Abstract
Arbuscular mycorrhizal fungi (AMF) form symbiotic associations with most vascular plants and play an important role in immobilizing heavy metals in soil. Urban green space ecosystems are increasingly affected by heavy metal pollution; however, how different types of green spaces influence AMF diversity, stability, and coexistence mechanisms under heavy metal stress remains unclear. Here, heavy metal-contaminated soil samples were collected from Zhengzhou, China—a large city in the warm temperate monsoon zone of the North China Plain—to conduct high-throughput sequencing and analyze AMF community assembly. (1) AMF community composition varied significantly among green space types, with higher diversity in park green spaces (Shannon = 21.24 ± 2.24) than in street green spaces (Shannon = 11.36 ± 1.17). (2) Heavy metals were the primary factors driving AMF community assembly. Stochastic processes, mainly dispersal limitation, dominated AMF assembly across sites, with a stronger influence in street green spaces. (3) Specialist taxa (mainly Glomus and Claroideoglomus) exhibited higher network connectivity and stability in park green spaces, whereas generalist taxa maintained network resilience in street green spaces. This study elucidates the ecological processes shaping AMF communities in urban ecosystems and provides a scientific basis for AMF-based approaches to heavy metal remediation and sustainable management of urban green spaces.
1. Introduction
Urban green spaces are important buildings block of urban ecosystems, and their ecological role contributes to the purification of air, protection of biodiversity, and carbon and oxygen balance maintenance [1,2]. With the rapid development of urbanization, frequent human activities have introduced large quantities of heavy metal pollutants into the urban soil environment, such as industrial activities, traffic emissions, coal-burning emissions, etc., which causes pollution in urban green spaces [3,4]. The effect of heavy metal pollution on urban green space ecosystem is an urgent problem that remains to be solved [5,6,7].
Distinct types of urban green spaces, including park green spaces, are mainly open to the public for leisure, and street green spaces fall within the scope of street lands with environmental protection functions. Soil bacteria, fungi, and protozoa exhibit variations in diversity across different green space types [8]. The diversity and aggregation of fungal communities are influenced by the type of green space [9]. Litao Lin et al. found that the Cd had a significant positive effect on the composition of AM fungal communities [10]. In addition, soil available phosphorus, available potassium, and total nitrogen also significantly influence fungal community composition [11,12]. While most research on AMF communities has focused on natural ecosystems such as forests [13,14] and grasslands [15], studies on urban green space ecosystems remain relatively scarce.
Arbuscular mycorrhizal fungi (AMF) are a group of soil fungi that form symbiotic associations with the roots of most terrestrial plants [16]. The survival and reproduction of these forms are closely linked to their host plants [17] and show a high susceptibility to environmental conditions, such as heavy-metal contamination [18]. Currently, the aggregation of AMF communities is influenced by both deterministic (niche) and stochastic (neutral) processes [19]. However, significant differences in AMF community aggregation processes arise due to variations in soil properties and spatial factors across different regions [20]. Xieluyao Wei et al. found that under conditions of heavy metal and microplastic pollution, stochastic processes dominate the assembly of AMF communities [21]. To date, there has been limited research on how different types of green spaces affect the assembly mechanisms and co-occurrence patterns of AMF species in urban ecosystems contaminated by heavy metals.
Microorganisms can be classified as generalists or specialists depending on environmental heterogeneity [22,23]. Generalists adapt widely, and specialists are selective about specific environments [24]. Although both coexist in the soil, they influence community dynamics in distinct ways [25]. In favorable environmental conditions, specialists demonstrate stronger competitiveness than generalists, while generalists prevail in environments characterized by significant spatial and temporal heterogeneity [26]. Within natural ecosystems, these two groups fulfill distinct roles and exhibit varied responses to environmental gradients [27,28]. Generalists tolerate diverse environments, and specialists thrive in those with a certain specificity. In terms of adaptation mechanisms to environmental constraints and physiological metabolism, specialists exhibit higher plasticity under environmental selection, whereas generalists are more inclined to aggregate through neutral processes [29]. Therefore, the investigation of the aggregation mechanisms of generalist and specialist AMF in soils of various green space types under heavy metal contamination is important to gain insights into the assembly of soil microbial communities in urban green spaces with heavy metal contamination.
The co-occurrence patterns of AMF are critical determinants of community stability and ecosystem functioning [30,31]. In recent years, network analysis has emerged as a powerful tool for investigating microbial interactions and computing network topological properties, and it has been widely applied in various ecosystems, including agricultural fields [32,33], wetlands [34], and lakes [35]. AMF co-occurrence network patterns exhibit considerable variability across different study locations and land-use types [36,37]. Wang Huan et al. demonstrated that the network complexity in park green spaces was higher than that in squares, campuses, and street green spaces, with distinct key species contributing to network stability in each type of green space [38]. While the interaction patterns of AMF in response to environmental changes have been extensively studied in natural ecosystems, there remains a lack of detailed assessments on the response of AMF network structures to different green space types within heavy metal-contaminated urban ecosystems.
This study considered Zhengzhou, a large and rapidly developing city in Central China, as the research object. Survey data from urban green spaces in Zhengzhou city were analyzed to reveal the effects of various green spaces on AMF diversity and coexistence mechanisms based on the comprehension of heavy-metal pollution status in urban green spaces. We hypothesized that the following: (1) the diversity of AMF is greater in park green spaces than in street green spaces; (2) stochastic processes prevail during the community assembly of AMF in different types of green spaces; (3) specialists perform a critical role in the AMF networks across various types of green spaces.
2. Materials and Methods
2.1. Study Site
Zhengzhou, located in Central China (34°16′–34°58′ N, 112°42′–114°13′ E) [39], is a rapidly developing metropolis with a permanent population of over 12.8 million as of 2022 [40]. This location lies within the transition zone between warm temperate and subtropical regions and exhibits a typical warm temperate continental monsoon climate. The city features an average annual temperature of 15.6 °C and receives approximately 542.15 mm of precipitation annually, with most of the rain falling from June to September. The study area which has a temperate monsoon climate (Cwa) according to the Köppen climate classification, with hot, rainy summers and cold, dry winters.
Zhengzhou City has a large green space area, with a green space rate of 36.81% as of 2022 [41]. Its vegetation can be classified under the warm temperate deciduous broad-leaved forest category, which comprises rich botanical resources [42]. The dominant tree species included Ligustrum lucidum and Platanus acerifolia, while the primary shrubs consisted of Prunus cerasifera “Atropurpurea” and Punica granatum. The most common herbaceous plants were Ophiopogon japonicus and Chenopodium album.
This study investigated Zhengzhou, where research on AMF communities remains limited. The city represents a typical case of urbanization in the region. It features diverse urban green spaces and significant heavy metal contamination, making it an ideal site to examine the effects of urbanization on AMF diversity and community assembly.
2.2. Sampling and Analysis
To mitigate human interference and potential disturbances from transplanted vegetation, we selected green spaces with an area exceeding 1 ha and a vegetation establishment period of over 3 years as survey sites. The distance between survey plots exceeded 1 km. Based on the sampling locations, street and park green spaces were delineated, with each green space type consisting of 20 survey sites (Figure 1A).

Figure 1.
(A). Distribution of sample plots in the street (blue circles) and park green spaces (yellow circles). A total of 40 sample plots were obtained, with 20 plots in each of street and park green spaces. The asterisk in the figure denotes the sampling site location within China. (B). Bar chart showing the soil heavy metal (Cd, Pb, Cu, Zn, and Fe) contents in various types of green spaces compared with the HBV (Background values of soil heavy metal pollution in Henan Province) in Henan Province.
At each survey site, plant survey and soil sample collection required the establishment of a quadrat measuring 10 m × 10 m. Measurements were conducted on individual count, height, and diameter at the breast height of all woody plants within each 10 m × 10 m quadrat. In addition, four quadrats measuring 1 m × 1 m were randomly established within each 10 m × 10 m quadrat for the measurement and recording of the individual count, average height, and coverage of all herbaceous plants. Flora of China and the Chinese Virtual Herbarium were used to verify the identity of all observed plants.
Four points were randomly selected within each 10 m × 10 m quadrat. At each sampling location, surface litter and stones were removed, and soil samples from the top 0–20 cm were collected and combined to create composite samples [43]. The soil samples were initially cleaned to remove coarse roots, stones, and plant material before being passed through a 2 mm sieve. The samples were then divided into two portions: one was stored at 4 °C for the analysis of soil physicochemical properties, while the other was frozen at −80 °C for AMF assessment. This study focused on the investigation of 40 soil samples (20 park green spaces and 20 street green spaces).
2.3. Soil Physical and Chemical Analysis
For each 10 m × 10 m plot, eleven soil physicochemical properties were measured. These properties included soil organic matter (SOM), soil available phosphorus (AP), soil available potassium (AK), soil water content (SWC), soil pH, and soil total nitrogen (TN) content [44,45,46,47,48,49]. After the soil samples were digested with a mixture of hydrochloric acid, hydrofluoric acid, nitric acid, and perchloric acid [50], heavy metals (Cd, Pb, Cu, Zn, and Fe) were determined by atomic absorption spectrophotometry (ISSCAS 1978).
2.4. DNA Extraction, Amplification, and Sequencing
DNA extraction from soil samples was carried out using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). Electrophoresis was performed on a 1% agarose gel at 5 V/cm for 20 min to assess DNA integrity [51]. The V4–V5 region of the fungal 18S rRNA gene was amplified using specific primers. The initial amplification employed primers AML1F and AML2R, followed by a second round using AMF-specific primers AMV4-5NF and AMDGR, conducted on a GeneAmp 9700 PCR system (GeneAmp 9700, ABI, Carlsbad, CA, USA) [52,53]. PCR products were verified through 2% agarose gel electrophoresis and purified using the AxyPrep DNA Gel Extraction Kit (AXYGEN, Union City, CA, USA) [54]. Based on preliminary electrophoresis results, PCR products were quantified and normalized with the QuantiFluor™-ST Blue Fluorescent Quantification System (Promega, Madison, WI, USA). Paired-end sequencing was performed on an Illumina MiSeq PE300 platform (Illumina Inc., San Diego, CA, USA), with sequencing libraries prepared using the TruSeq DNA Sample Prep Kit (Illumina, San Diego, CA, USA). Raw sequencing reads were analyzed using QIIME v1.9.1. Singleton sequences were removed, and the remaining unique reads were clustered into OTUs at a 97% similarity level [55]. Representative sequences for each OTU were then taxonomically assigned using the RDP Classifier v2.11 with a Bayesian approach. The classification was performed against the maar-jam081/AM database, using a confidence cutoff of 0.7 [53].
2.5. Identification of Generalists and Specialists
Levins’ ecological niche width index was used as a basis for determining the habitat specialization of AMF across various types of green spaces, which resulted in the classification of two functional groups: generalists and specialists [56]. The “spaa (version 4.1.2)” package in R was used to calculate the ecological niche width [57]. In addition, the permutation method implemented in the EcolUtils (version 4.1.2) package was utilized to conduct 1000 random permutations of species occurrence frequencies and compute the null distribution of the indices of species ecological niche width. According to the principle that generalists have wider fundamental niches than specialists, OTUs were classified based on ecological niche width indices. Those exceeding the upper 95% confidence interval of the null distribution were identified as generalists, while those below the lower limit were categorized as specialists [58,59].
2.6. Statistical Analysis
To examine AMF community composition, stacked bar plots were used to visualize the relative abundance of taxa at the family and genus levels across different green space types [60,61]. Alpha diversity (Shannon index) was calculated to assess species diversity, and statistical differences among green space types were evaluated using nonparametric tests [62]. Beta diversity patterns were explored using principal coordinate analysis (PCoA), and significance in community differences was tested by permutational multivariate analysis of variance (PERMANOVA) [63]. The influence of environmental factors, including soil properties, vegetation characteristics, and heavy metal concentrations, on AMF community composition was assessed through redundancy analysis (RDA) and Mantel tests [64,65].
Null model analysis based on the beta nearest taxon index (βNTI) was performed to quantify the relative contributions of deterministic and stochastic processes to AMF community assembly [65,66]. Deterministic processes were considered dominant when |βNTI| > 2, whereas |βNTI| < 2 indicated stochastic assembly [67]. The relationship between the construction of AMF communities and environmental factors across various types of green spaces was evaluated via random forest analysis. The Euclidean distance matrix of each environmental factor and βNTI values, which involved the construction of 999 decision trees and 99 permutations, was used in the analysis [29,67]. The “random Forest (version 4.1.2)” package R was implemented to carry out the analysis.
The results of random forest (RF) analysis were used as a basis to perform linear regression analysis in the examination of the relationship between βNTI values of various types of green space AMF (all, specialists, and generalists) and the top three driving factors [29,65]. Variance decomposition analysis was performed through the construction of Venn diagrams using the “Venn Diagram (version 4.1.2)” package in R language [68,69,70]; the diagrams were used to compare the correlation between βNTI values of various types of green space AMF (all, specialists, and generalists) and soil, vegetation, and heavy metals.
The internal structure of AMF was visualized using a co-occurrence network diagram, and the results revealed patterns of species co-occurrence [71]. Network complexity was characterized through the elucidation of network topological features, including nodes, edges, and average path lengths [72,73]. Within-module connectivity (Zi) and among-module connectivity (Pi) were used to identify key species maintaining the stability of urban green space AMF co-occurrence networks [74]. Analysis was performed using the “igraph (version 4.1.2)” package in R language.
All statistical analyses were conducted using R software (version 4.1.2).
3. Results
3.1. Composition and Diversity of AMF Under Heavy-Metal Pollution Across Various Types of Green Spaces
Street and park green spaces were found in a polluted state in Zhengzhou, with average concentrations of soil heavy metals Cd, Pb, Cu, Zn, and Fe exceeding the Henan background values (HBVs) (Figure 1B).
The composition of AMF communities—including all taxa, specialists, and generalists—varied notably in terms of family and genus representation across different urban green space types (Figure 2). At the family level, Gigasporaceae and Diversisporaceae were uniquely associated with park green spaces. In both parks and street green spaces, Glomeraceae and unclassified groups emerged as the predominant families. However, Glomeraceae showed relatively greater abundance in park settings, whereas unclassified taxa appeared more prevalent in street green spaces (Figure 2A). At the genus level, Archaeospora and Scutellospora were exclusively detected within park ecosystems. In both street and park green spaces, the dominant AMF genera were Glomus and unclassified taxa. The relative abundance of Glomus was higher in park green spaces than in street green spaces, whereas unclassified taxa were more abundant in street green spaces than in park green spaces (Figure 2B).
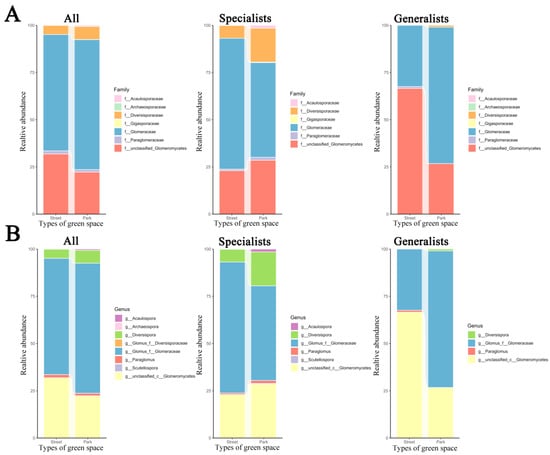
Figure 2.
Composition of all, specialist, and generalist AMF from different green space types at the family and genus levels in urban ecosystems contaminated by heavy metals. (A): Proportion of AMF at the family level across various green space types. (B): Proportion of AMF at the genus level across different green space types. The relative abundances of AMF at the family (A) and genus (B) levels in various green space types.
The diversity of all AMF and generalized AMF showed a significant difference between the two types of green spaces (p < 0.05), with park green spaces having a higher Shannon diversity index than street ones. However, specialists exhibited no significant differences in their diversity (Figure 3A). All AMF and generalized AMF also exhibited significantly varying community structure between the two types of green spaces (Figure 3B) (all: F = 4.675, p = 0.034; generalists: F = 3.717, p = 0.041). However, no significant difference was found in the community structure of specialist species (F = 0.653, p = 0.429).
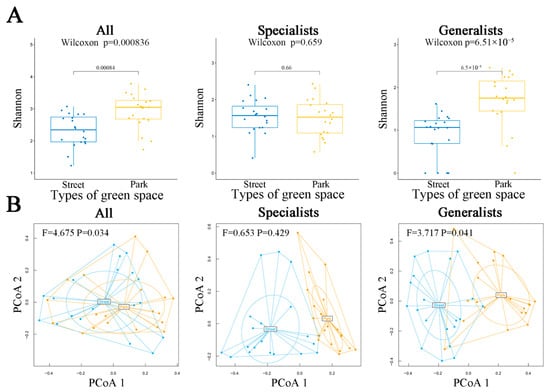
Figure 3.
Diversity and species composition of AMF (all, specialists, and generalists) across different green space types in urban ecosystems contaminated by heavy metals. (A): Box plot displaying the Shannon diversity indices of AMF in various green space types, with significant differences tested using the Wilcoxon method at p ≤ 0.05. (B): PCoA analysis of AMF species in different green space types, with PCoA 1 and PCoA 2 representing the two main coordinate axes.
3.2. Influence of Environmental Factors on AMF Communities Under Heavy-Metal Pollution Across Various Types of Green Spaces
RDA results indicated that the distribution of AMF communities in park green spaces was more strongly influenced by environmental factors than in street green spaces. Environmental factors explained 33.02% of the variation in AMF community distribution in park green spaces, while in street green spaces, they explained 27.33% of the variation (Figure 4A). The environmental factors affecting AMF communities differed between the two green space types. According to the Mantel test results, the significant environmental factors influencing AMF communities in park green spaces were available potassium (AK) and total nitrogen (TN), whereas in street green spaces, the significant factor was the heavy metal zinc (Zn) (Figure 4B).
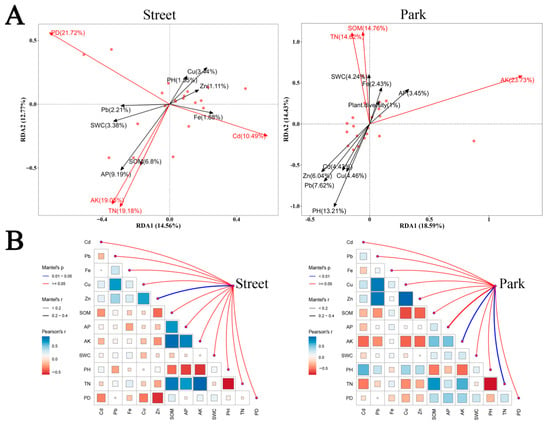
Figure 4.
The influence of environmental factors on AMF communities in different types of green spaces. (A): Redundancy Analysis (RDA) of AMF community composition and environmental factors in street and park green spaces. Red arrows indicate significant environmental factors (p < 0.05). (B): Environmental associations of AMF communities in street and park green spaces. The thickness of the lines represents the strength of the correlations. Note: PD represents plant diversity.
3.3. Community Assembly Processes of AMF Under Heavy-Metal Pollution Across Various Types of Green Spaces
AMF (all, specialists, and generalists) had βNTI values distributed between −2 and 2, and RCBray values between −0.95 and 0.95 (Figure S1). These numbers collectively indicated that stochastic processes primarily mediated the assembly of AMF communities in street and park green spaces. A null model-based framework was used to further quantify the relative importance of AMF community assembly processes (Figure 5). The results showed that stochastic processes predominated in both types of urban green spaces, with dispersal limitation being the most prominent. All AMF in street green spaces were more influenced by stochastic processes than those in park green spaces (Figure 5A). The RF analysis revealed that Zn had the greatest effect on the βNTI of AMF in street environments, followed by AK and Cu. Similarly, the most significant influence on the βNTI of AMF in park environments was displayed by Cu, followed by Pb and Cd (Figure 5B).

Figure 5.
The community assembly process shaping AMF (all, specialists, and generalists) across different green space types in urban ecosystems contaminated by heavy metals. (A): Ecological processes involved in AMF across various green spaces. The inner circle represents the contributions of stochastic and deterministic processes to community formation, while the outer ring details the specific ecological processes associated with each type. (B): The importance of RF means predictors (percentage increase in mean square error) in explaining the influence of soil property differences on βNTI across various green space types. (RF = Random Forest; NTI = Nearest Taxon Index).
In street green spaces, the Zn and Cu concentrations formed significant positive correlations with the βNTI of all AMF and generalized AMF, which suggested that the increased levels of Zn and Cu resulted in the transition from stochastic to deterministic processes in the assembly of all AMF and generalized AMF in street environments. Park green spaces presented significant positive correlations of the concentrations of Cu, Pb, and Cd with the βNTI of all AMF, which indicated that these elevated concentrations of these heavy metals prompted a shift in the AMF community assembly from stochastic to deterministic processes (Figure 6A). Furthermore, according to variance partitioning analysis, all environmental factors—including plants, heavy metals, and soil physicochemical properties—collectively explained 28.3% and 17.4% of the total variation in the βNTI of all AMF in street and park green spaces, respectively. Notably, heavy metals accounted for a higher proportion of variation in AMF community assembly than plant and soil variables in both street and park green spaces, contributing 21.3% and 18.4%, respectively (Figure 6B).
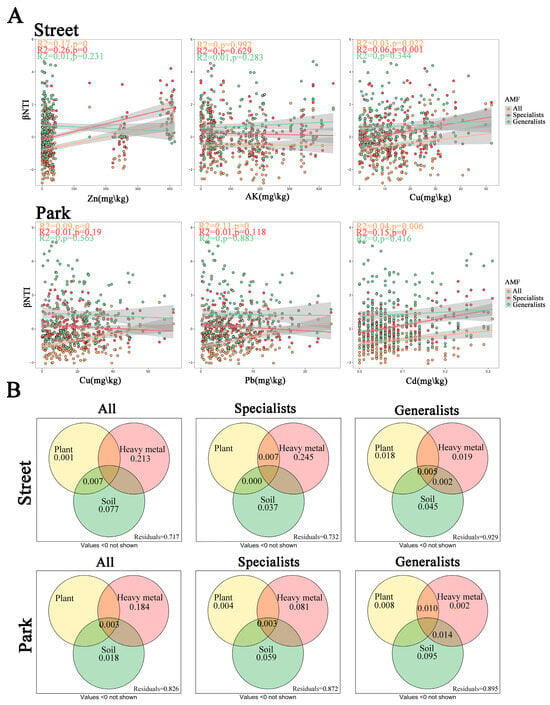
Figure 6.
Influence of environmental factors on the βNTI across various types of green spaces in heavy metal-polluted urban green space ecosystems. (A). Relationship between βNTI and the top three important environmental factors for various types of green spaces. (B): Variance decomposition plots of the effects of plants, heavy metals, and soil on βNTI in streets (top row) and parks (bottom row) for all, specialists, and generalists. Numbers denote the proportion of explained variation (adjusted R2 values). Values below zero are not shown. Note: Plants: species Shannon diversity; Heavy metals: Cd, Pb, Fe, Cu, Zn; Soil: SOM, AP, AK, SWC, PH, and TN.
3.4. Co-Occurrence Networks and Stability of AMF Under Heavy-Metal Pollution Across Various Types of Green Spaces
Using the full AMF dataset, a co-occurrence network was constructed and subsequently partitioned into six primary modules, collectively representing 59.61% of the total network (Figure 7A). Network modularization reflects the complexity and stability of a network structure. The AMF showed preferable distribution in modules 1, 2, 3, 4, and 6 in park green spaces and preferable distribution in module 5 and other modules in street green spaces. These results suggest that a greater number of AMF network nodes are present in park green spaces, which contributes to a more stable network. The Zi-Pi plot (Figure 7B) showed that compared with generalists, specialists were more likely to be network hubs (specialists:generalists = 14:2), which played a crucial role in the network. Most crucial taxa belonged to the Glomeraceae family, and no network or module hubs were detected in the network (Figure 7C).
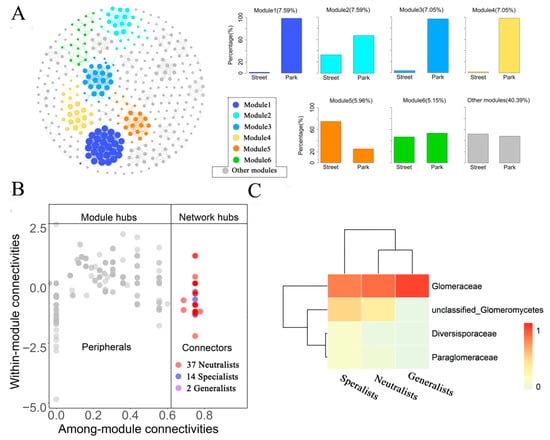
Figure 7.
Stability of network modules across various types of green spaces in heavy metal-polluted urban green space ecosystems. (A): Network of modular associations between AMFs (left). The relative abundance of AMF in major network modules of street and park green spaces (right). Nodes are colored based on modularity, with each node representing a specific OTU. Links indicate strong (SparCC |r| > 0.7) and significant (p < 0.05) associations. (B): Zi-Pi diagram revealing the distribution of topological properties of all AMFs. Each dot corresponds to an OTU. Neutralists, specialists, and generalists are indicated by red, blue, and purple dots, respectively. (C): Heat map showing the family to which the connection points belong.
4. Discussion
4.1. Community Structure and Diversity of AMF Vary Significantly Among Different Types of Green Spaces
In environments affected by heavy metal pollution, the diversity of all AMF and generalized AMF in park green spaces was significantly higher compared to those found in street green spaces (Figure 3). This outcome was observed because various green space types showed variations in plant diversity and soil physicochemical properties, which formed distinct microhabitats [75]. Compared with less intensively managed street green spaces, park green spaces—which receive refined anthropogenic management such as fertilization and pruning—typically exhibit higher vegetation diversity and greater soil nutrient concentrations [76]. Nutrient enrichment may promote AMF proliferation, thereby influencing community abundance and diversity [77].
In this study, Glomeraceae dominated across various green space types in heavy metal-contaminated urban green space ecosystems. Glomeraceae communities show a wide distribution in heavy metal-contaminated soils and can more easily develop survival capabilities in environments disturbed by heavy metal contamination compared with other species [78,79]. However, in this study, various green space types exhibited a nonsignificant effect on the diversity and community structure of specialist species. This finding was obtained possibly because specialists evolved to adapt to the environmental conditions of various green spaces under heavy-metal contamination [80]. These findings indicate that in heavy-metal-contaminated urban ecosystems, green space type significantly affects AMF diversity and structure, with these attributes shaped by distinct environmental drivers.
4.2. Different Environmental Drivers Shaped the AMF Community Composition in Various Types of Green Spaces
The AMF communities in park and street green spaces differed due to the influence of distinct environmental factors (Figure 4). In park green spaces, available phosphorus (AP) and total nitrogen (TN) were identified as the primary drivers of AMF community variation, whereas soil zinc (Zn) emerged as the most influential factor in street green spaces. Soil organic matter, nitrogen, and phosphorus directly regulate microbial growth and metabolic activity [81]. Changes in AP and TN may affect AMF spore germination, mycelial growth, and the efficiency of mycorrhizal symbiosis [82]. Moreover, due to exhaust emissions during vehicle transportation, soil heavy metal contamination in street green spaces is very severe [83], causing Zn to significantly influence the structure and composition of AMF communities. This is consistent with previous research findings. Ma et al. also found that Zn was a key factor influencing the composition of AMF communities in the rhizosphere of Stipa species [84].
4.3. Stochastic Processes Dominate the Community Assembly of AMF in Greenbelts Contaminated by Heavy Metals
Null model analysis revealed that under heavy metal contamination, the assembly of AMF communities was influenced more by stochastic processes than by deterministic ones (Figure 5A). This finding is consistent with the results of Mao et al., who also observed that stochastic processes dominated the assembly of microbial communities in Pb- and Zn-contaminated soils at smelting sites [85]. Similarly, in grasslands grazed by cattle and sheep, the assembly of AMF communities was primarily driven by stochastic processes [86]. Compared to park green spaces, AMF communities in street green spaces were more heavily influenced by stochasticity. This may be because street green spaces are relatively resource-poor, with lower plant coverage and diversity, which reduces environmental selection pressure (i.e., deterministic processes) on AMF. As a result, AMF colonization and survival rely more on random events, leading to a greater role of stochastic processes in community assembly in street green spaces.
The findings of RF analysis revealed heavy metals as critical factors affecting the assembly of AMF communities (Figure 5B). Specifically, soil Zn and Cu contents serve as key factors determining the assembly process of AMF communities in street green spaces, and Cu, Pb, and Cd contents contribute to the assembly process of AMF communities in park green spaces. These findings are in agreement with those of previous studies. Tao Sun et al. observed that heavy metals act as primary drivers of fungal community assembly processes [87]. As heavy-metal concentrations vary, microbial community aggregation also exhibits alterations [88,89]. This may have been due to the disrupted stability of microbial communities caused by residual heavy metals in the soil, which affected their assembly processes [90]. In addition, variance partitioning analysis revealed the strong correlation of the βNTI values of AMF in various types of green spaces with heavy metals and highlighted heavy metals as key factors influencing AMF community assembly. These results suggest that within heavy-metal contaminated urban green space ecosystems, the content of soil heavy metals determines the transitions between deterministic and stochastic processes in the assembly of the AMF community. Moreover, different kinds of heavy metals affect the assembly processes governing AMF communities in diverse types of green spaces.
4.4. Specialists Play a More Crucial Role in Network Stability than Generalists
According to network analysis, six network modules revealed a high consistency with the two types of green spaces, with park green spaces generally featuring more network nodes than street green spaces (Figure 6A). This result indicated that the structural modularity or characteristics of AMF communities are responsive to changes in green space types within environments affected by heavy metal contamination. The modularity of AMF can be attributed to the environmental heterogeneity arising from the diverse nature of green spaces [91]. Moreover, network modules could serve as important indicators for identifying key ecological processes in various green environment types. For a given network, all AMF and specialized AMF showed higher natural connectivity in park green spaces than in street green spaces, indicating a greater prevalence of nonrandom interactions in park green spaces. This condition may be more conducive to maintaining community network stability. Conversely, nonrandom interactions influence generalist species in street green spaces. In conclusion, the type of urban green spaces significantly impacts the stability of AMF networks in ecosystems affected by heavy metal pollution. Specifically, both all AMF and specialized AMF demonstrated enhanced network stability in parks compared to street green spaces. In contrast, the network stability of generalist species may be superior in street green spaces relative to parks.
Based on AMF network modules (Figure 6B), compared with generalists, specialists played a critical role in the network (connectivity points = specialists: generalists = 14:2). This finding indicates the crucial role played by specialists in the stability of AMF networks. Similar results were revealed in studies on microbial communities in urban drinking water [92], agricultural soil [93], and activated sludge ecosystems [94]. The majority of network keystone OTUs belong to Glomeraceae. This finding indicates the broad adaptability of Glomeraceae and its survival in various extreme environments, including heavy metal-contaminated areas and saline–alkali soils [52,95], which aligns with previous conclusions. In conclusion, specialists play a crucial role in sustaining the stability of urban green space networks.
5. Conclusions
This study found that AMF diversity and community structure differed markedly between park and street green spaces. Soil nutrients, particularly TN and AP, were the primary factors shaping AMF communities in park soils, whereas multiple environmental variables jointly influenced AMF patterns in street soils. Heavy metals regulated the balance between deterministic and stochastic processes, with stochastic processes playing the dominant role overall. Under heavy-metal stress, specialist taxa contributed more than generalists to maintaining AMF network stability. These findings clarify the ecological processes governing AMF assembly in urban soils and provide guidance for the management and restoration of heavy metal-contaminated green spaces.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17120810/s1, Figure S1: Boxplots of βNTI and RCbray for AMF in various types of green spaces (using 0.5 as the threshold).
Author Contributions
Z.Y.: Supervision, Project administration, Funding acquisition. Y.S.: Investigation, Formal analysis, Data curation, Methodology. L.N.: Investigation, Software, Validation, Formal analysis, Writing—original draft. Y.F.: Methodology, Writing—review & editing. J.L.: Formal analysis, Data curation. Z.G.: Formal analysis, Data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Biodiversity Conservation Research (GZS2023006). Natural Science Foundation of Henan (No. 252300420189), the Henan Province University Young Backbone Teachers Training Program (No. 2024GGJS026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw sequence data that support the findings of this study were uploaded to NCBI, Sequence Read Archive (SRA) under BioProject accession number PRJNA1147431. [NCBI] [Https://www.ncbi.nlm.nih.gov] [PRJNA1147431].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Groffman, P.M.; Williams, C.O.; Pouyat, R.V.; Band, L.E.; Yesilonis, I.D. Nitrate Leaching and Nitrous Oxide Flux in Urban Forests and Grasslands. J. Environ. Qual. 2009, 38, 1848–1860. [Google Scholar] [CrossRef]
- Astell-Burt, T.; Feng, X.Q. Urban green space, tree canopy and prevention of cardiometabolic diseases: A multilevel longitudinal study of 46 786 Australians. Int. J. Epidemiol. 2020, 49, 926–933. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, T.; Fu, Y.; Chen, B.; Crotty, F.; Murray, P.J.; Yu, S.; Xu, C.; Liu, W. Spatial change in glomalin-related soil protein and its relationships with soil enzyme activities and microbial community structures during urbanization in Nanchang, China. Geoderma 2023, 434, 116476. [Google Scholar] [CrossRef]
- Zeng, Y.; Bi, C.; Jia, J.; Deng, L.; Chen, Z. Impact of intensive land use on heavy metal concentrations and ecological risks in an urbanized river network of Shanghai. Ecol. Indic. 2020, 116, 106501. [Google Scholar] [CrossRef]
- Kabir, M.H.; Rashid, M.H.; Wang, Q.; Wang, W.; Lu, S.; Yonemochi, S. Determination of Heavy Metal Contamination and Pollution Indices of Roadside Dust in Dhaka City, Bangladesh. Processes 2021, 9, 1732. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Wong, C.U.I.; Li, F.; Xie, S. Assessment of Heavy Metal Contamination and Ecological Risk in Soil within the Zheng-Bian-Luo Urban Agglomeration. Processes 2024, 12, 996. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, X.; Zhu, C. Characteristics of Soil Heavy Metal Pollution and Health Risks in Chenzhou City. Processes 2024, 12, 623. [Google Scholar] [CrossRef]
- Grierson, J.; Flies, E.J.; Bissett, A.; Ammitzboll, H.; Jones, P. Which soil microbiome? Bacteria, fungi, and protozoa communities show different relationships with urban green space type and use-intensity. Sci. Total Environ. 2023, 863, 160468. [Google Scholar] [CrossRef]
- Huang, X.-R.; Neilson, R.; Yang, L.-Y.; Deng, J.-J.; Zhou, S.-Y.-D.; Li, H.; Zhu, Y.-G.; Yang, X.-R. Urban greenspace types influence the microbial community assembly and antibiotic resistome more in the phyllosphere than in the soil. Chemosphere 2023, 338, 139533. [Google Scholar] [CrossRef]
- Lin, L.; Chen, Y.; Qu, L.; Zhang, Y.; Ma, K. Cd heavy metal and plants, rather than soil nutrient conditions, affect soil arbuscular mycorrhizal fungal diversity in green spaces during urbanization. Sci. Total Environ. 2020, 726, 138594. [Google Scholar] [CrossRef]
- Lin, X.Y.; Han, X.M.; Yang, J.D.; Liu, F.Y.; Li, Y.Y.; Chen, Z.J. Network of Soil Fungi and the Microfauna Community under Diverse Anthropic Disturbances under Chrysopogon zizanioides Planting in the Reservoir. Plants 2024, 13, 393. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Si, Y.J.; Li, B.Q.; Chen, P.; Wu, L.L.; Guo, P.; Ji, R.Q. The Diverse Mycorrizal Morphology of Rhododendron dauricum, the Fungal Communities Structure and Dynamics from the Mycorrhizosphere. J. Fungi 2024, 10, 65. [Google Scholar] [CrossRef]
- Egan, C.P.; Callaway, R.M.; Hart, M.M.; Pither, J.; Klironomos, J. Phylogenetic structure of arbuscular mycorrhizal fungal communities along an elevation gradient. Mycorrhiza 2017, 27, 273–282. [Google Scholar] [CrossRef]
- Liu, Z.; Fang, J.; Song, B.; Yang, Y.; Yu, Z.; Hu, J.; Dong, K.; Takahashi, K.; Adams, J.M. Stochastic processes dominate soil arbuscular mycorrhizal fungal community assembly along an elevation gradient in central Japan. Sci. Total Environ. 2023, 855, 158941. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Fitter, A.H.; Helgason, T. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef]
- Xie, T.; Lin, Y.; Li, X. Responses of the arbuscular mycorrhizal fungi community to warming coupled with increased drought in an arid desert region. Geoderma 2024, 441, 116744. [Google Scholar] [CrossRef]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of Arbuscular Mycorrhizal (AM) Fungi on Growth, Cadmium Uptake, Osmolyte, and Phytochelatin Synthesis in Cajanus cajan (L.) Millsp Under NaCl and Cd Stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Pholchan, M.K.; Baptista, J.d.C.; Davenport, R.J.; Sloan, W.T.; Curtis, T.P. Microbial community assembly, theory and rare functions. Front. Microbiol. 2013, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, S.; Wu, B.; Wang, Y.; Ji, N.; Yao, H.; Cai, H.; Shi, M.; Zhang, D. Mainland and island populations of Mussaenda kwangtungensis differ in their phyllosphere fungal community composition and network structure. Sci. Rep. 2020, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.L.Y.; Tian, X.R.; Zhao, K.; Yu, X.M.; Chen, Q.; Zhang, L.Z.; Liao, D.C.; Penttinen, P.; Gu, Y.F. Bacterial community in the buckwheat rhizosphere responds more sensitively to single microplastics in lead-contaminated soil compared to the arbuscular mycorrhizal fungi community. Ecotoxicol. Environ. Saf. 2024, 281, 116683. [Google Scholar] [CrossRef]
- Bell, T.H.; Bell, T. Many roads to bacterial generalism. Fems Microbiol. Ecol. 2021, 97, fiaa240. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, H.; Huang, Y.; Dong, B.; Fu, S.; Zhang, C.; Li, X.; Tan, Y.; Zhang, X.; Zhang, X. Driving mechanisms of soil bacterial α and (3 diversity under long-term nitrogen addition: Subtractive heterogenization based on the environment selection. Geoderma 2024, 445, 116886. [Google Scholar] [CrossRef]
- Sexton, J.P.; Montiel, J.; Shay, J.E.; Stephens, M.R.; Slatyer, R.A. Evolution of Ecological Niche Breadth. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: San Mateo, CA, USA, 2017; Volume 48, pp. 183–206. [Google Scholar]
- Xu, Q.; Luo, G.; Guo, J.; Xiao, Y.; Zhang, F.; Guo, S.; Ling, N.; Shen, Q. Microbial generalist or specialist: Intraspecific variation and dormancy potential matter. Mol. Ecol. 2022, 31, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Leung, P.M.; Wood, J.L.; Bay, S.K.; Hugenholtz, P.; Kessler, A.J.; Shelley, G.; Waite, D.W.; Franks, A.E.; Cook, P.L.M.; et al. Metabolic flexibility allows bacterial habitat generalists to become dominant in a frequently disturbed ecosystem. ISME J. 2021, 15, 2986–3004. [Google Scholar] [CrossRef]
- Szekely, A.J.; Langenheder, S. The importance of species sorting differs between habitat generalists and specialists in bacterial communities. Fems Microbiol. Ecol. 2014, 87, 102–112. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, W.; Wilkinson, D.M.; Yu, Z.; Xiao, P.; Yang, J. Biogeography and co-occurrence patterns of bacterial generalists and specialists in three subtropical marine bays. Limnol. Oceanogr. 2021, 66, 793–806. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, G.; Liang, K.; Wang, T.; Lv, F.; Zhang, X.; Li, Z.; Zhai, B. SOC mediates the contribution of generalists and specialists to changes in soil nirK bacterial diversity: Evidence from apple orchards in main production areas of China. Appl. Soil Ecol. 2023, 182, 104713. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Castillo, C.G.; Borie, F.; Oehl, F.; Sieverding, E. Arbuscular mycorrhizal fungi biodiversity: Prospecting in Southern-Central zone of Chile. A review. J. Soil Sci. Plant Nutr. 2016, 16, 400–422. [Google Scholar] [CrossRef]
- Xu, Q.; Vandenkoornhuyse, P.; Li, L.; Guo, J.; Zhu, C.; Guo, S.; Ling, N.; Shen, Q. Microbial generalists and specialists differently contribute to the community diversity in farmland soils. J. Adv. Res. 2022, 40, 17–27. [Google Scholar] [CrossRef]
- Hu, A.; Wang, H.; Cao, M.; Rashid, A.; Li, M.; Yu, C.-P. Environmental Filtering Drives the Assembly of Habitat Generalists and Specialists in the Coastal Sand Microbial Communities of Southern China. Microorganisms 2019, 7, 598. [Google Scholar] [CrossRef]
- Sun, M.; Li, M.; Zhou, Y.; Liu, J.; Shi, W.; Wu, X.; Xie, B.; Deng, Y.; Gao, Z. Nitrogen deposition enhances the deterministic process of the prokaryotic community and increases the complexity of the microbial co-network in coastal wetlands. Sci. Total Environ. 2023, 856, 158939. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, H.; Yu, Y.; Huang, J.; Zhou, Z.; Zeng, J.; Chen, P.; Xiao, F.; He, Z.; Yan, Q. Ecological stability of microbial communities in Lake Donghu regulated by keystone taxa. Ecol. Indic. 2022, 136, 108695. [Google Scholar] [CrossRef]
- Ceola, G.; Goss-Souza, D.; Alves, J.; Alves da Silva, A.; Sturmer, S.L.; Baretta, D.; Sousa, J.P.; Klauberg-Filho, O. Biogeographic Patterns of Arbuscular Mycorrhizal Fungal Communities Along a Land-Use Intensification Gradient in the Subtropical Atlantic Forest Biome. Microb. Ecol. 2021, 82, 942–960. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Wang, D.; Guo, X.; Yang, T.; Xiang, X.; Walder, F.; Chu, H. Differential Responses of Arbuscular Mycorrhizal Fungal Communities to Long-Term Fertilization in the Wheat Rhizosphere and Root Endosphere. Appl. Environ. Microbiol. 2021, 87, e00349-21. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Zhang, Q.; Zou, M.; Li, T.; Ai, L.; Wang, H. Urban greenspace types and climate factors jointly drive the microbial community structure and co-occurrence network. Sci. Rep. 2024, 14, 16042. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, L.; Hu, J.; Yang, H.; Guo, W. Effect of Urbanization on the River Network Structure in Zhengzhou City, China. Int. J. Environ. Res. Public Health 2022, 19, 2464. [Google Scholar] [CrossRef]
- Jiang, L.G.; Liu, Y. Spatiotemporal Dynamics of COVID-19 Pandemic City Lockdown: Insights From Nighttime Light Remote Sensing. Geohealth 2024, 8, e2024GH001034. [Google Scholar] [CrossRef]
- News, Z. Zhengzhou’s New Green Space Has Fallen for the First Time, and It’s Because…. Available online: https://new.qq.com/rain/a/20230227A0310P00 (accessed on 23 May 2023).
- Yang, Y.; Ma, J.; Liu, H.; Song, L.; Cao, W.; Ren, Y. Spatial Heterogeneity analysis of urban forest ecosystem services in Zhengzhou City. PLoS ONE 2023, 18, e0286800. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.K.; Li, L.; Zhao, M.N.; Yan, P.S.; Liu, Y.; Li, W.; Ding, S.Y.; Zhao, Q.H. Soil respiration and carbon sequestration response to short-term fertilization in wheat-maize cropping system in the North China Plain. Soil Tillage Res. 2025, 251, 106536. [Google Scholar] [CrossRef]
- Lu, N.; Xu, X.; Wang, P.; Zhang, P.; Ji, B.; Wang, X. Succession in arbuscular mycorrhizal fungi can be attributed to a chronosequence of Cunninghamia lanceolata. Sci. Rep. 2019, 9, 18057. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Frey, B.; Yang, L.; Li, M.-H.; Ni, H. Land use change effects on diversity of soil bacterial, Acidobacterial and fungal communities in wetlands of the Sanjiang Plain, northeastern China. Sci. Rep. 2019, 9, 18535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, P.; Liu, Y.; Du, W.; Jing, H.; Nie, C. Soil properties and microbial abundance explain variations in N2O fluxes from temperate steppe soil treated with nitrogen and water in Inner Mongolia, China. Appl. Soil Ecol. 2021, 165, 103984. [Google Scholar] [CrossRef]
- Fu, Q.; Shao, Y.; Wang, S.; Liu, F.; Tian, G.; Chen, Y.; Yuan, Z.; Ye, Y. Soil Microbial Distribution Depends on Different Types of Landscape Vegetation in Temperate Urban Forest Ecosystems. Front. Ecol. Evol. 2022, 10, 858254. [Google Scholar] [CrossRef]
- Li, P.K.; Ding, S.Y.; Xin, X.L.; Zhu, A.N.; Ding, S.P.; Mei, Y.; Liu, Y.; Wu, X.Y.; Lu, K.X.; Zhao, Q.H. Ecological niche differentiation of detritivores dominates soil mesofaunal community assembly in a 33-year fertilized cropland. Soil Tillage Res. 2025, 252, 106605. [Google Scholar] [CrossRef]
- Page, A.L. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties. In Methods of Soil Analysis; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965. [Google Scholar]
- Ekere, N.R.; Ugbor, M.C.J.; Ihedioha, J.N.; Ukwueze, N.N.; Abugu, H.O. Ecological and potential health risk assessment of heavy metals in soils and food crops grown in abandoned urban open waste dumpsite. J. Environ. Health Sci. Eng. 2020, 18, 711–721. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, W.; Liu, Q.; Zhu, M.; Chen, Q.; Xu, Y.; Zhou, C. Association of Gut Microbiota Composition in Pregnant Women Colonized with Group B Streptococcus with Maternal Blood Routine and Neonatal Blood-Gas Analysis. Pathogens 2022, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Wang, Z.K.; He, Y.L.; Li, G.F.; Lv, X.H.; Zhuang, L. High-throughput sequencing analysis of the rhizosphere arbuscular mycorrhizal fungi (AMF) community composition associated with Ferula sinkiangensis. BMC Microbiol. 2020, 20, 355. [Google Scholar] [CrossRef]
- Zhang, R.; Mu, Y.; Li, X.; Li, S.; Sang, P.; Wang, X.; Wu, H.; Xu, N. Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci. Total Environ. 2020, 740, 139810. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Qiao, B.; Peng, M.; Deng, N.; Yu, R.; Tan, Z. Gut-Kidney Impairment Process of Adenine Combined with Folium sennae-Induced Diarrhea: Association with Interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and Acetic Acid, Inflammation, and Kidney Function. Cells 2022, 11, 3261. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Levins, R.A. Evolution in Changing Environments: Some Theoretical Explorations. (MPB-2); Princeton University Press: Princeton, NJ, USA, 1968. [Google Scholar]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Wilson, B.; Hayek, L.-A.C. Distinguishing relative specialist and generalist species in the fossil record. Mar. Micropaleontol. 2015, 119, 7–16. [Google Scholar] [CrossRef]
- Wu, W.; Logares, R.; Huang, B.; Hsieh, C.-h. Abundant and rare picoeukaryotic sub-communities present contrasting patterns in the epipelagic waters of marginal seas in the northwestern Pacific Ocean. Environ. Microbiol. 2017, 19, 287–300. [Google Scholar] [CrossRef]
- Ogola, H.J.O.; Selvarajan, R.; Tekere, M. Local Geomorphological Gradients and Land Use Patterns Play Key Role on the Soil Bacterial Community Diversity and Dynamics in the Highly Endemic Indigenous Afrotemperate Coastal Scarp Forest Biome. Front. Microbiol. 2021, 12, 592725. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, Z.; Liu, W.X.; Fu, Q.J.; Shao, Y.Z.; Liu, F.Q.; Ye, Y.Z.; Chen, Y.; Yuan, Z.L. Distribution Pattern of Woody Plants in a Mountain Forest Ecosystem Influenced by Topography and Monsoons. Forests 2022, 13, 957. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Y.; He, H.; Hu, P.; Sun, W.; Jin, M.; Wang, L.; Xu, X. Gut Microbiota Correlates With Clinical Responsiveness to Erythropoietin in Hemodialysis Patients With Anemia. Front. Cell. Infect. Microbiol. 2022, 12, 919352. [Google Scholar] [CrossRef]
- Xing, Y.; Cheng, L.; Zheng, L.; Wu, H.; Tan, Q.; Wang, X.; Tian, Q. Brownification increases the abundance of microorganisms related to carbon and nitrogen cycling in shallow lakes. Environ. Res. 2024, 257, 119243. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.J.; Shao, Y.Z.; Li, Z.H.; Zhao, P.F.; Ye, Y.Z.; Li, W.; Chen, Y.; Yuan, Z.L. Distribution of Woody Plant Species Among Different Disturbance Regimes of Forests in a Temperate Deciduous Broad-Leaved Forest. Front. Plant Sci. 2021, 12, 618524. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.X.; Li, X.Y.; Liu, F.Q.; Tian, X.Y.; Shao, Y.Z.; Yuan, Z.L.; Chen, Y. Differences in Soil Microbial Communities across Soil Types in China’s Temperate Forests. Forests 2024, 15, 1110. [Google Scholar] [CrossRef]
- Kuiper, J.J.; van Altena, C.; de Ruiter, P.C.; van Gerven, L.P.A.; Janse, J.H.; Mooij, W.M. Food-web stability signals critical transitions in temperate shallow lakes. Nat. Commun. 2015, 6, 7727. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Peng, F.; Gao, X.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.; Xue, Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 21. [Google Scholar] [CrossRef]
- Djotan, A.K.G.; Matsushita, N.; Fukuda, K. Year-round dynamics of arbuscular mycorrhizal fungi communities in the roots and surrounding soils of Cryptomeria japonica. Mycorrhiza 2024, 34, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Li, X.; Xing, W.; Zhang, X.; Chen, G. Mechanism insights into amendments enhanced dendroremediation for Cd and Zn-polluted soil: Bacterial co-occurrence networks’ complexity and stability. Geoderma 2024, 451, 117088. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, S.; Li, P.K.; Jia, H.R.; Wang, H.L.; Ye, Y.Z.; Yuan, Z.L. Stand Structure and Substrate Diversity as Two Major Drivers for Bryophyte Distribution in a Temperate Montane Ecosystem. Front. Plant Sci. 2017, 8, 874. [Google Scholar] [CrossRef]
- Guo, Y.; Bei, Q.; Dzomeku, B.M.; Martin, K.; Rasche, F. Genetic diversity and community composition of arbuscular mycorrhizal fungi associated with root and rhizosphere soil of the pioneer plant Pueraria phaseoloides. iMeta 2022, 1, e51. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Liao, X.; Yi, Q.; Zhang, W.; Lin, H.; Liu, K.; Peng, P.; Wang, K. Long-term returning agricultural residues increases soil microbe-nematode network complexity and ecosystem multifunctionality. Geoderma 2023, 430, 116340. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, Y.-M.; Yang, B.; Han, W.-X.; Zhao, W.-H.; Chai, H.-L.; Zhang, Z.-S.; Zhan, Y.-J.; Wang, L.-F.; Xing, Y.; et al. Comparative analysis of microbial communities in different growth stages of Dermacentor nuttalli. Front. Vet. Sci. 2022, 9, 1021426. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Chen, Y.; Shao, Y.; Xi, J.; Yuan, Z.; Ye, Y.; Wang, T. Community Preferences of Woody Plant Species in a Heterogeneous Temperate Forest, China. Front. Ecol. Evol. 2020, 8, 165. [Google Scholar] [CrossRef]
- de Mendonça, B.A.F.; Fernandes, E.I.; do Amaral, E.F.; Schaefer, C. Soils, Geoenvironments and Ecosystem Services of a Protected Area in Western Brazilian Amazonia. An. Acad. Bras. Cienc. 2023, 95, e20221071. [Google Scholar] [CrossRef]
- Xu, D.L.; Yu, X.W.; Chen, J.; Liu, H.J.; Zheng, Y.X.; Qu, H.T.; Bao, Y.Y. Arbuscular Mycorrhizae Fungi Diversity in the Root-Rhizosphere-Soil of Tetraena mongolica, Sarcozygium xanthoxylon, and Nitraria tangutorum Bobr in Western Ordos, China. Agronomy 2023, 13, 1485. [Google Scholar] [CrossRef]
- Ban, Y.; Jiang, Y.; Li, M.; Zhang, X.; Zhang, S.; Wu, Y.; Xu, Z. Homogenous stands of a wetland grass living in heavy metal polluted wetlands harbor diverse consortia of arbuscular mycorrhizal fungi. Chemosphere 2017, 181, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; You, F.; Wu, Z.; Bond, P.; Hall, M.; Huang, L. Molecular diversity of arbuscular mycorrhizal fungal communities across the gradient of alkaline Fe ore tailings, revegetated waste rock to natural soil sites. Environ. Sci. Pollut. Res. 2020, 27, 11968–11979. [Google Scholar] [CrossRef]
- Akhtar, N.; Mannan, M.A.-U. Mycoremediation: Expunging environmental pollutants. Biotechnol. Rep. 2020, 26, e00452. [Google Scholar] [CrossRef]
- Zhang, W.W.; Han, J.G.; Molla, A.; Zuo, S.D.; Ren, Y. The Optimization Strategy of the Existing Urban Green Space Soil Monitoring System in Shanghai, China. Int. J. Environ. Res. Public Health 2021, 18, 4820. [Google Scholar] [CrossRef]
- Weber, S.E.; Diez, J.M.; Andrews, L.V.; Goulden, M.L.; Aronson, E.L.; Allen, M.F. Responses of arbuscular mycorrhizal fungi to multiple coinciding global change drivers. Fungal Ecol. 2019, 40, 62–71. [Google Scholar] [CrossRef]
- Li, D.Y.; Liao, Y.L. Spatial Characteristics of Heavy Metals in Street Dust of Coal Railway Transportation Hubs: A Case Study in Yuanping, China. Int. J. Environ. Res. Public Health 2018, 15, 2662. [Google Scholar] [CrossRef]
- Ma, X.D.; Li, J.P.; Ding, F.C.; Zheng, Y.X.; Chao, L.M.; Liu, H.J.; Liu, X.Y.; Qu, H.T.; Bao, Y.Y. Changes of Arbuscular Mycorrhizal Fungal Community and Glomalin in the Rhizosphere along the Distribution Gradient of Zonal Stipa Populations across the Arid and Semiarid Steppe. Microbiol. Spectr. 2022, 10, e0148922. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.L.; Zheng, Z.K.; Ma, L.Y.; Wang, H.M.; Wang, X.J.; Zhu, F.; Xue, S.G.; Srivastava, P.; Sapsford, D.J. Polymetallic contamination drives indigenous microbial community assembly dominated by stochastic processes at Pb-Zn smelting sites. Sci. Total Environ. 2024, 947, 174575. [Google Scholar] [CrossRef]
- Zhou, J.Q.; Wang, P.S.; Wei, L.; Zhang, J.G.; Li, X.X.; Huang, N.; Liu, G.; Zou, K.; Fan, R.; Liu, L.; et al. Grazing increases the complexity of networks and ecological stochastic processes of mycorrhizal fungi. J. Environ. Manag. 2025, 373, 123933. [Google Scholar] [CrossRef]
- Sun, T.; Li, G.; Mazarji, M.; Delaplace, P.; Yang, X.; Zhang, J.; Pan, J. Heavy metals drive microbial community assembly process in farmland with long-term biosolids application. J. Hazard. Mater. 2024, 468, 133845. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Zhou, L.; Lou, W.; Zeng, W.; Liu, T.; Yin, H.; Liu, H.; Liu, X.; Mathivanan, K.; et al. Soil microbial community assembly model in response to heavy metal pollution. Environ. Res. 2022, 213, 113576. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Ding, K.; Liu, W.-S.; Baker, A.J.M.; Fei, Y.-H.; He, H.; Wang, Y.; Jin, C.; Wang, S.; et al. Heavy metal contamination affects the core microbiome and assembly processes in metal mine soils across Eastern China. J. Hazard. Mater. 2023, 443, 130241. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Y.; Chen, J.; Zhang, Z.; Cao, H.; Li, W.; Ye, M. Interspecific barrier effect driven by heavy metals makes soil bacterial functional assembly more stochastic. Environ. Res. 2024, 253, 119153. [Google Scholar] [CrossRef] [PubMed]
- Rottjers, L.; Faust, K. From hairballs to hypotheses-biological insights from microbial networks. Fems Microbiol. Rev. 2018, 42, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Hwang, C.; LeChevallier, M.W.; Andersen, G.L.; Liu, W.-T. Core-satellite populations and seasonality of water meter biofilms in a metropolitan drinking water distribution system. ISME J. 2016, 10, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Xu, Y.; Zhang, J.; Hao, X.; Lu, Y. Core Microbiota in Agricultural Soils and Their Potential Associations with Nutrient Cycling. Msystems 2019, 4, e00313-18. [Google Scholar] [CrossRef]
- Saunders, A.M.; Albertsen, M.; Vollertsen, J.; Nielsen, P.H. The activated sludge ecosystem contains a core community of abundant organisms. ISME J. 2016, 10, 11–20. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Wang, Z.; Su, X.; Liu, F.; Tian, X.; Ye, Y.; Shao, Y.; Yuan, Z. Cd contamination determined assembly processes and network stability of AM fungal communities in an urban green space ecosystem. Sci. Total Environ. 2023, 899, 166372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).