plot |
| FOR | Formentera |
| FstGBS | Fixation IndexGenotyping-by-Sequencing |
| GI | Gymnesic Islands |
| IBI | Ibiza |
| LGM | Last Glacial Maximum |
| MCH | Menorca Channel |
| MCMC | Markov Chain Monte Carlo |
| MEDITS | Mediterranean International bottom trawl survey |
| MEN | Menorca |
| mtDNA | mitochondrial DNA |
| NML | Northern Mallorca |
| SMLSNP | Southern MallorcaSingle Nucleotide Polymorphism |
References
- Darnaude, A.M.; Arnaud-Haond, S.; Hunter, E.; Gaggiotti, O.; Sturrock, A.; Beger, M.; Volckaert, F.A.M.; Pérez-Ruzafa, A.; López-López, L.; Tanner, S.E.; et al. Unifying approaches to Functional Marine Connectivity for improved marine resource management: The European SEA-UNICORN COST Action. Res. Ideas Outcomes 2022, 8, e80223. [Google Scholar] [CrossRef]
- Fragkopoulou, E.; Serrão, E.A.; De Clerck, O.; Assis, J. Global intraspecific diversity of marine forests of brown macroalgae predicted by past climate conditions. Commun. Biol. 2025, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, K.A.; Toonen, R.J. Marine connectivity: A new look at pelagic larval duration and genetic metrics of dispersal. Mar. Ecol. Prog. 2011, 436, 291–305. [Google Scholar] [CrossRef]
- Pascual, M.; Rives, B.; Schunter, C.; Macpherson, E. Impact of life history traits on gene flow: A multispecies systematic review across oceanographic barriers in the Mediterranean Sea. PLoS ONE 2017, 12, e0176419. [Google Scholar] [CrossRef]
- Quattrocchi, F.; Fiorentino, F.; Gargano, F.; Garofalo, G. The role of larval transport on recruitment dynamics of red mullet (Mullus barbatus) in the Central Mediterranean Sea. Mar. Environ. Res. 2024, 202, 106814. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.P.; Aschan, M.; Rasmussen, T.; Tande, K.S.; Slagstad, D. Larval dispersal and mother populations of Pandalus borealis investigated by a Lagrangian particle-tracking model. Fish. Res. 2003, 65, 173–190. [Google Scholar] [CrossRef]
- Basterretxea, G.; Jordi, A.; Catalan, I.A.; Sabatés, A.N.A. Model-based assessment of local-scale fish larval connectivity in a network of marine protected areas. Fish. Oceanogr. 2012, 21, 291–306. [Google Scholar] [CrossRef]
- Habib, A.; Ahmad, N.A.H.; Ariffin, N.A.; Seah, Y.G.; Alam, M.M.M.; Jaafar, T.N.A.M.; Fadli, N.; Nor, S.A.M.; Rahman, M.M. Mitochondrial control region sequences show high genetic connectivity in the brownstripe snapper, Lutjanus vitta (Quoy and Gaimard, 1824) from the east coast of Peninsular Malaysia. J. Genet. 2024, 103, 35. [Google Scholar] [CrossRef]
- Hadi, S.; Andayani, N.; Muttaqin, E.; Simeon, B.M.; Ichsan, M.; Subhan, B.; Madduppa, H. Genetic connectivity of the scalloped hammerhead shark Sphyrna lewini across Indonesia and the Western Indian Ocean. PLoS ONE 2020, 15, e0230763. [Google Scholar] [CrossRef]
- Francisco, S.M.; Castilho, R.; Lima, C.S.; Almada, F.; Rodrigues, F.; Šanda, R.; Vudić, J.; Pappalardo, A.M.; Ferrito, V.; Robalo, J.I. Genetic hypervariability of a Northeastern Atlantic venomous rockfish. PeerJ 2021, 9, e11730. [Google Scholar] [CrossRef]
- Beaumont, M.A. Approximate Bayesian computation in evolution and ecology. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 379–406. [Google Scholar] [CrossRef]
- Csillery, K.; Blum, M.G.B.; Gaggiotti, O.E.; Francois, O. Approximate Bayesian Computation (ABC) in practice. Trends Ecol. Evol. 2010, 25, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.C.; Roland, K.; Calves, I.; Austerlitz, F.; Friso, F.P.; Tolley, K.A.; Ryan, S.; Ferreira, M.; Jauniaux, T.; Llavona, A.; et al. Postglacial climate changes and rise of three ecotypes of harbour porpoises, Phocoena phocoena, in western Palearctic waters. Mol. Ecol. 2014, 23, 3306–3321. [Google Scholar] [CrossRef] [PubMed]
- Boissin, E.; Hurley, B.; Wingfield, M.J.; Vasaitis, R.; Stenlid, J.; Davis, C.; De Groot, P.; Ahumada, R.; Carnegie, A.; Goldarazena, A.; et al. Retracing the routes of introduction of invasive species: The case of the Sirex noctilio woodwasp. Mol. Ecol. 2012, 21, 5728–5744. [Google Scholar] [CrossRef] [PubMed]
- Pauls, S.U.; Nowak, C.; Balint, M.; Pfenninger, M. The impact of global climate change on genetic diversity within populations and species. Mol. Ecol. 2013, 22, 925–946. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation dynamics. Nature 1998, 396, 41–49. [Google Scholar] [CrossRef]
- Treml, E.A.; Halpin, P.N. Marine population connectivity identifies ecological neighbors for conservation planning in the Coral Triangle. Conserv. Lett. 2012, 5, 441–449. [Google Scholar] [CrossRef]
- Harmelin-Vivien, M.L.; Kaim-Malka, R.A.; Ledoyer, M.; Jacob-Abraham, S.S. Food partitioning among scorpaenid fishes in Mediterranean seagrass beds. J. Fish Biol. 1989, 34, 715–734. [Google Scholar] [CrossRef]
- Russell, B.C. The food and feeding habits of rocky reef fish of northeastern New-Zealand. New Zeal. J. Mar. Freshw. 1983, 17, 121–145. [Google Scholar] [CrossRef]
- La Mesa, M.; La Mesa, G.; Micalizzi, M. Age and growth of madeira scorpionfish, Scorpaena maderensis Valenciennes, 1833, in the central Mediterranean. Fish. Res. 2005, 74, 265–272. [Google Scholar] [CrossRef]
- Muñoz Frigola, M.; Lloret Romañach, J.; Vila Espuña, S. Effects of artisanal fisheries on the scorpaenids (Scorpaena spp.) reproduction in the marine protected area of Cap de Creus (NW Mediterranean). Fish. Res. 2013, 138, 146–151. [Google Scholar] [CrossRef]
- Hureau, J.C.; Litvinenko, N.I. Scorpaenidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; pp. 1211–1229. [Google Scholar]
- Ordines, F.; Massutí, E. Relationships between macro-epibenthic communities and fish on the shelf grounds of the western Mediterranean. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 370–383. [Google Scholar] [CrossRef]
- Muñoz, M.; Sàbat, M.; Vila, S.; Casadevall, M. Annual reproductive cycle and fecundity of Scorpaena notata (Teleostei, Scorpaenidae). Sci. Mar. 2005, 69, 555–562. [Google Scholar] [CrossRef]
- Ordines, F.; Quetglas, A.; Massutí, E.; Moranta, J. Habitat preferences and life history of the red scorpion fish, Scorpaena notata, in the Mediterranean. Est. Coast. Shelf Sci. 2009, 85, 537–546. [Google Scholar] [CrossRef]
- Acosta, J.; Canals, M.; López-Martınez, J.; Munoz, A.; Herranz, P.; Urgeles, R.; Palomo, C.; Casamor, J.L. The Balearic Promontory Mediterranean international trawl survey (MEDITS). Aquat. Living Resour. 2002, 12, 207–217. [Google Scholar]
- Lehucher, P.M.; Beautier, L.; Chartier, M.; Martel, F.; Mortier, L.; Brehmer, P.; Millot, C.; Alberola, C.; Benzhora, M.; Taupierletage, I.; et al. Progress from 1989 to 1992 in understanding the circulation of the Western Mediterranean Sea. Oceanol. Acta 1995, 18, 255–271. [Google Scholar]
- Bosc, E.; Bricaud, A.; Antoine, D. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Glob. Biogeochem. Cycles 2024, 18, 1–17. [Google Scholar] [CrossRef]
- Monserrat, S.; López-Jurado, J.L.; Marcos, M. A mesoscale index to describe the regional circulation around the Balearic Islands. J. Mar. Syst. 2008, 71, 413–420. [Google Scholar] [CrossRef]
- Guijarro, B.; Fanelli, E.; Moranta, J.; Cartes, J.E.; Massutí, E. Small-scale differences in the distribution and population dynamics of pandalid shrimps in the western Mediterranean in relation to environmental factors. Fish. Res. 2012, 119–120, 33–47. [Google Scholar] [CrossRef]
- Guijarro, B.; Massutí, E.; Moranta, J.; Cartes, J.E. Short spatio-temporal variations in the population dynamics and biology of the deep-water rose shrimp Parapenaeus longirostris (Decapoda: Crustacea) in the western Mediterranean. Sci. Mar. 2009, 73, 183–197. [Google Scholar] [CrossRef]
- Guijarro, B.; Massutí, E.; Moranta, J.; Díaz, P. Population dynamics of the red-shrimp Aristeus antennatus in the Balearic Islands (western Mediterranean): Spatio-temporal differences and influence of environmental factors. J. Mar. Syst. 2008, 71, 385–402. [Google Scholar] [CrossRef]
- Hidalgo, M.; Massutí, E.; Moranta, J.; Cartes, J.E.; Lloret, J.; Oliver, P.; Morales-Nin, B. Seasonal and short spatial patterns in European hake (Merluccius merluccius L.) recruitment process at the Balearic Islands (western Mediterranean): The role of environment on distribution and condition. J. Mar. Syst. 2008, 71, 367–384. [Google Scholar] [CrossRef]
- Massutí, E.; Monserrat, S.; Oliver, P.; Moranta, J.; López-Jurado, J.L.; Marcos, M.; Hidalgo, M.; Guijarro, B.; Carbonell, A.; Pereda, P. The influence of oceanographic scenarios on the population dynamics of demersal resources in the western Mediterranean: Hypothesis for hake and red shrimp of Balearic Islands. J. Mar. Syst. 2008, 71, 421–438. [Google Scholar] [CrossRef]
- Rueda, L.; Moranta, J.; Abelló, P.; Balbín, R.; Barberá, C.; Fernández de Puelles, M.L.; Olivar, M.P.; Ordines, F.; Ramón, M.; Torres, A.P.; et al. Body condition of the deep water demersal resources at two adjacent oligotrophic areas of the western Mediterranean and the influence of the environmental features. J. Mar. Syst. 2014, 138, 194–202. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Ivanova, N.V.; Zemlak, T.S.; Hanner, R.H.; Hebert, P.D.N. Universal primer cocktails for fish DNA barcoding. Mol. Ecol. Notes 2007, 7, 544–548. [Google Scholar] [CrossRef]
- Ostellari, L.; Bargelloni, L.; Penzo, E.; Patarnello, P.; Patarnello, T. Optimization of single-strand conformation polymorphism and sequence analysis of the mitochondrial control region in Pagellus bogaraveo (Sparidae, Teleostei): Rationalized tools in fish population biology. Anim. Genet. 1996, 27, 423–427. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Bio. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D.; Nakagawa, S. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Beerli, P.; Mashayekhi, S.; Sadeghi, M.; Khodaei, M.; Shaw, K. Population genetic inference with MIGRATE. Curr. Protoc. Bioinform. 2019, 68, 87. [Google Scholar] [CrossRef]
- Bouckaert, R.; Heled, J.; Kuhnert, D.; Vaughan, T.; Wu, C.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014, 10, 1–6. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, X.; Xu, S.; Yang, T.; Yanagimoto, T.; Gao, T. Comparative analysis of the complete mitochondrial genomes of three rockfishes (Scorpaeniformes, Sebastiscus) and insights into the phylogenetic relationships of Sebastidae. Biosc. Rep. 2020, 40, BSR20203379. [Google Scholar] [CrossRef]
- Crandall, E.D.; Sbrocco, E.J.; DeBoer, T.S.; Barber, P.H.; Carpenter, K.E. Expansion dating: Calibrating molecular clocks in marine species from expansions onto the Sunda Shelf following the Last Glacial Maximum. Mol. Biol. Evol. 2012, 29, 707–719. [Google Scholar] [CrossRef]
- Gwak, W.S.; Roy, A. Genetic diversity and population structure of brown croaker (Miichthys miiuy) in Korea and China inferred from mtDNA control region. Genes 2023, 14, 1692. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.A.; Xie, D.; Drummond, A.J. Tracer v1.6. 2014. Available online: http://beast.bio.ed.ac.uk/Tracer (accessed on 28 February 2025).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 28 February 2025).
- Massutí, E.; Reñones, O. Demersal resource assemblages in the trawl fishing grounds off the Balearic Islands (western Mediterranean). Sci. Mar. 2005, 69, 167–181. [Google Scholar] [CrossRef]
- Petit-Marty, N.; Liu, M.; Tan, I.Z.; Chung, A.; Terrasa, B.; Guijarro, B.; Ordines, F.; Ramírez-Amaro, S.; Massutí, E.; Schunter, C. Declining Population Sizes and Loss of Genetic Diversity in Commercial Fishes: A Simple Method for a First Diagnostic. Front. Mar. Sci. 2022, 9, 872537. [Google Scholar] [CrossRef]
- Pasini, N.; Riera, J.; Tudurí, A.; Bassitta, M.; Ferragut, J.F.; Picornell, A.; Ramírez-Amaro, S. Estudios Genéticos Como Una Herramienta de Diagnóstico Para Evaluar el Efecto de Las Zonas de Protección Pesquera en el Canal de Menorca Sobre Los Recursos Pesqueros Demersales. In Proceedings of the XIV Reunión del Foro Científico de Pesca Española en el Mediterráneo, Palma, Spain, 20–21 September 2023; Sánchez-Lizaso, J.L., Ed.; Universidad de Alicante: Alicante, Spain, 2024. [Google Scholar]
- Petit-Marty, N.; Vázquez-Luis, M.; Hendriks, I.E. Use of the Nucleotide Diversity in COI Mitochondrial Gene as an Early Diagnostic of Conservation Status of Animal Species. Conserv. Lett. 2021, 14, e12756. [Google Scholar] [CrossRef]
- Ferragut-Perello, F.; Ramírez-Amaro, S.; Tsikliras, A.C.; Petit-Marty, N.; Dimarchopoulou, D.; Massutí, E.; Serrat, A.; Ordines, F. Exploitation and conservation status of the thornback ray (Raja clavata) in the Balearic Islands (western Mediterranean). Fishes 2023, 8, 117. [Google Scholar] [CrossRef]
- Ordines, F.; Farriols, M.T.; Lleonart, J.; Guijarro, B.; Quetglas, A.; Massutí, E. Biology and population dynamics of by-catch fish species of the bottom trawl fishery in the western Mediterranean. Mediterr. Mar. Sci. 2014, 15, 613–625. [Google Scholar] [CrossRef]
- General Fisheries Commission for the Mediterranean (GFCM). Working Group on Stock Assessment of Demersal Species (WGSAD); General Fisheries Commission for the Mediterranean (GFCM): Rome, Italy, 2024. [Google Scholar]
- Farré, M.; Joher, S.; Farriols, M.F.; Ferragut, F.; Pasini, N.; Ramírez, S.; Valls, M.; Guijarro, B.; Massutí, E.; Ordines, F. Informe Campaña MEDITS_ES_2023 (GSA 5E, Eastern Balearic Islands); Centre Oceanogràfic de les Balears, Instituto Español de Oceanografía (IEO-CSIC): Muelle de Poniente, Spain, 2023. [Google Scholar]
- Song, C.Y.; Sun, Z.C.; Gao, T.X.; Song, N. Structure analysis of mitochondrial DNA control region sequences and its applications for the study of population genetic diversity of Acanthogobius ommaturus. Russ. J. Mar. Biol. 2020, 46, 292–301. [Google Scholar] [CrossRef]
- Cunha, D.B.D.; da Martins, K.C.P.; Júnior, J.N.S.; Moreira, E.C.O.; Sampaio, I.; Queiroz, C.C.S.; Leite, M.A.; de Souza, A.A.; dos Santos, C.A.; Vallinoto, M. High genetic diversity detected in the mitochondrial Control Region of the Serra Spanish Mackerel, Scomberomorus brasiliensis (Collette, Russo & Zavala, 1978) along the Brazilian coast. Mitochondrial DNA Part A 2021, 32, 178–185. [Google Scholar]
- Martin, A.P.; Naylor, G.J.P.; Palumbi, S.R. Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature 1992, 357, 153–157. [Google Scholar] [CrossRef]
- Martínez, S.; Carrillo, L.; Marinone, S.G. Potential connectivity between marine protected areas in the Mesoamerican Reef for two species of virtual fish larvae: Lutjanus analis and Epinephelus striatus. Ecol. Indic. 2019, 102, 10–20. [Google Scholar] [CrossRef]
- Hague, M.T.J.; Routman, E.J. Does population size affect genetic diversity? A test with sympatric lizard species. Heredity 2016, 116, 92–98. [Google Scholar] [CrossRef]
- Schunter, C.; Carreras-Carbonell, J.; Macpherson, E.; Tintoré, J.; Vidal-Vijande, E.; Pascual, A.; Guidetti, P.; Pascual, M. Matching genetics with oceanography: Directional gene flow in a Mediterranean fish species. Mol. Ecol. 2011, 20, 5167–5181. [Google Scholar] [CrossRef]
- Schunter, C.; Carreras-Carbonell, J.; Planes, S.; Sala, E.; Ballesteros, E.; Zabala, M.; Harmelin, J.G.; Harmelin-Vivien, M.; Macpherson, E.; Pascual, M. Genetic connectivity patterns in an endangered species: The dusky grouper (Epinephelus marginatus). J. Exp. Mar. Biol. Ecol. 2011, 401, 126–133. [Google Scholar] [CrossRef]
- Boissin, E.; Micu, D.; Janczyszyn-Le, M.G.; Neglia, V.; Bat, L.; Todorova, V.; Panayotova, M.; Kruschel, C.; Macic, V.; Milchakova, N.; et al. Contemporary genetic structure and postglacial demographic history of the black scorpionfish, Scorpaena porcus, in the Mediterranean and the Black Seas. Mol. Ecol. 2016, 25, 2195–2209. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Picornell, A.; Arenas, M.; Castro, J.A.; Massutí, E.; Ramon, M.M.; Terrasa, B. Contrasting evolutionary patterns in populations of demersal sharks throughout the western Mediterranean. Mar. Biol. 2018, 165, 3. [Google Scholar] [CrossRef]
- Galarza, J.A.; Carreras-Carbonell, J.; Macpherson, E.; Pascual, M.; Roques, S.; Turner, G.F.; Rico, C. The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Natl. Acad. Sci. USA 2009, 106, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.H.; Reed, D.C. Conceptual issues relevant to marine harvest refuges: Examples from temperate reef fishes. Can. J. Fish. Aquat. Sci. 1993, 50, 2019–2028. [Google Scholar] [CrossRef]
- Macpherson, E.; Raventós, N. Relationship between pelagic larval duration and geographic distribution of Mediterranean littoral fishes. Mar. Ecol. Prog. Ser. 2006, 327, 257–265. [Google Scholar] [CrossRef]
- López-Jurado, J.L.; Marcos, M.; Monserrat, S. Hydrographic conditions affecting two fishing grounds of Mallorca island (western Mediterranean): During the IDEA project (2003–2004). J. Mar. Syst. 2008, 71, 303–315. [Google Scholar] [CrossRef][Green Version]
- Gaggiotti, O.E.; Bekkevold, D.; Jørgensen, H.B.; Foll, M.; Carvalho, G.R.; Andre, C.; Ruzzante, D.E. Disentangling the effects of evolutionary, demographic, and environmental factors influencing genetic structure of natural populations: Atlantic herring as a case study. Evolution 2009, 63, 2939–2951. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Tanner, S.E.; Aboim, M.A.; Vasconcelos, R.P.; Laroche, J.; Charrier, G.; Pérez, M.; Presa, P.; Gillanders, B.M.; Cabral, H.N. Reconciling differences in natural tags to infer demographic and genetic connectivity in marine fish populations. Sci. Rep. 2018, 8, 10343. [Google Scholar] [CrossRef]
- Hoareau, T.B.; Boissin, E.; Berrebi, P. Evolutionary history of a widespread Indo-Pacific goby: The role of Pleistocene sealevel changes on demographic contraction/expansión dynamics. Mol. Phylogenet. Evol. 2012, 62, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Banguera-Hinestroza, E.; Evans, P.G.H.; Mirimin, L.; Reid, R.J.; Mikkelsen, B.; Couperus, A.S.; Deaville, R.; Rogan, E.; Hoelzel, A.R. Phylogeography and population dynamics of the white-sided dolphin (Lagenorhynchus acutus) in the North Atlantic. Conserv. Genet. 2014, 15, 789–802. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Castilho, R.; Stevens, J.R. Meta-analysis of northeast Atlantic marine taxa shows contrasting phylogeographic patterns following post-LGM expansions. PeerJ 2018, 6, e5684. [Google Scholar] [CrossRef]
- Muhs, D.R. Evidence for the timing and duration of the last interglacial period from high-precision uranium-series ages of corals on tectonically stable coastlines. Quat. Res. 2002, 58, 36–40. [Google Scholar] [CrossRef]
- Dahl-Jensen, D.; Albert, M.R.; Aldahan, A.; Azuma, N.; Balslev-Clausen, D.; Baumgartner, M.; Berggren, A.M.; Bigler, M.; Binder, T.; Blunier, T.; et al. Eemian interglacial reconstructed from a Greenland folded ice core. Nature 2013, 493, 489–494. [Google Scholar]
- Thunell, R.C. Pliocene-Pleistocene paleotemperature and paleosalinity history of the Mediterranean Sea: Results from DSDP Sites 125 and 132. Mar. Micropaleontol. 1979, 4, 173–187. [Google Scholar] [CrossRef]
- Landini, W.; Sorbini, C. Evolutionary dynamics in the fish faunas of the Mediterranean basin during the Plio-Pleistocene. Quat. Int. 2005, 140, 64–89. [Google Scholar] [CrossRef]
- Mikolajewicz, U. Modeling mediterranean ocean climate of the last glacial maximum. Clim. Past. 2011, 7, 161–180. [Google Scholar] [CrossRef]
- Li, W.H.; Tanimura, M. The molecular clock runs more slowly in man than in apes and monkeys. Nature 1987, 326, 93–96. [Google Scholar] [CrossRef]
- Wolfe, K.Y.; Sharp, P.M.; Li, W.H. Mutation rate among regions of mammalian genome. Nature 1989, 337, 283–285. [Google Scholar] [CrossRef]
- Nabholz, B.; Glémin, S.; Galtier, N. The erratic mitochondrial clock: Variations of mutation rate, not population size, affect mtDNA diversity across birds and mammals. BMC Evol. Biol. 2009, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.S.; Liu, M.; Gao, T.; Yanagimoto, T. Limits of Bayesian skyline plot analysis of mtDNA sequences to infer historical demographies in Pacific herring (and other species). Mol. Phylogenet. Evol. 2012, 65, 203–212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).