Influence of Bird Behavioural Traits and Habitat in Predicting Haemoparasite Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bird Sampling
2.3. Molecular Screening and Parasite Detection
2.4. Data Analysis
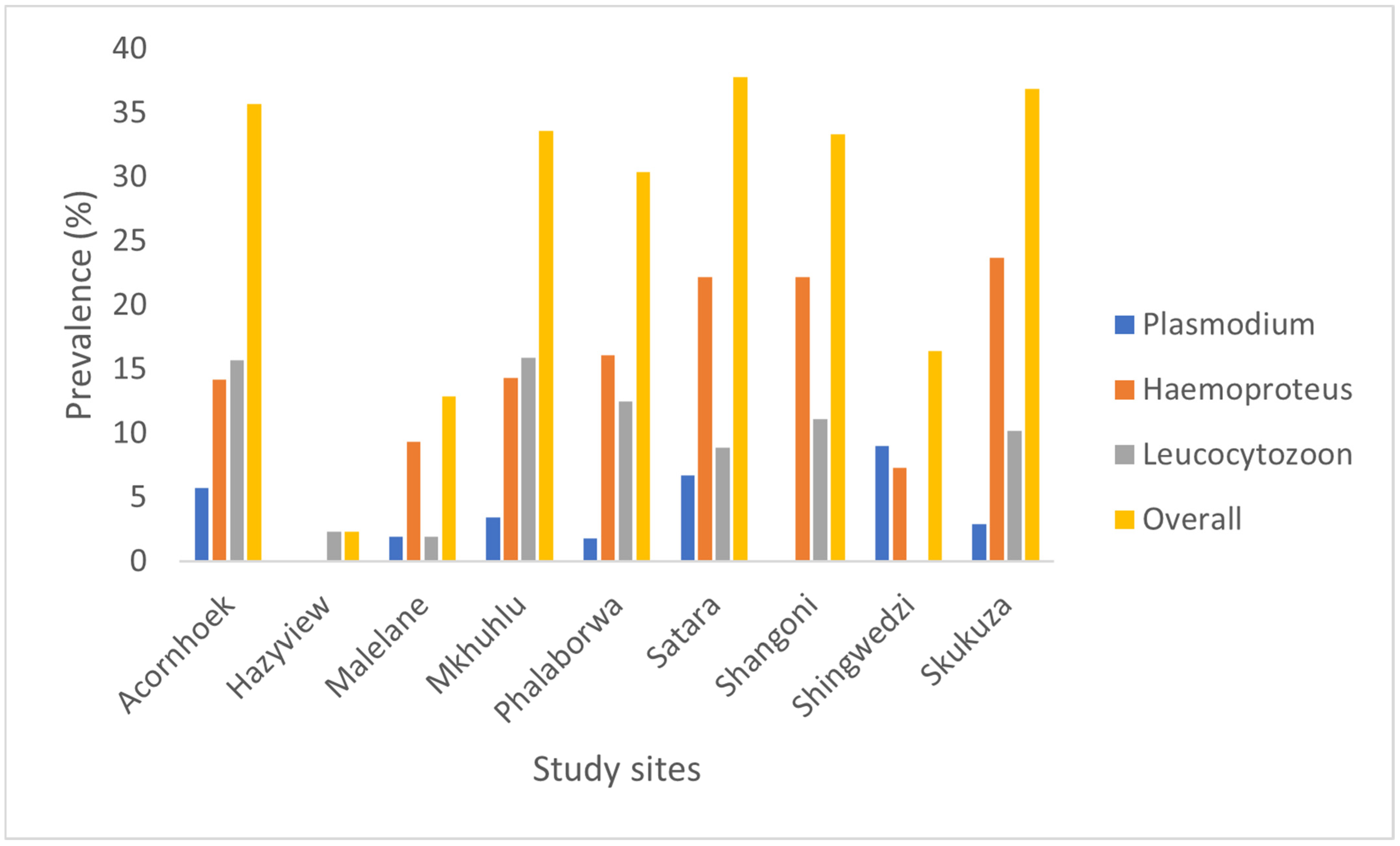
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paquette, S.-J.; Simon, A.Y.; Xiii, A.; Kobinger, G.P.; Shahhosseini, N. Medically significant vector-borne viral diseases in Iran. Microorganisms 2023, 11, 3006. [Google Scholar] [CrossRef]
- Lutz, H.L.; Hochachka, W.M.; Engel, J.I.; Bell, J.A.; Tkach, V.V.; Bates, J.M.; Hackett, S.J.; Weckstein, J.D. Parasite Prevalence Corresponds to Host Life History in a Diverse Assemblage of Afrotropical Birds and Haemosporidian Parasites. PLoS ONE 2015, 10, e0121254. [Google Scholar]
- Mora-Rubio, C.; Ferraguti, M.; Magallanes, S.; Bravo-Barriga, D.; Hernandez-Caballero, I.; Marzal, A.; de Lope, F. Unravelling the Mosquito-Haemosporidian Parasite-Bird Host Network in the Southwestern Iberian Peninsula: Insights into Malaria Infections, Mosquito Community and Feeding Preferences. Parasites Vectors 2023, 16, 395. [Google Scholar] [CrossRef]
- Norris, K.; Evans, M.R. Ecological Immunology: Life History Trade-Offs and Immune Defense in Birds. Behav. Ecol. 2000, 11, 19–26. [Google Scholar] [CrossRef]
- Clark, N.J.; Wells, K.; Dimitrov, D.; Clegg, S.M. Co-infections and Environmental Conditions Drive the Distributions of Blood Parasites in Wild Birds. J. Anim. Ecol. 2016, 85, 1461–1470. [Google Scholar] [CrossRef]
- Janousek, W.M.; Marra, P.P.; Kilpatrick, A.M. Avian Roosting Behavior Influences Vector-Host Interactions for West Nile Virus Hosts. Parasites Vectors 2014, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Chiles, R.E.; Fang, Y.; Barker, C.M.; Reisen, W.K. Role of Nestling Mourning Doves and House Finches as Amplifying Hosts of St. Louis Encephalitis Virus. J. Med. Entomol. 2004, 41, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Lindsay, L.R.; Hanincová, K.; Barker, I.K.; Bigras-Poulin, M.; Charron, D.F.; Heagy, A.; Francis, C.M.; O’Callaghan, C.J.; Schwartz, I. Role of Migratory Birds in Introduction and Range Expansion of Ixodes Scapularis Ticks and of Borrelia Burgdorferi and Anaplasma Phagocytophilum in Canada. Appl. Environ. Microbiol. 2008, 74, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- César, P.A. Bird-Parasite Interactions in Tropical Forests: An Ecological and Evolutionary Perspective. Ph.D. Thesis, Universidad de los Andes, Bogotá, Colombia, 2018. [Google Scholar]
- Scheuerlein, A.; Ricklefs, R.E. Prevalence of Blood Parasites in European Passeriform Birds. Proc. R. Soc. Lond. B Biol. Sci. 2019, 271, 1363–1370. [Google Scholar] [CrossRef]
- Jasinskiene, N.; Coleman, J.; Ashikyan, A.; Salampessy, M.; Marinotti, O.; James, A.A. Genetic Control of Malaria Parasite Transmission: Threshold Levels for Infection in an Avian Model System. Am. J. Trop. Med. Hyg. 2007, 76, 1072–1078. [Google Scholar] [CrossRef]
- Valkiunas, G. Avian Malaria Parasites and other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Clark, N.J.; Clegg, S.M.; Lima, M.R. A Review of Global Diversity in Avian Haemosporidians (Plasmodium and Haemoproteus: Haemosporida): New Insights from Molecular Data. Int. J. Parasitol. 2014, 44, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.C.; Santiago-Alarcon, D.; Braga, É.M. Diptera Vectors of Avian Haemosporidians: With Emphasis on Tropical Regions. In Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics; Springer International Publishing: Cham, Switzerland, 2020; pp. 185–250. [Google Scholar]
- Ganser, C.; Monadjem, A.; McCleery, R.A.; Ndlela, T.; Wisely, S.M. Is it Best on the Nest? Effects of Avian Life-History on Haemosporidian Parasitism. Int. J. Parasitol. Parasites Wildl. 2020, 13, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Mkongewa, V.J.; Newmark, W.D.; Stanley, T.R. Breeding Biology of an Afrotropical Forest Understory Bird Community in Northeastern Tanzania. Wilson J. Ornithol. 2013, 125, 260–267. [Google Scholar] [CrossRef]
- Chahad-Ehlers, S.; Fushita, A.T.; Lacorte, G.A.; Assis, P.C.P.d.; Del Lama, S.N. Effects of Habitat Suitability for Vectors, Environmental Factors and Host Characteristics on the Spatial Distribution of the Diversity and Prevalence of Haemosporidians in Waterbirds from Three Brazilian Wetlands. Parasites Vectors 2018, 11, 276. [Google Scholar] [CrossRef]
- Pori, T.; Ndlovu, M.; Markus, M.B. Influence of season and other factors on avian Trypanosoma spp. and microfilarial prevalence in the Lowveld, South Africa. S. Afr. J. Sci. 2023, 119, 1–6. [Google Scholar] [CrossRef]
- Hoppe, I.R. Social and Ecological Correlates of Avian Infection by Haemosporidian Blood Parasites. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2021. [Google Scholar]
- Gonzalez, A.D.; Matta, N.E.; Ellis, V.A.; Miller, E.T.; Ricklefs, R.E.; Gutierrez, H.R. Mixed Species Flock, Nest Height, and Elevation Partially Explain Avian Haemoparasite Prevalence in Colombia. PLoS ONE 2019, 9, e100695. [Google Scholar] [CrossRef]
- Svensson-Coelho, M.; Blake, J.G.; Loiselle, B.A.; Penrose, A.S.; Parker, P.G.; Ricklefs, R.E. Diversity, Prevalence, and Host Specificity of Avian Plasmodium and Haemoproteus in a Western Amazon Assemblage. Ornithol. Monogr. 2013, 76, 1–47. [Google Scholar] [CrossRef]
- Rivero de Aguilar, J.; Castillo, F.; Moreno, A.; Penafiel, N.; Browne, L.; Walter, S.T.; Karubian, J.; Bonaccorso, E. Patterns of Avian Haemosporidian Infections Vary with Time, but not Habitat, in a Fragmented Neotropical Landscape. PLoS ONE 2018, 13, e0206493. [Google Scholar] [CrossRef]
- Meixell, B.W.; Arnold, T.W.; Lindberg, M.S.; Smith, M.M.; Runstadler, J.A.; Ramey, A.M. Detection, Prevalence, and Transmission of Avian Hematozoa in Waterfowl at the Arctic/Sub-Arctic Interface: Co-Infections, Viral Interactions, and Sources of Variation. Parasites Vectors 2016, 9, 390. [Google Scholar] [CrossRef]
- Dubiec, A.; Da Silva, A.; Celej, M. Low Prevalence of Haemosporidian and Trypanosome Infections in the Eurasian Nightjar (Caprimulgus Europaeus). J. Ornithol. 2023, 164, 445–453. [Google Scholar] [CrossRef]
- Ndlovu, M.; Wardjomto, M.B.; Pori, T.; Nangammbi, T.C. Diversity and Host Specificity of Avian Haemosporidians in an Afrotropical Conservation Region. Animals 2024, 14, 2906. [Google Scholar] [CrossRef]
- Pori, T.; Ndlovu, M.; Markus, M.; Govender, D. Avian Haemoparasite Prevalence in Kruger National Park and the Surrounding Human Settlements. In Proceedings of the 15th Annual Savanna Science Network Meeting, Skukuza, South Africa, 12–16 March 2017. [Google Scholar]
- Wardjomto, M.B.; Ndlovu, M.; Pérez-Rodríguez, A.; Pori, T.; Nangammbi, T. Avian haemosporidia in native and invasive sparrows at an Afrotropical region. Parasitol. Res. 2021, 120, 2631–2640. [Google Scholar] [CrossRef]
- Malherbe, J.; Smit, I.P.; Wessels, K.J.; Beukes, P.J. Recent Droughts in the Kruger National Park as Reflected in the Extreme Climate Index. Afr. J. Range Forage Sci. 2020, 37, 1–17. [Google Scholar] [CrossRef]
- Barnes, K.; Behrens, K. Birds of Kruger National Park. In Birds of Kruger National Park; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Ndlovu, M. Birdcall Lures Improve Passerine Mist-net Captures at a Sub-tropical African Savanna. PLoS ONE 2018, 13, e0199595. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Weckstein, J.D.; Fecchio, A.; Tkach, V.V. A New Real-time PCR Protocol for Detection of Avian Haemosporidians. Parasites Vectors 2015, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Örjan, Ö.; Hannson, B.; Westerdahl, H.; Pinheiro, R.T. Host Specificity in Avian Blood Parasites: A Study of Plasmodium and Haemoproteus Mitochondrial DNA Amplified from Birds. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef]
- Hellgren, O.; Križanauskiene, A.; Valkiūnas, G.; Bensch, S. Diversity and Phylogeny of Mitochondrial Cytochrome B Lineages from Six Morphospecies of Avian Haemoproteus (Haemosporida: Haemoproteidae). J. Parasitol. 2007, 93, 889–896. [Google Scholar] [CrossRef]
- Okanga, S.; Cumming, G.S.; Hockey, P.A. Avian Malaria Prevalence and Mosquito Abundance in the Western Cape, South Africa. Malar. J. 2013, 12, 370. [Google Scholar] [CrossRef]
- Turcotte, A.; Bélisle, M.; Pelletier, F.; Garant, D. Environmental Determinants of Haemosporidian Parasite Prevalence in a Declining Population of Tree Swallows. Parasitology 2018, 145, 961–970. [Google Scholar] [CrossRef]
- Dubiec, A.; Podmokła, E.; Harnist, I.; Mazgajski, T.D. Haemoparasites of the Pied Flycatcher: Inter-Population Variation in the Prevalence and Community Composition. Parasitology 2018, 145, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Okanga, S.; Cumming, G.S.; Hockey, P.A.; Nupen, L.; Peters, J.L. Host Specificity and Co-Speciation in Avian Haemosporidia in the Western Cape, South Africa. PLoS ONE 2014, 9, e86382. [Google Scholar] [CrossRef] [PubMed]
- Paaijmans, K.P.; Thomas, M.B. The Influence of Mosquito Resting Behaviour and Associated Microclimate for Malaria Risk. Malar. J. 2011, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.T.; Van Riper, C. Vectors, Epizootiology, and Pathogenicity of Avian Species of Haemoproteus (Haemosporina: Haemoproteidae). Bull. Soc. Vector Ecol. 1991, 16, 109–126. [Google Scholar]
- Rodríguez-Hernández, K.; Álvarez-Mendizábal, P.; Chapa-Vargas, L.; Escobar, F.; Dattilo, W.; Santiago-Alarcon, D. Infection Intensity Shapes Specialization and Beta Diversity of Haemosporidian–Bird Networks Across Elevations. Ecosphere 2023, 14, e4481. [Google Scholar] [CrossRef]
- Fallon, S.M.; Fleischer, R.C.; Graves, G.R. Malarial Parasites as Geographical Markers in Migratory Birds? Biol. Lett. 2006, 2, 213–216. [Google Scholar] [CrossRef]
- Sehgal, R.N. Manifold Habitat Effects on the Prevalence and Diversity of Avian Blood Parasites. Int. J. Parasitol. Parasites Wildl. 2015, 4, 421–430. [Google Scholar] [CrossRef]
- Schumm, Y.R.; Lederer-Ponzer, N.; Masello, J.F.; Quillfeldt, P. High Prevalence of Haemosporidian Parasites in Eurasian Jays. Parasitol. Res. 2024, 123, 182. [Google Scholar] [CrossRef]
- Beadell, J.S.; Ishtiaq, F.; Covas, R.; Melo, M.; Warren, B.H.; Atkinson, C.T.; Bensch, S.; Graves, G.R.; Jhala, Y.V.; Peirce, M.A. Global Phylogeographic Limits of Hawaii’s avian Malaria. Proc. R. Soc. B Biol. Sci. 2006, 273, 2935–2944. [Google Scholar] [CrossRef]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A Three-Genome Phylogeny of Malaria Parasites (Plasmodium and Closely Related Genera): Evolution of Life-History Traits and Host Switches. Mol. Phylogenet. Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef]
- Medeiros, M.C.; Ricklefs, R.E.; Brawn, J.D.; Hamer, G.L. Plasmodium Prevalence Across Avian Host Species is Positively Associated with Exposure to Mosquito Vectors. Parasitology 2015, 142, 1612–1620. [Google Scholar] [CrossRef]
- Fecchio, A.; Ellis, V.A.; Bell, J.A.; Andretti, C.B.; D’horta, F.M.; Silva, A.M.; Tkach, V.V.; Weckstein, J.D. Avian Malaria, Ecological Host Traits and Mosquito Abundance in Southeastern Amazonia. Parasitology 2017, 144, 1117–1132. [Google Scholar] [CrossRef]
- Lachish, S.; Knowles, S.C.; Alves, R.; Wood, M.J.; Sheldon, B.C. Fitness Effects of Endemic Malaria Infections in a Wild Bird Population: The Importance of Ecological Structure. J. Anim. Ecol. 2011, 80, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Lotta, I.A.; Valkiūnas, G.; Pacheco, M.A.; Escalante, A.A.; Hernández, S.R.; Matta, N.E. Disentangling Leucocytozoon Parasite diversity in the Neotropics: Descriptions of Two New Species and Shortcomings of Molecular Diagnostics for Leucocytozoids. Int. J. Parasitol. Parasites Wildl. 2019, 9, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lara, C.; González-García, F.; Santiago-Alarcon, D. Spatial and Seasonal Variation of Avian Malaria Infections in Five Different Land Use Types Within a Neotropical Montane Forest Matrix. Landsc. Urban Plan. 2017, 157, 151–160. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Wardjomto, M.B.; Ndlovu, M.; Pérez-Rodríguez, A.; Pori, T.; Nangammbi, T.C. Comparative performance of microscopy, nested PCR, and real-time PCR for screening avian haemosporidian parasites in Afrotropical starlings (family Sturnidae). Parasitol. Res. 2023, 122, 2393–2404. [Google Scholar] [CrossRef]

| Species | n | Behaviour | Infected | Prevalence (%) | H | P | L | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Association | Foraging | Movement | Sociality | |||||||
| Actophilornis africanus | 5 | Wild | Ground | Migratory | Solitary | 0 | 0 | 0 | 0 | 0 |
| Anaplectes rubriceps | 10 | Wild | Aerial | Resident | Solitary | 6 | 60 | 4 | 0 | 2 |
| Buphagus erythrorhynchus | 30 | Mixed | Aerial | Resident | Gregarious | 14 | 43 | 0 | 0 | 13 |
| Colius striatus | 6 | Wild | Aerial | Resident | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Creatophora cinerea | 38 | Wild | Ground | Resident | Gregarious | 16 | 42 | 13 | 3 | 0 |
| Crithagra mozambica | 25 | Anthropogenic | Ground | Resident | Gregarious | 17 | 56 | 9 | 1 | 7 |
| Dicrurus adsimilis | 14 | Mixed | Mixed | Resident | Solitary | 2 | 29 | 2 | 0 | 0 |
| Euplectes orix | 9 | Wild | Ground | Resident | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Halcyon albiventris | 5 | Wild | Aerial | Migratory | Solitary | 1 | 20 | 1 | 0 | 0 |
| Hirundo rustica | 28 | Anthropogenic | Aerial | Migratory | Gregarious | 1 | 7 | 1 | 0 | 0 |
| Hirundo smithii | 22 | Anthropogenic | Aerial | Migratory | Gregarious | 4 | 18 | 4 | 0 | 0 |
| Lamprotornis chalybaeus | 191 | Mixed | Ground | Resident | Gregarious | 64 | 34 | 45 | 18 | 2 |
| Lamprotornis nitens | 15 | Mixed | Ground | Resident | Gregarious | 5 | 33 | 5 | 0 | 0 |
| Merops bullockoides | 6 | Mixed | Aerial | Nomadic | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Motacilla aguimp | 7 | Mixed | Ground | Resident | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Passer diffusus | 31 | Wild | Ground | Resident | Solitary | 20 | 65 | 14 | 1 | 10 |
| Passer domesticus | 84 | Anthropogenic | Ground | Resident | Gregarious | 18 | 21 | 3 | 0 | 16 |
| Ortygornis sephaena | 10 | Wild | Ground | Nomadic | Solitary | 5 | 50 | 0 | 3 | 3 |
| Ploceus capensis | 7 | Mixed | Ground | Resident | Gregarious | 4 | 57 | 3 | 1 | 0 |
| Ploceus cucullatus | 69 | Wild | Mixed | Resident | Gregarious | 38 | 55 | 18 | 15 | 8 |
| Ploceus intermedius | 23 | Wild | Mixed | Resident | Gregarious | 4 | 17 | 4 | 0 | 0 |
| Ploceus velatus | 8 | Mixed | Mixed | Resident | Gregarious | 3 | 38 | 2 | 1 | 0 |
| Prinia subflava | 7 | Wild | Ground | Resident | Solitary | 1 | 14 | 1 | 0 | 0 |
| Prionops plumatus | 5 | Wild | Ground | Resident | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Pycnonotus tricolor | 17 | Anthropogenic | Ground | Nomadic | Gregarious | 8 | 47 | 4 | 1 | 7 |
| Quelea quelea | 65 | Mixed | Ground | Migratory | Gregarious | 1 | 2 | 1 | 0 | 0 |
| Spermestes cucullata | 5 | Mixed | Ground | Resident | Gregarious | 0 | 0 | 0 | 0 | 0 |
| Spilopelia senegalensis | 102 | Anthropogenic | Ground | Nomadic | Solitary | 35 | 34 | 22 | 0 | 20 |
| Streptopelia decipiens | 54 | Wild | Ground | Nomadic | Solitary | 2 | 4 | 2 | 0 | 0 |
| Streptopelia semitorquata | 8 | Wild | Ground | Resident | Solitary | 3 | 38 | 3 | 0 | 1 |
| Terpsiphone viridis | 9 | Wild | Mixed | Migratory | Solitary | 1 | 11 | 1 | 0 | 1 |
| Tockus erythrorhynchus | 40 | Wild | Ground | Resident | Gregarious | 3 | 5 | 1 | 1 | 0 |
| Tockus leucomelas | 30 | Wild | Ground | Nomadic | Gregarious | 1 | 3 | 1 | 0 | 0 |
| Trachyphonus vaillantii | 5 | Mixed | Ground | Resident | Solitary | 2 | 40 | 2 | 0 | 1 |
| Turdoides jardineii | 6 | Wild | Ground | Resident | Gregarious | 3 | 50 | 0 | 0 | 3 |
| Uraeginthus angolensis | 7 | Mixed | Mixed | Resident | Gregarious | 1 | 14 | 1 | 0 | 0 |
| Total | 1003 | 283 | 167 | 45 | 94 | |||||
| Avian Family | n | Infected | Infection Prevalence % | |||
|---|---|---|---|---|---|---|
| Overall | Haemoproteus | Plasmodium | Leucocytozoon | |||
| Fringillidae | 25 | 14 | 56.00 | 36.00 | 4.00 | 28.00 |
| Buphagidae | 30 | 13 | 43.33 | - | - | 43.33 |
| Sturnidae | 244 | 86 | 35.25 | 25.82 | 8.61 | 0.82 |
| Passeridae | 115 | 38 | 33.04 | 14.78 | 0.87 | 22.61 |
| Ploceidae | 191 | 56 | 29.32 | 16.75 | 8.90 | 5.24 |
| Columbidae | 164 | 40 | 24.39 | 16.46 | - | 12.80 |
| Hirundinidae | 50 | 5 | 12.00 | 10.00 | - | - |
| Bucerotidae | 70 | 3 | 4.29 | 2.86 | 1.43 | - |
| Common Name | Species | n | Detected Haemoparasites |
|---|---|---|---|
| Dark-capped Bulbul | Ploceus capensis | 7 | P (1), H (3), L (7) |
| Greater Blue-eared Starling | Lamprotornis chalybaeus | 191 | P (18), H (45), L (2) |
| Village Weaver | Ploceus cucullatus | 69 | P (15), H (18), L (7) |
| Yellow-fronted Canary | Crithagra mozambica | 25 | P (1), H (8), L (7) |
| Total | 302 |
| Infection Genus | Prevalence (%) | Mann–Whitney U | p Value | |
|---|---|---|---|---|
| Gregarious | Solitary | |||
| Plasmodium | 5.52 (41/743) | 1.54 (4/260) | 88,380 | 0.005 |
| Haemoproteus | 15.48 (115/743) | 20.00 (52/260) | 87,049 | 0.039 |
| Leucocytozoon | 7.54 (56/743) | 14.61 (38/260) | 87,865 | 0.025 |
| Overall | 29.82 (205/743) | 30.00 (78/260) | 87,722 | 0.141 |
| Parasite | Prevalence (%) | Kruskal–Wallis (x2, df, p) | ||
|---|---|---|---|---|
| Aerial | Ground | Mixed | ||
| Plasmodium | 0 (0/107) | 3.66 (28/766) | 11.54 (15/130) | 23.50, 2, <0.001 |
| Haemoproteus | 10.28 (11/107) | 16.01 (123/766) | 23.08 (30/130) | 8.26, 2, 0.016 |
| Leucocytozoon | 14.02 (15/107) | 8.09 (62/766) | 6.92 (9/130) | 5.02, 2, 0.081 |
| Overall | 24.30 (26/107) | 25.72 (197/766) | 39.23 (51/130) | 16.06, 2, <0.001 |
| Parasite | Prevalence (%) | Kruskal–Wallis (x2, df, p) | ||
|---|---|---|---|---|
| Resident | Nomadic | Migratory | ||
| Plasmodium | 6.00 (39/650) | 1.83 (4/219) | 0 (0/134) | 13.7, 2, 0.001 |
| Haemoproteus | 19.69 (128/650) | 12.33 (27/219) | 6.72 (9/134) | 16.0, 2, <0.001 |
| Leucocytozoon | 8.64 (57/660) | 12.79 (28/219) | 0.75 (1/134) | 14.7,2, <0.001 |
| Overall | 33.23 (216/650) | 22.37 (49/219) | 6.72 (9/134) | 47.0, 2, <0.001 |
| Parasite | Prevalence (%) | Kruskal–Wallis (x2, df, p) | ||
|---|---|---|---|---|
| Anthropogenic | Mixed | Wild | ||
| Plasmodium | 0.72 (2/278) | 5.56 (20/360) | 5.75 (21/365) | 11.815, 2, 0.003 |
| Haemoproteus | 15.10 (42/278) | 17.50 (63/360) | 16.16 (59/365) | 0.409, 2, 0.815 |
| Leucocytozoon | 16.91 (47/278) | 4.44 (16/360) | 6.30 (23/365) | 32.831, 2, <0.001 |
| Overall | 28.84 (79/278) | 26.94 (97/360) | 26.84 (98/365) | 2.718, 2, 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyathi, G.; Ndlovu, M.; Nangammbi, T.C. Influence of Bird Behavioural Traits and Habitat in Predicting Haemoparasite Infection. Diversity 2025, 17, 731. https://doi.org/10.3390/d17100731
Nyathi G, Ndlovu M, Nangammbi TC. Influence of Bird Behavioural Traits and Habitat in Predicting Haemoparasite Infection. Diversity. 2025; 17(10):731. https://doi.org/10.3390/d17100731
Chicago/Turabian StyleNyathi, Grace, Mduduzi Ndlovu, and Tshifhiwa C. Nangammbi. 2025. "Influence of Bird Behavioural Traits and Habitat in Predicting Haemoparasite Infection" Diversity 17, no. 10: 731. https://doi.org/10.3390/d17100731
APA StyleNyathi, G., Ndlovu, M., & Nangammbi, T. C. (2025). Influence of Bird Behavioural Traits and Habitat in Predicting Haemoparasite Infection. Diversity, 17(10), 731. https://doi.org/10.3390/d17100731






