Abstract
Understanding species distribution and environmental niches is crucial for conserving endangered taxa. The recent taxonomic split of the European freshwater mussels U. crassus and U. nanus into distinct species requires a reassessment of their distinct ecologies for conservation. This study uses species distribution models (SDMs) to define and compare the environmental niches and reconstruct the distributions across six past time periods, starting from the mid-Pliocene Warm Period (mPWP, ca. 3.205 Ma) to the present. Our results reveal significant environmental niche differentiation between the two species, with U. crassus occupying a broader environmental niche primarily influenced by annual mean temperature and precipitation in the warmest quarter. In contrast, U. nanus shows a narrower niche shaped by temperature seasonality, mean diurnal range, annual mean temperature, and precipitation seasonality. Paleodistribution models indicate that during the Last Glacial Maximum (LGM, ca. 21 ka), U. crassus persisted in multiple southern refugia, whereas U. nanus was restricted to a single western refugium. These contrasting glacial histories led to divergent post-glacial colonization routes, explaining their current genetic patterns and partially overlapping ranges. By identifying present environmental hotspots, this research provides an essential framework for developing targeted, species-specific conservation strategies for these freshwater mussels.
1. Introduction
Knowledge of species distribution patterns in relation to environmental conditions is fundamental for understanding the ecology, evolution, and conservation of endangered taxa [1,2]. Species distribution models (SMD) are common tools for predicting suitable and unsuitable habitats, a critical step in conservation management [3]. While widely used for predicting current and future distributions of species, their application to reconstructing past distributions is less common. Understanding historical distribution is important for interpreting phylogeography and current genetic patterns but is often overlooked [4]. The SDM approach offers new possibilities for an enhanced contribution of paleobiology to ecology and conservation biology, e.g., for assessing climate change impacts and for informing management actions [5]. To date, past distribution models have mainly focused on a plethora of immobile plant and tree species [6,7,8,9,10,11]. Mollusk species are tightly linked to environmental conditions, making them excellent biological indicators often used for biomonitoring [12,13,14]. Since freshwater mussels are sessile filter feeders with no or little active movement, they are ideal subjects for studying how climatic changes have influenced the genetic patterns and distributions of species [15]. Despite their suitability for such studies, investigations in this direction remain rare [16]. Existing research has primarily focused on modeling present and future distributions—for instance, predicting genetic structure under future climate change or spatial prioritizing—while largely excluding historical ones [17,18,19,20].
Recent taxonomic studies revised the Unio crassus aggregate, a complex of European freshwater mussels, establishing 12 distinct species [21], making them an ideal target for re-assessing potential differences in the past and present distribution in relation to environmental niche partitioning. The empirical basis for this taxonomic partitioning was derived from comprehensive phylogenetic analyses utilizing a multi-locus molecular approach to establish lineage divergence. Specifically, phylogenetic inference was initially performed using a mitochondrial marker, cytochrome oxidase subunit I (COI), across a wide geographical and numerical sampling scale (815 specimens derived from 182 populations). This was supplemented, in a subset of specimens, by sequencing whole mitogenomes and, critically, by employing Anchored Hybrid Enrichment (AHE) to generate high-resolution data from approximately 600 independent nuclear loci.
Two of these newly defined species, U. nanus and U. crassus, have broad distributions that frequently overlap across temperate Europe [21]. This new understanding potentially has critical conservation implications, as both species have declined by over 50% in the last 60 years and are therefore listed as ‘Endangered’ in the IUCN Red List of Threatened Species [22,23]. Since earlier studies on the ecology, biology, and conservation of these species did not differentiate U. nanus from U. crassus (e.g., [24,25,26,27]), their findings require reassessment using the new species definition presented herein. Moreover, the similar shell morphology of U. crassus and U. nanus often hinders correct species identification [21,28] and makes fossil records unreliable for investigating the distribution history of these cryptic species (Figure 1). Given these limitations, a modeling approach appears most useful to reconstruct their distinct environmental requirements and historical biogeography.
Figure 1.
Typical morphotypes for (a) U. crassus; (b) U. nanus. Note that the two species are morphologically cryptic and cannot be reliably distinguished by shell characteristics alone.
This study addresses this research gap regarding the environmental requirements and the history of the two freshwater mussels U. crassus and U. nanus. To provide a clear conceptual foundation, we address the ambiguity in the ecological literature by following the Grinnellian concept of the environmental niche, defined as the precise set of abiotic conditions governing a species’ geographic distribution [29,30]. Specifically, we investigate the differences in their environmental niches to determine whether these two species have distinct and fundamentally different habitat requirements, which may have important implications for conservation and explain their distinct distributional patterns. We use SDMs to reconstruct the historical distributions of U. crassus and U. nanus over time, providing insights into how past climate fluctuations may have shaped their current genetic and geographic patterns. We further hypothesize that their currently overlapping distributions are a recent phenomenon resulting from post-glacial expansion starting around 11,700 years ago from separate glacial refugia. Using the example, this work establishes a novel framework for understanding the biogeography of cryptic species by integrating a revised taxonomy with paleodistribution modeling. While the former literature did not differentiate between the two species U. crassus and U. nanus—except for genetic studies [21,28]—this approach offers critical insights for developing effective, species-specific conservation strategies. Crucially, the application of this framework aims to identify present-day environmental hotspots for both U. crassus and U. nanus.
2. Materials and Methods
Presence point data for U. crassus and U. nanus were downloaded from the most recently available trans-European distribution monitoring effort (Supplementary data in [21]). Data points were extracted for each specific species, and duplicate entries were subsequently removed. This yielded a total of 118 unique presence points, comprising 54 for U. crassus and 64 for U. nanus. Species identification for these occurrence records was validated using COI gene sequences [21]. A map of presence points of U. crassus and U. nanus used in this study (Figure 2a) and their current distribution range (Figure 2b) were created using QGIS (version 3.34.5).
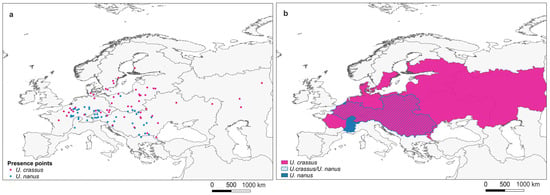
Figure 2.
(a) Presence point data set for U. crassus and U. nanus used in this study; (b) Current distribution map of U. crassus and U. nanus, redrawn from [21].
Nineteen bioclimatic variables were extracted from the Paleoclim database (http://www.paleoclim.org/) at a spatial resolution of 2.5 arc-minutes (approximately 5 km). These variables corresponded to the following key time periods, representing significant climatic shifts:
- Current (1979–2013): Anthropocene [31]
- LH: Late-Holocene, Meghalayan (4.2–0.3 ka) [32]
- EH: Early-Holocene, Greenlandian (11.7–8.326 ka) [32]
- LGM: Last Glacial Maximum (21 ka) [31]
- LIG: Last Interglacial (130 ka) [33]
- MIS19: Marine Isotope Stage 19 (787 ka) [34]
- mPWP: Mid-Pliocene Warm Period (3.205 Ma) [35]
The divergence of U. crassus and U. nanus dates to the Miocene (10 Ma) or Early Pliocene (5.3–3.6 Ma) [21]. Therefore, mPWP was selected for our analysis, as it is the oldest available time frame by which these two species had certainly split into distinct lineages. The modelling approach is based on the assumption of niche conservatism over deep time [36]. This approach is supported by paleobiological evidence from marine mollusks, which have demonstrated stable ecological niches over the past three million years [37].
To address multicollinearity among the bioclimatic variables, a two-step approach was employed. First, the Variance Inflation Factor (VIF) was calculated using the vif.step function within the usdm package in R (version 4.5.1). Variables with a VIF exceeding a threshold of 5 were iteratively removed, indicating high correlation with other predictors [38]. In the second step, Pearson’s correlation coefficients (PCC) were calculated for the remaining variables, and the resulting correlation matrix was visualized using the corrplot package in R (version 4.5.1). Variables exhibiting a PCC greater than 0.6 were then excluded from the dataset to ensure independence among predictors.
For compatibility with MaxEnt (version 3.4.4), the downloaded bioclimatic variables, initially in TIFF format, were reprojected and converted to ASCII file format using the raster package in R (version 4.5.1). Species Distribution Models (SDMs) were developed using MaxEnt (version 3.4.4) to infer the environmental niche and potential distribution of U. crassus and U. nanus. Initially, models were calibrated using the selected bioclimatic variables from the current time period (1979–2013) to infer the present environmental niche of each species and compare it with their known current distributions. Model settings included a logistic output format, ten replicates and cross-validation to ensure robustness. Model performance was evaluated by measuring the Area Under the Receiver Operating Characteristic curve (AUC). Generally, AUC values > 0.9 indicate excellent performance and AUC values ranging from 0.8–0.9 indicate reliable performance, whereas AUC values ranging from 0.7–0.8 are categorized as fair and AUC values < 0.7 are considered as poor (Swets, 1988 [39]). Subsequently, these calibrated MaxEnt models were projected onto bioclimatic layers from the six past time periods. These resulting Paleo species distribution models (PaleoSDMs) were used to reconstruct potential historical distributions and identify putative glacial refugia, thereby understanding shifts in suitable habitat driven by past climate change. The same modeling methods and algorithms were consistently applied for both present and past distribution projections.
3. Results
3.1. Bioclimatic Variable Selection and Model Performance
The VIF method yielded a set of seven bioclimatic variables with no collinearity issues (Table 1). Although bio19 had a low VIF = 2.35, it was negatively correlated with bio4 (PCC = −0.64) and therefore excluded from further analyses (Figure A1). Both VIF and PCC assessments resulted in a selection of six appropriate bioclimatic variables that were used in the subsequent modelling procedure. For the time period MIS19 and mPWP bio2 data was not available, and so five variables were used for those modelling predictions.

Table 1.
Bioclimatic variables from the Paleoclim database and calculated VIF; selected variables for the model are written in bold.
The performance of both MaxEnt models was robust, as indicated by their high AUC values. The U. crassus model achieved an average AUCtest of 0.859 ± 0.059 SD, while the U. nanus model exhibited an even higher average AUCtest of 0.951 ± 0.021 SD. These values suggest high discriminatory power for both models in distinguishing suitable from unsuitable habitats (Table 2).

Table 2.
Selected variables and model performance. Percent variable contribution for U. crassus and U. nanus.
3.2. Species Distribution Model (SDM)
The environmental suitability models for U. crassus and U. nanus identified distinct sets of highly influential bioclimatic variables (Table 2).
For U. crassus, annual mean temperature (41.3%) emerged as the most significant predictor of habitat suitability, followed by precipitation of the warmest quarter (30.9%) and temperature seasonality (19.6%). In contrast, the model for U. nanus was primarily driven by temperature seasonality (27.1%), followed by annual mean temperature (21.6%) and mean diurnal range (17.4%). Precipitation seasonality (11.5%) also contributed notably to the U. nanus model. While the SDM for U. crassus is highly influenced by two bioclimatic variables (annual mean temperature and precipitation of the warmest quarter), the SDM for U. nanus is defined by a more complex combination of multiple bioclimatic variables. Interestingly, the bioclimatic variable mean diurnal range achieved a low contribution to the SDM of U. crassus (2.7%), while for U. nanus this variable contributed substantially to the model (17.4%). The mean diurnal range, which represents the difference between the maximum and minimum temperature for each month, serves as a more critical environmental factor for U. nanus than for U. crassus, indicating that U. nanus is more dependent on temperature fluctuations. In contrast, the high percentage contribution of precipitation of the warmest quarter for U. crassus indicates that the species is more dependent on a specific rainfall regime during its warmest season than for U. nanus.
The potential distribution predictions for U. crassus showed a core distribution in central Europe extending to the Baltic in the East and the Atlantic in the West (Figure 3a). Intermediate suitable habitats can be found around the Baltic Sea, the Black Sea and most of the Danube basin. The Iberian and Balkan Peninsulas constitute no suitable habitats for U. crassus, while the Apennine Peninsula displays suitable habitats for this species. The potential distribution predictions for U. crassus represent a high concordance with its actual current distribution (Figure 2b and Figure 3a). However, the model identifies high-potential habitats on the Apennine Peninsula and the British Isles, regions from which no current occurrences of this species have been documented. The distribution pattern, extending to the Ural mountains, indicates that U. crassus is adapted to a wide range of continental and maritime European river systems.
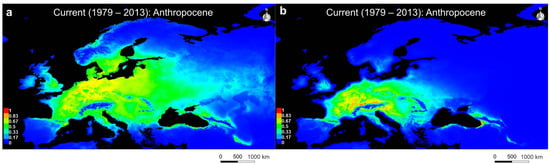
Figure 3.
SDM for (a) U. crassus and (b) U. nanus for the Current (1979–2013): Anthropocene; Warmer colors indicate higher suitability, while blue indicates unsuitable areas.
The potential distribution predictions for U. nanus show highly suitable habitats in central Europe around the Alps, extending to the Rhine and Upper Danube basin, as well as the Western coast around the Black Sea (Figure 3b). Intermediate suitable habitats can be found around the Baltic Sea, the Black Sea and most of the Danube basin. Similar to U. crassus, the Iberian and the southern Balkan Peninsula were not identified as suitable habitats for U. nanus, while the Apennine Peninsula and the British Isles provide a small proportion of suitable habitats for U. nanus, i.e., regions where no current occurrence of this species is known. Despite the British Isles and the Apennine Peninsula, the potential SDM of U. nanus is in congruence with its actual current distribution (Figure 2b and Figure 3a). In general, the overall potential distribution of U. nanus is a more restricted and fragmented distribution compared to U. crassus, despite partial overlapping in their predicted distribution.
3.3. Paleo Species Distribution Models (PaleoSDM)
During the Mid-Pliocene Warm Period (mPWP, 3.205 Ma), the potential distribution of U. crassus was widespread across Europe, extending from France in the west to the Ural Mountains in the east (Figure 4a). Suitable habitats were also present in parts of Scandinavia, the British Isles, and the coastal regions around the Black Sea. The PaleoSDM for U. nanus shows a similar distribution pattern, but with more highly suitable habitats concentrated in southern Germany around the Alpine foothills, the North European Plain, and the Western coast of the Black Sea (Figure 4d). Interestingly, the potential distribution predictions for both U. crassus and U. nanus during the mPWP indicate a significantly broader range than their actual current distribution.
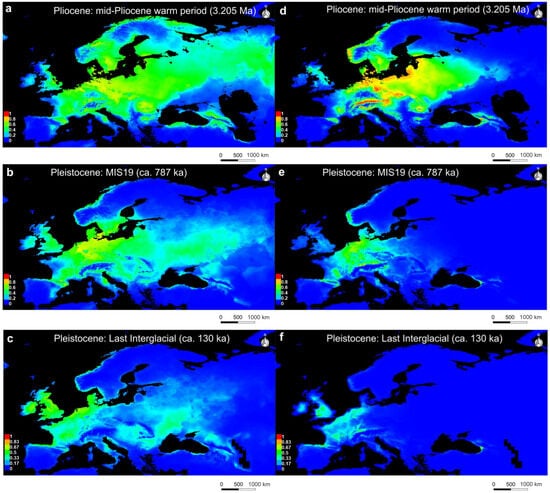
Figure 4.
PaleoSDM for U. crassus (a–c) and U. nanus (d–f) over time: (a) U. crassus during mid-Pliocene Warm period (3.205 Ma); (b) U. crassus during Marine Isotope Stage 19 (787 ka); (c) U. crassus during Last Interglacial Period (130 ka); (d) U. nanus during mid-Pliocene Warm period (3.205 Ma); (e) U. nanus during Marine Isotope Stage 19 (787 ka); (f) U. nanus during Last Interglacial Period (130 ka); warmer colors indicate higher suitability, while blue indicates unsuitable areas.
During the Marine Isotope Stage 19 (MIS19, 787 ka), the predicted distribution pattern of U. crassus was more restricted than during mPWP, but still suitable habitats could be found in various parts across Europe, e.g., Central, Western, Eastern (Figure 4b). Notably, a distribution shift southward was detected in the East. While the British Isles or parts of Scandinavia were identified as suitable habitats for U. crassus, this was not the case for U. nanus. The PaleoSDM during MIS19 for U. nanus showed a condensed core distribution in Central and Western Europe, while regions in the Eastern and Southern parts of Europe constituted unfavorable conditions, except for a small proportion of suitable habitat, e.g., on the western coast of the Black Sea (Figure 4e).
During the Last Interglacial (LIG, 130 ka), the PaleoSDM for U. crassus predicted a loss of suitable habitats compared to MIS19, resulting in a distribution division into a Western and Eastern part including parts of the Danube basin (Figure 4c). For U. nanus, suitable habitat loss was even more pronounced, e.g., in vast parts of Eastern Europe. The PaleoSDM during LIG for U. nanus showed few suitable habitats in the Western part of Europe including the British Isles and a small spot in the western coast of the Black Sea (Figure 4f).
The most profound range contraction for both species was revealed to have occurred during the Last Glacial Maximum (LGM), where a near-complete disappearance of suitable habitats was predicted. During the LGM, model predictions showed only small, fragmented suitable habitats for both U. crassus (Figure 5a) and U. nanus (Figure 5b). These remaining areas, likely corresponding to major glacial refugia, were located in the Balkans (e.g., the Danube basin), the coastline around the Black Sea, and southern parts of France. For U. nanus, the glacial refugia were likely located in Western Europe, in France, consistent with its core distribution being found in this region during the LIG.
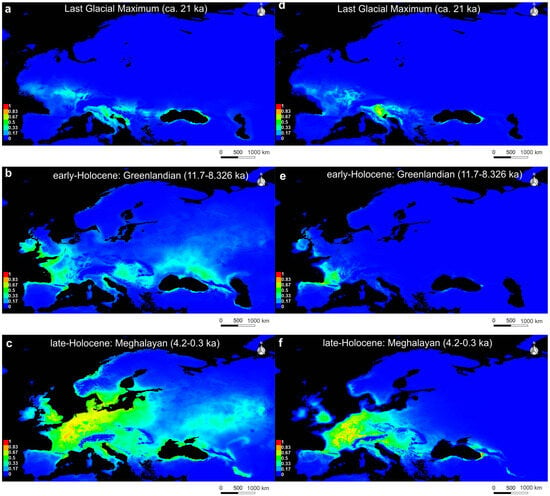
Figure 5.
PaleoSDM for U. crassus (a–c) and U. nanus (d–f) over time: (a) U. crassus during Last Glacial Maximum (21 ka); (b) U. crassus during early-Holocene, Greenlandian (11.7–8.326 ka); (c) U. crassus during late-Holocene, Meghalayan (4.20.3 ka); (d) U. nanus during Last Glacial Maximum (21 ka); (e) U. nanus during early-Holocene, Greenlandian (11.7–8.326 ka); (f) U. nanus during late-Holocene, Meghalayan (4.20.3 ka); Warmer colors indicate higher suitability, while blue indicates unsuitable areas.
During the Early Holocene (EH; 11.7–8.326 ka), model predictions for U. crassus showed an expansion of suitable habitats northwards from its potential glacial refugia. This expansion encompassed the regions of Western Europe, the Carpathians, and the Pontic-Caspian region (Figure 5d). In contrast, suitable habitats for U. nanus were limited to a single restricted region in Western Europe during the EH, expanding northwards from the Pyrenees into the Rhône basin (Figure 5e).
During the Late-Holocene (LH, 4.20.3 ka), the PaleoSDM for U. crassus revealed a massive expansion of highly suitable habitats in Central Europe, the Western Atlantic coast regions, and the North European plain (Figure 5c). Suitable habitats could also be found around the Baltic Sea, as well as the Carpathians and parts of the East European plain. For U. nanus, model predictions during LH showed suitable habitats concentrated in Western and Central Europe, the Alpine foothills, and parts of the Carpathians, indicating a range expansion from West to East (Figure 5f). Suitable habitats for both species were found in the British Isles and the Apennine peninsula during this period, regions where neither species is currently recorded. A comparison of the LH and present-day distributions revealed that both U. crassus and U. nanus had achieved near-maximal range expansion during LH, resulting in a potential distribution highly similar to their current extent.
The paleodistribution patterns of U. crassus and U. nanus demonstrated a clear response to past climatic changes with several range expansions and contractions across the seven investigated time periods. Both species exhibited a significantly broader potential range during warmer periods such as the mPWP compared to the present day. Subsequent colder glacial periods, such as MIS19 and the LGM, led to severe fragmentations and a notable southward contraction of their distributions. Interestingly, range contraction was not exclusively induced by glacial events. Interglacial periods similarly influenced their distribution, resulting in a more contracted and reduced range, which is especially highlighted by the predicted distributions of U. nanus during LIG. After the LGM, both species began to expand northwards, with U. nanus starting from a single refugium in the West, while U. crassus initiated expansion from several southern refugia, ultimately leading to its wider present-day distribution. Crucially, the significant range overlap resulting from these differing post-glacial movements establishes present-day contact zones across Europe, which potentially contributed to the sympatric populations observed today.
4. Discussion
This study reconstructed the distinct environmental niches and paleodistributions of the recently split freshwater mussels U. crassus and U. nanus, thereby elucidating the ecological and biogeographic disparities that likely contributed to their evolutionary divergence. Despite their overlapping geographic distribution, substantial differences in the environmental niches between U. crassus and U. nanus were found. Overall, U. crassus shows a significantly broader environmental niche than U. nanus, a characteristic which defines it as a climatic generalist and is directly reflected by its vast geographic distribution across Europe. The significant contribution of annual mean temperature and precipitation of the warmest quarter to the U. crassus model suggests that the species is critically dependent on thermal averages and constant rainfall during its warmest season, likely indicating a crucial period for metabolic activity and somatic growth. In contrast, the model for U. nanus is defined by multiple distinct variables, indicating that this species exhibits a more complex and restricted niche than U. crassus. Since temperature seasonality was the most influential factor, this species appears to be adapted to a specific annual thermal regime. This is likely a requisite for crucial life-history events such as reproduction, as low winter temperatures are necessary for gametogenesis and subsequent rising temperatures between April and July trigger reproduction [40,41]. Hence, future elevated water temperatures could have negative effects on gametogenesis and the timing in reproduction. The shift of brooding periods and glochidia release to earlier times of the year due to global warming is known to lead to a reduction in the reproductive output and therefore to the decline of species [42]. Although the annual mean temperature and the precipitation of the warmest quarter influence the model for U. nanus, their contribution is substantially lower than in the model for U. crassus. Critically, the environmental variables mean diurnal range and temperature seasonality play a substantial role in the model for U. nanus, whereas these variables have little to no effect on the U. crassus model. These findings indicate that U. nanus is particularly vulnerable to decline driven by climate change. These findings suggest that they may have adapted to different microclimates or even heterogeneous parts of the same river system, which is of particular relevance for river systems where both species occur sympatrically.
Consequently, key distinctions in past distributions and colonization history were found between U. crassus and U. nanus. Especially U. crassus surviving in multiple refugia during LGM, recolonized Europe more rapidly during EH than U. nanus. For U. crassus, high genetic diversity was revealed [28], which can be explained by harboring genetic variation in multiple refugia. Conversely, U. nanus survived in a single refugium during LGM, experiencing a delayed postglacial recolonization from westwards. This colonization history and the dramatic impact of range contraction during LGM and LIG could have led to the low genetic diversity observed in U. nanus compared to U. crassus [28,43]. This is likely a natural consequence of the species originating from a single source area. The alignment between the genetic evidence and the PaleoSDM findings provides a robust validation for the reconstructed colonization histories. Former genetic studies revealed two distinct post-glacial colonization routes: a Western route for U. nanus originating from a single refugium, and an Eastern route for U. crassus originating from multiple Ponto-Caspian refugia [28]. These divergent pathways, which converge in a secondary contact zone in Central Europe, are highly consistent with the independent findings of the modelled reconstructions. The models not only provide a plausible climatic explanation for why these specific refugia existed but also confirm the different post-glacial expansion dynamics that led to the currently observed genetic structure.
Though the model’s reliance on bioclimatic variables introduces several fundamental limitations. First, the model does not account for critical biotic interactions, most notably the obligate parasitic relationship with host fish, which serves as the primary dispersal vector. Second, it overlooks alternative dispersal pathways such as passive downstream drift, river captures, or connections formed by glaciations and dispersal barriers. Finally, the model does not account for anthropogenic factors, including artificial canals or human-mediated introductions via infected fish, which could also have shaped modern distributions. Further, a fundamental limitation of this modeling approach is that it is designed to investigate the potential environmental niche, not the realized niche. For example, some dispersal routes appear very unlikely based on the contemporary geography, e.g., the dispersion of U. nanus from northern Italy to southwestern France or from France to the British Isles. While the SDM for U. nanus identified high climatic suitability in regions like the Apennine peninsula, this species is not known to occur there. This is a significant finding as it demonstrates that while the climate was suitable, physical dispersal barriers (such as the Alps) and the lack of hydrological connections or appropriate host–fish routes likely prevented U. nanus from ever colonizing this region.
Despite these model limitations, the models provide a plausible ecological reason for the speciation and maintenance of U. crassus and U. nanus as distinct species. The limitation regarding biotic interactions is particularly relevant for obligate parasitic organisms such as freshwater mussels, whose colonization routes are fundamentally constrained by the presence and movement of suitable host fish.
In the context of the host–fish relationship, the divergent colonization patterns predicted for U. crassus and U. nanus align with the known phylogeographic histories of their most common host fish, the European chub (Squalius cephalus). During the LGM, S. cephalus was eradicated from most of Europe, with different genetic lineages surviving in several putative southern refugia, including the southern Danube (Western–Danubian lineage), the Black Sea periphery (Eastern lineage), and the Rhone basin (Western–Atlantic lineage) [44,45]. A strong association emerges between these distinct host fish lineages and the refugia predicted for the two mussel species. Specifically, the Western–Atlantic lineage of the European chub, which survived LGM in the Rhone basin, corresponds precisely to the single western refugium predicted for U. nanus during LGM. In parallel, the Western–Danubian and Eastern lineages of S. cephalus match the multiple Balkan and Black Sea refugia identified for U. crassus. This striking congruence suggests a deep co-dispersal history, providing biological validation for the divergent post-glacial colonization pathways identified by the PaleoSDMs.
The past reconstructions are based on the assumption of niche conservatism, which is, in general, a well-established hypothesis and has been demonstrated for diverse taxa [46]. However, it is necessary to state that this assumption is theoretical, as ecological requirements may have evolved or shifted over the longer time spans analyzed, particularly across major climatic transitions [47]. Our model reconstructions therefore represent the potential habitat under the assumption that the present-day climatic niche is conserved and reflects the historical one.
The fact that the past literature did not distinguish between U. crassus and U. nanus represents a significant limitation in past ecological research. This historical conflation has likely obscured true species-specific habitat requirements and conservation needs. The present study, by analyzing these species separately, rectifies this issue and highlights the critical importance of treating them as distinct evolutionary and ecological units. Establishing their unique environmental niches is a prerequisite for developing effective, species-specific conservation strategies.
The divergence of U. crassus and U. nanus in historical dispersal, shaped by climatic fluctuations, may have played a significant role in shaping their current genetic structure and geographic distribution, as observed for many freshwater mussel species [15,48,49]. The divergent environmental niches of U. crassus and U. nanus support their classification as distinct species. Hence, this taxonomic separation should be maintained. Crucially, this study identifies present-day environmental hotspots for both U. crassus and U. nanus, which can serve as essential templates for prioritizing future conservation regions.
Author Contributions
Conceptualization, S.E. and J.G.; methodology, S.E.; validation, S.E. and R.K.; formal analysis, S.E. and R.K.; investigation, S.E.; writing—original draft preparation, S.E.; writing—review and editing, S.E., J.G. and R.K.; visualization, S.E.; supervision, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area Under the Receiver Operating Characteristic Curve |
| EH | Early-Holocene, Greenlandian (11.7–8.326 ka) |
| ka | kilo-annum = thousand years ago |
| IUCN | International Union for Conservation of Nature |
| LGM | Last Glacial Maximum (21 ka) |
| LH | Late-Holocene, Meghalayan (4.2–0.3 ka) |
| LIG | Last Interglacial (130 ka) |
| Ma | Mega-annum = million years ago |
| MIS19 | Marine Isotope Stage 19 (787 ka) |
| mPWP | Mid-Pliocene Warm Period (3.205 Ma) |
| PaleoSDM | Paleo species distribution model |
| PCC | Pearson correlation coefficient |
| SDM | Species distribution model |
| temp | Temperature |
Appendix A
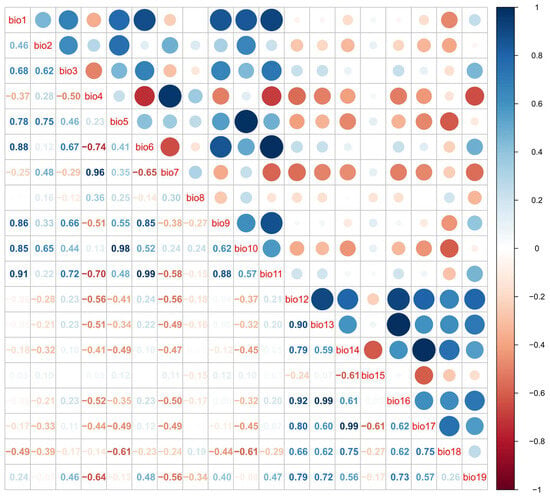
Figure A1.
Pearson correlation matrix among the 19 bioclimatic variables; variables with a PCC > 0.6 were excluded from the MaxEnt projections.
References
- Araújo, M.B.; Williams, P.H. Selecting areas for species persistence using occurrence data. Biol. Conversat. 2000, 96, 331–345. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Chen, Y.; Lembrechts, J.J.; Hautier, Y.; Xu, D.; Li, Y.; Dong, Y.; Mao, L. Identifying divergence time and paleoclimate change hotspots for better conservation under future climate change. Biol. Conserv. 2025, 312, 111499. [Google Scholar] [CrossRef]
- Svenning, J.C.; Fløjgaard, C.; Marske, K.A.; Nógues-Bravo, D.; Normand, S. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 2011, 30, 2930–2947. [Google Scholar] [CrossRef]
- Svenning, J.C.; Normand, S.; Kageyama, M. Glacial refugia of temperate trees in Europe: Insights from species distribution modelling. J. Ecol. 2008, 96, 1117–1127. [Google Scholar] [CrossRef]
- Worth, J.R.; Williamson, G.J.; Sakaguchi, S.; Nevill, P.G.; Jordan, G.J. Environmental niche modelling fails to predict Last Glacial Maximum refugia: Niche shifts, microrefugia or incorrect palaeoclimate estimates? Glob. Ecol. Biogeogr. 2014, 23, 1186–1197. [Google Scholar] [CrossRef]
- Mayol, M.; Riba, M.; González-Martínez, S.C.; Bagnoli, F.; de Beaulieu, J.L.; Berganzo, E.; Burgarella, C.; Dubreuil, M.; Krajmerová, D.; Paule, L.; et al. Adapting through glacial cycles: Insights from a long-lived tree (Taxus baccata). New Phytol. 2015, 208, 973–986. [Google Scholar] [CrossRef]
- Domic, A.I.; Capriles, J.M. Distribution shifts in habitat suitability and hotspot refugia of Andean tree species from the last glacial maximum to the Anthropocene. Neotrop. Biodivers. 2021, 7, 297–309. [Google Scholar] [CrossRef]
- Majeský, Ľ.; Vaculná, L.; Kobrlová, L.; Bonomi, C.; Akopian, J.A.; Aymerich, P.; Barlog, M.; Bryndzová, Š.; Delahaye, T.; Dentant, C.; et al. Tracing the evolutionary history of Dracocephalum austriacum: Insight from population genomics and species distribution modelling. Conserv. Genet. 2025; submitted. [Google Scholar] [CrossRef]
- Cruz-Jiménez, I.; Delgado-Sánchez, P.; Guerrero-González, M.D.L.L.; Puente-Martínez, R.; Flores, J.; De-Nova, J.A. Predicting geographic distribution and habitat suitability of Opuntia streptacantha in paleoclimatic, current, and future scenarios in Mexico. Ecol. Evol. 2023, 13, e10050. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, H. Mollusks: Tools in Environmental and Climate Research. Am. Malacol. Bull. 2015, 33, 310–324. [Google Scholar] [CrossRef]
- Moraitis, M.L.; Tsikopoulou, I.; Geropoulos, A.; Dimitriou, P.D.; Papageorgiou, N.; Giannoulaki, M.; Valavanis, V.; Karakassis, I. Molluscan indicator species and their potential use in ecological status assessment using species distribution modeling. Mar. Environ. Res. 2018, 140, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Krepski, T.; Cieplok, A.; Spyra, A. Predictors of Distribution and Diversity of Rare, Protected, and Endangered Freshwater Mollusks in Rivers with Various Land Use in the Context of Environmental Changes. Ecol. Evol. 2025, 15, e71209. [Google Scholar] [CrossRef]
- Inoue, K.; Lang, B.K.; Berg, D.J. Past climate change drives current genetic structure of an endangered freshwater mussel species. Mol. Ecol. 2025, 24, 1910–1926. [Google Scholar] [CrossRef]
- Kozak, K.H.; Graham, C.H.; Wiens, J. Integrating GIS-based environmental data into evolutionary biology. Trends Ecol. Evol. 2008, 23, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kiser, A.H.; Craig, C.A.; Bonner, T.H.; Littrell, B.; Smith, C.H.; Robertson, C.R.; Wang, H.-H.; Grant, W.E.; Johnson, M.S.; Lopes, R.; et al. Creating a systematic prioritization of stream reaches for conservation of aquatic species. Ecosphere 2024, 15, e4772. [Google Scholar] [CrossRef]
- Barnes, M.A.; Patiño, R. Predicting suitable habitat for dreissenid mussel invasion in Texas based on climatic and lake physical characteristics, Manag. Biol. Invasion 2020, 11, 63–79. [Google Scholar] [CrossRef]
- O’Brien, R.S.; DiRenzo, G.V.; Roy, A.H.; Carmignani, J.; Quinones, R.M.; Rogers, J.B.; Swartz, B.I. Catchment prioritization for freshwater mussel conservation in the Northeastern United States based on distribution modelling. PLoS ONE 2025, 20, e0324387. [Google Scholar] [CrossRef] [PubMed]
- Vikhrev, I.V.; Kuehn, R.; Geist, J.; Kondakov, A.V.; Ieshko, E.P.; Chelpanovskaya, O.A.; Bolotov, I.N. Conservation genetic units under future climate change scenarios: A case of the threatened freshwater pearl mussel (Margaritifera margaritifera). Biodivers. Conserv. 2025, 34, 105–129. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Geist, J.; Egg, S.; Beran, L.; Bikashvili, A.; Van Bocxlaer, B.; Bogan, A.E.; Bolotov, I.N.; Chelpanovskaya, O.A.; Douda, K.; et al. Integrative phylogenetic, phylogeographic and morphological characterisation of the Unio crassus species complex reveals cryptic diversity with important conservation implications. Mol. Phyl. Evol. 2024, 195, 108046. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Prié, V. Unio nanus. The IUCN Red List of Threatened Species 2024: e.T215447310A215447388. Available online: https://www.iucnredlist.org/species/215447310/215447388 (accessed on 12 October 2025).
- Lopes-Lima, M.; Prié, V.; Österling, M.; Zając, T.A. Unio crassus. The IUCN Red List of Threatened Species 2024: e.T210291828A215467836. Available online: https://www.iucnredlist.org/species/210291828/215467836 (accessed on 12 October 2025).
- Denic, M.; Stoeckl, K.; Gum, B.; Geist, J. Physicochemical assessment of Unio crassus habitat quality in a small upland stream and implications for conservation. Hydrobiologia 2014, 735, 111–122. [Google Scholar] [CrossRef]
- Schneider, L.D.; Nilsson, P.A.; Österling, E.M. Evaluating temperature-and host-dependent reproduction in the parasitic freshwater mussel Unio crassus. Hydrobiologia 2018, 810, 283–293. [Google Scholar] [CrossRef]
- Stoeckl, K.; Geist, J. Hydrological and substrate requirements of the thick-shelled river mussel Unio crassus (Philipsson 1788). Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 456–469. [Google Scholar] [CrossRef]
- Vaessen, Q.; Houbrechts, G.; Van Campenhout, J.; Hambuckers, A. Which environmental factors influence the distribution patterns of an endangered freshwater mussel (Unio crassus)? Geomorphology 2024, 454, 109180. [Google Scholar] [CrossRef]
- Egg, S.; Lopes-Lima, M.; Bayerl, H.; Froufe, E.; Stoeckle, B.C.; Kuehn, R.; Geist, J. The Impact of Glacial Disturbance History Upon the Genetic Diversity of Unio crassus and Unio nanus in Europe and Implications for Conservation. Ecol. Evol. 2025, 15, e72113. [Google Scholar] [CrossRef]
- Sales, L.P.; Hayward, M.W.; Loyola, R. What do you mean by “niche”? Modern ecological theories are not coherent on rhetoric about the niche concept. Acta Oecol. 2021, 110, 103701. [Google Scholar] [CrossRef]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 2017, 34, 427–433. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, P.; Kessler, M. Climatologies at high resolution for the Earth land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Fordham, D.A.; Saltré, F.; Haythorne, S.; Wigley, T.M.; Otto-Bliesner, B.L.; Chan, K.C.; Brook, B.W. PaleoView: A tool for generating continuous climate projections spanning the last 21,000 years at regional and global scales. Ecography 2017, 40, 1348–1358. [Google Scholar] [CrossRef]
- Otto-Bliesner, B.L.; Marshall, S.J.; Overpeck, J.T.; Miller, G.H.; Hu, A.; CAPE Last Interglacial Project members. Simulating Arctic climate warmth and icefield retreat in the last interglaciation. Science 2006, 311, 1751–1753. [Google Scholar] [CrossRef]
- Brown, J.L.; Hill, D.J.; Dolan, A.M.; Carnaval, A.C.; Haywood, A.M. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci. Data 2018, 5, 180254. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J. The non-analogue nature of Pliocene temperature gradients. Earth Planet. Sci. Lett. 2015, 425, 232–241. [Google Scholar] [CrossRef]
- Stigall, A.L. Using ecological niche modelling to evaluate niche stability in deep time. J. Biogeogr. 2012, 39, 772–781. [Google Scholar] [CrossRef]
- Saupe, E.E.; Hendricks, J.R.; Portell, R.W.; Dowsett, H.J.; Haywood, A.; Hunter, S.J.; Lieberman, B.S. Macroevolutionary consequences of profound climate change on niche evolution in marine molluscs over the past three million years. Proc. R. Soc. B Proc. Biol. Sci. 2014, 281, 20141995. [Google Scholar] [CrossRef] [PubMed]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Sousa, R.; Geist, J.; Aldridge, D.C.; Araujo, R.; Bergengren, J.; Bespalaya, Y.; Bódis, E.; Burlakova, L.; Van Damme, D.; et al. Conservation status of freshwater mussels in Europe: State of the art and future challenges. Biol. Rev. 2017, 92, 572–607. [Google Scholar] [CrossRef]
- Hochwald, S. Plasticity of Life-History Traits in Unio crassus. In Ecology and Evolution of the Freshwater Mussels Unionoida; Ecological Studies, Bauer, G., Wächtler, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 145, pp. 132–134. [Google Scholar] [CrossRef]
- Zając, T.A.; Zając, K. Spawning in a threatened freshwater mussel shifts to earlier dates as a result of increasing summer mortality. Sci. Rep. 2025, 15, 7733. [Google Scholar] [CrossRef]
- Feind, S.; Geist, J.; Kuehn, R. Glacial perturbations shaped the genetic population structure of the endangered thick-shelled river mussel (Unio crassus, Philipsson 1788) in Central and Northern Europe. Hydrobiologia 2018, 810, 177–189. [Google Scholar] [CrossRef]
- Durand, J.D.; Persat, H.; Bouvet, Y. Phylogeography and postglacial dispersion of the chub (Leuciscus cephalus) in Europe. Mol. Ecol. 1999, 8, 989–997. [Google Scholar] [CrossRef]
- Seifertová, M.; Bryja, J.; Vyskočilová, M.; Martínková, N.; Šimková, A. Multiple Pleistocene refugia and post-glacial colonization in the European chub (Squalius cephalus) revealed by combined use of nuclear and mitochondrial markers. J. Biogeogr. 2012, 39, 1024–1040. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Stigall, A.L. When and how do species achieve niche stability over long time scales? Ecography 2014, 37, 1123–1132. [Google Scholar] [CrossRef]
- Hewitt, T.L.; Bergner, J.L.; Woolnough, D.A.; Zanatta, D.T. Phylogeography of the freshwater mussel species Lasmigona costata: Testing post-glacial colonization hypotheses. Hydrobiologia 2018, 810, 191–206. [Google Scholar] [CrossRef]
- Vikhrev, I.V.; Ieshko, E.P.; Kondakov, A.V.; Mugue, N.S.; Bovykina, G.V.; Efremov, D.A.; Bulakov, A.G.; Tomilova, A.A.; Yunitsyna, O.A.; Bolotov, I.N. Postglacial expansion routes and mitochondrial genetic diversification of the freshwater pearl mussel in Europe and North America. Diversity 2022, 14, 477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).