Abstract
Caudal autotomy is a well-known and widely used defensive strategy among lizards, but its impact on social behaviour, particularly in intramale interactions and dominance hierarchies, has been relatively understudied. In this study, we examined the effects of tail autotomy and subsequent regeneration on the social hierarchy of male Aegean wall lizards (Podarcis erhardii), a territorial species of lacertid lizards. We found that tail loss significantly reduced the ability of adult males to dominate same-sized conspecifics, hinting at a possible difficulty in securing high-quality territories, potentially reducing access to mating opportunities and increasing mortality risks. Both autotomised dominant and intact subordinate males in our interactions adjusted their behaviour following autotomy, with dominant males reducing their aggression and subordinate ones increasing theirs. Surprisingly, tail regeneration did not restore social status or aggression levels to pre-autotomy conditions, suggesting that social costs of tail loss could extend beyond the associated decrease in body size and may persist even after the tail has fully regenerated. These results indicate that caudal autotomy could have lasting consequences on male social interactions and potentially significant implications for reproductive success and survival. Our study highlights the complex role of tail condition in the social dynamics of territorial lizards and calls for further research on the long-term impacts of autotomy on fitness and social structures.
1. Introduction
Caudal autotomy, the instantaneous shedding of the tail in response to mechanical stimuli, is a defensive mechanism of numerous saurian families that allows escape from attempted predation [1,2,3,4,5]. Additionally, tail loss may also be driven by intraspecific aggression, which is particularly intense on certain island environments [6,7,8]. Nevertheless, tail loss comes with severe costs. Tailless lizards cannot reuse autotomy to escape and also experience impaired locomotor performance [9,10,11], thus risking higher vulnerability to subsequent predatory attacks [12,13,14]. They may also suffer from lower reproductive success [15,16] and reduced survival [17,18,19]. Through tail shedding, lizards may lose important caudal energy reserves [20,21,22,23], while at the same time they may have to increase their metabolism [24,25,26], food intake [27] or digestive efficiency [28] to fuel the regeneration process.
Moreover, autotomised individuals may adopt behavioural changes to compensate for tail loss, including adjustments in habitat and refuge use [29,30], activity and movement patterns [15,31,32], foraging tactics [31,33] or thermoregulatory behaviour [34,35]. Tailless lizards can be at a disadvantage during social interactions as well, since tails play an important role in territorial disputes and courtship. Tailed males have been shown to hold dominant status [36,37,38,39] and manage higher mating success than their tailless counterparts [16,38]. Thus, dominant males may fall in social rank after autotomy and become restricted to territories of lower quality, where food availability and access to females might be limited [36,37,40].
Tail regeneration comes swiftly after autotomy in most species, and the formation of the new tail is completed within a few weeks [19,41,42]. Though regenerated tails may differ from the originals in traits such as length [43,44,45] or histological composition [46,47], most of the tail’s primary functions are restored upon full regeneration [43,48]. For instance, experimental evidence suggests that locomotor capacities of lizards with regenerated tails match those of their conspecifics with intact ones [45,49]. On the contrary, the anti-predator effectiveness of the tail is only partially retrieved after regeneration, most notably due to the inability of the cartilaginous regenerated portion of the new tail to autotomise again [50], but also the reduced intensity of post-autotomic movements of regenerated tails [26,49]. Concerning the social role of the tail, very limited data exists on the post-regeneration period. Some observations suggest that dominant males may recover their position in social hierarchy once part of their tail has been regenerated [38,51]. However, Fox et al. [37] failed to detect restoration of social status when the tails of previously dominant males were experimentally restored. Additionally, males with regenerated tails were found to have smaller home ranges than males with complete tails, one or even several years after autotomy [52], revealing potential long-lasting effects of tail loss on fitness.
Although tail regeneration may offset an important part of the significant costs of autotomy, its effect on social structures remains understudied. Here, we aimed to investigate the impact of tail autotomy and subsequent regeneration on male social hierarchy in the Aegean wall lizard (Podarcis erhardii). Males of this species establish small territories of a few square metres and defend them fiercely against conspecific rivals [53,54,55]. These fights may result in caudal amputation and even consumption of the tail [56,57,58]. Since tail condition is known to affect the outcomes of saurian social behaviour [16,59], we presumed autotomy may alter, temporarily or permanently, the status of males engaging in agonistic encounters, especially in such a territorial species. First, we anticipated that tail loss would lead to loss of social status in dominant males. Second, we predicted that both dominant and subordinate individuals would modify their behaviour accordingly. Third, we hypothesised that the previous social hierarchy would be restored after tail regeneration.
2. Materials and Methods
2.1. Study System
Podarcis erhardii (Bedriaga, 1882) is a small diurnal lacertid (maximum snout-to-vent length—SVL—of about 10 cm). In Greece, it is found in vast areas of the mainland and most of the Cyclades and Sporades Islands [60]. It is the most common and widely distributed lacertid lizard in the Aegean Archipelago [61], displaying a remarkable diversification, encompassing 21 subspecies [62]. In cases of elevated intraspecific competition, P. erhardii may display cannibalistic behaviour, consuming parts or even the entire body of conspecifics [57,63]. The species widely uses tail autotomy as a main antipredator strategy [64,65,66].
In spring 2021, we conducted fieldwork on Mount Parnitha, a mountain range located at the northern limits of the Athens Metropolitan Area. The focal location (N 38°9′48″, E 23°43′23″), a moderate slope at an elevation of approximately 1100 m above sea level, features low shrubby vegetation and a rocky substrate. The area is typically covered with Greek fir forests (Abies cephalonica). However, the location we chose suffered a large wildfire that completely destroyed the forest in 2007. As a result, the lizards that were once restricted in rocky openings within the fir forest [67] presently occupy the whole area and have established dense populations [68].
We captured 22 adult males using a noose and transported them to the laboratory facilities of the National and Kapodistrian University of Athens. Lizards were housed individually in plastic terraria (36 × 27 × 16 cm) featuring a sand substrate, brick shelters and ad libitum water access. Incandescent heat lamps (40 W) were placed above the terraria and were programmed to provide a daily nine-hour photoperiod, allowing the animals to freely thermoregulate inside their terraria. Lizards were fed Tenebrio molitor larvae coated with a vitamin supplement three times a week. The room temperature was maintained constant at approximately 28 °C.
2.2. Staged Encounters
The captured lizards were measured using digital callipers (Silverline 380244, Toolstream Ltd., Yeovil, UK, accurate within 0.01 mm) and weighed using spring scales (Pesola 50 g, division 0.5 g, Pesola Präzisionswaagen AG, Chur, Switzerland, accurate within ±0.3%). Each male was then assigned to another male of similar size in order to eliminate possible effects of body size differences on social interactions [69,70,71,72,73]. The pairs that were formed consisted of individuals displaying maximum SVL differences of 2 mm, maximum total length differences of 16 mm and maximum weight differences of 0.6 g.
Once the animals were acclimated to laboratory conditions for a week, agonistic encounters were staged between the lizards in each pair, in accordance with previous studies on dominance in Podarcis lizards [55,74,75]. These encounters took place in a neutral arena (80 × 80 × 35 cm) with a sand substrate and white blinded sidewalls. The arena was equipped with a heating lamp (60 W) and a video camera (Microsoft LifeCam HD-3000, Microsoft Corporation, Redmond, WA, USA). Prior to each trial, the two lizards were allowed to thermoregulate inside the arena for 10 min, separated from each other with a detachable separator. Once the separator was removed, a 15 min encounter was recorded, during which the lizards interacted freely with each other. All encounters took place in the morning, while three encounters were performed for each pair, always on separate days.
The recorded videos were subsequently analysed, and a list of discrete, repeatedly observed behaviours was generated (Table 1). The documented behaviours were then scored according to the degree of aggression or submission displayed. A score of “+1” was given for behaviours we assessed as highly aggressive, a score of “+0.5” was given for behaviours of moderate aggressiveness, while a score of “−1” was given for the only observed behaviour we evaluated as submissive, when one of the lizards rapidly retreated in the face of an aggressive display from its rival (Table 1). Each lizard then received a total aggression (TA) score, which was calculated as the sum of all score points obtained during each contest, averaged over the three completed contests of the pair. The lizard within each pair with the highest TA score was thereby identified as the dominant of the pair. Additionally, each pair received a social disparity (SD) score, calculated by subtracting the TA score of the subordinate individual within each pair from that of the dominant of the pair. We used this index as a measure of the disparity in aggression (and thus dominance) between the members of each pair of lizards [36,37].

Table 1.
Observed agonistic behaviours.
The dominant lizard of each pair was subsequently autotomised, using a pair of callipers to simulate predation [76]. The tail was grasped at exactly 20 mm from the lizard’s vent. The process of regeneration that follows autotomy can be schematically divided into four stages or phases: the wound-healing phase, which lasts for about 10 days; the formation of a blastema, which occurs from approximately days 10 to 15 post-amputation; a differentiating phase, during which an elongating cone is formed and the tail starts growing rapidly in length; and finally, a maturing phase, during which the regenerated tail reaches its maximum length and becomes fully scaled [77]. In our case, the autotomised animals were allowed to recover for a period of 10 days, coinciding with the wound-healing phase, after which new agonistic encounters were realised every 20 days between the same pairs of individuals as before. Once again, three encounters were performed for each pair, on separate days. This time interval in-between contests allowed us to evaluate potential hierarchy shifts during the different phases of the regeneration process, leading up to its completion, approximately 10 weeks after autotomy (Figure 1).
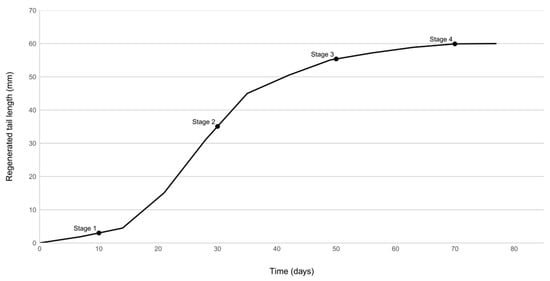
Figure 1.
Mean regenerated tail length of autotomised dominant males over time. Agonistic encounters were carried out 10 days after autotomy was induced and every 20 days thereafter, up to 70 days post-autotomy.
In all, four post-autotomy sets of encounters took place, hereafter called “stages”: stage 1 encounters were completed during the early days of blastema formation, and as a result, tail length of the autotomised individuals was at its minimum. Stage 2 encounters took place during the phase of rapid tail elongation, while stage 3 encounters were performed well into the maturing phase, when the growth rate of the tail had significantly decreased (Figure 1). Finally, stage 4 encounters approximately coincided with the completion of regeneration, when maximum tail length was reached. At this point, we must acknowledge an innate flaw in our study: the lack of control agonistic encounters between non-autotomised lizards throughout the duration of the experiment. The dominance or aggressiveness of a male could be altered as a result of captivity or seasonality. To minimise the effects of these parameters, we made sure that all trials were completed within the shortest possible period with respect to the time required for caudal regeneration. None of the encounters resulted in any observable injuries, and all the animals were returned to the capture site upon completion of the experiment.
The recorded videos were once again analysed, and all agonistic behaviours were documented and scored following the same protocol as before. For all four stages, new TA scores were calculated for all individuals, and new SD scores were estimated for all pairs. SD scores were obtained by again subtracting the TA scores of the original subordinate individuals from those of the original dominant ones, disregarding possible changes in dominance within the pairs, so that potential changes in SD would be evaluated relatively to pre-autotomy encounters.
2.3. Data Analysis
We compared mean SD scores between the five sets of trials that were carried out in total, one before tail autotomy was induced and four during the regeneration process. Additionally, to better understand the drivers behind possible changes in social hierarchy, the behaviour of the dominant and subordinate individuals was also analysed separately. Since SD scores were calculated using the TA scores of both members of a lizard pair, shifts in SD could be driven by changes in the behaviour of one or both of the participants of an encounter [37]. For instance, a decrease in SD could result from an increase in aggressiveness by the subordinate individual, a decrease in aggressiveness by the dominant, or a combination of the two. Therefore, we also compared independently mean TA scores of the dominant and subordinate lizards between the five sets of encounters.
Repeated-measures ANOVAs were performed to test for differences in SD, TA of the dominant, and TA of the subordinate individuals between sets of trials. The Shapiro–Wilk test was used to test for normality and Mauchly’s test to check for departure from sphericity. No corrections were applied since neither assumption was violated. Pairwise post hoc testing using least-squares means was performed to locate differences between sets of trials [78]. A p-value adjustment was used to control the false discovery rate (FDR) due to multiple testing [79]. All statistical analyses were performed using the software R 4.2.1 [80].
3. Results
Social disparity significantly decreased after autotomy (Univariate Type-III Repeated-Measures ANOVA: F(4,40) = 5.610, p = 0.001). In other words, the gap in aggressiveness between now-tailless dominant and tailed subordinate males was considerably diminished. However, full reversal of dominance (when a previously subordinate individual became the dominant) was recorded in only 27.3% of lizard pairs (3 out of 11).
Overall, SD did not significantly vary during the regeneration period (pairwise comparisons: all p-values > 0.05 between regeneration stages) (Table 2), as it remained consistently low compared to pre-autotomy levels (all p-values < 0.05) (Figure 2; Table 2). Out of the three pairs where dominance was fully reversed following tail loss, only one previously dominant individual regained its rank in subsequent trials. This occurred at stage 4 of tail regeneration, when the regenerate tail had reached its maximum length.

Table 2.
p-values for post hoc pairwise comparisons between stages. Significant differences (p < 0.05) appear in black.
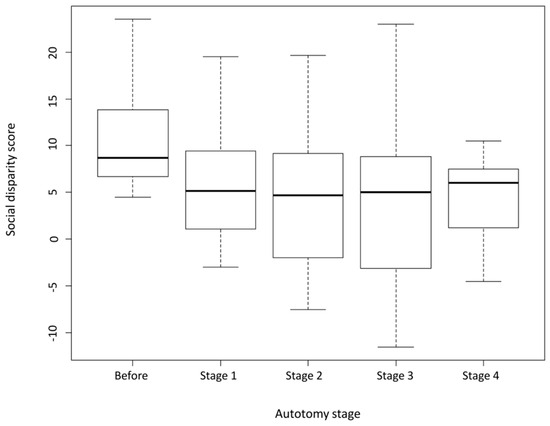
Figure 2.
Mean social disparity (SD) scores before and after autotomy. SD significantly declined post-autotomy and remained at lower levels throughout the tail regeneration process.
When TA scores were examined separately for dominant and subordinate individuals, we found that the observed changes in SD were driven by behavioural adjustments from both dominants and subordinates. Like SD, aggression of dominant males significantly declined post-autotomy (Univariate Type-III Repeated-Measures ANOVA: F(4,40) = 7.922, p < 0.001). Additionally, pairwise comparisons revealed that their TA scores stayed at a relatively low level during regeneration (all p-values > 0.05 between regeneration stages, all p-values < 0.05 compared to pre-autotomy) (Table 2), following a pattern closely resembling that of SD (Figure 3).
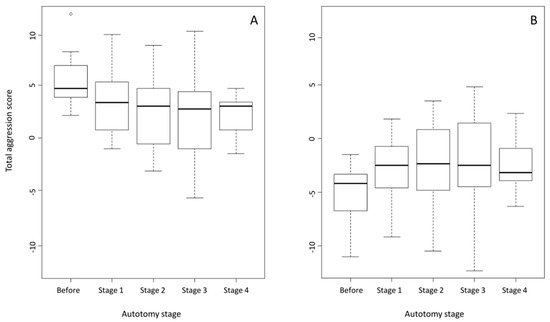
Figure 3.
Mean total aggression scores (TA) for dominant (A) and subordinate (B) individuals. ΤA of dominant males significantly decreased after tail loss and remained at lower levels during tail regeneration. ΤA of subordinates significantly increased right after autotomy and remained at higher levels even 70 days after tail loss.
On the contrary, subordinate aggression followed a less clear pattern. Once again, TA scores significantly varied between stages (Univariate Type-III Repeated-Measures ANOVA: F(4,40) = 3.497, p = 0.015). However, post hoc analysis revealed that, while they significantly increased right after autotomy (stage 1, p = 0.023), statistical significance was not reached for the differences between pre-autotomy and stages 2 (p = 0.072) and 3 (p = 0.072) of the regeneration process, namely 30 and 50 days after tail loss (Table 2). On the contrary, TA scores at stage 4 were significantly higher than before tail loss (p = 0.001), while overall variation between the four post-autotomy stages was not significant (all p-values > 0.05) (Table 2). This discrepancy is probably due to a higher variability in the responses of the subordinate individuals during the encounters of stages 2 and 3 (Figure 3), leading to limited statistical significance. Nevertheless, a significant increase in subordinate aggressiveness was observed at the moment of complete lack of tail by their dominant opponents. This effect, although slightly confounded in the following regeneration stages, probably persisted throughout the regeneration period, leading to significantly higher aggressiveness, even 70 days after autotomy, when dominants’ tails were fully regenerated.
4. Discussion
According to our results, the disparity in aggressiveness between adult male P. erhardii lizards significantly declined once the tail was removed from dominant males. This observation was in accordance with previous studies [36,37]. However, contrary to our prediction, this decline reversed dominance in only 27% of cases. Nevertheless, our results suggest that caudal autotomy significantly decreased the ability of an adult male to dominate a conspecific of the same SVL. Inability to achieve dominant status may lead tailless males to fail to secure high-quality territories and, in turn, experience reduced access to mating opportunities [38,40,81] and increased risk of death [18,19,36,82]. Since we used social disparity to measure dominance, a composite index encompassing the behaviour of both members of an interaction [37], we also deemed it necessary to evaluate behavioural changes separately for dominant and subordinate individuals in order to fully comprehend the observed changes in social disparity. As we predicted, adjustments in aggressiveness were made by both dominant and subordinate males.
Dominant males significantly reduced their aggressiveness after tail loss. This response might be driven by energetic constraints, namely, the necessity to conserve energy that could be allocated to cover other needs. Tail regeneration is an energetically expensive process [9,26], utilising an important fraction of a lizard’s metabolic expenditure [28]. Thus, the need for rapid regeneration may lead lizards to reduce costs related to agonistic behaviours. Territorial defence requires high energy output as well [83,84,85], meaning that lizards in the process of regenerating their tails might be compelled to limit their engagement in territorial activities. Furthermore, additional energetic requirements, such as somatic growth or reconstitution of caudal energy reserves lost along with the tail, may also lead individuals to limit other activities. Martín & Salvador [38] observed that tailless Lacerta monticola males had a higher weight gain than tailed ones during the reproductive period, most probably due to reduced aggression costs. While energetic allocations for tail regeneration are high, male lizards might avoid fighting to conserve energy, thus limiting aggressive behaviours.
Another possible explanation for this observed reduction in aggressiveness may be rooted in a decreased reproductive potential of tailless male lizards. Even though costs of intraspecific male aggression are high, since fights are both energetically costly and perilous in terms of risk of injury, these costs are nevertheless largely offset by the fact that successful fighting may allow dominant individuals to mate with multiple females but also limit the probabilities that these females will mate with other males. Males actively engaging in intraspecific agonistic encounters are thus competing for current or future mating opportunities, as well as the possibility to prevent conspecific males from reproducing [86]. However, if a male’s reproductive chances are already impaired, assuming the costs of aggressive behaviours would no longer be pertinent. As a consequence, any factors that could cause an a priori reduction in the reproductive potential of tailless males, independently from territorial disputes with other males, could lead to the adoption of less aggressive and less costly strategies during these agonistic interactions. For instance, females might prefer males with intact tails [16], leading tailless males, faced with an already diminished mating potential, to curtail their aggression during male-male interactions in order to minimise associated costs.
On the other hand, the need for adjustments in anti-predator behaviour could also be a driver behind reduced aggressiveness following tail loss. For as long as their locomotor abilities are limited and the use of autotomy is not available to them, male lizards may adapt their anti-predator behaviour in ways that are not fully compatible with high-risk social behaviours, such as the courtship of females or the defence of a territory against other males [30,86,87]. These behaviours might need to be restricted or altered following autotomy. Anti-predator behaviour has been shown to vary when territorial males are actively engaged in female courtship or in agonistic encounters with other males, because the cost of abandoning these activities once they have been initiated is high [86]. As a consequence, during social interactions, males might assume higher risks in terms of predator avoidance and probability of detection. For instance, flight initiation distance may be shortened and re-emergence time reduced, in comparison with isolated males [88,89,90]. Conversely, lower levels of aggression may benefit tailless individuals by reducing their detectability by predators [91]. The evolution of anti-predator behaviour in an impaired state, such as the one faced by tailless male lizards, could have driven adaptations reducing needless costs associated with aggressive behaviour.
We also observed a post-autotomy increase in aggression in subordinate males, although this effect was somewhat muddled during the subsequent stages of tail regeneration. It is nonetheless worth noting that Fox et al. [37], who conducted a similar experiment on both male and female subadult individuals of Uta stansburiana, did not observe such an increase in subordinate aggression. More specifically, our results agree with theirs in terms of dominant male responses to tail loss but significantly diverge from theirs in regard to the responses of subordinate individuals. This disparity in responses from subordinate males could potentially be explained by the fact that we used fully mature adult males, while Fox et al. used subadults. The escalation in subordinate aggressiveness we observed could be attributed to a possible increase in the reproductive potential of these males. The cost of assuming a subordinate social role for adult male lizards might be high [92], since they may be confined to small territories of limited quality and suffer both reproductive and survival costs [38,40]. So, individuals may constantly test their ability to dominate and thus be able to detect potential changes in their opponent’s capacities. However, our results showed that full reversal of dominance was seldom achieved, as behavioural adjustments following autotomy were, on most occasions, not sufficient to overturn previously established hierarchy. Comparably, Salvador et al. [91] observed that large Psammodromus algirus males managed to maintain their dominant status after autotomy, even though they had to reduce the range of their territories. We conclude that adult subordinate males may increase their aggressiveness when faced with autotomised opponents because of the high fitness benefits in terms of reproductive success they could potentially gain if they managed to obtain dominant status.
Contrary to our prediction, neither social disparity nor aggressiveness was restored to pre-autotomy levels following tail regeneration. These results reinforce the findings by Fox et al. [37], where experimental restoration of the tail led to restoration of previous rank for formerly dominant female lizards, but not for dominant males. The hypothesis that tail regeneration leads to a mere rebalancing of body sizes and thus of social hierarchy does not seem to be appropriate for male lizards. These authors hypothesised that the role of the tail of female Uta stansburiana individuals differed from that of males because females may be able to adopt an alternative, subordinate social role when autotomised, allowing them to retain some reproductive success. On the contrary, males might not have this possibility because of their need to occupy large, high-quality territories to gain mating opportunities. Our results validate the hypothesis that, in the case of male hierarchy in a territorial species, other factors associated with autotomy are responsible for the loss of status, acting jointly with or independently from the loss of body size. It is possible, for instance, that lizards with regenerated tails are forced to adopt predator avoidance behaviours that more closely resemble those of tailless lizards rather than ones with original tails [26].
Additionally, some potentially severe social costs incurred by tailless males through the loss of mating opportunities appear to be long-lasting and to not necessarily be relieved via tail regeneration. Salvador et al. [52] found that males with regenerated tails suffered reduced access to females, due to contracted home ranges, years after autotomy had occurred. In contrast with our initial hypothesis, we observed decreased aggressiveness from autotomised individuals, lasting even when their tails were almost fully regenerated. In natural conditions, such an aggression deficit could possibly lead individuals with regenerated tails to surrender space to neighbouring males with complete tails and suffer the resulting decrease in reproductive success. At this point, we must stress that our findings sketch out the particular conditions of staged encounters in an arena. Factors such as the high quality of a given territory may affect the stamina and resolve with which a resident male would protect its position in the wild.
In conclusion, we demonstrated that tail autotomy reduced the ease with which male P. erhardii dominated same-sized conspecifics. Behavioural shifts by both autotomised dominant males and tailed subordinates were observed. On the other hand, tail regeneration failed to restore previous social circumstances. These findings emphasise the tail’s importance for male lizards in a social context and reveal a potentially considerable long-lasting impact of its loss on male reproductive success and survival. Future studies should attempt to validate our conclusions regarding possible long-term adverse effects of autotomy on social interactions and determine the reasons why tail regeneration might not fully alleviate them.
Author Contributions
Conceptualization, A.D.-T. and P.P.; methodology, A.D.-T.; software, not applicable; validation, A.D.-T., E.D.V. and P.P.; formal analysis, A.D.-T.; investigation, A.D.-T. and C.K.-E.; resources, A.D.-T., C.K.-E. and P.P.; data curation, A.D.-T.; writing—original draft preparation, A.D.-T.; writing—review and editing, A.D.-T. and P.P.; visualisation, A.D.-T. and P.P.; supervision, A.D.-T., E.D.V. and P.P.; project administration, A.D.-T. and P.P.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Department of Biology of the National and Kapodistrian University of Athens.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Lizard sampling was conducted under a special permit (ΥΠΕΝ/ΔΔΔ/28501/968) issued by the Greek Ministry of the Environment and Energy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arnold, E.N. Evolutionary aspects of tail shedding in lizards and their relatives. J. Nat. Hist. 1984, 18, 127–169. [Google Scholar] [CrossRef]
- Arnold, E.N. Caudal autotomy as a defence. In Biology of the Reptilia; Gans, C., Huey, R., Alan, R., Eds.; Liss Inc.: New York, NY, USA, 1988; Volume 16, pp. 235–273. [Google Scholar]
- Daniels, C.B. Economy of autotomy as a lipid conserving mechanism: An hypothesis rejected for the gecko Phyllodactylus marmoratus. Copeia 1985, 1985, 468–472. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Pérez-Mellado, V.; Vitt, L.J. Ease and effectiveness of costly autotomy vary with predation intensity among lizard populations. J. Zool. 2004, 262, 243–255. [Google Scholar] [CrossRef]
- Naidenov, L.A.; Allen, W.L. Tail autotomy works as a pre-capture defense by deflecting attacks. Ecol. Evol. 2021, 11, 3058–3064. [Google Scholar] [CrossRef]
- Jennings, W.B.; Thompson, G.G. Territorial behaviour in the Australian scincid lizard Ctenotus fallens. Herpetologica 1999, 55, 352–361. [Google Scholar]
- Cooper, W.E., Jr.; Dimopoulos, I.; Pafilis, P. Sex, age, and population density affect aggressive behaviors in island lizards promoting cannibalism. Ethology 2015, 121, 260–269. [Google Scholar] [CrossRef]
- Itescu, Y.; Schwarz, R.; Meiri, S.; Pafilis, P. Intraspecific competition, not predation, drives lizard tail loss on islands. J. Anim. Ecol. 2017, 86, 66–74. [Google Scholar] [CrossRef]
- Lin, Z.H.; Qu, Y.F.; Ji, X. Energetic and locomotor costs of tail loss in the Chinese skink, Eumeces chinensis. Comp. Biochem. Physiol. A 2006, 143, 508–513. [Google Scholar] [CrossRef] [PubMed]
- McElroy, E.J.; Bergmann, P.J. Tail autotomy, tail size, and locomotor performance in lizards. Physiol. Biochem. Zool. 2013, 86, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Savvides, P.; Stavrou, M.; Pafilis, P.; Sfenthourakis, S. Tail autotomy affects bipedalism but not sprint performance in a cursorial Mediterranean lizard. Sci. Nat. 2017, 104, 3. [Google Scholar] [CrossRef]
- Downes, S.; Shine, R. Why does tail loss increase a lizard’s later vulnerability to snake predators. Ecology 2001, 82, 1293–1303. [Google Scholar] [CrossRef]
- Medger, K.; Verburgt, L.; Bateman, P.W. The influence of tail autotomy on the escape response of the Cape dwarf gecko, Lygodactylus capensis. Ethology 2008, 114, 42–52. [Google Scholar] [CrossRef]
- Domínguez-López, M.E.; Ortega-León, Á.M.; Zamora-abrego, G.J. Tail autotomy effects on the escape behavior of the lizard Gonatodes albogularis (Squamata: Sphaerodactylidae), from Córdoba, Colombia. Rev. Chil. Hist. Nat. 2015, 88, 1. [Google Scholar] [CrossRef][Green Version]
- Martín, J.; Salvador, A. Effects of tail loss on the time-budgets, movements, and spacing patterns of Iberian rock lizards, Lacerta monticola. Herpetologica 1997, 53, 117–125. [Google Scholar][Green Version]
- Langkilde, T.; Alford, R.A.; Schwarzkopf, L. No behavioural compensation for fitness costs of autotomy in a lizard. Austral Ecol. 2005, 30, 713–718. [Google Scholar] [CrossRef]
- Wilson, B.S. Tail injuries increase the risk of mortality in free-living lizards (Uta stansburiana). Oecologia 1992, 92, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.F.; McCoy, J.K. The effects of tail loss on survival, growth, reproduction, and sex ratio of offspring in the lizard Uta stansburiana in the field. Oecologia 2000, 122, 327–334. [Google Scholar] [CrossRef]
- Lin, J.W.; Chen, Y.R.; Wang, Y.H.; Hung, K.C.; Lin, S.M. Tail regeneration after autotomy revives survival: A case from a long-term monitored lizard population under avian predation. Proc. R. Soc. B 2017, 284, 20162538. [Google Scholar] [CrossRef]
- Daniels, C.B. The importance of caudal lipid in the gecko Phyllodactylus marmoratus. Herpetologica 1984, 40, 337–344. [Google Scholar]
- Russell, A.P.; Lynn, S.E.; Powell, G.L.; Cottle, A. The regenerated tail of juvenile leopard geckos (Gekkota: Eublepharidae: Eublepharis macularius) preferentially stores more fat than the original. Zoology 2015, 118, 183–191. [Google Scholar] [CrossRef]
- Price, E.R. The physiology of lipid storage and use in reptiles. Biol. Rev. 2017, 92, 1406–1426. [Google Scholar] [CrossRef]
- Eberle, P.; Haro, D.; Rekevics, K.; Liwanag, H.E. Physiological Effects of Tail Regeneration following Autotomy in Italian Wall Lizards, Podarcis siculus. J. Herpetol. 2022, 56, 434–443. [Google Scholar] [CrossRef]
- Dial, B.E.; Fitzpatrick, L.C. The energetic costs of tail autotomy to reproduction in the lizard Coleonyx brevis (Sauria: Gekkonidae). Oecologia 1981, 51, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Naya, D.E.; Božinović, F. The role of ecological interactions on the physiological flexibility of lizards. Funct. Ecol. 2006, 20, 601–608. [Google Scholar] [CrossRef]
- Naya, D.E.; Veloso, C.; Muñoz, J.L.; Božinović, F. Some vaguely explored (but not trivial) costs of tail autotomy in lizards. Comp. Biochem. Physiol. A 2007, 146, 189–193. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, I.; Braña, F. Allocation costs of regeneration: Tail regeneration constrains body growth under low food availability in juvenile lizards. Oecologia 2022, 198, 853–864. [Google Scholar] [CrossRef]
- Sagonas, Κ.; Deimezis-Tsikoutas, A.; Reppa, A.; Domenikou, I.; Papafoti, M.; Synevrioti, K.; Moschona, A.; Polydouri, I.; Voutsela, A.; Dagonaki, A.; et al. Tail regeneration alters the digestive performance of lizards. J. Evol. Biol. 2021, 34, 671–679. [Google Scholar] [CrossRef]
- Martín, J.; Salvador, A. Tail loss consequences on habitat use by the Iberian rock lizard, Lacerta monticola. Oikos 1992, 65, 328–333. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr. Compensatory changes in escape and refuge use following autotomy in the lizard Sceloporus virgatus. Can. J. Zool. 2007, 85, 99–107. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr. Shifted balance of risk and cost after autotomy affects use of cover, escape, activity, and foraging in the keeled earless lizard (Holbrookia propinqua). Behav. Ecol. Sociobiol. 2003, 54, 179–187. [Google Scholar] [CrossRef]
- Michelangeli, M.; Melki-Wegner, B.; Laskowski, K.; Wong, B.B.; Chapple, D.G. Impacts of caudal autotomy on personality. Anim. Behav. 2020, 162, 67–78. [Google Scholar] [CrossRef]
- Martín, J.; Salvador, A. Tail loss and foraging tactics of the Iberian rock-lizard, Lacerta monticola. Oikos 1993, 66, 318–324. [Google Scholar] [CrossRef]
- Martín, J.; Salvador, A. Thermoregulatory behaviour of rock lizards in response to tail loss. Behaviour 1993, 124, 123–136. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, I.; Barroso, F.M.; Carretero, M.A. An integrative analysis of the short-term effects of tail autotomy on thermoregulation and dehydration rates in wall lizards. J. Therm. Biol. 2021, 99, 102976. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.F.; Rostker, M.A. Social cost of tail loss in Uta stansburiana. Science 1982, 218, 692–693. [Google Scholar] [CrossRef]
- Fox, S.F.; Heger, N.A.; Delay, L.S. Social cost of tail loss in Uta stansburiana: Lizard tails as status-signalling badges. Anim. Behav. 1990, 39, 549–554. [Google Scholar] [CrossRef]
- Martín, J.; Salvador, A. Tail loss reduces mating success in the Iberian rock-lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 1993, 32, 185–189. [Google Scholar] [CrossRef]
- Perry, G.; LeVering, K.; Girard, I.; Garland, T. Locomotor performance and social dominance in male Anolis cristatellus. Anim. Behav. 2004, 67, 37–47. [Google Scholar] [CrossRef]
- Fox, S.F.; Rose, E.; Myers, R. Dominance and the acquisition of superior home ranges in the lizard Uta stansburiana. Ecology 1981, 62, 888–893. [Google Scholar] [CrossRef]
- Jacyniak, K.; McDonald, R.P.; Vickaryous, M.K. Tail regeneration and other phenomena of wound healing and tissue restoration in lizards. J. Exp. Biol. 2017, 220, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Lizard tail regeneration as an instructive model of enhanced healing capabilities in an adult amniote. Connect. Tissue Res. 2017, 58, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Maginnis, T.L. The costs of autotomy and regeneration in animals: A review and framework for future research. Behav. Ecol. 2006, 17, 857–872. [Google Scholar] [CrossRef]
- Simou, C.; Pafilis, P.; Skella, A.; Kourkouli, A.; Valakos, E.D. Physiology of original and regenerated tails in Aegean Wall Lizard (Podarcis erhardii). Copeia 2008, 2008, 504–509. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J.; Rubiño-Hispán, M.V.; Reguera, S.; Moreno-Rueda, G. Does tail regeneration following autotomy restore lizard sprint speed? Evidence from the lacertid Psammodromus algirus. Herpetol. J. 2016, 26, 213–220. [Google Scholar]
- Alibardi, L. Morphological and Cellular Aspects of Tail and Limb Regeneration in Lizards: A Model System with Implications for Tissue Regeneration in Mammals; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gilbert, E.A.; Payne, S.L.; Vickaryous, M.K. The anatomy and histology of caudal autotomy and regeneration in lizards. Physiol. Biochem. Zool. 2013, 86, 631–644. [Google Scholar] [CrossRef]
- Luís, C.; Rodrigues, I.; Guerreiro, S.G.; Fernandes, R.; Soares, R. Regeneration in the Podarcis bocagei model organism: A comprehensive immune-/histochemical analysis of the tail. Zoomorphology 2019, 138, 399–407. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, I.; Braña, F. The movement dynamics of autotomized lizards and their tails reveal functional costs of caudal autotomy. Integr. Zool. 2020, 15, 511–521. [Google Scholar] [CrossRef]
- Barr, J.I.; Boisvert, C.A.; Somaweera, R.; Trinajstic, K.; Bateman, P.W. Re-regeneration to reduce negative effects associated with tail loss in lizards. Sci. Rep. 2019, 9, 18717. [Google Scholar] [CrossRef]
- Berry, K.H. The Ecology and Social Behavior of the Chuckwalla, Sauromalus obesus obesus Baird; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1974. [Google Scholar]
- Salvador, A.; Martín, J.; López, P.; Veiga, J.P. Long-term effect of tail loss on home-range size and access to females in male lizards (Psammodromus algirus). Copeia 1996, 1996, 208–209. [Google Scholar] [CrossRef]
- Valakos, E.D. The Ecology of the Lizard Podarcis erhardii (Bedriaga, 1882) (Sauria: Lacertidae) in a Typical Insular Ecosystem on Naxos Island. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 1990. [Google Scholar]
- Tsasi, G.; Pafilis, P.; Simou, C.; Valakos, E.D. Predation pressure, density-induced stress and tail regeneration: A casual-nexus situation or a bunch of independent factors. Amphibi. Reptil. 2009, 30, 471–482. [Google Scholar] [CrossRef]
- Brock, K.M.; Chelini, M.C.; Ayton, C.; Madden, I.; Ramos, C.; Pafilis, P.; Blois, J.; Edwards, D.L. Colour morph predicts social behaviour and contest outcomes in a polymorphic lizard (Podarcis erhardii). Anim. Behav. 2022, 191, 91–103. [Google Scholar] [CrossRef]
- Deem, V.; Hedman, H. Potential cannibalism and intraspecific tail autotomization in the Aegean wall lizard, Podarcis erhardii. Hyla 2014, 2014, 33–34. [Google Scholar]
- Donihue, C.M.; Brock, K.M.; Foufopoulos, J.; Herrel, A. Feed or fight: Testing the impact of food availability and intraspecific aggression on the functional ecology of an island lizard. Funct. Ecol. 2016, 30, 566–575. [Google Scholar] [CrossRef]
- BeVier, G.T.; Brock, K.M.; Foufopoulos, J. Ecology and home range of the Aegean wall lizard (Podarcis erhardii). Herpetol. Conserv. Biol. 2021, 16, 394–404. [Google Scholar]
- Bateman, P.W.; Fleming, P.A. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Pafilis, P.; Maragou, P. (Eds.) Atlas of Amphibians and Reptiles of Greece; Broken Hill Publishers: Nicosia, Cyprus, 2020; Available online: http://herpatlas.gr/wp-content/uploads/2021/02/Atlas_Amphibians_and_Reptiles_of_Greece.pdf (accessed on 9 September 2025).
- Lymberakis, P.; Pafilis, P.; Poulakakis, N.; Sotiropoulos, K.; Valakos, E.D. The Amphibians and Reptiles of the Aegean Sea. In Biogeography and Biodiversity of the Aegean. In Honour of Prof. Moysis Mylonas; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K.A., Eds.; Broken Hill Publishers: Nicosia, Cyprus, 2018; pp. 169–189. [Google Scholar]
- The Reptile Database. Available online: http://www.reptile-database.org (accessed on 9 September 2025).
- Madden, I.E.; Brock, K.M. An extreme case of cannibalism in Podarcis erhardii mykonensis (Reptilia: Lacertidae) from Siros Island, Cyclades, Greece. Herpetol. Notes 2018, 11, 291–292. [Google Scholar]
- Pafilis, P.; Valakos, E.D.; Foufopoulos, J. Comparative post-autotomy tail activity in six Mediterranean lacertid lizard species. Physiol. Biochem. Zool. 2005, 78, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.M.; Bednekoff, P.M.; Pafilis, P.; Foufopoulos, J. Evolution of antipredator behavior in an island lizard species, Podarcis erhardii (Reptilia: Lacertidae): The sum of all fears. Evolution 2015, 69, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Pafilis, P.; Sagonas, K.; Kapsalas, G.; Foufopoulos, J.; Valakos, E.D. Sex does not affect tail autotomy in lacertid lizards. Acta Herpetol. 2017, 12, 19–27. [Google Scholar]
- Pafilis, P.; Simou, C. The southernmost geographic distribution of Podarcis erhardii. Herpetol. Rev. 2006, 37, 361–362. [Google Scholar]
- Gkourtsouli-Antoniadou, I.; Deimezis-Tsikoutas, A.; Vassaki, K.; Vezyrakis, A.; Pafilis, P. A tail where it shouldn’t be: A morphological anomaly in Podarcis erhardii. Herpetol. Notes 2017, 10, 233–234. [Google Scholar]
- Cooper, W.E., Jr.; Vitt, L.J. Deferred agonistic behavior in a long-lived scincid lizard Eumeces laticeps. Oecologia 1987, 72, 321–326. [Google Scholar] [CrossRef]
- Tokarz, R.R. Mate choice in lizards: A review. Herpetol. Monogr. 1995, 9, 17–40. [Google Scholar] [CrossRef]
- Torr, G.A.; Shine, R. Patterns of dominance in the small scincid lizard Lampropholis guichenoti. J. Herpetol. 1996, 30, 230–237. [Google Scholar] [CrossRef]
- Sacchi, R.; Pupin, F.; Gentilli, A.; Rubolini, D.; Scali, S.; Fasola, M.; Galeotti, P. Male-male combats in a polymorphic lizard: Residency and size, but not color, affect fighting rules and contest outcome. Aggress. Behav. 2009, 35, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Titone, V.; Marsiglia, F.; Mangiacotti, M.; Sacchi, R.; Scali, S.; Zuffi, M.A.L. Better to be resident, larger or coloured? Experimental analysis on intraspecific aggression in the ruin lizard. J. Zool. 2017, 304, 260–267. [Google Scholar] [CrossRef]
- Abalos, J.; Pérez i de Lanuza, G.; Carazo, P.; Font, E. The role of male coloration in the outcome of staged contests in the European common wall lizard (Podarcis muralis). Behaviour 2016, 153, 607–631. [Google Scholar] [CrossRef]
- Names, G.; Martin, M.; Badiane, A.; Le Galliard, J.-F. The relative importance of body size and UV coloration in influencing male-male competition in a lacertid lizard. Behav. Ecol. Sociobiol. 2019, 73, 98. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Corti, C.; Lo Cascio, P. Tail autotomy and extinction in Mediterranean lizards. A preliminary study of continental and insular populations. J. Zool. 1997, 243, 533–541. [Google Scholar] [CrossRef]
- Alibardi, L. Histochemical, biochemical and cell biological aspects of tail regeneration in lizard, an amniote model for studies on tissue regeneration. Prog. Histochem. Cytochem. 2014, 48, 143–244. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 3 September 2022).
- Schwartz, A.M.; Baird, T.A.; Timanus, D.K. Influence of age and prior experience on territorial behavior and the costs of defense in male collared lizards. Ethology 2007, 113, 9–17. [Google Scholar] [CrossRef]
- Fox, S.F. Natural selection on behavioral phenotypes of the lizard Uta stansburiana. Ecology 1978, 59, 834–847. [Google Scholar] [CrossRef]
- Pough, F.H.; Andrews, R.M. Use of anaerobic metabolism by free-ranging lizards. Physiol. Zool. 1985, 58, 205–213. [Google Scholar] [CrossRef]
- Marler, C.A.; Walsberg, G.; White, M.L.; Moore, M. Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav. Ecol. Sociobiol. 1995, 37, 225–231. [Google Scholar] [CrossRef]
- Ord, T.J. Costs of territoriality: A review of hypotheses, meta-analysis, and field study. Oecologia 2021, 197, 615–631. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr. Social behavior and antipredatory defense in lizards. In Lizard Social Behavior; Fox, S.F., McCoy, J.K., Baird, T.A., Eds.; The John Hopkins University Press: Baltimore, MD, USA, 2003; pp. 107–141. [Google Scholar]
- Formanowicz, D.R., Jr.; Brodie, E.D., Jr.; Bradley, P.J. Behavioural compensation for tail loss in the ground skink, Scincella lateralis. Anim. Behav. 1990, 40, 782–784. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr. Tradeoffs between courtship, fighting, and antipredatory behavior by a lizard, Eumeces laticeps. Behav. Ecol. Sociobiol. 1999, 47, 54–59. [Google Scholar] [CrossRef]
- Díaz-Uriarte, R. Anti-predator behaviour changes following an aggressive encounter in the lizard Tropidurus hispidus. Proc. R. Soc. B 1999, 266, 2457–2464. [Google Scholar] [CrossRef]
- Cooper, W.E., Jr.; Wilson, D.S. Sex and social costs of escaping in the striped plateau lizard Sceloporus virgatus. Behav. Ecol. 2007, 18, 764–768. [Google Scholar] [CrossRef]
- Salvador, A.; Martín, J.; López, P. Tail loss reduces home range size and access to females in male lizards, Psammodromus algirus. Behav. Ecol. 1995, 6, 382–387. [Google Scholar] [CrossRef]
- Riley, J.L.; Noble, D.W.; Byrne, R.W.; Whiting, M.J. Early social environment influences the behaviour of a family-living lizard. R. Soc. Open Sci. 2017, 4, 161082. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).