Abstract
Black band disease (BBD), which overgrows and kills scleractinian coral, is known to have various phases. The initial phase is the cyanobacterial patches (CPs) phase, followed by the intermediate phase (IP), and finally the mature BBD phase. Here, we hypothesize, pending further evidence, that when coral fragments infected with mature BBD are placed under shaded conditions, the BBD band shifts into a CP-like condition, with the shading causing a complex shift in the microbial consortium. While these microbial changes are beyond the scope of this paper, the photographs within provide interesting potential insights into this transition.
Black band disease (BBD) is a form of multi-microbial coral disease, which is seasonal in nature and actively progresses through live zooxanthellate scleractinian corals, causing tissue loss and death [1,2]. Fully mature and active BBD is generally black in color; however, studies have reported the color to vary at different locations and depths, and BBD is also known to be affected by other associated environmental parameters, e.g., [3,4,5] light and temperature [1,2,6,7]. The various developmental stages of BBD have been studied on the reefs of Great Barrier Reef (GBR), where it was divided into three phases: (1) cyanobacterial patches (CPs), (2) intermediate phase (IP), and (3) mature BBD [8,9]. These stages have distinct microbial compositions [8]. Similar initial infection phases have also been recently observed in situ in the reefs of Taiwan [10], but no demonstration that they are successional stages was given.
Several important aspects of the nature of BBD have been confirmed in tank experiments [7], further strengthening its relationship to light and temperature. However, the visual interpretation of the nature of the band is still elusive. Thus, it is essential to report unique and/or particular observations that may provide additional insights into the ecology and behavior of BBD. Herein, we report on photographic observations during a large-scale experiment. In two BBD-infected fragments of encrusting Montipora corals maintained in flow-through aquaria, we observed the transition of virulent BBD via shading. Although this may be a rare phenomenon requiring very specific lighting conditions (Figure S1), these data provide additional potential knowledge of BBD.
A total of 36 fragments from a single Montipora colony (to reduce genetic variability) were collected using a hammer and chisel from the reef of Sesoko Island, Okinawa, Japan, in September 2020. These fragments were trimmed to approximately 10 × 10 cm using a Dremel 3000 cutting drill (Dremel, Mount Prospect, IL, USA). Fragments were kept in outdoor aquarium facilities (Sesoko Station, University of the Ryukyus, Japan); they were later (after a week) transferred to the glasshouse facilities within the same marine station to initiate artificial infection. The fragments were kept under natural sunlight protected by shade to avoid excessive exposure to sunlight, which may lead to shock and bleaching. The fragments were then artificially infected by direct contact with BBD, using a 1 × 1 cm vector coral piece, which had signs of an active BBD lesion by direct contact. Once each healthy fragment showed evident signs of BBD lesions, the vector fragment was removed. We observed that the BBD lesion started progressing towards the direction of the live tissue over the course of a week. The infected fragments were then divided into two conditions depicting two different lighting stages, representing low light (shading) and natural light (control). All treatments were kept in triplicates (i.e., three separate flow-through aquarium tanks; six fragments in each treatment, i.e., a total of 18 fragments in shade and another 18 in natural light). The infected fragments were then photographed in the afternoon and post-midnight to observe any changes in the active band conditions. Two Montipora fragments (Shade 1/S1; Shade 2/S2) (Figure 1) under shaded conditions rapidly grew and eventually showed signs of transition, indicating the rarity of the event. The distinct black band progressively lost its sharp boundary and sulfide-rich front and instead developed into a diffuse cyanobacterial mat-like characteristic of CP (Figure 1). Additionally, BBD-infected fragments (control) without any signs of transition were considered for comparison (n = 5). Photographs of coral fragments were taken with an Olympus TG-5 camera, set at ISO 200. Light and temperature measurements were performed with HOBO Pendant temp/light loggers (Onset Co., Cape Cod, Bourne, MA, USA). Light intensity was calculated between 8:00 and 18:00 and was converted to µmol m−2 s−1 [2] (Tables S1 and S2).

Figure 1.
Specimen S1 (a–c): (a) Fragment shows BBD infection (20 Sept; 15:42). (b) BBD progresses, and greenish transition state can be observed (24 Sept; 3:30). (c) Limited light causes BBD to expand, and transitioned state moves ahead of BBD (24 Sept; 13:30); Specimen S2 (d–f): (d) Fragment shows BBD infection (21 Sept; 08:55). (e) BBD progresses without any form of transition (24 Sept; 03:30). (f) Transition of BBD band evident, as observed on 24 Sept; 13:30; Specimen S3 (g–i): (g) Fragment shows BBD infection (21 Sept; 08:56). (h) BBD progresses, killing live tissue (24 Sept; 03:33). (i) Dead skeleton visible, and BBD band still active (24 Sept; 13:40); Scale: 1 cm; white arrow: black band disease (BBD); yellow arrow: cyanobacterial-patch-like (CP-like); red arrow: dead tissue; yellow line: delineates CP-like condition.
Disease progression was measured through open-sourced ImageJ ver. 1.54p (Table S3). As the data were not normally distributed (Shapiro–Wilk test), differences between shade and control treatments were assessed using a Wilcoxon rank-sum test in R (ver. 2025.05.0+496).
The mean temperature over the experimental period was 28.71 ± 1.08 °C, with a maximum mid-day temperature of 32.35 °C. Temperature conditions were relatively constant across all three replicate tanks (Table S1). Light intensity, however, differed to a great extent (Table S2). The BBD progression rate differed significantly between treatments (Wilcoxon rank-sum test, p < 0.001) (Figure 2). In the control treatment, the BBD progression rate was 0.35 cm/day (±0.2), ranging from 0.01 to 0.7 cm/day, whereas in the shade treatment, the progression was higher, at 1.11 cm/day (±0.2), ranging from 0.9 to 1.3 cm/day (Figure 2; Table S3).
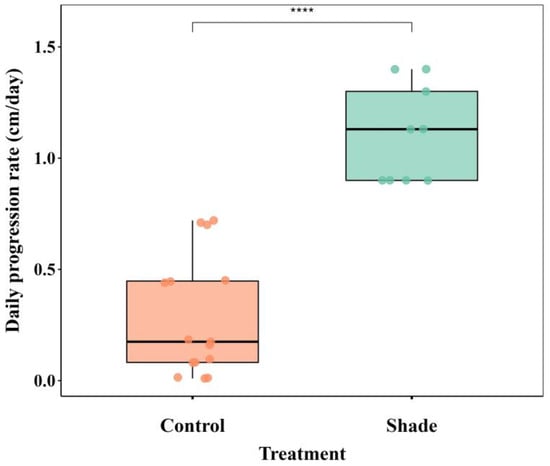
Figure 2.
Daily progression rate of black band disease (BBD) in encrusting Montipora corals under control and shade treatments. Box plots show the median and interquartile range (IQR), with whiskers extending to 1.5 × IQR. Individual points represent each replicate fragment (control N = 5 and Shade N = 3). Significance of differences between treatments was examined using a Wilcoxon rank-sum test; **** p < 0.001.
BBD is known to rapidly progress if placed under a shaded environment [11], which likely explains the high progression rates of BBD on the shaded fragments (Figure 1). Control fragments, however, were exposed to a much higher intensity of sunlight reaching up to >400 µmol m−2 s−1 (Figure S1), which should have propelled various behavioral mechanisms, as reported earlier [12]. The cyanobacteria associated with BBD in Okinawa has been identified as Roseofilum reptotaenium, which is known to show clumping behavior at 50 µmol m−2 s−1 in laboratory conditions [4]. However, in shaded treatments, we were able to see a higher bandwidth (Table S3), and increased progression, ~100 µmol m−2 s−1, is still considered low by the standards suggested by Muller and van Woesik (2011) [11] (Table S2).
Chen et al. (2017) [9] modeled several transition phases in BBD in the genus Montipora. In our case, we hypothesize, pending further evidence, that BBD was able to transform into CP-like cyanobacteria due to the reduction in light intensity as a result of shading. We further hypothesize that there was a change in the microbial consortium composition and abundance between the two phases. These observations thus call for future similar experiments that investigate the successional mechanism of BBD, including microbial diversity assessment through the use of next-generation sequencing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100732/s1. Table S1: Sea water temperatures obtained from Hobo temperature loggers; Table S2: Light intensity obtained from Hobo light sensors; Table S3: Disease progression comparative to control. * BBD showing transition effect. Figure S1: Light intensity retrieved from Logger 2 (Shade/Control) from Tank 2, where samples showed signs of BBD transition. Red arrows = time of observation of each BBD-infected Montipora fragment.
Author Contributions
Conceptualization, R.R.D.; investigation, R.R.D., P.T.-K.; methodology, R.R.D.; resources, R.R.D., J.D.R.; software, R.R.D., P.T.-K.; writing—original draft, R.R.D.; writing—reviewing and editing, R.R.D., P.T.-K., S.-L.T., J.D.R.; visualization, R.R.D., P.T.-K.; supervision, J.D.R.; project administration, R.R.D.; funding acquisition, R.R.D., P.T.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by MEXT (no. 170021/2017~2021) provided by the Ministry of Education, Culture, Sports, Science & Technology (MEXT), Japan to R.R.D. P.T.-K. was supported by Suzuki Shohei Marine Biological Research Grant from the University of the Ryukyus.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are grateful to the staff of Sesoko Marine Station (University of the Ryukyus) for providing the necessary facilities. The authors are grateful to the three anonymous reviewers for enhancing an earlier version of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sato, Y.; Bourne, D.G.; Willis, B.L. Dynamics of seasonal outbreaks of black band disease in an assemblage of Montipora species at Pelorus Island (Great Barrier Reef, Australia). Proc. R. Soc. B 2009, 276, 2795–2803. [Google Scholar] [CrossRef]
- Das, R.R.; Wada, H.; Masucci, G.D.; Singh, T.; Tavakoli-Kolour, P.; Wada, N.; Tang, S.L.; Yamashiro, H.; Reimer, J.D. Four-year field survey of black band disease and skeletal growth anomalies in encrusting Montipora spp. corals around Sesoko Island, Okinawa. Diversity 2022, 14, 32. [Google Scholar] [CrossRef]
- Wada, N.; Mano, N.; Yanagisawa, Y.; Mori, T. Occurrence of coral diseases at Akajima, Okinawa, Japan in 2010 and 2011. Galaxea J. Coral Reef Stud. 2017, 19, 35–44. [Google Scholar] [CrossRef][Green Version]
- Hutabarat, P.; Nguyen, X.H.; Suda, S. Black band disease-related (BBD) cyanobacterium from Okinawan corals. J. Appl. Phycol. 2018, 30, 3197–3203. [Google Scholar] [CrossRef]
- Kubomura, T.; Yamashiro, H.; Reimer, J.D. Appearance of an anomalous black band disease at the upper mesophotic depths after coral bleaching. Dis. Aquat. Org. 2018, 131, 245–249. [Google Scholar] [CrossRef]
- Boyett, H.V.; Bourne, D.G.; Willis, B.L. Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar. Biol. 2007, 151, 1711–1720. [Google Scholar] [CrossRef]
- Sato, Y.; Bourne, D.G.; Willis, B.L. Effects of temperature and light on the progression of black band disease on the reef coral, Montipora hispida. Coral Reefs 2011, 30, 753–761. [Google Scholar] [CrossRef]
- Sato, Y.; Willis, B.L.; Bourne, D.G. Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J. 2010, 4, 203–214. [Google Scholar] [CrossRef]
- Chen, C.C.M.; Bourne, D.G.; Drovandi, C.C.; Mengersen, K.; Willis, B.L.; Caley, M.J.; Sato, Y. Modelling environmental drivers of black band disease outbreaks in populations of foliose corals in the genus Montipora. PeerJ 2017, 5, e3438. [Google Scholar] [CrossRef][Green Version]
- Huang, C.Y.; Hwang, J.S.; Yamashiro, H.; Tang, S.L. Spatial and cross-seasonal patterns of coral disease in reefs of Taiwan: High prevalence and regional variation. Dis. Aquat. Org. 2021, 146, 145–156. [Google Scholar] [CrossRef]
- Muller, E.M.; van Woesik, R. Black-band disease dynamics: Prevalence, incidence, and acclimatization to light. J. Exp. Mar. Biol. Ecol. 2011, 397, 52–57. [Google Scholar] [CrossRef]
- Myers, J.L.; Sekar, R.; Richardson, L.L. Molecular detection and ecological significance of the cyanobacterial genera Geitlerinema and Leptolyngbya in black band disease of corals. Appl. Environ. Microbiol. 2007, 73, 5173–5182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).