Epibenthic Dinoflagellates in the Southern Gulf of California: Species Composition and Abundance
Abstract
1. Introduction
2. Materials and Methods
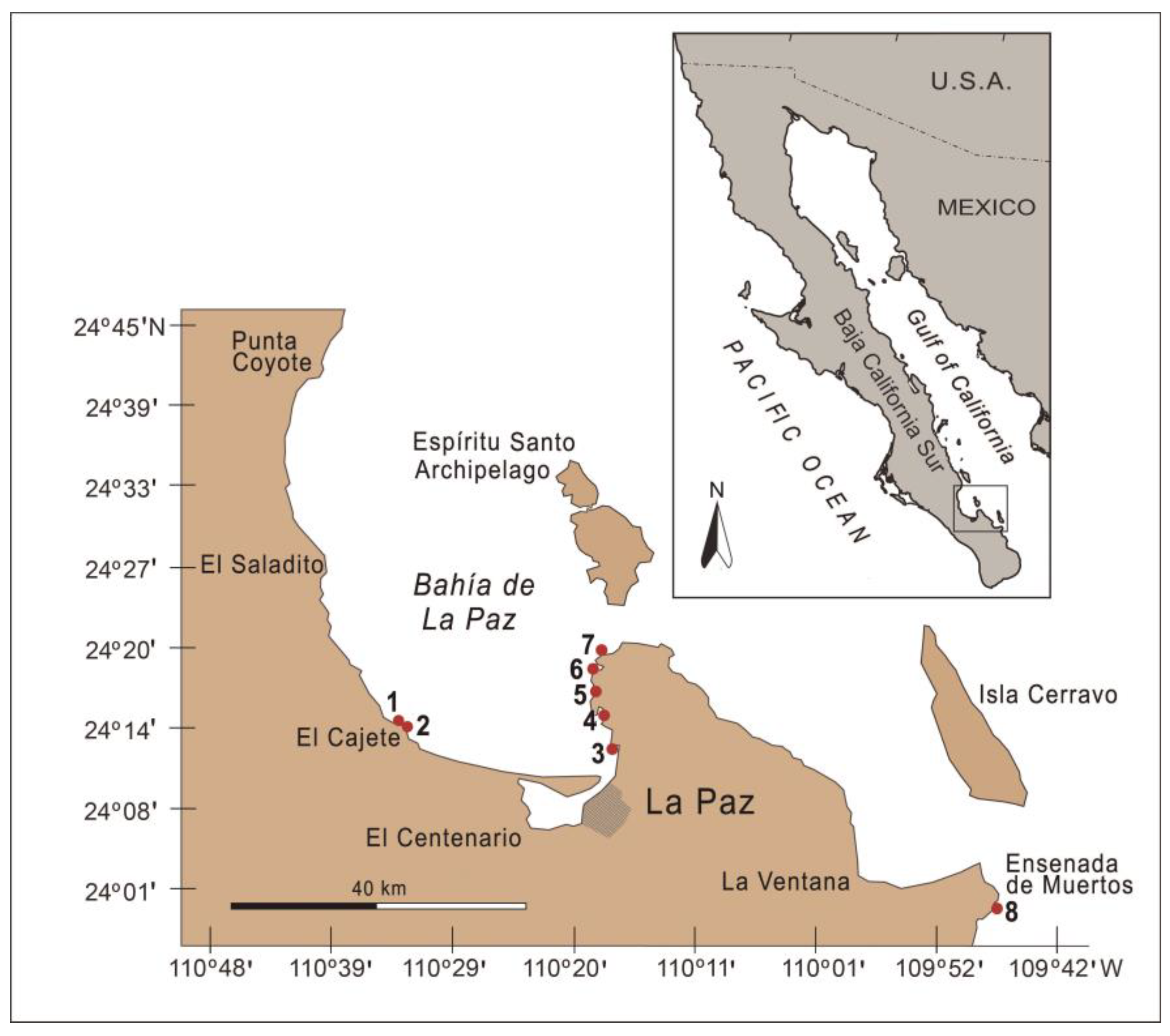
2.1. Study Area
2.2. Sampling
2.3. Laboratory Analyses
2.4. Data Analysis
3. Results
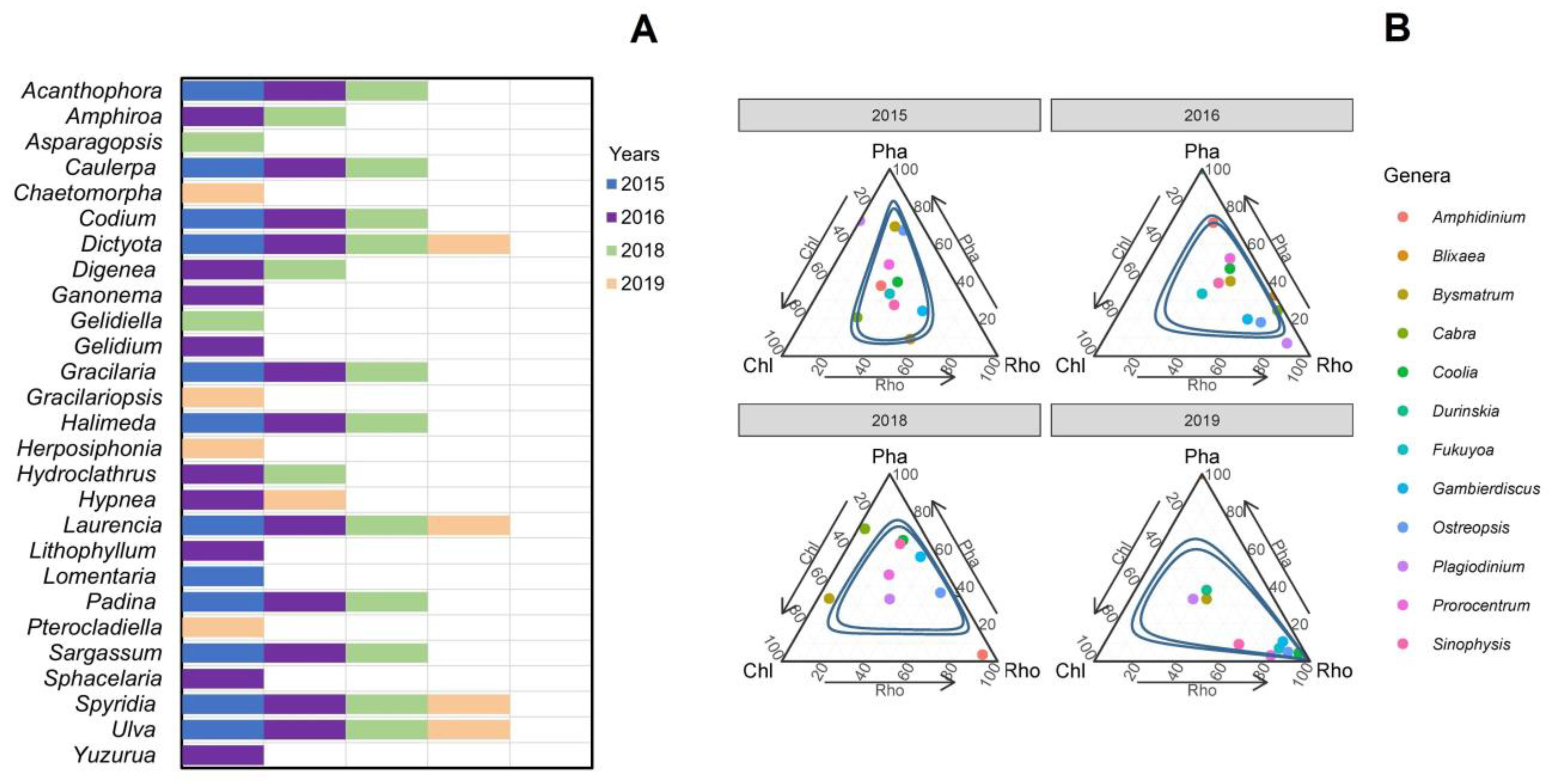
3.1. Species Composition of Macroalgae
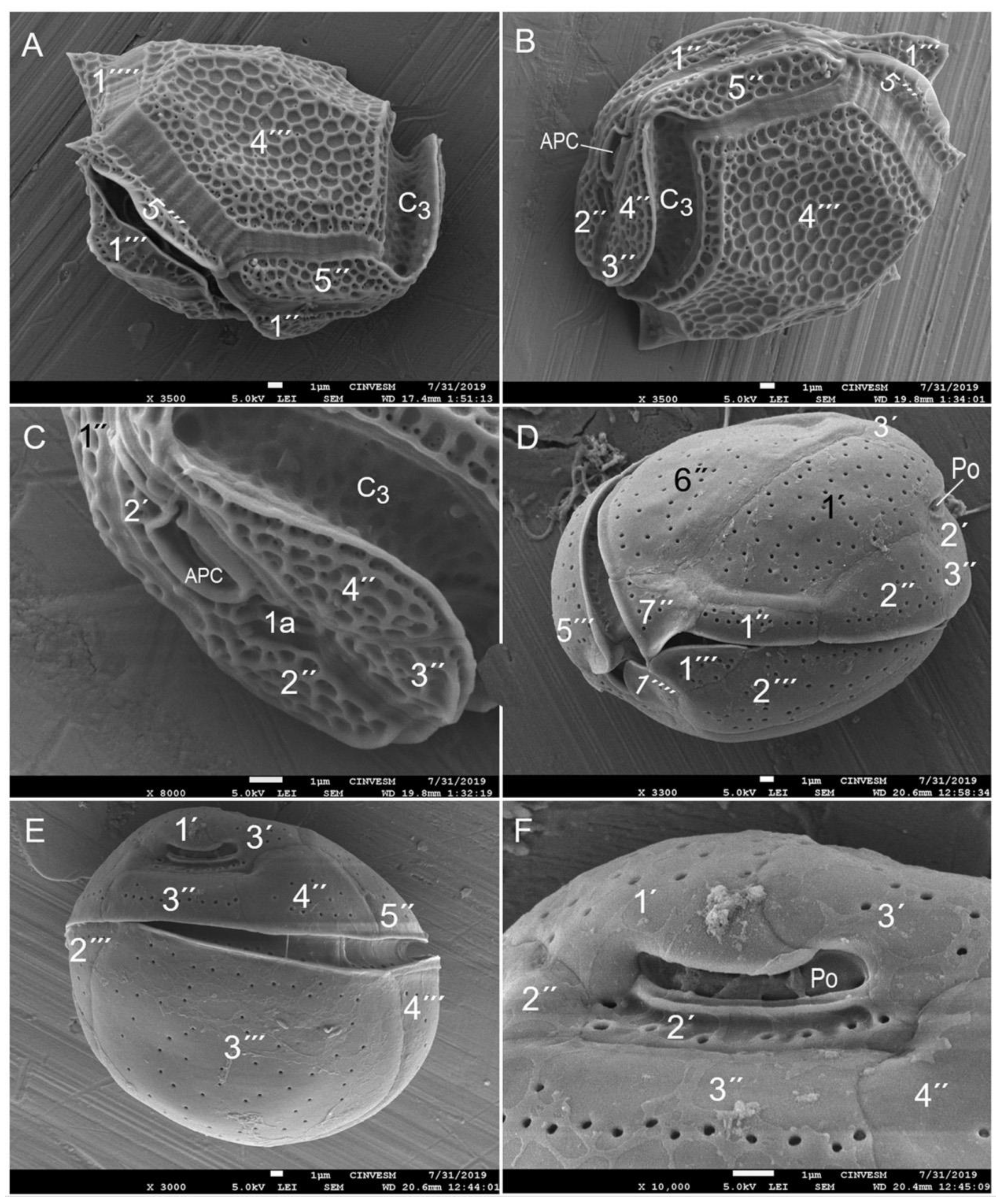
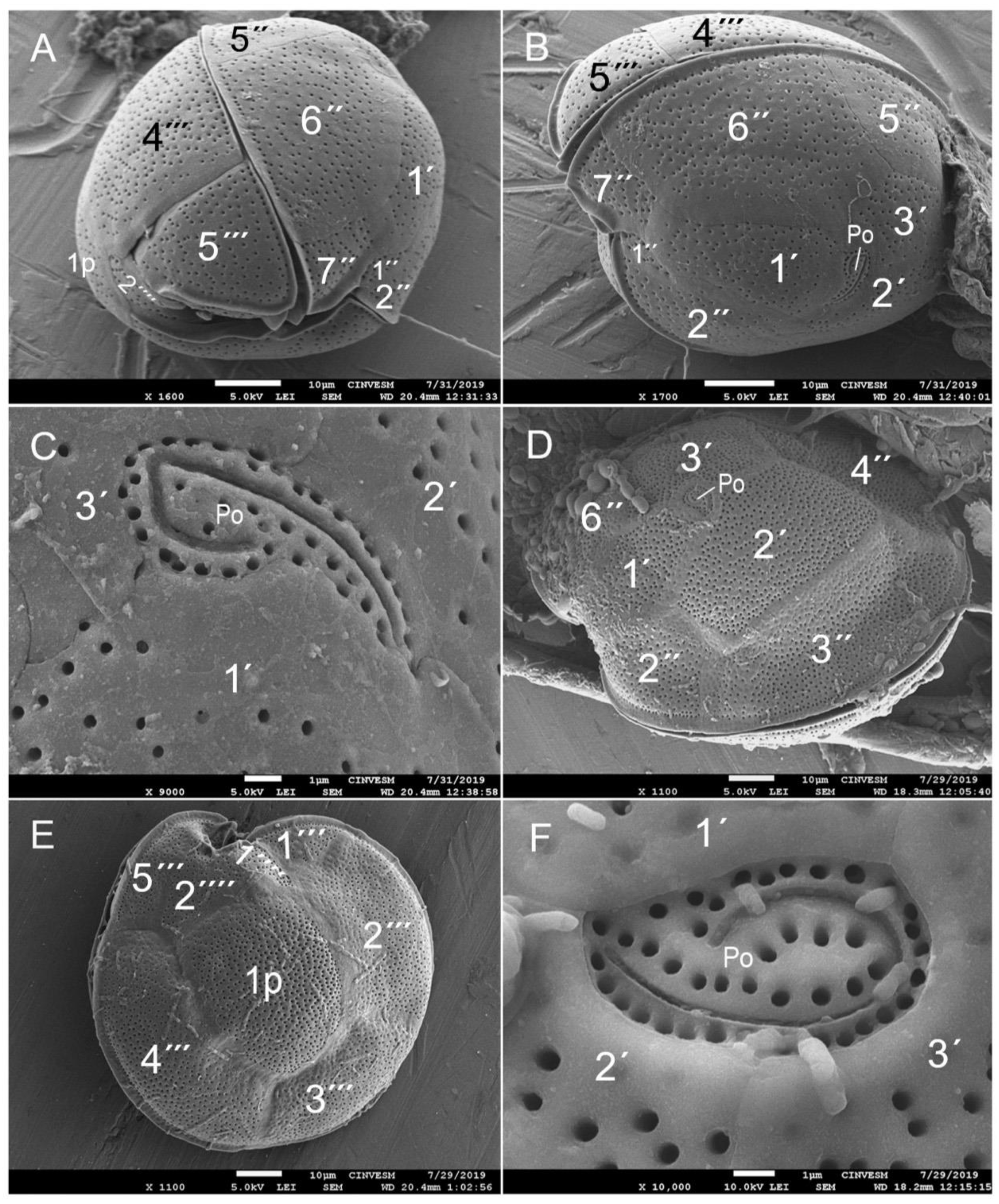
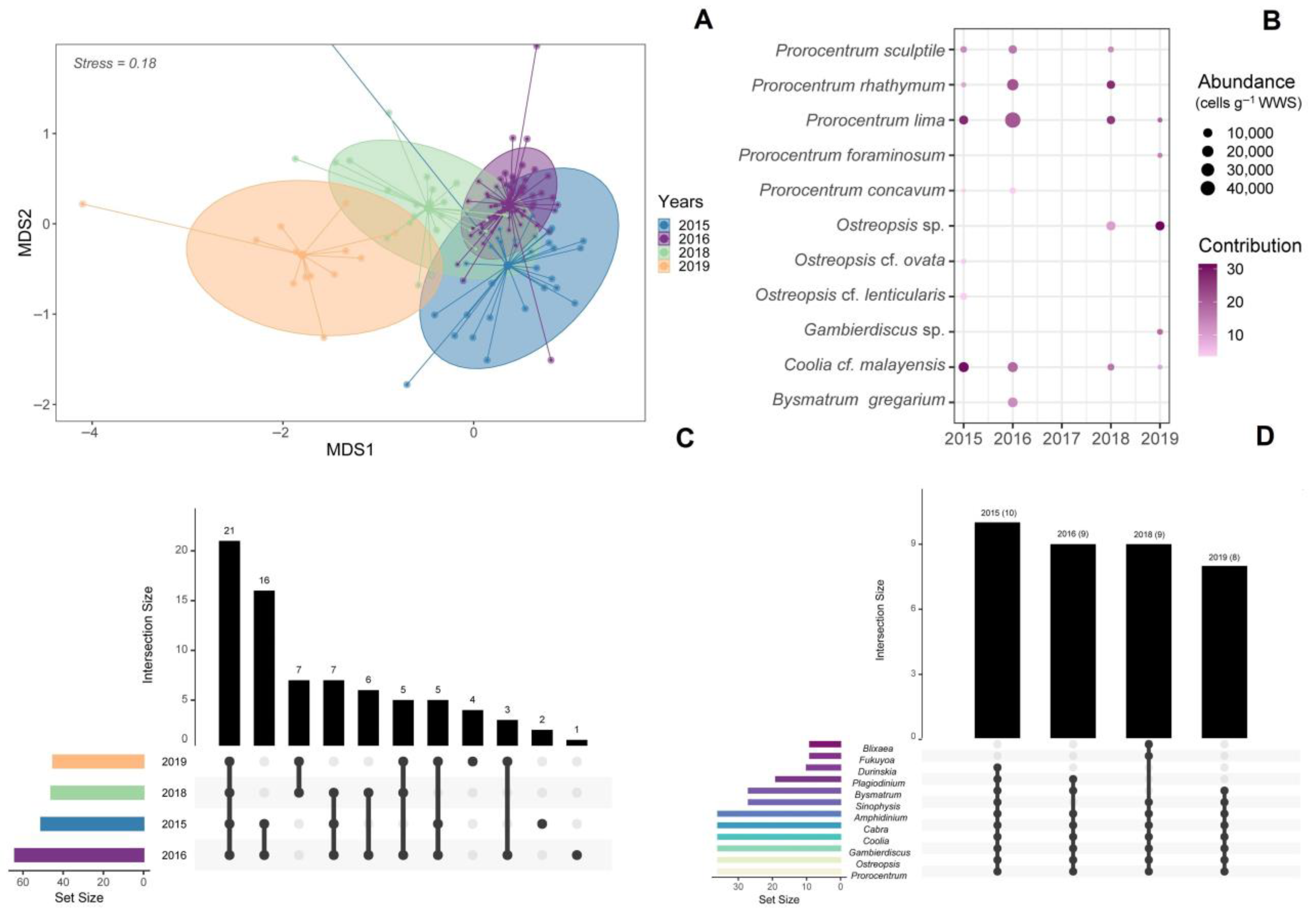
3.2. Species Composition of Dinoflagellates
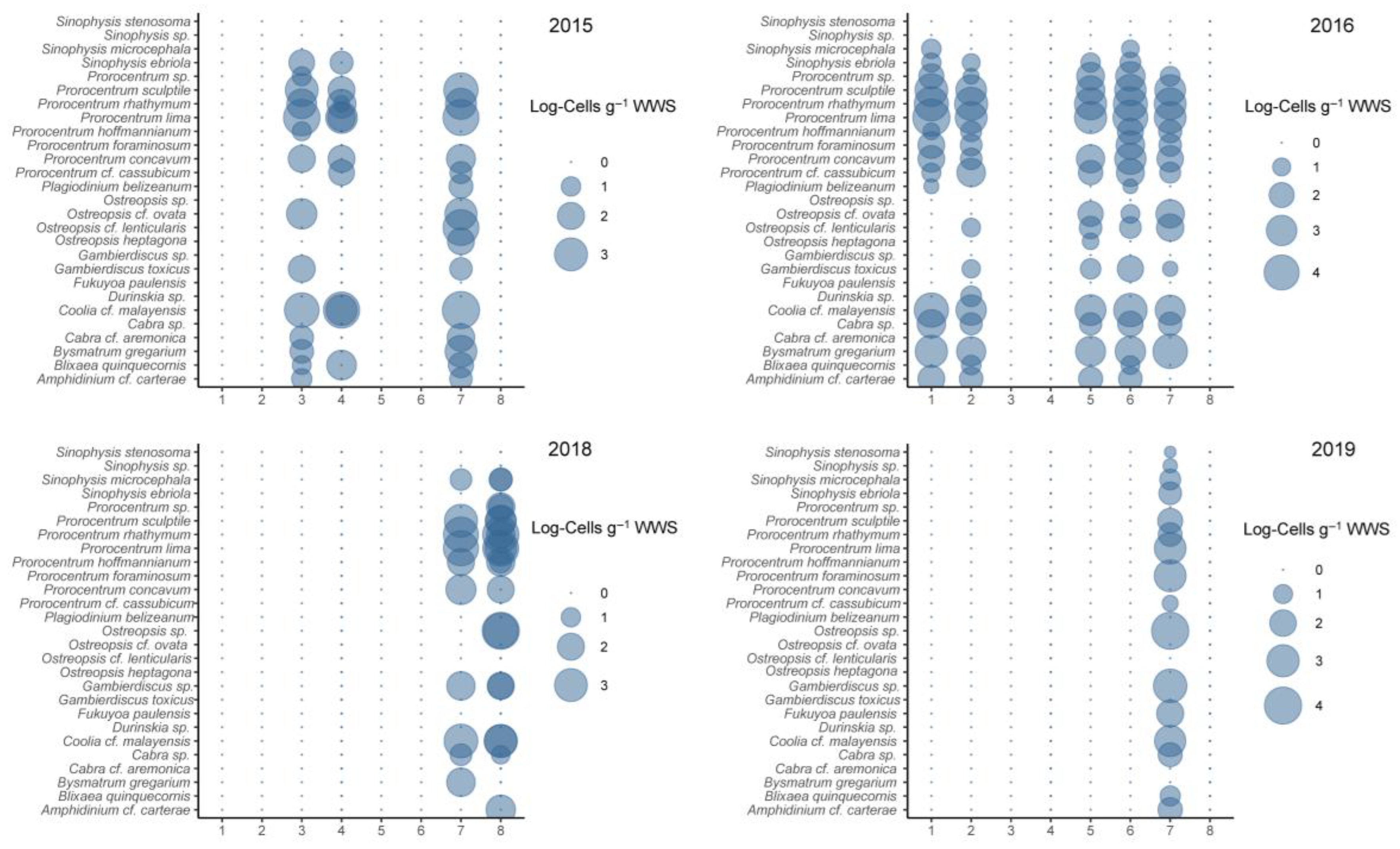
3.3. Abundance of Dinoflagellates
3.4. Interannual and Spatial Changes in Cell Abundance of Dinoflagellates
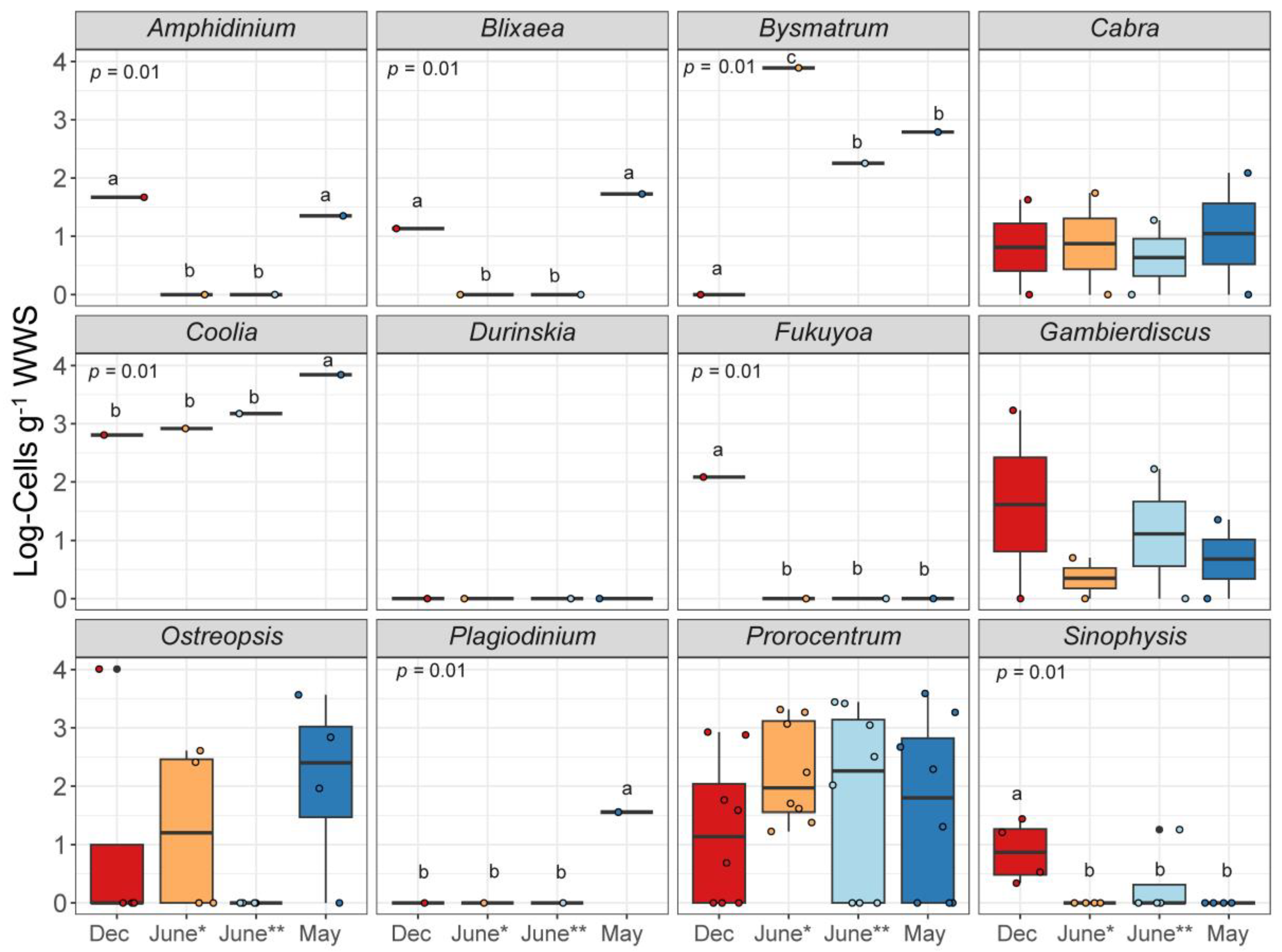
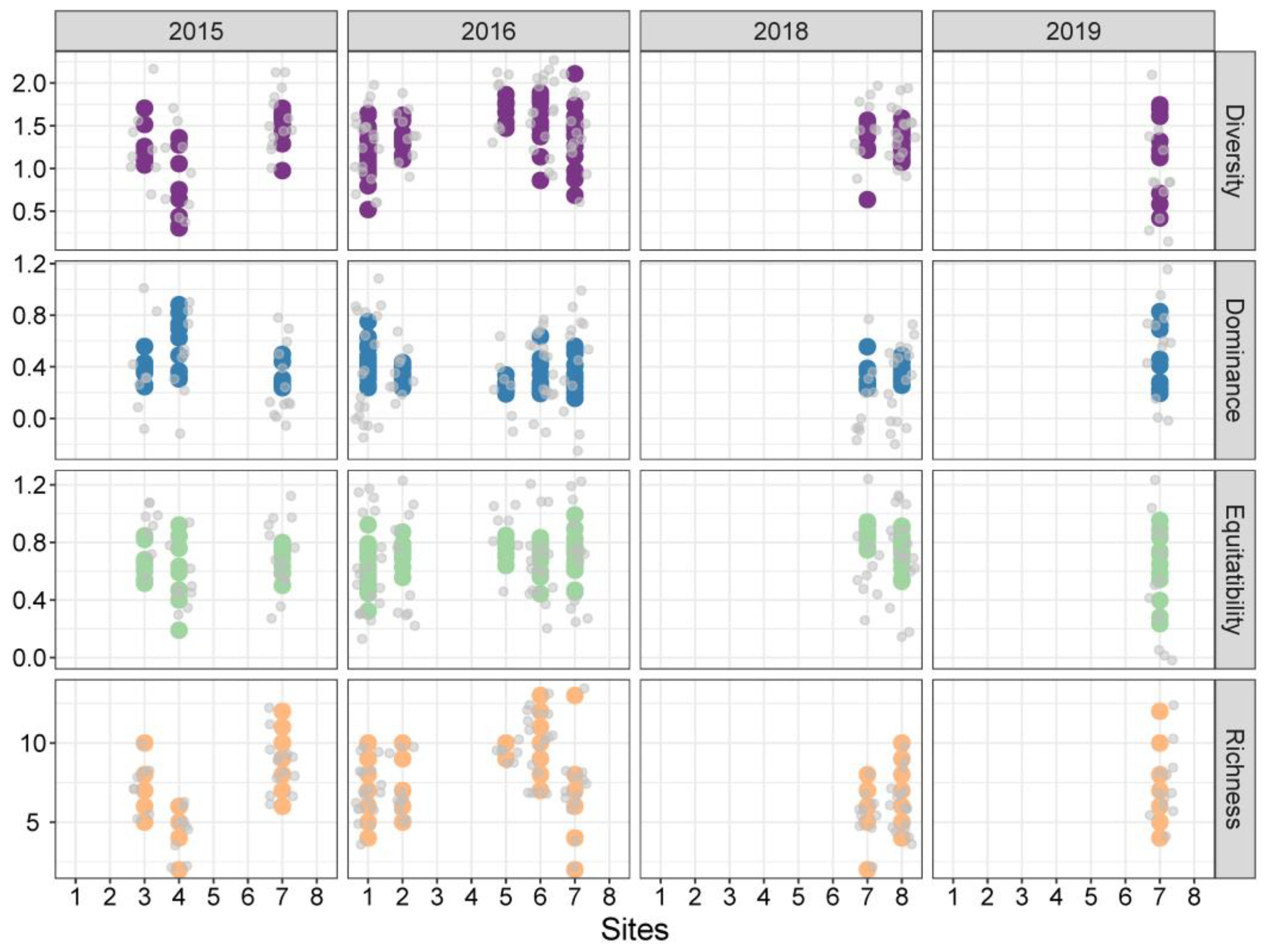
3.5. Seasonal Variation in Epibenthic Dinoflagellates
3.6. Alpha and Beta Diversity of Epiphytic Dinoflagellates
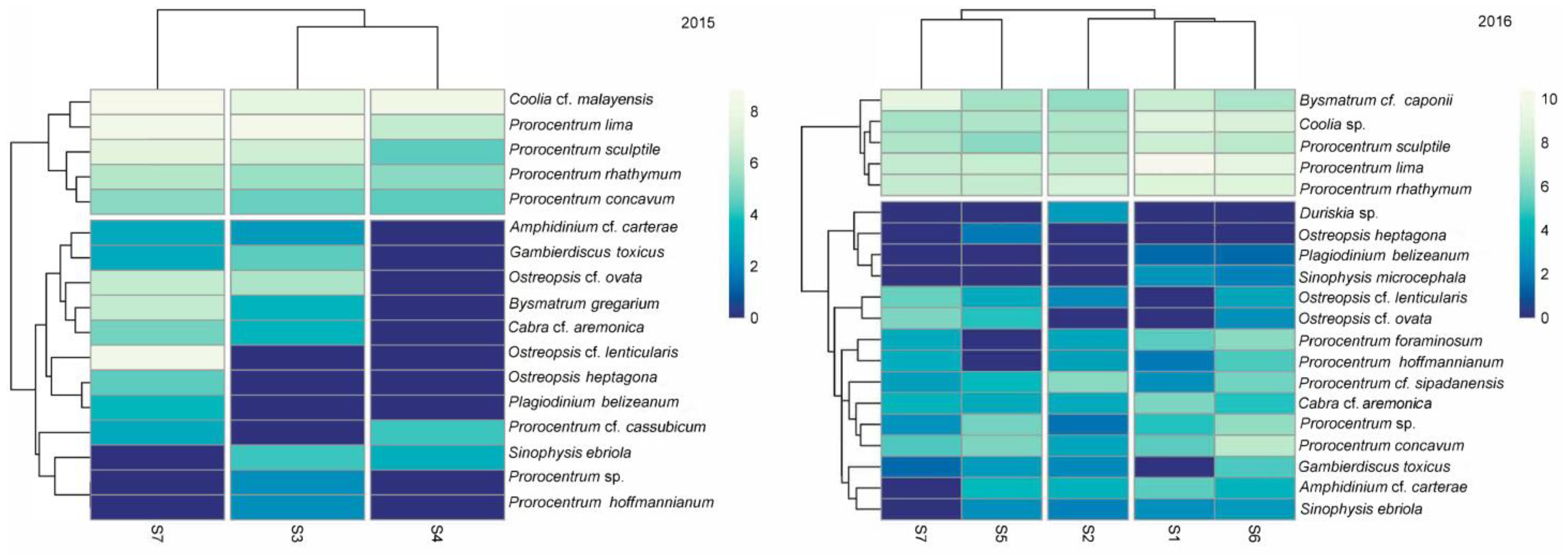
3.7. Dinoflagellate-Macroalgae Associations
4. Discussion
4.1. Dinoflagellate-Macroalgae Associations
4.2. Potentially Toxic Dinoflagellates
4.3. Areas with the Highest Potential Toxic Impact
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HABs | harmful algal blooms |
| CFP | ciguatera fish poisoning |
| BHABs | benthic harmful algal blooms |
| DSP | diarrhetic shellfish poisoning |
| HPLC | high-performance liquid chromatography |
| Ifremer | Institut Français de Recherche pour l’Exploitation de la Mer |
| WWS | wet weight substrate |
| FESEM | field emission scanning electron microscope |
| PERMANOVA | permutational multivariate analysis of variance |
| NMDS | non-metric multidimensional scaling |
| ANOSIM | analysis of similarities |
| SIMPER | similarity percentage analysis |
| UpSetR | a package for visualization of set intersections |
| CINVESTAV-IPN | Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional |
| FOMIX | Fondos Mixtos |
| CONACyT | Consejo Nacional de Ciencia y Tecnología (Mexico) |
| COFAA | Comisión de Operación y Fomento de Actividades Académicas del IPN |
References
- Anderson, D.M. Toxic algal bloom and red tides: A global perspective. In Red tides: Biology, Environmental Science and Technology; Okaichi, T., Anderson, D.M., Nemoto, T., Eds.; Elsevier: New York, NY, USA, 1989; pp. 11–16. [Google Scholar]
- Anderson, D.M. HABs in a changing world: A perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climatic and environental change. In Harmful Algae 2012: Proceedings of the 15th International Conference on Harmful Algae; Kim, H.-G., Reguera, B., Hallegraeff, G.M., Eds.; International Scociety for the Study of Harmful Algae, and IOC of UNESCO: Paris, France, 2014; pp. 3–17. [Google Scholar]
- Smayda, T.J. Novel and nuisance phytoplankton booms in the sea: Evidence for a global epidemic. In Toxic Marine Phytoplankton; Graneli, E., Sundström, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 29–40. [Google Scholar]
- Hallegraeff, G. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Dechraoui Bottein, M.-Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms in attributable to intensified monitoring bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef] [PubMed]
- Hoppenrath, M.; Chomérat, N.; Horiguchi, T.; Murray, S.A.; Rhodes, L. Marine Benthic Dinoflagellates—Their Relevance for Science and Society, 2nd ed.; Senckenberg Gesellschaft für Naturforschung: Frankfurt am Main, Germany, 2023. [Google Scholar]
- Joniver, C.F.H.; Photiades, A.; Moore, P.J.; Winters, A.L.; Woolmer, A.; Adams, J.M.M. The global problem of nuisance macroalgal blooms and pathways to its use in the circular economy. Algal Res. 2021, 58, 102407. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Hoppenrath, M.; Saldarriaga, J.F. Dinoflagellate diversity and distribution. Biodivers. Conserv. 2008, 17, 407–418. [Google Scholar] [CrossRef]
- Hoppenrath, M. Dinoflagellate taxonomy—A review and proposal of a revised classification. Mar. Biodivers. 2017, 47, 381–403. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Durán-Riveroll, L.M.; Band-Schmidt, C.J.; Leyva-Valencia, I.; Gárate-Lizárraga, I.; Cembella, A.D. A review on marine benthic dinoflagellates in Mexico. Hidrobiológica 2022, 32, 183–210. [Google Scholar] [CrossRef]
- Mafra, L.L., Jr.; Sunesen, I.; Pires, E.; Nascimento, S.M.; Álvarez, G.; Mancera-Pineda, J.E.; Torres, G.; Carnicer, O.; Huamaní-Galindo, J.A.; Sánchez-Ramirez, S.; et al. Benthic harmful microalgae and their impacts in South America. Harmful Algae 2023, 127, 102478. [Google Scholar] [CrossRef]
- Alabarces Álvarez, E.; Klemm, K.; Hoppenrath, M.; Cembella, A.; John, U.; Karlton, B. Temporal and spatial distribution of epibenthic dinoflagellates in the Kattegat-Skagerrak, NE Atlantic—Focus on Prorocentrum lima and Coolia monotis. Harmful Algae 2002, 118, 102312. [Google Scholar] [CrossRef]
- Piñón-Gimate, A.; Chávez-Sánchez, T.; Mazariegos-Villarreal, A.; Balart, E.F.; Serviere-Zaragoza, E. Species richness and composition of macroalgal assemblages of a disturbed coral reef in the Gulf of California, Mexico. Acta Bot. Mex. 2020, 127, e1653. [Google Scholar] [CrossRef]
- Hernández-Castro, J.E. Dinoflagelados y Toxinas Lipofílicas en Bancos Naturales de Bivalvos al sur de la Bahía de la Paz, B.C.S., México. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico, 2017. [Google Scholar]
- Sepúlveda-Villarraga, M. Dinoflagelados Potencialmente Tóxicos Asociados a Macroalgas en la Bahía de la Paz. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico, 2017. [Google Scholar]
- Herrera-Herrera, N.V. Interacciones Alelopáticas de Dinoflagelados Epibentónicos de los Géneros Amphidinium y Coolia (Dinophyceae) en Condiciones de Cultivo. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico, 2022. [Google Scholar]
- Ramos-Santiago, A.E. Taxonomía Morfológica y Molecular de Dinoflagelados Epibentónicos del Golfo de California. Master’s Thesis, Instituto Politécnico Nacional, Centro Interdisciplinario de Ciencias Marinas, La Paz, Mexico, 2023. [Google Scholar]
- Espinoza-Espíritu, V.A. Morfología, Filogenia Molecular y Toxicología de Sepas del Género Ostreopsis (Dinophyceae) Aisladas de la Región Central y Sur Oeste del Golfo de California. Master’s Thesis, Centro de Investigaciones Biológicas del Noroeste, S.C., La Paz, Mexico, 2024. [Google Scholar]
- Núñez-Vásquez, E.J.; Almazán-Becerril, A.; López-Cortés, D.J.; Heredia-Tapia, A.; Hernández-Sandoval, F.E.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Gárate-Lizárraga, I.; García-Mendoza, E.; Salinas-Zavala, C.A.; et al. Ciguatera in Mexico (1984–2013). Mar. Drugs 2019, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Heredia-Tapia, A.; Arredondo-Vega, B.O.; Núñez-Vásquez, E.J.; Yasumoto, T.; Yasuda, M.; Ochoa, J.L. Isolation of Prorocentrum lima (Syn. Exuviaella lima) and diarrhetic shellfish poisoning (DSP) risk assessment in the Gulf of California, Mexico. Toxicon 2002, 40, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Gárate-Lizárraga, I.; Muñetón-Gómez, M.S. Bloom of Peridinium quinquecorne Abé in la Ensenada de la Paz, Gulf of California (July 2003). Acta Bot. Mex. 2008, 83, 33–47. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; González-Armas, R.; Verdugo-Díaz, G.; Okolodkov, Y.B.; Pérez-Cruz, B.; Díaz-Ortíz, J.A. Seasonality of the dinoflagellate Amphidinium cf. carterae (Dinophyceae: Amphidiniales) in Bahía de La Paz, Gulf of California. Mar. Pollut. Bull. 2019, 146, 532–541. [Google Scholar] [CrossRef]
- Gómez-Valdés, J.G.; Delgado, J.A.; Dworak, J.A. Overtides, compound tides, and tidal residual current in Ensenada de la Paz lagoon, Baja California Sur. Mexico. Geofís. Int. 2003, 42, 623–634. [Google Scholar] [CrossRef]
- Martínez-López, A.; Cervantes-Duarte, R.; Reyes-Salinas, A.; Valdez-Holguín, J.E. Cambio estacional de clorofila a en la Bahía de La Paz, B.C.S., México. Hidrobiológica 2001, 11, 45–52. [Google Scholar]
- Cervantes-Duarte, R.; Aguirre-Bahena, F.; Reyes-Salinas, A.; Valdez-Holguín, J.E. Caracterización hidrológica de una laguna costera de Baja California Sur, México. Oceánides 2001, 16, 93–105. [Google Scholar]
- Urcádiz-Cásarez, F.J.; Cruz-Escalona, V.H.; Nava-Sánchez, E.H.; Ortega-Rubio, A. Clasificación de unidades del fondo marino a partir de la distribución espacial de los sedimentos superficiales de la Bahía de La Paz, Golfo de California. Hidrobiológica 2017, 17, 399–409. [Google Scholar]
- Ocean Virtual Laboratory. Available online: https://ovl.oceandatalab.com/ (accessed on 15 July 2025).
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Chomérat, N.; Couté, A.; Nézan, E. Further investigations on the sand-dwelling genus Cabra (Dinophyceae, Peridiniales) in South Brittany (northwestern France), including the description of C. aremonica sp. nov. Mar. Biodiv. 2010, 40, 131–142. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Chomérat, N.; Horiguchi, T.; Schweikert, M.; Nagahaama, Y.; Murray, S. Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—A proposal and review. Harmful Algae 2013, 27, 1–28. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2025; Available online: https://www.algaebase.org (accessed on 15 July 2025).
- Fritz, L.; Triemer, R.E. A rapid simple technique utilizing Calcofluor White M2R for the visualization of dinoflagellate thecal plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Lebour, M.V. The Dinoflagellates of Northern Seas; The Marine Biological Association of the United Kingdom: Plymouth, UK, 1925. [Google Scholar]
- Taylor, F.J.R. Dinoflagellates. Identification problems. Some specific preparations, Chapter 6. In Phytoplankton Manual. Monographs on Oceanographic Methodology; Sournia, A., Ed.; UNESCO: Paris, France, 1978; pp. 143–147. [Google Scholar]
- Abbot, I.A.; Hollenberg, G.J. Marine Algae of California; Stanford University Press: Stanford, CA, USA, 1992. [Google Scholar]
- Littler, D.S.; Littler, M.M. Caribbean Reef Plants. An Identification Guide to the Reef Plants of the Caribbean, Bahamas, Florida and Gulf of Mexico; Offshore Graphics, Inc.: Washington, DC, USA, 2000. [Google Scholar]
- Norris, J.N. Marine Algae of the Northern Gulf of California: Chlorophyta and Phaeophyceae; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2010; Volume 94, pp. 1–274. [Google Scholar] [CrossRef]
- Norris, J.N. Marine Algae of the Northern Gulf of California II: Rhodophyta; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2014; Volume 96, pp. 1–555. [Google Scholar] [CrossRef]
- Wynn, M.J. A Checklist of Benthic Marine Algae of the Tropical and Subtropical Western Atlantic, 4th ed.; Nova Hedwigia. Beiheft, 145; Borntraeger Science Publishers: Stuttgart, Germany, 2017; Volume 145, pp. 1–203. [Google Scholar]
- Posit Team. RStudio: Integrated Development Environment for R; Posit Software, PBC: Boston, MA, USA, 2023; Available online: http://www.posit.co/ (accessed on 3 February 2025).
- Anderson, M.J. Permutational multivariate analysis of variance (PERMANOVA). In Wiley StatRref: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–15. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology. Developments in Environmental Modeling, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24. [Google Scholar]
- Turki, S. Distribution of toxic dinoflagellates along the leaves of seagrass Posidonia oceanica and Cymodocea nodosa from the Gulf of Tunis. Cah. Biol. Mar. 2005, 46, 29–34. [Google Scholar]
- Boisnoir, A.; Pascal, P.-Y.; Cordonnier, S.; Lemée, R. Depth distribution of benthic dinoflagellates. J. Sea Res. 2018, 135, 74–83. [Google Scholar] [CrossRef]
- Yasumoto, T.; Inoue, A.; Bagnis, R. Ecological survey of a toxic dinoflagellate associated with ciguatera. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H., Eds.; Elsevier/North-Holland: New York, NY, USA, 1979; pp. 221–224. [Google Scholar]
- Withers, N.W. Toxin production, nutrition, and distribution of Gambierdiscus toxicus (Hawaiian strain). In Proceedings of the Fourth International Coral Reef Symposium, Manila, Philippines, 18–22 May 1981. [Google Scholar]
- Shimizu, Y.; Shimizu, H.; Scheuer, P.; Hokama, Y.; Oyama, O.; Miyahara, J.T. Gambierdiscus toxicus, a ciguatera causing dinoflagellate from Hawaii. Bull. Jpn. Soc. Sci. Fish 1982, 48, 811–813. [Google Scholar] [CrossRef]
- Mustapa, N.I.; Yong, H.L.; Lee, L.K.; Lim, Z.F.; Lim, H.C.; Teng, S.; Luo, Z.; Gu, H.; Leaw, C.P.; Lim, P.T. Growth and epiphytic behavior of three Gambierdiscus species (Dinophyceae) associated with various macroalgal substrates. Harmful Algae 2019, 89, 101671. [Google Scholar] [CrossRef]
- Parsons, M.L.; Aligizaki, K.; Dechraoui Bottein, M.-Y.; Fraga, S.; Morton, S.L.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Bomber, J.W.; Rubio, M.G.; Norris, D.R. Epiphytism of dinoflagellates associated with the disease ciguatera: Substrate specificity and nutrition. Phycologia 1989, 28, 360–368. [Google Scholar] [CrossRef]
- Mitchell, L.E. Ecological Studies of Benthic Dinoflagellates Associated with Ciguatera in the Florida Keys: The 0-38 Micrometer Size Fraction. Master’s Thesis, Florida Institute of Technology, Melbourne, FL, USA, 1985. [Google Scholar]
- Bomber, J.W.; Norris, D.R.; Mitchell, L.E. Benthic dinoflagellates associated with ciguatera from the Florida Keys. II. Temporal, spatial and substrate heterogeneity of Prorocentrum lima. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier Science: New York, NY, USA, 1989; pp. 45–50. [Google Scholar]
- Ichinotsubo, D.; Asahina, A.Y.; Titus, E.; Chun, S.; Hong, T.W.; Shirai, J.L.; Hokama, Y. Survey for ciguatera fish poisoning in west Hawaii. Mem. Queensl. Mus. 1994, 34, 513–522. [Google Scholar]
- Morton, S.L.; Faust, M. Survey of toxic epiphytic dinoflagellates from the Belizean barrier reef ecosystem. Bull. Mar. Sci. 1997, 61, 899–906. [Google Scholar]
- Vila, M.; Garcés, E.; Masó, M. Potentially toxic epiphytic dinoflagellate assemblages on macroalgae in the NW Mediterranean. Aquat. Microb. Ecol. 2001, 26, 51–60. [Google Scholar] [CrossRef]
- Delgado, G.; Lechuga-Devéze, C.H.; Popowski, G.; Troccoli, L.; Salinas, C.A. Epiphytic dinoflagellates associated with ciguatera in the northwestern coast of Cuba. Rev. Biol. Trop. (Int. J. Trop. Biol.) 2006, 54, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Noor, N.; Daugbjerg, N.; Moestrup, Ø.; Anton, A. Marine epibenthic dinoflagellates from Malaysia—A study of live cultures and preserved samples based on light and scanning electron microscopy. Nordic J. Bot. 2007, 24, 629–690. [Google Scholar] [CrossRef]
- Parsons, M.L.; Preskitt, L.B. A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai‘i. Harmful Algae 2007, 6, 658–669. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Campos-Bautista, G.; Gárate-Lizárraga, I.; González-González, J.A.G.; Hoppenrath, M.; Arenas, V. Seasonal changes of benthic and epiphytic dinoflagellates in the Veracruz reef zone, Gulf of Mexico. Aquat. Microb. Ecol. 2007, 47, 223–237. [Google Scholar] [CrossRef]
- Aguilar-Trujillo, A.C.; Okolodkov, Y.B.; Herrera-Silveira, J.A.; Merino-Virgilio, F.d.C.; Galicia-García, C. Taxocoenosis of epibenthic dinoflagellates in the coastal waters of the northern Yucatan Peninsula before and after the harmful algal bloom event in 2011–2012. Mar. Pollut. Bull. 2017, 119, 396–406. [Google Scholar] [CrossRef]
- Kim, H.S.; Yih, W.; Kim, J.H.; Myung, G.; Heong, H.J. Abundance of epiphytic dinoflagellates from coastal waters off Jeju Island, Korea during autumn 2009. Ocean Sci. J. 2011, 46, 205–209. [Google Scholar] [CrossRef][Green Version]
- Selina, M.S.; Morozova, T.V.; Vyshkvartsev, D.I.; Orlova, T.Y. Seasonal dynamics and spatial distribution of epiphytic dinoflagellates in Peter the Great Bay (Sea of Japan) with special emphasis on Ostreopsis species. Harmful Algae 2014, 32, 1–10. [Google Scholar] [CrossRef]
- Almazán-Becerril, A.; Escobar-Morales, S.; Rosiles-González, G.; Valadez, F. Benthic epiphytic dinoflagellates from the northern portion of the Mesoamerican Reef System. Bot. Mar. 2015, 58, 115–128. [Google Scholar] [CrossRef]
- Hachani, M.A.; Dhib, A.; Fathalli, A.; Ziadi, B.; Turki, S.; Aleya, L. Harmful epiphytic dinoflagellate assemblages on macrophytes in the Gulf of Tunis. Harmful Algae 2018, 77, 29–42. [Google Scholar] [CrossRef]
- Irola-Sansores, E.D.; Delgado-Pech, B.; García-Mendoza, E.; Núñez-Vázquez, E.J.; Olivos-Ortiz, A.; Almazán-Becerril, A. Population dynamics of benthic-epiphytic dinoflagellates on two macroalgae from coral reef systems of the northern Mexican Caribbean. Front. Mar. Sci. 2018, 5, 487. [Google Scholar] [CrossRef]
- Yong, H.L.; Mustapa, N.I.; Lee, L.K.; Lim, Z.F.; Tan, T.H.; Usup, G.; Gu, H.; Litaker, R.W.; Tester, P.A.; Lim, P.T.; et al. Habitat complexity affects benthic harmful dinoflagellate assemblages in the fringing reef of Rawa Island, Malaysia. Harmful Algae 2018, 78, 56–68. [Google Scholar] [CrossRef]
- Boisnoir, A.; Pascal, P.-Y.; Cordonnier, S.; Lemée, R. Spatio-temporal dynamics and biotic substrate preferences of benthic dinoflagellates in the Lesser Antilles, Caribbean Sea. Harmful Algae 2019, 81, 18–29. [Google Scholar] [CrossRef]
- Boisnoir, A.; Pascal, P.-Y.; Marro, S.; Lemée, R. First spatial distribution of potentially toxic benthic dinoflagellates in the Lesser Antilles (Guadeloupe and Martinique), Caribbean Sea. Bot. Mar. 2019, 62, 309–322. [Google Scholar] [CrossRef]
- Lee, L.K.; Lim, Z.F.; Gu, H.; Chan, L.L.; Litaker, R.W.; Tester, P.A.; Leaw, C.P.; Lim, P.T. Effects of substratum and depth on benthic harmful dinoflagellate assemblages. Sci. Rep. 2020, 10, 11251. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.S.; Jeong, H.J. Benthic dinoflagellates in Korean waters. Algae 2021, 36, 91–109. [Google Scholar] [CrossRef]
- Tang, J.-Q.; Shen, Q.-H.; Han, Y.-Y.; Wu, Y.; He, X.-F.; Li, D.-W.; Huang, Y. Analysis of research status and trends on marine benthic dinoflagellate toxins: A bibliometric study based on web of science database and VOSviewer. Environ. Res. 2023, 238, 117179. [Google Scholar] [CrossRef]
- Lundholm, N.; Bernard, C.; Churro, C.; Escalera, L.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Murray, S. (Eds.) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae; World Register of Marine Species: Oostende, Belgium, 2009. [Google Scholar] [CrossRef]
- Totti, C.; Accoroni, S.; Cerino, F.; Cucchiari, E.; Romagnoli, T. Ostreopsis ovata bloom along the Conero Riviera (northern Adriatic Sea): Relationships with environmental conditions and substrata. Harmful Algae 2010, 9, 233–239. [Google Scholar] [CrossRef]
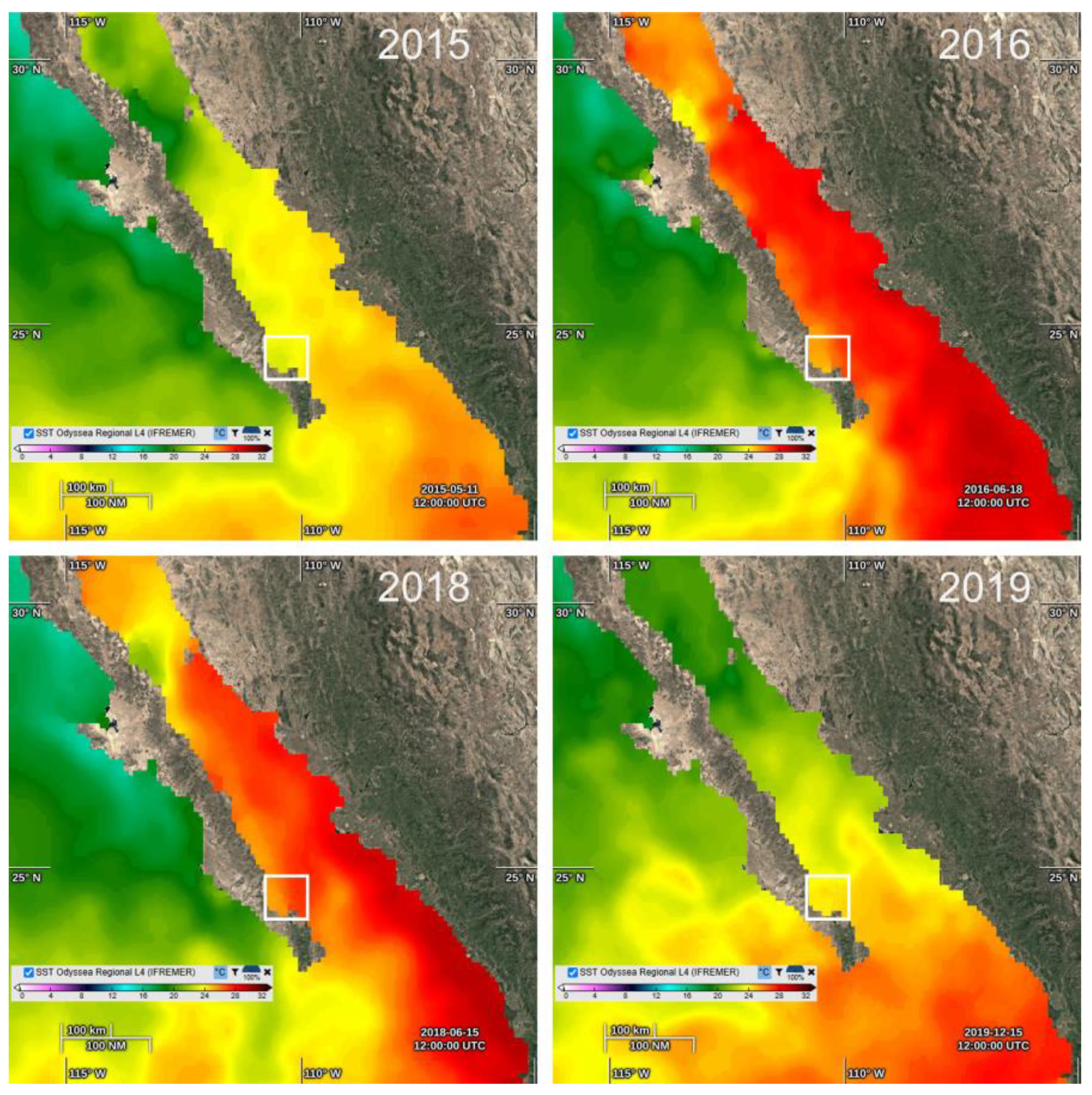
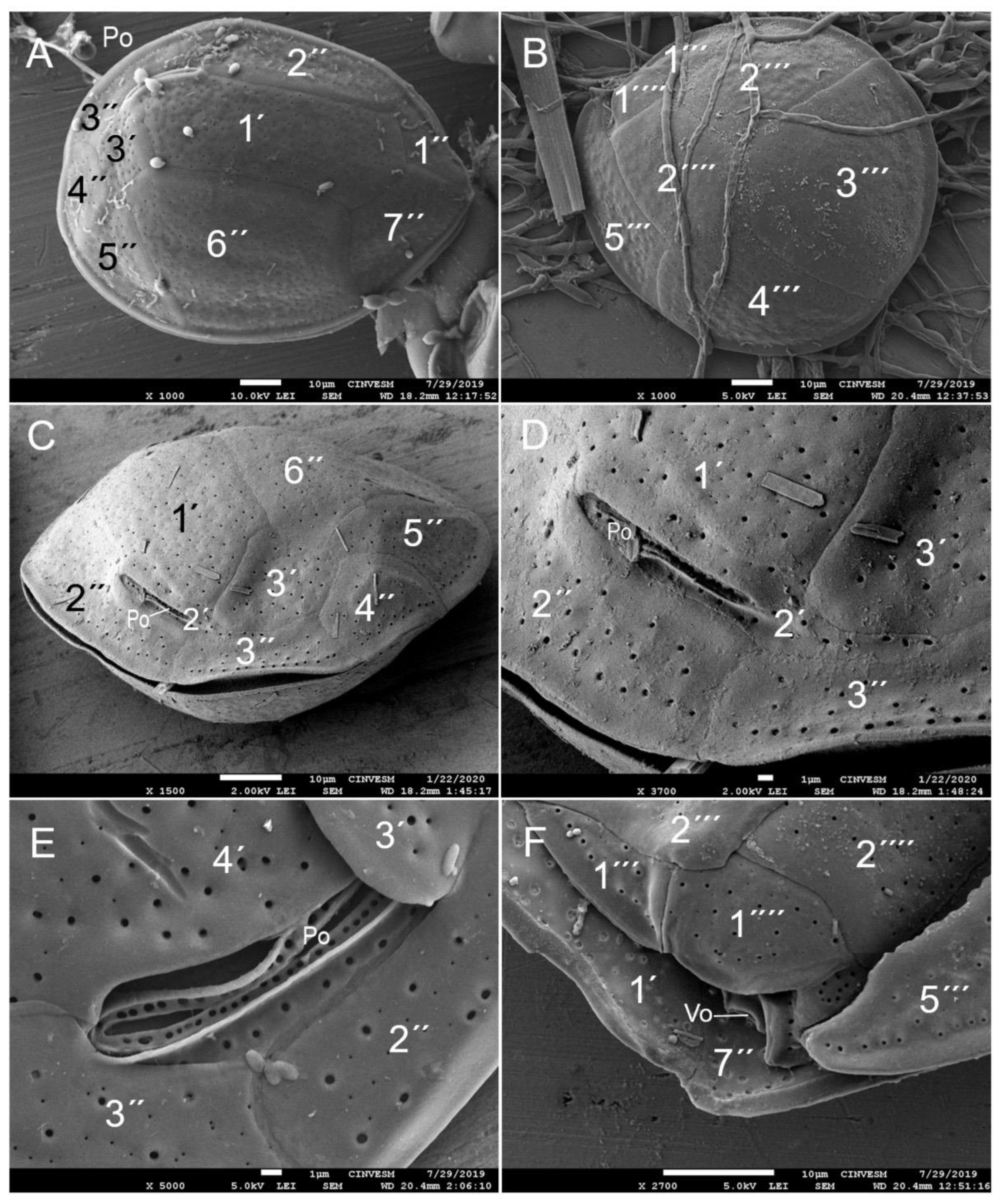
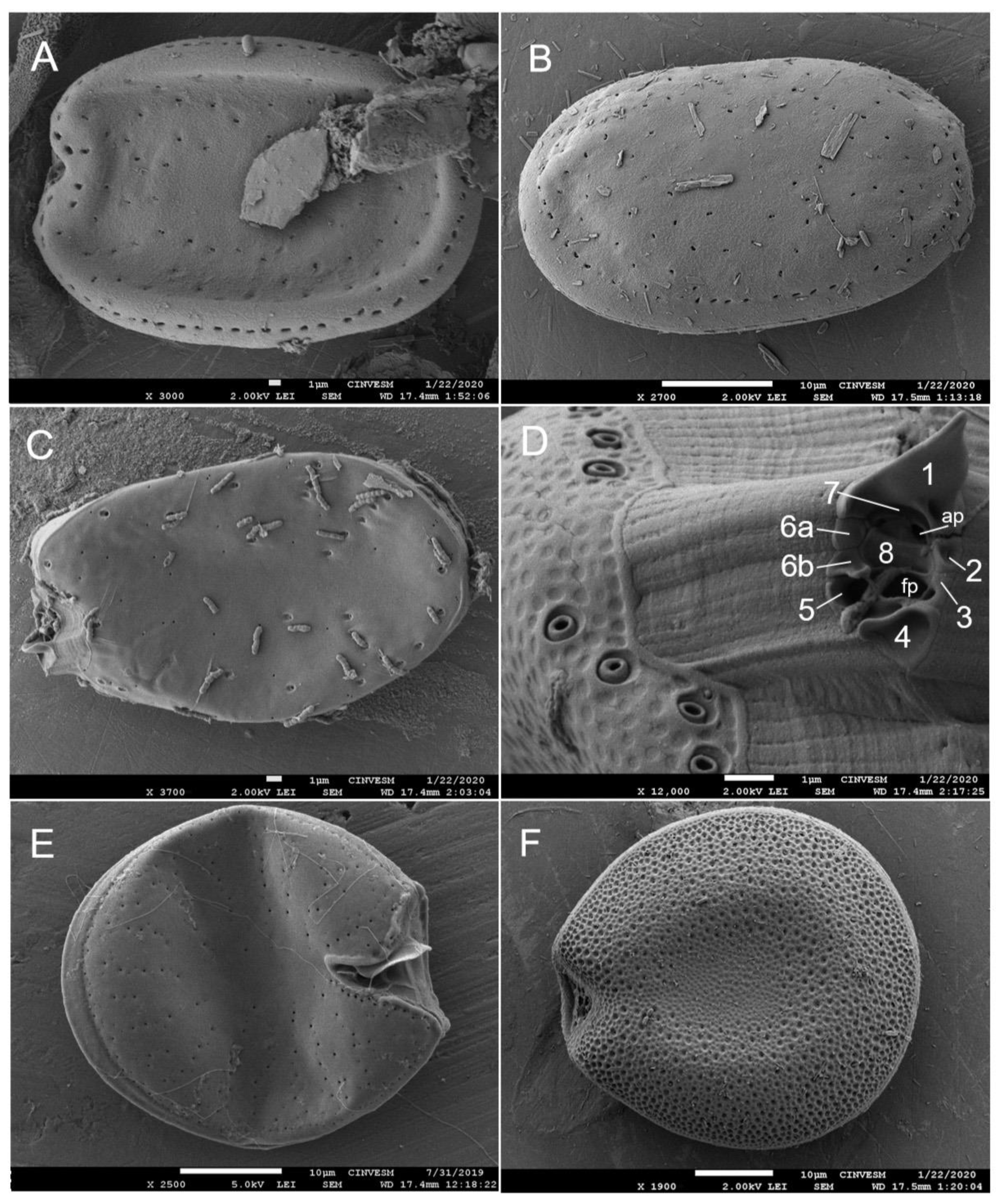
| Taxa | May 2015 | June 2016 | June 2018 | December 2019 |
|---|---|---|---|---|
| Rhodophyta | ||||
| Acanthophora spicifera (Vahl) Børgesen | 3, 4b | 1, 2, 6a,b, 7 | 7 | |
| Amphiroa cf. vanbosseae Me. Limoine | 7 | |||
| Amphiroa sp. | 7 | 8 | 7 | |
| Asparagopsis sp. | 8 | |||
| Bostrychia radicans (Montagne) Montagne | 4a * | |||
| Callithamnion sp. | 7 | |||
| Centroceras sp. | 4a | |||
| Ceramium sp. | 2, 7 | 7 | 7 | |
| Champia sp. | 7 | |||
| Corallinaceae gen. sp. | 4b, 7 | |||
| Crouania sp. | 7 | |||
| Digenea simplex (Wulfen) C. Agardh | 5, 8 | 8 | ||
| Ganonema cf. farinosum (J.V. Lamouroux) | ||||
| K.-C. Fan et Y.-C. Wang | 7 | |||
| Gayliella sp. | 2 | |||
| Gelidiella sp. | 8 | |||
| Gelidium sp. | 6a | |||
| Gigartinaceae gen. sp. | 8 | |||
| Gracilaria sp. | 2 | 8 | ||
| (?)Gracilariopsis sp. | 7 | |||
| Herposiphonia cf. littoralis Hollenberg | 7 | |||
| Herposiphonia sp. | 7 | |||
| Hypnea valentiae (Turner) Montagne | 1, 2, 6b, 7 | |||
| Hypnea sp. | 3 | 5, 8 | 8 | 7 |
| Laurencia cf. masonii Setchell et N.L. Gardner | 2 | |||
| Laurencia spp. | 3, 7 | 1, 2, 5, 6a,b, 7, 8 | 7 | 7 |
| Lithophyllum sp. | 7 | |||
| Lomentaria sp. | 4b | |||
| Neosiphonia sp. | 3 | 1 | 7, 8 | 7 |
| (?)Pterocladia sp. | 7 | |||
| Spyridia filamentosa (Wulfen) Harvey | 3, 7 | 2, 6b, 7 | ||
| Spyridia sp. | 4a,b, 7 | 2, 5, 6a, 8 | 7 | 7 |
| Wrangelia sp. | 7 | |||
| Yuzurua sp. | 6a | |||
| Phaeophyceae | ||||
| Chnoospora sp. | 8 | |||
| Colpomenia sp. | 7 | |||
| Dictyopteris sp. | 7 | |||
| Dictyota spp. | 7 | 1, 2, 7 | 8 | 7 |
| Hydroclathrus clathratus (C. Agardh) M. Howe | 1, 2, 6b | |||
| Hydroclathrus sp. | 7 | |||
| Myrionema sp. | 1, 2 ** | |||
| Padina mexicana E.Y. Dawson var. erecta | ||||
| Avila-Ortíz | 1 | 5 | ||
| Padina spp. | 3, 7 | 2, 8 | 7, 8 | |
| Sargassum agardhianum Farlow | 1, 2, 7 | |||
| Sargassum sp. | 3, 7 | 1, 5, 8 | 7, 8 | 7 |
| Sphacelaria furcigera Kützing | 1, 2 *** | |||
| (?)Taonia sp. | 1 | |||
| Chlorophyta | ||||
| Caulerpa racemosa (Forsskål) J. Agardh | 5, 8 | 8 | ||
| Caulerpa sertularioides (S.G. Gmelin) R. Howe | 2 | 8 | ||
| Chaetomorpha cf. californica Collins | 7 | |||
| Cladophora cf. stimpsonii Harvey | 7 | |||
| Cladophora sp. | 8 | 7 | ||
| Codium cuneatum (Setchell) N.L. Gardner | 1, 2, 6b | |||
| Codium sp. | 3, 4b, 7 | 6a,b, 7 | 7, 8 | |
| Halimeda sp. | 3, 7 | 6a, 7 | 7 | 7 |
| Ulva clathrata (Roth) C. Agardh | 6b | |||
| Ulva intestinalis Linnaeus | 7 | |||
| Ulva sp.1 (laminar) | 5, 6b, 7, 8 | 7, 8 | 7 | |
| Ulva sp.2 (tubular) | 4b | 6b | 7 | |
| Factor | Pseudo-F | p (Perm) | R2 |
|---|---|---|---|
| Density | |||
| Years | 1.36 | 0.001 | 0.02 |
| Sites | 3.23 | 0.001 | 0.12 |
| Years * Sites | 3.62 | 0.912 | 0.01 |
| Shannon diversity | |||
| Years | 0.45 | 0.508 | 0.003 |
| Sites | 7.60 | 0.007 | 0.05 |
| Years * Sites | 15.78 | 0.001 | 0.11 |
| Richness | |||
| Years | 8.09 | 0.001 | 0.15 |
| Sites | 4.47 | 0.032 | 0.03 |
| Years * Sites | 15.28 | 0.001 | 0.10 |
| Equitability index | |||
| Years | 14.09 | 0.001 | 0.10 |
| Sites | 4.26 | 0.010 | 0.03 |
| Years * Sites | 2.76 | 0.106 | 0.01 |
| Simpson index (λ) | |||
| Years | 4.29 | 0.048 | 0.03 |
| Sites | 6.35 | 0.016 | 0.04 |
| Years * Sites | 11.28 | 0.002 | 0.08 |
| Groups | Global R | Significance Level (%) |
|---|---|---|
| All years | 0.570 | 0.0001 |
| Year 2015 vs. 2016 | 0.463 | 0.0006 |
| Year 2015 vs. 2018 | 0.386 | 0.0006 |
| Year 2015 vs. 2019 | 0.795 | 0.0006 |
| Year 2016 vs. 2018 | 0.485 | 0.0012 |
| Year 2016 vs. 2019 | 0.96 | 0.0006 |
| Year 2018 vs. 2019 | 0.638 | 0.0006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okolodkov, Y.B.; Gárate-Lizárraga, I.; Cervantes-Urieta, V.A.; Martínez-Cruz, M.E.; Galicia-García, C. Epibenthic Dinoflagellates in the Southern Gulf of California: Species Composition and Abundance. Diversity 2025, 17, 674. https://doi.org/10.3390/d17100674
Okolodkov YB, Gárate-Lizárraga I, Cervantes-Urieta VA, Martínez-Cruz ME, Galicia-García C. Epibenthic Dinoflagellates in the Southern Gulf of California: Species Composition and Abundance. Diversity. 2025; 17(10):674. https://doi.org/10.3390/d17100674
Chicago/Turabian StyleOkolodkov, Yuri B., Ismael Gárate-Lizárraga, Victor A. Cervantes-Urieta, Manuel E. Martínez-Cruz, and Citlalli Galicia-García. 2025. "Epibenthic Dinoflagellates in the Southern Gulf of California: Species Composition and Abundance" Diversity 17, no. 10: 674. https://doi.org/10.3390/d17100674
APA StyleOkolodkov, Y. B., Gárate-Lizárraga, I., Cervantes-Urieta, V. A., Martínez-Cruz, M. E., & Galicia-García, C. (2025). Epibenthic Dinoflagellates in the Southern Gulf of California: Species Composition and Abundance. Diversity, 17(10), 674. https://doi.org/10.3390/d17100674










