Abstract
The majority of research on food webs has focused on temperate lakes, and little is known about the food web of lakes in polar regions. Subarctic lakes are particularly sensitive to climate change, which affects their stability. Therefore, the trophic structure of the food web in such lakes was considered as the object of this study. We studied a clear-water oligotrophic lake located in the subarctic region of Eurasia, specifically in northern Karelia and the White Sea coast of Russia. The study examined both open water periods (summer–autumn) and ice-covered periods (winter–spring) in this lake. Stable isotope analysis of carbon (13C/12C ratio or δ13C value) and nitrogen (15N/14N, δ15N) in producers and consumers was applied and revealed significant seasonal variations in the structure of the food web. The results indicate the presence of both pelagic and littoral/benthic food web compartments, with a notable contribution of autochthonous carbon derived from benthic sources. Omnivorous fish (perch, Perca fluviatilis; vendace, Coregonus albula; nine-spined sticklebacks, Pungitius pungitius) and some benthic invertebrates (mayfly, Ephemera vulgata; bivalves, Sphaerium corneum) had intermediate δ13C values, integrating these compartments by obtaining resources from both. Planktonic invertebrates had significantly depleted 13C, with the lowest δ13C value reaching −41.7‰, indicating an important contribution of methane-derived carbon. The study also revealed close trophic relationships between lake invertebrates and cyanobacteria, namely with planktonic Dolichospermum lemmermannii and benthic Phormidium sp. Seasonal changes in δ15N values and in trophic position have been observed among predacious omnivorous fish and crustaceans (amphipods, Gammaracanthus loricatus, and copepods, Cyclops scutifer), which are capable of a generalist feeding strategy depending on food availability. Using the example of this lake, it can be concluded that polar lake ecosystems are characterized by different seasonal intakes of allochthonous organic carbon from wetland catchment (humic compounds) and nitrogen because of nitrogen fixation in the air by cyanoprocaryotes. Alternative energy sources, such as carbon derived from methane, can also contribute to the energy balance of lake ecosystems. This study contributes to our understanding of energy flow and connectivity between producers and consumers in high-latitude lakes.
1. Introduction
The biological communities in lakes in northern high latitudes are simpler than those found in more temperate environments due to environmental limitations (low water temperatures, excessive moisture conditions in the catchment, and a short vegetation period), and they are extremely sensitive to global change [1]. Because of the poor photosynthetic production of phytoplankton, aquatic food webs in northern lakes may heavily rely on allochthonous organic matter [2,3,4] and alternative energy sources [5]. Warming trends in high-latitude regions lead to changes in lake catchment chemistry caused by increased precipitation and mobilization of organic matter, resulting in an increased influx of allochthonic dissolved organic matter [3,6]. Allochthony—the important role of terrestrial organic matter in maintaining aquatic food webs—is considered important for brown-water lakes. However, in recent years, the overall content of dissolved organic carbon, including humic compounds, has notably increased in oligotrophic arctic lakes with wetland catchments [6,7]. The composition of humic substances in lake sediments from cold-water tundra lakes and other Arctic lakes is regionally specific; humic acids have low carbon and nitrogen content but high oxygen content [8]. This peculiarity and dominating aliphatic fragments in the humic acid structure indicate that mineralization processes of organic substances in lake sediments prevail over humification processes [8].
Earlier research [5,9] identified methane-derived carbon as an alternate energy pathway in aquatic ecosystems, which can contribute to the flux of carbon through both benthic and pelagic food webs in freshwater lakes. Methane is produced either in the anaerobic sediment layers or in the hypolimnion of stratified lakes. It serves as a source of carbon for aerobic methanotrophic bacteria, as well as providing substantial energy for the lake’s food web. The microbial loop transfers carbon from this source to protists and micro-metazoans in the food web of lakes, increasing the number of trophic links between sources and consumers [10]. Some studies suggest that there is substantial methane-derived carbon transfer to higher trophic levels, such as zooplankton, benthic invertebrates, and fish [11,12,13].
The use of stable isotope analysis (SIA) of carbon (13C/12C ratio or δ13C value) and nitrogen (15N/14N, δ15N) in producers and consumers is a useful tool for assessing the relative contribution of different sources of nutrients to food webs and the linkages between consumers [14]. In particular, carbon isotopes are used to determine the sources of organic matter, and nitrogen isotopes are used to identify an organism’s trophic position in the food web [14,15,16]. Values of δ13C may also indicate the contribution of allochthonous and autochthonous carbon supplies to the food web.
We applied SIA to analyze the food web in a subarctic cold-water lake on the White Sea coast (Lake Krivoe). We studied the structure of the food web during different times of the year, including the under-ice period. Although the biotic communities (plankton, benthos, and fish) in the lake have been investigated for over 50 years [17,18,19], these studies were mainly conducted during the open water period (from June to September). These previous studies were based mostly on pelagic food web compartments fueled by phytoplankton and allochthonous organic matter transformed by heterotrophic microorganisms [17,18], but benthic producers have not received much attention. At the same time, it has been found that benthic production plays an important role in maintaining higher trophic levels of consumers, such as fish [20,21]. The zoobenthyphagy are widespread, supporting fish production and causing competition and disruption of trophic dynamics in the pelagic zone [20].
This work aimed to find the most important sources of carbon and nitrogen that feed the northern lake ecosystem, which is a model of oligotrophic clear-water lakes, and to estimate the contribution of photosynthetic carbon (produced by phytoplankton and benthic/periphytic algae) and alternative food resources (methane-derived carbon, diazotroph-derived nitrogen) to support the lake’s food web. Isotope studies of nitrogen-fixing aquatic organisms (such as cyanoprokaryotes) may help detect changes in nitrogen dynamics in the food web during nitrogen deficits. They may fix atmospheric nitrogen and integrate it into the food chains, restoring the trophic reserves. As atmospheric nitrogen has a δ15N value of 0, its presence in the food web can affect consumer isotopic ratios [22,23,24]. For example, cyanoprokaryotes Aphanizomenon flos-aquae fix atmospheric nitrogen (N2), and their nitrogen isotope ratio (δ15N) is closer to the atmospheric value of 0 than the values of δ15N in species that are unable to fix nitrogen N2. Their values of δ15N range from −1‰ to −2‰ [23]. This can lead to a decrease in the 15N content in the tissues of invertebrates that consume cyanobacteria. Consumers associated with carbon derived from methanotrophs also have depleted δ15N, as documented in lake chironomid larvae [5,10]. At the same time, they are characterized by depleted δ13C values (<−30‰) compared to other consumers in the food web [5,13].
We assumed that periphytic producers, methane-derived carbon, and nitrogen derived from diazotrophs will notably contribute as primary sources of nutrients to the lake’s food web. Furthermore, we hypothesized that during the dark ice period (which has received little attention from limnologists), when terrestrial allochthonous food sources and availability are reduced and heterotrophic food pathways dominate, trophic relationships between producers and consumers will change. In particular, these changes may affect the trophic position of predatory consumers by increasing their omnivorousness. Most predacious consumers in low-production environments are omnivores (i.e., feed at multiple trophic levels), either at a certain stage of ontogeny [25] or at a certain time of year [26]. Under stressful or variable conditions, generalist feeding strategies are preferable to specialized ones [27]. Because prey abundance and quality, as well as the productivity of the lake’s ecosystem, can vary seasonally, adaptive changes in the trophic position of fish in the food web may occur.
2. Materials and Methods
2.1. Study Lakes
Sample collection was conducted at Lake Krivoe (hereafter referred to as the lake), a small, deep, stratified, oligotrophic lake located near the Arctic Circle on the shore of the White Sea in northern Karelia (66.3435 N, 33.6375 E) (Figure 1). The lake is fed by both precipitation and runoff from a swampy catchment area, and a rather notable brown-water stream flows into its southwest part (close to site 3, Figure 1). This stream temporarily dries up in the warm season but becomes full of water during snowmelt and in autumn, when there is intense water exchange between the lake and a smaller, dystrophic, brown-water lake (Figure 1). The catchment has many depressions with small ponds and marshes, and water from them sporadically enters the lake, for example, with rain flows (Figure S1).
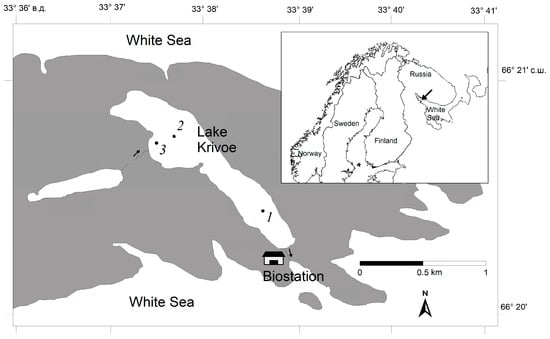
Figure 1.
Lake location with marked sites of sampling (1–3): 1—depth 30 m, 2—8 m, 3—3 m. The arrows show the direction of water flow from the upper swamp and the flow from the lake into the sea.
Table 1 presents the physical and chemical properties of the lake, which is ice-free from mid-May or early June to late November.

Table 1.
Physical, chemical, and production characteristics of the lake. Minimal and maximal values of transparency are shown for the open water season (site 1). Other characteristics are given for all periods combined for sites 1–3. Designations in the table: PP is primary production, C is carbon, Chl is chlorophyll, Phe is phaeopigments, and SD is standard deviation.
At the end of the ice-covered period, oxygen deficiency develops in the benthic zone. Lake sediments consist of gray and brown silt, clay, and organic detritus. Sand, stones, and rocky substrates covered with a 3–4 cm film of benthic algae (Figure 2) predominate in the littoral zone (0–4 m). Cyanobacterial blooms (caused by Dolichospermum lemmermannii) periodically occur in surface waters in calm weather from June to September. Phytobenthic organisms (algae and prokaryotes) emerge as major autochthonous carbon sources, exceeding the contribution of planktonic algae (Table 1).
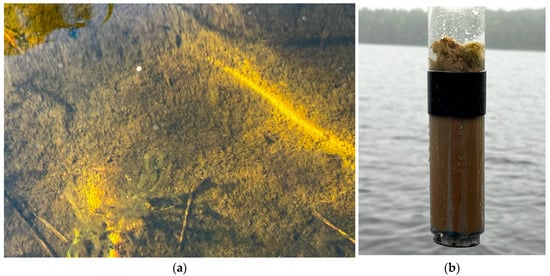
Figure 2.
Photographs of the bottom at a depth of 2.5 m (a) and corer with sediments, showing a 2–4 cm layer of benthic (bottom and periphytic) algae and cyanoprokaryotes (b), the main producers of autochthonous organic matter in the lake.
2.2. Field Sampling and Laboratory Procedures
Planktonic, benthic, and fish samples were collected during five months (February, April, June, September, November) in 2019 and 2020 at three lake stations at depths of 3, 8, and 30 m (Figure 1). A 2.2 L Ruttner bathometer (Borok, Moscow, Russia) was used to sample seston (phytoplankton and microzooplankton) from the water column between 5 m and the lake’s surface (for deep areas). At shallow water stations, seston was collected from the water’s surface.
Planktonic crustaceans were gathered using a Juday net (0.17 m diameter, 125 micron mesh size) from both the surface (0–5 m) and the deep-water zone (15–30 m). Phytoperiphyton and benthic animals were collected with a bottom grab and hand net (in a shallow zone), whereas fish and large nektonic animals were caught at a depth of 3–8 m with monofilament nets (mesh sizes 16–24 cm) and traps at a deeper site. The larvae of aquatic insects at larval stages 3–4 were collected for SIA. In the case of other arthropods, such as the amphipods Gammarus lacustris and Monoporeia affinis, which have one generation a year, we collected individuals with prevailing body sizes. The smallest specimens were found in September in the case of G. lacustris and in June in the case of M. affinis.
To separate phytoplankton from zooplankton, a water sample was illuminated in a vertical cylinder using light. Then, the upper (phytoplankton) and lower (zooplankton) fractions were carefully collected onto paper filters. Because the carbon content of crustacean exoskeletons differs by 1–2% compared to body tissues [28], we considered it possible to analyze cladocerans, copepods, and small amphipods as a whole. Legs or head capsules were taken from arthropods, and dorsal muscles were taken from fish. The bodies of mollusks were separated from their shells, and soft muscle tissue was taken for analysis. All categories of organisms were collected in at least three replicates.
All samples were transferred to 10%HCl for several minutes [21] to remove carbonates before being thoroughly rinsed with deionized water and dried. The samples were then dried at 60 °C for at least 48 h. The prepared samples were kept at −20 °C prior to stable isotope analysis.
2.3. Stable Isotope Analysis
Carbon and nitrogen stable isotope analysis (SIA) in organism tissues was performed using a set of equipment (Elementar, Cheadle, UK) including an Isoprime Vision isotope mass spectrometer and a Vario ISOTOPE select elemental analyzer, located at the Joint Use Center of the A.N. Severtsov Institute of Ecology and Evolution (Moscow, Russia). Approximately 300 μg of animal material and 1000 μg of plant material were wrapped in tin foil and weighed using a Mettler Toledo MX5 (Mettler-Toledo, Columbus, OH, USA).
The isotopic compositions of carbon (C) and nitrogen (N) are given in per mils (δ, ‰) relative to the international standard using the formula
where X is an element (nitrogen or carbon) and R is the atomic ratio of heavy and light isotopes (13C/12C or 15N/14N) of an element in the sample and the standard. Certified batches of casein and alfalfa powder (Elemental Microanalysis Ltd., Okehampton, UK) were used as working laboratory standards. Quality control measures included determining the magnitude of CO2 and N2 peaks on detectors (not less than 1 nA) and calculating the total content of C and N in the sample. The standard deviations of δ15N and δ13C values in laboratory standards were <0.15‰.
δX = [(Rsample/Rstandard) − 1]
Trophic groups analyzed in the present study were separated according to their possible food resources according to [17]. As lipids are strongly depleted in 13C, lipid-corrected δ13C′ values for consumers with C:N > 3.5 were used [29].
The 15N enrichment in animals relative to their food is mostly due to fractionation during deamination and transamination, which transfers nitrogen from amino acids to urea for elimination of the isotopically lighter nitrogen in urine [15]. Because nitrogen levels increase with trophic level, δ15N can help determine an organism’s position in the food web. Usually, the heavier isotope, 15N, is enriched in predators by 3–5‰ relative to their diet [14], but this trophic fractionation factor (Δ15N) may vary depending on alternative energy pathways and nutrient sources [24].
The trophic position of predacious consumers (mainly fish) was determined by comparing their δ15N values with those of herbivores (basic consumers) in cases where a trophic link was established between them [14].
We used a general formula,
where δ15Nc is the ratio of nitrogen isotopes in predacious consumers (the taxon in question), Δ15N is the trophic enrichment factor (fractionation per trophic level), and δ15Nb and TPb are the average nitrogen isotope and trophic position of baseline, with corresponding constants of TPb = 2 (trophic level 2 [14]).
TPc = (δ15Nc − δ15Nb)/Δ15N + TPb
The δ15N value of cladoceran crustaceans and the amphipod crustacean M. affinis was taken as the value of herbivores/detritivores (basic consumers) in pelagic compartments of the food web for the fish C. albula and the predaceous crustaceans C. scutifer and G. loricatus. In littoral areas, the δ15N values of chironomids and gammarids were chosen as the baseline for the fish P. pungutius and P. fluviatilis. The Δ15N was calculated by the ratio of basic consumers to potential producers (phytoplankton in pelagic compartments, phytoperiphyton in benthic compartments) if the relationships between them were clear.
All data are presented as arithmetic means with standard deviations (SDs). Spearman rank correlation (Spearman R) was used for an analysis of the association between isotope signatures of producers and their consumers. Coefficient variation was calculated as the ratio of SD to mean values, expressed in %. Differences in studied variables between seasons within groups of organisms were analyzed using ANOVA (if data had normal distribution, confirmed by Shapiro–Wilk’s normality test) or a non-parametric Kruskal–Wallis test and Mann–Whitney paired comparisons (if the data were not normally distributed or the number of observations was less than 10), using the software packages Statistica 10.0 and PAST 3.4 (https://statistica.software.informer.com/; https://past.en.lo4d.com/, both accessed on 5 October 2025).
3. Results
3.1. Structure of the Lake’s Food Web
The biotic communities of the studied Lake were described in detail prior [19,21]. Briefly, green microalgae dinoflagellates, cryptophytes, and cyanobacteria constituted the phytoplankton community (Table 2). The filamentous benthic cyanobacteria and pennate diatoms periphytic dominated in benthic vegetation/phytoperiphyton (Table 2, Figure 2). The richest species and most abundant groups of heterotrophic organisms included planktonic protists, crustaceans, and rotifers and diverse benthic consumers (Table 2). Three species of fish, perch, P. fluviatilis, vendace, C. Albula, and nine-spined sticklebacks, P. pungitius, inhabited this lake.

Table 2.
Ecological and trophic groups in the lake’s food web separated according to their possible food resources.
3.2. Carbon and Nitrogen Stable Isotopes’ Composition
The δ13C values varied during all period of observation, from −41.7‰ to −15.5‰ (Figure 3). The lowest δ13C value was registered in plankton consumers in September and November (Figure 4, Table S1 with statistical significance of differences). Significant differences in δ13C values between months were observed for November and April compared to other months (p = <0.0001 to 0.02, Figure S1). Consumers had the highest δ15N values (11.3‰) under the ice and the lowest (−4.5‰) during the open water period, namely in September and November (Figure 3 and Figure S2). δ15N values were the most variable in September compared to other months and did not differ from others (p > 0.05, Figure S2).
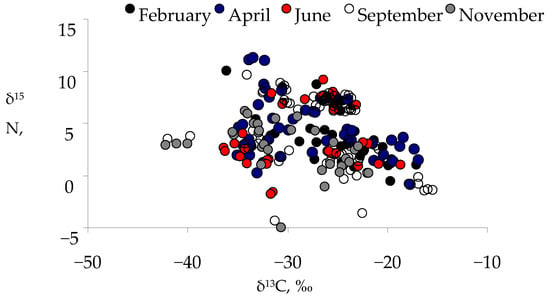
Figure 3.
Biplots of δ13C and δ15N values (‰) of lake food web participants in seasons of 2019–2020: under ice (February, April) and during open water (June–November). Number of observations (n) was 33 in February, 52 in April, 33 in June, 56 in September, and 34 in November.
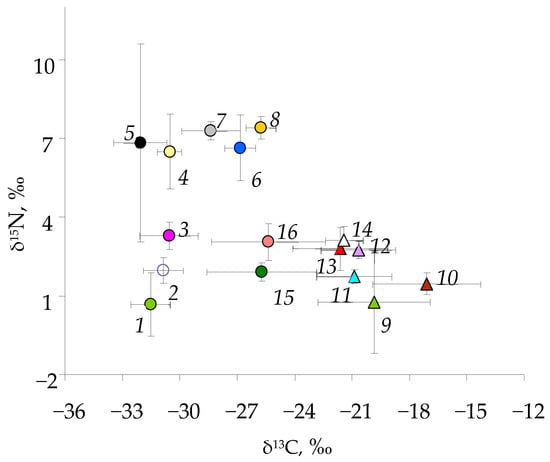
Figure 4.
Biplots of mean values (±SD) of isotopic ratios δ13C and δ15N (‰) for phytoplankton (1), cladoceran Bosmina longirostris (2), amphipod Monoporeia affinis (3), amphipod Gammaracanthus loricatus (4), copepod Cyclops scutifer (5), sticklebacks Pungitius pungitius (6), vendace Coregonus albula (7), perch Perca (8), benthic algae/Phytoperiphyton (9), gastropod Lymnaea stagnalis (10), Gammarus lacustris (11), Chironomidae Sergentia coracina (12), megalopteran Sialis sp. (13), trichopteran Phryganea bipunctata (14), bivalve mollusks Sphaerium corneum (15), and ephemeropteran Ephemera vulgata (16).
The wide range of δ15N values (−2 to 11‰) implies the presence of four trophic levels in the lake’s food web (Figure 4 and Figure 5). The lowest δ15N values (−2 to 2‰) were typical of detritus and aquatic plants/cyanobacteria (producers), with higher values for predatory invertebrates (5–11‰) and fish (5–9‰). Pelagic carbon sources are more negative than benthic carbon sources; the difference in mean values of δ13C between representatives of two compartments was approximately 8–10‰ (Figure 4). Therefore, two food web compartments can be distinguished in the lake’s food web; one group is fueled by pelagic carbon sources, and the second group is fueled by benthic sources. Some consumers (such as ephemeropteran Ephemera vulgata and bivalves Sphaerium corneum) were characterized by a very wide range of δ13C values (Figure 4), obviously mixing resources from both compartments and using alternative sources. All fish species also occurred at intermediate positions relative to primary sources and their possible prey (Figure 4).
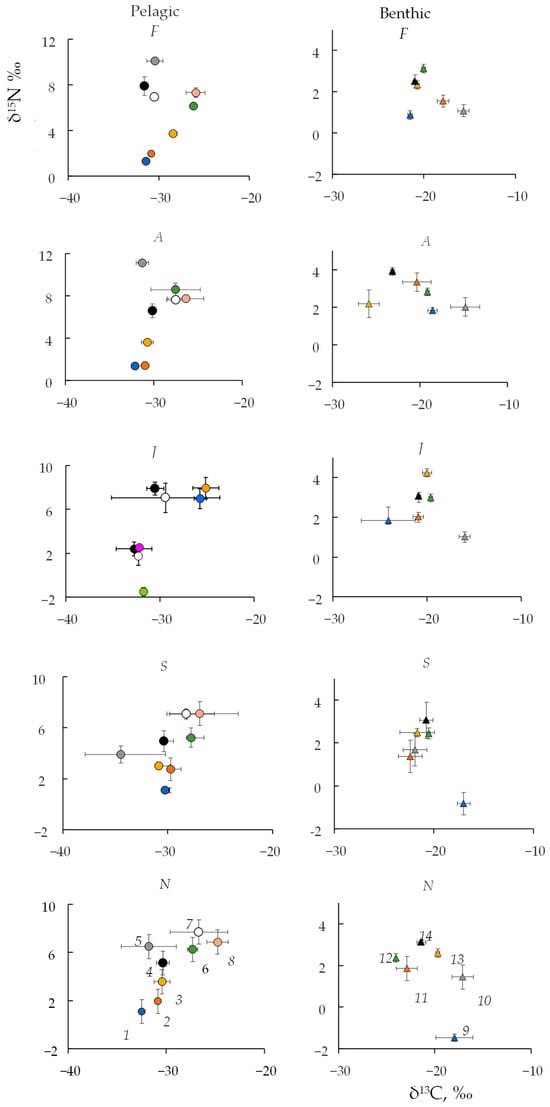
Figure 5.
Biplots of δ13C and δ15N mean values (‰) ±1 SD for pelagic and benthic/littoral food web compartments in different seasons (F—February, A—April, J—June, S—September, N—November). The numbers of food web participants are the same as in Figure 4.
3.3. Seasonal Changes in Carbon and Nitrogen Stable Isotopes
Figure 5 shows values of δ13C and δ15N of lake producers and consumers in different months, reflecting the seasonal change in the food web. Although all fish species could use both pelagic and benthic carbon sources in the lake, we put signatures of the fish within the pelagic compartment Figure 5 (left side) to trace them. Depleted δ13C values were found in planktonic crustaceans (Copepoda and Cladocera) during all studied months. During the open water period, there was a decrease in the mean δ13C values compared to the ice-covered period of the lake (Table S1). In particular, carbon-depleted values (δ13C < −40‰) were found for copepods (Cyclops scutifer) in September (Figure 5, Table S1). A small but statistically significant increase in phytoplankton δ13C values was noted in June and September compared to November and April (Table S1), the periods of limited photosynthesis. The values of the isotopic signature δ13C of Cladocera positively correlated with the δ13C values in phytoplankton (Spearman R = 0.69).
Significant seasonal changes in δ15N values were observed in both food web compartments (Figure 5, Table S1). The lowest (−1.5‰) for plankton producers were recorded in June and in the benthic food web in autumn (Figure 5, Table S1). A statistically significant decrease in δ15N values of benthic producers (−0.8 to −1.5‰), dominated by benthic cyanoprokaryote Phormidium spp., was noted in September and November compared to other months (Table S1). At the same time, in April–June, the highest δ15N values were recorded for benthic producers (3.0‰), as well as for gastropods directly fed on them. We found significant positive correlations of lowest and highest values of δ15N in planktonic producers and cladocerans, the amphipod Monoporeia affinis and vendace, benthic producers and bivalve mollusks (Sphaerium), as well as benthic producers and perch (Spearman R, Table S2). Furthermore, we obtained significant seasonal differences in the values of δ15N for omnivorous consumers capable of predation (Gammaracanthus loricatus, Cyclops scutifer, and all species of fish). These omnivorous consumers were directly related to consumers at a basic level, but not producers, so they will be discussed later.
A notable overlap of isotope signatures (both δ13C and δ15N values) was noted in the studied fish species (Figure S3), especially in sticklebacks and vendace. Perch was associated to a greater degree with carbon sources from benthic and littoral sources, while vendace was associated mostly with pelagic sources and distinguished by lower δ13C values (Figure 4). In terms of δ13C values, perch showed the greatest seasonal variability (coefficient variation is 40%) compared to vendace (24%) and sticklebacks (21%, Table S3). Differences in the mean values of δ13C were significant (p < 0.01, Table S3) between perch and other fish species (vendace and sticklebacks).
3.4. Trophic Position of Predatory Consumers
The mean δ15N values differed between sticklebacks and two other fish species (Table S3, all p < 0.05), while there were no significant differences in the mean δ15N values between perch and vendace. Trophic positions (TPs, Figure 6) of fish and crustaceans (Gammaracanthus loricatus and Cyclops scutifer) were calculated relative to their preferred food sources, taking into account the enrichment factor (Table 3). In the pelagic food web compartment, the enrichment factor (Δ15N) between phytoplankton and zooplankton (primarily Cladocera) averages 1.7‰, ranging from 0.7 to 3.4‰ (Table 3). The benthic food web compartment showed similar results, with differences between periphytic algae and basic consumers (Gammarus) ranging from 0.1 to 3.9‰ (Table 3). For gastropods, this difference was very low (<0–0.21‰) during the ice-covered period and in June, increasing in autumn (2.5–2.9‰).
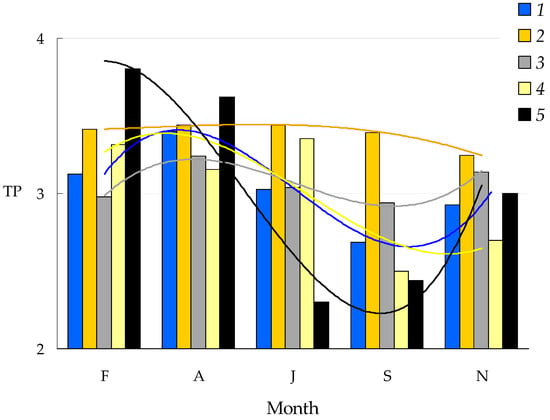
Figure 6.
Seasonal change in trophic position of predatory consumers depending on the nitrogen isotope in their food source (i.e., the basic consumer). 1—stickleback, 2—perch, 3—vendace, 4—Gammaracanthus, and 5—Cyclops. The designations of months are as in Figure 5.

Table 3.
Trophic enrichment factor (Δ15N) for primary consumers and predatory consumers in plankton and benthic compartments of the lake’s food web during each season.
TPs of predatory consumers varied depending on the month and the species (Figure 6). Perch had roughly comparable TP ranges around 3.4 (predator II), while the TP of vendace ranged from 2.9 to 3.1, with the lowest TP value in September. Other predatory consumers, such as sticklebacks and crustaceans, had a considerably higher TP than perch and vendace during the under-ice period but switched to trophic positions of omnivorous/herbivorous consumers (2 < TP < 3) during summer and autumn.
4. Discussion
4.1. Structural Specificity of the Lake’s Food Web
The structure of the food web, from producers (phytoplankton and phytobenthos) to higher-order consumers (fish), was investigated in a subarctic lake using stable carbon and nitrogen isotope analysis. This revealed a distinct and unique trophic web structure, with a strong seasonal pattern, as has been found in other studies [30]. Seasonal variations in the ice-covered lake are generated through modifications to the organization of the food chain [31,32,33]. In summer, the lake’s food web is mainly based on the production of phototrophs and aerobic heterotrophs. By the end of November, when the lake is covered in ice and snow, a reductive metabolic process takes place.
The specificity of the ecosystem of the studied lake is that most of the planktonic algae (chrysophytes and cryptophytes) in the studied lake are mixotrophs (Table 2), which means that they receive part of the carbon and phosphorus from bacteria [34]. In some oligotrophic lakes, planktonic communities (Lake Skärlen, Sweden) account for a significant part of the phytoplankton biomass (18–31%), while mixotrophic phytoflagellates are present throughout the year [35]. The mixotrophic type of feeding is thought to be especially beneficial when inorganic nutrients are few, which is typical in polar lakes [19]. Some mixotrophic species, such as chrysophytes (Dinobryon), have an efficient mechanism of phosphate absorption, which is especially noticeable in waters with a low concentration of phosphates [36].
During winter, low light and snow covering the lake’s ice limit photosynthesis, so phytoplankton rely primarily on bacteriophage or heterotroph feeding as a major source of carbon flow. Many taxa of Chrysophyta [37] and Cryptophyta [38] can consume bacteria directly, but some diatoms [39] and cyanobacteria [40] can metabolize dissolved organic substances in dark places beneath the ice. With large amounts of dissolved organic matter, heterotrophic algae growth can beat autotrophic growth. Cryptomonas and Dinobryon have shown significant bacterial ingestion rates [41]. The bacterial ingestion rates in these algae vary throughout the year, with the peak in winter (0.57 bacteria cells/hour), most likely due to the low light [36].
4.2. Possible Reasons for Seasonal Changes in δ13C Values
This study provides valuable insights into the food web structure over the course of the year in boreal systems (subarctic lake), where seasonality is pronounced. The allochthonous organic matter in the lake is formed from humic compounds and swampy waters that drain from the catchment area into open waters. There are two periods of intensive influx of water from wetland basins into the lake: snowmelt (late May and June) and autumn, which is characterized by a lot of precipitation. During these times, marsh waters are supplied, enriched with humic substances and microbial biofilms, which develop over the summer vegetation season. This community includes organisms like methanotrophs and methanogens, characteristic of swamp ecosystems. These substances can be transported from the catchments to the lake ecosystem through complex microbial food webs, and carbon from methane enters the food web through methane-oxidizing bacteria in both pelagic compartments and bottom sediments. Methanotrophs (genus Methylocella) and methanogens (genus Methanobacterium), as well as other microorganisms, are able to decompose complex organic matter and provide methanogenic substrates for methane synthesis. Therefore, they participate in the methane cycle in lakes fed by wetland catchment waters [42].
Low δ13C values (close to −30‰) in aquatic food webs are common in pelagic consumers of many lakes and likely reflect autochthonous production [43]. Lower δ13C values obtained for the case study and others [9,10,11,12,13] may suggest microbial activity, including methanotroph-mediated carbon uptake as a food source for the lake’s food web. The photoautotrophic basal food resources have δ13C values of −35 to −25‰, with the most negative values in that range resulting from algal photosynthesis [15]. Most of the methane carbon present in the lakes is methanogenic or microbially produced in anaerobic conditions when other substrates, such as sulphate, nitrate, and ferric iron, are depleted [13,44]. The methane carbon produced from these processes is heavily fractionated and therefore depleted in 13C, having values ranging from −50‰ to −110‰ [45]. Methanogenic methane-derived carbon becomes available to higher trophic levels when it is consumed by methane-oxidizing bacteria (methanotrophs) [4]. Carbon in the biomass of methanotrophs shows further fractionation, having δ13C reductions of up to −30‰ and below [9,46]. The values of δ13C for planktonic invertebrates (lowest as −42‰) in the studied lake may demonstrate the presence of carbon derived from methane in the pelagic compartment of the food web. Carbon derived from methane, unlike other carbon sources, undergoes strong isotopic fractionation during methanogenesis. Therefore, consumers of this methane carbon are also characterized by a depleted isotopic composition in consumers, close to −40‰ and even lower, as in the case of the studied lake. Low δ13C values (less than −40‰) were previously noted for chironomids feeding on methanotrophic bacteria [47,48]. The methanotrophs were detected in the guts of chironomid larvae during winter in an ice-covered lake [49].
In small boreal forest lakes, the δ13C values of zooplankton decreased from −35‰ to −45‰ with an increase in humic substance concentrations [9,50]. This could be associated with methanotrophic bacteria feeding on methane, including light carbon isotopes. Similarly, results from a boreal humic lake (Lake Mekkojarvi, Finland) enclosure during autumn revealed a decrease in δ13C values in adult Daphnia from −40.5‰ to −50.3‰, reflecting its extensive consumption of 13C-depleted methanotrophic bacteria [51]. Stable isotope analysis and PCR-based molecular methods have shown close relationships between Daphnia and methane-oxidizing bacteria in the pelagic compartment of a humic lake (Michigan, USA) [9].
Significant but weak depletion of stable carbon isotopes in zooplankton and other lake consumers also suggests the presence of several indirect cascade trophic interactions between them and methanotrophs, i.e., the use of intermediate methane consumers, such as heterotrophic flagellates and protozoa [10]. Another explanation is that zooplankton forms symbiotic interactions with different bacteria and microorganisms, including epibiotic mixotrophs [52] and endosymbiotic plastids [53]. Carbon derived from methane appears to be used by intermediate consumers able to ingest carbon from methane-oxidizing bacteria. These linkages were not included in our study and warrant further study, as they obviously play an important role in lake food web functioning.
4.3. Seasonal Variations in δ15N Values and Trophic Positions of Consumers
We found high variability in the δ15N of benthic producers, which are characterized by high productivity (Table 1) and species richness of primary producers (Table 2). High variability of producers and related grazers, such as gammarids (Gammarus lacustris), gastropods (Lymnaea stagnalis), and insect larvae (Chironomidae, Ephemeroptera, Trichoptera, Megaloptera), is understandable, and it is precisely related to this heterogeneity in the composition of producers and the mixing of different algae species in the consumer diet. Establishing correlative relationships requires further detailed studies on trophic links between producers and their consumers.
At the same time, positive correlations between the signatures of producers and some consumers were revealed, as well as depleted δ15N values in the tissues of different consumers during the open water period compared to the ice-covered period of the lake (see Figure 5). These decreases in the δ15N values coincide with the summer and autumn periods, when mass development of cyanoprokaryotes can be observed on the lake’s surface [19]. These depleted δ15N values in the consumers’ tissues may indicate the influence of N-depleted alternative sources, such as nitrogen derived from diazotrophs (nitrogen-fixing cyanoprocaryotes). The cyanobacteria, planktonic Dolichospermum lemmermannii and benthic Phormidium sp., were the dominant producers in the studied lake. These species belong to a group of gas-vacuolated cyanobacteria that can regulate their position in the water column (buoyancy) to optimize the use of resources, such as nutrients, temperature, and light [54]. Ammonia and dissolved organic nitrogen are released into water during nitrogen fixation by dying bacterial cells, and this available nitrogen is used directly by phytoplankton or bacteria [54,55]. Nitrogen from cyanobacterial cells enters the primary consumers and the entire food web, not only through direct consumption of cyanobacteria but also through grazing on phytoplanktonic organisms and members of the microbial loop (heterotrophic bacteria and flagellates) and ciliates that feed directly on cyanobacteria, which may contribute to variability in δ15N [55]. Depleted δ15N values were notable in planktonic crustaceans (cladocerans) and short-lived benthic invertebrates (amphipods), which have a relatively rapid turnover time for elements in tissues [24]. However, significant correlations in δ15N values were also found between producers and fish, which can be considered a sign of the incorporation of cyanobacterial nitrogen into the food web [55].
4.4. Predators’ Omnivory and Seasonal Changes in Trophic Position
15N enrichment (Δ15N) in animals relative to their food is mostly due to fractionation during deamination and transamination, which transfers nitrogen from amino acids to urea for the elimination of the isotopically lighter nitrogen in urine [14]. Because nitrogen levels increase with each trophic level, δ15N can help determine the position of an organism in the food web. It has been found that in predators, the heavier isotope, 15N, is enhanced by 3–5‰ (averaging 3.4‰) relative to their diet [14]. In the studied lake, Δ15N varied between 4.1 and 5.3‰ (averaging 4.7‰). The enrichment between sources of organic matter and consumers mainly depends on the degree of omnivory of the predacious consumers (fish and crustaceans in the case study) and the fact that the invertebrates fed by fish may be also omnivorous consumers with diverse sources in their diet. Δ15N is usually lower when there is a greater contribution of easily digestible animal food to the diet and higher when there is a predominance of plant and detritus diets [56].
The higher δ15N values in winter compared to summer in the same species (herein Cyclops scutifer, Gammaracanthus loricatus, and Pingutius pingutius) may indicate mixing of food resources with the prevalence of a predatory strategy, as well as the presence of functionally different groups of food items. Surprising changes in the nitrogen isotope composition of nine-spined sticklebacks in winter, leading to some increase in their trophic position, was a consequence of their consumption of vendace eggs and embryos [57]. On the contrary, the winter trophic position level of perch and vendace did not change notably due to their opportunistic feeding strategy [21,58].
High values of δ15N in copepod C. scutifer during winter are associated with the fact that they can consume mixotrophic microzooplankton, i.e., based on both autotrophic and heterotrophic feeding. Pelagic copepods often bridge classical and microbial food webs by feeding on microzooplankton (e.g., ciliates) in lakes, and this consumption can trigger trophic cascades in the microbial food web [59] and influence the efficiency of carbon transfer in food webs [60]. The species of genus Cyclops are polyphagous organisms characterized by high food plasticity [61]. They consume plant food, detritus, protozoa, rotifers, diatoms, and dinoflagellates and even eat their own copepodites [61,62].
The fish species showed a distinct overlap in carbon and nitrogen isotopic ratios. At the same time, the mean δ13C values of vendace were closer to pelagic sources than benthic sources. Perch had intermediate values, but their δ13C values were closer to periphytic and benthic sources, with mean values significantly different from that of vendace. Polar lakes (including the studied lake) are characterized by a peculiarity, low pelagic production (oligotrophic status) and scarce pelagic sources insufficient for fish, so available producers may occur mainly in the benthic food web compartment. It was revealed earlier [21] that only small-sized vendace (less than 80 mm in length) are planktivore consumers based mainly on daphniids (genera Ceriodaphnia and Bosmina), while larger vendace individuals tend to be omnivore consumers based on a variety of benthic organisms. Perch feed on zooplankton in the early stages of life, and the diet of one-year-old perch consists mainly of bottom organisms. Two- and three-year-olds (100–180 cm) in this lake do not become predatory fish, based on benthic invertebrates [58]. The food spectrum of sticklebacks (P. pingutius) differs between months. During June–September, sticklebacks prefer benthic larvae of aquatic insects (chironomids, ephemeropterans) as food (up to 90% of their stomach content mass), while from November to April the mass percentage of chironomid larvae decreases to 50% of stomach content, and vendace egg/embryos and copepods contribute an important part of the stickleback’s diet [57]. All fish species are characterized by opportunistic feeding behavior and dietary seasonal changes that can be related to prey availability throughout the year, with important contributions of benthic sources to the diets of all species. The omnivorous feeding habits of the fish species under study demonstrate their significance as key species connecting the pelagic and benthic components of the food web and facilitating the transfer of energy and carbon between these ecological niches [20,63]. In addition, larvae of Ephemera vulgata and bivalve sphaeriid mollusks were found to be opportunistic species with very wide variability of carbon signatures and possibly acting as benthic–pelagic integrators. At the same time, their feeding habits are poorly understood and require more detailed investigation to recognize their role in the food web.
5. Conclusions
This study focused on the trophic structure of food webs using stable isotope analysis in a clear oligotrophic lake located in the subarctic region of Eurasia (Karelia, Russia). The study analyzed both the open water (summer–autumn) and the ice-covered (winter–spring) periods. Changes in food web structure were observed during the ice-covered period compared to the open water season as a result of changes in organic matter input and alterations in biotic relationships within the lake’s ecosystem. Mixotrophs play an important role in carbon and nutrient cycling in this polar environment. Depleted δ13C values (close to −40‰) may indicate that carbon is derived from methanotrophs. This may be one of the alternative sources of carbon in subarctic lake ecosystems with depleted autochthonous carbon sources. Fish and some invertebrates (ephemeropterans and bivalve mollusks) occupy an intermediate position in the food web, fueled by energy sources from both the benthic and pelagic compartments and serving as important integrators of carbon within the lake’s food web. Dominating cyanobacteria influence the δ15N values in grazers and primary consumers, resulting in negative δ15N values in their tissues and some decrease in δ15N values in higher consumers, indicating possible diazotrophic nitrogen assimilation into the lake’s food web. Fish and predatory crustaceans showed the greatest seasonal differences in δ15N values, leading to changes in their trophic positions. We associate this seasonal decline (in open water compared to the period when the lake is under ice) with two factors: the feeding of these consumers at different trophic levels (high degree of omnivory) and the summer–autumn incorporation of nitrogen from cyanobacteria, which are the dominant taxa of phytoplankton, phytobenthos, and periphyton in this lake. The advantage of this study is that energy flows from pelagic and benthic sources are considered together throughout all of the seasons, allowing us to obtain a more realistic picture of energy flow in the lake’s ecosystem. This study contributes to our understanding of food web connectivity and ecosystem functioning in the sensitive environment of high-latitude lakes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17110799/s1, Figure S1: Lake catchment with swampy hollows and streams in summer (left) and autumn (right) with bacterial microfilm on the surface of the water in September; Figure S2: Isotopic signatures of δ13C (a) and δ15N (b) values in tissues of different representatives of the lake’s food web during the study period (2019–2020). Designations on the abscissa axis: F—February, A—April, J—June, S—September, N—November. Differences in δ13C values between months were high (Figure S2a, Kruskal–Wallis, H = 48.9, p < 0.0001). According to post hoc comparisons, there were no significant differences in δ13C values across open water months (between June and September) (p > 0.05). However, δ13C values in November and April differed from other months (p ≤ 0.0001 to 0.02). Seasonal fluctuations in δ15N levels were significant (Kruskal–Wallis, H = 22.1, p = 0.0002;). δ15N values in September were more variable than in other months, so they did not differ significantly from other months (p > 0.05); Figure S3: Isotopic signatures of δ13C and δ15N values in tissues of different species of fish in the lake’s food web (all data for the study period). Letters a, b show significant differences (at p < 0.05) between study species; Table S1: Letters indicating significant differences (a–b, c–d, e–f,…) in carbon (δ13C) and nitrogen (δ15N) values between months for producers and consumers in the lake’s food web; Table S2: Spearman coefficient correlation (and p) between δ15N values of producers and consumers in the lake’s food web; Table S3: Basic statistical values of δ13C and δ15N averaged per season for studied fish. Conf.—confidence, Min—minimum, Max—maximum. Different letters show the significance of the differences at p < 0.05.
Author Contributions
Conceptualization, N.A.B.; investigation, N.A.B., Y.I.G., and A.A.M.; formal analysis, N.A.B.; writing—original draft preparation, N.A.B.; writing—review and editing, N.A.B., Y.I.G., and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Education of the Russian Federation (No 125012800888-5; 125012800889-2) and the Russian Foundation of Basic Research under grant number 19-04-0100 (supporting the stable isotope analysis).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to express our gratitude to the staff of the Kartesh Biological Station (Zoological Institute of the Russian Academy of Sciences) for laboratory and accommodation facilities and technical support. The authors cordially thank Andrey N. Sharov (IBIW RAS) and Piotr M. Terentiev (KNC RAS) for help with sampling and Alexei V. Tiunov (Severtsov Institute of Ecology and Evolution, RAS, Moscow) for stable isotope analysis and valuable comments on the first version of the manuscript and linguistic improvement.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huser, B.J.; Futter, M.N.; Bogan, D.; Brittain, J.E.; Culp, J.M.; Goedkoop, W.; Lento, J. Spatial and temporal variation in Arctic freshwater chemistry—Reflecting climate-induced landscape alterations and a changing template for biodiversity. Freshw. Biol. 2022, 67, 14–29. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.; Bade, D.L.; Bastviken, D.; Gille, C.M.; Hodgson, J.R.; Kitchell, J.F.; Kritzberg, E.S. Ecosystem subsidies: Terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 2005, 86, 2737–2750. [Google Scholar] [CrossRef]
- Karlsson, J.; Bergström, A.-K.; Byström, P.; Gudasz, C.; Rodríguez, P.; Hein, C. Terrestrial organic matter input suppresses biomass production in lake ecosystems. Ecology 2015, 96, 2870–2876. [Google Scholar] [CrossRef]
- Taipale, S.J.; Galloway, A.W.; Aalto, S.L.; Kahilainen, K.K.; Strandberg, U.; Kankaala, P. Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Sci. Rep. 2016, 6, 30897. [Google Scholar] [CrossRef]
- Grey, J. The incredible lightness of being methane-fuelled: Stable isotopes reveal alternative energy pathways in aquatic ecosystems and beyond. Front. Ecol. Evol. 2016, 4, 8. [Google Scholar] [CrossRef]
- de Wit, H.A.; Valinia, S.; Weyhenmeyer, G.A.; Futter, M.N.; Kortelainen, P.; Austnes, K.; Hessen, D.O.; Räike, A.; Laudon, H.; Vuorenmaa, J. Current browning of surface waters will be further promoted by wetter climate. Environ. Sci. Technol. Lett. 2016, 3, 430–435. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; De Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Guzeva, A.V.; Slukovskii, Z.I. Geochemical characterization of humic acids isolated from tundra lakes sediments of Murmansk Region, Russia. Water Sect. Russia Prob. Technol. Manag. 2023, 1, 78–92. (In Russian) [Google Scholar] [CrossRef]
- Jones, R.I.; Grey, J. Biogenic methane in freshwater food webs. Freshw. Biol. 2011, 56, 213–229. [Google Scholar] [CrossRef]
- Jones, S.E.; Lennon, J.T. Evidence for limited microbial transfer of methane in a planktonic food web. Aquat. Microb. Ecol. 2009, 58, 45–53. [Google Scholar] [CrossRef]
- Schilder, J.; van Hardenbroek, M.; Bodelier, P.; Kirilova, E.P.; Leuenberger, M.; Lotter, A.F.; Heiri, O. Trophic state changes can affect the importance of methane-derived carbon in aquatic food webs. Proc. R. Soc. B 2017, 284, 20170278. [Google Scholar] [CrossRef]
- Deines, P.; Bodelier, P.L.; Eller, G. Methane-derived carbon flows through methane oxidizing bacteria to higher trophic levels in aquatic systems. Environ. Microbiol. 2007, 9, 1126–1134. [Google Scholar] [CrossRef]
- DelVecchia, A.G.; Stanford, J.A.; Xu, X. Ancient and methane-derived carbon subsidizes contemporary food webs. Nat. Commun. 2016, 7, 13163. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Winberg, G.G.; Alimov, A.F.; Boulion, V.V.; Ivanova, M.B.; Korobtzova, E.V.; Kuzmitzkaya, N.K.; Nikulina, V.N.; Finogenova, N.P.; Fursenko, M.V. Biological productivity of two subarctic lakes. Freshw. Biol. 1973, 3, 177–197. [Google Scholar] [CrossRef]
- Bouillon, V.V. Relationship between growth rates of planktonic algae and bacteria. Sci. South Russia 2023, 19, 61–72. (In Russian) [Google Scholar] [CrossRef]
- Litvinchuk, L.F.; Sharov, A.N.; Chernova, E.N.; Smirnov, V.V.; Berezina, N.A. Mutual links between microcystins-producing cyanobacteria and plankton community in clear and brown northern lakes. Food Webs 2023, 35, e00279. [Google Scholar] [CrossRef]
- Zanden, J.; Vadeboncoeur, Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology 2002, 83, 2152–2161. [Google Scholar] [CrossRef]
- Berezina, N.A.; Terentjev, P.M.; Zubova, E.M.; Tsurikov, S.M.; Maximov, A.A.; Sharov, A.N. Seasonal diet changes and trophic links of cold-water fish (Coregonus albula) within a northern lake ecosystem. Animals 2024, 14, 394. [Google Scholar] [CrossRef]
- Mayer, B.; Wassenaar, L.I. Isotopic characterization of nitrate sources and transformations in Lake Winnipeg and its contributing rivers, Manitoba, Canada. J. Great Lakes Res. 2012, 38, 135–146. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Duberg, J.; Motwani, N.H.; Hogfors, H.; Klawonn, I.; Ploug, H.; Svedén, J.B.; Garbaras, A.; Sundelin, B.; Hajdu, S.; et al. Nitrogen fixation by cyanobacteria stimulates production in Baltic food webs. AMBIO 2015, 44, 413–426. [Google Scholar] [CrossRef]
- Berezina, N.A.; Tiunov, A.V.; Tsurikov, S.M.; Kurbatova, S.A.; Korneva, L.G.; Makarova, O.S.; Bykova, S.N. Cyanobacteria as a food source for invertebrates: Results of a model experiment. Russ. J. Ecol. 2021, 52, 247–252. [Google Scholar] [CrossRef]
- Davis, A.M.; Blanchette, M.L.; Pusey, B.; Jardine, T.D.; Pearson, R.G. Gut content and stable isotope analyses provide complementary understanding of ontogenetic dietary shifts and trophic relationships among fishes in a tropical river. Freshw. Biol. 2012, 57, 2156–2172. [Google Scholar] [CrossRef]
- Rautio, M.; Mariash, H.; Forsström, L. Seasonal shifts between autochthonous and allochthonous carbon contributions to zooplankton diets in a subarctic lake. Limnol. Oceanogr. 2011, 56, 1513–1524. [Google Scholar] [CrossRef]
- Laske, S.; Rosenberger, A.; Wipfli, M.; Zimmerman, C. Generalist feeding strategies in Arctic freshwater fish: A mechanism for dealing with extreme environments. Ecol. Freshw. Fish 2018, 27, 767–784. [Google Scholar] [CrossRef]
- Perga, M.E. Potential of δ13C and δ15N of cladoceran subfossil exoskeletons for paleo-ecological studies. J. Paleolimnol. 2010, 44, 387–395. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Bazin, S.; Domaizon, I.; Barouillet, C.; Frossard, V.; Sentis, A. Seasonal variations in planktonic food web structure affect stability by shifting the distribution of energy fluxes. Oikos 2025, e11528. [Google Scholar] [CrossRef]
- Gaedke, U.; Li, X.; Guill, C.; Hemerik, L.; de Ruiter, P.C. Seasonal shifts in trophic interaction strength drive stability of natural food webs. Ecol. Lett. 2025, 28, e70075. [Google Scholar] [CrossRef]
- Bertilsson, S.; Burgin, A.; Carey, C.C.; Fey, S.B.; Grossart, H.-P.; Grubisic, L.M.; Jones, I.D.; Kirillin, G.; Lennon, J.T.; Shade, A. The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr. 2013, 58, 1998–2012. [Google Scholar] [CrossRef]
- Vigneron, A.; Lovejoy, C.; Cruaud, P.; Kalenitchenko, D.; Culley, A.; Vincent, W.F. Contrasting winter versus summer microbial communities and metabolic functions in a permafrost thaw lake. Front. Microbiol. 2019, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pålsson, C.; Graneli, W. Diurnal and seasonal variations in grazing by bacterivorous mixotrophs in an oligotrophic clearwater lake. Arch. Hydrobiol. 2003, 157, 289–307. [Google Scholar] [CrossRef]
- Sanders, R.; Porter, K.G. Phagotrophic Phytoflagellates. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Springer: Boston, MA, USA, 1988; Volume 10. [Google Scholar] [CrossRef]
- Pereira, I.; Rangel, A.; Chagas, B.; de Moura, B.; Urbano, S.; Sassi, R.; Castro, C. Microalgae Growth Under Mixotrophic Condition Using Agro-Industrial Waste: A Review; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kristiansen, J.; Skaloud, P. Chrysophyta; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Roberts, E.; Laybourn-Parry, J. Mixotrophic cryptophytes and their predators in the Dry Valley lakes of Antarctica. Freshwat. Biol. 2001, 41, 737–746. [Google Scholar] [CrossRef]
- Tuchman, N.C.; Schollett, M.A.; Rier, S.T.; Geddes, P. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 2006, 561, 167–177. [Google Scholar] [CrossRef]
- Pelroy, R.A.; Bassham, J.A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch. Für Mikrobiologie. 1972, 86, 25–38. [Google Scholar] [CrossRef]
- Flöder, S.; Hansen, T.; Ptacnik, R. Energy–dependent bacterivory in ochromonas minima–A strategy promoting the use of substitutable resources and survival at insufficient light supply. Protist 2006, 157, 291–302. [Google Scholar] [CrossRef]
- Glagolev, M.V. Methane emission: Ideology and methodology of the “standard model” for Western Siberia. Dyn. Environ. Glob. Clim. Change 2008, S1, 176–190. [Google Scholar] [CrossRef]
- Grosbois, G.; Power, M.; Evans, M.; Koehler, G.; Rautio, M. Content, composition, and transfer of polyunsaturated fatty acids in an Arctic lake food web. Ecosphere 2022, 13, e03881. [Google Scholar] [CrossRef]
- Conrad, R. Control of microbial methane production in wetland rice fields. Nutr. Cycl. Agro-Ecosyst. 2002, 64, 59–69. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and hydrogen isotope systematics of bacterial formation and oxidation of methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Summons, R.E.; Jahnke, L.L.; Roksandic, Z. Carbon isotopic fractionation in lipids from methanotrophic bacteria—Relevance for interpretation of the geochemical record of biomarkers. Geochim. Cosmochim. Acta 1994, 58, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Kankaala, P.; Taipale, S.; Grey, J.; Sonninen, E.; Arvola, L.; Jones, R.I. Experimental delta 13C evidence for a contribution of methane to pelagic food webs in lakes. Limnol. Oceanogr. 2006, 51, 2821–2827. [Google Scholar] [CrossRef]
- Deines, P.; Grey, J.; Richnow, H.H.; Eller, G. Linking larval chironomids to methane: Seasonal variation of the microbial methane cycle and chironomid delta C-13. Aquat. Microb. Ecol. 2007, 46, 273–282. [Google Scholar] [CrossRef]
- Agasild, H.; Zingel, P.; Tuvikene, L.; Tuvikene, A.; Timm, H.; Feldmann, T.; Salujõe, J.; Toming, K.; Jones, R.I.; Nõges, T. Biogenic methane contributes to the food web of a large, shallow lake. Freshw. Biol. 2014, 59, 272–285. [Google Scholar] [CrossRef]
- Jones, R.I.; Carter, C.E.; Kelly, A.; Ward, S.; Kelly, D.J.; Grey, J. Widespread contribution of methane-cycle bacteria to the diets of lake profundal chironomid larvae. Ecology 2008, 89, 857–864. [Google Scholar] [CrossRef]
- Peter, H.; Sommaruga, R. An evaluation of methods to study the gut bacterial community composition of fresh-water zooplankton. J. Plankton Res. 2008, 30, 997–1006. [Google Scholar] [CrossRef]
- Barea-Arco, J.; Perez-Martinez, C.; Morales-Baquero, R. Evidence of a mutualistic relationship between an algal epibiont and its host, Daphnia pulicaria. Limnol. Oceanogr. 2001, 46, 871–881. [Google Scholar] [CrossRef]
- Chang, N.; Jenkins, D.G. Plastid endosymbionts in the freshwater crustacean Daphnia obtusa. J. Crustac. Biol. 2000, 20, 231–238. [Google Scholar] [CrossRef][Green Version]
- Montoya, J.P.; Carpenter, E.J.; Capone, D.G. Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol. Oceanogr. 2002, 47, 1617–1628. [Google Scholar] [CrossRef]
- Motwani, N.H.; Duberg, J.; Svedén, J.B.; Gorokhova, E. Grazing on cyanobacteria and transfer of diazotrophic nitrogen to zooplankton in the Baltic Sea: Cyanobacteria blooms support zooplankton growth. Limnol. Oceanogr. 2018, 63, 672–686. [Google Scholar] [CrossRef]
- Mehner, T.; Attermeyer, K.; Brauns, M.; Brothers, S.; Hilt, S.; Scharnweber, K.; van Dorst, R.M.; Vanni, M.J.; Gaedke, U. Trophic Transfer Efficiency in Lakes. Ecosystems 2022, 25, 1628–1652. [Google Scholar] [CrossRef]
- Berezina, N.A.; Zhgareva, N.N.; Strelnikova, A.P. Feeding features of the nine-spined stickleback Pungitius pungitius (Gasterosteidae) in water bodies of the North-West of Russia. J. Ichthyol. 2023, 63, 308–317. [Google Scholar] [CrossRef]
- Terentjev, P.M.; Berezina, N.A. Ecological and morphological characteristics and feeding of perch (Perca fluviatilis) in the autumn–winter period in dystrophic and oligotrophic lakes of northern Karelia (Russia). Inland Water Biol. 2022, 15, 915–928. [Google Scholar] [CrossRef]
- Bundy, M.H.; Vanderploeg, H.A.; Lavrentyev, P.J.; Kovalcik, P.A. The importance of microzooplankton versus phytoplankton to copepod populations during late winter and early spring in Lake Michigan. Can. J. Fish. Aquat. Sci. 2005, 62, 2371–2385. [Google Scholar] [CrossRef]
- Moore, M.; DeStasio, B.; Huizenga, K.; Silow, E. Trophic coupling of a microbial and classical food web in Lake Baikal, Siberia. Freshw. Biol. 2018, 64, 1–14. [Google Scholar] [CrossRef]
- Makarewicz, J.C.; Likens, G.E. Niche analysis of a zooplankton community. Science 1975, 190, 1000–1003. [Google Scholar] [CrossRef]
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters II—Cyclopiformes; Backhuys Publishers: Leiden, The Netherlands, 2006; 354p. [Google Scholar]
- Williams, R.J.; Martinez, N.D. Limits to trophic levels and omnivory in complex food webs: Theory and data. Am. Nat. 2004, 163, 458–468. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).