Abstract
Halarachne halichoeri mites are respiratory parasites of pinnipeds that have been recorded from different locations around the world, but not from the coast of Argentina. In this study, the presence of H. halichoeri on the southern elephant seal, Mirounga leonina, is documented for the first time in the Argentinean mainland, specifically in the Paraná River on the Buenos Aires coast. A total of 4130 adult and immature mites (90.4% larvae, 0% nymphs, 9.2% females and 0.4% males) were collected from the nose and nasopharyngeal cavity of a stranded juvenile male during a necropsy. The species was identified using scanning electron microscopy (SEM) and stereomicroscope photography, and the morphological characteristics of the females and males are shown. Also, images of the male are shown for the first time, with observable details of traits. We also propose a method for simple sex recognition. Finally, we provide a revision of global records of geographic distribution of H. halichoeri in wild marine mammals. The present record of occurrence of the species H. halichoeri in Argentina expands our knowledge about the distribution area known to date for this species of marine parasite.
1. Introduction
The parasitic arthropods of marine mammals have been poorly studied, particularly in the southern hemisphere. There are scarce works that include the study of marine mites on the South Atlantic and Pacific coasts [1,2,3,4,5,6,7].
Mites belonging to the family Halarachnidae Oudemans 1906 (Acari, Mesostigmata) are obligate parasites of the respiratory tract of some mammals [8,9] and are usually found in the nasal cavity mucosa, upper respiratory tract, and lungs. The species of the genus Halarachne have been registered infesting phocids (seals and elephant seals) and non-pinniped species as lutrinids. To date, four species of Halarachne have been described that affect marine mammals: H. halichoeri Allman, 1847, H. americana Banks, 1899, H. miroungae Ferris, 1925, and H. laysanae Furman & Dailey, 1980.
Halarachne halichoeri host range reports are scarce but it has been documented as a parasite of eight species of pinnipeds in different parts of the world, but not for mainland Argentina. Adults and larvae are found in the upper respiratory tract of their hosts. The gray seals Halichoerus grypus (Fabricius, 1791), harbor seals Phoca vitulina (Linnaeus, 1758) and sea otters Enhydra lutris (Linnaeus, 1758) are within the most recorded species parasitized. In terms of the taxonomic status of H. halichoeri, this mite species was first described on Halichoerus grypus (Allman 1847) from the coast of Dublin, Ireland. Regarding synonymy, the geographic records of H. halichoeri in this study were examined relative to all four synonyms. Of these, H. taita was reported as a synonym of H. miroungae by Eichler (1958), but it has been argued that it corresponds to H. halichoeri [10,11]. Conversely, H. otariae was misidentified by Steding (1923) as a parasite of an otariid [12], while H. erratica was originally listed as a synonym of H. miroungae but is actually a synonym of H. halichoeri [10,11]. So, the synonyms of H. halichoeri include H. erratica Fain & Mortelmans, 1959, H. taita Eichler, 1958 and H. otariae Steding, 1923 [10].
Larvae are small-sized, measuring on average 1.2 mm in length. Adults are big mites with bodies measuring on average up to 2.8 mm length and 0.9 mm width. These mites have no motility and live inside the host [10,13,14,15]. Males and females present sexual dimorphism and can be identified by examining the morphology of the chelicerae. However, H. halichoeri males are often difficult to find and females are generally the only sex available to study. Only a few studies have observed H. halichoeri males [10,16] and none of these have photographed it.
Given the difficulty to find males, a rapid way to identify potential males when analyzing samples would provide a useful tool. However, no sexual trait easily distinguishable under a stereomicroscope has been suggested in the literature. Nonetheless, it is possible to analyze conspicuous morphological characters using just a stereomicroscope.
In the present article, we report for the first time the presence of H. halichoeri in mainland Argentina parasitizing Mirounga leonina Linnaeus, 1758. Through scanning electronic and optical photography, we examined the morphology of both female and less abundant male specimens. We also provide a simple method to identify sexes. Finally, we present a revised global distribution of H. halichoeri mites in wild marine mammals, with reference to the scientific literature on records of this species.
2. Materials and Methods
2.1. Methods of Collection
Mites were collected from a stranded Southern Elephant Seal, M. leonina (Carnivora, Phocidae) (Figure 1a), which was rescued from the coast of Buenos Aires Province, at the locality of Tigre in the Río Paraná in Argentina (34°25′00″ S; 58°35′00″ W) in March 2024. The animal was transferred to Mundo Marino Foundation’s Rescue and Rehabilitation Centre, where it was assisted (ID M6524, juvenile male). Following its death, a necropsy was performed, and the upper respiratory system was removed. The elephant seal’s respiratory organs were stored in a freezer, dissected, and washed in preparation for manual collection of mites. During the dissection of the respiratory organs, the nasal cavities, turbinates, nasopharynx, pharynx, and trachea were separated. All mites present in the respiratory tissues were then collected manually using forceps and a paintbrush (Figure 1b). These specimens were counted as larvae or adults, then fixed in 96% alcohol and stored in a freezer at −18 °C until used for taxonomic identification.

Figure 1.
Host marine mammals and mite collection process: (a) Subadult male of M. leonina (ID M6524), host of the collected mites; (b) Extracted nasopharyngeal tissues containing a H. halichoeri larva in the turbinates (74x).
2.2. Specimen Handling and Morphological Identification of H. halichoeri
The specimens for optical observation were fixed in ethanol and placed in a Petri dish [17]. Images were captured using a Leica M205A stereo microscope (Leica Microsystems, Wetzlar, Germany). For scanning electron microscopy, the specimens were dehydrated according to described methods [18]. They were then processed in amyl acetate and ethanol, mounted with copper adhesive tape, sputter-coated with gold-palladium (60:40) and examined with a FE-SEM ZEISS Supra 40 (Zeiss, Oberkochen, Germany).
The morphological characteristics observed for the taxonomic identification of mites follow previous descriptions for this genus of mites [10,11,19,20]. Adults were identified by the morphology of the dorsal shield, disposition and number of body setae, and the disposition and number of setae on the femur of leg III and the genu of leg IV. Females and males were distinguished by the shape of opisthosoma and chelicerae morphology. However, as these traits are not always easily observed at low magnification, we propose adding the coxal insertion pattern as a new observable trait. Coxal insertion pattern was examined under a stereomicroscope to assess sex differences for rapid identification, as well as the differential development of the chelicerae and body size and shape. Drawings of coxal insertion patterns and body shape for females and males were made to provide an illustrated key for sex differences. Also, we performed measurements on 10 males and 10 females of the intercoxal distance of the leg II and leg IV to analyze the differences in the coxal insertion patterns. We then calculated the ratio between these distances as a quick measurement of the pattern. These characteristics could be considered to quickly separate females from males. Larvae were identified by observing the morphology of the idiosoma and the size and arrangement of the postanal and adanal setae. All specimens present in the host were counted.
The studied material is housed in the Colección de Artrópodos, Laboratorio de Entomología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires (LEE-FCEN-UBA).
2.3. Review of Worldwide Geographic Records of H. halichoeri
An exhaustive bibliographic search was conducted to assess the worldwide occurrence of H. halichoeri and its taxonomic synonyms in the species of marine mammals known to date. All scientific papers, conference proceedings, and thesis were reviewed. Based on the information obtained, a compilation of the marine mammal species sampled at each location together with their bibliographic citations was made, along with a map that shows the documented geographic distribution of this mite species to date.
3. Results
3.1. Identification of H. halichoeri Specimens and Sex Recognition
A total of 4130 mites collected from the nasal cavities, turbinates and nasopharynx: 3733 larvae and 397 adults (379 females and 18 males) (Figure 2). Based on the distance of the opisthosoma vs. the podosoma, the size of the dorsal shield relative to the idiosoma and its rigidity compared to that of adults, no nymphs were found. No specimens were found attached in the pharynx or trachea. Based on leg chaetotaxy, idiosoma dimensions, body chaetotaxy, and dorsal shield shape, the specimens were identified as H. halichoeri.
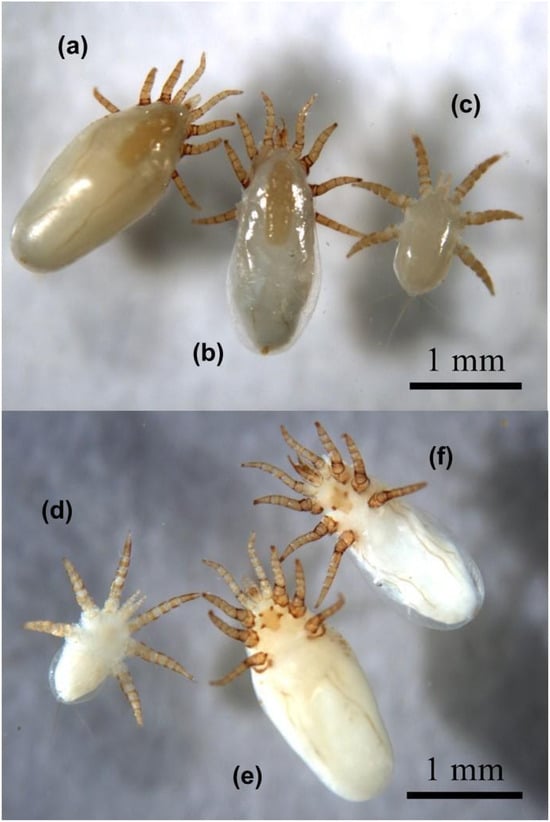
Figure 2.
Marine mammal mites collected: (a) H. halichoeri, female, dorsal habitus; (b) H. halichoeri, male, dorsal habitus; (c) H. halichoeri, larva, dorsal habitus; (d) H. halichoeri, larva, ventral habitus; (e) H. halichoeri, female, ventral habitus; (f) H. halichoeri, male, ventral habitus. Note in (e,f) the sclerotized sternal cornua present in the sternal shield. Mites are preserved and stored in the LEE-FCEN-UBA.
Adult female and male specimens were identified based on the observation of a subcylindrical idiosoma measuring approximately 2.2 mm in length, a quadrangular linguiform dorsal shield of approximately 0.7 mm in length and 0.3 mm in width with an obtuse posterior part wider than the anterior part (Figure 2a,b,e,f and Figure 3a,b), a posteriorly truncated sternal shield with the third pair of sternal setae off the sternal shield (Figure 3f) and the disposition of six setae on the femur of leg III and the genu of leg IV (Figure 3c–e) [10,14,20,21]. The morphological features of females, males and larvae of H. halichoeri from Argentina in this study are consistent with the descriptions for H. halichoeri described in the literature [10,11,16,19,20]. Females and males could be differentiated by the shape of the idiosoma, the morphology of the chelicerae, and the coxal insertion pattern. The idiosoma of females is subcylindrical, more rounded than that of males, with a slight constriction after the IV legs (Figure 4b,d and Figure 5d). Males have a pear-shaped idiosoma and are slightly narrower than females (Figure 4a,c and Figure 5c). The female chelicerae are not conspicuous and show a slight asymmetry, as in other Parasitiformes (Figure 4f), whereas the male chelicerae are highly developed distally, distinctive and with a prominent spermatodactyl where the chela has the movable digit tubular, sinuate, not forming a discrete claw, and with a small dorsal lamella (Figure 4e). Furthermore, for males, the idiosoma measures 1875–1909 µm in length, with a dorsal shield measuring 788–956 µm. The genital opening is subcircular in shape. The length of the legs is comparable to that observed in the larva and female (Figure 2). Male structures such as the subcuticular apodemes and tarsal claws are similar to those found in the female. The morphological characteristics of the males found in this host are consistent with those reported in the original descriptions made for the males of this species [10,11,16], including a degree of intraspecific variation between specimens of the same host [10]. Regarding the coxal insertion pattern, the coxae of the male are arranged in a shape nearly oval, with the last pair of legs inserted more internally than the previous pair (Figure 4a and Figure 5a). In females, the coxae show a shape similar to a trapeze, with the last pair of legs inserted more externally than the previous two pairs (Figure 4b and Figure 5b). This pattern is evinced by a larger intercoxal distance of leg IV than leg II in females ([Lower Confidence Interval; mean; Upper Confidence Interval] = [0.319; 0.341; 0.364] vs. [0.208; 0.226; 0.244] mm). In turn, the intercoxal distance of leg IV for the males was similar to leg II ([LCI; mean; UCI] = [0.259; 0.278; 0.296] vs. [0.233; 0.234; 0.235] mm). Furthermore, the ratio between these distances (leg IV/leg II) is significantly larger for females than for males ([LCI; mean; UCI] = [1.415; 1.518; 1.621] vs. [1.109; 1.186; 1.263]). In light of the huge difference in the sex ratio in H. halichoeri found in host’s nasal cavity, the shape outlined by coxae is proposed as an easy and fast method to identify potential males in samples observed under a stereomicroscope.
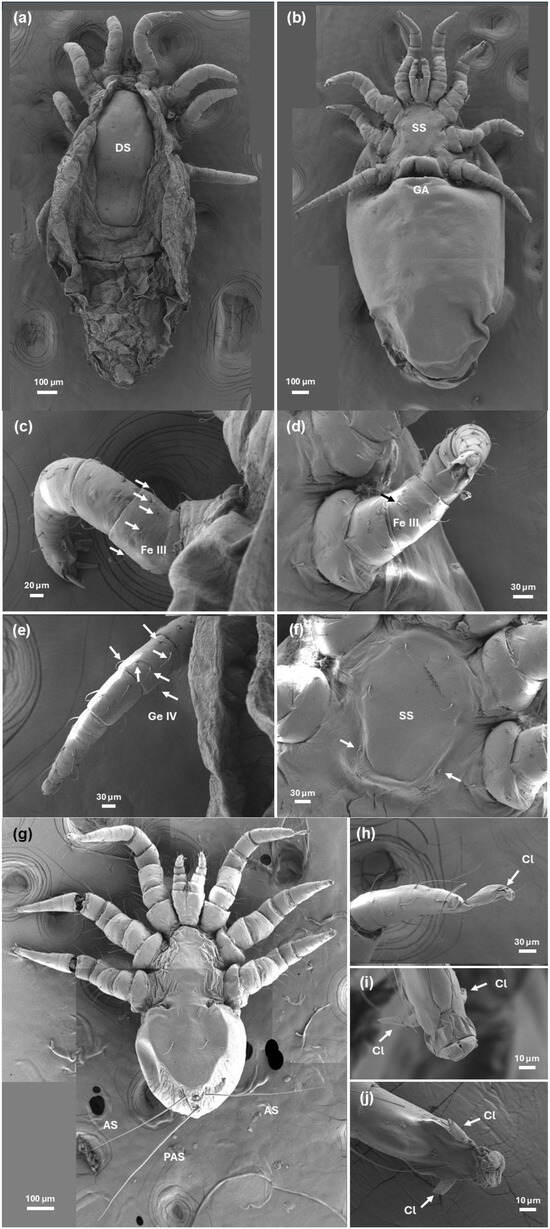
Figure 3.
Scanning electron microscopy photographs of H. halichoeri: (a) Dorsal view of adult female showing the characteristic linguiform shield (DS); (b) Ventral view of adult female showing the sternal shield (SS) and genital aperture (GA) a gaping transverse slit between coxae IV; (c,d) Femur of leg III of the adult with the typical six setae disposition (arrows); (e) Genu of leg IV of the adult showing the six setae disposition (arrows); (f) Detail of adult ventral view showing the posteriorly truncated sternal shield and the third pair of sternal setae (arrows) off the sternal shield; (g) Larva general ventral view showing the elliptical idiosoma and a detail of the post-anal setae (PAS) longer than adanals (AS); (h) Lateral view of tarsus of leg I of the larva showing a stalked claw (arrow); (i) Ventral view of tarsus of leg II of the larva showing two large and sessile claws (arrows); (j) Ventral view of tarsus of leg III of the larva showing two large and sessile claws (arrows).

Figure 4.
Halarachne halichoeri sexual differences: (a) Ventral habitus male; (b) Ventral habitus female; (c) Lateral habitus male, pear-shaped; (d) Lateral habitus female, subcylindrical shape; (e) Male, detail of anterior part, distinctive chelicerae; (f) Female, detail of anterior part, normal chelicerae.
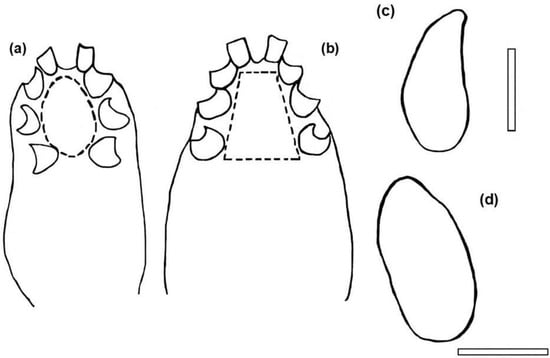
Figure 5.
Halarachne halichoeri diagram for the rapid determination of sexual differences: (a) Male, coxal insertion pattern, note that they are arranged in an oval shape, the insertion of the last pair of legs is further inside than the previous one. (b) Female, coxal insertion pattern, note that they are arranged in a trapeze, the insertion of the last pair of legs is further outside than the previous one. (c) Male, lateral contour shape. (d) Female, lateral contour shape. Bars: 1 mm.
Larvae show a short, subcylindrical idiosoma of approximately 1.2 mm in length and 0.7 mm in width [10,16] (Figure 2c,d and Figure 3g). The tarsi of legs II and III has large and sessile claws (Figure 3i,j), while leg I has an elongated stalked ambulacrum (Figure 3h). The hypostoma exhibits two lobes between the base of the palpi, and the postanal seta is longer than the adanal setae (Figure 3g).
Male H. halichoeri specimens are difficult to obtain. For the first time, we present photographs of the male, showing its general appearance and details of its distinctive features on the ventral gnathosoma and idiosoma (Figure 2b,f and Figure 4a,c,e). Based on these characteristics, we developed a rapid identification scheme for males, which illustrates body shape, chelicerae development and coxae insertion patterns and compare them with those of females (Figure 5).
3.2. Developmental Parameters of H. halichoeri
A total of 4130 adult and immature mites were collected, of which 90.4% were larvae, 9.2% were females and 0.4% were males. Among adults, females comprised around 96% of total individuals counted, with males being very scarce. Nymphs were not observed in this study, probably because this stage is either extremely short or absent [13].
3.3. Worldwide Geographic Records of H. halichoeri
The compilation of all occurrence records for H. halichoeri shows that there are records for Germany, Poland, the United Kingdom, Ireland, Spain, the Netherlands, Greece, the United States, Australia, and in the territories of Alaska and Antarctica. Our study adds Argentina as a new locality for this species (Table 1, Figure 6).

Table 1.
Phocid and non-pinniped marine species documented as wild hosts of H. halichoeri mites in different parts of the world.

Figure 6.
Halarachne halichoeri worldwide distribution, based on records in scientific literature and considering the species synonyms. The red dots represent the localities where wild hosts infested with this mite species were found (see details in Table 1).
4. Discussion
In this study, we report the occurrence of H. halichoeri parasitizing M. leonina in mainland Argentina for the first time, thereby extending its known geographical distribution. We also presented a photograph of a male for the first time and provided observable traits for simple sex recognition, observing structural differences in the coxal insertion pattern and body shape. Finally, we provide an up-to-date revision of the global records of the worldwide geographical distribution of H. halichoeri in wild marine mammals.
Regarding the occurrence of this mite, 38 geographic records were found on wild hosts for seven species of marine mammals (Table 1). The only record reported in Cystophora cristata was not considered because the geographic evidence of its presence was not detailed enough [10]. Of the 38 records, only three studies, other than this one, have identified M. leonina as a host of H. halichoeri. The southern elephant seal has a wide distribution throughout the southern hemisphere below 35° S. It is therefore expected that the geographic distribution of H. halichoeri coincides with that of its host.
We found an extremely high ratio of H. halichoeri larvae to adults. Studies analyzing the biological and ecological reasons for the abundance of developmental stages of dermanyssoid mites are scarce [5,10,48,49]. The high abundance of H. halichoeri larvae found in the nasopharynx—the most external anatomical area of the host’s respiratory system—could be related to their dispersal strategy in the environment [5,13]. In contrast, adult mites are sedentary and are mostly found in the turbinates and choanae. Unfortunately, we did not collect nymphs of this species. However, it has been reported that halarachnid nymphs are very difficult to find due to the absence or extremely short duration of the protonymphal and deutonymphal stages, an adaptation to the parasitic lifestyle [13,20].
We found an extremely low ratio of H. halichoeri males to females and there is a lack of information on the males of this species in the literature. The scarcity of males in populations of mites from the Halarachnidae family is a common and intriguing pattern found in other mite groups [10,48,49]. One possible explanation is a sex ratio bias, whereby females produce more females than males. This bias may be determined by genetic mechanisms and may be adaptive if fertilization can be achieved with few males and a high reproductive rate is ensured. Also, there may be an evolutionary advantage to the female bias. In populations where dispersal is a risky process involving high individual mortality, it makes sense to maximize female production, ensuring that more dispersing individuals are available to colonize new hosts.
Finally, in conclusion, this work provides a simple method for identifying H. halichoeri males in samples, as well as updating the research carried out worldwide on this species. There is a lack of research on Halarachnidae mites, which is particularly evident in the southern hemisphere.
Author Contributions
Conceptualization, M.K.C.; methodology, M.K.C. and J.P.L.; investigation, M.K.C. and J.P.L.; resources, M.K.C. and J.P.L.; data curation, M.K.C.; writing—original draft preparation, M.K.C.; writing—review and editing, M.K.C.; visualization, M.K.C.; supervision, M.K.C.; project administration, M.K.C. and J.P.L.; funding acquisition, M.K.C. and J.P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundación Mundo Marino, the Consejo Nacional de Investigaciones Científicas y Técnicas, and the Universidad de Buenos Aires, grant number UBACyT 2020 20020190100059BA, as well as the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación—FONCyT, grant number PICT 2019-I-A-00975.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank the veterinarians and technicians of the Fundación Mundo Marino, especially Dolores Erviti and Juana Caferri, for their assistance and collaboration in collecting the samples, as well as Claudia Marchi and Silvio Ludueña of FCEN-UBA for help with the microscopy service. We would also thank David Verón for his contributions to the fieldwork and assistance with necropsies, as well as for providing access to material. Finally, we would like to thank José Crespo for his valuable comments on the manuscript and Iván Crespo for his help with logistics.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Duarte-Benvenuto, A.; Sacristán, C.; Reisfeld, L.; Santos-Costa, P.C.; Fernandes, N.C.D.; Ressio, R.A.; Mello, D.M.D.; Favero, C.; Groch, K.R.; Diaz-Delgado, J.; et al. Clinico-pathologic findings and pathogen screening in fur seals (Arctocephalus australis and Arctocephalus tropicalis) stranded in southeastern Brazil, 2018. J. Wildl. Dis. 2022, 58, 86–99. [Google Scholar] [CrossRef]
- Gastal, S.B.; Mascarenhas, C.S.; Ruas, J.L. Infection rates of Orthohalarachne attenuata and Orthohalarachne diminuata (Acari: Halarachnidae) in Arctocephalus australis (Zimmermann, 1783) (Pinipedia: Otariidae). Comp. Parasitol. 2016, 83, 245–249. [Google Scholar] [CrossRef]
- Gomez-Puerta, L.A.; Gonzales-Viera, O. Ectoparásitos del lobo marino sudamericano (Otaria flavescens) de la costa peruana. Rev. Peru. Biol. 2015, 22, 259–262. [Google Scholar] [CrossRef]
- Katz, H.; Morgades, D.; Castro-Ramos, M. Pathological and Parasitological Findings in South American Fur Seal Pups (Arctocephalus australis) in Uruguay. ISRN Zool. 2012, 2012, 586079. [Google Scholar] [CrossRef]
- Porta, A.O.; Loureiro, J.P.; Castelo, M.K. First record of Orthohalarachne attenuata in Arctocephalus australis in mainland Argentina (Parasitiformes, Mesostigmata, Dermanyssoidea, Halarachnidae) with observations on its ambulacral morphology. ZooKeys 2024, 1207, 355. [Google Scholar] [CrossRef]
- Rivera-Luna, H.; Kniha, E.; Muñoz, P.; Painean, J.; Balfanz, F.; Hering-Hagenbeck, S.; Prosl, H.; Walochnik, J.; Taubert, A.; Hermosilla, C.; et al. Non-invasive detection of Orthohalarachne attenuata (Banks, 1910) and Orthohalarachne diminuata (Doetschman, 1944) (Acari: Halarachnidae) in free-ranging synanthropic South American sea lions Otaria flavescens (Shaw, 1800). Int. J. Parasitol. Parasites Wildl. 2023, 21, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Seguel, M.; Calderón, K.; Colegrove, K.; Adkesson, M.; Cárdenas-Alayza, S.; Paredes, E. Helminth and respiratory mite lesions in Pinnipeds from Punta San Juan, Peru. Acta Parasitol. 2018, 63, 839–844. [Google Scholar] [CrossRef]
- Lindquist, E.E.; Krantz, G.W.; Walter, D.E. Order Mesostigmata. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 124–232. [Google Scholar]
- Newell, I.M. Studies on the morphology and systematics of the family Halarachnidae Oudemans 1906 (Acari, Parasitoidea). Bull. Bingham Oceanogr. Collect. 1947, 10, 235–266. [Google Scholar]
- Furman, D.P.; Dailey, M.D. The genus Halarachne (Acari: Halarachnidae), with the description of a new species from the Hawaiian Monk Seal. J. Med. Entomol. 1980, 17, 352–359. [Google Scholar] [CrossRef]
- Domrow, R. Halarachne miroungae Ferris redescribed. Pac. Insects 1962, 4, 859–863. [Google Scholar]
- Ferris, G.F. On two species of the genus Halarachne (Acarina; Gamasidae). Parasitology 1925, 17, 163–167. [Google Scholar] [CrossRef]
- Furman, D.P.; Smith, A.W. In vitro development of two species of Orthohalarachne (Acarina: Halarachnidae) and adaptations of the life cycle for endoparasitism in mammals. J. Med. Entomol. 1973, 10, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Farré, J.M.; D’Silva, J.D.; Gestal, C. Naso-pharyngeal mites Halarachne halichoeri (Allman, 1847) in grey seals stranded on the NW Spanish Atlantic Coast. Vet. Parasitol. 2012, 183, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Reckendorf, A.; Wohlsein, P.; Lakemeyer, J.; Stokholm, I.; von Vietinghoff, V.; Lehnert, K. There and back again–The return of the nasal mite Halarachne halichoeri to seals in German waters. Int. J. Parasitol. Parasites Wildl. 2019, 9, 112–118. [Google Scholar] [CrossRef]
- Oudemans, A.C. Halarachne-Studien. Arch. Naturgesch. A. 1926, 91, 48–108. [Google Scholar]
- Walter, D.E.; Krantz, G.W. Collecting, rearing, and preparing specimens. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Pérez-Benavides, A.L.; Ospina-Peñuela, E.; Gamboa, J.; Duran-Bautista, E.H. Amyl acetate: An alternative technique to dry mount Chalcidoidea (Hymenoptera) from alcohol, faster and inexpensively. J. Insect Sci. 2023, 23, 4. [Google Scholar] [CrossRef]
- Allman, G.J. Description of a new genus and species of tracheary arachnidans. Ann. Mag. Nat. Hist. 1847, 20, 47–52. [Google Scholar] [CrossRef][Green Version]
- Shields, M.M.; Roth, T.; Pesapane, R. A pictorial key to the adult and larval nasal mites (Halarachnidae) of marine mammals. ZooKeys 2024, 1216, 101. [Google Scholar] [CrossRef]
- Pesapane, R.; Archibald, W.; Norris, T.; Fontaine, C.; Halaska, B.; Duignan, P.; Javeed, N.; Miller, M.; Foley, J. Nasopulmonary mites (Halarachnidae) of coastal Californian pinnipeds: Identity, prevalence, and molecular characterization. Int. J. Parasitol. Parasites Wildl. 2021, 16, 113–119. [Google Scholar] [CrossRef]
- Pesapane, R.; Dodd, E.; Javeed, N.; Miller, M.; Foley, J. Molecular characterization and prevalence of Halarachne halichoeri in threatened southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. Parasites Wildl. 2018, 7, 386–390. [Google Scholar] [CrossRef]
- Pesapane, R.; Chaves, A.; Foley, J.; Javeed, N.; Barnum, S.; Greenwald, K.; Dodd, E.; Fontaine, C.; Duignan, P.; Murray, M.; et al. Nasopulmonary mites (Acari: Halarachnidae) as potential vectors of bacterial pathogens, including Streptococcus phocae, in marine mammals. PLoS ONE 2022, 17, e0270009. [Google Scholar] [CrossRef] [PubMed]
- Shocking Dent, C.E.; Miller, M.A.; Batac, F.; Dodd, E.; Smith, W.; Pesapane, R.; Foley, J. Pathology and epidemiology of nasopulmonary acariasis (Halarachne sp.) in southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. Parasites Wildl. 2019, 9, 60–67. [Google Scholar] [CrossRef]
- Alonso, J.M.; Gestal, C.; López, A. Identificación de ácaros naso-faríngeos Halarachne sp. (Acari: Halarachnidae) en focas grises Halichoerus grypus varadas en Galicia. In Proceedings of the II Simposium de la Sociedad Española de Cetáceos (SEC), Valsaín, Segovia, Spain, 2–3 November 2001. [Google Scholar]
- Anderson, S.S.; Bonner, W.N.; Baker, J.R.; Richards, R. Grey seals, Halichoerus grypus, of the Dee Estuary and observations on a characteristic skin lesion in British seals. J. Zool. 1974, 174, 429–440. [Google Scholar] [CrossRef]
- Lehnert, K.; Seibel, H.; Hasselmeier, I.; Wohlsein, P.; Iversen, M.; Nielsen, N.H.; Siebert, U. Increase in parasite burden and associated pathology in harbour porpoises (Phocoena phocoena) in West Greenland. Polar Biol. 2014, 37, 321–331. [Google Scholar] [CrossRef]
- Rolbiecki, L.; Izdebska, J.N.; Bidziński, K.; Jankowska-Jarek, M. Nasopharyngeal mites Halarachne halichoeri (Allman, 1847) parasitizing the gray seal Halichoerus grypus (Fabricius, 1791) in the Baltic Sea with notes on other parasitic Halarachnidae associated with marine mammals. Oceanol. Hydrobiol. St. 2018, 47, 398–404. [Google Scholar] [CrossRef]
- Rolbiecki, L.; Izdebska, J.N.; Kozina, P.; Dzido, J.; Kuczkowski, T.; Fryderyk, S.; Pawlik, I.P.V.; Cierocka, K. Current data on the parasitofauna of the grey seal Halichoerus grypus (Carnivora: Phocidae) from the southern Baltic Sea. In Proceedings of the 14th European Multicolloquium of Parasitology (EMOP XIV), Wrocław, Poland, 26–30 August 2024. [Google Scholar]
- Rolbiecki, L.; Izdebska, J.N.; Kuczkowski, T.; Dzido, J.; Fryderyk, S.; Kozina, P.; Pawliczka, I.; Cierocka, K. Preliminary data on the parasitofauna of seals (Carnivora: Phocidae) from the Baltic Sea. In Proceedings of the XXVth Congress of the Polish Parasitological Society, Warsaw, Poland, 9–12 September 2019. [Google Scholar]
- Pugh, P.J.A. The structure and function of the tarsus I sensillar field in mites of the genus Halarachne (Halarachnidae: Gamasida). J. Nat. Hist. 1996, 30, 1069–1086. [Google Scholar] [CrossRef]
- Baker, J.R. The pathology of the grey seal (Haliochoerus grypus). II. Juveniles and adults. Brit. Vet. J. 1980, 136, 443–447. [Google Scholar] [CrossRef]
- Baker, J.R. Causes of mortality and morbidity in wild juvenile and adult grey seals (Halichoerus grypus). Brit. Vet. J. 1987, 143, 203–220. [Google Scholar] [CrossRef]
- Barnett, J.E.; Gilbertson, J.A.; Arrow, N.; Gillmore, J.D.; Hawkins, P.N.; Larbalestier, L.; Jarvis, D.; Sayer, S.; Wessels, M.E. Hepatic amyloidosis in a chronically entangled grey seal (Halichoerus grypus). J. Comp. Pathol. 2022, 199, 1–7. [Google Scholar] [CrossRef]
- Nijenhuis, I. Analysis of Stranding Data and Pathological Findings in Stranded Harbor Seals and Grey Seals on Texel and the North-West Coast of the Netherlands between 2009 and 2012. Master’s Thesis, Utrecht University, Utrecht, Netherlands, 2012. [Google Scholar]
- Wigman, S. Pathological Findings in Stranded Common Seals (Phoca vitulina) and Grey Seals (Halichoerus grypus) in North-Holland Including Texel, During 2009–2012. Master’s Thesis, Utrecht University, Utrecht, Netherlands, 2013. [Google Scholar]
- Herzog, I.C. Prevalence, Adaptation and Impact of Arthropod Parasites on Seals in the German North and Baltic Sea. Ph.D. Thesis, University of Veterinary Medicine Hannover, Hannover, Germany, 12 May 2025. [Google Scholar]
- Herzog, I.C.; Siebert, U.; Wohlsein, P.; Lehnert, K. From Nose to Nose: Prevalence, Lifecycle, and Health Impact of Halarachne halichoeri in Grey Seals of the North and Baltic Sea. In Proceedings of the Joint Parasitology Spring Meeting, Würzburg, Germany, 11–14 March 2025. [Google Scholar]
- Reckendorf, A.; Siebert, U.; Wohlsein, P.; Lehne, K. First record of the nasal mite Halarachne halichoeri in a grey seal from the German Wadden Sea. In Proceedings of the 30th Conference of the European Cetacean Society, Funchal, Madeira, Portugal, 14–16 March 2016. [Google Scholar]
- Domrow, R. Some dermanyssid mites (Acari), mostly from Australasian rodents. Proc. Linn. Soc. N. S. W. 1981, 103, 189–208. [Google Scholar]
- Wilson, N. Mesostigmata: Rhinonyssidae, Halarachnidae (nasal mites); Metastigmata: Ixodidae (ticks). In Entomology of Antarctica; Gressitt, J.L., Ed.; American Geophysical Union: Washington, DC, USA, 1967; Volume 10, pp. 41–49. [Google Scholar] [CrossRef]
- Athinaiou, N.; Sarantopoulou, J.; Komnenou, A.; Papadopoulos, E.; Exadactylos, A.; Gkafas, G.A. From Atlantic to Greece. The Case of Nasal Mite in Mediterranean Monk Seal. EIMBO 2023, 6, 000640. [Google Scholar] [CrossRef]
- Koitsanou, E.; Akritopoulou, E.; Athinaiou, N.; Sarantopoulou, J.; Komnenou, A.; Dendrinos, P.; Exadactylos, A.; Gkafas, G.A. Molecular identification of a parasitic mite found in the respiratory system of a stranded mediterranean monk seal in the area of Pagasitikos Gulf. In Proceedings of the Marine and Inland Waters Research Symposium, Porto Heli, Argolida, Greece, 16–20 September 2022. [Google Scholar]
- Fay, F.H.; Furman, D.P. Nasal mites (Acari: Halarachnidae) in the spotted seal, Phoca largha PaIlas, and other pinnipeds of Alaskan waters. J. Wildl. Dis. 1982, 18, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ferris, G.F. Observations on some ectoparasitic mites (Arachnida: Acarina: Dermanyssidae). Microentomology 1942, 7, 77–83. [Google Scholar]
- Munro, R.; Ross, H.; Cornwell, C.; Gilmour, J. Disease conditions affecting common seals (Phoca vitulina) around the Scottish mainland, September–November 1988. Sci. Total Environ. 1992, 115, 67–82. [Google Scholar] [CrossRef]
- Scheffer, V.B.; Slipp, J.W. The harbor seal in Washington State. Am. Midl. Nat. 1944, 32, 373–416. [Google Scholar] [CrossRef]
- Kim, K.C. Coevolution of Parasitic Arthropods and Mammals; Wiley: New York, NY, USA, 1985; pp. 3–82. [Google Scholar]
- Domrow, R. Acari Astigmata (excluding feather mites) parasitic on Australian vertebrates: An annotated checklist, keys and bibliography. Invertebr. Syst. 1992, 6, 1459–1606. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).