Abstract
Eyespots are functionally complex and highly variable elements of butterfly wing patterns. The Meadow Brown, Maniola jurtina, is a classic model species studied for variation in eyespots as an index of evolutionary divergence and adaptation. However, the role of fine-scale ecogeographic conditions on eyespot variation remains poorly understood. In this study, we examined hindwing eyespot number, distribution, and combination patterns in male M. jurtina across climatically and topographically diverse north-western Balkans. Compared to the species average, males in this region displayed greater spottiness and phenotypic diversity. While the typical two-spot phenotype was dominant and stable, in some populations, three-spotted and even four-spotted males occurred at similar frequencies. Rare six-spotted individuals were recorded only at mountain localities above 1200 m. Geographic and climatic factors together influenced this variation: higher altitudes and cooler, thermally stable environments promoted increased eyespot number and greater phenotypic plasticity than warmer, more variable environments. This pattern contrasts with large-scale latitudinal trends previously described for the species, emphasizing the importance of local climatic heterogeneity. Our findings suggest the north-western Balkans as a possible transitional zone where environmental complexity promotes elevated eyespot variability, contributing to the understanding of adaptive morphological plasticity in M. jurtina.
1. Introduction
Eyespots are among the most intensively studied elements of butterfly wing patterns and striking examples of morphological diversity. Though their developmental positions are conserved, eyespots exhibit substantial variation in size, number, shape, and arrangement both between and within species [1,2,3]. They are especially conspicuous and abundant in butterflies of the family Nymphalidae, in which they form serially repeated patterns along the anterior–posterior axis of the wings [3]. As visual signals, eyespots mediate predator avoidance and sexual signaling [1,4,5]. Small peripheral eyespots on ventral wing surfaces are thought to direct predator attacks away from vital body parts, increasing survival, whereas large dorsal eyespots function in intimidation or mate choice [1,3,6,7]. Ventral eyespots, more exposed when at rest, tend to be less sexually dimorphic and predominantly subject to natural selection, while dorsal and forewing elements are often more dimorphic and influenced by sexual selection [5,8].
Because of functional complexity and high variability, eyespots are model traits for investigating phenotypic plasticity, adaptation, and evolutionary developmental mechanisms in Lepidoptera [1,2,3]. One of the most frequently studied species of nymphalid butterflies is the Meadow Brown butterfly, Maniola jurtina Linnaeus, 1758. This species has been used in early ecological genetics studies in Europe [9,10,11] due to its extensive variability in eyespot patterns, widespread distribution, abundance, and availability [12]. Variation in the number of small hindwing eyespots in M. jurtina has been used as an index of evolutionary divergence and adaptation [13,14] and became a classic example of genetic polymorphism [10]. The number of the ventral hindwing eyespots in M. jurtina ranges from zero to six, though two is typical. Males tend to be more spotted than females and with less variable spotting. On a large geographic scale, a latitudinal pattern of variability in the number of eyespots, with a clinal increasing gradient from north to south, has been suggested [15]. Stability of wing-spot frequencies has been reported throughout the center of the species range (Western Europe) with greater variability towards the periphery [16]. While wing-spot patterns can remain unchanged for several decades [17], significant temporal changes can appear and have been linked to ecological factors [10,18]. The number of eyespots may vary or remain stable across and/or within seasons [11,19,20,21,22,23]. Such seasonal variation, particularly a decline in spotting, has been recently correlated to higher field temperature during larval/pupal development in females [23]. This thermal plasticity contrasts with the previous belief that the wing-spot polymorphism in M. jurtina is the result of differential survival of genetically predisposed wing-spot phenotypes [13,24,25].
Despite extensive research, the influence of fine-scale ecogeographic conditions on natural variation in eyespot patterns in M. jurtina remains poorly understood. On the one hand, wing spotting can change after environmental alteration [23], and on the other, uniformity in spotting can persist despite environmental heterogeneity [14,26]. Research predominantly comes from the British Isles [13,14,17,21,22,23,26,27,28,29,30], with studies also conducted in Spain [20], Portugal [31], Sweden [18], and Belgium [32]. The north-western part of the Balkan Peninsula remains a major gap in M. jurtina eyespot research, despite its importance as a glacial refugium for one of the two phylogenetic lineages of M. jurtina [33], and as a biodiversity hotspot [34]. Data on M. jurtina eyespot variability in this region remains scarce [35,36]. Moreover, the pronounced climatic heterogeneity and steep elevational gradients in the region provide ideal conditions for investigating how environmental variability drives phenotypic variation in natural populations.
In this study, we examine hindwing eyespot number, distribution, and combination patterns in male M. jurtina across multiple localities in the north-western Balkans. By integrating morphometric and climatic data, our work provides the first comprehensive assessment of climate-associated eyespot variability and plasticity in M. jurtina across fine-scale gradients in the north-western Balkans, offering new insights into the mechanisms underlying environmentally induced morphological variation in butterflies.
2. Materials and Methods
2.1. Species and Sample
The Meadow Brown, Maniola jurtina Linnaeus, 1758, is a butterfly from the family Nymphalidae (Figure 1), distributed across the Palearctic region. Its habitats include grasslands, forest edges, shrublands, and woodland clearings. Its altitudinal range spans from sea level up to approximately 2000 m, and it is active from May to September [12].

Figure 1.
Male of the Meadow Brown butterfly, Maniola jurtina, with one eyespot on underside of the forewing, and two eyespots on underside of the hindwing in positions S2 and S5. Foto P. Jakšić.
The wings are dark brown, and there is sexual dimorphism in coloration, with females exhibiting lighter tones. In the upper third of the forewings, there is a distinctive color pattern in the form of a large eyespot. On the underside of the hindwings, there is a pattern of small eyespots. The number of these eyespots is variable, ranging from zero to six, though two is typical. Males tend to be more spotted than females.
Samples of Maniola jurtina were collected in June and July at eight locations in Serbia, Bosnia and Herzegovina, and Montenegro (Section 3.4, Table 1). Only male specimens were used in this study. The specimens were preserved in a dry state in the private collection of the co-author, P. Jakšić.

Table 1.
Geographic samples of Maniola jurtina with ID numbers, location names, region, country, geographic coordinates, altitude, and number (N) of male specimens used in the study.
2.2. Number of Eyespots and Occurrence by Candidate Position
The posterior part of the ventral side of the hindwing of M. jurtina is divided into ten cells by veins (Figure 2). Eyespots are located on a distinct fold between the veins, with no more than one per fold [27]. These cells are candidate positions where we checked for the presence of spots or eyespots. Spots are defined as patches of dark-colored scales of a single color that contrast with the background color, whereas eyespots consist of concentric rings of different colors [3,37]. In our sample, most spots had a yellow-orange ring, but spots without a ring were also recorded, and we made no distinction between them. For each of the 609 male specimens, the ventral side of the right hindwing was photographed. The presence of eyespots in the expected position was determined from the photographs at 8× magnification. To avoid subjective errors, the eyespot determinations were carried out by the same person. The presence of an eyespot at a candidate position was recorded as 1, and its absence as 0.
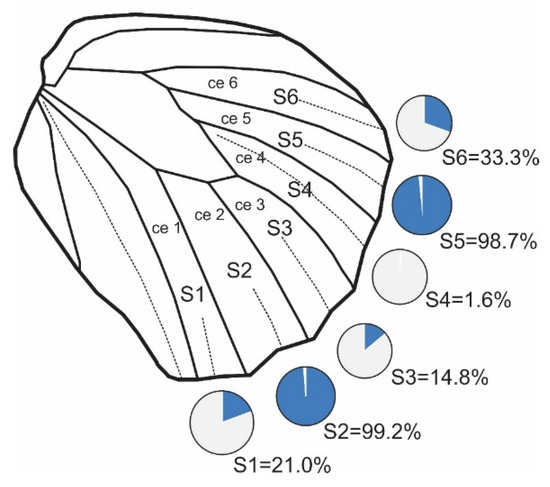
Figure 2.
Underside of the hindwing of Maniola jurtina with cell arrangement and eyespot labels. Cells (ce) are labeled from ce1 to ce6, which represent candidate positions for eyespots. The eyespot positions are labeled from S1 to S6. Pie charts provide information on the frequency of each eyespot across the sample of 609 male individuals.
The naming of the eyespots is not standardized, and in the literature, we found six different naming systems [15,23,31,38,39,40,41,42,43]. In this study, we used the British system terminology for naming candidate positions [42]. Eyespots were named using a combination of the uppercase letter “S” for “spot” and the cell number in which the eyespot was located (Figure 2). The eyespots were numbered starting from the anal region and moving towards the costal region [40]. The data with measurements of eyespots on 609 butterfly wings are provided in the Supplementary Materials (Table S1).
To describe the variability in the number of eyespots on the hindwing, we calculated the mean, standard deviation, median, minimum, and maximum number of eyespots for each geographic locality as well as for the total sample. To assess the relative variability in the number of eyespots across localities, we calculated the coefficient of variation (CVm; [44]) for the number of eyespots for each locality. To describe the occurrence of eyespots at candidate positions, we calculated the frequency of occurrence for each eyespot for each geographic locality and the whole sample.
2.3. Combinations of Eyespots
Based on the presence of eyespots in the hindwing cells, we recorded all eyespot combinations present in the hindwings of M. jurtina and labeled them with a sequence of present eyespots (e.g., S1-S2-S5, when S1, S2, and S5 were present). The frequency of each eyespot pattern was calculated for the whole sample and for each geographic locality.
2.4. Geographic Variability
To simplify the morphometric data and identify patterns of variability in M. jurtina eyespots, we performed a Principal Coordinates Analysis (PCoA) in R version 4.4.3 [45]. Six variables representing the presence or absence of individual eyespots (S1–S6) were used to calculate Principal Coordinates (PCos), which reduced the dimensionality of the dataset and were referred to as Morphological Principal Coordinates (MPCos; Table S3). The analysis was conducted using the dudi.pco() function from the “ade4” package [46] using the Euclidean distance for binary data via the vegdist() function from the “vegan” package [47]. Euclidean distance symmetrically accounts for both presence and absence of eyespots, enabling interpretation of overall morphological dissimilarity.
We calculated the correlation coefficients r between the first few MPCos and the number of eyespots, the proportions of individual eyespots (spots 1–6), and the eyespot combinations.
Differences in morphometric variability according to geographic locality were tested using MANOVA using the manova() function in R version 4.4.3. MPCo1 and MPCo2 were included in the analysis as dependent variables, and geographic locality as a factor. Due to multiple comparisons between localities, posterior testing was performed using the Bonferroni correction.
The morphometric variability, explained by the first two MPCos, was presented with a violin plot, generated using the “ggplot2” package [48] in R version 4.4.3 with the geom_violin() function.
To test whether geographic factors are associated with morphometric characters, we calculated the correlation coefficients r between altitude, latitude, and longitude and the number of eyespots, the proportions of individual eyespots (spots 1–6), the proportions of eyespot combinations, the first two MPCos, and coefficients of variation (CVm) for the number of eyespots.
2.5. Ecomorphological Variability
To analyze ecomorphological variability of eyespots in M. jurtina, we obtained bioclimatic data (Bio1–19) for the eight geographic samples (Table S4) using the “raster” package [49] in R from the WorldClim website. We correlated the proportions of individual eyespots, the number of eyespots, and the first two MPCos to the bioclimatic variables.
To reduce the dimensionality of the bioclimatic data, we conducted a Principal Component Analysis (PCA) using the prcomp() function in R version 4.4.3 [45] on standardized values of the bioclimatic variables. We labeled them Climatic Principal Components (CPCs). The first climatic principal component (CPC1) accounted for 90% of the total variation, while the second (CPC2) explained an additional 5.7% (Table S5). Both CPC1 and CPC2 scores were included in subsequent correlation and regression analyses.
We examined the relationship between eyespot variability in M. jurtina, summarized by the first two MPCos, and the variability of bioclimatic traits, summarized by the CPC1, using correlation and regression analyses [50,51]. Linear effects of variables in the regression model were assessed using a general linear model implemented via the lm() function in R version 4.4.3. To explore potential non-linear relationships, we used the gam() function from the “mgcv” package version 1.9-3 [52]. After fitting initial models that included all covariates, model selection was performed through backward elimination based on AICc values [53], using the “MuMIn” package version 1.48.11 [54].
3. Results
3.1. Number of Eyespots
The number of eyespots on the underside of the hindwing of male M. jurtina ranged from 0 to 6, with an average of 2.69 ± 0.88 eyespots per wing, and a median of 2 (Table 2). Nearly half (48.77%) of the specimens had two eyespots, 33.17% had three, and 12.97% had four (Figure 3, Table S2). Total numbers of zero, one, five, and six eyespots were rare, each occurring in less than 3% of individuals. The mean number of eyespots by locality ranged from 2.50 ± 0.81 (median 2) at Baština to 3.21 ± 1.13 (median 3) at Slatina (Table 2). Proportions of specimens with a specific number of eyespots varied across the localities (Figure 3). In Slatina, Grabovo, Bojanine Vode, and Zasavica, two- and three-spotted butterflies were similarly abundant. Specimens with two eyespots were the most common in Milošev Do (64.47%) and the least in Slatina (30.55%). The coefficient of variation (CVm) for the total sample was 32.88, with the lowest variation observed at Zasavica (CVm = 27.77) and the highest at Milošev Do (CVm = 37.42) (Table 2).

Table 2.
Descriptive statistics of the number of eyespots on the hindwing of male Maniola jurtina by locality and in total sample, CVm—coefficient of variation in the number of eyespots per locality and total sample.
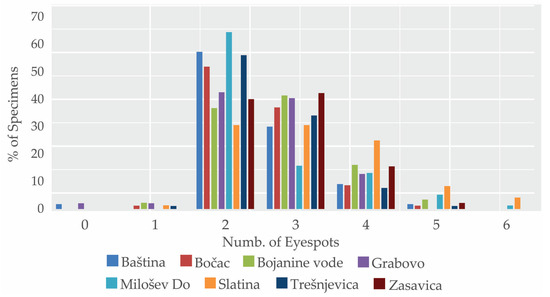
Figure 3.
Proportion (in %) of specimens with a specific number of eyespots (0—no eyespots, 6—max. recorded number of eyespots) on the hindwing of male Maniola jurtina by locality.
3.2. Occurrence of Individual Eyespots
Six different eyespots (S1 to S6) appeared in six cells, with one eyespot per cell. Across the entire sample, the frequencies of individual eyespots varied on average from 1.64% for S4 to 99.18% for S2 (Figure 2, Table 3). Nearly complete presence was observed for S2 and S5, while S1, S3, and S6 were present in 15% to 33% of all specimens.

Table 3.
Frequencies (in %) of occurrence of individual eyespots (spots 1–6) on the hindwing of male Maniola jurtina by locality and in total sample.
3.3. Combinations of Eyespots
The number of eyespots and occurrence across the cells formed distinct eyespot patterns on the hindwings of M. jurtina. The most common was the two-spot combination of S2 and S5 (S2-S5), found in nearly half of the cases (48.6%) (Figure 4). From 64 (26) possible combinations of the six eyespots, we observed only 17, or 26.6% of all possible combinations. Combinations present in more than 5% of the cases were S2-S5 (48.6%), S2-S5-S6 (19.4%), S1-S2-S5 (7.6%), S1-S2-S5-S6 (7.6%), and S2-S3-S5 (6.2%) (Figure 4). The pair of S2 and S5 was present in 98.5% of all individuals. Frequencies of all combinations of eyespots are listed in Table S6. The frequencies of individual eyespot patterns across localities are presented with a heat map (Figure 4). Of the 17 recognized combinations, five were present in all samples: S2-S5, S2-S5-S6, S1-S2-S5, S1-S2-S5-S6, S2-S3-S5. Three combinations were present only in one sample: S1-S2 in Bojanine Vode, S2-S3-S4-S5-S6 in Slatina, and the combination S2-S4-S5-S6 in Trešnjevica.
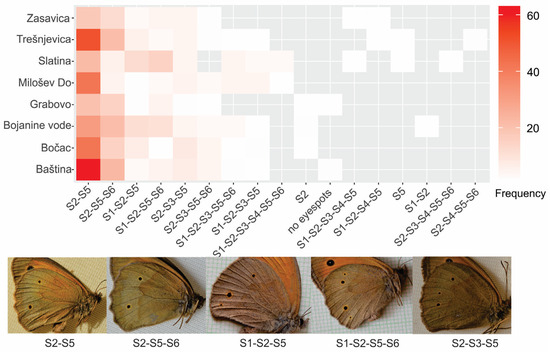
Figure 4.
Frequencies (in %) of 17 different combinations of eyespots (e.g., S2-S5—eyespots S2 and S5) on the hindwing of male Maniola jurtina by locality. The arrangement of combinations is from the most frequent on the left to the least frequent on the right. Photographs of specimens present examples of the five most common eyespot combinations.
3.4. Geographic Variability
The first three MPCos together explained 93.48% of the total variation in the number of eyespots and their distribution across cells (Table S3). An increase in the first MPCo (explaining 44.44% of total variation) was characterized most strongly (|r| > 0.6) by a decrease in the number of eyespots, in the proportion of eyespots S1, S4, S6, and the S1-S2-S5, and S1-S2-S5-S6 combination (Table 4). Its increase was also characterized by the increase in the S2-S5 combination. The second MPCo (explaining 28.11% of total variation) was negatively correlated to the number of eyespots and the proportions of eyespots S1, S3, S4, combinations S1-S2-S5 and S1-S2-S5-S6, and positively to S2-S5-S6 (Table 4). MPCo3 was positively correlated to S6 and S1-S2-S5-S6, and negatively to S2-S3-S5.

Table 4.
Correlation coefficients r between morphometric characters of male Maniola jurtina (number of eyespots, proportions (in %) of individual eyespots, and the proportions (in %) of the five most common eyespot combinations) and the first three Morphological Principal Coordinates (MPCos). Strong correlations (|r| > 0.6) are indicated in bold. Statistically significant correlations at p < 0.05 are marked with an asterisk *.
Geographic variability captured with the first two MPCos was statistically significant (Wilks’ λ = 0.88, F14, 1200 = 5.7, p < 0.001). After the Bonferroni correction for pairwise comparisons, only the sample from Slatina was statistically significantly different from other localities. Comparison of violin plots across geographic samples revealed Slatina had the widest range and the narrowest spread of the violin plot along the MPCo1 axis and the most negative mean MPCo1 (Figure 5). Also, projection of the mean MPCo1 values for geographic localities onto the geographic map (Figure 6) did not form a recognizable spatial pattern. Two of the geographically closest locations, Slatina and Milošev Do, had the most different mean MPCo1 values. The MPCo2 showed a strong negative correlation to altitude and a positive correlation to latitude (Table 5). Decreasing latitude was connected to increasing altitude (r = −0.75, p = 0.034), which means the more southern locations were general at higher elevation. Increasing altitude showed a strong positive correlation to the proportion of eyespot S3 and the S1-S2-S5-S6 combination. There was an absence of correlation between the two most frequently represented eyespots, and their combination (S2, S5, and S2-S5), to the altitude. Increasing altitude was strongly correlated to increasing variation in the number of eyespots (CVm).
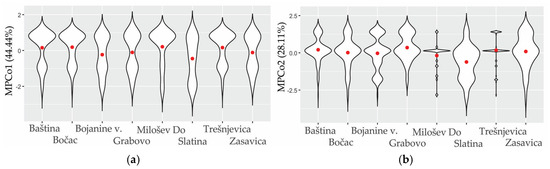
Figure 5.
Morphological variability of eyespots in male M. jurtina for MPCo1 (a) and MPCo2 (b) across eight localities. The shape of each plot represents the frequency at a given MPCo value, while the range illustrates the morphometric variation within the sample. The red dot indicates the group mean.
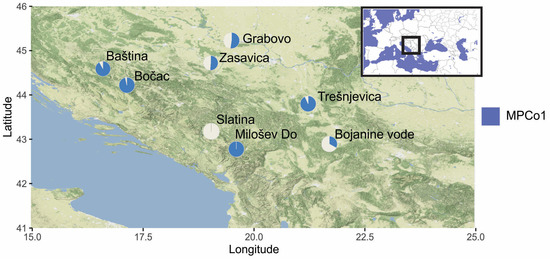
Figure 6.
Map of the north-western part of Balkan Peninsula showing eight locations from which samples of Maniola jurtina were collected. The description of the locations is provided in Table 1. The pie charts display the average value of MPCo1 for each sample, with the blue color representing the MPCo1 value. A filled blue circle represents the maximum value, while a filled white circle represents the minimum value of MPCo1.

Table 5.
Correlation coefficients r between morphometric characters of male Maniola jurtina (number of eyespots, proportions (in %) of individual eyespots, proportions (in %) of the five most common eyespot combinations, the first three MPCos, and coefficient of variation (CVm) in the number of eyespots) and altitude, latitude, and longitude. Strong correlations (|r| > 0.6) are in bold. Statistically significant correlations at p < 0.05 are marked with an asterisk *.
3.5. Ecomorphological Variability
The number of eyespots on the hindwing of males in M. jurtina was, among others, negatively associated with the mean annual temperature (Bio1), diurnal and annual temperature range (Bio2 and Bio7), and temperature seasonality (Bio4) (Table 6). These associations were moderately strong. The proportion of S1 showed a negative correlation to temperature. Similarly, the proportion of S3 showed a negative correlation to temperature, but a positive correlation to precipitation, except precipitation seasonality. Eyespots showing very low variability, namely S2 and S5 (present in approximately 99% of the specimens) and S4 (present in less than 2%), were excluded from the correlation analysis. MPCo2 showed strong positive correlations with temperature (except Bio9) and negative correlations with precipitation (except participation seasonality, Bio15).

Table 6.
Correlations between morphometric characters in male M. jurtina (number of eyespots, proportion of eyespots S1, S3, and S6, and first three MPCos) and 19 bioclimatic variables. Strong correlations (|r| > 0.6) are in bold. Statistically significant correlations at p < 0.05 are marked with an asterisk *.
The first CPC, explaining 90.0% of the total variation, showed, in general, very strong positive correlations (r > 0.8, p < 0.05) with temperature variables and very strong negative correlations with precipitation variables, except for a positive correlation with the precipitation seasonality (Table S5). This means that an increase in CPC1 described a warmer and drier climate with pronounced seasonal precipitation, while a decrease in CPC1 described cooler and wetter conditions. Increase in CPC1 was strongly connected to a decrease in altitude (r = −0.85, p = 0.008). In contrast to CPC1, the second climatic principal component CPC2 was not strongly correlated to any climatic variable (r < 0.6, p > 0.05) (Table S5).
The correlation between MPCo1 and CPC1 was weak (r = 0.12, p = 0.770). The correlation between MPCo2 and CPC1 was positive and strong (r = 0.80, p = 0.017).
The morphological variability of MPCo1 in relation to climate was best explained by a generalized additive model, MPCo1~CPC1. The climate variable CPC1 explained 73.2% of the variation in MPCo1 (adjusted R2 = 0.63). A non-linear relationship is illustrated in Figure 7. The general linear model MPCo2~CPC1 was statistically significant (F1, 6 = 10.6, p = 0.017), and climate variable CPC1 explained 64% of the variation in MPCo2 (Adjusted R2 = 0.578) (Table S7, Figure 7).
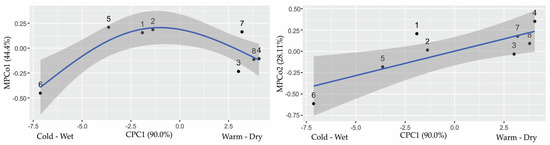
Figure 7.
Regression of morphometric characters (MPCo1 and MPCo2) of the hindwing of male Maniola jurtina onto climatic conditions summarized by CPC1. The description of the locations is in Table 1.
4. Discussion
This study provides the first comprehensive assessment of hindwing eyespot variation in M. jurtina across the climatically and topographically diverse north-western Balkan region. Overall, wing spottiness in the region was high compared to the species averages. In half of the sampled populations, males with two and three eyespots occurred in similar proportions, while the number of eyespots ranged from none to extremely rare, a maximum of six per individual. Three of the six potential eyespot positions were nearly invariant, with eyespots either present or absent in nearly 99% of all individuals. Eyespot variation in male M. jurtina could be explained by a combination of geographic factors (latitude, altitude) and climatic conditions. Colder and thermally more stable environments at higher altitudes produced phenotypes with more eyespots than warmer and thermally more variable environments.
The number of hindwing eyespots in male M. jurtina across our study area showed a lower dominance of the two-spot phenotype than reported in earlier studies from the region [16,35,36], with less than half of the males exhibiting the two-spot phenotype. In some populations, three-spotted males were more common, and in Slatina, three- and four-spotted individuals occurred in almost equal frequencies as the two-spotted. The overall coefficient of variation (CVm = 32.88) indicated relatively high plasticity when contrasted with the characteristic unimodal stability of two-spotted males across much of the species’ range [16]. Our results, therefore, suggest elevated phenotypic diversity in this south-eastern area of the M. jurtina range. This is partly in line with the reported stability in the center and increased variability toward the periphery of the species’ range [16]; however, the true southern periphery lies farther south. The north-western Balkans may represent a transitional zone where local climatic and topographic heterogeneity promotes elevated variability, as well as high spottiness characteristic of southern populations [15].
Despite high variability in eyespot number in our sample, two eyespots, S2 and S5, were almost universally present (>98%), indicating their role as core elements of the M. jurtina hindwing pattern [32,40]. In contrast, the intermediate eyespots were generally rare: S4 was almost always absent, and S3 occurred in only 14% of individuals, resulting in a visible spotless gap between the consistently present S2 and S5. The spots at the beginning and end of the spot series, S1 and S6, occurred in 21% and 33% of individuals, respectively. This pattern suggests relative symmetry in the eyespot arrangement, with a slightly greater costal positioning of the eyespots [27]. Moreover, out of 64 possible combinations of eyespots, only 17 were observed, with a sharp dominance of the two-spot S2-S5 pattern (48.6%). This shows that despite developmental potential and flexibility of butterfly eyespots [1], only a limited number of stable phenotypes are realized in nature. Though eyespot size was not considered in this study, it must be mentioned that in M. jurtina eyespots S2 and S5 are not only the most frequent but usually also the largest eyespots in the hindwing spot series [38,40]. The size of the individual hindwing spots also increases with spot number [38,40]. Functionally, ventral eyespots in butterflies might increase survival by deflecting predator attacks away from the vital body parts, act as visual signals in mate attraction, or enhance crypsis [1,3,55]. The potential evolutionary significance of the dominant two-eyespot pattern in males may represent a balance between predator avoidance and sexual selection. Different environmental conditions can favor different phenotypes [2]. In this study, the frequency of eyespots S1 and S3 increased with altitude and decreasing annual temperature. A higher frequency of S3 was also associated with wetter environments. However, the occurrence of eyespot S6 appeared to be independent of both geographic and climatic factors.
A general pattern in male M. jurtina in this study showed that colder environments at higher altitudes produced phenotypes with more eyespots than warmer environments at lower altitudes. This finding of a negative association between eyespot number and temperature contrasts with the large-scale latitudinal pattern reported by Frazer and Willcox [15], in which spotting in males increased from cooler northern to warmer southern regions of the M. jurtina distribution range. Also in our study area, decreasing latitude was associated with increased spotting, but mostly because of increasing altitude, so southern populations often experienced cooler conditions, helping to explain the increased spotting observed in these sites. This discrepancy suggests that fine-scale environmental effects—such as local temperature extremes, seasonal variability, and precipitation—may override broad latitudinal trends in affecting eyespot expression. It should be noted, however, that inter-annual climatic variation was not considered in this study and should be added for more detailed research. Eyespot reduction in warmer conditions is in concordance with studies of M. jurtina reporting temporal eyespot reduction in unusually warm and dry seasons [18] or within seasons along with increasing temperatures [20,21,23]. However, seasonal spot variability appears to be rare in males but common in females, in which spottiness is, contrary to males, strongly influenced by the temperature experienced during development [23]. In females, reduced spotting over the season may enhance crypsis [23]. Rare seasonal spot reduction in males has been interpreted as differential survival of the low- and high-spotted morphs because of avian predation and selection against high-spotted morphs [31]. Similar seasonal plasticity has been observed in other butterfly species. In the tropical butterfly Bicyclus anynana, eyespots on exposed wing surfaces are reduced in the dry season and reappear in the wet season [56]. Small eyespots are thought to function cryptically, reducing detection by vertebrate predators, whereas large eyespots are conspicuous and serve to deflect attacks from invertebrate predators [1,57,58]. Males of M. jurtina are more spotted and more active than females [32], and because small eyespots are thought to be more effective in decoying predators during flight [58], this higher activity likely enhances the anti-predator function of higher spotting in males. In our study, increasing altitude was associated not only with a higher number of hindwing eyespots but also with a greater variation in eyespot number, including males with the exceptionally rare maximum of six eyespots per wing, observed only at two sites above 1200 m a.s.l. This pattern may reflect shifts in predator communities along the altitudinal gradient, maintaining plasticity in eyespot patterns to optimize survival under different predator types or abundances.
In conclusion, our study reveals that male M. jurtina in the north-western Balkans exhibit unusually high hindwing eyespot variability compared to the species average. While the S2-S5 pair of eyespots is the dominant and stable pattern, higher altitudes, with wetter and cooler environments, were associated with both increased spot number and greater phenotypic plasticity, including the rare occurrence of six-spotted individuals. These findings suggest that local climatic and topographic heterogeneity of the north-western Balkan region promotes elevated variability, highlighting the role of fine-scale environmental factors in shaping eyespot expression.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100675/s1, Table S1: Eyespot presence/absence data for 609 male Maniola jurtina butterfly wings; Table S2: Number of eyespots (0—no eyespots, 6—max. recorded number of eyespots) on the hindwing of male Maniola jurtina by locality and in total sample. Total N—total number of specimens with the specific number of eyespots; Total %—proportion of specimens with the specific number of eyespots. Table S3: Morphological principal coordinates (MPCos1–3) with proportion of explained total variance; Table S4: Geographic coordinates, elevation, and 19 bioclimatic variables (bio1–19) for the sampled localities; Table S5: Climatic principal components (CPCs1–2) with proportion of explained total variance and correlation coefficients between bio1–19 and CPCs1–2; Table S6: Distribution of 17 eyespots patterns across localities and relative frequencies of the patterns; Table S7: Ranking of regression models based on AICc values and relation of MPCo1–2 to CPC1–2 and altitude.
Author Contributions
Conceptualization, F.J. and P.J.; field work, specimen collection, determination, photographing, P.J.; data collection and methodology, F.J.; validation, T.K. and P.J.; original draft preparation, F.J.; data interpretation, manuscript writing, T.K.; visualization, F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Slovenian Research and Innovation Agency (ARIS) and research program Computationally intensive complex systems (P1-0403) and Infrastructure program (I0-0029).
Data Availability Statement
The dataset used during this study is available in the Supplementary Materials of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| PCoA | Principal Coordinates Analysis |
| PCos | Principal Coordinates |
| MPCo | Morphological Principal Coordinate |
| CPC | Climatic Principal Component |
| CVm | Coefficient of variation |
| S | Spot |
| Bio | Bioclimatic variable |
References
- Monteiro, A. Origin, Development, and Evolution of Butterfly Eyespots. Annu. Rev. Entomol. 2015, 60, 253–271. [Google Scholar] [CrossRef]
- Beldade, P.; Brakefield, P.M. The genetics and evo-devo of butterfly wing patterns. Nat. Rev. Genet. 2002, 3, 442–452. [Google Scholar] [CrossRef]
- Beldade, P.; Monteiro, A. Eco-evo-devo advances with butterfly eyespots. Curr. Opin. Genet. Dev. 2021, 69, 6–13. [Google Scholar] [CrossRef]
- Stevens, M. The role of eyespots as anti-predator mechanisms, principally demonstrated in the Lepidoptera. Biol. Rev. 2005, 80, 573–588. [Google Scholar] [CrossRef]
- Oliver, T.; Hill, K.J.; Thomas, D.C.; Brereton, T.; Roy, B.D. Changes in habitat specificity of species at their climatic range boundaries. Ecol. Lett. 2009, 12, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Vallin, A.; Jakobsson, S.; Lind, J.; Wiklund, C. Prey survival by predator intimidation: An experimental study of peacock butterfly defence against blue tits. Proc. R. Soc. B Biol. Sci. 2005, 272, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Schachat, R.S.; Piel, H.W.; Monteiro, A. Attack risk for butterflies changes with eyespot number and size. R. Soc. Open Sci. 2016, 3, 150614. [Google Scholar] [CrossRef] [PubMed]
- Tokita, C.K.; Oliver, J.C.; Monteiro, A. A Survey of Eyespot Sexual Dimorphism across Nymphalid Butterflies. Int. J. Evol. Biol. 2013, 2013, 926702. [Google Scholar] [CrossRef]
- Dowdeswell, W.H.; Ford, E.B. Ecological genetics of Maniola jurtina L. on the Isles of Scilly. Heredity 1955, 9, 265–272. [Google Scholar] [CrossRef]
- Ford, E.B. Ecological Genetics; Springer: Dordrecht, The Netherlands, 1964. [Google Scholar]
- Creed, E.R.; Ford, E.B.; McWhirter, K.G. Evolutionary studies on Maniola jurtina: The Isles of Scilly, 1958–59. Heredity 1964, 19, 471–488. [Google Scholar] [CrossRef]
- Brakefield, P.M. Case studies in ecological genetics. In Butterflies of Europe; Kudrna, O., Ed.; AULA Verlag: Wiesbaden, Germany, 1990; Volume 2, pp. 307–331. [Google Scholar]
- Dowdeswell, W.H.; Fisher, R.A.; Ford, E.B. The quantitative study of populations in the Lepidoptera II. Maniola jurtina L. Heredity 1949, 3, 67–84. [Google Scholar] [CrossRef]
- Dowdeswell, W.H.; Ford, E.B. The distribution of spot number as an index of geographical variation in the butterfly Maniola jurtina, L. Heredity 1952, 6, 99–109. [Google Scholar] [CrossRef]
- Frazer, J.F.D.; Willcox, H.N.A. Variation in spotting among the close relatives of the butterfly, Maniola jurtina. Heredity 1975, 34, 305–322. [Google Scholar] [CrossRef]
- Dowdeswell, W.H.; McWhirter, K.G. Stability of spot distribution in Maniola jurtina throughout its range. Heredity 1967, 22, 187–210. [Google Scholar] [CrossRef]
- Baxter, S.W.; Hoffman, J.I.; Tregenza, T.; Wedell, N.; Hosken, D.J. EB Ford revisited: Assessing the long- term stability of wing- spot patterns and population genetic structure of the meadow brown butterfly on the isles of Scilly. Heredity 2017, 118, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, S.-A. Spot-Distribution in Maniola jurtina (Lepidoptera) on Small Islands in Southern Sweden. Ecography 1978, 1, 54–61. [Google Scholar] [CrossRef]
- Ford, E.B. Ecological Genetics, 4th ed.; Chapman and Hall: New York, NY, USA, 1975. [Google Scholar]
- Garcia Pereira, P.; Garcia-Barros, E. Spot distribution in Maniola jurtina (Linnaeus, 1758) (Lepidoptera, Nymphalidae, Satyrinae) in Central Spain. Nota Lepidopterol. 1996, 19, 152–164. [Google Scholar]
- Goulson, D. Variation in the genitalia of the butterfly Maniola jurtina (Lepidoptera: Satyrinae). Zool. J. Linn. Soc. 1993, 107, 65–71. [Google Scholar] [CrossRef]
- Tudor, O.; Parking, D. Studies on phenotypic variation in Maniola jurtina (Lepidoptera: Satyridae) in the wyre forest, England. Heredity 1979, 42, 91–104. [Google Scholar] [CrossRef]
- Mowbray, S.; Bennie, J.; Rhodes, M.W.; Smith, D.A.S.; French-Constant, R.H. Eyespot variation and field temperature in the Meadow Brown butterfly. Ecol. Evol. 2024, 14, e10842. [Google Scholar] [CrossRef]
- Thomson, G. Geographical variation in Maniola jurtina (L.). Tijdschr. Voor Entomol. 1973, 116, 185–226. [Google Scholar]
- Brakefield, P.M.; Shreeve, T.G. Case studies in Evolution. In The Ecology of Butterflies in Britain; Dennis, R.L.H., Ed.; University Press: Oxford, UK, 1992; pp. 197–216. [Google Scholar]
- Shreeve, T.G.; Dennis, R.L.H.; Williams, W.R. Uniformity of wing spotting of Maniola jurtina (L.) (Lepidoptera: Satyrinae) in relation to environmental heterogeneity. Nota Lepidopterol. 1996, 96, 77–92. [Google Scholar]
- McWhirter, K.G.; Creed, E.R. An analysis of spot placing in the meadow brown butterfly Maniola jurtina. In Ecological Genetics and Evolution: Essays in Honour of E.B. Ford; Creed, E.R., Ed.; Blackwell: Oxford, UK, 1971; pp. 275–289. [Google Scholar]
- Brakefield, P. Spot-number in Maniola jurtina—Variation between generations and selection in marginal populations. Heredity 1979, 42, 259–266. [Google Scholar] [CrossRef]
- Brakefield, P.M. Ecological studies on the butterfly Maniola jurtina in Britain. II. Population dynamics: The present position. J. Anim. Ecol. 1982, 51, 727–738. [Google Scholar] [CrossRef]
- Brakefield, P.M. Geographical variability in, and temperature effects on, the phenology of Maniola jurtina and Pyronia tithonus (Lepidoptera, Satyrinae) in England and Wales. Ecol. Entomol. 1987, 12, 139–148. [Google Scholar] [CrossRef]
- Owen, D.F.; Smith, D.A.S. Interpopulation variation and selective predation in the meadow brown butterfly, Maniola jurtina (L.) (Lepidoptera: Satyridae) in the Canary Islands. Biol. J. Linn. Soc. 1990, 39, 251–267. [Google Scholar] [CrossRef]
- Merckx, T.; Van Dyck, H. Interrelations Among Habitat Use, Behavior, and Flight-Related Morphology in Two Cooccurring Satyrine Butterflies, Maniola jurtina and Pyronia tithonus. J. Insect Behav. 2002, 15, 541–561. [Google Scholar] [CrossRef]
- Schmitt, T. Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Front. Zool. 2007, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, T.L.; Steyvers, M. Finding Scientific Topics. Proc. Natl. Acad. Sci. USA 2004, 101, 5228–5235. [Google Scholar] [CrossRef]
- Jakšić, P. Pregled utvrđenih vrsta dnevnih leptira Borskog područja (Lepidoptera: Hesperioidea i Papilionoidea). Zb. Rad. O Fauni Srb. 2006, 7, 35–86. [Google Scholar]
- Parker, R.; Jakšić, P. The butterflies of Stara Planina (Serbia) with emphasis on M. jurtina Linnaeus. Br. J. Entomol. Nat. Hist. 1996, 9, 93–101. [Google Scholar]
- Bhardwaj, S.; Si-Hui Jolander, L.; Wenk, M.R.; Oliver, J.C.; Nijhout, H.F.; Monteiro, A. Origin of the mechanism of phenotypic plasticity in satyrid butterfly eyespots. eLife 2020, 9, e49544. [Google Scholar] [CrossRef] [PubMed]
- Brakefield, P.M. The ecological genetics of quantitative characters in Maniola jurtina and other butterflies. In The Biology of Butterflies; Vane-Wright, R.I., Ackery, P.R., Eds.; Academic Press: London, UK, 1984; pp. 167–190. [Google Scholar]
- Brakefield, P.M. A decline of melanism in the peppered moth Biston betularia in The Netherlands. Biol. J. Linn. Soc. 1990, 39, 327–334. [Google Scholar] [CrossRef]
- Brakefield, P.M.; van Noordwik, A.J. The genetics of spot pattern characters in the meadow brown buttertfly Maniola jurtina (Lepidoptera: Satyrinae). Heredity 1985, 54, 275–284. [Google Scholar] [CrossRef]
- Owen, F.D.; Smith, S.A.D. Spot variation in Maniola jurtina (L.) (Lepidoptera: Satyridae) in southern Portugal and a comparison with the Canary Islands. Biol. J. Linn. Soc. 1993, 49, 355–365. [Google Scholar] [CrossRef]
- Scott, J.A. Morphology: Adult structure and function. In Butterflies of Europe; Kudrna, O., Ed.; AULA Verlag: Wiesbaden, Germany, 1990; Volume 2, pp. 108–151. [Google Scholar]
- Tauber, A.F. Stammesgeschichtlich interessante Extremvarianten bei Manioliden (Lep. Satyridae). Z. Der Arbeitsgemeinschaft Osterr. Entomol. 1969, 21, 29–37. [Google Scholar]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.r-project.org/ (accessed on 20 January 2025).
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.7-1. 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 June 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Hijmans, R. raster: Geographic Data Analysis and Modeling. R Package Version 3.6-32. 2025. Available online: https://CRAN.R-project.org/package=raster (accessed on 13 June 2025).
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Zuur, A.F.; Hilbe, J.M.; Ieno, E.N. A Beginner’s Guide to GLM and GLMM with R; Highland Statistics: Newburgh, NY, USA, 2013. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC Texts in Statistical Science: New York, NY, USA, 2017. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.48.11. 2025. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 13 June 2025).
- Debat, V.; Chazot, N.; Jarosson, S.; Blandin, P.; Llaurens, V. What Drives the Diversification of Eyespots in Morpho Butterflies? Disentangling Developmental and Selective Constraints from Neutral Evolution. Front. Ecol. Evol. 2020, 8, 112. [Google Scholar] [CrossRef]
- Brakefield, P.M.; Larsen, T.B. The evolutionary significance of dry and wet season forms in some tropical butterflies. Biol. J. Linn. Soc. 1984, 22, 1–12. [Google Scholar] [CrossRef]
- Prudic, K.L.; Jeon, C.; Cao, H.; Monteiro, A. Developmental plasticity in sexual roles of butterfly species drives mutual sexual ornamentation. Science 2011, 331, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Lyytinen, A.; Brakefield, P.M.; Mappes, J. Significance of butterfly eyespots as an anti-predator device in ground-based and aerial attacks. Oikos 2003, 100, 373–379. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).