Differences in Habitat Use, Thermal Ecology, and Behavior of the Semiaquatic Lizard Anolis aquaticus at a High- and Low-Elevation Site
Abstract
1. Introduction
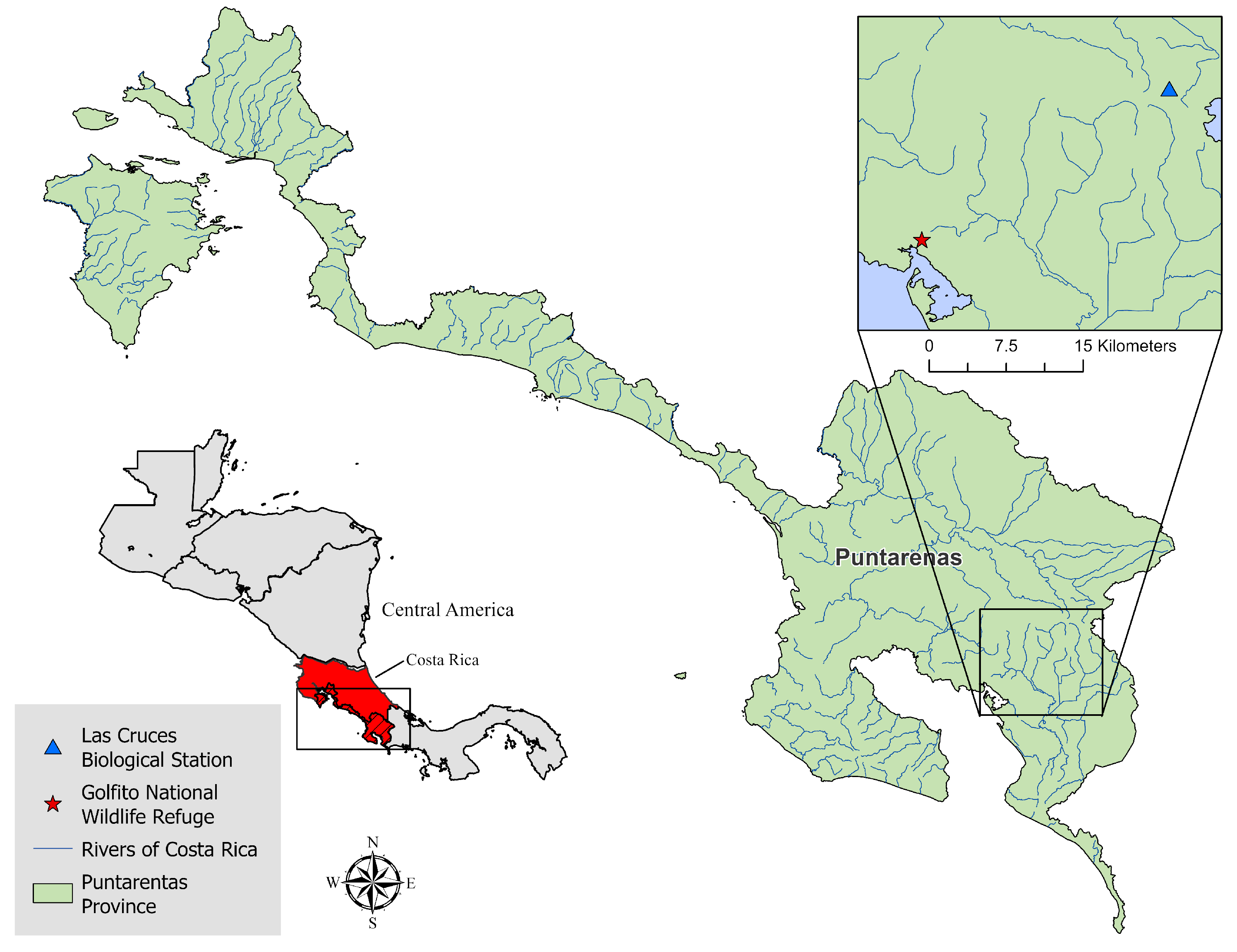
2. Materials and Methods
3. Results
3.1. Population Characteristics
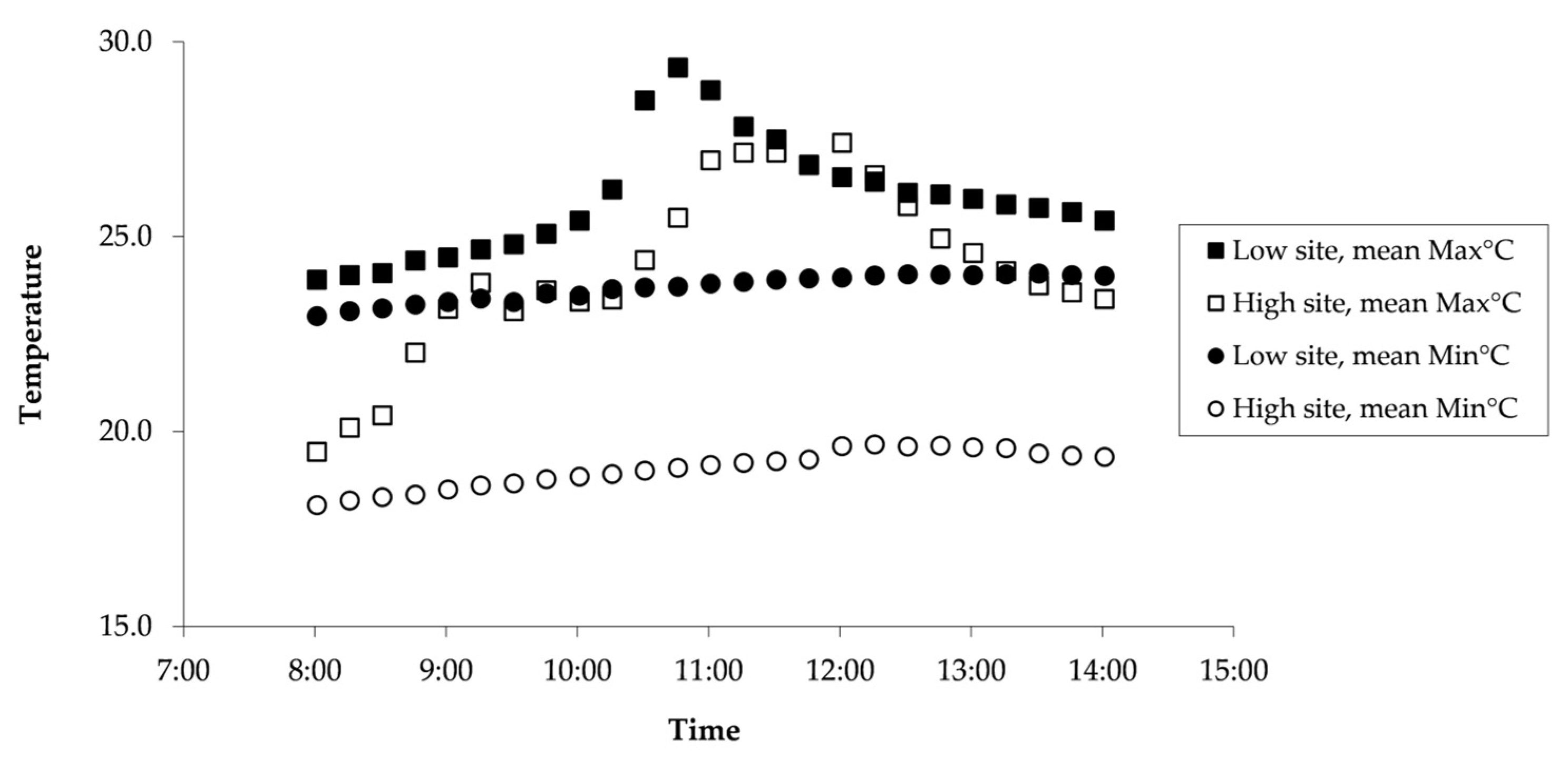
3.2. Thermal Ecology
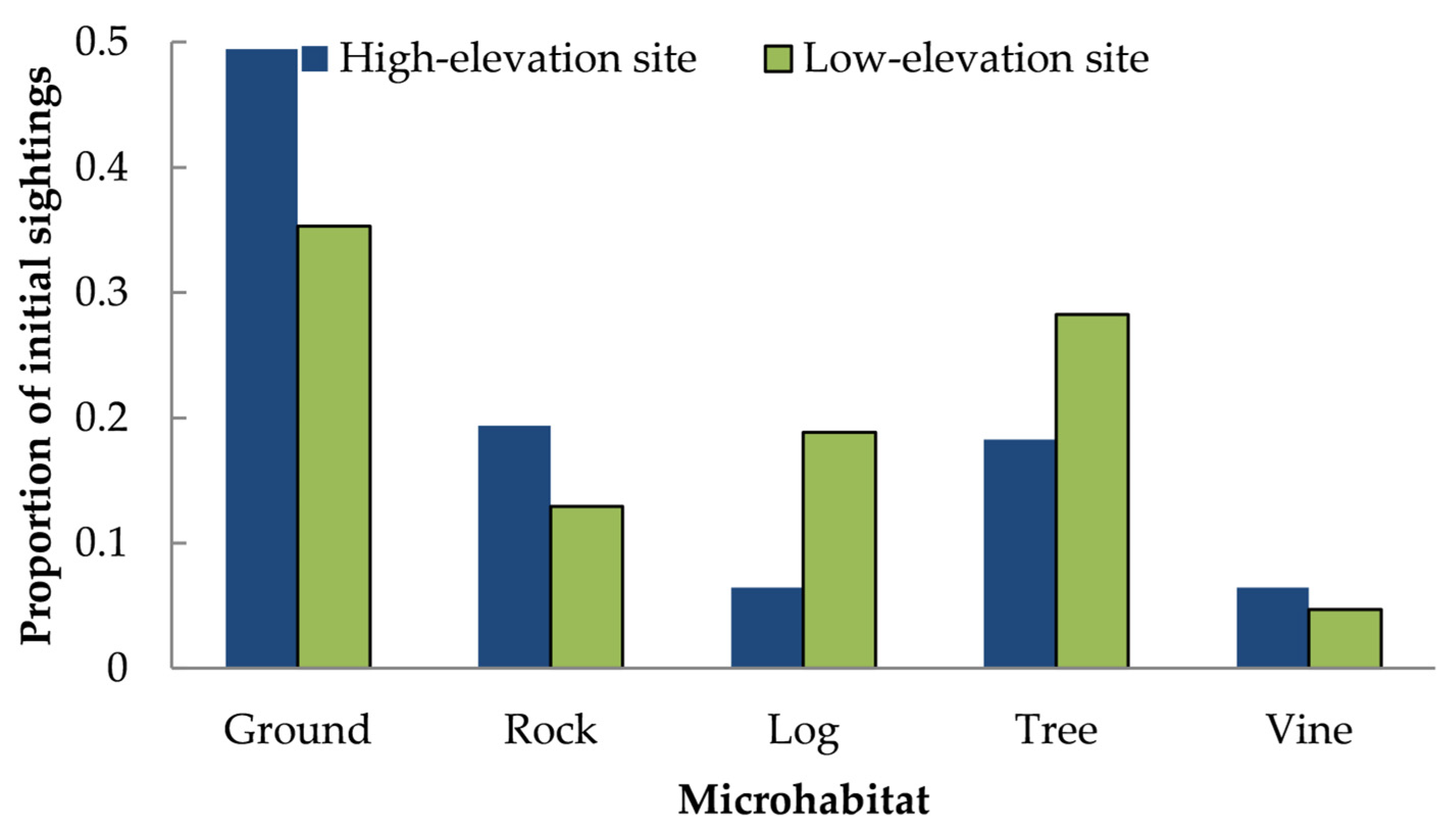
3.3. Habitat Use
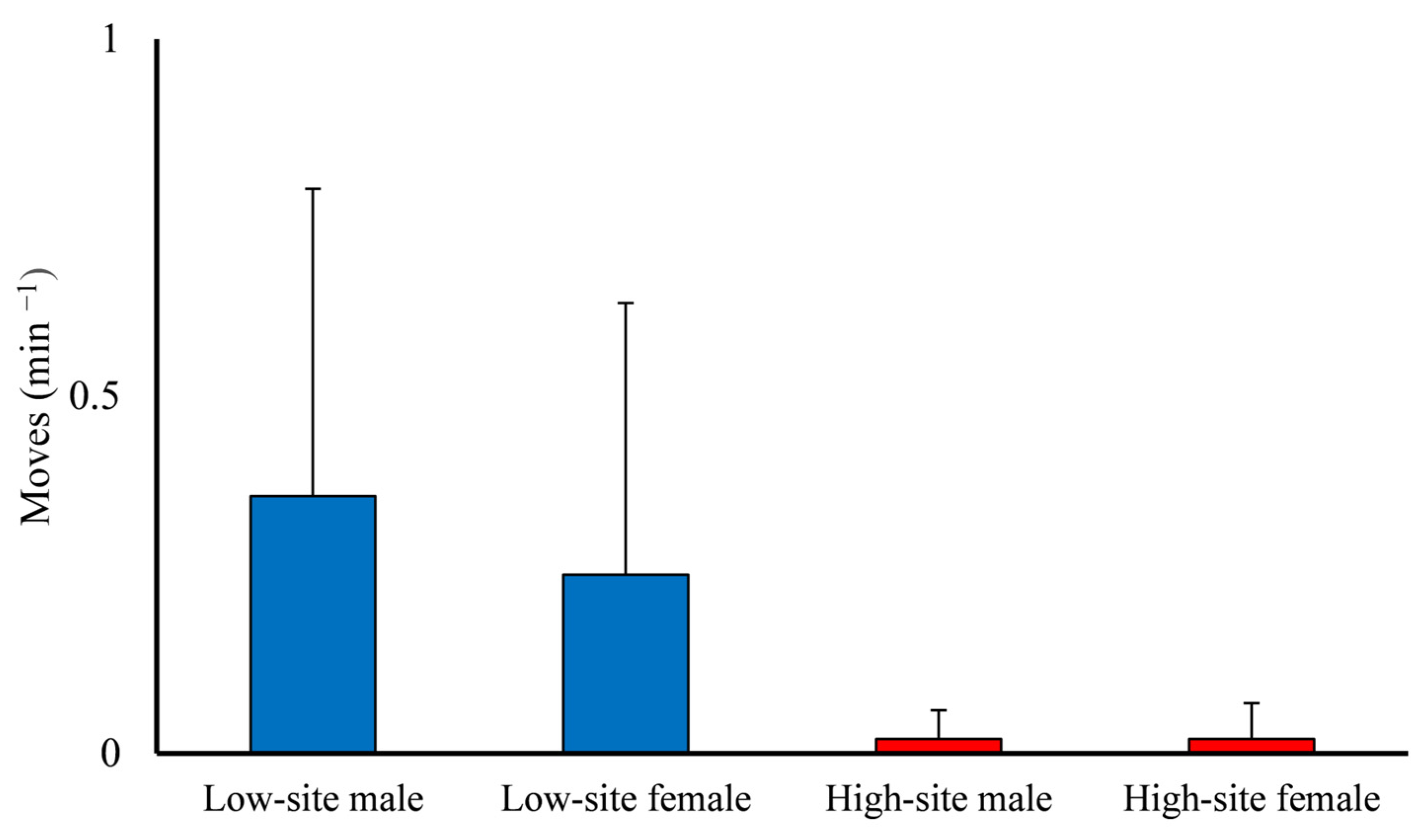
3.4. Behavioral Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbons, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The global decline of reptiles, déjá vu amphibians. Bioscience 2000, 50, 653–666. [Google Scholar] [CrossRef]
- Huey, R.B.; Deutsch, C.A.; Tewksbury, J.J.; Vitt, L.J.; Hertz, P.E.; Álvarez Pérez, H.J.; Garland, T., Jr. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B Biol. Sci. 2009, 276, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef]
- Theisinger, O.; Berg, W.; Dausmann, K.H. Compensation of thermal constraints along a natural environmental gradient in a Malagasy iguanid lizard (Oplurus quadrimaculatus). J. Therm. Biol. 2017, 68, 21–26. [Google Scholar] [CrossRef]
- Johnson, M.A.; Kirby, R.; Wang, S.; Losos, J.B. What drives variation in habitat use by Anolis lizards: Habitat availability or selectivity? Can. J. Zool. 2006, 84, 877–886. [Google Scholar] [CrossRef]
- Losos, J.B. Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles; University of California Press: Berkeley, CA, USA, 2009. [Google Scholar]
- Losos, J.B. Ecomorphology, performance capability, and scaling of West Indian Anolis lizards: An evolutionary analysis. Ecol. Monogr. 1990, 60, 369–388. [Google Scholar] [CrossRef]
- Inger, R.F. Temperature responses and ecological relations of two Bornean lizards. Ecology 1959, 40, 127–136. [Google Scholar] [CrossRef]
- Ruibal, R. Thermal relations of five species of tropical lizards. Evolution 1961, 15, 98–111. [Google Scholar] [CrossRef]
- Hertz, P.E. Thermal passivity of a tropical forest lizard, Anolis polylepis. J. Herpetol. 1974, 8, 323–327. [Google Scholar] [CrossRef]
- Huey, R.B.; Webster, T.P. Thermal biology of a solitary lizard: Anolis marmoratus of Guadeloupe, Lesser Antilles. Ecology 1975, 56, 445–452. [Google Scholar] [CrossRef]
- Huey, R.B.; Webster, T.P. Thermal biology of Anolis lizards in a complex fauna: The cristatellus group of Puerto Rico. Ecology 1976, 57, 985–994. [Google Scholar] [CrossRef]
- Hertz, P.E.; Huey, R.B. Compensation for altitudinal changes in the thermal environment by some Anolis lizards on Hispaniola. Ecology 1981, 62, 515–521. [Google Scholar] [CrossRef]
- Huey, R.B.; Slatkin, M. Cost and benefits of lizard thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef]
- Leal, M.A.; Knox, A.K.; Losos, J.B. Lack of convergence in aquatic Anolis lizards. Evolution 2002, 56, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.M.; Crandell, K.E.; Campbell-Staton, S.C.; Fenstermacher, K.; Frank, H.K.; Van Middlesworth, P.; Sasa, M.; Losos, J.B.; Herrel, A. Multiple paths to aquatic specialisation in four species of Central American Anolis lizards. J. Nat. Hist. 2015, 49, 1717–1730. [Google Scholar] [CrossRef]
- Baeckens, S.; Temmerman, M.; Gorb, S.N.; Neto, C.; Whiting, M.J.; Van Damme, R. Convergent evolution of skin surface microarchitecture and increased skin hydrophobicity in semi-aquatic anole lizards. J. Exp. Biol. 2021, 224, jeb242939. [Google Scholar] [CrossRef]
- Meyer, J.R. The ecological significance of feeding behavior in the Mexican lizard, Anolis barkeri. Bull. South. Calif. Acad. Sci. 1968, 67, 255–262. [Google Scholar]
- Campbell, H.W. Ecological observations on Anolis lionotus and Anolis poecilopus (Reptilia, Sauria) in Panama. Am. Mus. Novit. 1973, 2516, 1–29. [Google Scholar]
- Vitt, L.J.; Zani, P.A.; Durtsche, R.D. Ecology of the lizard Norops oxylophus (Polychrotidae) in lowland forest of southeastern Nicaragua. Can. J. Zool. 1995, 73, 1918–1927. [Google Scholar] [CrossRef]
- Birt, R.A.; Powell, R.; Greene, B.D. Natural history of Anolis barkeri: A semiaquatic lizard from southern México. J. Herpetol. 2001, 35, 161–166. [Google Scholar] [CrossRef]
- Eifler, D.A.; Eifler, M.A. Habitat use in the semiaquatic lizard, Norops aquaticus. Southwest. Nat. 2010, 55, 470–473. [Google Scholar] [CrossRef]
- Savage, J.M. The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas; University of Chicago Press: Chicago, IL, USA, 2002. [Google Scholar]
- Goodman, D. Differential selection of immobile prey among terrestrial and riparian lizards. Am. Midl. Nat. 1971, 86, 217–219. [Google Scholar] [CrossRef]
- Márquez, C.; Mora, J.M.; Bolaños, F.; Rea, S. Aspectos de la biología poblacional en el campo de Anolis aquaticus, Sauria: Polychridae en Costa Rica. Ecol. Appl. 2005, 4, 59–69. [Google Scholar] [CrossRef][Green Version]
- Swierk, L. Novel rebreathing adaptation extends dive time in a semi-aquatic lizard. Biol. Lett. 2024, 20, 20240371. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Boccia, C.K.; Swierk, L. Diving behavior in semi-aquatic Anolis lizards results in heat loss with sex-specific cooling tolerance. Behav. Ecol. Sociobiol. 2024, 78, 33. [Google Scholar] [CrossRef]
- Vitt, L.J.; Sartorius, S.S. HOBOs, Tidbits and lizard models: The utility of electronic devices in field studies of ectotherm thermoregulation. Funct. Ecol. 1999, 13, 670–674. [Google Scholar] [CrossRef]
- Shine, R.; Kearney, M. Field studies of reptile thermoregulation: How well do physical models predict operative temperatures. Funct. Ecol. 2001, 15, 282–288. [Google Scholar] [CrossRef]
- McAlpine-Bellis, E.; Utsumi, K.; Diamond, K.; Klein, J.; Gilbert-Smith, S.; Garrison, G.; Eifler, M.; Eifler, D. Movement patterns and habitat use for the sympatric species Gambelia wislizenii and Aspidoscelis tigris. Ecol. Evol. 2023, 13, e10422. [Google Scholar] [CrossRef] [PubMed]
- Petelo, M.; Swierk, L. Trait allometries generate super-honesty in Anolis dewlaps and may underlie sexual dimorphism. Integr. Zool. 2017, 12, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Putman, B.J.; Azure, K.R.; Swierk, L. Dewlap size in male water anoles associates with consistent inter-individual variation in boldness. Curr. Zool. 2019, 65, 189–195. [Google Scholar] [CrossRef]
- Agresti, A. Categorical Data Analysis, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hertz, P.E. Adaptation to altitude in two West Indian anoles (Reptilia: Iguanidae): Field thermal biology and physiological ecology. J. Zool. 1981, 195, 25–37. [Google Scholar] [CrossRef]
- Hertz, P.E. Temperature regulation in Puerto Rican Anolis lizards: A field test using null hypotheses. Ecology 1992, 73, 1405–1417. [Google Scholar] [CrossRef]
- Hertz, P.E.; Arce-Hernandez, A.; Ramirez-Vazquez, J.; Tirado-Rivera, W.; Vazquez-Vives, L. Geographical variation of heat sensitivity and water loss rates in the tropical lizard, Anolis gundlachi. Comp. Biochem. Physiol. A 1979, 62, 947–953. [Google Scholar] [CrossRef]
- Rogowitz, G.L. Evaluation of thermal acclimation and altitudinal variation of metabolism in a neotropical lizard, Anolis gundlachi. Copeia 1996, 1996, 535–542. [Google Scholar] [CrossRef]
- Lopera, D.; Guo, K.C.; Putman, B.J.; Swierk, L. Keeping it cool to take the heat: Tropical lizards have greater thermal tolerance in less disturbed habitats. Oecologia 2022, 199, 819–829. [Google Scholar] [CrossRef]
- Hertz, P.E.; Huey, R.B.; Stevenson, R.D. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. Am. Nat. 1993, 142, 796–818. [Google Scholar] [CrossRef]
- Dzialowski, E.M. Use of operative temperature and standard operative temperature models in thermal biology. J. Therm. Biol. 2005, 30, 317–334. [Google Scholar] [CrossRef]
- Huey, R.B.; Pianka, E.R. Lizard thermal biology: Do genders differ? Am. Nat. 2007, 170, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lailvaux, S.P. Interactive effects of sex and temperature on locomotion in reptiles. Integr. Comp. Biol. 2007, 47, 189–199. [Google Scholar] [CrossRef]
- Huey, R.B. Temperature, physiology, and the ecology of reptiles. In Biology of the Reptilia; Gans, C., Pough, F.H., Eds.; Academic Press: New York, NY, USA, 1982; Volume 12, pp. 25–91. [Google Scholar]
- Angilletta, M.J., Jr. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Baldellou, M.; Henzi, S. Vigilance, predator detection and the presence of supernumerary males in vervet monkey troops. Anim. Behav. 1992, 43, 451–461. [Google Scholar] [CrossRef]
- Mathot, K.J.; van den Hout, P.J.; Piersma, T. Differential responses of red knots, Calidris canutus, to perching and flying sparrowhawk, Accipiter nisus, models. Anim. Behav. 2009, 77, 1179–1185. [Google Scholar] [CrossRef][Green Version]
- Monclús, R.; Anderson, A.M.; Blumstein, D.T. Do yellow-bellied marmots perceive enhanced predation risk when they are farther from safety? An experimental study. Ethology 2015, 121, 831–839. [Google Scholar] [CrossRef]
- Beaupre, S.J. (Ed.) Guidelines for Use of Live Amphibians and Reptiles in Field and Laboratory Research, 2nd ed.; American Society of Ichthyologists and Herpetologists: Miami, FL, USA, 2004. [Google Scholar]




| Low Males (46) | Low Females (40) | High Males (63) | High Females (33) | |
|---|---|---|---|---|
| SVL (mm) s,l | 64.8 ± 3.8 | 58.6 ± 2.2 | 67.9 ± 5.7 | 61.8 ± 3.5 |
| Mass (g) s,l | 5.6 ± 1.1 | 4.6 ± 0.5 | 6.9 ± 1.7 | 5.9 ± 1.1 |
| Air °C s,l | 25.1 ± 0.6 | 24.8 ± 0.6 | 20.8 ± 0.6 | 20.5 ± 0.7 |
| Substrate °C s,l | 24.9 ± 0.6 | 24.6 ± 0.6 | 20.5 ± 0.8 | 20.3 ± 0.7 |
| Body °C s,l | 25.4 ± 0.8 | 25.0 ± 0.5 | 21.0 ± 1.0 | 20.4 ± 1.1 |
| Perch height (cm) | 99 ± 81 | 92 ± 68 | 97 ± 57 | 89 ± 51 |
| Perch distance (cm) l | 44 ± 52 | 38 ± 41 | 86 ± 88 | 104 ± 98 |
| Visual noise l | 2.2 ± 1.0 | 2.0 ± 1.0 | 1.6 ± 1.1 | 1.2 ± 0.9 |
| Low-Elevation Site | ||||
| Body °C | Air °C | Substrate °C | Max °C | |
| Air °C | 0.86 | |||
| Substrate °C | 0.84 | 0.92 | ||
| Max °C | 0.5 | 0.53 | 0.51 | |
| Min °C | 0.82 | 0.87 | 0.85 | 0.55 |
| High-Elevation Site | ||||
| Air °C | 0.74 | |||
| Substrate °C | 0.69 | 0.72 | ||
| Max °C | 0.27 * | 0.37 | 0.45 | |
| Min °C | 0.62 | 0.56 | 0.7 | 0.42 |
| Low-Site Males (27) | Low-Site Females (19) | High-Site Males (26) | High-Site Females (8) | |
|---|---|---|---|---|
| Head turns * | 1.89 ± 1.54 | 1.79 ± 1.10 | 0.42 ± 0.40 | 0.49 ± 0.71 |
| Moves * | 0.36 ± 0.43 | 0.25 ± 0.38 | 0.02 ± 0.04 | 0.02 ± 0.05 |
| Feeding * | 0.02 ± 0.08 | 0.03 ± 0.06 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Head bobs | 0.08 ± 0.17 | 0.03 ± 0.06 | 0.04 ± 0.15 | 0.00 ± 0.00 |
| Dewlap displays | 0.03 ± 0.10 | 0.02 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eifler, D.A.; Dittmer, D.E.; Dick, L.; Rowe, B.; Johnson, J.J.; Stanley, D.R.; Eifler, M.A. Differences in Habitat Use, Thermal Ecology, and Behavior of the Semiaquatic Lizard Anolis aquaticus at a High- and Low-Elevation Site. Diversity 2025, 17, 673. https://doi.org/10.3390/d17100673
Eifler DA, Dittmer DE, Dick L, Rowe B, Johnson JJ, Stanley DR, Eifler MA. Differences in Habitat Use, Thermal Ecology, and Behavior of the Semiaquatic Lizard Anolis aquaticus at a High- and Low-Elevation Site. Diversity. 2025; 17(10):673. https://doi.org/10.3390/d17100673
Chicago/Turabian StyleEifler, Douglas A., Drew E. Dittmer, Leann Dick, Barbara Rowe, Jesse James Johnson, Dahtiya R. Stanley, and Maria A. Eifler. 2025. "Differences in Habitat Use, Thermal Ecology, and Behavior of the Semiaquatic Lizard Anolis aquaticus at a High- and Low-Elevation Site" Diversity 17, no. 10: 673. https://doi.org/10.3390/d17100673
APA StyleEifler, D. A., Dittmer, D. E., Dick, L., Rowe, B., Johnson, J. J., Stanley, D. R., & Eifler, M. A. (2025). Differences in Habitat Use, Thermal Ecology, and Behavior of the Semiaquatic Lizard Anolis aquaticus at a High- and Low-Elevation Site. Diversity, 17(10), 673. https://doi.org/10.3390/d17100673







