Mitochondrial Genome Diversity of Schistura McClelland, 1838 (Teleostei, Nemacheilidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Mitochondrial Data Retrieval
2.2. Mitochondrial Genome Sequencing of S. fasciolata
2.3. Data Analysis
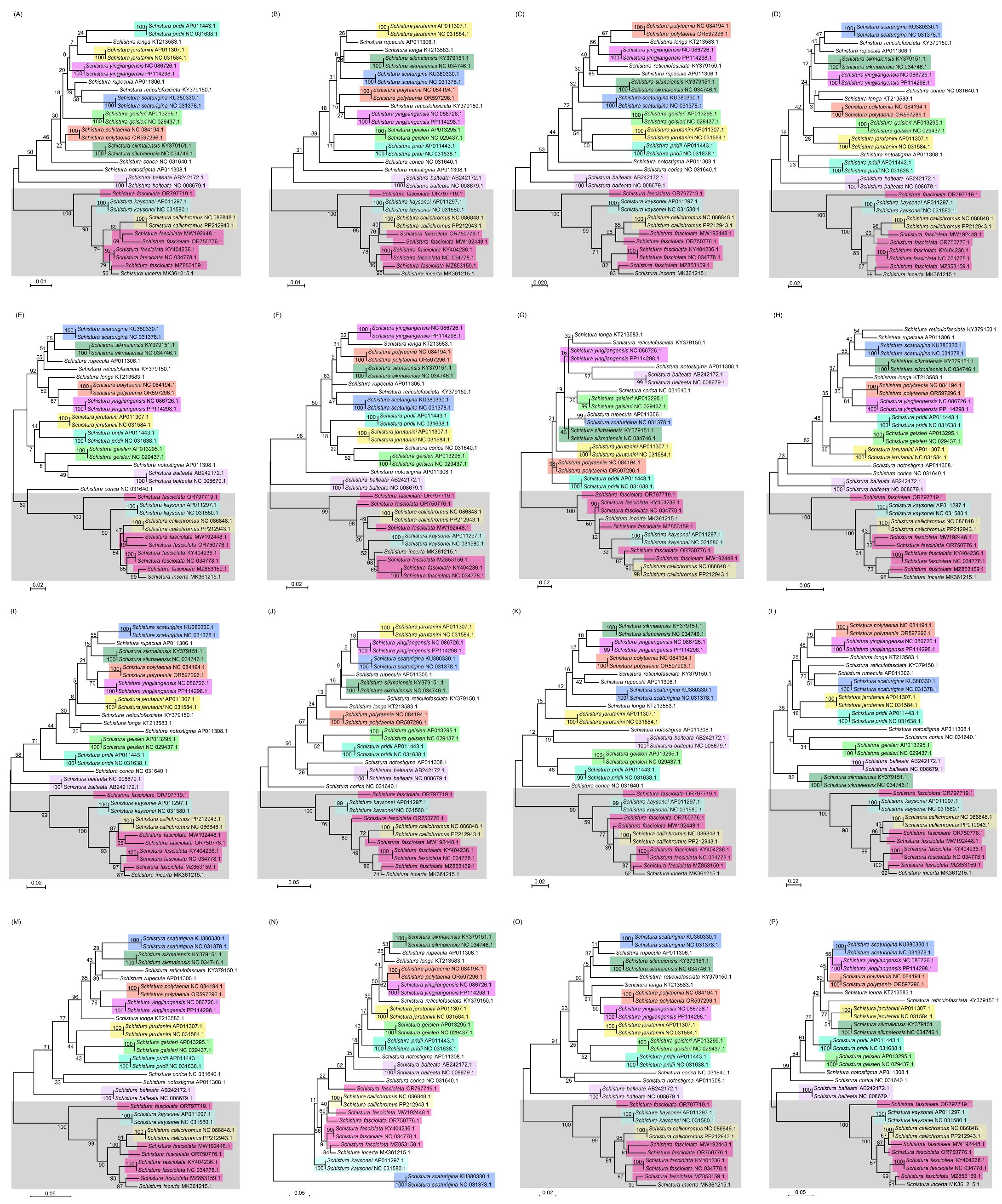
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Yang, J.; Li, Y.; Li, X. Exploring Taxonomic Diversity and Biogeography of the Family Nemacheilinae (Cypriniformes). Ecol. Evol. 2019, 9, 10343–10353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kong, D.; Yang, J. Schistura cryptofasciata, a New Loach (Cypriniformes: Balitoridae) from Salween Drainage in Yunnan, Southwestern China. Raffles Bull. Zool. 2005, 13, 27–32. Available online: https://eurekamag.com/research/023/561/023561462.php (accessed on 30 October 2023).
- Bohlen, J.; Dvořák, T.; Šlechta, V.; Šlechtová, V. Resolving an unnoticed diversity within the Schistura robertsi species complex (Teleostei: Nemacheilidae) using molecules and morphology. Mol. Phylogen. Evol. 2020, 151, 106894. [Google Scholar] [CrossRef] [PubMed]
- Dvořák, T.; Bohlen, J.; Kottelat, M.; Šlechtová, V. Revision of the Schistura cincticauda species group (Teleostei, Nemacheilidae) using molecular and morphological markers. Sci. Rep. 2023, 13, 16996. [Google Scholar] [CrossRef] [PubMed]
- Kottelat, M. Conspectus Cobitidum: An Inventory of the Loaches of the World (Teleostei: Cypriniformes: Cobitoidei). Raffles Bull. Zool. 2012, 26, 1–199. [Google Scholar]
- Siva, C.; Kumar, R.; Sharma, L.; Laskar, M.A.; Sumer, S.; Barat, A.; Sahoo, P.K. The Complete Mitochondrial Genome of a Stream Loach (Schistura reticulofasciata) and Its Phylogeny. Conserv. Genet. Resour. 2018, 10, 829–832. [Google Scholar] [CrossRef]
- Liu, S.; Mayden, R.L.; Zhang, J.; Yu, D.; Tang, Q.; Deng, X.; Liu, H. Phylogenetic Relationships of the Cobitoidea (Teleostei: Cypriniformes) Inferred from Mitochondrial and Nuclear Genes with Analyses of Gene Evolution. Gene 2012, 508, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, S.; Abdoli, A.; Sherafatian, M.; Golzarianpour, K. Analysis of Mitochondrial DNA Sequences of Turcinoemacheilus genus (Nemacheilidae Cypriniformes) in Iran. Iran. J. Fish. Sci. 2013, 12, 592–604. [Google Scholar] [CrossRef]
- Sgouros, K.; Page, L.M.; Orlofske, S.A.; Jadin, R.C. A Revised Molecular Phylogeny Reveals Polyphyly in Schistura (Teleostei: Cypriniformes: Nemacheilidae). Zootaxa 2019, 4559, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Eschmeyer, W.; Fricke, R.; Van der Laan, R. Catalog of Fishes: Classification. Catalog of Fishes, Electronic Version; 2018. Available online: http://www.calacademy.org/scientists/projects/eschmeyers-catalog-of-fishes (accessed on 17 May 2018).
- Deng, H.; Wen, H.; Xiao, N.; Zhou, J. A New Blind Species of the Cave Genus Oreonectes from Guizhou, China (Nemacheilinae). Zookeys 2016, 637, 47–59. [Google Scholar] [CrossRef]
- Xiong, F.; Shu, L.; Zeng, H.; Gan, X.; He, S.; Peng, Z. Methodology for fish biodiversity monitoring with environmental DNA metabarcoding: The primers, databases and bioinformatic pipelines. Water Biol. Secur. 2022, 1, 100007. [Google Scholar] [CrossRef]
- Satoh, T.P.; Miya, M.; Mabuchi, K.; Nishida, M. Structure and Variation of the Mitochondrial Genome of Fishes. BMC Genom. 2016, 17, 719. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.C.; Maloy, A.P.; Rees, C.B.; Bartron, M.L. Fish Mitochondrial Genome Sequencing: Expanding Genetic Resources to Support Species Detection and Biodiversity Monitoring Using Environmental DNA. Conserv. Genet. Resour. 2020, 12, 433–446. [Google Scholar] [CrossRef]
- Wang, I.-C.; Lin, H.-D.; Liang, C.-M.; Huang, C.-C.; Wang, R.-D.; Yang, J.-Q.; Wang, W.-K. Complete Mitochondrial Genome of the Freshwater Fish Onychostoma lepturum (Teleostei, Cyprinidae): Genome Characterization and Phylogenetic Analysis. Zookeys 2020, 1005, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Siva, C.; Ali, S.; Sahoo, P.K.; Nath, R.; Laskar, M.A.; Sarma, D. The Complete Mitochondrial Genome of the Medicinal Fish, Cyprinion semiplotum: Insight into Its Structural Features and Phylogenetic Implications. Int. J. Biol. Macromol. 2020, 164, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Qin, H.; Zhao, Z.; Liao, J.; Chen, H.; Jiang, L.; Dayananda, B. The Mitochondrial Genome and Phylogenetic Analysis of Rhacophorus rhodopus. Sci. Rep. 2022, 12, 13693. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, M.; D’Elia, A.K.P.; Rocha, G.; Arantes, C.A.; Henning, F.; de Vasconcelos, A.T.R.; Solé-Cava, A.M. Mitochondrial Genome Structure and Composition in 70 Fishes: A Key Resource for Fisheries Management in the South Atlantic. BMC Genom. 2024, 25, 215. [Google Scholar] [CrossRef] [PubMed]
- Muhala, V.; Guimarães-Costa, A.; Bessa-Silva, A.R.; Rabelo, L.P.; Carneiro, J.; Macate, I.E.; Watanabe, L.; Balcázar, O.D.; Gomes, G.E.; Vallinoto, M.; et al. Comparative Mitochondrial Genome Brings Insights to Slight Variation in Gene Proportion and Large Intergenic Spacer and Phylogenetic Relationship of Mudskipper Species. Sci. Rep. 2024, 14, 3358. [Google Scholar] [CrossRef] [PubMed]
- Huckstorf, V. Schistura fasciolata. IUCN Red List. Threat. Species 2012, 2012, e.T166080A1110341. [Google Scholar] [CrossRef]
- Arseneau, J.-R.; Steeves, R.; Laflamme, M. Modified Low-Salt CTAB Extraction of High-Quality DNA from Contaminant-Rich Tissues. Mol. Ecol. Resour. 2017, 17, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Pan, Q.; Liu, Y.; et al. SOAPdenovo2: An Empirically Improved Memory-Efficient Short-Read de Novo Assembler. Gigascience 2012, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Madan, A. CAP3: A DNA Sequence Assembly Program. Genome Res. 1999, 9, 868–877. [Google Scholar] [CrossRef]
- Delcher, A. Improved Microbial Gene Identification with GLIMMER. Nucleic Acids Res. 1999, 27, 4636–4641. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. TRNAscan-SE: A Program for Improved Detection of Transfer RNA Genes in Henomic Sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Stærfeldt, H.-H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and Rapid Annotation of Ribosomal RNA Genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. Rfam: Annotating Non-Coding RNAs in Complete Genomes. Nucleic Acids Res. 2004, 33, D121–D124. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Genomic Sequence with Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Minhas, B.F.; Beck, E.A.; Cheng, C.-H.C.; Catchen, J. Novel Mitochondrial Genome Rearrangements Including Duplications and Extensive Heteroplasmy Could Underlie Temperature Adaptations in Antarctic Notothenioid Fishes. Sci. Rep. 2023, 13, 6939. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Dong, X.-L.; Wang, Z.-M.; Miao, X.-G.; Wang, S.-Y.; Kong, X.-Y. Complete Mitogenome Sequences of Four Flatfishes (Pleuronectiformes) Reveal a Novel Gene Arrangement of L-Strand Coding Genes. BMC Evol. Biol. 2013, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Cheng, C.-H.C. ND6 gene “lost” and found: Evolution of mitochondrial gene rearrangement in Antarctic Notothenioids. Mol. Biol. Evol. 2010, 27, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.P.; Peer, A.C.; Miller, T.J.; Secor, D.H.; Place, A.R. A phylogeny of the temperate seabasses (Moronidae) characterized by a translocation of the mt-nd6 gene. J. Fish Biol. 2012, 80, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Attardi, G. The mtDNA-encoded ND6 subunit of mitochondrial NADH dehydrogenase is essential for the assembly of the membrane arm and the respiratory function of the enzyme. EMBO J. 1998, 17, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Miya, M.; Nishida, M. The Mitogenomic Contributions to Molecular Phylogenetics and Evolution of Fishes: A 15-Year Retrospect. Ichthyol. Res. 2015, 62, 29–71. [Google Scholar] [CrossRef]
- Sember, A.; Bohlen, J.; Šlechtová, V.; Altmanová, M.; Symonová, R.; Ráb, P. Karyotype Differentiation in 19 Species of River Loach Fishes (Nemacheilidae, Teleostei): Extensive Variability Associated with RDNA and Heterochromatin Distribution and Its Phylogenetic and Ecological Interpretation. BMC Evol. Biol. 2015, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Tikochinski, Y.; Carreras, C.; Tikochinski, G.; Vilaça, S.T. Population-specific signatures of intra-individual mitochondrial DNA heteroplasmy and their potential evolutionary advantages. Sci. Rep. 2020, 10, 211. [Google Scholar] [CrossRef]
- Miyazaki, J.-I.; Dobashi, M.; Tamura, T.; Beppu, S.; Sakai, T.; Mihara, M.; Hosoya, K. Parallel evolution in eight-barbel loaches of the genus Lefua (Balitoridae, Cypriniformes) revealed by mitochondrial and nuclear DNA phylogenies. Mol. Phylogenet. Evol. 2011, 60, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Sakai, T.; Nakao, K.; de Oliveira Martins, L.; Hosoya, K.; Miyazaki, J.-I. Phylogeography of loaches of the genus Lefua (Balitoridae, Cypriniformes) inferred from mitochondrial DNA sequences. Zool. Sci. 2005, 22, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-J.; Li, G.-H.; Jin, F.-P.; Zhao, J.-X.; Lei, C.-Y.; Gao, H.-T.; Fu, S.-W.; Zhou, R.; Luo, Y.-X.; Xue, S.-W.; et al. Genetic diversity analysis of mitochondrial D-loop region of Anabarilius graham in Fuxian Lake. Acta Hydrobiol. Sin. 2022, 46, 385–394. [Google Scholar] [CrossRef]
- Padhi, A. Geographic variation within a tandemly repeated mitochondrial DNA D-loop region of a North American freshwater fish, Pylodictis olivaris. Gene 2014, 538, 63–68. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Xu, B.; Chen, C.; Xiao, T.; Su, J. Mitochondrial Genome Diversity of Schistura McClelland, 1838 (Teleostei, Nemacheilidae). Diversity 2024, 16, 494. https://doi.org/10.3390/d16080494
Peng X, Xu B, Chen C, Xiao T, Su J. Mitochondrial Genome Diversity of Schistura McClelland, 1838 (Teleostei, Nemacheilidae). Diversity. 2024; 16(8):494. https://doi.org/10.3390/d16080494
Chicago/Turabian StylePeng, Xiaohuang, Baohong Xu, Changjun Chen, Tiaoyi Xiao, and Jianming Su. 2024. "Mitochondrial Genome Diversity of Schistura McClelland, 1838 (Teleostei, Nemacheilidae)" Diversity 16, no. 8: 494. https://doi.org/10.3390/d16080494
APA StylePeng, X., Xu, B., Chen, C., Xiao, T., & Su, J. (2024). Mitochondrial Genome Diversity of Schistura McClelland, 1838 (Teleostei, Nemacheilidae). Diversity, 16(8), 494. https://doi.org/10.3390/d16080494




