Abstract
A single specimen of a new genus and species of marine palaemonid shrimp was collected from the outer slope of the barrier reef of Koumac, northwest New Caledonia. The new shrimp is remarkable due to its unique series of diagnostic characters, i.e., the presence of compressed supraorbital teeth, a well-developed orbital cavity, closely set antennal and hepatic teeth on the carapace, swollen eyestalks, fully reduced endites on the maxilla, an anteriorly produced lobe on the fourth thoracic sternite, relatively small second chelipeds, and strongly biunguiculate ambulatory dactyli. This specimen occupies an isolated phylogenetic position among the symbiotic palaemonid shrimp analyzed, only demonstrating a feeble morphological inclination towards the “gnathophyllid” section of these. However, neither the main diagnostic characters nor genetic data allow for further clarification of a closer relationship between this new shrimp and any currently known palaemonid genus as of yet. The new genus and species is described in this report, and its phylogenetic position is discussed based on molecular and morphological characters.
1. Introduction
Palaemonidae Rafinesque, 1815, with almost 160 currently valid genera and over 1000 known species, most of which belong to a wide assemblage of predominantly marine symbiotic shrimp earlier recognized as the traditional subfamily Pontoniinae Kingsley, 1879 (recently synonymized with Palaemonidae; [1]), is the most speciose family of caridean shrimp. Among these genera, a large number of species are still placed in the genus Periclimenes O.G. Costa, 1844. Due to the generally “palaemon-like” body shape of these species and historically broad generic diagnosis, the genus now comprises quite a heterogeneous and speciose assemblage of more or less unrelated taxa; moreover, the polyphyletic state of the genus has been confirmed by recent molecular studies (e.g., [2,3,4,5]).
A major revision of the genus Periclimenes is still in preparation by the present team. Due to previous partial revisions, especially after the last millennium break, a wide series of Periclimenes species was transferred into reinstated historical genera, e.g., Ancylocaris Schenkel, 1902; Cuapetes Clark, 1919; and Laomenes Clark, 1919 [6,7,8], or established as representatives of new genera, e.g., Actinimenes Ďuriš & Horká, 2017; Ancylomenes Okuno & Bruce, 2010; Brucecaris Marin & Chan, 2006; Cristimenes Ďuriš & Horká, 2017; Leptomenaeus Bruce, 2007; Michaelimenes Okuno, 2017; Phycomenes Bruce, 2008; Rapimenes Ďuriš & Horká, 2017; and Unguicaris Marin & Chan, 2006 [6,8,9,10,11,12,13]. The introduction of combined bulk collecting techniques in recent marine biodiversity expeditions (e.g., [14,15]), as well as the broad application of molecular analytic methods, has revealed a quantity of data highly important for taxonomic and faunistic studies, and these have allowed for the discovery of unique specimens as potential representatives of new taxa as well.
Recently, a single tiny specimen of an undescribed palaemonid shrimp, initially identified as a Periclimenes, was collected during the Koumac 2.3 expedition to New Caledonia (Figure 1). It was caught by means of a “brushing” technique (e.g., [15]) from dead corals and stones at the outer slope of the barrier reef. Due to the unique set of morphological characters of the specimen observed via closer examination in the lab and a subsequent DNA comparison with a series of palaemonid taxa, it was confirmed as a representative of a new genus with a separate phylogenetic position among symbiotic palaemonid shrimp. Thus, the aim behind this study was to describe and illustrate this new genus and species, as well as discuss its unique phylogenetic position.
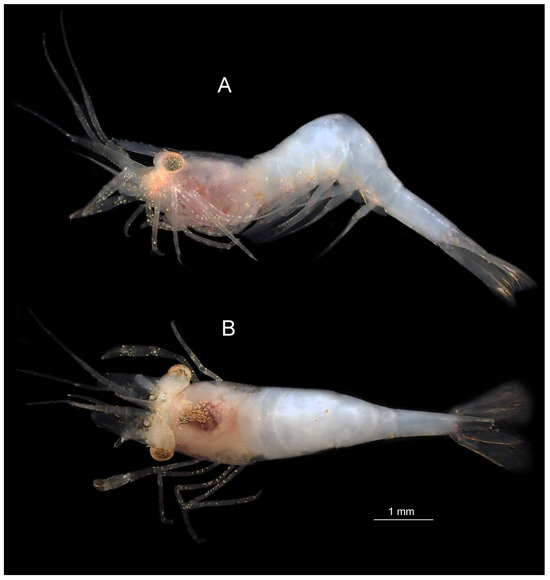
Figure 1.
Koumacella neocaledonica, gen. et sp. nov., holotype, female (MNHN-IU-2019-3593), habitus and color pattern: (A) lateral aspect; (B) dorsal aspect. Photos: Z. Ďuriš.
2. Materials and Methods
2.1. Morphological Analysis
A single specimen of a new genus and species was collected during an international biodiversity expedition of the Muséum National d’Histoire Naturelle, Paris (MNHN), to New Caledonia (KOUMAC Expedition 2018) within the program Our Planet Reviewed/La Planète Revisitée, in which the first author (ZĎ) participated. The specimen was caught via a bulk collecting method (brushing of dead corals) and was separated (already dead) from sieved material, photographed post mortem by means of sequence photography using an OLYMPUS TG4 photo camera, and preserved in 80% ethanol. Drawings of the shrimp and its body parts were created using a drawing tube attached to the stereomicroscope WILD M8. Final figures were redrawn using the Wacom tablet Cintiq 13HD. The biometric parameter pocl (=post-orbital carapace length) was measured in the dorsal midline of the carapace, from the posterior orbital margin to the posterior margin. The rostral formula (RF) used signifies the number of postorbital teeth on carapace + number of teeth on the rostrum proper/number of ventral rostral teeth.
2.2. Molecular Analyses
A series of Indo-West Pacific (IWP), western Atlantic (WA), and eastern Atlantic (EA) symbiotic palaemonid shrimp, comprising members of Periclimenes, in addition to representatives of genera previously separated from Periclimenes-like species of other genera (e.g., Diapontonia Bruce, 1986), and some other palaemonids (Altopontonia Bruce, 1990; Pliopontonia Bruce, 1973; and previous “gnathophyllid” and “hymenocerid” shrimp) were selected for use as a molecular background in the search for the phylogenetic position of Koumacella, gen. nov. among palaemonid shrimp. All of the analyzed species represented an assemblage of selected taxa from Clades 3, 4, and 7 in the study of Horká et al. [3] or the Pon-II group of Chow et al. [4,5]. The genera from the Palaemonella/Cuapetes complex by Frolová et al. [16] or the Pon-I group of Chow et al. [4,5] and most of the deeply specialized symbiotic genera (e.g., spongobionts and corallobionts) were excluded from the analysis in this study as they share an evolutionarily more remote lineage.
A total of 34 palaemonid shrimp species from 17 genera were used in the molecular analysis performed in this study. Of these, seven Periclimenes species from two biogeographic regions (four from EA; three from IWP) and one specimen of Koumacella neocaledonica, gen. et sp. nov. were newly sequenced. Exoclimenella maldivensis Ďuriš & Bruce, 1995, and Stenopus hispidus (Olivier, 1811), were selected as outgroup taxa. Details of the analyzed specimens, biogeographic areas, voucher identification numbers, sampling locations, and GenBank accession numbers are provided in Table 1.

Table 1.
Newly obtained DNA sequences used in the phylogenetic analyses with details on biogeographic region, voucher ID, sampling locations, and GenBank accession numbers of analyzed specimens. Abbreviations: IWP, Indo-West Pacific; Mart., Martinique; N/A, not available; N.Cal, New Caledonia; WA, western Atlantic; pres, present study.
The used sequences retrieved from GenBank are listed in Table 2. In total, 24 sequences for four genes, i.e., 6 for the cytochrome c oxidase subunit I (COI), 7 for the histone 3 (H3), 8 for 16S rRNA (16S), and 3 for 18S rRNA (18S), were obtained from newly analyzed specimens (Table 1). In addition, 83 sequences in total for the above-mentioned genes (Table 2) were retrieved from GenBank [2,3,5,17,18,19,20]. The final combined dataset of four genes (COI, 16S, H3 and 18S) consisted of 1774 bp (420 for COI, 410 for 16S, 290 for H3, and 654 for 18S).

Table 2.
DNA sequences retrieved from GenBank and used in the present analyses.
The total genomic DNA was isolated from pleopods or pleonal muscle tissue using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The mitochondrial genes for COI and 16S were amplified using the primer pairs LCO1490/HCO2198 [21], 16Sar/16Sbr [22], and 1471B/1472B [23], respectively. The nuclear genes H3 and 18S were amplified using the primer pairs H3af/H3ar [24] and 18Sa2.0/18S9r [25], respectively. Subsequent PCRs were performed in 20 μL volumes containing 1 μL of DNA template, 0.4 μM of both forward and reverse primers, Milli-Q water, MyTaq Red PCR buffer, and 1 U of MyTaq™ Red DNA polymerase (Bioline Reagents, London, UK). The thermal cycling profiles were as follows: 16S, according to Bracken-Grissom et al. [26], H3, according to Li et al. [27], and COI, 18S, according to the protocol of Horká et al. [3]. The amplified products were purified using the Gel/PCR DNA Fragments Extraction Kit (Geneaid Biotech Ltd., New Taipei City, Taiwan) following the manufacturer’s protocol. Sequencing reactions were performed using an ABI3730XL DNA Sequencer at Macrogen, Amsterdam. The chromatograms were checked and edited in the software Chromas v2.6.6 (Technelysium, South Brisbane, Australia). All newly obtained 16S, 18S, COI, and H3 gene sequences were deposited in GenBank (Table 1).
Multiple sequence alignments were constructed using the MUSCLE algorithm [28] in MEGA v10 software [29]. In MEGA v10, the protein-coding genes (H3, COI) were translated to amino acids to control frameshift mutation and stop codons. The substitution saturation for COI and H3 genes was tested in DAMBE v7.3.11 [30] according to an index by Xia et al. [31]. In the COI gene, a saturation was detected at the third position and, thus, the latter was excluded from further analyses. The alignments for non-coding genes 16S and 18S were cleaned of highly divergent blocks within the software Gblocks v0.91 [32] with default parameters, but allowing half-gap positions.
Maximum Likelihood analysis (ML) with 1000 replicates was performed using IQ-TREE v1.6.12 [33]. The TPM3 + F + G4 model, according to BIC, was selected as the best-fit model according to ModelFinder [34] on the IQ-TREE web server available at http://iqtree.cibiv.univie.ac.at (accessed on 1 June 2024) [35].
The Bayesian phylogeny (BI) was inferred using parameters selected by PartitionFinder v2.1.1 [36] and conducted in MrBayes on XSEDE v3.2.7a [37] via the online CIPRES Science Gateway v3.3 [38]. The analysis was run for 20 million generations saving a tree every 1000 generations, with 25% of trees discarded as burn-in. The convergence of the Bayesian analysis was confirmed by an analysis of output parameters in Tracer v1.7.2 [39]. Both ML and BI trees were displayed in the online application iTOL v6 [40].
3. Results
3.1. Molecular Analysis
Based on the molecular analysis conducted (Figure 2) on a series of predominantly Periclimenes or Periclimenes-like species, Koumacella, gen. nov. is well nested within the family Palaemonidae in the assemblage of “predominantly symbiotic palaemonid shrimps” (Clades 2–7 of Horká et al. [3] or the “Pon-II” group of Chow et al. [4,5]). In the phylogenetic tree, the new species is well nested within the main variety of the IWP, as well as the Atlantic (Atl.) and deep-water genera, being separately positioned between two main clades there. These comprise Clade A and the combined Clade B–E.
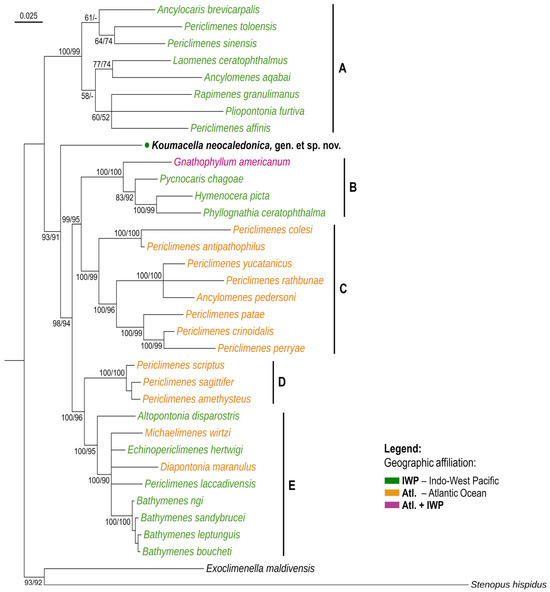
Figure 2.
Phylogenetic tree of Palaemonidae shrimp taxa resolved by Bayesian inference based on the combined dataset of four genes (COI, 16S, H3, 18S) showing the position of Koumacella neocaledonica, gen. et sp. nov. Bayesian posterior probabilities and Maximum Likelihood IQTREE bootstrap supports are marked as percentages, respectively, indicated on nodes (BI/ML). A dash (-) indicates values < 50. Geographic affiliations of species are highlighted by color. Main clades are marked with letters (A–E).
The former (Clade A) is composed of the IWP cnidarian and crinoid-associated species of the genera Ancylocaris, Ancylomenes, Laomenes, and Rapimenes, and also two members of the Periclimenes incertus/obscurus group and P. affinis (Zehntner, 1894). The latter clade (combined Clade B–E) comprises the IWP “gnathophyllid/hymenocerid” shrimp (Clade B), a series of western Atlantic cnidarian and crinoid-associated Periclimenes, one Ancylomenes species (Clade C), three eastern Atlantic Periclimenes anemone shrimp (Clade D), and the IWP and Atlantic mid- or deep-water shrimp, including representatives of Altopontonia, Diapontonia, Echinopericlimenes Marin & Chan, 2014, Michaelimenes, and Bathymenes Kou, Li & Bruce, 2015, as well as one Periclimenes, i.e., P. laccadivensis (Alcock & Anderson, 1894) (Clade E).
None of the examined species or genera exhibits a close molecular relationship with the new genus; thus, no distinct sister taxon has yet been detected.
3.2. Systematics
Decapoda Latreille, 1802
Infraorder Caridea Dana, 1852
Family Palaemonidae Rafinesque, 1815
3.2.1. Koumacella, gen. nov.
(urn:lsid:zoobank.org:act:047E7465-94B9-4CFC-84FA-6974A0246309)
Generic Diagnosis. Small-sized palaemonid shrimp. Carapace with depressed triangular supraorbital teeth and antennal and hepatic teeth, epigastric tooth lacking; hepatic tooth set closely posterior and slightly below antennal tooth. Rostrum straight, with seven low dorsal teeth, distinct midrib, and two subterminal ventral teeth; inferior orbital angle triangular, orbit with distinct dorsal and ventral ridges, obscured posteriorly. Thoracic sternites broad, fourth sternite produced anteriorly, depressed, trapezoid. Antennula and antenna normal, without special features, scaphocerite moderately broad, with small distolateral tooth. Eyes held obliquely backwards, with small globular cornea, without accessory spot, stalk larger than cornea, swollen, depressed. Mandible without palp, incisor process with four teeth, molar process slender, oblique and dentate distally. Maxillula with bilobed palp, well developed upper lacinia and small lower lacinia. Maxilla with well-developed palp and scaphognathite, endites fully reduced, non-setose. First maxilliped with simple palp, distal endite broad, exopod well developed, caridean lobe normal, epipod bilobed. Second maxilliped with normal endopod and exopod, epipod small, simple, without podobranch. Third maxilliped with moderately broad antepenultimate segment; exopod well developed, coxa with stout hooked lateral plate, arthrobranch absent. First pereiopods stout, fingers narrow, simple, slightly longer than palm, coxa with medial setose process. Second pereiopods similar and equal, greatly reduced in size (compared to most palaemonids), only moderately larger than first pereiopods, fingers distinctly shorter than palm, cutting edges with three (dactylus) or two (fixed finger) small recurved proximal teeth. Ambulatory pereiopods slender, dactyli broadly biunguiculate, with strong accessory tooth on corpus, propodus with pair of distoventral spines and two spaced minute spinules on ventral margin. Pleon with anterior pleurae rounded, pleurae IV and V posteroventrally acutely produced; telson narrow, with at least one pair of dorsal spinules around midlength. Pleopods normal. Uropods with narrow rami, distolateral angle of exopod with small tooth and movable spine.
Etymology. The generic name is derived from the type locality, the Koumac region, New Caledonia; gender: feminine.
Type species. Koumacella neocaledonica, gen. et sp. nov., by present designation.
Included species. Koumacella neocaledonica, sp. nov.
Systematic position. The new genus is characterized by the presence of the following main diagnostic features: (1) the presence of supraorbital teeth, (2) swollen eyestalks, (3) a prominent depressed lobe positioned anteriorly on the fourth thoracic sternite, (4) fully reduced maxillar endites, (5) moderately reduced second pereiopods, and (6) ambulatory legs with well-developed biunguiculate dactyli. Most of those characters are rarely present among symbiotic palaemonids (i.e., traditional Pontoniinae). The only palaemonid genus whose current diagnosis [10,41] might formally qualify as having these characters is Periclimenes. Historically, a series of diagnostic characters allowed for the acquisition of a multivariate state, which allowed the genus to turn into a highly heterogeneous polyphyletic assemblage of numerous, often unrelated, taxa (e.g., [2,3,4,5]). All of the characters mentioned above (simultaneously with the biunguiculate ambulatory dactyli) immediately distinguish Koumacella, gen. nov. from the core group of Periclimenes (s.s.), i.e., the eastern Atlantic P. amethysteus group of the anemone-associated species [42,43], thus distinguishing it as a representative of a separate genus.
Based on the molecular comparison performed, the new genus is affiliated to the main assemblage of symbiotic palaemonids (i.e., Clades 2–7 of Horká et al. [3] or the “Pon-II” group of Chow et al. [4,5]). Within that assemblage, well-developed supraorbital teeth are present in the following genera: Anchistioides Paulson, 1975; Apopontonia Bruce, 1976; Araiopontonia Fujino & Miyake, 1970; Climeniperaeus Bruce, 1994; Cristimenes Ďuriš & Horká, 2017; Laomenes Clark, 1919; Pontonides Borradaile, 1917; Notopontonia Bruce, 1991; some Periclimenaeus spp.; and in one species of Hamodactylus Holthuis, 1952, i.e., H. boschmai Holthuis, 1952.
From those taxa, only some share two or three of the above-mentioned characters with Koumacella, gen. nov.; Laomenes nudirostris (Bruce, 1968) and Hamodactylus boschmai possess eyestalks moderately swollen basally, with the eyes usually held retracted. The latter also has second pereiopods reduced in size (but the reduction is extreme, with those legs even smaller than the first chelipeds); L. nudirostris differs from the new genus with well-developed strong second chelipeds, with dentate fingers, and with ambulatory dactyli bearing a minute accessory unguis ([44]: Figure 103; [45]: Figure 26).
Swollen eyestalks may also be present in some Periclimenaeus Borradaile, 1915, species (e.g., P. rastrifer Bruce, 1980; [46]) or small shrimp of Onycocaris Nobili, 1904 (e.g., [47,48]), but their stout eyes and bodies are strongly adapted to the endo-spongobiotic habit, and all of them widely differ from the “Periclimenes-like” appearance of Koumacella, gen. nov.
Deeply or fully reduced maxillar endites are frequently present in the palaemonid genus Anchistioides and in shrimp of the previously recognized families Gnathophyllidae Dana, 1852, and Hymenoceridae Ortmann, 1890 (now synonymized with Palaemonidae; [1]). Anchistioides species also usually possess supraorbital teeth, as in the new genus, but lack the hepatic tooth and exopods on maxillipeds I and III, and have stout second pereiopods and simple or feebly biunguiculate ambulatory legs instead [49,50,51]. “Gnathophyllid” and “hymenocerid” shrimp differ from the new genus by their lack of mandibular incisor, whereas the incisors are well developed in Koumacella, gen. nov. and in Anchistioides.
A rarely occurring character, the depressed and anteriorly produced linguiform lobe on the fourth thoracic sternite, which has only been reported in Periclimenes difficilis Bruce, 1976 ([52]: 111, Figure 16B), and, of a lesser size, in the genus Phycomenes ([53]: Figure 2C [as Periclimenes]; [11]: Figures 2I and 6E). Both of these taxa also have biunguiculate ambulatory dactyli ([52]: Figure 17B; [11]: Figure 4F–J), as in the new genus, with the Phycomenes species also having second pereiopods partly reduced in size. Nevertheless, both lack supraorbital teeth, and their maxillae have a developed distal endite (e.g., [52,53,54]).
Thus, generally, Koumacella, gen. nov. does not demonstrate a close relationship with any of the currently known taxa, and it occupies an isolated position among the genera of symbiotic palaemonid shrimp.
3.2.2. Koumacella neocaledonica, sp. nov.
(urn:lsid:zoobank.org:act:C381F775-6B9B-437C-B30B-8BF63397876D)
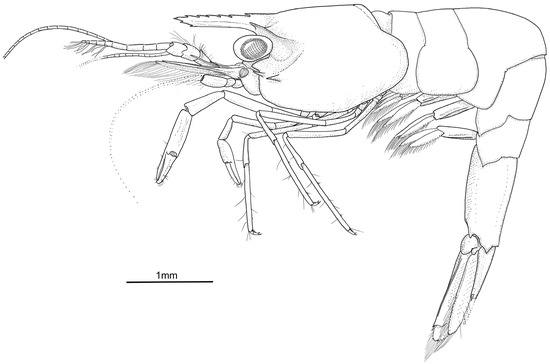
Figure 3.
Koumacella neocaledonica, gen. et sp. nov., holotype, female (MNHN-IU-2019-3593), habitus, lateral view.
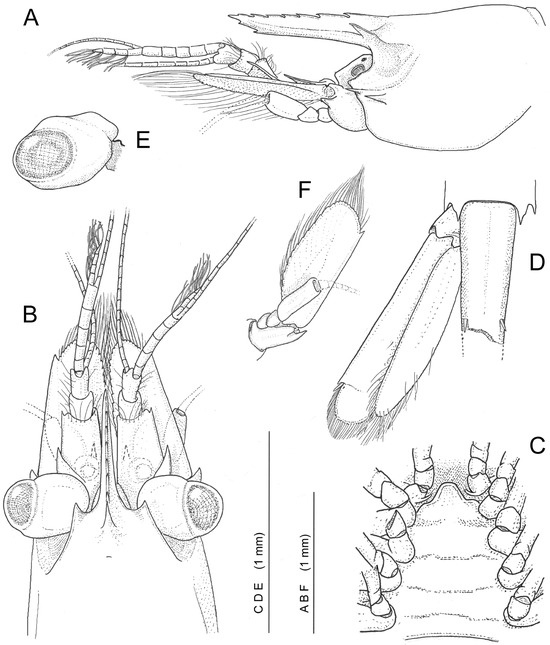
Figure 4.
Koumacella neocaledonica, gen. et sp. nov., holotype, female (MNHN-IU-2019-3593): (A) cephalothorax and antennae, lateral view (eye not shown); (B) carapace, antennae, and eyes, dorsal view; (C) thoracic sternum, 4th to 8th segments, ventral view; (D) telson (broken) and left uropod, dorsal view; (E) left eye, posterolateral view; (F) right antenna, ventral view.
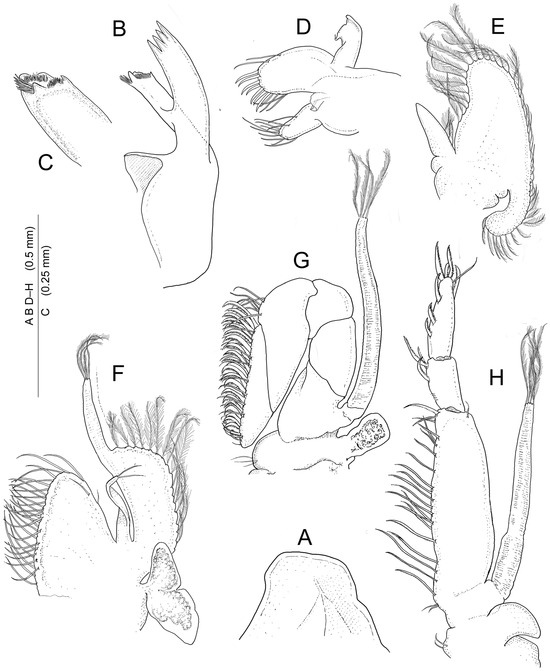
Figure 5.
Koumacella neocaledonica, gen. et sp. nov., holotype, female (MNHN-IU-2019-3593), mouthparts (left appendages dissected): (A) paragnaths; (B) mandible; (C) same, molar process; (D) maxillula; (E) maxilla; (F) first maxilliped; (G) second maxilliped; (H) third maxilliped.
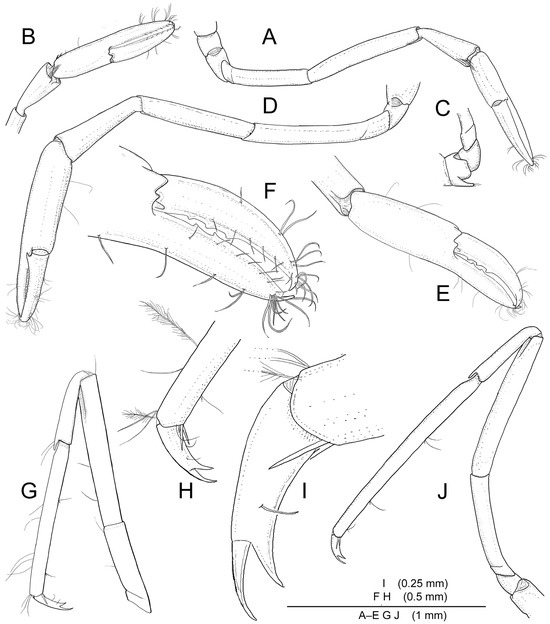
Figure 6.
Koumacella neocaledonica, gen. et sp. nov., holotype, female (MNHN-IU-2019-3593): (A) right first pereiopod, outer view; (B) same, chela and carpus; (C) same, proximal segments; (D) left second pereiopod, outer view; (E) same, chela, lateral side; (F) same, fingers; (G) left third pereiopod, lateral view; (H) same, dactylus and distal propodus; (I) same, dactylus; (J) left fifth pereiopod, lateral view.
Type material. Holotype: female, pocl 1.15 mm, RF 7/2, MNHN-IU-2019-3593, KOUMAC 2.3, KB650, New Caledonia, off Koumac, 20°46.6′ S, 164°15.7′ E, barrier reef, overhang and boulders on outer slope, depth 33 m, 18 November 2019, collected by “brushing” team of the expedition.
Description (female holotype). Small sized shrimp with cephalothorax somewhat compressed, pleon subcylindrical (Figure 1 and Figure 3). Carapace (Figure 3 and Figure 4A,B) smooth, with supraorbital, antennal and hepatic teeth, postrostral and epigastric teeth lacking (small pit indicated on carapace behind posteriormost rostral tooth); antennal tooth submarginal, failing to reach half-length of dorsal length of antennal basicerite, hepatic tooth stronger and situated immediate posteriorly and slightly below level of antennal tooth. Rostrum 0.9 of pocl, straight, anteriad, with distinct midrib; dorsal lamina bearing seven low teeth with horizontal dorsal margin, ventral lamina lacking, with two minute subapical teeth. Lateral ridges narrow, basally expanded into strong depressed and anteriad triangular supraorbital teeth failing to reach level of apex of posteriormost dorsal rostral tooth; supraorbital tooth extending posterolaterally into distinct dorsal orbital ridge. Orbital cavity extending to 0.3 of pocl, feebly subdivided into deeper anterior and shallower posteroventral chambers to accommodate eyestalk and cornea (respectively) when flexed back, dorsal and posteroventral ridges distinct, postorbital ridge incomplete, obscured posterodorsally. Inferior orbital angle triangular, subacute. Pterygostomian angle of carapace rounded.
Ophthalmic somite without interocular process. Thoracic sternites (Figure 4C) broad, fourth thoracic sternite with strong depressed obtusely trapezoid plate, posterior sterna unarmed.
Pleonal segments (Figure 3) with tergites non-carinate or posteriorly produced; pleura of first to third pleomeres posteroventrally rounded; pleura of fourth and fifth pleomeres acutely produced; sixth pleomere 0.9 of pocl, dorsally approximately twice longer than fifth pleomere, and twice longer than deep anteriorly, posterolateral and posteroventral lobes subtriangular, acutely produced. Telson broken, posterior part lost; anterior part narrow, broken at level of first pair of small dorsal spinules (on length respective to 0.6 of sixth pleomere dorsal length).
Eyes (Figure 4E) held obliquely backwards, cornea small, rounded, diameter 0.25 of pocl, eyestalk swollen, wider, and longer than corneal length, additional pigment spot absent.
Antennula (Figure 4A,B) well developed; basal segment approximately 1.6 times as long as wide; stylocerite acute, overreaching middle of basal segment, distolateral tooth small, acute, slightly overreaching small rounded anterior margin, ventral tooth situated on middle of medial margin; intermediate and distal segment combined length approximately half of basal segment length; intermediate segment robust, subquadrate; distal segment narrower but slightly longer than preceding segment; upper flagellum biramous, fused part with five segments, shorter free ramus consisting of four segments, with five groups of aesthetascs, longer free ramus and lower flagellum slender, filiform.
Antenna (Figure 4A,B,F) with basicerite bearing distolateral tooth; carpocerite reaching midlength of scaphocerite; scaphocerite exceeding antennular peduncle and rostrum, 2.5 times as long as maximum width, lateral margin straight, distolateral tooth small, falling short of distal margin of lamina.
Mouthparts with paragnaths (Figure 5A) simple, obtusely trapezoid, without alae; left mouth appendages dissected (Figure 5B–H).
Mandible (Figure 5B,C) without palp, incisor process (Figure 5B) elongate, with four terminal teeth, molar process slender, elongate, occlusal field oblique with four teeth and three fields of setal brushes.
Maxillula (Figure 5D) with palp feebly bilobed, lower lobe with small hooked seta, upper lacinia larger than lower lacinia, broadly rounded distally, with seven strong distal spines and several setae; lower lacinia tapering distally to rounded apex bearing group of slender spines.
Maxilla (Figure 5E) with well-developed palp three times longer than wide basally, tapering to non-setose apex, basally with three lateral plumose setae; coxal and basal endites fully reduced, non-setose; scaphognathite narrow posteriorly, broad anteriorly, approximately 2.2 times longer than wide, with plumose marginal setae.
First maxilliped (Figure 5F) with slender palp bearing two apical setae; basal endite well developed, broad and rounded anteriorly, with numerous spiniform setae along medial margin (coxal endite broken, form unknown); exopod with caridean lobe well developed, overreaching level of basal endite, with plumose marginal setae; flagellum shorter than caridean lobe (latter measured from base of palp), with four terminal plumose setae; epipod broadly triangular, bilobed.
Second maxilliped (Figure 5G) stout, dactylar segment elongately triangular, 3.6 times longer than broad, with numerous stout curved serrulate setae along medial margin; medial angle of propodus broadly rounded, with several slender setae; carpus and merus short, ischium and basis fused; coxa rounded medially, epipod short, simple, without podobranch; exopod elongate, overreaching flexed endopod by distal third, apex with four plumose setae.
Third maxilliped (Figure 5H) stout; coxa with proximolaterally hooked lateral plate, rounded laterally, arthrobranch absent; basis and ischiomerus fused, ischio-basal suture feebly demarcated, compound antepenultimate segment compressed, broad, 3.3 times as long as wide basally, with long serrulate setae medially, penultimate segment short, 1.5 times as long as wide and 0.25 of antepenultimate segment length, with three stout serrate setae distomedially; ultimate segment 1.5 times as long as penultimate one, and three times as long as wide, with several stout serrate setae; exopod only slightly overreaching ischiomerus, apically with four plumose setae.
First pereiopod (Figure 6A–C) slender, unarmed, falling short of end of scaphocerite; chela 0.6 of pocl, fingers 0.55 of chela length; fingers (Figure 6A,B) slender, five times as long as deep, tapering distally to hooked acute tip on movable finger and double tip on fixed finger, cutting edges simple, several tufts of long setae on distal halves of fingers; palm subcylindrical, twice longer than deep, with two short rows of cleaning setae proximally; carpus half of chela length and approximately twice as long as distal width, with several long cleaning setae distoventrally, tapering proximally; merus subequal to chela length and eight times longer than uniform depth; ischium as deep as and 0.8 of merus length; basis and coxa short, coxa with small setose proximomedial process (Figure 6C).
Second pereiopods short, approximately 1.35 times longer than first pereiopods, and overreaching scaphocerite by half of palm, similar in shape and equal in size (Figure 6D–F); chelae held with dactyli laterally, length 0.7 of pocl; fingers moderately stout, four times as long as deep, slightly curved, cutting edges narrow laminar, entire distally; dactylus with three low recurved teeth on proximal half, tip sharp, hooked; fixed finger with two recurved proximal teeth, laminar edge incised near base of oblique tip to accommodate hooked tip of dactylus; palm 1.5 times longer than fingers, slightly compressed, approximately 2.3 times as long as deep; carpus 0.8 of palm length and 3.3 times as long as proximal width, tapering proximally; merus subequal to palm length and five times longer than uniform depth; ischium as deep as and 1.2 times longer than merus; basis and coxa short.
Third pereiopod (Figure 6G–I) slender, reaching (if extended anteriorly) distal end of scaphocerite by apex of dactylus; dactylus biunguiculate, 3.5 times longer than deep basally, curved; unguis occupying distal third of dactylus, well demarcated from corpus, slender, with sharp curved tip; corpus compressed, distoventral accessory tooth stout, reaching two-thirds of unguis length, ventral margin of corpus smoothly concave; propodus 0.6 of pocl, four times longer than dactylus and 10 times as long as uniform depth, with pair of distoventral slender spines (as long as basal dactylar depth) near sides of dactylus, and with two minute single spinules on distal quarter and proximal third of ventral margin; carpus 0.5 of propodus length and four times as long as distal depth, tapering proximally; merus slightly shorter than propodus, twice longer than carpus and seven times as long as uniform depth; ischium slightly deeper and approximately 0.7 of merus length, basis and coxa short.
Fourth and fifth pereiopods generally similar to third leg but gradually longer, fifth pereiopod (Figure 6J) 1.15 times longer than third pereiopod, propodus without grasping setae.
Uropods (Figure 4D) narrow, elongate; lateral border of uropodal exopod straight, with small distal tooth and slender mobile spine 2.5 times longer than tooth.
Measurements. Female holotype: total body length (from tip of rostrum to posterior margin of telson) ~6.7 mm; rostrum length 1.0 mm; pocl 1.15 mm; scaphocerite length × width, 1.0 × 0.4 mm; first pereiopod length 2.2 mm; first pereiopod chela length 0.6 mm; second pereiopod length 3.0 mm; second pereiopod chela length 0.9 mm; third pereiopod length 2.7 mm; fifth pereiopod length 3.1 mm; sixth pleomere dorsal length × depth 1.15 × 0.6 mm; telson (broken).
Coloration. Single available specimen (post mortem photographed) generally colorless, whitish, with yellowish tinge on eyestalks, bases of antennae, thoracic and anterior pleonal bottom; internal thoracic organs dark, densely white-dotted dorsally. Some recognizable remains of in-life coloration present, i.e., antennular statocysts white; eye cornea and eyestalks white-dotted on yellowish background; interocular band diffusely white; carapace with several scattered red and white dots anteriorly and ventrally on branchiostegites; three red dots in “V” figure dorsally on third pleomere; pereiopods white-dotted, more densely near articulations.
Etymology. The specific name is derived from New Caledonia, the country of the type locality of the species, with the prefix neo– from neos (Greek), = new.
Host and habitat. Host unknown; the species was collected from dead corals.
Distribution. Known only from the barrier reef off Koumac at New Caledonia.
4. Discussion
As pointed out in the “Systematic position” paragraph under the genus (above), Koumacella neocaledonica, gen. et sp. nov. is nested well within a wide assemblage of marine palaemonid shrimps composed of genera containing predominantly symbiotic species (Clades 2–7 in the phylogeny of Horká et al. [3], or “Pon-II” group of Chow et al. [4,5]). Nevertheless, its sister clade encompasses a range of different lifestyles, making it difficult to infer the mode of life of Koumacella.
As pointed out by Bruce ([55]: 1269), useful clues to both the systematic relationships and the host animals might be inferred from the shape of the dactyli of the ambulatory legs in associated shrimps. However, except for the non-simple (here biunguiculate) ambulatory dactyli, which generally allows inference of a symbiotic mode of life, the body of Koumacella, gen. nov. does not provide any further evidence for a potential host group of the shrimp.
The well-developed biunguiculate ambulatory dactyli with two widely set and subequal terminal “ungui” are typical of a number of sponge-dwellers, such as the Indo-West Pacific Thaumastocaris streptopus Kemp, 1922, inhabiting cavities and larger channels in sponges [56], and Periclimenes incertus Borradaile, 1915, a frequent ectobiont [57]. On the other hand, Brachycarpus biunguiculatus (Lucas, 1846), a pantropical shrimp positioned remotely from the Pon-II group among palaemonids (after Chow et al. [4]), also possesses similar ambulatory dactyli. This is regarded as a free-living species, although has also been occasionally observed as a fish cleaner [58,59] or an inhabitant of large cavities in sponges or on dead bases of scleractinian corals (ZĎ, pers. observ.).
Koumacella neocaledonica, sp. nov. is most likely not a sponge-endosymbiont due to its elongate and dentate rostrum; a pair of large, widely set supraorbital teeth; and feeble second chelipeds. However, sponge-ectobiosis cannot be excluded; the supraorbital teeth are present in Apopontonia and Climeniperaeus, and a dentate rostrum occurs in the following ectobiotic spongobionts: species of Anchistioides, adult Thaumastocaris Kemp, 1922, and P. incertus (cf. [4,56,60]); the latter also possesses the anteriorly produced lobe on the fourth thoracic sternite, as in the new genus. In P. incertus, a minute body and slender pereiopods are also similarly present. Nevertheless, the analyzed species of the P. incertus/obscurus group are positioned remotely from the new genus in the present molecular tree.
In conclusion, the new species, Koumacella neocaledonica, belongs to a new genus of shrimp of the family Palaemonidae. None of the currently known genera share a large number of morphological features with Koumacella, gen. nov. The phylogenetic position of the new genus is not yet satisfactorily clarified, as the sister genus has not been identified. Koumacella neocaledonica, gen. et sp. nov. is phylogenetically nested among the majority of symbiotic taxa of the family, yet occupies an isolated position.
Author Contributions
Conceptualization, Z.Ď.; internal funding acquisition, Z.Ď. and K.F.; investigation, Z.Ď. and K.F.; resources, Z.Ď.; writing original draft, Z.Ď. and K.F.; writing—review and editing, Z.Ď. and K.F.; photographs and hand-drawn figures, Z.Ď.; DNA analyses and evolutionary tree figure, K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially financed by the student grant projects SGS01/PřF/2023 and SGS06/PřF/2024 of University of Ostrava (KF); from the MSK/SMO scholarship from the Moravian–Silesian Region (KF); and from institutional support from University of Ostrava (ZĎ).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All newly produced sequences were deposited in GenBank.
Acknowledgments
The described specimen was collected during the Koumac 2.3 expedition to New Caledonia in 2019 under the “Our Planet Reviewed/La Planète Revisitée” global initiative founded in 2007 by Muséum National d’Histoire Naturelle (MNHN), Paris, and Pro-Natura International. The New Caledonia expedition (2016–2019) is a project of MNHN (PI: Philippe Bouchet) in partnership with Conservatoire d’Espaces Naturels de Nouvelle-Calédonie. The Koumac part of the expedition was funded mainly by the Gouvernement de la Nouvelle-Calédonie, Province Nord, Agence Française de la Biodiversité, the Lounsbery Foundation, Office des Postes et Télécommunications, Maison de la Nouvelle-Calédonie, and in-kind support from Mairie de Koumac, Société Nationale de Sauvetage en Mer, Régiment du Service Militaire Adapté de Koumac, Base Navale de Nouméa, AirCalin, Avis Nouvelle-Calédonie, and Socalait. The expedition operated under permits num. 609002-31/2018/DEPART/YM and 609011-55/2019/DEPART/JJC issued, respectively, on the 27 August 2018 and 22 October 2019 by the Direction du Développement Economique et de l’Environnement of Province Nord. Geraldine Vallejo (Groupe Kering) generously sponsored the description of the new species at a charity dinner held at MNHN, Nov. 5, 2019. We extend our deep gratitude to P. Bouchet for inviting the first author (ZĎ) to the Koumac expeditions, and to the MNHN staff, especially Laure Corbari as the coordinator of the Crustacea team, and international participants, for their logistic help and friendly atmosphere. Charles H.J.M. Fransen (Naturalis Biodiversity Center, Leiden), Shane Ahyong (Australian Museum), and Anna Šobáňová (University of Ostrava, UO) read the draft manuscript and kindly provided valuable remarks. Ivona Kočárková (UO) is acknowledged for consultation regarding the DNA methodology.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Grave, S.; Fransen, C.H.J.M.; Page, T.J. Let’s be pals again: Major systematic changes in Palaemonidae (Crustacea: Decapoda). PeerJ 2015, 3, e1167. [Google Scholar] [CrossRef] [PubMed]
- Kou, Q.; Li, X.; Chan, T.-Y.; Chu, K.H.; Huang, H.; Gan, Z. Phylogenetic relationships among genera of the Periclimenes complex (Crustacea: Decapoda: Pontoniinae) based on mitochondrial and nuclear DNA. Mol. Phylogenet. Evol. 2013, 68, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Horká, I.; De Grave, S.; Fransen, C.H.J.M.; Petrusek, A.; Ďuriš, Z. Multiple host switching events shape the evolution of symbiotic palaemonid shrimps (Crustacea: Decapoda). Sci. Rep. 2016, 6, e26486. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.H.; De Grave, S.; Tsang, L.M. The family Anchistioididae Borradaile, 1915 (Decapoda: Caridea) is a synonym of Palaemonidae Rafinesque, 1815 based on molecular and morphological evidence. J. Crustacean Biol. 2020, 40, 277–287. [Google Scholar] [CrossRef]
- Chow, L.H.; De Grave, S.; Tsang, L.M. Evolution of protective symbiosis in palaemonid shrimps (Decapoda: Caridea) with emphases on host spectrum and morphological adaptations. Mol. Phylogenet. Evol. 2021, 162, e107201. [Google Scholar] [CrossRef] [PubMed]
- Okuno, J.; Fujita, Y. Resurrection of the genus Laomenes A. H. Clark, 1919 (Decapoda, Caridea, Palaemonidae). Crustaceana 2007, 80, 113–124. [Google Scholar] [CrossRef]
- Okuno, J. Cuapetes Clark, 1919, a senior synonym of Kemponia Bruce, 2004 (Crustacea: Decapoda: Palaemonidae). Zootaxa 2009, 2028, 67–68. [Google Scholar] [CrossRef]
- Ďuriš, Z.; Horká, I. Towards a revision of the genus Periclimenes: Resurrection of Ancylocaris Schenkel, 1902, and designation of three new genera (Crustacea, Decapoda, Palaemonidae). ZooKeys 2017, 646, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Chan, T.-Y. Two new genera and a new species of crinoid-associated pontoniine shrimps (Decapoda: Caridea: Palaemonidae). J. Crustac. Biol. 2006, 26, 524–539. [Google Scholar] [CrossRef]
- Bruce, A.J. Leptomenaeus gen. nov., a new name for Leptomenes Bruce, 2006 (Crustacea: Decapoda: Pontoniinae). Cah. Biol. Mar. 2007, 48, 411–412. [Google Scholar]
- Bruce, A.J. Phycomenes zostericola gen. nov., sp. nov., a new pontoniine shrimp (Crustacea: Decapoda: Palaemonidae) from Moreton Bay, Queensland. In The Marine Fauna and Flora of Moreton Bay, Queensland. Proceedings of the Thirteenth International Marine Biological Workshop; Davie, P.J.F., Phillips, J.A., Eds. Mem. Qld Mus. 2008, 54, 219–232. [Google Scholar]
- Okuno, J.; Bruce, A.J. Designation of Ancylomenes gen. nov., for the ‘Periclimenes aesopius species group’ (Crustacea: Decapoda: Palaemonidae), with the description of a new species and a checklist of congeneric species. In: De Grave, S. & C.H.J.M. Fransen (ed.), Contributions to Shrimp Taxonomy. Zootaxa 2010, 2372, 85–105. [Google Scholar]
- Okuno, J. Designation of a new genus Michaelimenes (Decapoda: Caridea: Palaemonidae), with new host record and range extension of its type species, M. perlucidus (Bruce, 1969). Nauplius 2017, 25, e2017013. [Google Scholar] [CrossRef]
- Bouchet, P.; Loyouet, P.; Maestrati, P.; Heros, V. Assessing the magnitude of species richness in tropical marine environments: Exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc. 2002, 75, 421–436. [Google Scholar] [CrossRef]
- Albano, P.G.; Sabelli, B.; Bouchet, P. The challenge of small and rare species in marine biodiversity surveys: Microgastropod diversity in a complex tropical coastal environment. Biodivers. Conserv. 2011, 20, 3223–3237. [Google Scholar] [CrossRef]
- Frolová, P.; Horká, I.; Ďuriš, Z. Molecular phylogeny and historical biogeography of marine palaemonid shrimps (Palaemonidae: Palaemonella–Cuapetes group). Sci. Rep. 2022, 12, e15237. [Google Scholar] [CrossRef] [PubMed]
- Kou, Q.; Li, X.; Chan, T.-Y.; Chu, K.H.; Gan, Z. Molecular phylogeny of the superfamily Palaemonoidea (Crustacea: Decapoda: Caridea) based on mitochondrial and nuclear DNA reveals discrepancies with the current classification. Invertebr. Syst. 2013, 27, 502–514. [Google Scholar] [CrossRef]
- Aznar-Cormano, L.; Brisset, J.; Chan, T.-Y.; Corbari, L.; Puillandre, N.; Utge, J.; Zbinden, M.; Zuccon, D.; Samadi, S. An improved taxonomic sampling is a necessary but not sufficient condition for resolving inter-families relationships in Caridean decapods. Genetica 2015, 143, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.B.; Li, X.Z.; Chan, T.-Y.; Chu, K.H.; Kou, Q. Phylogeny of Indo-West Pacific pontoniine shrimps (Crustacea: Decapoda: Caridea) based on multilocus analysis. J. Zool. Syst. Evol. Res. 2015, 53, 282–290. [Google Scholar] [CrossRef]
- Chen, C.-L.; Goy, J.W.; Bracken-Grissom, H.D.; Felder, D.L.; Tsang, L.M.; Chan, T.-Y. Phylogeny of Stenopodidea (Crustacea: Decapoda) shrimps inferred from nuclear and mitochondrial genes reveals non-monophyly of the families Spongicolidae and Stenopididae and most of their composite genera. Invertebr. Syst. 2016, 30, 479–490. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech 1994, 3, 294–299. [Google Scholar] [CrossRef]
- Palumbi, S.; Martin, A.; Romaro, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR. Version 2.0; University of Hawaii: Honolulu, HI, USA, 2002. [Google Scholar]
- Liu, M.Y.; Cai, Y.X.; Tzeng, C.S. Molecular systematics of the freshwater prawn genus Macrobrachium Bate, 1868 (Crustacea: Decapoda: Palaemonidae) inferred from mtDNA sequences, with emphasis on east Asian species. Zool. Stud. 2007, 46, 272–289. [Google Scholar]
- Colgan, D.J.; Hutchings, P.A.; Beacham, E. Multi-gene analyses of the phylogenetic relationships among the Mollusca, Annelida, and Arthropoda. Zool. Stud. 2008, 47, 338–351. [Google Scholar]
- Whiting, M.F. Mecoptera is paraphyletic: Multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool. Scr. 2002, 31, 93–104. [Google Scholar] [CrossRef]
- Bracken-Grissom, H.D.; Robles, R.; Felder, D. Molecular phylogenetics of American snapping shrimps allied to Alpheus floridanus Kingsley, 1878 (Crustacea: Decapoda: Alpheidae). Zootaxa 2014, 3895, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; De Grave, S.; Lei, H.C.; Chan, T.-Y.; Chu, K.H. Molecular systematics of caridean shrimps based on five nuclear genes: Implications for superfamily classification. Zool. Anz. 2011, 250, 270–279. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Phylogenet. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Phylogenet. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z.; Salemi, M.; Chen, L.; Wang, Y. An index of substitution saturation and its application. Mol. Phylogenet. Evol. 2003, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Phylogenet. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Phylogenet. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Gateway Computing Environments Workshop (GCE 2010), New Orleans; IEEE: Piscataway, NJ, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J. Tracer. MCMC Trace Analysis Tool Version 1.7.2. 2009. Available online: https://github.com/beast-dev/tracer/releases/tag/v1.7.2 (accessed on 15 February 2024).
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.J. A synopsis of the Indo-West Pacific genera of the Pontoniinae (Crustacea: Decapoda: Pontoniinae). Thes. Zool. 1995, 25, 1–172. [Google Scholar]
- Grippa, G.B.; d’Udekem d’Acoz, C. The genus Periclimenes Costa, 1844 in the Mediterranean Sea and the Northeastern Atlantic Ocean: Review of the species and description of Periclimenes sagittifer aegylios subsp. nov. (Crustacea, Decapoda, Caridea, Pontoniinae). Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Natur. Milano 1996, 135, 401–412. [Google Scholar]
- Fransen, C.H.J.M.; Wirtz, P. A new Octocorallia-associated shrimp of the genus Periclimenes (Crustacea, Caridea, Palaemonidae) from West Africa. Arthropoda 2023, 1, 420–431. [Google Scholar] [CrossRef]
- Holthuis, L.B. A general revision of the Palaemonidae (Crustacea Decapoda Natantia) of the Americas. II. The subfamily Palaemoninae. Occ. Pap. A. Hancock Found. 1952, 12, 1–396. [Google Scholar]
- Bruce, A.J. Two new species of Periclimenes (Crustacea: Decapoda: Palaemonidae) from Lizard Island, Queensland, with notes on related taxa. Rec. Aust. Mus. 1992, 44, 45–84. [Google Scholar] [CrossRef][Green Version]
- Ďuriš, Z.; Šobáňová, A.; Lin, C.-W. Redescription and new record of the spongobiotic shrimp Periclimenaeus rastrifer Bruce, 1980 (Crustacea: Decapoda: Palaemonidae) from Taiwan. Zootaxa 2021, 5081, 566–578. [Google Scholar] [CrossRef]
- Bruce, A.J. Notes on some Indo-Pacific Pontoniinae, XLIX. Onycocaris balssi sp. nov., from northern Australia, with the designation of O. fujinoi sp. nov. Crustaceana 2011, 84, 477–490. [Google Scholar] [CrossRef]
- Marin, I. New records of pontoniine shrimp genus Onycocaris Nobili, 1904 (Crustacea: Decapoda: Palaemonidae) from the Indo-Pacific with the description of two new species from French Polynesia. Arthrop. Selecta 2015, 24, 283–302. [Google Scholar] [CrossRef]
- Gordon, I. On new or imperfectly known species of Crustacea Macrura. Zool. J. Linn. Soc. 1935, 39, 307–351. [Google Scholar] [CrossRef]
- Fransen, C.H.J.M. New records of the sponge-symbiotic shrimp genus Anchistioides from the Indo-West Pacific (Decapoda, Caridea). Crustaceana 2020, 93, 423–1447. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, D.; Lee, S.H.; De Grave, S. First record of the sponge-dwelling palaemonid shrimp, Anchistioides compressus (Crustacea: Decapoda: Palaemonidae) in Korea. Anim. Syst. Evol. Div. 2020, 36, 319–329. [Google Scholar] [CrossRef]
- Bruce, A.J. A report on some pontoniid shrimps collected from the Seychelle Islands by the F.R.V. Manihine, 1972, with a review of the Seychelles pontoniid shrimp fauna. Zool. J. Linn. Soc. 1976, 59, 89–153. [Google Scholar] [CrossRef]
- Ďuriš, Z.; Horká, I.; Marin, I. Periclimenes sulcatus sp. nov., a new pontoniine shrimp (Crustacea: Decapoda: Palaemonidae) from Vietnam. Zootaxa 2008, 1860, 35–50. [Google Scholar] [CrossRef]
- Bruce, A.J. Additions to the genus Phycomenes Bruce, 2008 (Crustacea: Decapoda: Pontoniinae). In Contributions to Shrimp Taxonomy; De Grave, S., Fransen, C.H.J.M., Eds. Zootaxa 2010, 2372, 367–368. [Google Scholar] [CrossRef]
- Bruce, A.J. Two new palaemonid shrimps (Crustacea: Decapoda) from the Australian North West shelf. J. Nat. Hist. 1988, 22, 1263–1276. [Google Scholar] [CrossRef]
- Bruce, A.J. Shallow–water palaemonid shrimps from New Caledonia (Crustacea: Decapoda). In: Le Benthos des Fonds Meubles des Lagons de Nouvelle–Calédonie (R. de Forges, ed.). Étud. Thès. ORSTOM 1991, 1, 221–279. [Google Scholar]
- Bruce, A.J. On some pontoniine shrimps from Noumea, New Caledonia. Cah. Indo-Pacif. 1980, 2, 1–39. [Google Scholar]
- Corredor, L. Notes on the behavior and ecology of the new fish cleaner shrimp Brachycarpus biunguiculatus (Lucas) (Decapoda, Natantia, Palaemonidae). Crustaceana 1978, 35, 35–40. [Google Scholar] [CrossRef]
- Horká, I.; De Grave, S.; Fransen, C.H.J.M.; Petrusek, A.; Ďuriš, Z. Multiple origins and strong phenotypic convergence in fish-cleaning palaemonid shrimp lineages. Mol. Phylogenet. Evol. 2018, 124, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S. Notes on Crustacea Decapoda in the Indian Museum, XV. Pontoniinae. Rec. Indian Mus. 1922, 24, 113–288, Pls. 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).