The Relationship Between Abundance and Actual Spatial Distribution of Terrestrial Isopods (Oniscidea)
Abstract
1. Introduction
2. Materials and Methods
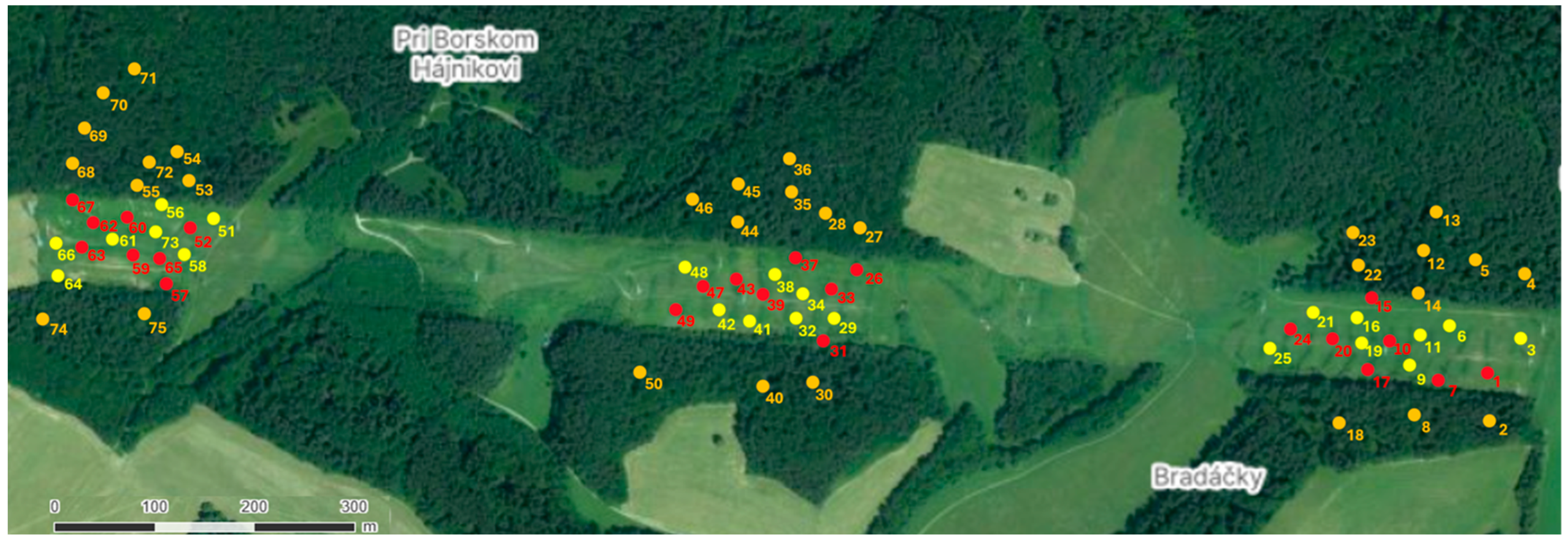
2.1. Study Area
2.2. Sampling Design
2.3. Statistical Analyses
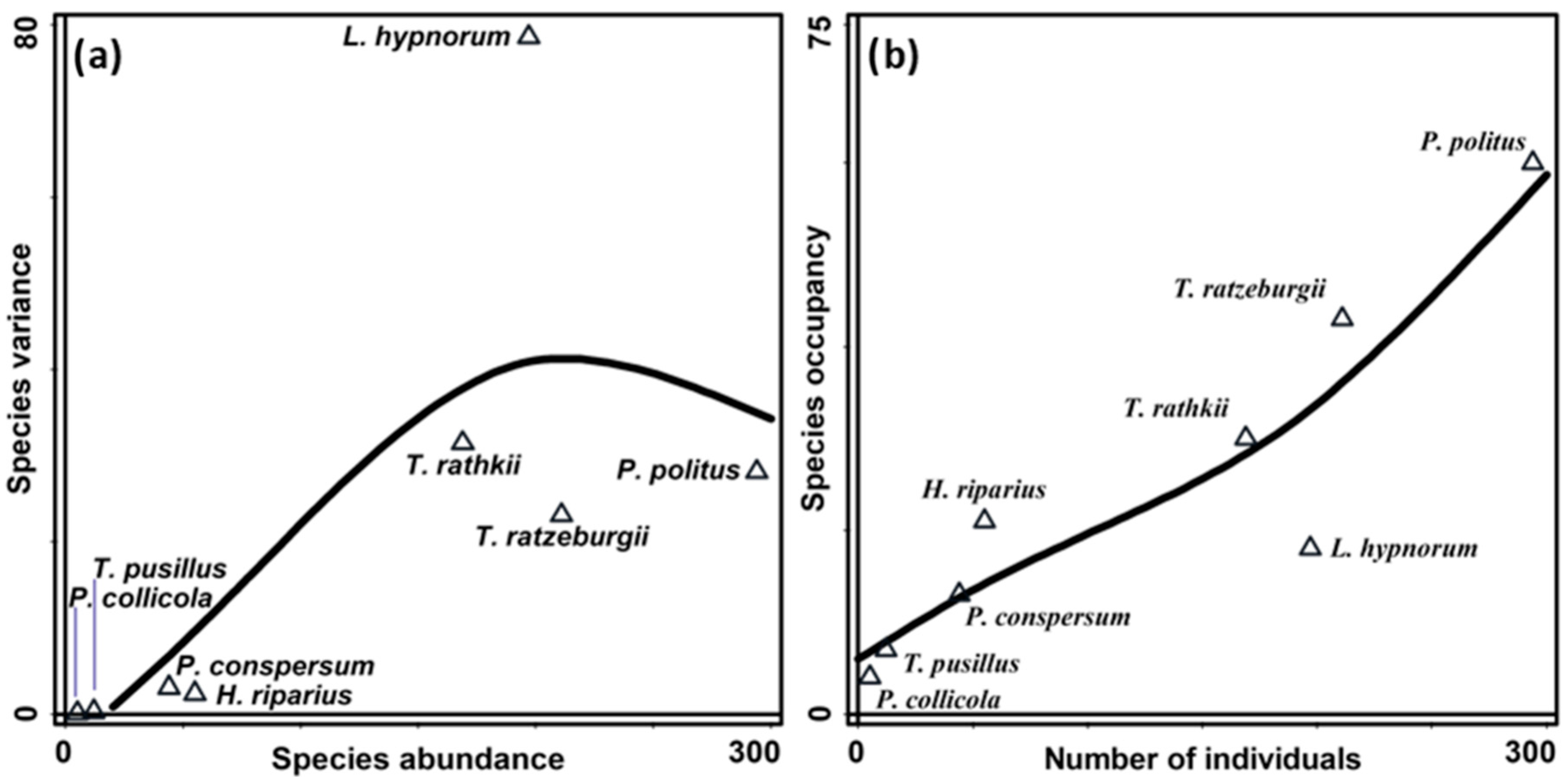
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of pattern and scale in ecology: The Robert H. MacArthur award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Horne, J.K.; Schneider, D.C. Spatial variance in ecology. Oikos 1995, 74, 18–26. [Google Scholar] [CrossRef]
- Nielsen, U.N. Soil Fauna Assemblages: Global to Local Scales; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar] [CrossRef]
- Sutton, S. Woodlice; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Hornung, E. Evolutionary adaptation of oniscidean isopods to terrestrial life: Structure, physiology and behavior. Terr. Arthropod Rev. 2011, 4, 95–130. [Google Scholar] [CrossRef]
- Allee, W.C. Studies in animal aggregations: Causes and effects of bunching in land isopods. J. Exp. Zool. 1926, 45, 255–277. [Google Scholar] [CrossRef]
- David, J.-F.; Handa, I.T. The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol. Rev. 2010, 85, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.T.C.; Krab, E.J.; Mariën, J.; Zimmer, M.; Cornelissen, J.H.C.; Ellers, J.; Wardle, D.A.; Berg, M.P. Traits underpinning desiccation resistance explain distribution patterns of terrestrial isopods. Oecologia 2013, 172, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Csonka, D.; Halasy, K.; Buczkó, K.; Hornung, E. Morphological traits—Desiccation resistance—Habitat characteristics: A possible key for distribution in woodlice (Isopoda, Oniscidea). ZooKeys 2018, 801, 481–499. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, P.; Baeten, L.; Berg, M.P.; Gallet-Moron, E.; Brunet, J.; Cousins, S.A.O.; Decocq, G.; Diekmann, M.; Giffard, B.; De Frenne, P.; et al. Desiccation resistance determines distribution of woodlice along forest edge-to-interior gradients. Eur. J. Soil Biol. 2018, 85, 1–3. [Google Scholar] [CrossRef]
- De Smedt, P.; Baeten, L.; Proesmans, W.; Berg, M.P.; Brunet, J.; Cousins, S.A.O.; Decocq, G.; Deconchat, M.; Diekmann, M.; Gallet-Moron, E.; et al. Linking macrodetritivore distribution to desiccation resistance in small forest fragments embedded in agricultural landscapes in Europe. Landsc. Ecol. 2018, 33, 407–421. [Google Scholar] [CrossRef]
- Sfenthourakis, S.; Hornung, E. Isopod distribution and climate change. ZooKeys 2018, 801, 25–61. [Google Scholar] [CrossRef]
- Stroud, J.T.; Bush, M.R.; Ladd, M.C.; Nowicki, R.J.; Shantz, A.A.; Sweatman, J. Is a community still a community? Reviewing definitions of key terms in community ecology. Ecol. Evol. 2015, 5, 4757–4765. [Google Scholar] [CrossRef]
- Schmalfuss, H. Eco-morphological strategies in terrestrial isopods. Symp. Zool. Soc. Lond. 1984, 53, 49–63. [Google Scholar]
- Kight, S. Reproductive ecology of terrestrial isopods (Crustacea: Oniscidea). Terr. Arthropod Rev. 2009, 1, 95–110. [Google Scholar] [CrossRef]
- Upton, G.J.G.; Cook, I. A Dictionary of Statistics: The Invaluable Guide to All Aspects of Statistics, 2nd ed.; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Taylor, L.R. Aggregation, variance and the mean. Nature 1961, 189, 732–735. [Google Scholar] [CrossRef]
- He, F.; Gaston, K.J. Occupancy, spatial variance, and the abundance of species. Am. Nat. 2003, 162, 366–375. [Google Scholar] [CrossRef]
- Gaston, K.J.; Borges, P.A.V.; He, F.; Gaspar, C. Abundance, spatial variance and occupancy: Arthropod species distribution in the Azores. J. Anim. Ecol. 2006, 75, 646–656. [Google Scholar] [CrossRef]
- Python Software Foundation. Python: A Programming Language for Statistical and Scientific Computing, Version 3.12; Python Software Foundation: Wilmington, DE, USA, 2025; Available online: https://www.python.org/ (accessed on 25 October 2025).
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using Canoco 5, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Tajovský, K.; Schlaghamerský, J.; Pižl, V.; Kučerová, A. Assemblages of Terrestrial Isopods (Isopoda, Oniscidea) in a Fragmented Forest Landscape in Central Europe. ZooKeys 2012, 176, 171–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tajovský, K. Terrestrial isopods (Isopoda: Oniscidea) of the Kokořínsko PLA. Bohemia Cent. 2006, 27, 189–197. [Google Scholar][Green Version]
- Tajovský, K. Terrestrial isopods (Isopoda: Oniscidea) of the Křivoklátsko PLA and BR. Bohemia Cent. 2011, 32, 275–283. [Google Scholar][Green Version]
- Tajovský, K. Terrestrial isopods (Isopoda: Oniscidea) of the Brdy Protected Landscape Area. Bohemia Cent. 2018, 39, 205–218. [Google Scholar][Green Version]
- Tuf, I.H.; Jeřábková, E. Diurnal epigeic activity of terrestrial isopods (Isopoda: Oniscidea). In Proceedings of the International Symposium of Terrestrial Isopod Biology—ISTIB-07; Zimmer, M., Charfi-Cheikhrouha, F., Taiti, S., Eds.; Shaker Verlag: Aachen, Germany, 2008; pp. 167–172. [Google Scholar][Green Version]
- Antonović, I.; Brigić, A.; Sedlar, Z.; Bedek, J.; Šoštarić, R. Terrestrial isopod community as indicator of succession in a peat bog. ZooKeys 2012, 176, 171–188. [Google Scholar] [CrossRef]
- Tajovský, K.; Štrichelová, J.; Tuf, I.H. Terrestrial isopods (Oniscidea) of the White Carpathians (Czech Republic and Slovakia). ZooKeys 2018, 801, 305–321. [Google Scholar] [CrossRef]
- Ilosvay, G. A talajfelszínen mozgó állatok napszakos aktivitásának vuzsgálata a farkasgyepüi bükkösben. Folia Musei Hist. Nat. Bakony. 1982, 1, 171–180. [Google Scholar]
- Lawton, J.H. Range, population abundance and conservation. Trends Ecol. Evol. 1993, 8, 409e413. [Google Scholar] [CrossRef] [PubMed]
- Rigal, F.; Whittaker, R.J.; Triantis, K.A.; Borges, P.A.V. Integration of non-indigenous species within the interspecific abundance-occupancy relationship. Acta Oecologica—Int. J. Ecol. 2013, 48, 69–75. [Google Scholar] [CrossRef]
- Korsós, Z.; Hornung, E.; Szlávecz, K.; Kontschán, J. Isopoda and Diplopoda of urban habitats: New data to the fauna of Budapest. Ann. Hist.-Nat. Musei Natl. Hung. 2002, 94, 193–208. [Google Scholar]
- Hornung, E.; Tóthmérész, B.; Magura, T.; Vilisics, F. Changes of isopod assemblages along an urban–suburban–rural gradient in Hungary. Eur. J. Soil Biol. 2007, 43, 158–165. [Google Scholar] [CrossRef]
- Tomescu, N.; Teodor, L.; Ferenti, S.; Covaciu-Marcov, S.-D. Trachelipus species (Crustacea, Isopoda, Oniscidea) in Romanian fauna: Morphology, ecology, and geographic distribution. North-West. J. Zool. 2015, 11 (Suppl. 1), S1–S106. [Google Scholar]
- Gerlach, A.; Russell, D.J.; Jaeschke, B.; Römbke, J. Feeding preferences of native terrestrial isopod species (Oniscoidea, Isopoda) for native and introduced leaf litter. Appl. Soil Ecol. 2014, 83, 95–100. [Google Scholar] [CrossRef]
- Harding, P.T.; Sutton, S.L. Woodlice in Britain and Ireland: Distribution and Habitat; Biological Records Centre, Institute of Terrestrial Ecology: Huntingdon, UK, 1985. [Google Scholar]
- Oliver, P.G.; Meechan, C.J. Woodlice; Field Studies Council: Shrewsbury, UK, 1993. [Google Scholar]
- Judas, M.; Hauser, H. Patterns of isopod distribution: From small to large scale. Isr. J. Zool. 1998, 44, 333–343. [Google Scholar]
- Orsavová, J.; Tuf, I.H. Woodlice: Distribution Atlas in the Czech Republic and Bibliography 1840–2018; Muzeum Regionu Valašsko & Muzeum Jihovýchodní Moravy: Zlín, Czech Republic, 2018. [Google Scholar]
- Inui, N.; Kimbara, R.; Yamaguchi, H.; Miura, T. Pleopodal lung development in a terrestrial isopod, Porcellio scaber (Oniscidea). Arthropod Struct. Dev. 2022, 71, 101210. [Google Scholar] [CrossRef]
- Boeraeve, P.; Arijs, G.; Segers, S.; De Smedt, P. Habitat and seasonal activity patterns of the terrestrial isopods (Isopoda: Oniscidea) of Belgium. Belg. J. Entomol. 2021, 116, 1–95. [Google Scholar]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Vilisics, F.; Elek, Z.; Lövei, G.L.; Hornung, E. Composition of terrestrial isopod assemblages along an urbanisation gradient in Denmark. Pedobiologia 2007, 51, 45–53. [Google Scholar] [CrossRef]
- Adis, J. Problems of interpreting arthropod sampling with pitfall traps. Zool. Anz. 1979, 202, 177–184. [Google Scholar]
- Topping, C.J.; Sunderland, K.D. Limitations to the use of pitfall traps in ecological studies exemplified by a study of spiders in a field of winter wheat. J. Appl. Ecol. 1992, 29, 485–491. [Google Scholar] [CrossRef]


| P. politus | T. ratzeburgii | L. hypnorum | T. rathkii | H. riparius | P. conspersum | T. pusillus | P. collicola | |
|---|---|---|---|---|---|---|---|---|
| All individuals | 294 | 211 | 197 | 169 | 55 | 44 | 12 | 5 |
| Dominance | 30 | 21 | 20 | 17 | 6 | 4 | 1 | 1 |
| Frequency | 80 | 57 | 24 | 40 | 28 | 17 | 9 | 5 |
| Constancy | 25 | 19 | 7 | 13 | 7 | 5 | 2 | 1 |
| Min. per trap | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Max. per trap | 32 | 25 | 51 | 30 | 9 | 11 | 3 | 2 |
| Mean | 3.9 | 2.8 | 2.6 | 2.3 | 0.7 | 0.6 | 0.2 | 0.1 |
| Standard Error | 0.6 | 0.6 | 1.0 | 0.6 | 0.2 | 0.2 | 0.1 | 0.0 |
| Variance | 28.2 | 23.1 | 78.6 | 31.5 | 2.4 | 3.1 | 0.3 | 0.1 |
| Standard Dev. | 5.3 | 4.8 | 8.9 | 5.6 | 1.5 | 1.8 | 0.5 | 0.3 |
| Median | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 percentile | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 75 percentile | 5 | 3 | 0 | 2 | 1 | 0 | 0 | 0 |
| Mode | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| VMRt | 7.2 | 8.2 | 29.9 | 14.0 | 3.3 | 5.3 | 1.9 | 1.4 |
| VMRh | 11.8 | 3.2 | 2.9 | 10.5 | 4.6 | 6.8 | 2 | 2 |
| VMRs | 5.9 | 11.6 | 34.7 | 16.4 | 1.8 | 3.8 | 1.8 | 0.1 |
| VMRf | 4.5 | 5.6 | 28.3 | 1 | 2.7 | 4.3 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinka, M.; Tuf, I.H. The Relationship Between Abundance and Actual Spatial Distribution of Terrestrial Isopods (Oniscidea). Diversity 2025, 17, 790. https://doi.org/10.3390/d17110790
Martinka M, Tuf IH. The Relationship Between Abundance and Actual Spatial Distribution of Terrestrial Isopods (Oniscidea). Diversity. 2025; 17(11):790. https://doi.org/10.3390/d17110790
Chicago/Turabian StyleMartinka, Martin, and Ivan Hadrián Tuf. 2025. "The Relationship Between Abundance and Actual Spatial Distribution of Terrestrial Isopods (Oniscidea)" Diversity 17, no. 11: 790. https://doi.org/10.3390/d17110790
APA StyleMartinka, M., & Tuf, I. H. (2025). The Relationship Between Abundance and Actual Spatial Distribution of Terrestrial Isopods (Oniscidea). Diversity, 17(11), 790. https://doi.org/10.3390/d17110790






