Abstract
Seven species of Hemiscorpius Peters, 1861 have been recorded in Iran. Due to the medical importance of this genus, the scorpion populations of southern Kerman Province, in the south of Iran, were studied. Scorpion specimens were collected in 2023 and 2024 from mountainous areas in Bam and Jiroft counties. Morphological, morphometric, and molecular data revealed a new species, described herein as Hemiscorpius jiroftensis sp. n. The genetic distances between the new species and other species of Hemiscorpius varied from 0.105 with samples of H. lepturus from Iran to 0.138 with samples of H. enischnochela. The Jebal Barez Mountains appear to have provided a geographical barrier, separating the new species from its closest relative, Hemiscorpius acanthocercus Monod & Lourenço, 2005. It is important to understand the geographical distributions and morphological differences among the species of Hemiscorpius to implement appropriate medical responses to envenomation by these scorpions.
1. Introduction
Genus Hemiscorpius Peters, 1861, currently comprising 18 described species distributed from northeastern Africa, through the Arabian Peninsula, to Iran [1], is the sole representative of family Hemiscorpiidae Pocock, 1893, the sister group of Hormuridae Laurie, 1896 (2,3). Diagnostic characters of this genus include a pentagonal sternum, pedipalps with Type C trichobothrial pattern, lateroapical margins of the leg telotarsi straight, a single ventromedian carina on metasomal segments I–IV, and a double hook on the male hemispermatophore [2,3,4].
Seven species of Hemiscorpius have been recorded in Iran: Hemiscorpius acanthocercus Monod & Lourenço, 2005; Hemiscorpius enischnochela Monod & Lourenço, 2005; Hemiscorpius gaillardi (Vachon, 1974); Hemiscorpius kashkayi Karataş & Moradi-Gharkheloo, 2013; Hemiscorpius lepturus Peters, 1861; Hemiscorpius persicus (Birula, 1903); and Hemiscorpius shahii Kovařík et al., 2017 [4,5].
Due to the medical importance of members of this genus, including the report of a death due to envenomation by H. acanthocercus in Hormozgan Province [6], and the broad distribution of Hemiscorpius in southern Iran [5], populations of this genus were studied in Bam and Jiroft counties of Kerman Province. Kerman Province is situated southwest of Kavir-e Lut, in southern Iran. The lowest point in Kerman Province is 143 m at the southern margin of the Jazmourian lowland and its highest point is at 3884 m in the Bahr-e-Asman and Jebal Barez mountains. The climate of the area is cold and dry in the highlands, and hot and humid to dry in the plains [7].
Hemiscorpius acanthocercus and H. enischnochela were previously reported from Manujan, in southern Kerman [8], whereas only H. acanthocercus was reported from Jiroft County [9].
Morphological, morphometric, and molecular data obtained from scorpion specimens collected in mountainous areas of Bam and Jiroft counties revealed a new species, described herein as Hemiscorpius jiroftensis sp. n.
2. Material and Methods
2.1. Morphology
Scorpions were collected in 2023 and 2024 by searching at night with portable ultraviolet lights [10] at Dehbakri (Bam County) as well as Hishin and Mohammad Abad (Jiroft County) in the Jebal Barez Mountains of Kerman Province (Figure 1). Material was deposited in the Medical Entomology Collection of Jiroft University of Medical Science, Iran (JMU), and the Research Institute of Zabol, Iran (RIZ).
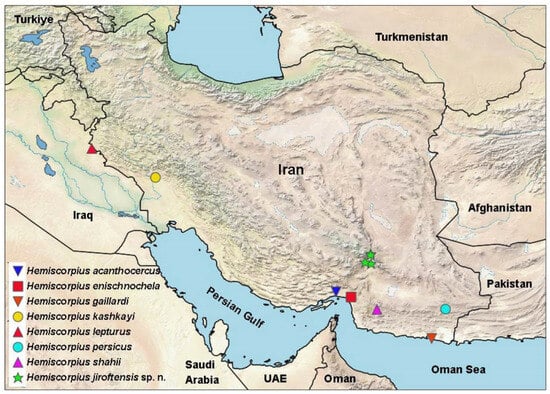
Figure 1.
Geographical distribution of Hemiscorpius jiroftensis sp. n. and type localities of other species of Hemiscorpius Peters, 1861 recorded in Iran.
Material was examined using a Labomed Luxeo 6Z stereomicroscope and measured with Accud (d = 0.01 mm) digital point calipers. Species were identified using keys by Shahi and Barahoei [4] and Barahoei et al. [5]. Morphological terminology follows Vachon [11] for trichobothria, Loria and Prendini [12] for lateral ocelli, González-Santillán and Prendini [13] for pedipalpal and metasomal carinae, and Stahnke [14], Sissom [15], and Prendini [2] for general nomenclature.
2.2. Molecular Systematics
DNA was extracted from up to 25 mg of pedipalp muscle tissue using a FavorPrep™ Tissue Genomic DNA Extraction Mini Kit. The mitochondrial Cytochrome c Oxidase I (COI) gene was PCR amplified using the HCO2198 and LCO1490 primers [16] at a specific temperature cycle [17,18]. PCR products were sent to Niagen Noor Company (Tehran, Iran) for sequencing. The five newly generated COI sequences were analyzed together with eleven sequences—ten Hemiscorpius sequences representing three species, and Scorpio palmatus (Ehrenberg, 1828) as an outgroup—retrieved from GenBank. Maximum Likelihood analysis was conducted with PhyML 3.0 [19] using the TN93+G substitution model, under the Akaike Information Criterion (4823,39612), for 10,000 iterations. Bayesian inference was conducted with MrBayes 3.2.7a [20] on the CIPRES Science Gateway (https://phylo.org accessed on 4 April 2025) for 50,000,000 generations and 5000 burn in. The phylogenetic trees obtained were visualized with FigTree 1.4.4 (https://tree.bio.ed.ac.uk/software/figtree accessed on 4 April 2025), and the final editing conducted using Photoshop 15.2.3.
3. Results
3.1. Molecular Systematics
The new COI gene sequences were 624 bp long, containing 438 conserved (70.19%), 186 variable (29.81%), and 117 parsimony informative (18.75%) sites. The phylogenetic tree obtained from analysis of the sequences (Figure 2) placed H. enischnochela (clade A) as the basalmost species, sister to the other species (clade B). Hemiscorpius lepturus was placed sister to a group comprising H. acanthocercus and H. jiroftensis sp. n. The low bootstrap support for clade B and the group comprising H. acanthocercus and H. jiroftensis sp. n. may be attributed to the relatively small sample of sequences from a single gene locus.
The genetic distances between the new species and other species of Hemiscorpius varied from 0.105 with H. lepturus from Iran to 0.138 with H. enischnochela (Table 1). The genetic distance between the new species and its closest relative, H. acanthocercus, was 0.130.

Table 1.
Genetic distances (average Kimura 2-parameter) in the Cytochrome Oxidase c Subunit I gene among and within (bold) four species of Hemiscorpius Peters, 1861.
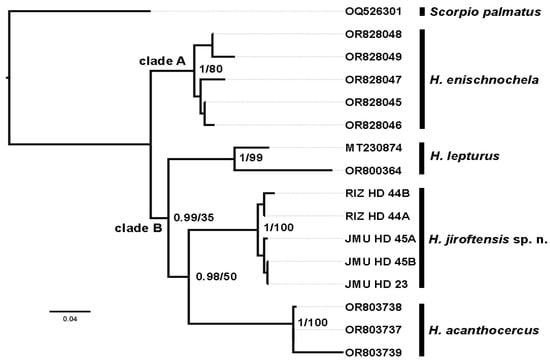
Figure 2.
Phylogeny of four species of Hemiscorpius Peters, 1861 with Scorpio palmatus (Ehrenberg, 1828) as outgroup, based on Maximum Likelihood and Bayesian inference of the Cytochrome Oxidase c Subunit I gene. Posterior probabilities and bootstrap values at nodes.
3.2. Taxonomy
Family Hemiscorpiidae Pocock, 1893
Genus Hemiscorpius Peters 1861
Hemiscorpius jiroftensis sp. n.
urn:lsid:zoobank.org:act:D636E8F7-2479-4ADE-835B-7E5B74F3B27C
Type Material. Holotype ♂ (RIZ HD 44A), IRAN: Kerman Prov.: Jiroft Co.: Hishin village, 28°37′48″ N 57°56′24″ E, 10.ix.2024, H. Dehghan and E. Amiri. Paratypes: IRAN: Kerman Prov.: Bam Co.: Sharik Abad village, 29°04′12″ N 57°55′12″ E, 22.viii.2023, 1 ♂ (JMU HD 23), 1 ♀ (JMU HD 43), 27.viii.2024, 3 ♂ (JMU HD 45A–C). Jiroft Co.: Hishin village, 28°37′48″ N 57°56′24″ E, 10.ix.2024, H. Dehghan and E. Amiri, 1 ♂, 1 ♀ (RIZ HD 44B, C).
Diagnosis. Hemiscorpius jiroftensis sp. n. differs from other species of Hemiscorpius recorded in Iran as follows. The new species differs from H. enischnochela, H. gaillardi, and H. shahii in possessing only three trichobothria on the ventral surface of the pedipalp patella (Figure 5C) unlike the other three species, each of which possess more than ten trichobothria on the ventral surface of the patella. Unlike H. kashkayi and H. persicus, the new species is markedly sexually dimorphic (Figure 3 and Figure 7), possessing an elongated metasoma and a pair of blunt projections posteriorly on the telson vesicle of the male (Figure 7A). The new species differs from H. lepturus in its more granular tegument. For example, the carapace lateral margins bear small spiniform granules ventral to the lateral ocelli (Figure 5A) and the metasomal dorsosubmedian carinae each comprise numerous prominent spiniform granules (Figure 7) in H. jiroftensis sp. n. whereas the carapace lateral margins are smooth and the metasomal dorsosubmedian carinae comprise few, small spiniform granules, especially on the anterior segments, in H. lepturus.
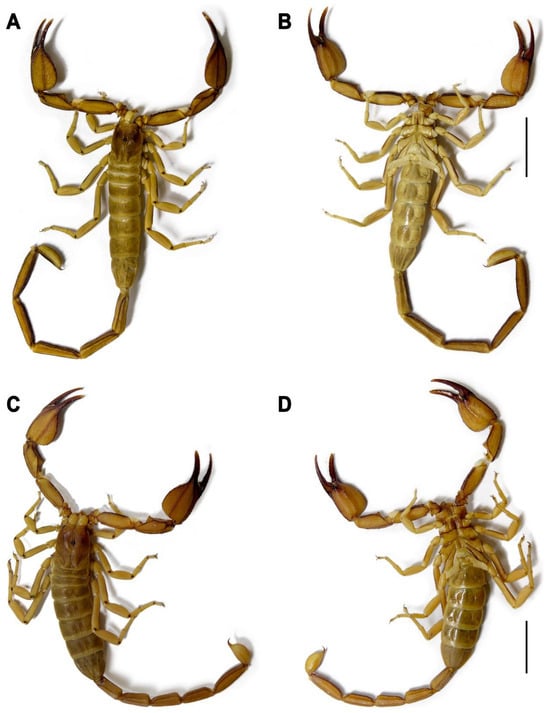
Figure 3.
Hemiscorpius jiroftensis sp. n., habitus, dorsal (A,C) and ventral (B,D) aspects. (A,B). Holotype ♂ (RIZ HD 44A). (C,D). Paratype ♀ (RIZ HD 44C). Scale bars = 10 mm.
Hemiscorpius jiroftensis sp. n. is most closely related to H. acanthocercus, from which it differs as follows. The new species is larger (more than 65 mm in total length in the male and 60 mm in total length in the female; Table 2) than H. acanthocercus (less than 65 mm in total length in the male and 55 mm in total length in the female [4]). Its pedipalp patella is 2.7–2.9 times longer than wide in the male and its pedipalp chela movable finger is longer than the chela manus (Table 2) whereas the pedipalp patella is 2.4–2.6 times longer than wide, and the movable finger shorter than the manus in H. acanthocercus. The new species is also darker in color, with the dorsal surface of the telson vesicle infuscate (Figure 7A) rather than immaculate, as in H. acanthocercus. Whereas metasomal segment V is immaculate in the female of the new species (Figure 3), it is infuscate in the female of H. acanthocercus.

Table 2.
Morphometric data (measurements in mm) for type material of Hemiscorpius jiroftensis sp. n., deposited in the Medical Entomology Collection of Jiroft University of Medical Science, Iran (JMU), and the Research Institute of Zabol, Iran (RIZ).
Etymology. The new species is named after Jiroft County where the type locality is situated. Jiroft (noun) comprises two words, jeer, meaning low, and oft, meaning fallen, and refers to a lowland alluvial plain.
Description. The following description is based on the holotype ♂ (RIZ HD 44A) and paratype ♀ (RIZ HD 44C) (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, Table 2).
Total length: Total length 80.94 mm (♂) or 69.16 mm (♀).
Colour: Base color brown (Figure 3); chelicerae yellow, teeth of fingers light brown; carapace brown, median and lateral ocular tubercles black; pedipalp dark brown, carinae darker, chela brown with reddish brown fingers; legs yellow; tergites light brown; sternites dark yellow; metasomal segments brown, segments IV and V darker than I–III, carinae dark brown to black; dorsal surface of telson vesicle and aculeus black, ventral and lateral surfaces yellow (Figure 3).
Chelicerae: Fixed finger, median and basal teeth bifurcate; movable finger with one subdistal tooth and one basal tooth in external series; distal external tooth smaller than distal internal tooth; cheliceral teeth without secondary serrations.
Carapace: Carapace subrectangular, lateral sides almost parallel; surfaces finely granular with two smooth areas above the lateral eyes, longer than wide; small median ocular tubercle located in anterior half of carapace (Figure 6A), with finely granular superciliary carinae; three pairs of lateral ocelli, posterior ocellus (PDMi) smaller than others (Type 3A); frontal notch semicircular; lateral margins with large spiniform granules ventral to lateral ocular tubercles; anteromedian longitudinal sulcus narrow, suturiform, bifurcated anteriorly, continuous from anterior margin to ocular tubercle; posteromedian sulcus forming deep depression; posterolateral sulcus deep (Figure 6A).
Pedipalps: Femur elongate, 2.86 times longer than wide (Table 2); pentacarinate, with four distinct carinae; prodorsal, retrodorsal, proventral, and retroventral carinae each comprising prominent spiniform granules; ventromedian carina reduced to few granules proximally (Figure 5); dorsal surface finely and densely granular (Figure 5A); prolateral surface with few prominent spiniform granules (Figure 5B); retrolateral surface shagreened; ventral surface shagreened to sparsely granular, becoming smooth distally (Figure 5B).
Patella elongate, 2.98 times longer than wide (Table 2); seven carinae, six distinct (Figure 5); prodorsal carina comprising prominent spiniform granules; retrodorsal carina obsolete, granular; proventral, retroventral, and retromedian carinae granular; dorsal and ventral surfaces sparsely granular (Figure 5); prolateral process pronounced, separated into prodorsal and proventral tubercles, prodorsal tubercle with single spiniform granule.
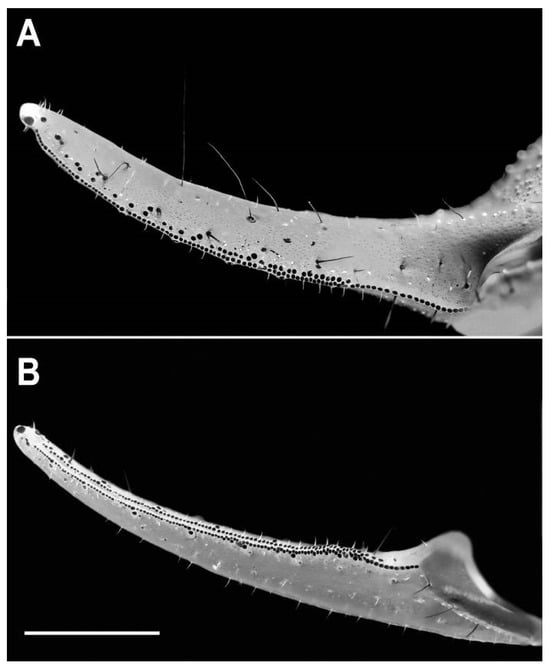
Figure 4.
Hemiscorpius jiroftensis sp. n., holotype ♂ (RIZ HD 44A), pedipalp chela fixed finger, proventral aspect (A) and movable finger, dorsal aspect (B). Scale bar = 2.5 mm.

Figure 5.
Hemiscorpius jiroftensis sp. n., holotype ♂ (RIZ HD 44A), pedipalp dorsal (A), retrolateral (B), and ventral (C) aspects, illustrating trichobothria. Scale bar = 5 mm.
Chela stout, 1.29 times longer than wide (Table 2); five distinct carinae (Figure 5); dorsomedian carina granular; prodorsal carina coarsely granular; retrodorsal carina granular, becoming smooth distally; retromedian carina finely granular; proventral carina comprising spiniform granules; manus surfaces finely and densely granular (Figure 5); fingers, dorsal and ventral surfaces predominantly smooth and finely punctate; movable finger longer than manus (Table 2); fixed finger with proximal notch (Figure 4A); movable finger with shallow median lobe (Figure 4B); movable finger with two rows of denticles, interrupted at regular intervals by seven larger denticles, each with accessory denticle (Figure 4B); fixed finger with retrolateral row complete, prolateral row comprising separate granules merging proximally into row near fifth large denticle (Figure 4B); fingertips each with pronounced terminal hook (Figure 4).
Type C trichobothrial pattern (Figure 5). Femur with three trichobothria: i retroproximal on prolateral surface; d dorsoproximal on dorsal surface; e dorsoproximal on retrolateral surface (Figure 5A). Patella with 19 trichobothria: i dorsal in distal half of prolateral surface; d1 retroproximal to prodorsal carina; d2 situated midway (Figure 5A); five eb trichobothria; two esb and em trichobothria; est trichobothrium near et series (Figure 5B); three et (Figure 5B) and v trichobothria (Figure 5C). Chela manus with 15 trichobothria: Db proximal on dorsal surface; three Eb trichobothria proximal on retrolateral surface; Esb near Eb series (Figure 5B); Est near Et series; five Et trichobothria, Et1 on ventral surface (Figure 5C); four V trichobothria, V3 and V4 in proximal half of manus, V1 and V2 distal (Figure 5C). Fixed finger with 11 trichobothria: Dt proximal on dorsal surface; db dorsal on prolateral surface (Figure 5A), in proximal half of fixed finger; dsb, dst, and dt on dorsal surface, in distal half of fixed finger; eb on retrolateral surface, in basal third of fixed finger, opposite db; esb, est, and et in distal half of fixed finger, opposite dsb, dst, and dt, respectively (Figure 5B); it and ib in median third of fixed finger (Figure 5C).
Legs: Ventral surfaces smooth; trochanter and femur, dorsal surfaces finely and sparsely granular; telotarsus with ventromedian row of spinules and paired ventrosubmedian rows of rigid spiniform macrosetae; tarsal ungues of equal length.
Sternum: Sternum pentagonal, longer than wide (Figure 6C); median sulcus deep, more pronounced in posterior half; posterior pit absent.
Genital operculum: Operculum comprising two subtriangular sclerites (♂) or single, triangular sclerite (♀); genital papillae short, not protruding from beneath operculum (♂; Figure 6C) or absent (♀).
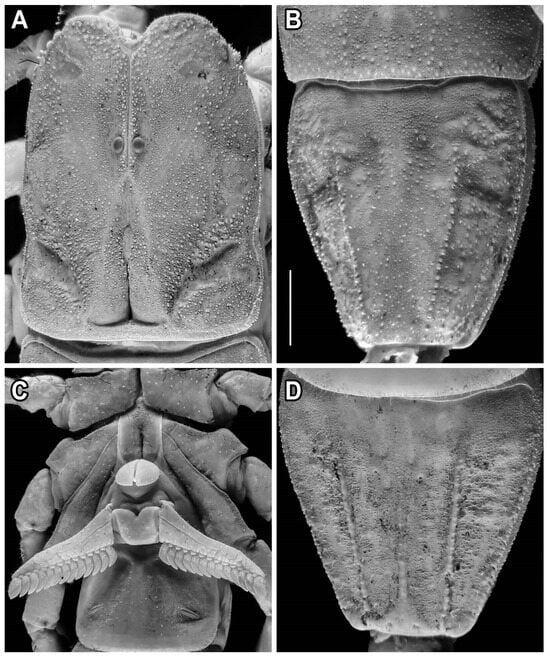
Figure 6.
Hemiscorpius jiroftensis sp. n., holotype ♂ (RIZ HD 44A), carapace (A), tergite VII (B), sternum and pectines (C), sternite VII (D). Scale bar = 2.5 mm.
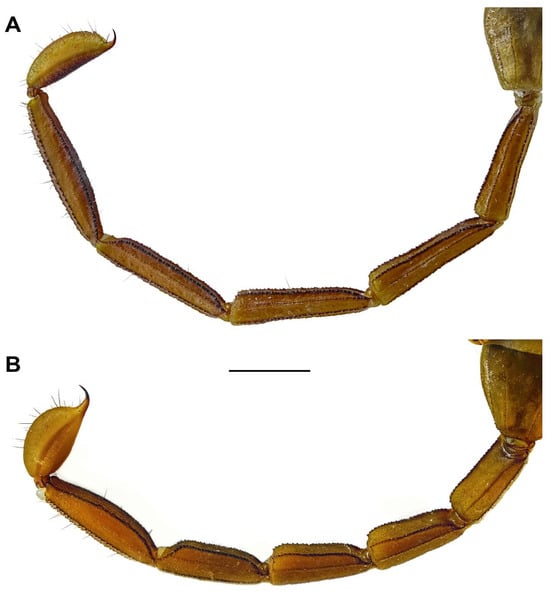
Figure 7.
Hemiscorpius jiroftensis sp. n., metasoma and telson, lateral aspect. (A) Holotype ♂ (RIZ HD 44A). (B) Paratype ♀ (RIZ HD 44C). Scale bar = 5 mm.
Mesosoma: Tergites finely granular, each with shallow median to submedian depressions; median carina absent from tergites I–III, obsolete on IV–VII; VII longer than wide (♂) or wider than long (♀), with lateral and sublateral carinae costate-granular, restricted to posterior three-quarters (Figure 6B). Sternites III–VI smooth and finely punctate; III with pair of large, finely and densely granular depressions, covered by pectines; IV–VI each with pair of deep median sulci; VII longer than wide (♂) or wider than long (♀), with fine granules medially, becoming coarser laterally (♂) or entirely finely and sparsely granular (♀), pair of costate-granular lateral carinae in posterior three-quarters (Figure 6D), median carina restricted to anterior half; spiracles crescent-shaped.
Metasoma: Segments elongated and slender (♂) (Figure 3A,B and Figure 7A) or short and stout (♀) (Figure 3C,D and Figure 7B); surfaces finely and sparsely granular. Segments I–IV each with seven distinct carinae (Figure 7); paired dorsosubmedian carinae each comprising spiniform granules; paired median lateral carinae costate-granular on I and II, granular on III and IV; paired ventrolateral carinae costate, almost smooth on I and II, each comprising spiniform granules on III and IV (Figure 7); ventromedian carina granular on I–IV. Segment V with five distinct carinae; paired dorsosubmedian carinae each comprising spiniform granules; paired median lateral carinae obsolete, each comprising sparse row of fine granules in anterior two-thirds; ventromedian carina and paired ventrolateral carinae each comprising spiniform granules (Figure 7).
Telson: Vesicle elongate (Figure 7), length/width = 3.6 (♂; Table 2) or oval, length/with = 2.4 (♀; Table 2), with (♂) or without (♀) pair of blunt tubercles posteriorly at base of aculeus (Figure 7B); posterior tubercles (♂) each with small spiniform granules; dorsal surface densely granular with shallow median depression; lateral surfaces granular; ventral surfaces granular with few macrosetae anteriorly, more numerous near base of aculeus; ventrolateral sulci and ventromedian carina absent; aculeus short and stout, strongly curved, becoming narrower midway (Figure 7A).
Intraspecific Variation. The metasomal segments are much longer in males, with the length/width of metasomal segment V greater than 6, than in females, with the length/width of segment V less than 4.1 (Table 2, Figure 7). The pectines of males and females possess 12–14 and 9 or 10 teeth, respectively (Table 2). Unlike adult males, females and immature males do not possess an elongated metasoma nor a pair of blunt tubercles posteriorly on the telson vesicle. These characters only appear in the final instar [2].
Distribution. The new species is currently known only from the Jebal Barez Mountains in the Bam and Jiroft counties of Kerman Province, southern Iran (Figure 1).
4. Discussion
In the present study, morphological and molecular evidence confirmed the presence of a new species of Hemiscorpius from Kerman Province in southern Iran, raising the number of Hemiscorpius species recorded in Iran to eight (Figure 1). It is important to understand the geographical distributions and morphological differences among the species of Hemiscorpius to implement appropriate medical responses to envenomation by these scorpions, which may prove fatal [6].
The Hemiscorpius species of Iran may be divided into two groups. The first group, including H. enischnochela, H. gaillardi, and H. shahii, bears more than nine trichobothria on the ventral surface of the pedipalp patella. The second group, including H. acanthocercus, H. kashkayi, H. jiroftensis, H. lepturus, and H. persicus, bears only three trichobothria on the ventral surface of the patella. Whether or not these groups are monophyletic, however, remains to be tested by phylogenetic analysis with a broader sample of species from across the known distribution of the genus.
Hemiscorpius jiroftensis sp. n. is most closely related to H. acanthocercus, from Hormozgan Province [3]. Specimens previously identified as H. acanthocercus by Shahi and Barahoei [4] from Jiroft County, Kerman Province, are conspecific with H. jiroftensis sp. n.
The altitude of Jebal Barez ranges from 1800 to 2700 m above sea level, and its average annual precipitation reaches 140 mm. Asteraceae with 39 species is the most diverse family of plants in this region [21]. Hemiscorpius jiroftensis sp. n. was collected from the highland areas of the Jebal Barez Mountains including Hishin village (southern part) and Sharik Abad village (northern part). This Mountains appear to have provided a geographical barrier, separating the new species from its closest relative, H. acanthocercus.
Another four scorpion species have also been reported in Jiroft County [9,22,23]: Hottentotta navidpouri Kovarik et al., 2018; Hottentotta sistanensis Kovarik et al., 2018; Mesobuthus kirmanensis (Birula, 1900); and Orthochirus kucerai Kovařík & Navidpour, 2020. In addition to H. jiroftensis sp. n., two other species of Hemiscorpius, i.e., H. acanthocercus and H. enischnochela, have also been recorded in Kerman Province.
The large intraspecific genetic distance (0.089) between the Iranian (OR800364) and Iraqi (MT230874) COI sequences of H. lepturus suggests that the Iranian sample may represent another new species. This is not particularly surprising, considering the great distance between the localities at which these samples were collected and the presence of numerous geographical barriers between them, which may have resulted in speciation. The species limits of H. lepturus will need to be studied with a more comprehensive sample.
Author Contributions
Conceptualization, E.A.G.S. and H.B.; methodology, H.B. and H.D.; software, H.B.; validation, L.P.; formal analysis, H.B. and L.P.; investigation, E.A.G.S. and H.D.; resources, J.R. and M.A.O.; data curation, E.A.G.S., H.D. and H.B.; writing—original draft preparation, H.D. and H.B.; writing—review and editing, M.A.O., J.R., A.A. and L.P.; visualization, H.B.; supervision, H.D. and J.R.; project administration, E.A.G.S. and J.R.; funding acquisition, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tehran University of Medical Sciences, project number 40111260002.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available from GenBank (National Center for Biotechnology Information) at https://www.ncbi.nlm.nih.gov accessed on 4 April 2025.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lourenço, W.R. The genus Hemiscorpius Peters, 1861 (Scorpiones: Hemiscorpiidae) in East Africa, and description of a new species from Somalia. Entomol. Mitt. zool. Mus. Hamburg. 2011, 15, 275–285. [Google Scholar]
- Prendini, L. Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): An exemplar approach. Cladistics 2000, 16, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Monod, L.; Lourenço, W.R. Hemiscorpiidae (Scorpiones) from Iran, with descriptions of two new species and notes on biogeography and phylogenetic relationships. Rev. suisse Zool. 2005, 112, 869–941. [Google Scholar] [CrossRef]
- Shahi, M.; Barahoei, H. Morphological study of Hemiscorpius Peters, 1861 (Scorpiones: Hemiscorpiidae) in Hormozgan Province, southern Iran. Arch. Razi Inst. 2023, 78, 1588–1600. [Google Scholar] [CrossRef]
- Barahoei, H.; Navidpour, S.; Aliabadian, M.; Siahsarvie, R.; Mirshamsi, O. Scorpions of Iran (Arachnida: Scorpiones): Annotated checklist, DELTA database and identification key. J. Insect Biodivers. Syst. 2020, 6, 375–474. [Google Scholar] [CrossRef]
- Shahi, M.; Rafinejad, J.; Az-Khosravi, L.; Moosavy, S. First report of death due to Hemiscorpius acanthocercus envenomation in Iran: Case report. Electron. Physician 2015, 7, 1234–1238. [Google Scholar]
- Akbari, H.; Mesbahzadeh, T.; Zehtabian, G. Prediction of climate change in arid and semi-arid regions of the western basin of Jazmourian wetland. Environ. Res. 2021, 11, 27–43. [Google Scholar]
- Salari, M.; Sampour, M. First two records of Hemiscorpius species (Scorpiones: Hemiscorpiidae) from Kerman Province, southeast of Iran. Asian J. Appl. Sci. 2017, 5, 101–108. [Google Scholar]
- Adeli-Sardou, M.; Shahi, M.; Dehghan, H.; Ahmadyousefi-Sarhadi, M.; Falah, G.; Barahoei, H. Geographical distribution of scorpions (Arachnida: Scorpiones) in southern regions of Kerman Province, Iran. Biol. Bull. 2024, 51, 644–654. [Google Scholar] [CrossRef]
- Dehghani, R.; Mazaheri Tehrani, A.; Ghadami, F.; Sanaei-Zadeh, H. Methods for collecting and capturing scorpions: A review. Int. J. Zool. Res. 2016, 4, 65–72. [Google Scholar]
- Vachon, M. Études des caractères utilisés pour classer les familles et les genres des scorpions (Arachnides). 1. La trichobothriotaxie en arachnologie. Sigles trichobothriaux et types de trichobothriotaxie chez les scorpions. Bull. Mus. Natl. Hist. Nat. 1974, 140, 857–958. [Google Scholar]
- Loria, S.F.; Prendini, L. Homology of the lateral eyes of Scorpiones: A six-ocellus model. PLoS ONE 2014, 9, e112913. [Google Scholar] [CrossRef]
- González-Santillán, E.; Prendini, L. Redefinition and generic revision of the north American vaejovid scorpion subfamily Syntropinae Kraepelin, 1905, with descriptions of six new genera. Bull. Am. Mus. Nat. Hist. 2013, 382, 1–71. [Google Scholar] [CrossRef]
- Stahnke, H.L. Scorpion nomenclature and mensuration. Entomol. News 1970, 81, 297–316. [Google Scholar]
- Sissom, W.D. Systematics, biogeography and paleontology. In The Biology of Scorpions; Polis, G.A., Ed.; Stanford University Press: Stanford, CA, USA, 1990; pp. 31–80. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome c Oxidase Subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Barahoei, H.; Prendini, L.; Navidpour, S.; Tahir, H.M.; Aliabadian, M.; Siahsarvie, R.; Mirshamsi, O. Integrative systematics of the tooth-tailed scorpions, Odontobuthus (Buthidae), with descriptions of three new species from the Iranian Plateau. Zool. J. Linn. Soc. 2022, 195, 355–398. [Google Scholar] [CrossRef]
- Shahi, M.; Barahoei, H. Molecular study of Hemiscorpius Peters (Scorpiones: Hemiscorpiidae) in Hormozgan Province, south of Iran. Arch. Razi Inst. 2024, 79, 216–222. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Vakili Shahrbabaki, S.M.A.; Alikhani, T. Introduction of the flora, life form and chorotypes of plants in Jeball Barez (Jiroft). J. Plant Environ. Physiol. 2013, 8, 66–79. [Google Scholar]
- Kovařík, F.; Navidpour, S. Six new species of Orthochirus Karsch, 1892 from Iran (Scorpiones: Buthidae). Euscorpius 2020, 132, 1–41. [Google Scholar]
- Kovařík, F.; Fet, V.; Gantenbein, B.; Graham, M.R.; Yağmur, E.A.; Šťáhlavský, F.; Poverennyi, N.M.; Novruzov, N.E. A revision of the genus Mesobuthus Vachon, 1950, with a description of 14 new species (Scorpiones: Buthidae). Euscorpius 2022, 348, 1–189. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).