Abstract
A partial ichthyosaur skeleton from the Toarcian (Lower Jurassic) bituminous shales of the ‘Schistes Carton’ unit of southern Luxembourg is described and illustrated. In addition, associated remnant soft tissues are analyzed using a combination of imaging and molecular techniques. The fossil (MNHNL TV344) comprises scattered appendicular elements, together with a consecutive series of semi-articulated vertebrae surrounded by extensive soft-tissue remains. We conclude that TV344 represents a skeletally immature individual (possibly of the genus Stenopterygius) and that the soft parts primarily consist of fossilized skin, including the epidermis (with embedded melanophore pigment cells and melanosome organelles) and dermis. Ground sections of dorsal ribs display cortical microstructures reminiscent of lines of arrested growth (LAGs), providing an opportunity for a tentative age determination of the animal at the time of death (>3 years). It is further inferred that the exceptional preservation of TV344 was facilitated by seafloor dysoxia/anoxia with periodical intervals of oxygenation, which triggered phosphatization and the subsequent formation of a carbonate concretion.
1. Introduction
Since the original description in 1699 [1], ichthyosaurs (marine ’fish lizards‘ of the Mesozoic) have been the subject of extensive research. Owing to numerous fossil findings, many of which are preserved in a pristine condition, our understanding of ichthyosaur ecology and phylogeny is relatively comprehensive [2]. In addition, recent structural and molecular analyses of ichthyosaur soft tissues have enabled inferences to be made about certain aspects of their biology [3], thereby strengthening hypotheses regarding similarities between derived ichthyosaurs and extant toothed whales [4].
Ichthyosaur soft-tissue remains were first described in 1836 by William Buckland, in the form of presumed skin associated with a Lower Jurassic specimen from Lyme Regis, southern England. Since then, numerous finds of ichthyosaur soft parts have been made, mainly from Jurassic Konservat-Lagerstätten in Germany and the United Kingdom [2]. These findings comprise several different types of organs and tissues, including skin [3,5,6,7], connective tissue [8] and internal organs (e.g., liver, intestines and stomach) [3,7,9]. Regarding skin, all three layers of the amniote integument, i.e., the epidermis, dermis and hypodermis, have been documented in ichthyosaur fossils [2,3]. Furthermore, remnant cells and endogenous biomolecular traces, including melanophores (pigment cells) and melanosomes (melanin-bearing cellular organelles), have been retrieved from some specimens [3,10]. The distribution of melanophores and melanosomes in ichthyosaur fossils additionally has shown that at least some species were countershaded in life [3]. This color scheme likely served a variety of functions, including camouflage, thermoregulation and protection against harmful ultraviolet (UV) radiation [3]. Blubber is another type of peripheral tissue that has recently been documented in ichthyosaurs [3], emphasizing the evolutionary convergence between these ancient marine reptiles and modern odontocetes (toothed whales).
In this contribution, an ichthyosaur fossil (hereafter referred to by its accession number, MNHNL TV344), represented by both incomplete postcranial elements and extensive soft tissues, from Toarcian (Lower Jurassic) strata of southern Luxembourg is described and illustrated.
Institutional abbreviations. MH, Urweltmuseum Hauff, Holzmaden, Germany; MNHNL, National Museum of Natural History Luxembourg.
2. Geological Setting
TV344 was collected from a temporary outcrop exposing Lower Toarcian sediments near the town of Dudelange in southern Luxembourg (Figure 1). In this area, the Jurassic marine strata are sub-divided into the Dactylioceras tenuicostatum, Harpoceras serpentinum and Hildoceras bifrons ammonite zones.
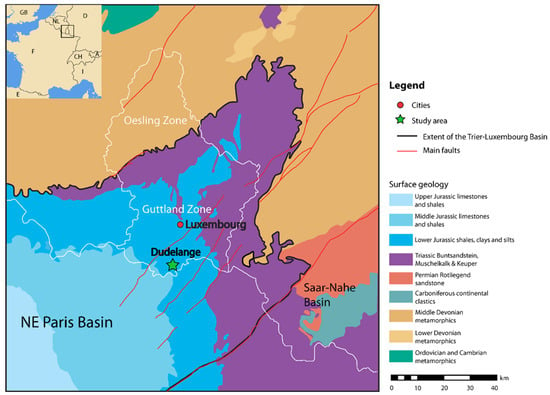
Figure 1.
Geological map of Luxembourg and its surroundings, with the study area indicated by a green star (modified from Figure 1 in Ref. [11]). White lines indicate national borders.
Global oceanic anoxia occurred periodically during the Early Jurassic, promoting the formation of organic-rich black shales across large parts of what is now Europe [12]. The Toarcian Oceanic Anoxic Event (hereafter referred to as the T-OAE) is thought to be one of many environmental perturbations caused by the Karoo–Ferrar Large Igneous Province in present-day Southern Africa and Antarctica [13].
The Posidonienschiefer Formation of Germany comprises an extensive accumulation of bituminous and highly fossiliferous black shales with intercalated limestone horizons, formed during the T-OAE [14]. Roughly contemporaneous and lithostratigraphically equivalent to the Posidonienschiefer Formation is the ‘Schistes Carton’ unit of southern Luxembourg [15,16]. The strata of the ‘Schistes Carton’ unit have been subjected to extensive compression and are in at least three levels rich in carbonate concretions, which have yielded numerous vertebrate and invertebrate remains [15,17,18]. The fossil-producing nodules are generally grey, ovoid, of decimeter-size or more, and have a finely laminated internal texture. The lithological similarity between these nodules and the surrounding host rock implies that the formation of the former took place at about the same time as the main sediments were deposited. This, in turn, suggests that fossilization within the nodules occurred synsedimentary [18,19]. It has been argued that the formation of the nodules was initiated as a result of sulfate-reducing bacteria locally increasing the pH of the pore waters, causing intense local carbonate precipitation [20].
The ‘Schistes Carton’ unit contains a diverse fossil macrofauna. In addition to numerous ichthyosaur specimens, plesiosaurs and metriorhynchoids have also been recorded from these strata [20,21]. Aside from marine reptiles, fish, squids, belemnites, ammonites, gastropods, bivalves, and insects have further been documented [20]. The presence of abundant insect fossils supports an interpretation that the sediments of the ‘Schistes Carton’ unit accumulated in a relatively nearshore environment [20,21].
3. Materials and Methods
TV344 was acquired from an amateur paleontologist who collected the fossil from a temporary exposure of marine strata of the ‘Schistes Carton’ unit at a construction site near the town of Dudelange, Luxembourg, in the early 1990s. No data concerning the exact position or orientation of the nodule in the stratigraphic succession is available. However, lithological comparisons with similarly-looking nodules suggests that it originates from a bed within to the Harpoceras serpentinum ammonite Zone (Harpoceras exaratum ammonite Subzone), immediately below a level interpreted to be stratigraphic equivalent to ‘Unterer Stein’ in south-western Germany and roughly corresponding to the base of the Jet Rock series of Yorkshire, England [15,17,22,23,24]. The specimen was initially prepared by the collector; however, the methods employed were not documented. Nonetheless, it can be inferred that mechanical preparation beyond initial cracking of the nodule was minimal, and that the exposed surfaces were treated with an organic preservative.
We photographed the ichthyosaur fossil under both polarized and UV light using a Nikon D3500 camera equipped with a 18–55 mm zoom lens and a Laowa 60 mm macro lens. Images were produced using the methods outlined in [25] for polarized light photography and [26] for UV photography.
To gain a better understanding of the structural layering of the various tissues, two petrographic sections were produced from an area with both soft tissues and skeletal elements (Figure 2A). A sample was removed from the fossil and embedded in epoxy. Two slices were then produced using a slow-speed diamond saw and ground using a Struers® (Copenhagen, Denmark) RotoPol-25 with a 600 grit diamond plate. The rock chips were mounted on glass slides using epoxy resin and ground to adequate thickness (70–100 µm) using the Struers RotoPol-25 and alternating between 600 and 1200 grit diamond plates. Finally, the ground sections were polished using 1 μm and then 0.25 μm diamond paste.
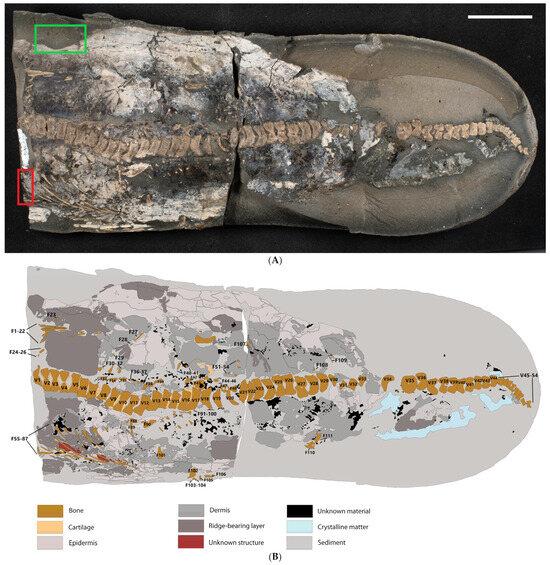
Figure 2.
TV344, a partial ichthyosaur from the ‘Schistes Carton’ unit of Luxembourg. (A): Photograph taken under polarized light. The sample site for the petrographic sections is marked with a red rectangle, whereas the samples used in our molecular analyses are demarcated by a green rectangle. The scale bar represents 5 cm. (B): Sketch map outlining the different components of the fossil and its surrounding matrix.
Soft-tissue samples for ultrastructural and molecular analyses were collected by removing some of the surrounding sediment, thereby revealing a previously unexposed part of the fossil. From there, two soft-tissue samples were extracted; these were demineralized using 0.5 M ethylenediaminetetraacetic acid (EDTA) and then rinsed with Milli-Q water. Ultrastructural and molecular analyses were conducted using a combination of scanning electron microscopy and mass spectrometry, as described below.
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) was employed to assess the molecular composition of the soft-tissue samples. The sample which provided the most distinct molecular signal was then selected for a subsequent scanning electron microscopy investigation.
Field Emission Gun Scanning Electron Microscopy (FEG-SEM) was employed in two ways: firstly, it was used to analyze one of the samples that had previously undergone ToF-SIMS analysis. This was done using a Zeiss® (Jena, Germany) Supra 40VP FEG-SEM at RISE in Borås, Sweden. Before being placed in the microscope, the sample was coated with 15 nm chromium. Secondly, FEG-SEM coupled with EDX was employed to investigate the petrographic sections and to obtain an overview of the chemical composition of the various skin layers and bones. A Tescan® (Brno, Czechia) Mira3 High Resolution Schottky FEG-SEM at the Department of Geology, Lund University, was used for producing micrographs, and a Oxford Instruments® X-MaxN 80, linked to this instrument, was used for the EDX analysis. Before being placed in the microscope, the petrographic sections were coated with 5 nm of a platinum/palladium mixture.
4. Systematic Paleontology
ICHTHYOSAURIA de Blainville, 1835 [27].
NEOICHTHYOSAURIA Sander, 2000 [28].
5. Results
5.1. General Description
The carbonate nodule that houses TV344 measures approximately 38 cm in total length and 16 cm in maximum width. The nodule has been cracked into part and counterpart sections, revealing a semi-articulated vertebral column that comprises 54 consecutive vertebral centra (Figure 2). The largest of these measure ~1.9 cm in height and ~0.6 cm in length, whereas the smallest ones measure ~0.5 cm in height and ~0.3 cm in length. Towards the posterior end of the specimen, the vertebral centra rapidly decrease in size. Vertebrae and associated bone fragments are hereafter referred to by the following numbers: V1–V54 (referring to vertebrae) and F1–F111 (other bone fragments), respectively (Figure 2B). V45–V54 bend sharply downward relative to V1–V44.
Owing to the presence of one set of ribs on either side of the vertebral column, and that the backbone is mostly articulated, the animal is interpreted to have settled on the seafloor on its back. Therefore, the area below the vertebral column (when the anterior end is facing towards the left; Figure 2) is hereafter referred to as the left side of the animal, whereas the area above the vertebral column is considered to be the right side. It should be noted, however, that this is only a preliminary assessment as there are no accompanying field data indicating which direction is stratigraphically up in the nodule.
5.2. Vertebral Column
As a result of the nodule being broken into part and counterpart sections, the vertebral centra have been split along their longitudinal axis. The bony tissues have a spongious inner texture. Moreover, the centra have an amphicoelous shape characteristic of ichthyosaurs, with a notochordal foramen visible in eight of these skeletal elements (Figure 2). F38–39 and F42–48 are located directly above and between the vertebral centra (Figure 2B). Based on their shape and distribution, they likely represent remains of neural arches. Between V1–10 and V13–17, as well as above F44–46, ossified matter is preserved that resembles bone in color and luminescence but is not readily identifiable as vertebrae (Figure 2, A1). This material is tentatively interpreted as cartilage (Figure 2B).
The ribs of TV344 transition from longer to shorter between V11 and V17. Based on this, the trunk–tail transition likely occurs in the area between V11 and V17. Consequently, F88–F90, and possibly also F101, likely constitute parts of the pelvic girdle. This assessment is corroborated by the number of caudal vertebrae present in the fossil. Vertebral columns of the common Early Jurassic genera Ichthyosaurus and Stenopterygius generally have 25 to 35 vertebrae in the region between the pelvis and onset of the tail bend [29]. TV344 has 33 vertebral centra between the inferred pelvic girdle and tail bend, which is consistent with these numbers. This inference would mean that V1–V11 are all dorsal vertebrae, whereas V12–V54 represent caudal ones.
5.3. Ribs
Two sets of ribs are present in TV344, one on either side of the vertebral column. The ribs positioned on the left side of the body (F55–87) are exposed at lower angles than those on the right side (F1–26). F1–26 and F55–87 presumably represent dorsal ribs, owing to their elongate appearance and distance to the vertebrae, whereas the shorter F30–37 and F91–100 likely are the remains of sacral and caudal ribs.
F33–37 and F30–32 are oriented in the same direction as the dorsal ribs, and positioned immediately to the right of the vertebral column. F33 and F34 both have a dichocephalous shape at the end facing the centra, to suggest that they—alongside the fragments surrounding them—represent smaller rib fragments. F91–100 are located directly to the left of the vertebral column. They are of sub-centimeter size, and mostly oriented at the same angle as the rib section F55–87; thus, they likely also represent remnant ribs. F88–90 are positioned next to the left set of ribs, and directly to the left of the vertebral column, and the individual elements measure between 1 and 2 cm in length. F101 is located near these fragments and is of similar size as the former elements.
Twelve dorsal ribs are visible in cross-section in the petrographic slides; two occur near the surface of the sections and ten in a gently bent row underneath (Figure A3). The two surficial ribs are sectioned more proximally than are those in the underlying row. While the two upper ribs are entirely cancellous and sub-circular in cross-section (Figure 3B), the ribs of the underlying row display circular cortical bone, an inner ring of cancellous bone, and a medullary cavity (Figure 3A,C). In the lower rib sections, at least three, but possibly four, regularly spaced, concentric lines can be seen in the peripheral cortex (Figure 3C). Osteocyte lacunae additionally occur throughout the peripheral bony tissues (Figure 3C).
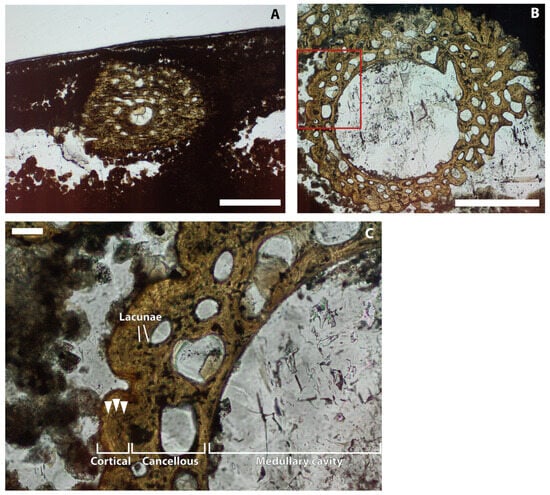
Figure 3.
Petrographic section produced from a set of ribs on the left-hand side of TV344. (A): Upper part of the section, showing a more proximally located section through a rib. Scale bar represents 1 mm. (B): Lower part of the petrographic slide, showing a distal cross-section of a rib. Scale bar represents 500 µm. (C): Magnification of (B), as indicated by red frame. Cortical bone, cancellous bone and the medullary cavity are all indicated. Circumferential lines in the cortex are highlighted by arrowheads. Lacunae are indicated by white lines. Scale bar represents 50 µm.
5.4. Soft Tissues
Most of the soft-tissue structures preserved in TV344 are identifiable as traces of the animal’s integument (see also Section 6.1), and are therefore treated as such in the following description. Assuming that the animal came to rest on its back (see above), the integumentary tissues surrounding the skeleton all likely derive from the dorsal side. The externalmost layer (facing the sediment) is light in color (Figure 4B), highly luminescent under UV-light (Figure A1), and is herein interpreted as the epidermis. The inner termination of the epidermis interfingers with an underlying material, a boundary that is evident in the elemental maps where the epidermis can be seen as a phosphorus-enriched layer into which calcium- and magnesium-rich ridges (~50 µm in diameter) protrude (Figure 5). These ridges have localized concentrations of iron and sulfur, likely stemming from pyrite, and correlate with dense, angular grains visible in the backscatter electron images (Figure 5). The undulating surface at the bottom of the epidermis corresponds to a layer of ridges that is observable under light microscopy (Figure 4A,B). These ridges extend parallel to the longitudinal axis of the fossil, thereby corresponding in orientation to dermal ridges of odontocetes [30].

Figure 4.
Detailed photographs of TV344. (A): Ribs from the left side of the fossil covered by rippled skin (indicated by an arrowhead). (B): Close-up view of skin covering the ribs, showing the transition between the epidermis and underlying ridged layer (indicated by arrowheads).
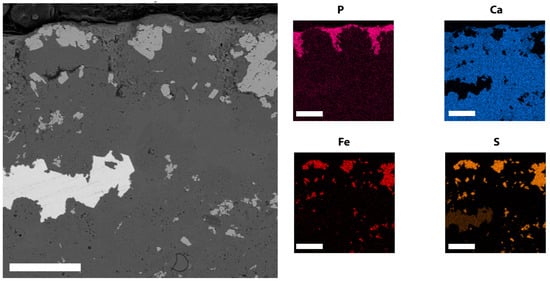
Figure 5.
FEG-SEM and EDX micrographs of TV344 integument. Back-scattered electron image to the left and EDX elemental maps to the right. Note dermal ridges protruding into the phosphatized epidermis, and localized concentrations of iron and sulfur in the ridges. Scale bars represent 50 μm.
At higher magnification, dark, branching bodies about 10–20 μm in diameter can be seen (Figure 6A). These also occur in SEM micrographs as clusters of small (~1 µm), oblong bodies at and near the boundary between the epidermis and dermis (Figure A4). ToF-SIMS analysis of TV344 did reveal a chemical signature broadly comparable to that of eumelanin at two sites in one of our samples (Figure A2); however, the mass spectra also revealed peaks characteristic of phthalates and other components that can be assigned to epoxy resins, indicating contamination by the preservative used to consolidate the fossil. Under FEG-SEM, microbodies were identified at the sites from where the eumelanin-like signal originated (Figure 6C,D). The similarity in shape and size between these microbodies and previously identified remnant melanosomes [3,31] suggests that these microstructures likewise are fossilized pigment organelles. Wherever melanosomes were found, they were always bunched together into dense clusters. However, these aggregations were sparsely distributed, which might explain the ambiguity of the ‘melanin’ signal.
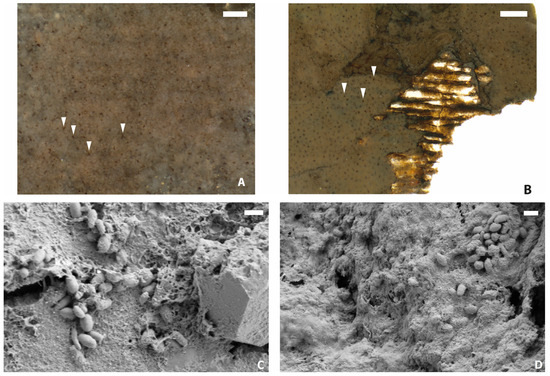
Figure 6.
Pigmentation in ichthyosaur fossils. (A): Epidermis of TV344 displaying dark, branching bodies (highlighted by arrowheads) with a diameter of ~10–20 μm. Scale bar represents 100 μm. (B): Epidermis of Stenopterygius specimen MH 432 similarly displaying dark, branching melanophores (see [3]). Melanophores highlighted by arrowheads. Scale bar represents 200 μm. (C,D): FEG-SEM micrographs of demineralized TV344 integument showing remnant melanosomes embedded in amorphous organic matter. Scale bars represent 1 μm.
Visible in Figure 4A,B are ripple-like structures interpreted as skin folds, similar to what have previously been described from preserved ichthyosaur integument [3,32]. Such ripples might form when the integument loses its structural integrity during the initial stages of decomposition [3].
6. Discussion
6.1. Integument of TV344
Based on both ultrastructural and compositional similarities with previously documented soft-tissue ichthyosaurs [3], the topographically outermost layer in TV344 most likely represents the epidermis. Furthermore, in the lower part of this stratum, along its undulating inner termination, clusters of small, oblong bodies occur (Figure A4). These are consistent with melanosomes in both size and shape [3,31].
Immediately underlying the epidermis is a layer of ridge-like structures oriented longitudinally to the main axis of the body (Figure 3A,B and Figure 6A,C). Similar structures have been observed in other ichthyosaur specimens and interpreted as integumental fibers [33,34,35]. In the aforementioned studies, the authors also reported additional fiber layers extending in opposing directions, collectively forming a cross-layered structure. Alternatively, ridges in ichthyosaur skin have been interpreted as representing the interface between the epidermis and dermis [3]. Owing to the position of this layer in TV344 directly underneath the epidermis and the lack of structures running in opposing directions, we here favor the second interpretation (which is also consistent with the architecture of odontocete integument [30]).
TV344 exhibits signs of what could be a dark skin pigmentation, similar to what has previously been documented in other ichthyosaur fossils [3,6,10]. Under light microscopy, dark, branching bodies that bear superficial resemblance to melanophores are observed (Figure 6A,B). Melanosomes were subsequently identified using FEG-SEM, occurring as small clusters (Figure 6C,D). Based on these observations, the integumental layers have been reconstructed in Figure 7.
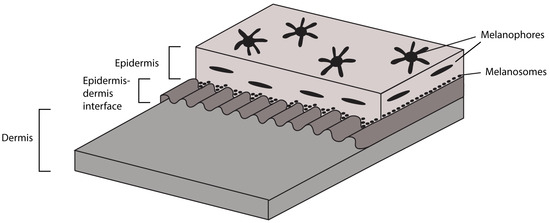
Figure 7.
Principal sketch of the inferred layering of the integument in TV344.
6.2. Ontogenetic Stage
When considering the age of the animal at the time of death, there is plenty of evidence that TV344 represents a juvenile individual, including the appearance of the vertebral centra. In juvenile ichthyosaurs, the spongiosa tend to grow continuously denser during ontogeny [36]. The osseous trabeculae of the vertebral centra in TV344 are set widely apart relative to larger and thus presumably skeletally more mature ichthyosaurs. Furthermore, the open notochordal foramen and absence of secondary osteons are additional indicators of skeletal immaturity in TV344.
A more precise age could be determined by analyzing the peripheral lines visible in one of the rib sections (Figure 3C). If these markings represent annual lines of arrested growth (LAGs), then the animal was at least three years old at the time of death. Notably, LAGs have been previously reported in ichthyosaur cortical bone, e.g., in Mixosaurus [37]. Annuli have also been observed in a number of ichthyosaurs, e.g., in Ichthyosaurus and Stenopterygius [38,39,40].
The notion that the animal which ultimately gave rise to TV344 could have been at least three years old when it died would imply that this individual exhibited a long-lasting juvenile stage and slow growth rates for at least the first few years of its life. Growth rates of derived Jurassic ichthyosaurs are generally considered to have been rapid, as indicated by their highly vascularized fibrolamellar bone [41] and purported absence of LAGs. Nonetheless, slow growth in juveniles followed by more rapid growth as adulthood approaches have been previously documented in many vertebrates, both extant and extinct ones [42].
6.3. Implications of Rib Anatomy
In contrast to previously described Early–Middle Jurassic ichthyosaurs, such as Stenopterygius and Mollesaurus [40,43], the ribs of TV344 have an open medullary cavity. This cavity is rather small at the proximal end but considerably larger towards the distal termination. An open medullary cavity has been previously observed in the Triassic ichthyosaur Omphalosaurus [41], as well as in both juvenile and adult specimens of the Late Jurassic to Early Cretaceous genus Caypullisaurus [44]. However, this condition has hitherto not been reported in any Early Jurassic ichthyosaur. Instead, published histological studies of Early Jurassic ichthyosaurs have noted an absence of a medullary cavity (for instance, the medullary region in the ribs of a Stenopterygius quadriscissus has a rounded intertrabecular region [40]). Since TV344 represents an immature individual, this could imply that an open medullary cavity is a juvenile feature in Early Jurassic ichthyosaurs. Secondary bone would then deposit in the medullary region as the animal aged.
In S. quadriscissus, it has also been noted that the internal morphology of the rib varies, with thicker cortical bone and fewer vascular canals near the mid-shaft than at the proximal and distal ends [40]. This parallels with the rib morphology of TV344, which shows that the mid-distal portion is less spongy than the proximal part.
6.4. Taphonomy
In cases where the integument survives degradative processes, fossilization usually is facilitated by rapid mineral precipitation [31]. Phosphatization, i.e., replacement of organic matter with phosphatic minerals, is a common mode of fossilization of soft tissues [45]. For phosphatization to occur, the depositional environment must be supersaturated with calcium and phosphate ions and a pH below 6.4 must prevail to prevent the formation of carbonates [46]. Microbes, specifically sulfate-oxidizing bacteria, are presumed to have a significant function in this process [47]. As a carcass decomposes, the bacteria lower the pH of the surrounding environment, thereby contributing to the release of phosphate from the decaying body—a process that is enhanced under dysoxic and anoxic conditions [48,49]. The combined effect of dysoxia/anoxia and a lowered pH is enhanced precipitation of calcium phosphate. Furthermore, when these processes take place during early taphonomic stages, they can even facilitate the preservation of endogenous biomolecules [31].
In the ‘Schistes Carton’ unit, the first phase of fossilization presumably took place during a period of seawater oxygenation in the otherwise dysoxic/anoxic Tethys Ocean. Reactive iron oxides can cycle phosphate ions into the bottom waters by reacting with ions in the upper water column, subsequently releasing phosphorous into the bottom waters in a process known as the ‘iron pumping cycle’ [31]. This cycling of phosphates was enhanced by seasonal contributions of oxygenated waters. These caused the redox chemocline to move from the water column into the sediment, which enabled sulfate-oxidizing bacteria to absorb phosphates [31]. The build-up of phosphate ions below the chemocline was further increased by downfall of organic detritus materials, such as plankton, from the surface. Sulfate-oxidizing bacteria in the soupy substrate locally lowered the pH to enable precipitation of calcium phosphate, which then rapidly encrusted and entombed the carcass, thereby facilitating the preservation of soft parts. Phosphate precipitation may have been further facilitated by an overall lowered seawater pH during the T-OAE [50]. The phosphate and calcium ions in the seawater and/or sediment stemmed both from the decaying carcass itself and, in the case of phosphate ions, from phosphates participating in the iron pumping cycle (Figure 8). This specific mode of preservation, where seasonal input of oxygenated waters facilitates mineralization, has been described in several Toarcian formations across the Tethys and Panthalassa oceans [31,51]. Carbonate ions might also have been present, originating from decaying organic matter and calcium carbonate shells [52], but were not favored in the precipitation process because of the relatively low pH. Precipitation of calcium phosphate likely occurred during a relatively brief time span [53] and during one of the oxygenation intervals that led to phosphatization also in other Early Toarcian strata.
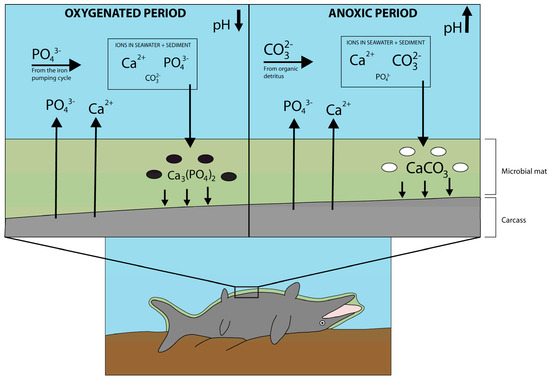
Figure 8.
Model for the taphonomic conditions that enabled the fossilization of TV344. The carcass is covered by a microbial mat containing both sulphate-oxidizing and sulphate-reducing bacteria. During brief oxygenated periods (upper left half of figure), sulphate-oxidizing bacteria (black ovals) locally lower the pH, thereby enabling ions in the seawater and sediment to form a sheet of calcium phosphate on top of the carcass. During dysoxic/anoxic periods (upper right half of figure), sulphate-reducing bacteria (white ovals) locally elevated the pH, thereby facilitating the formation of a calcium carbonate nodule from ions in the seawater and sediment.
The second fossilization phase followed in between periods of oxygenation, when the bottom waters were again dysoxic/anoxic. During this phase, sulfate-reducing bacteria locally elevated the pH, enabling precipitation of calcium carbonate (Figure 8). Phosphate, calcium and carbonate ions were still available, but carbonate ions were favored over phosphate ions due to the elevated pH. The concentration of phosphate ions might further have been lower, as the redox chemocline would have been lifted out of the sediment and into the water column, thereby reducing the amount of trapped phosphates. Hence, the combined effects of an elevated pH and a relatively higher concentration of carbonate ions caused the precipitation of calcium carbonate [23]. Furthermore, it has been suggested that nodule beds in shales formed during the T-OAE correspond to a general rise in oceanic pH caused by higher fluxes of organic matter [51]. The formation of carbonate concretions was by all likelihood rapid. In addition to altering the pH of the seawater surrounding a carcass, microorganisms might also have shielded the decomposing organic matter through the formation of microbial mats.
As a result of these taphonomic processes, the integument of TV344 was preserved as an outer phosphatized layer (the epidermis), underlain by the epidermis-dermis interface and the dermis, which are diagenetically altered to varying extent. During decomposition and subsequent fossilization, sediments likely infiltrated the carcass; this is inferred from the extensive fragmentation of the integument. This intrusion likely led to large portions of the lower epidermis being consumed by microbes, in turn leading to a concentration of melanosomes. Some epidermal melanophores remained, however, as evidenced in Figure 6A. Similarly, the dermis was extensively infiltrated by sediments, leading to large-scale replacement of the organic matter, as shown by the ridging remaining as impressions in the overlying epidermis. While several integumental layers are observable to the naked eye (Figure 4), they are not readily apparent in the petrographic sections. This is presumably because these layers are unevenly distributed across the body due to patchy preservation. In some parts, the dermis presumably was lost altogether.
The preservation of skeletal elements in TV344 is incomplete; the vertebral column is intact, but many of the appendicular elements have been lost, presumably due to post-depositional disturbance of the bones. Furthermore, a significant portion of the animal’s body is missing from the fossil. This can be attributed to the nodule being partial; the appearance of the nodule (one rounded edge and one edge which appears to have been broken off) suggests that there originally was another part present. The anterior continuation of the body likely was preserved in this remaining nodule; however, the MNHNL is not in possession of any additional nodule material.
7. Conclusions
This study presents the first thorough description of a soft-tissue ichthyosaur (TV344) from the Toarcian ‘Schistes Carton’ unit of southern Luxembourg. We conclude the following:
- TV344 represents an incomplete juvenile individual belonging to the clade Neoichthyosauria, and possibly the genus Stenopterygius;
- The fossil comprises a consecutive series of 54 vertebral centra, associated ribs, 20 bone fragments, as well as extensive soft-tissue remains;
- The soft tissues surrounding the vertebral column are largely integumentary in nature. In addition, there is a distinct layering of the skin, where the epidermis, dermis and epidermis–dermis interface are preserved. Furthermore, the epidermis contains melanophores and melanosomes;
- Concentric ring-like structures observed in sectioned ribs of TV344 may represent annual LAGs, to suggest that the animal was at least three years old at the time of death;
- The taphonomic conditions that enabled the exceptional preservation of TV344 included a combination of seafloor dysoxia/anoxia with periodical oxygenation, thus facilitating rapid phosphatization of the soft tissues during oxygenated periods and the formation of the carbonate nodule during dysoxic/anoxic intervals. Microbial activity aided by lowering the pH during the oxygenated phases, which further enhanced phosphate precipitation.
Author Contributions
Conceptualization, I.B.W., J.L., M.E.E. and B.T.; methodology, I.B.W. and J.L.; formal analysis, P.S. and I.B.W.; investigation, P.S., I.B.W., J.L. and R.G.D.L.G.; resources, B.T., P.S. and J.L.; writing—original draft preparation, I.B.W.; writing—review and editing, I.B.W., J.L., M.E.E., B.T., P.S. and R.G.D.L.G.; visualization, I.B.W.; supervision, J.L., M.E.E. and B.T.; project administration, J.L. and I.B.W.; funding acquisition, J.L. and M.E.E. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by project grants (#2020-03542), (#2019-03516) and (#2019-03731) from the Swedish Research Council to J.L., M.E.E. and P.S., respectively, and a research grant (#20220563) from the Crafoord Foundation to J.L.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank Martin Jarenmark (LU) for assistance preparing samples and Carl Alwmark (LU) for assistance with SEM/EDX analysis. The authors also wish to thank three anonymous reviewers for their rigorous review, which served to improve the finished manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
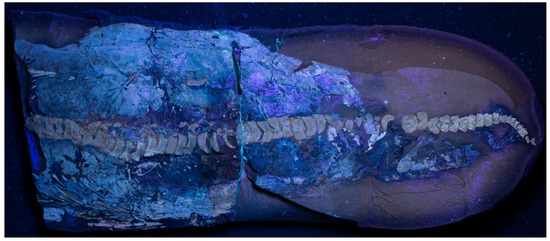
Figure A1.
TV344 photographed under ultraviolet light.
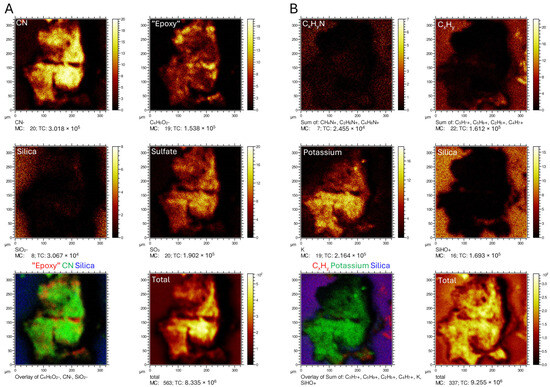
Figure A2.
(A): ToF-SIMS images of negative ions taken at Sample Point #5 on the right-hand side of the fossil (see Figure 2A), representative of nitrogen-containing organics (CN−), epoxy (C4H5O2−), silica (SiO2−) and sulfate (SO3−), respectively. The two panels in the bottom row depict an overlay of epoxy, CN− and silica together with the total signal intensity distribution. (B): ToF-SIMS images of positive ions acquired at Sample Point #5 on the right-hand side of the fossil (see Figure 2A), representative of amino acid-containing organics (CxHyN+), aliphatic hydrocarbons (CxHy+), potassium (K+) and silica (SiOH+). The two panels in the bottom row include an overlay of aliphatic hydrocarbons, potassium and silica, and the total signal intensity distribution.

Figure A3.
Overview of a complete petrographic section obtained from TV344. Scale bar represents 5 mm.
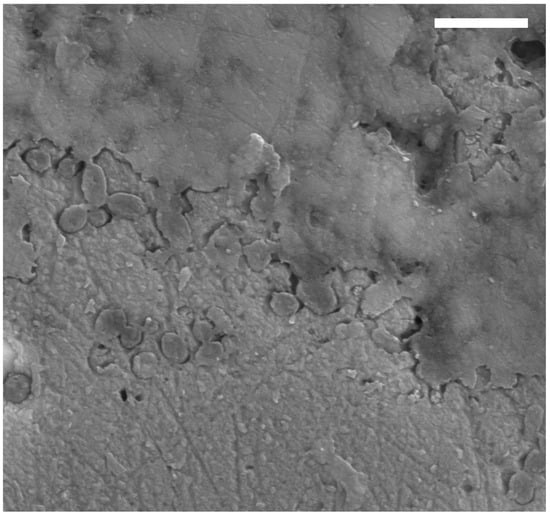
Figure A4.
FEG-SEM micrograph of TV344 integument, showing oblong microbodies clustered near the inner termination of the epidermis. Scale bar represents 2 μm.
References
- Lhuyd, E. Lithophylacii Brittannici Ichnographia, Sive Lapidum Aliorumque Fossilium Brittanicorum Singulari Figura Insignium; Gleditsch & Weidmann: London, UK, 1699. [Google Scholar]
- Eriksson, M.E.; De La Garza, R.; Horn, E.; Lindgren, J. A review of ichthyosaur (Reptilia, Ichthyopterygia) soft tissues with implications for life reconstructions. Earth-Sci. Rev 2022, 226, 103965. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Motani, R. Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints. Annu. Rev. Earth Planet. Sci. 2005, 33, 395–420. [Google Scholar] [CrossRef]
- Lingham-Soliar, T.; Plodowski, G. Taphonomic evidence for high-speed adapted fins in thunniform ichthyosaurs. Sci. Nat. 2007, 94, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.; Sjövall, P.; Carney, R.M.; Uvdal, P.; Gren, J.A.; Dyke, G.; Schultz, B.P.; Shawkey, M.D.; Barnes, K.R.; Polcyn, M.J. Skin pigmentation provides evidence of convergent melanism in extinct marine reptiles. Nature 2014, 506, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Renesto, S.; Dal Sasso, C.; Fogliazza, F.; Ragni, C. New findings reveal that the Middle Triassic ichthyosaur Mixosaurus cornalianus is the oldest amniote with a dorsal fin. Acta Palaeontol. Pol. 2020, 65, 511–522. [Google Scholar] [CrossRef]
- Martill, D.M. An ichthyosaur with preserved soft tissue from the Sinemurian of southern England. Paleontology 1995, 38, 897–903. [Google Scholar]
- Jacobs, M.L.; Martill, D.M. A new ophthalmosaurid ichthyosaur from the Upper Jurassic (Early Tithonian) Kimmeridge Clay of Dorset, UK, with implications for Late Jurassic ichthyosaur diversity. PLoS ONE 2020, 15, e0241700. [Google Scholar] [CrossRef]
- Whitear, M. XCVII.—On the colour of an ichthyosaur. Ann. Mag. Nat. Hist. 1956, 9, 742–744. [Google Scholar] [CrossRef]
- Schintgen, T.; Förster, A. Geology and basin structure of the Trier-Luxembourg Basin—Implications for the existence of a buried Rotliegend graben. ZDGG 2013, 164, 615–637. [Google Scholar] [CrossRef]
- Muscente, A.D.; Vinnes, O.; Sinha, S.; Schiffbauer, J.D.; Maxwell, E.; Schweigert, G.; Martindale, R.C. What role does anoxia play in exceptional fossil preservation? Lessons from the taphonomy of the Posidonia Shale (Germany). Earth-Sci. Rev. 2023, 238, 104323. [Google Scholar] [CrossRef]
- Fantasia, A.; Föllmi, K.B.; Adatte, T.; Spangenberg, J.E.; Montero-Serrano, J.-C. The Early Toarcian oceanic anoxic event: Paleoenvironmental and paleoclimatic change across the Alpine Tethys (Switzerland). Glob. Planet. Chang. 2018, 162, 53–68. [Google Scholar] [CrossRef]
- Ruebsam, W.; Schmid-Röhl, W.; Al-Husseini, M. Astronomical timescale for the early Toarcian (Early Jurassic) Posidonia Shale and global environmental changes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 623, 111619. [Google Scholar] [CrossRef]
- Hermoso, M.; Delsate, D.; Baudin, F.; Le Callonnec, L.; Minoletti, F.; Renard, M.; Faber, A. Record of Early Toarcian carbon cycle perturbations in a nearshore environment: The Bascharage section (easternmost Paris Basin). Solid Earth 2014, 5, 793–804. [Google Scholar] [CrossRef]
- Bechly, G. First record and a new species of the fossil dragonfly genus Proinogomphus (Odonata: Liassogomphidae) from the Early Jurassic of Bascharage in the Grand Duchy of Luxembourg. Zootaxa 2018, 4450, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Heads, S.W.; Thuy, B.; Tamarri, J. Two new species of Archelcana (Sharov (Orthoptera: Elcanidae) from the Lower Jurassic of Luxembourg. Kentiana 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Hanzo, M. A propos de nodules carbonatés du Toarcien inférieur de la région de Bettembourg (Grand-Duché de Luxembourg). In Proceedings of the 103e Congrès National de Sociétés Savants, Nancy-Metz, Nancy, France, 10-15 April 1978. [Google Scholar]
- Hanzo, M. Milieu de dépôt et évolution diagénétique des argilites toarciennes d’après l’etude nodules carbonatés des “Schistescarton” de Bettembourg (Grand-Duché de Luxembourg). Mém. Sci. Terre Nancy 1979, 23, 45–49. [Google Scholar]
- Henrotay, M.; Marques, D.; Paicheler, J.-C.; Gall, J.-C.; Nel, A. Le Toarcien inferieur des régions de Bascharage et de Bettembourg (Grand-Duché du Luxembourg): Évidences paléontologiques et sédimentologiques d’environnements restreints proches de l’émersion. Geodiversitas 1998, 20, 263–284. [Google Scholar]
- Johnson, M.M.; Young, M.T.; Brusatte, S.L.; Thuy, B.; Weis, R. A catalogue of teleosauroids (Crocodylomorpha: Thalattosuchia) from the Toarcian and Bajocian (Jurassic) of southern Luxembourg. Hist. Biol. 2019, 31, 1179–1194. [Google Scholar] [CrossRef]
- Riegraf, W.; Werner, G.; Lörcher, F. Der Posidonienschiefer. Biostratigraphie, Fauna und Fazies des südwestdeutschen Untertoarciums (Lias epsilon); Enke Verlag: Stuttgart, Germany, 1984; 195p. [Google Scholar]
- Frimmel, A. Hochauflösende Untersuchungen von Biomarkern an epikontinentalen Schwarzschiefern des Unteren Toarciums (Posidonienschiefer, Lias ε) von SW-Deutschland. Ph.D. Thesis, Eberhard-Karls-Universität Tübingen, Metzingen, Germany, 2003. [Google Scholar]
- Thibault, N.; Ruhl, M.; Ullmann, C.V.; Korte, C.; Kemp, D.B.; Gröcke, D.R.; Hesselbo, S.P. The wider context of the Lower Jurassic Toarcian oceanic anoxic event in Yorkshire coastal outcrops, UK. Proc. Geol. Assoc. 2018, 129, 372–391. [Google Scholar] [CrossRef]
- Crabb, P. The use of Polarised light in photography of macrofossils. Palaeontology 2001, 44, 659–664. [Google Scholar] [CrossRef]
- Tischlinger, H.; Arratia, G. Ultraviolet light as a tool of investigating Mesozoic fishes with a focus on the ichthyofauna of the Solnhofen Limestone. In Mesozoic Fishes 5. Global Diversity and Evolution, Proceedings of the International Meeting, Saltillo, Mexico, 2010; Arratia, G., Schultze, H.-P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2010; pp. 549–560. [Google Scholar]
- de Blainville, H.M.D. Description of some species of reptiles of California, preceded by the analysis of a general system of Herpetology and amphibiology. Ann. Mag. Nat. Hist. 1835, 4, 236–296. [Google Scholar]
- Sander, P.M. Ichthyosauria: Their diversity, distribution, and phylogeny. Palaontol. Z. 2000, 74, 1–35. [Google Scholar] [CrossRef]
- Romer, A.S. Osteology of the Reptiles; University of Chicago Press: Chicago, IL, USA, 1956; p. 772. [Google Scholar]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Anatomy of Dolphins: Insights into Body Structure and Function; Academic: Amsterdam, The Netherlands, 2017. [Google Scholar]
- De La Garza, R.; Sjövall, P.; Hauff, R.; Lindgren, J. Preservational modes of some ichthyosaur soft tissues (Reptilia, Ichthyopterygia) from the Jurassic Posidonia Shale of Germany. Palaeontology 2023, 66, e12668. [Google Scholar] [CrossRef]
- Smithwick, F. A Taphonomic and Paleoecological Approach to the Study of Paleocolour. Ph.D. Thesis, University of Bristol, Bristol, UK, 2019. [Google Scholar]
- Delair, J.B. Unusual preservation of fibrous elements in an ichthyosaur skull. Nature 1966, 212, 575–576. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. Rare soft tissue preservation showing fibrous structures in an ichthyosaur from the Lower Lias (Jurassic) of England. Proc. Biol. Sci. 1999, 266, 2367–2373. [Google Scholar] [CrossRef]
- Lingham-Soliar, T. The ichthyosaur integument: Skin fibers, a means for a strong, flexible and smooth skin. Lethaia 2001, 34, 287–302. [Google Scholar] [CrossRef]
- Houssaye, A.; Nakajima, Y.; Sander, P.M. Structural, functional, and physiological signals in ichthyosaur vertebral centrum microanatomy and histology. Geodiversitas 2018, 40, 161–170. [Google Scholar] [CrossRef]
- Kolb, C.; Sánchez-Villagra, M.R.; Scheyer, T.M. The palaeohistology of the basal ichthyosaur Mixosaurus Baur, 1887 (Ichthyopterygia, Mixosauridae) from the Middle Triassic: Palaeobiological implications. Comptes Rendus Palevol 2011, 10, 403–411. [Google Scholar] [CrossRef]
- Seitz, A.L.L. Vergleichende Studien über den Mikroskopischen Knochenbau Fossiler und Rezenter Reptilien und Dessen Bedeutung für das Wachstum und Umbildung des Knochengewebes im Allgemeinen; Druck von E. Karras: Halle, Germany, 1907; p. 172. [Google Scholar]
- Gross, W. Die Typen des mikroskopischen Knochenbaues bei fossilen Stegoccphalen und Reptilien. Z. Anat. Entwickl. 1934, 103, 731–764. [Google Scholar] [CrossRef]
- Anderson, K.L.; Druckenmiller, P.S.; Erickson, G.M.; Maxwell, E.E. Skeletal microstructure of Stenopterygius quadriscissus (Reptilia, Ichthyosauria) from the Posidonienschiefer (Posidonia Shale, Lower Jurassic) of Germany. Palaeontology 2019, 62, 433–449. [Google Scholar] [CrossRef]
- Houssaye, A. Bone histology of aquatic reptiles: What does it tell us about secondary adaptation to an aquatic life? Biol. J. Linn. Soc. 2012, 108, 3–21. [Google Scholar] [CrossRef]
- Horner, J.R.; Padian, K. Age and growth dynamics of Tyrannosaurus rex. Proc. Biol. Sci. 2004, 271, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Talevi, M.; Fernandez, M. Unexpected skeletal histology of an ichthyosaur from the Middle Jurassic of Patagonia: Implications for evolution of bone microstructure among secondary aquatic tetrapods. Naturwissenschaften 2012, 99, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Talevi, M.; Fernandez, M.; Salgado, L. Variación Ontogenética en la Histología Ósea de Caypullisaurus Bonapartei Fernández, 1997 (Ichthyosauria: Ophthalmosauridae). Ameghiniana 2012, 49, 38–46. [Google Scholar] [CrossRef]
- Briggs, D.E.G. The Role of Decay and Mineralization in the Preservation of Soft-Bodied Fossils. Annu. Rev. Earth Planet. Sci. 2003, 31, 275–301. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Wilby, P.R. The role of the calcium carbonate-calcium phosphate switch in the mineralization of soft-bodied fossils. J. Geol. Soc. 1996, 153, 665–668. [Google Scholar] [CrossRef]
- Iniesto, M.; Buscalioni, Á.D.; Carmen Guerrero, M.; Benzerara, K.; Moreira, D.; López-Archilla, A.I. Involvement of microbial mats in early fossilization by decay delay and formation of impressions and replicas of vertebrates and invertebrates. Sci. Rep. 2016, 6, 25716. [Google Scholar] [CrossRef]
- Peterson, J.E.; Lenczewski, M.E.; Scherer, R.P. Influence of microbial biofilms on the preservation of primary soft tissue in fossil and extant archosaurs. PLoS ONE 2010, 5, e13334. [Google Scholar] [CrossRef]
- Brock, J.; Schulz-Vogt, H.N. Sulfide induces phosphate release from polyphosphate in cultures of a marine Beggiatoa strain. ISME J. 2011, 5, 497–506. [Google Scholar] [CrossRef]
- Bomou, B.; Suan, G.; Schlögl, J.; Grosjean, A.-S.; Suchéras-Marx, B.; Adatte, T.; Spangenberg, J.E.; Fouché, S.; Zacaï, A.; Gibert, C.; et al. The paleoenvironmental context of Toarcian vertebrate-yielding shales of southern France (Hérault). Geol. Soc. Spec. Publ. 2021, 514, 121–152. [Google Scholar] [CrossRef]
- Muscente, A.D.; Martindale, R.C.; Schiffbauer, J.D.; Creighton, A.L.; Bogan, B.A. Taphonomy of the Lower Jurassic Konservat-Lagerstätte at Ya Ha Tinda (Alberta, Canada) and its significance for exceptional fossil preservation during oceanic anoxic events. Palaios 2019, 34, 515–541. [Google Scholar] [CrossRef]
- Berner, R.A. Calcium carbonate concretions formed by the decomposition of organic matter. Science 1968, 159, 195–197. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Kear, A.J. Fossilization of Soft Tissue in the Laboratory. Science 1993, 259, 1439–1442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).