New Records of Marine Mollusca from the Culuccia Peninsula (NW Sardinia, Italy)
Abstract
1. Introduction
2. Materials and Methods
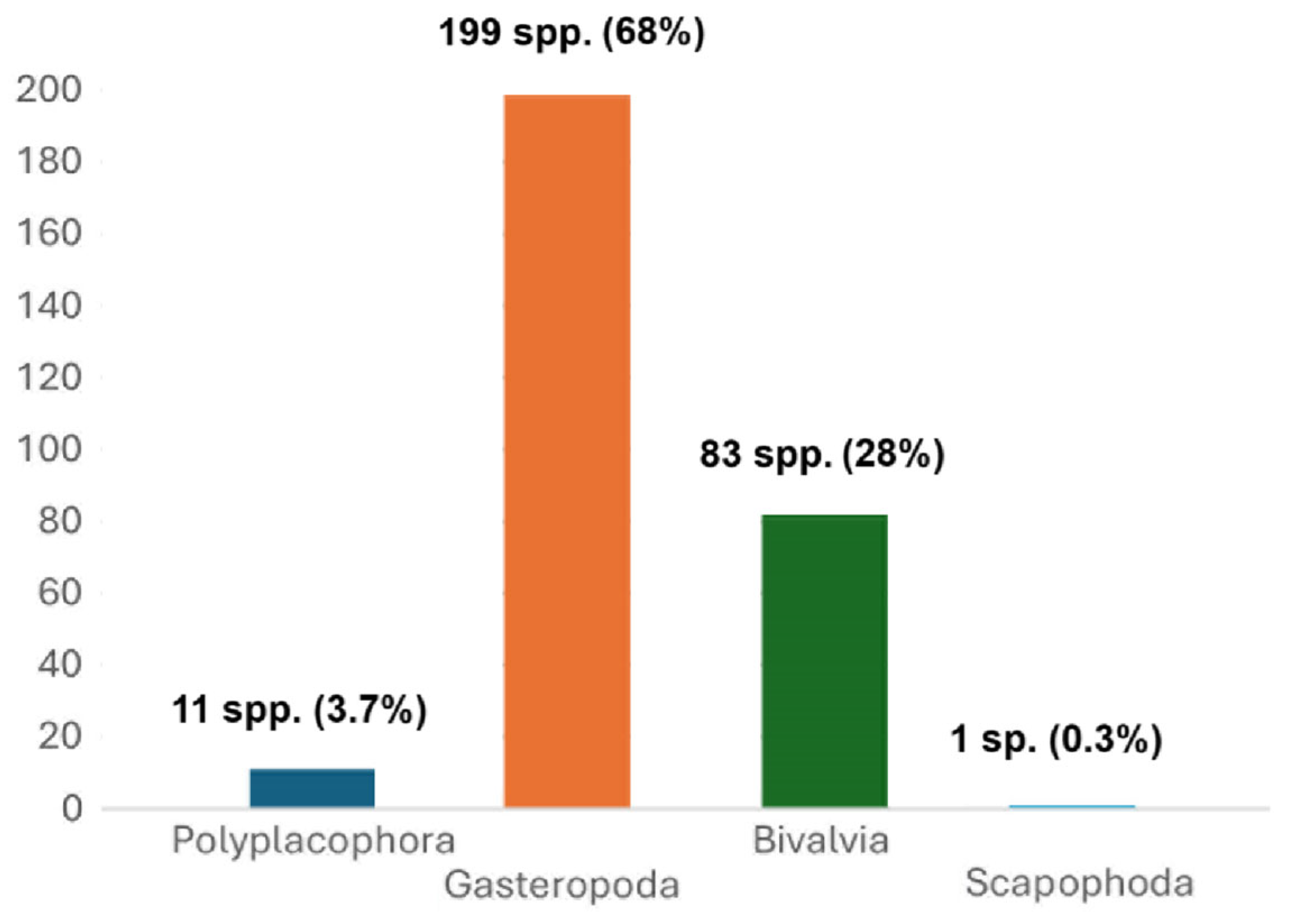
3. Results
3.1. New Records
- (1)
- The removal of Leptochiton asellus (Gmelin, 1791) from the inventory—valves of Leptochiton scabridus (Jeffreys, 1880) were misidentified;
- (2)
- Substitution of Vermetus granulatus (Gravenhorst, 1831) with Thylacodes arenarius (Linnaeus, 1758);
- (3)
- Substitution of Ebenomitra granum (Forbes, 1844) with Ebenomitra savignyi (Payraudeau, 1826);
- (4)
- Substitution of Parthenina alesii Micali, Nofroni & Perna, 2012 with Parthenina cf. suturalis (R. A. Philippi, 1844).
3.2. Non-Native Species (NNS)
4. Discussion
4.1. Jujubinus vulgaris (Risso, 1826)
4.2. Rissoa auriscalpium (Linnaeus, 1758)
4.3. Rissoa membranacea (J. Adams, 1800)
4.4. Gibberula caelata (Monterosato, 1877)
4.5. Doriopsilla areolata (Bergh, 1880)
4.6. Cyerce cristallina (Trinchese, 1881)
4.7. Bosemprella daniliana (Brusina, 1866)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NNS | Non-Native Species |
| SS | Sampling Site(s) |
References
- Soto, I.; Balzani, P.; Carneiro, L.; Cuthbert, R.N.; Macêdo, R.; Serhan Tarkan, A.; Ahmed, D.A.; Bang, A.; Bacela-Spychalska, K.; Bailey, S.A.; et al. Taming the terminological tempest in invasion science. Biol. Rev. 2024, 99, 1357–1390. [Google Scholar] [CrossRef] [PubMed]
- Renda, W.; Amati, B.; Bogi, C.; Bonomolo, G.; Capua, D.; Dell’Angelo, B.; Furfaro, G.; Giannuzzi-Savelli, R.; La Perna, R.; Nofroni, I.; et al. The new checklist of the Italian fauna: Marine Mollusca. Biogeogr.—J. Integr. Biogeogr. 2022, 37, ucl005. [Google Scholar] [CrossRef]
- Hassoun, A.E.R.; Mojtahid, M.; Merheb, M.; Lionello, P.; Gattuso, J.-P.; Cramer, W. Climate change risks on key open marine and coastal mediterranean ecosystems. Sci. Rep. 2025, 15, 24907. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U. Chapter 17 Molluscs as Bioindicators. In Trace Metals and Other Contaminants in the Environment; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 6, pp. 577–635. [Google Scholar]
- Bouchet, P. The magnitude of marine biodiversity. In The Exploration of Marine Biodiversity: Scientific and Technological Challenges; Duarte, C.M., Ed.; Fundación BBVA: Bilbao, Spain, 2006; p. 158. [Google Scholar]
- Chapman, M.G.; Blockley, D.J. Engineering novel habitats on urban infrastructure to increase intertidal biodiversity. Oecologia 2009, 161, 625–635. [Google Scholar] [CrossRef]
- Zhang, Z. Animal biodiversity: An introduction to higher-level classification and taxonomic richness. Zootaxa 2011, 3148, 7–12. [Google Scholar] [CrossRef]
- Zhang, Z. Phylum Arthropoda. In: Zhang, Z.-Q. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa 2013, 3703, 17–26. [Google Scholar] [CrossRef]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2020; Volume 2. [Google Scholar]
- MolluscaBase. World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=137914 (accessed on 15 October 2025).
- Galil, B.S.; Marchini, A.; Occhipinti-Ambrogi, A. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2018, 201, 7–16. [Google Scholar] [CrossRef]
- Zenetos, A.; Delongueville, C.; Scaillet, R. An Overlooked group of citizen scientists in NIS information: Shell collectors and their contribution to molluscan NIS diversity. Diversity 2024, 16, 299. [Google Scholar] [CrossRef]
- Guelorget, O.; Perthuisot, J.P. Paralic ecosystems biological organization and functioning. Vie et Milieu 1992, 42, 215–251. [Google Scholar]
- Novoa, L.; Aldea, C.; Andrade, C.; Guarda, B.; Holtheuer, J.; Schories, D. Unveiling hidden molluscan diversity: New species records in Bougainville Bay, Strait of Magellan. Biodivers. Data J. 2025, 13, e157304. [Google Scholar] [CrossRef]
- Underwood, A.J. The effects of grazing by gastropods and physical factors on the upper limits of distribution of intertidal macroalgae. Oecologia 1980, 46, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, P.; Smriglio, C.; Oliverio, M.; Rossi, S.; Di Giulio, A. Checklist of the marine malacofauna of Culuccia Peninsula (NW Sardinia, Italy), with notes on relevant species. Biodivers. Data J. 2024, 12, e115051. [Google Scholar] [CrossRef] [PubMed]
- Annessi, M.; Riccieri, A.; Marconi, M.; Rossi, S.; Di Giulio, A. Integrating taxonomic, genetic and ecological data to explore the diversity of wild bees (Hymenoptera: Apoidea: Anthophila) of the Culuccia Peninsula (NE Sardinia, Italy). J. Hymenopt. Res. 2025, 98, 117–145. [Google Scholar] [CrossRef]
- Annessi, M.; Marzialetti, F.; Marconi, M.; Acosta, A.T.R.; Di Giulio, A. Butterfly diversity in relation to the CORINE Land Cover of the Culuccia Peninsula (Sardinia, Italy). J. Insect Conserv. 2025, 29, 17. [Google Scholar] [CrossRef]
- Annessi, M.; Riccieri, A.; Di Giulio, A. A new species of Andrena (Hymenoptera, Andrenidae) from northern Sardinia (Italy). J. Hymenopt. Res. 2025, 98, 795–816. [Google Scholar] [CrossRef]
- Annessi, M.; Forte, F.; Di Giulio, A. Flower visitor insects of Myrtus communis L., 1753 in the Culuccia Peninsula (NE Sardinia, Italy). Fragm. Entomol. 2025, 57, 293–296. [Google Scholar]
- Garzia, M.; Doneddu, M.; Giacobbe, S.; Salvi, D.; Trainito, E.; Mariottini, P. Molecular and morphological data provide evidence for only one alien species of pearl oyster in the Mediterranean Sea. Sci. Mar. 2025, 88, e085. [Google Scholar] [CrossRef]
- Pezzotti, S. Il Genere Jujubinus (Gastropoda; Vetigastropoda; Trochidae): Un Approccio Molecolare. Master’s Thesis, University of Roma Tre, Rome, Italy, 2018. [Google Scholar]
- Colognola, R.; Masturzo, P.; Russo, G.F.; Scardi, M.; Vinci, D.; Fresi, E. Biometric and genetic analysis of the marine rissoid Rissoa auriscalpium (Gastropoda, Prosobranchia) and its ecological implications. Mar. Ecol. 1986, 7, 265–285. [Google Scholar] [CrossRef]
- Oliverio, M. Developmental vs genetic variation in two Mediterranean rissoid gastropod complexes. J. Molluscan Stud. 1994, 60, 461–465. [Google Scholar] [CrossRef]
- Russo, G.F.; Patti, F.P. Early life history of two closely related gastropods, Rissoa auriscalpium and Rissoa italiensis (Caenogastropoda: Rissoidae). Mar. Biol. 2005, 147, 429–437. [Google Scholar] [CrossRef]
- Munksgaard, C. Electrophoretic separation of morphologically similar species of the genus Rissoa (Gastropoda: Prosobranchia). Ophelia 1990, 31, 97–104. [Google Scholar] [CrossRef]
- van Aartsen, J.J.; Menkhorst, H.P.M.G.; Gittenberger, E. The marine mollusca of the Bay of Algeciras, Spain, with general notes on Mitrella, Marginellidae and Turridae. Basteria 1984, 1–135. Available online: http://natuurtijdschriften.nl/download?type=document;docid=645087 (accessed on 15 October 2025).
- Gofas, S.; Moreno, D.; Salas, C. Moluscos Marinos de Andalucía; Servicio de Publicaciones e Intercambio Científico, Universidad de Málaga: Málaga, Spain, 2011; Volume 1, pp. 1–342. ISBN 978-84-9747356-9. [Google Scholar]
- Appolloni, M.; Smriglio, C.; Amati, B.; Lugli, È.L.; Nofroni, I.; Tringali, L.P.; Mariottini, P.; Oliverio, M. Catalogue of the primary types of marine molluscan taxa described by Tommaso Allery Di Maria, Marquis of Monterosato, deposited in the Museo Civico di Zoologia, Roma. Zootaxa 2018, 4477, 1–138. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, G.; Schreier, C.; Trainito, E.; Pontes, M.; Madrenas, E.; Girard, P.; Mariottini, P. The Sea Slug Doriopsilla areolata Bergh, 1880 (Mollusca, Gastropoda) in the Mediterranean Sea: Another Case of Cryptic Diversity. Diversity 2022, 14, 297. [Google Scholar] [CrossRef]
- Moreno, K.; Rico, D.M.; Middlebrooks, M.; Medrano, S.; Valdés, Á.A.; Krug, P.J. A cryptic radiation of Caribbean Sea slugs revealed by integrative analysis: Cyerce ‘antillensis’ (Sacoglossa: Caliphyllidae) is six distinct species. Zool. J. Linn. Soc. 2024, 4, 940–979. [Google Scholar] [CrossRef] [PubMed]
- Brusina, S. Contribuzione Pella Fauna dei Molluschi Dalmatic; Imperiale e Reale Società Zoologico-Botanica: Vienna, Austria, 1866; p. 134. [Google Scholar]
- Strong, E.E.; Gargominy, O.; Ponder, W.F.; Bouchet, P. Global Diversity of Gastropods (Gastropoda; Mollusca) in Freshwater. Hydrobiologia 2008, 595, 149–166. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Voultsiadou, E.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple nonspecies-specific pathogens possibly triggered the mass mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef]
- Cossu, P.; Mura, L.; Dedola, G.L.; Lai, T.; Sanna, D.; Scarpa, F.; Azzena, I.; Fois, N.; Casu, M. Detection of genetic patterns in endangered marine species is affected by small sample sizes. Animals 2022, 12, 2763. [Google Scholar] [CrossRef]
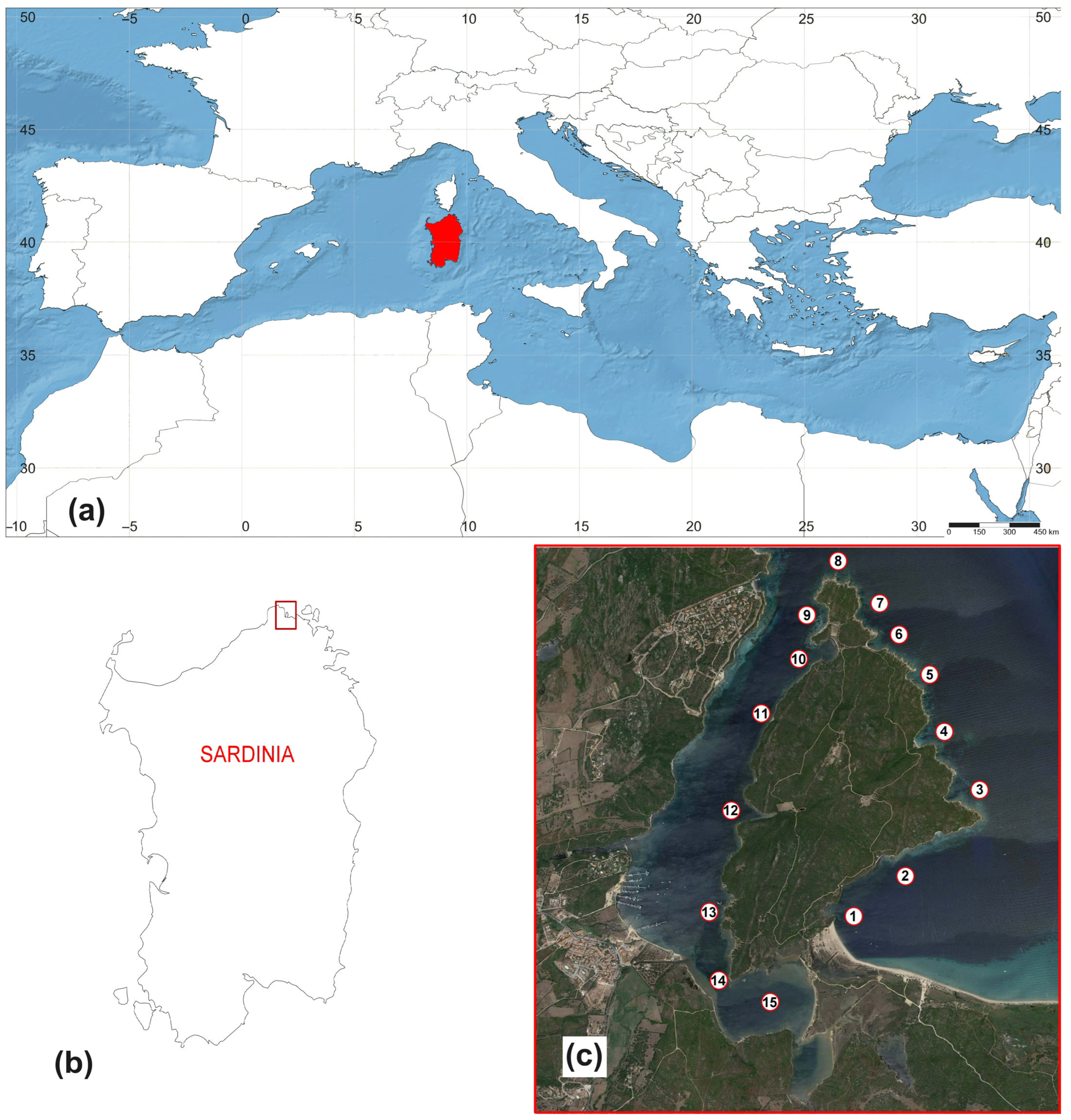




| Family | Species | Sampling Site | Shell/Alive |
|---|---|---|---|
| Patellidae | Patella caerulea Linnaeus, 1758 | #2,#3,#4,#5,#7,#8,#9,#10,#11 | alive |
| Patella ferruginea Gmelin, 1791 | #4,#5,#8 | alive | |
| Patella rustica Linnaeus, 1758 | #4,#5,#6,#7 | alive | |
| Patella ulyssiponensis Gmelin, 1791 | #1,#2,#3#,#7 | alive | |
| Lottiidae | Tectura virginea (Mueller O.F., 1776) | #8 | shell |
| Scissurellidae | Scissurella costata d’Orbigny, 1824 | #8 | shell |
| Haliotidae | Haliotis tuberculata Linnaeus, 1758 | #4,#5,#8,#12,#13 | alive |
| Fissurellidae | Emarginula adriatica OG Costa, 1830 | #12 | alive |
| Emarginula huzardii Payraudeau, 1826 | #14 | alive | |
| Emarginula pustula Thiele in Küster, 1913 | #8 | shell | |
| Emarginula sicula JE Gray, 1825 | #8 | shell | |
| Diodora dorsata (Monterosato, 1878) (Figure 3a) | #9 | alive | |
| Diodora gibberula (Lamarck, 1822) | #8 | shell | |
| Diodora graeca (Linnaeus, 1758) (Figure 3b) | #1,#13 | alive | |
| Diodora italica (Defrance, 1820) | #4 | alive | |
| Fissurella nubecula (Linnaeus, 1758) (Figure 3c) | #9 | alive | |
| Trochidae | Phorcus articulatus (Lamarck, 1822) | #13 | alive |
| Phorcus turbinatus (Born, 1778) | #4,#5,#9 | alive | |
| Gibbula ardens (Salis Marschlins, 1793) | #6 | alive | |
| Gibbula fanulum (Gmelin, 1791) | #8 | alive | |
| Gibbula guttadauri (Philippi, 1836) | #8 | shell | |
| Gibbula turbinoides (Deshayes, 1835) | #8 | shell | |
| Steromphala adansonii (Payraudeau, 1826) (Figure 3d) | #10 | shell | |
| Steromphala divaricata (Linnaeus, 1758) (Figure 3e) | #1,#2 | alive | |
| Steromphala umbilicaris (Linnaeus, 1758) | #4,#9,#10 | alive | |
| Steromphala varia (Linnaeus, 1758) (Figure 3f) | #10 | alive | |
| Jujubinus baudoni (Monterosato, 1891) | #8 | shell | |
| Jujubinus depictus (Deshayes, 1835) | #8 | shell | |
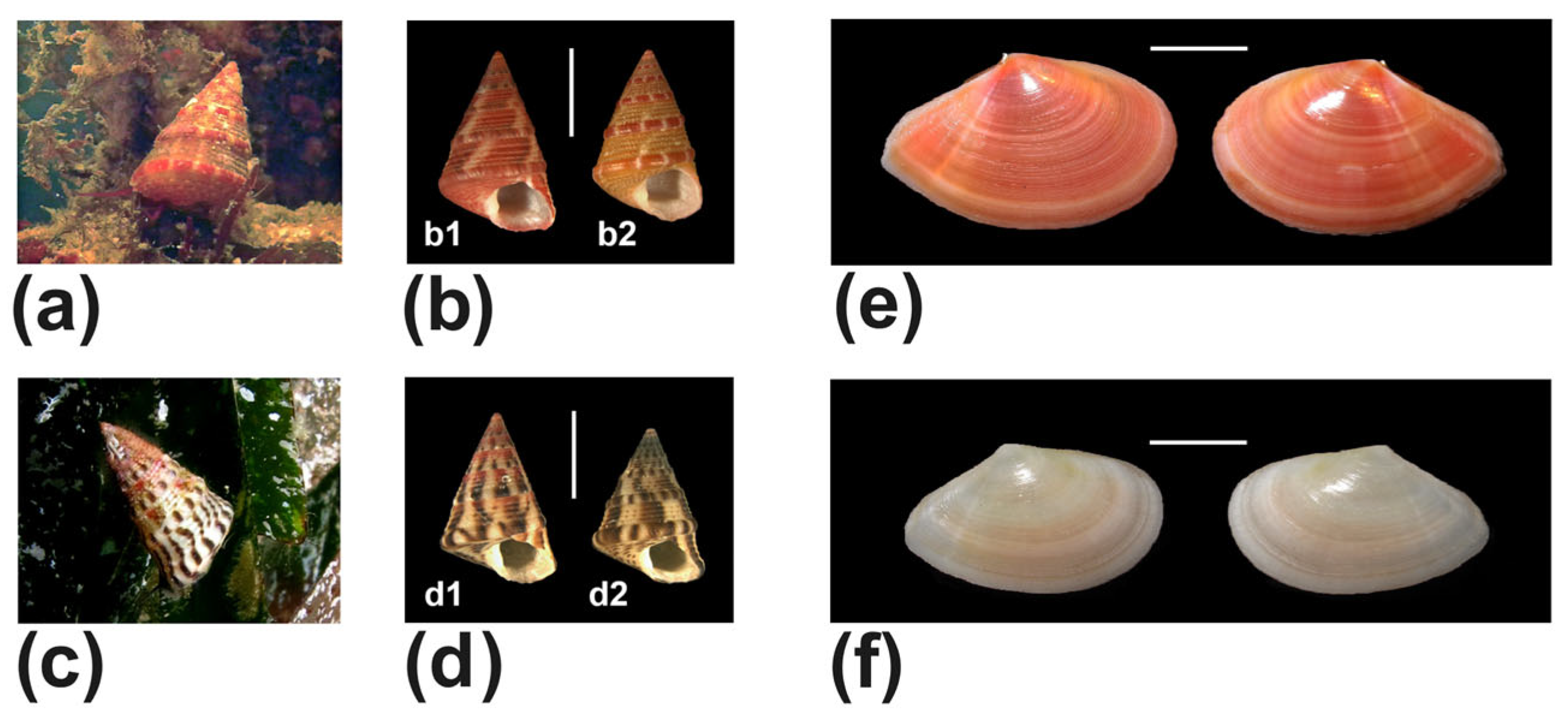
| Jujubinus exasperatus (Pennant, 1777) (Figure 12a,b) | #4,#5,#8 | alive | |
| Jujubinus striatus (Linnaeus, 1758) | #8 | shell | |
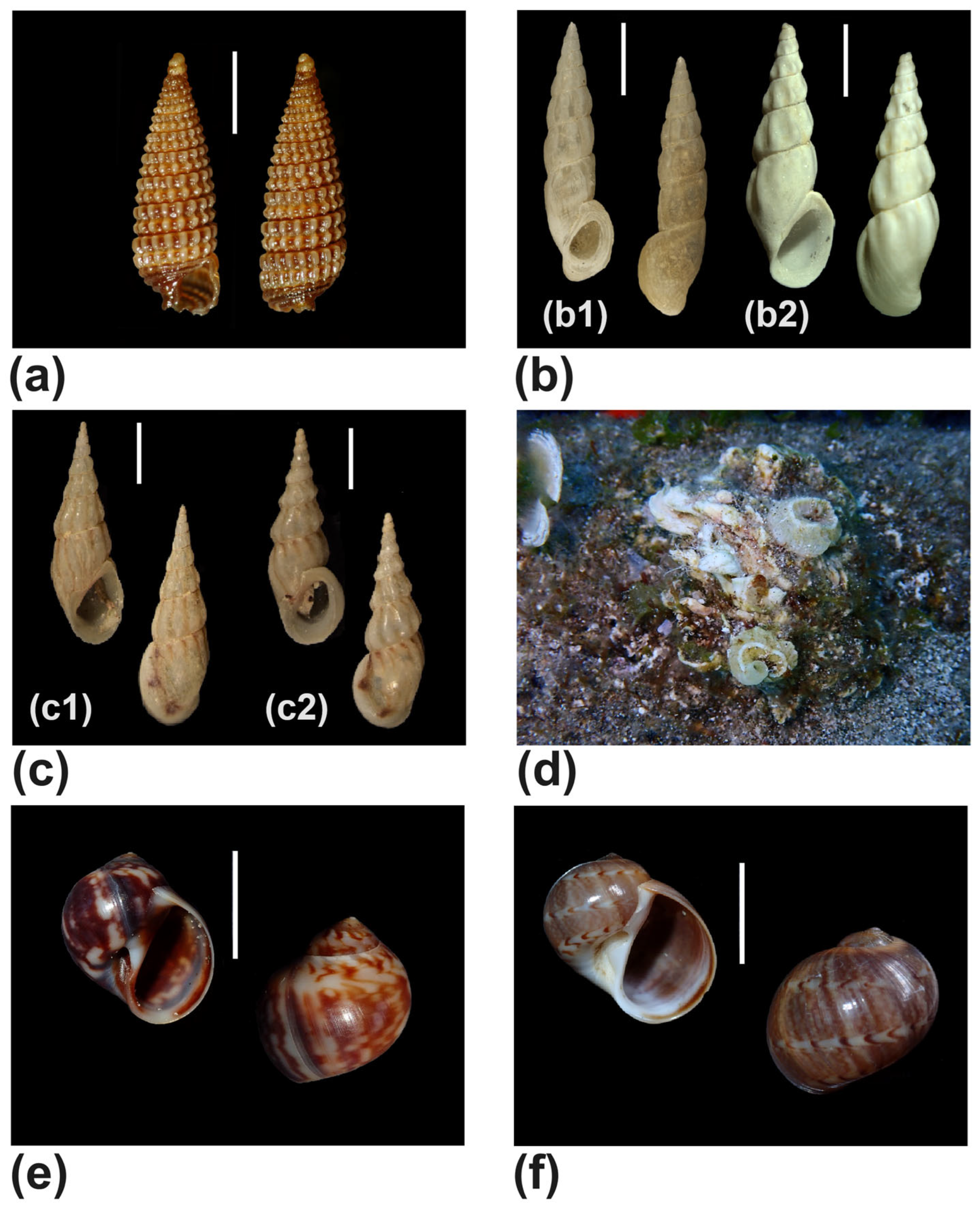
| Jujubinus vulgaris (Risso, 1826) (Figure 12c,d) | #4,#8,#12 | alive | |
| Clanculus corallinus (Gmelin, 1791) | #8 | shell | |
| Clanculus cruciatus (Linnaeus, 1758) | #4,#5,#10 | alive | |
| Clanculus jussieui (Payraudeau, 1826) | #4,#5,#9,#10 | alive | |
| Calliostomatidae | Calliostoma laugieri (Payraudeau, 1826) | #8 | alive |
| Calliostoma conulus (Linnaeus, 1758) | #8 | alive | |
| Skeneidae | Skenea serpuloides (Montagu, 1808) | #8 | shell |
| Turbinidae | Bolma rugosa (Linnaeus 1767) | #3,#4,#7 | alive |
| Homalopoma sanguineum (Linnaeus, 1758) | #8 | shell | |
| Phasianellidae | Tricolia pullus (Linnaeus, 1758) | #8 | shell |
| Tricolia speciosa (Von Muhelfeldt, 1824) | #8,#12 | alive | |
| Neritidae | Smaragdia viridis (Linnaeus, 1758) | #9,#10,#12 | alive |
| Cerithiidae | Bittium reticulatum (da Costa, 1778) | #4,#5,#6 | alive |
| Cerithium renovatum Monterosato, 1884 | #4,#5#8,12,#13,#14 | alive | |
| Cerithium vulgatum Bruguiere, 1792 | #2,#4,#5,#6 | alive | |
| Potamididae | Pirenella conica (Blainville, 1826) | #15 | alive |
| Turritellidae | Turritella turbona Monterosato, 1887 | #8 | shell |
| Triphoridae | Marshallora adversa (Montagu, 1803) | #8 | shell |
| Monophorus perversus (Linnaeus, 1758) | #8 | shell | |
| Metaxia metaxa (Delle Chiaje, 1828) | #8 | shell | |
| Cerithiopsidae | Dizoniopsis coppolae (Aradas, 1870) (Figure 4a) | #8 | shell |
| Cerithiopsis tubercularis (Montagu, 1803) | #8 | shell | |
| Epitoniidae | Epitonium clathrus (Linnaeus, 1758) | #8 | shell |
| Eulimidae | Eulima glabra (da Costa, 1778) | #8 | shell |
| Eulima sp. | #8 | shell | |
| Melanella polita (Linnaeus, 1758) | #8 | shell | |
| Parvioris ibizenca (F. Nordsieck, 1968) | #8 | shell | |
| Vanikoridae | Megalomphalus azoneus (Brusina, 1865) | #8 | shell |
| Rissoidae | Pusillina inconspicua (Alder, 1844) | #8 | shell |
| Pusillina marginata (Michaud, 1830) | #8 | shell | |
| Pusillina radiata (Philippi, 1836) | #8 | shell | |
| Rissoa auriscalpium (Linnaeus, 1758) (Figure 4b) | #8 | shell | |
| Rissoa lia (Monterosato, 1884) | #8 | shell | |
| Rissoa membranacea (J. Adams, 1800) (Figure 4c) | #8 | shell | |
| Rissoa variabilis (Megerle von Mühlfeld, 1824) | #8 | shell | |
| Rissoa ventricosa Desmarest, 1814 | #8 | shell | |
| Rissoa violacea Desmarest, 1814 | #8 | shell | |
| Alvania beanii (Hanley, 1844) | #8 | shell | |
| Alvania carinata (da Costa, 1778) | #8 | shell | |
| Alvania lactea (Michaud, 1830) | #8 | shell | |
| Alvania lineata Risso, 1826 | #8 | shell | |
| Alvania mamillata Risso, 1826 | #8 | shell | |
| Alvania scabra (Philippi, 1844) | #8 | shell | |
| Rissoina bruguieri (Payraudeau, 1826) | #8 | shell | |
| Crisilla semistriata (Montagu, 1808) | #8 | shell | |
| Manzonia crassa (Kanmacher, 1798) | #8 | shell | |
| Caecidae | Caecum subannulatum de Folin, 1870 | #8 | shell |
| Caecum trachea (Montagu, 1803) | #8 | shell | |
| Truncartellidae | Truncatella subcylindrica (Linnaeus, 1767) | #8 | shell |
| Calyptreidae | Calyptraea chinensis (Linnaeus, 1758) | #8 | shell |
| Crepidula unguiformis Lamarck, 1822 | #8 | shell | |
| Triviidae | Trivia arctica (Pulteney, 1799) | #8 | shell |
| Trivia mediterranea (Risso, 1826) | #8 | shell | |
| Vermetidae | Thylacodes arenarius (Linnaeus, 1758) | #2,#4,#5 | alive |
| Vermetus triquetrus Bivona e Bernardi, 1832 (Figure 4d) | #6 | alive | |
| Cypreaidae | Luria lurida (Linnaeus, 1758) | #4,#8,#9 | alive |
| Naria spurca (Linnaeus, 1758) | #4,#8 | alive | |
| Naticidae | Euspira intricata (Donovan, 1804) | #8 | shell |
| Euspira macilenta (Philippi, 1844) (Figure 4e) | #2 | shell | |
| Euspira nitida (Donovan, 1803) | #8 | shell | |
| Naticarius hebraeus (Martyn, 1786) | #1 | shell | |
| Notocochlis dillwynii (Payraudeau, 1826) (Figure 4f) | #2 | shell | |
| Neverita josephinia Risso, 1826 | #1,#2 | alive | |
| Cassidae | Semicassis granulata (Born, 1778) | #1 | shell |
| Bursidae | Talisman scrobilator (Linnaeus, 1758) | #8 | shell |
| Cymatiidae | Cabestana cutacea (Linnaeus, 1767) (Figure 5a) | #8 | alive |
| Monoplex parthenopeus (Salis-Marschlins, 1793) (Figure 5b,c) | #8 | shell, crabbed | |
| Muricidae | Bolinus brandaris (Linnaeus, 1758) | #14 | alive |
| Hexaplex trunculus (Linnaeus, 1758) | #1,#4,#5,#9,#14,#15 | alive | |
| Dermomurex scalaroides (de Blainville, 1829) | #8 | shell | |
| Ocenebra edwardsii (Payraudeau, 1826) | #8 | shell | |
| Ocinebrina corallina Scacchi, 1836 | #8 | shell | |
| Muricopsis cristata (Brocchi, 1814) | #1,#2,#4,#6,#8,#10,#13 | alive | |
| Typhinellus labiatus (de Cristofori & Jan, 1832) | #8 | shell | |
| Stramonita haemastoma (Linnaeus, 1767) | #2,#5,#6,#8 | alive | |
| Coralliophila meyendorffii (Calcara, 1845) | #5 | alive | |
| Cystiscidae | Granulina marginata (Bivona, 1832) | #8 | shell |
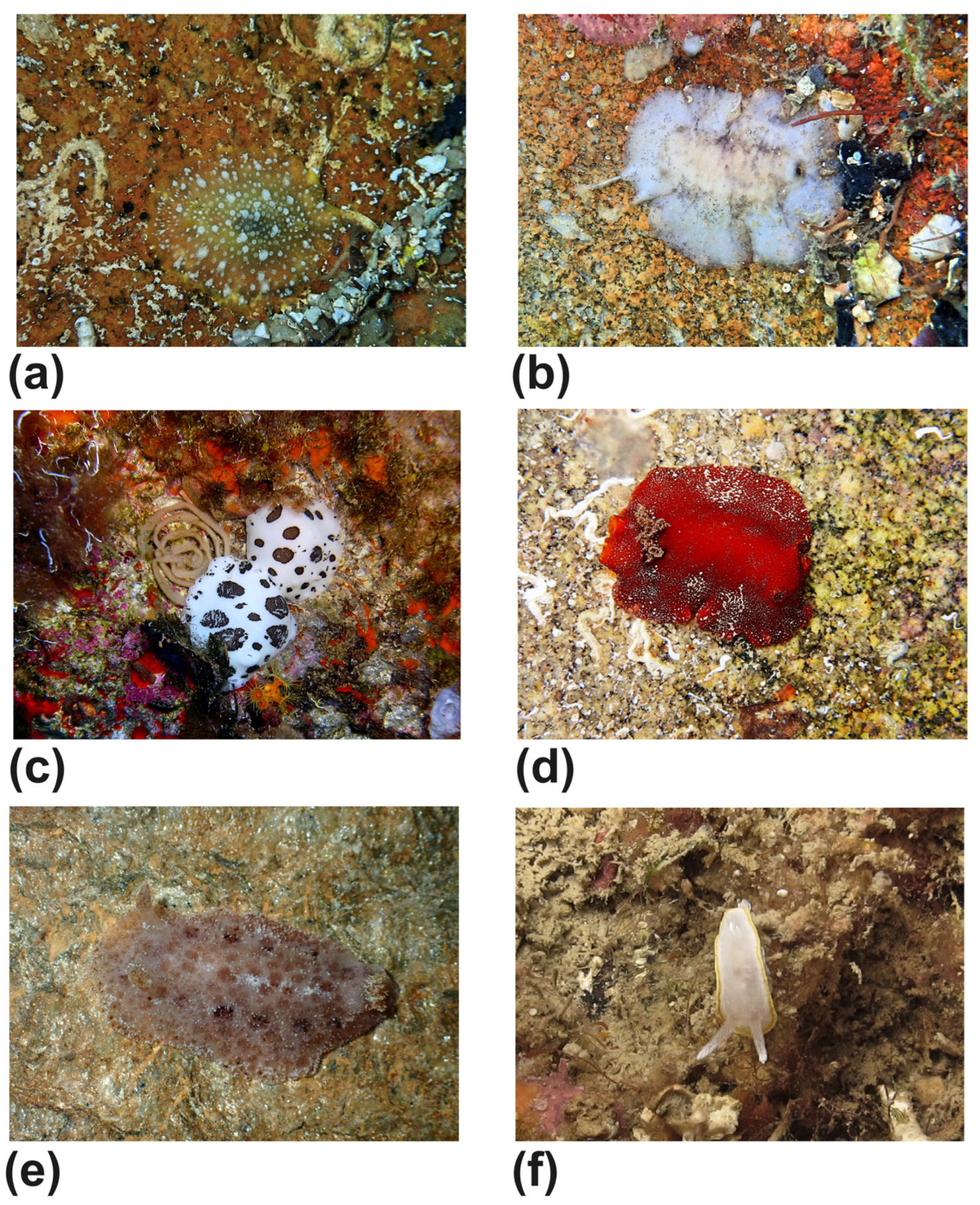
| Marginellidae | Gibberula caelata (Monterosato, 1877) (Figure 5d) | #8 | shell |
| Gibberula miliaria (Linnaeus, 1758) | #8 | shell | |
| Gibberula philippii (Monterosato, 1878) | #8 | shell | |
| Costellaridae | Ebenomitra ebenus (Lamark, 1811) | #9,#10 | shell |
| Ebenomitra savignyi (Payraudeau, 1826) | #8 | shell | |
| Ebenomitra tricolor (Gmelin, 1790) | #8 | shell | |
| Chauvetiidae | Chauvetia mamillata (Risso, 1826) | #8 | shell |
| Chauvetia turritellata (Deshayes, 1835) | #8 | shell | |
| Pisaniidae | Pisania striata (Gmelin, 1791) | #4,#5,#7,#10,#12 | alive |
| Aplus dorbigny (Payraudeau, 1826) | #4,#10,#12 | alive | |
| Aplus scaber (Locard, 1891) | #8 | shell | |
| Nassariidae | Tritia corniculum (Olivi, 1792) | #1,#2 | alive |
| Tritia incrassata (Strøm, 1768) | #8 | alive | |
| Tritia grana (Lamarck, 1822) (Figure 5e) | #8 | shell | |
| Tritia mutabilis (Linnaeus, 1758) | #1,#2 | shell | |
| Tritia neritea (Linnaeus, 1758) | #15 | alive | |
| Tritia pellucida (Risso, 1827) | #1,#4 | shell | |
| Tudiclidae | Euthria cornea (Linnaeus, 1758) | #2,#4,#9 | alive |
| Columbellidae | Columbella rustica (Linnaeus, 1758) | #1,#2,#3,#8 | alive |
| Mitrella minor (Scacchi, 1836) | #8 | shell | |
| Mitrella scripta (Linnaeus, 1758) | #12 | alive | |
| Mitridae | Episcomitra cornicula (Linnaeus, 1758) | #4,#7 | alive |
| Fasciolariide | Tarantinaea lignaria (Linnaeus, 1758) | #4,#5,#8,#10,#12 | alive |
| Pseudofusus pulchellus (R. A. Philippi, 1844) | #8 | shell | |
| Aptyxis syracusana (Linnaeus, 1758) | #1,#2 | shell | |
| Horaiclavidae | Haedropleura septangularis (Montagu, 1803) | #8 | shell |
| Mitromorphidae | Mitromorpha columbellaria (Scacchi, 1836) | #8 | shell |
| Conidae | Conus ventricosus Gmelin, 1791 | #1,#2,#4,#14 | alive |
| Raphitomidae | Cyrillia linearis (Montagu, 1803) | #8,#10 | alive |
| Leufroyia concinna (Scacchi, 1836) | #8 | shell | |
| Leufroyia leufroyi (Michaud, 1828) | #8 | shell | |
| Raphitoma bicolor (Risso, 1826) | #8 | shell | |
| Raphitoma densa (Monterosato, 1884) | #8 | shell | |
| Raphitoma horrida (Monterosato, 1884) | #8 | shell | |
| Raphitoma lineolata (Bucquoy, Dautzenberg & Dollfus, 1883) | #8 | shell | |
| Raphitoma locardi Pusateri, Giannuzzi-Savelli & Oliverio, 2013 | #8 | shell | |
| Raphitoma philberti (Michaud, 1829) | #8 | shell | |
| Mangeliidae | Lyromangelia taeniata (Deshayes, 1835) | #8 | shell |
| Mangelia multilineolata (Deshayes, 1835) | #8 | shell | |
| Mangelia unifasciata (Deshayes, 1835) | #8 | shell | |
| Pseudomangelia vauquelini (Payraudeau, 1827) | #8 | shell | |
| Pyrgocythara stosiciana (Brusina, 1869) | #8 | shell | |
| Smithiella costulata (Risso, 1826) (Figure 5f) | #8 | shell | |
| Pleurobranchidae | Berthella plumula (Montagu, 1803) | #12,#13,#14 | alive |
| Berthella aurantiaca (Risso, 1818) | #4,#5,#8,#9,#10 | alive | |
| Chromodorididae | Felimida elegantula (R. A. Philippi, 1844) | #12 | alive |
| Felimida krohni (Vérany, 1846) | #9,#10 | alive | |
| Felimida luteorosa (Rapp, 1827) | #1,#2 | alive | |
| Felimida purpurea (Risso, 1831) | #5,#8 | alive | |
| Felimare orsinii (Vérany, 1846) | #5 | alive | |
| Felimare picta (Schultz in Philippi, 1836) | #1 | alive | |
| Felimare villafranca (Risso, 1818) | #5,#12 | alive | |
| Phyllidiidae | Phyllidia flava Aradas, 1847 | #8 | alive |
| Dendrodorididae | Dendrodoris grandiflora (Rapp, 1827) | #8,#10 | alive |
| Dendrodoris limbata (Cuvier, 1804) | #8,#12,#13,#14 | alive | |
| Doriopsilla rarispinosa Pruvot-Fol, 1951 (Figure 6a) | #12 | alive | |
| Taringa armata Swennen, 1961 | #4,#12 | alive | |
| Discodorididae | Paradoris indecora (Bergh, 1881) (Figure 6b) | #13 | alive |
| Peltodoris atromaculata Bergh, 1880 (Figure 6c) | #7,#8 | alive | |
| Platydoris argo (Linnaeus, 1767) (Figure 6d) | #4 | alive | |
| Tayuva lilacina (A. Gould, 1852) (Figure 6e) | #4 | alive | |
| Calycidorididae | Diaphorodoris alba Portmann & Sandmeier, 1960 (Figure 6f) | #2 | alive |
| Diaphorodoris papillata Portmann & Sandmeier, 1960 (Figure 7a) | #2 | alive | |
| Janolidae | Antiopella cristata (Delle Chiaje, 1841) | #8 | alive |
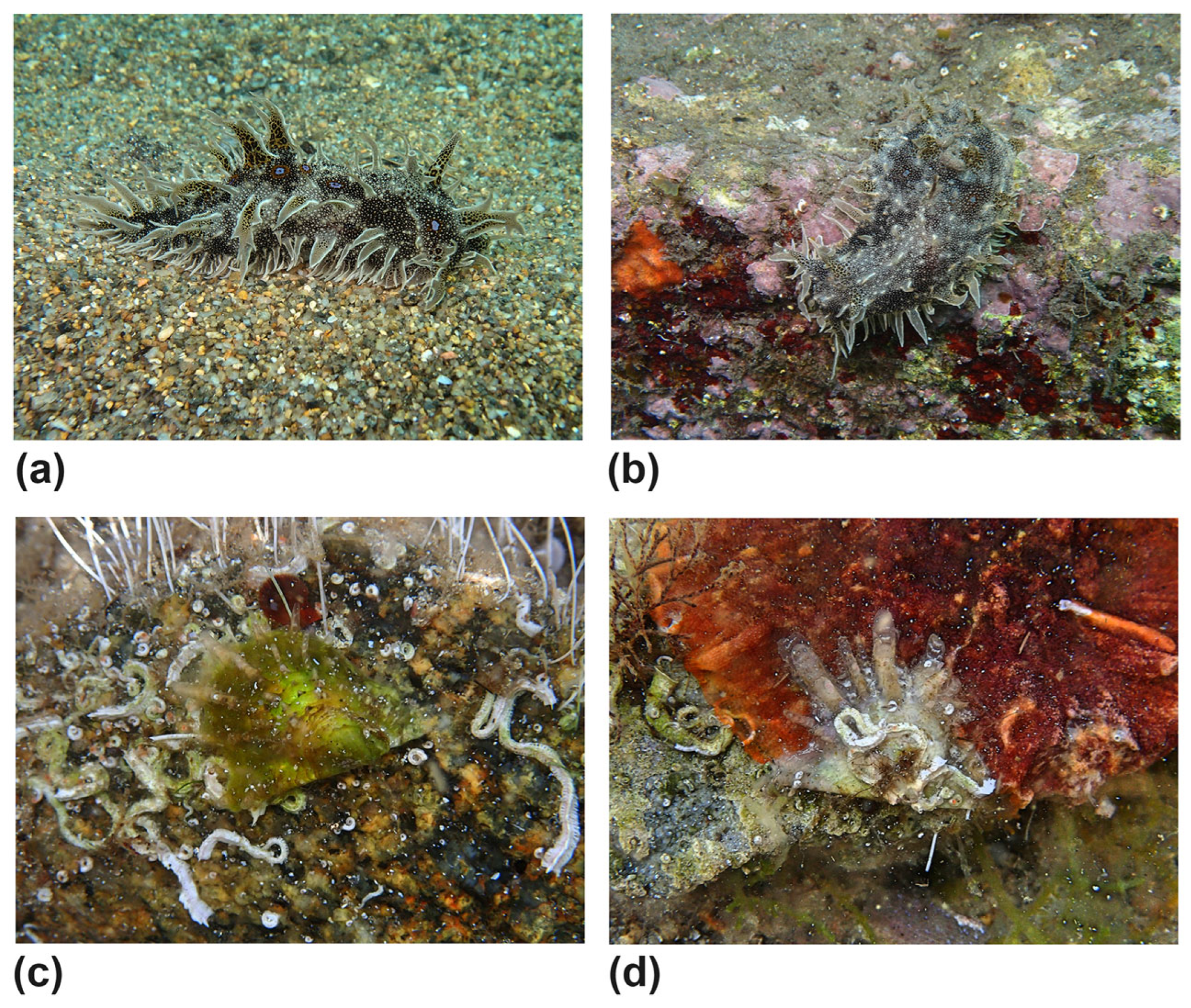
| Aeolidiidae | Aeolidiella alderi (Cocks, 1852) | #12,#14 | alive |
| Spurilla neapolitana (Delle Chiaje, 1841) | #1,#2,#9,#12 | alive | |
| Facelinidae | Caloria elegans (Alder & Hancock, 1845) (Figure 7b) | #8 | alive |
| Caloria quatrefagesi (Vayssière, 1888) (Figure 7c) | #2 | alive | |
| Cratena peregrina (Gmelin, 1791) | #1,#13,#14 | alive | |
| Tylodinidae | Tylodina perversa (Gmelin, 1791) | #8 | shell |
| Umbraculidae | Umbraculum umbraculum ([Lighfoot], 1786) | #10 | alive |
| Cylichnidae | Cylichna cylindracea (Pennant, 1777) | #8 | shell |
| Haminoeidae | Haminoea hydatis (Linnaeus, 1758) | #1,#14 | shell |
| Lamprohaminoea cyanomarginata (Heller & T. E. Thompson, 1983) * | #9 | alive | |
| Philinidae | Philine catena (Montagu, 1803) | #8 | shell |
| Aplysidae | Aplysia depilans Gmelin, 1791 | #10,#12 | alive |
| Aplysia fasciata Poiret, 1789 (Figure 7d) | #1 | alive | |
| Aplysia punctata (Cuvier, 1803) (Figure 7e) | #12 | alive | |
| Bursatella leachii Blainville, 1817 * (Figure 11a,b) | #12 | alive | |
| Petalifera petalifera (Rang, 1828) | #9 | alive | |
| Caliphyllidae | Cyerce cristallina (Trinchese, 1881) (Figure 7f) | #12 | alive |
| Plakobranchidae | Elysia timida (Risso, 1818) | #2,#4,#9,#12,#13 | alive |
| Thuridilla hopei (Vérany, 1853) | #9,#12 | alive | |
| Siphonariidae | Williamia gussoni (O. G. Costa, 1829) | #8 | shell |
| Pyramidellidae | Euparthenia humboldti (Risso, 1826) | #8 | shell |
| Folinella excavata (Philippi, 1836) | #8 | shell | |
| Parthenina cf. suturalis (R. A. Philippi, 1844) | #8 | shell | |
| Parthenina angulosa (Monterosato, 1889) | #8 | shell | |
| Parthenina monozona (Brusina, 1869) | #8 | shell | |
| Pyrgiscus jeffreysii (Forbes & Hanley, 1850) | #8 | shell | |
| Megastomia conoidea (Brocchi, 1814) | #8 | shell | |
| Turbonilla gradata Bucquoy, Dautzenberg & Dollfus, 1883 | #8 | shell |
| Family | Species | Sampling Site | Shell/Alive |
|---|---|---|---|
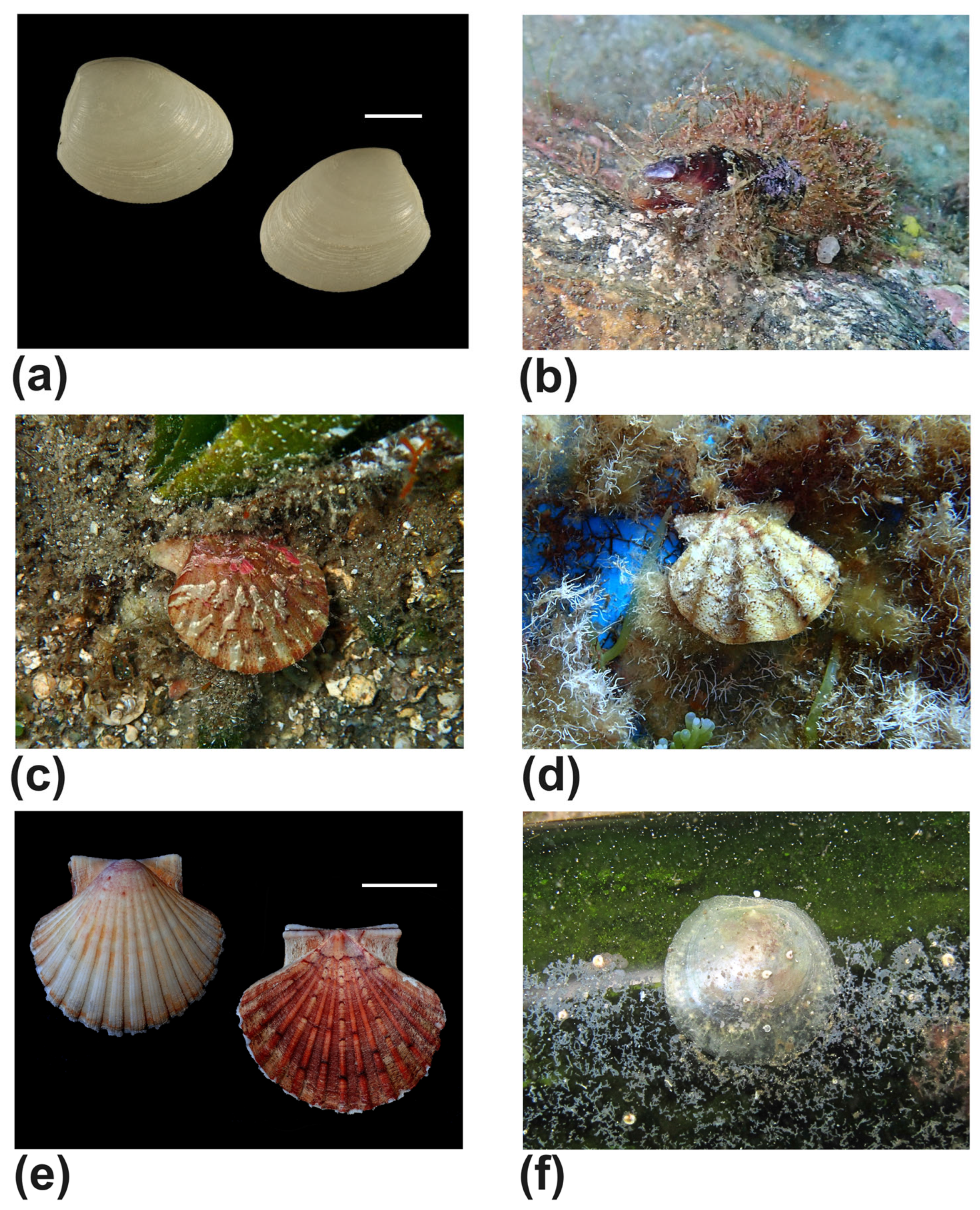
| Nuculidae | Nucula nitidosa Winckworth, 1930 (Figure 8a) | #8 | shell |
| Arcidae | Arca noae Linnaeus, 1758 | #12, | alive |
| Barbatia barbata (Linnaeus, 1758) | #4,#12,#13,#14,#15 | alive | |
| Noetiidae | Striarca lactea (Linnaeus, 1758) | #1,#2,#11,#14 | alive |
| Mytilidae | Litophaga lithophaga (Linnaeus, 1758) | #1,#2 | alive |
| Modiolus barbatus (Linnaeus, 1758) (Figure 8b) | #1,#2 | alive | |
| Mytilaster marioni (Locard, 1889) | #13 | alive | |
| Mytilus galloprovincialis Lamarck, 1819 | #1,#2,#15 | alive | |
| Musculus subpictus (Cantraine 1835) | #8 | shell | |
| Gregariella semigranata (Reeve, 1858) | #8 | shell | |
| Spondylidae | Spondylus gaederopus Linnaeus, 1758 | #2,#8 | alive |
| Pectinidae | Flexopecten hyalinus (Poli, 1795) (Figure 8c) | #8 | shell |
| #12 | alive (1) | ||
| Flexopecten flexuosus (Poli, 1795) (Figure 8d) | #8 | alive | |
| Mimachlamys varia (Linnaeus, 1758) | #10,#14,#15 | alive | |
| Manupecten pesfelis (Linnaeus, 1758) | #8 | alive | |
| Talochlamys multistriata (Poli, 1795) | #8,#14 | shell | |
| Palliolum incomparabile (Risso, 1826) | #8 | shell | |
| Pecten jacobeus (Linnaeus, 1758) (Figure 8e) | #1 | shell | |
| Anomiidae | Anomia ephippium Linnaeus, 1758 | #1,#2 | alive |
| Heteranomia squamula Linnaeus, 1759 | #8 | shell | |
| Pododesmus squama (Gmelin, 1791) (Figure 8f) | #10 | alive | |
| Limidae | Lima lima (Linnaeus, 1758) | #4,#9 | alive |
| Limaria hians (Gmelin, 1791) | #5, | alive | |
| Limaria tuberculata (Olivi, 1792) | #2,#12,#13,#14,#15 | alive | |
| Ostreidae | Ostrea edulis Linnaeus, 1758 | #1,#2,#10,#11,#13,#14 | alive |
| Ostrea stentina Payraudeau, 1826 | #2,#13,#14 | alive | |
| Magallana gigas (Thunberg, 1793) * | #12,#14 | shell | |
| Pinnidae | Pinna nobilis Linnaeus, 1758 | #10,#11 | shell |
| Pinna rudis Linnaeus, 1758 (Figure 9a) | #8 | alive | |
| Margaritidae | Pinctada radiata (Leach, 1814) * (Figure 11c,d) | #2 | alive (2) |
| #10 | shell | ||
| Lucinidae | Ctena decussata (O. G. Costa, 1829) | #8 | shell |
| Loripes orbiculatus Poli,1791 | #4 | shell | |
| Galeommatidae | Galeomma turtoni W. Turton, 1825 | #9 | shell |
| Carditidae | Glans trapezia (Linnaeus, 1767) | #8 | shell |
| Cardites antiquatus (Linnaeus, 1758) | #1,#2 | shell | |
| Cardita calyculata (Linnaeus, 1758) | #15 | alive | |
| Cardiidae | Acanthocardia paucicostata (G. B. Sowerby II, 1834) | #1,#2 | shell |
| Acanthocardia tuberculata (Linnaeus, 1758) | #1.#2,#4 | alive | |
| Cerastoderma glaucum (Brugui ère, 1789) | #14,#15 | alive | |
| Parvicardium exiguum (Gmelin, 1791) | #1 | shell | |
| Papillicardium papillosum (Poli, 1791) | #1 | shell | |
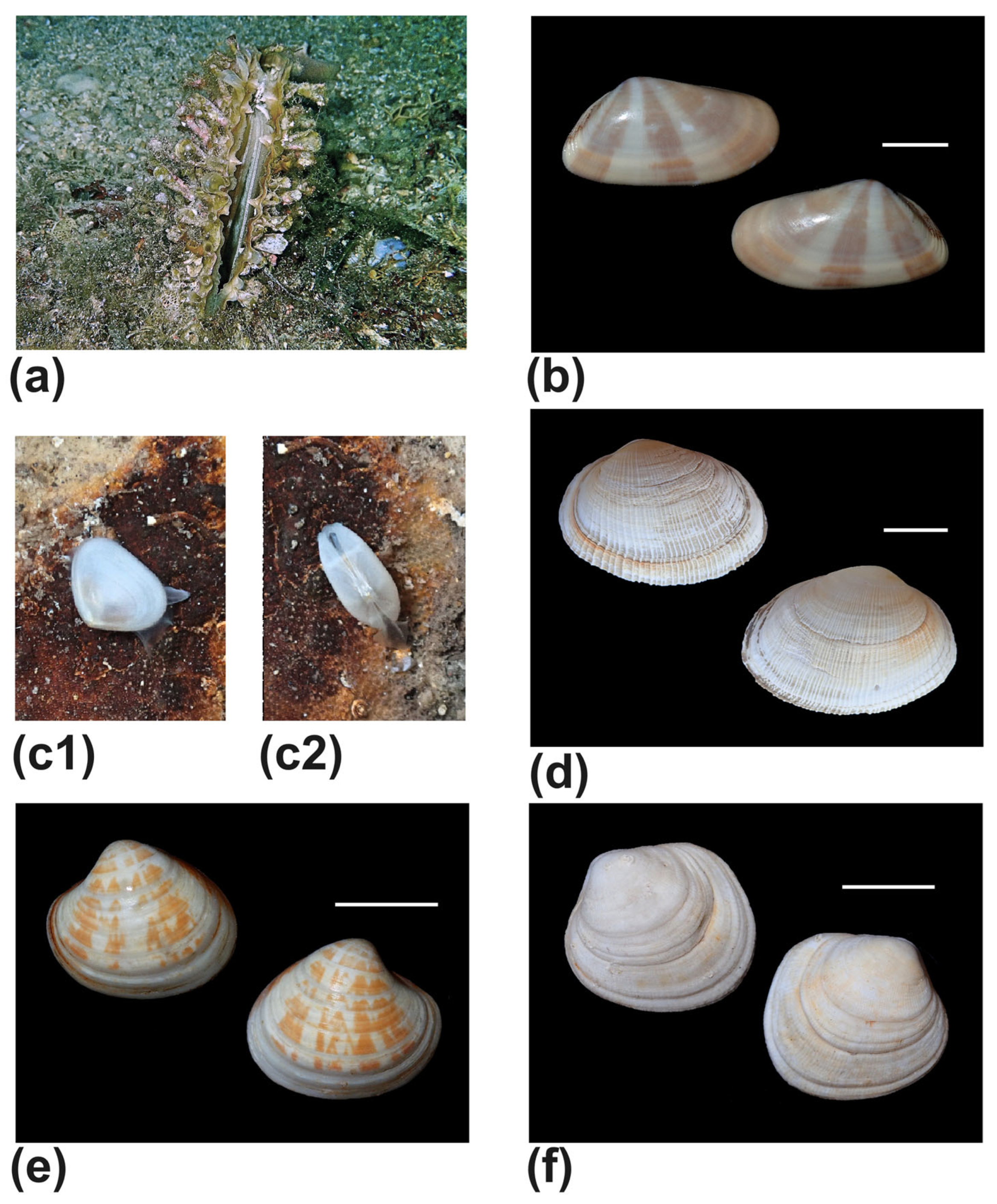
| Tellinidae | Bosemprella incarnata (Linnaeus, 1758) (Figure 12e) | #4 | shell |
| Bosemprella daniliana (Brusina, 1866) (Figure 12f) | #1 | beached | |
| Fabulina fabula (Gmelin, 1791) | #4 | shell | |
| Moerella donacina (Linnaues, 1758) | #1,#4,#8 | alive | |
| Moerella pulchella (Lamarck, 1818) | #1,#2 | shell | |
| Peronaea planata (Linnaeus, 1758) | #1,#2,#4 | alive | |
| Peronidia albicans (Gmelin, 1791) | #1, #2 | alive | |
| Arcopella balaustina (Linnaeus, 1758) | #8 | alive | |
| Gastrana fragilis (Linnaeus, 1758) | #1,#2,#3,#4 | alive | |
| Solecurtidae | Solecurtus strigilatus (Linnaeus, 1758) | #4 | shell |
| Donacidae | Donax trunculus Linnaeus, 1758 | #1,#2 | shell |
| Donax variegatus (Gmelin, 1791) | #1,#2,#8 | shell | |
| Donax venustus Poli, 1795 (Figure 9b) | #2 | shell | |
| Psammobiidae | Gari costulata (W. Turton, 1822) | #8 | shell |
| Gari depressa (Pennant, 1777) | #1,#2 | alive | |
| Chamidae | Chama circinata Monterosato, 1878 | #4, | alive |
| Chama gryphoides (Linnaeus, 1758) | #2,#4,#9 | alive | |
| Pseudochama gryphina (Lamarck, 1819) | #1,#2,#5 | alive | |
| Neoleptonidae | Neolepton sulcatulum (Jeffreys, 1859) | #8 | shell |
| Lasaeidae | Bornia sebetia (O. G. Costa, 1830) (Figure 9c) | #1 | alive |
| Mactridae | Eastonia rugosa (Helbling, 1779) (Figure 9d) | #1,#2 | shell |
| Mactra stultorum (Linnaeus, 1758) | #1,#2 | shell | |
| Spisula subtruncata (da Costa, 1778) | #1 | shell | |
| Lutraria oblonga (Gmelin, 1791) | #1 | shell | |
| Mesodesmatidae | Donacilla cornea (Poli, 1795) | #1,#15 | alive |
| Veneridae | Venus verrucosa Linnaeus, 1758 | #1 | alive |
| Dosinia exoleta (Linnaeus, 1758) | #1,#2 | alive | |
| Dosinia lupinus (Linnaeus, 1758) | #1 | alive | |
| Irus irus (Linnaeus, 1758) | #1 | shell | |
| Pitar rudis (Poli, 1795) (Figure 9e) | #1,#2 | shell | |
| Lajonkairialajonkairii (Payraudeau, 1826) (Figure 9f) | #1,#2 | shell | |
| Polititapes aureus (Gmelin, 1791) | #1,#2 | shell | |
| Polititapes lucens (Locard, 1886) | #8 | shell | |
| Ruditapes decussatus (Linnaeus, 1758) | #1 | shell | |
| Callista chione (Linnaeus, 1758) | #1 | shell | |
| Trapezidae | Coralliophaga lithophagella (Lamarck, 1819) | #8 | shell |
| Ungulinidae | Microstagon trigonum (Scacchi, 1835) | #8 | shell |
| Solenidae | Solen marginatus Pulteney, 1799 | #1 | shell |
| Pharidae | Ensis minor (Chenu, 1843) | #1 | shell |
| Myidae | Sphenia binghami W. Turton, 1822 | #8 | shell |
| Thraciidae | Thracia convexa (W. Wood, 1815) | #1,#2 | shell |
| Thracia phaseolina (Lamarck, 1818) | #1,#2 | shell | |
| Thracia villosiuscula (MacGillivray, 1827) | #1,#2 | shell |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariottini, P.; Smriglio, C.; Oliverio, M.; Rossi, S.; Di Giulio, A. New Records of Marine Mollusca from the Culuccia Peninsula (NW Sardinia, Italy). Diversity 2025, 17, 809. https://doi.org/10.3390/d17120809
Mariottini P, Smriglio C, Oliverio M, Rossi S, Di Giulio A. New Records of Marine Mollusca from the Culuccia Peninsula (NW Sardinia, Italy). Diversity. 2025; 17(12):809. https://doi.org/10.3390/d17120809
Chicago/Turabian StyleMariottini, Paolo, Carlo Smriglio, Marco Oliverio, Sabrina Rossi, and Andrea Di Giulio. 2025. "New Records of Marine Mollusca from the Culuccia Peninsula (NW Sardinia, Italy)" Diversity 17, no. 12: 809. https://doi.org/10.3390/d17120809
APA StyleMariottini, P., Smriglio, C., Oliverio, M., Rossi, S., & Di Giulio, A. (2025). New Records of Marine Mollusca from the Culuccia Peninsula (NW Sardinia, Italy). Diversity, 17(12), 809. https://doi.org/10.3390/d17120809








