Abstract
Invasive plants are capable of homogenizing both aboveground and belowground biota and, along with climate change, are recognized as one of the biggest threats to global biodiversity. Soil nematode communities reflect the surroundings they inhabit and are therefore frequently employed as biological indicators of soil condition. In this study, soil properties and nematode communities in Carpathian beech forest floor covered by dense vegetation of invasive Impatiens parviflora (small balsam) were investigated over two vegetation seasons. We assumed that the spread of invasive I. parviflora could influence soil fauna through litter accumulation when established and could also change several soil properties, consequently altering soil nematode communities. A total of 52 nematode species were found in the soil samples. The mean number of species varied from 18 to 31, but did not significantly differ between invaded and uninvaded plots across all sampling dates. However, redundancy analysis indicated that the nematode community in plots with small balsam differed significantly from that in uninvaded plots, reflecting different proportions of genera in the two communities. Invasion by small balsam significantly enhanced the relative abundance of bacterivores, whereas it decreased the abundance of plant parasites and root-fungal feeders, mainly in the spring and summer season. Ordination of nematode species along the structure index and enrichment index trajectories revealed a maturing food web, low to moderately disturbed in the I. parviflora invaded soils as well as in uninvaded forest plots. Decomposition channels of soil food webs in both plots were balanced and fungal–bacterial mediated, although low values of the channel index suggested prevailing bacterial decomposition. Our study reveals that the expansion of I. parviflora moderately influenced the composition of nematode communities and the soil food web, increased soil nitrogen, carbon and C/N ratio, but did not modify soil acidity.
1. Introduction
The Carpathian forests are among the most species-rich habitats in continental Central Europe with a temperate climate and adequate rainfall. The high level of biodiversity can be explained by the heterogeneous geomorphology of the mountain range and its geographical position bridging the Eastern Carpathians from Serbia and Romania through Ukraine; the Central Carpathians through Slovakia, Poland and Hungary; to the Western Carpathians in the Czech Republic and Austria. There are almost 36,000 km2 of protected areas and national parks that play a key role in biodiversity protection. However, such areas are subjected to negative pressure in the context of global climate change [1].
In invasion ecology, disturbance has been considered a key factor that facilitates the invasion of non-native species into various habitats [2]. Therefore, it is assumed that undisturbed closed-canopy forests are highly resistant to plant invasions [3]. However, a review [4] revealed that at least 139 exotic plant species have invaded deeply shaded forest understories in temperate and tropical regions around the world that have not undergone substantial disturbance; previous assumptions are thus not justified. One such invasive non-native species is small balsam Impatiens parviflora, a representative of the Balsaminaceae family of Central and East Asiatic origin.
I. parviflora was introduced to Europe in the 1830s as an ornamental plant to botanical gardens. At the end of the 19th century, it began its expansion, mainly into forest communities [5]. At present, the species is common in riparian forests, oak–hornbeam forests, beech woods, acidophilous beech forests and mixed coniferous forests; it is more rarely found in pine woods [6,7]. Several recent studies [8,9,10] confirm the tolerance and/or adaptability of small balsam to shady habitats, moisture, water stress, temperature and nutrients as well as its preference for acidic and non-compacted soil conditions [11].
The influence of I. parviflora on indigenous flora is ecologically significant, as it reduces other herbaceous forest species in good lighting conditions [12,13]. Population densities of small balsam were found to negatively correlate with the herb layer diversity of forest communities [14,15]. Nevertheless, an experiment by Hejda [16] suggests that I. parviflora does not suppress native vegetation but is more likely a ‘passenger’ of the ongoing changes in the invaded vegetation. In particular, the potential impact of Impatiens parviflora on soil-dwelling organisms is understudied despite their important role in ecosystem function [17,18].
Among the various soil inhabitants, nematodes represent one of the most abundant and diverse metazoan groups in terrestrial ecosystems [19]. They occupy several trophic levels by feeding on algae and plants, bacteria and fungi, or soil animals (in particular other nematodes), thus being a central element of the soil food web [20]. This makes them particularly suitable for studying global change effects on different trophic levels within the same faunistic group. All nematodes can be categorized into two main reproductive strategies: (1) K-strategists, which thrive in stable environments, are larger and have long life cycles with small population increases; and (2) r-strategists, whose abundance increases rapidly under favorable conditions, are quite small and have short life cycles and high reproductive rates [21]. Soil nematode communities are thus useful bioindicators, with their functional shifts providing valuable information on the state of an ecosystem, thus allowing inferences regarding other biotic groups and soil health [22]. The representation of nematode species/genera within a community or the abundance of trophic groups, as well as colonizer–persister values of taxa, allow the calculation of various ecological indices and ratios; these parameters facilitate functional interpretation concerning disturbance.
Wolfe and Klironomos [23] proposed three linkages that invasive species directly impact: plant community composition and ecosystem processes; plant and soil community composition; and soil community composition and ecosystem processes. Nevertheless, predicting the impacts of alien plant species and the response of native above- and/or belowground soil biota in natural habitats where invasion has taken place is difficult due to: (i) the unpredictability of invasion locations, (ii) lack of data on native organism communities in those locations prior the invasion and (iii) variability in traits of the invading species that are often new to ecosystems, i.e., individual size (biomass, root area, leaf area), presence of perennial tissue, clonal growth, salinization or the ability to fix nitrogen [24,25]. Therefore, for most invasive plant species, comparative studies remain the main source of information, while several removal experiments have been performed to assess the impact of invasive species such as I. glandulifera [26] or Mimulus guttatus [27] on native vegetation. Thus, we can only compare the community structure of nematodes inhabiting I. parviflora-invaded sites with communities in nearby uninvaded sites, assuming that both sites had similar nematode compositions before the entry of small balsam.
Several previous comparative studies by our research group, such as on the invasive Heracleum sosnowskyi [28,29] or Fallopia japonica [30,31] revealed significant shifts in plant species composition, which subsequently modified nematode assemblages. In contrast, the invasive Asclepias syriaca did not affect nematode communities or nematode species diversity and soil properties, despite considerably decreasing native plant species cover [32]. Unfortunately, there is a lack of data on typical below-ground animal groups with low mobility such as soil nematodes under invasive I. parviflora in Carpathian beech forests and elsewhere. Therefore, in the present study, we explored how the composition and structure of nematode communities were influenced by small balsam colonizing land surfaces in European beech Fagus sylvatica forest. The study aims to answer the following questions: Has the invasion by I. parviflora affected nematode abundance, species presence and diversity, functional guilds, feeding groups and feeding strategies of nematodes compared to plots without its occurrence? Will the changes in the nematode communities be permanent and similar across all seasons over the two-year investigation?
2. Materials and Methods
2.1. Study Site and Characteristics
The impact of I. parviflora, an invasive plant, on the communities of free-living soil and plant-parasitic nematodes was examined repeatedly during 2017–2018 in the deciduous forest of Slanské Hills (48°36′16″ N, 21°25′29″ E). These uplands belong to the Carpathian volcanic arch, an area characterized by a temperate or moderately temperate climate with a mean annual precipitation of 649 mm. The elevation of the study plots ranged from 700 to 728 m a.s.l. The dominant soil type is Cambisol. In terms of soil reaction, these are strongly acidic or acidic soils.
The beech forest colonized by I. parviflora (INV) was covered by 85% Fagus sylvatica L., 10% Quercus petraea (Matt.) Liebl. and 5% Acer caprense L. The understory vegetation was dominated by I. parviflora (80%), with sporadic occurrences of Galium odoratum L., Fragaria vesca L., Isopyrum thalictroides L., Dyopterix filix-mas (L.) Schott or Melica uniflora Retz. The estimated time of invasion was 10–15 years.
The control, non-invaded forest (CON) was covered by 80% F. sylvatica and 20% Q. petraea, with a 5–10 cm layer of dead leaf litter, while understory vegetation was absent.
We selected five permanent research plots (100 m2) in INV and five research plots (100 m2) in CON on the beech forest floor. The distance between permanent plots was established at 100 m, while the distance between invaded and uninvaded plots was set at 50 m. This distance was chosen to exclude possible water and nutrient fluxes between the invaded and uninvaded plots. Pairs of invaded and non-invaded plots did not differ in elevation, inclination or exposure.
2.2. Soil Collection, Analysis, Nematode Extraction and Identification
The soil samples were collected in spring (May), summer (July) and autumn (September) during two years (2017–2018) from each permanent research plot (100 m2). Soil samples were collected using a garden trowel in a systematic design along two independent diagonal transects due to spatial heterogeneity of soil abiotic and biotic characteristics. The soil was collected from a depth of 10 cm, excluding the surface layer, and the subsamples were combined to produce representative bulk samples (1 kg) for each plot, which were enclosed separately in zip-lock plastic bags. A total of 60 representative soil samples were collected, five in each season (May, July and September) in 2017 and 2018 in both INV and CON plots. All samples were transferred to the laboratory and stored at 5 °C until processing.
The contents of each bag were carefully homogenized, coarse vegetation was removed, and the entire sample was subjected to nematode extraction. Nematodes were extracted from each sample by a combination of sieving and a modified funnel technique [31]. This method allows nematodes in water suspension to actively swim and pass through the fine spaces in the filters into the water below for 48 h. Extracted nematodes in water suspension were heat-killed, and total abundance was counted under a stereomicroscope (LEICA S8APO, Wetzlar Germany, magnification up to 80×). After counting, nematodes were fixed with 4% formaldehyde. For identification, the first 10% of the nematodes were randomly selected, with a minimum of 100 individuals per sample, and identified to the species level using an Eclipse 90i light microscope (Nikon Instruments Europe BV, Amstelveen The Netherlands; 100, 200, 400, 600, and 1000× magnification). Total nematode abundance was expressed as the number of individuals/100 g dry soil.
Simultaneously, basic physico-chemical soil parameters were examined for each representative soil sample used for nematode analysis. Soil moisture content was measured from fresh soil gravimetrically by oven-drying at 105 °C to a constant weight overnight. Total organic C and N were measured by using a Vario MACRO Elemental Analyzer (CNS Version; Elementar, New York, NY, USA). Soil pH was estimated potentiometrically in 1 M KCl suspension and distilled water using a digital pH meter (Hanna instruments, Woonsocket, RI, USA). All studied soil properties were measured as co-variables.
2.3. Nematodes, Community Indices and Statistical Analysis
Nematode taxa were assigned to six trophic groups according to [33] and [20]. Additionally, they were categorized according to the colonization–persistence gradient (c–p values) into five colonizer–persister groups (1–5) following [21]. Group c–p1 includes ‘r-strategists’ with short generation times, small eggs and high fecundity, while c–p5 includes ‘k-strategists’ with the longest generation times, largest body sizes, lowest fecundity and greatest sensitivity to disturbance [22]. Nematode species diversity was assessed using the Shannon–Weaver diversity index [34].
Four maturity indices were computed [21,22,35] to indicate the successional stage of communities: the maturity index (MI) for free-living nematodes (c–p from 1 to 5); the maturity index (MI2–5) for free living nematodes (c–p from 2 to 5); the plant parasitic index (PPI) for plant-feeding nematodes; and the total maturity index (ΣMI) for cp–1 to cp–5 combined free-living and plant-parasitic nematodes. Three indices, namely the enrichment index (EI), structure index (SI) and channel index (CI) indicating structure and function of the soil food web, following the weighted faunal analysis concept of [22], were also calculated. Unlike the maturity index concept, nematode taxa are assigned to functional guilds, where all nematodes in a functional guild have the same feeding habit [33] and the same c–p value (e.g., functional guild Ba2 includes all bacterivores with a c–p value of 2). These functional guilds are indicators of ‘basal’ (b), ‘structured’ (s) and ‘enriched’ (e) soil food web conditions. Higher EI values indicate a greater proportion of enrichment microbivores, while higher SI values indicate a larger proportion of omnivores and predators within a community. Soils with high EI and SI are typically low to moderately disturbed (e.g., physical disturbance, pollutants), soils with high EI and lower SI are more heavily disturbed. Soils with low EI and high SI are generally undisturbed, and soils with both low EI and low SI indicate stressed systems [22]. The CI is essentially a weighted ratio of fungivores to bacterivores, indicating the predominant nature of decomposition ‘channels’ in the soil. A high CI (>50%) indicates a higher proportion of fungal decomposition, whereas a low CI (<50%) suggests prevailed bacterial decomposition channels [22].
Statistical analyses were performed using Tukey’s Honestly Significant Difference (HSD) post-hoc test from the PlotIt program for standard statistical analysis. All data were log-transformed prior to analysis to meet assumptions of normality. To identify the relationships between nematode community descriptors and abiotic soil properties, nonparametric Spearman’s correlation coefficient (rs) was computed for each sample using STATISTICA v9.0. Correlations with p < 0.05 and p < 0.01 were considered significant. To determine possible correlations between soil properties that could affect the distribution of nematode taxa, redundancy analysis (RDA) using relative abundance data, log-transformed prior to analyses, was applied. To facilitate such relationships, the position of the nematode genera was presented on the ordination diagram rather than the position of the nematode species. For multivariate analyses, CANOCO version 5 Software (version 5.04) was used [36].
3. Results
3.1. Soil Properties
Across all sampling seasons, no significant differences (p > 0.05) were found in soil pH and moisture (n = 5) between the INV and CON plots, except for soil moisture (SM) in summer 2017 (Table 1). INV plots had significantly higher N and C contents during the whole study and higher C/N ratio in the summer season of both years than CON plots (p < 0.05). Both C and N content increased from spring to autumn in INV plots towards the end of each year. In contrast, N content in the control plots decreased toward autumn. The results of RDA were consistent between 2017 and 2018, highlighting the effect of I. parviflora on soil properties, when INV plots were located at the site where C, N and the C/N ratio were higher. Moreover, RDA identified a positive correlation between C and N contents and C/N ratio. Soil pH showed a negative correlation with soil moisture (Figure 1).

Table 1.
Mean ± SD soil physicochemical properties associated with Impatiens parviflora-invaded (INV) and control plots (CON) in spring (V), summer (VII) and autumn (IX) 2017 and 2018.
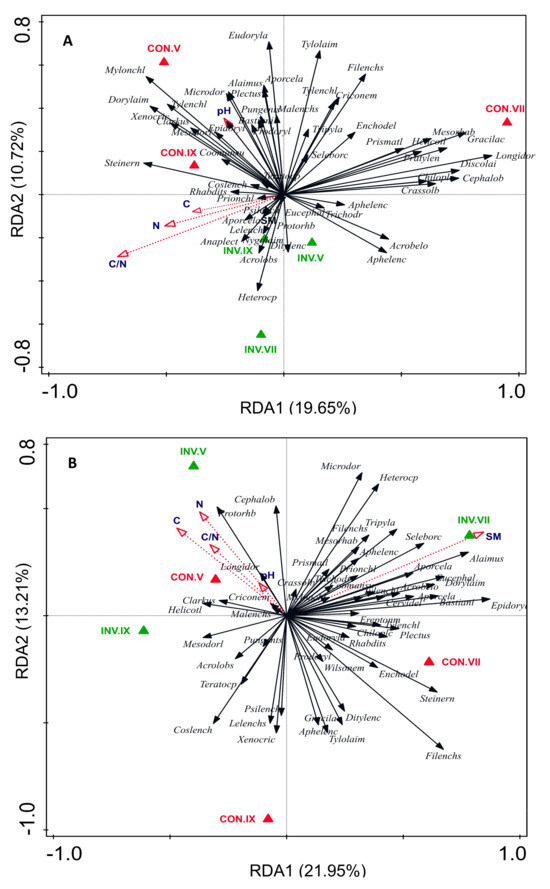
Figure 1.
Relationships between soil factors and genus-level composition of soil nematode communities in Impatiens parviflora-invaded (INV) and control plots (CON) in spring (V), summer (VII) and autumn (IX) 2017 (A) and 2018 (B) assessed by redundancy analysis (RDA). N: soil nitrogen content (% of dry weight); C: soil carbon content (% of dry weight); C/N: ratio of carbon to nitrogen; SM: soil moisture (% of initial weight); pH (KCl): soil acidity.
In general, the mean total abundance of nematodes was greater in CON than in INV plots (Table 2). However, nematode abundance was not significantly affected by I. parviflora invasion (HSD, p < 0.05), although it was negatively correlated with soil pH (HSD, p < 0.05) (Table 3).

Table 2.
Mean values (± SD) for total nematode abundance, species number, relative abundance of nematode trophic groups and community indices associated with Impatiens parviflora-invaded (INV) and control plots (CON) in spring (V), summer (VII) and autumn (IX) 2017 and 2018 (n = 5).

Table 3.
Spearman’s rank correlation between nematode abundance, species number, nematode trophic groups, ecological indices and soil properties.
Across all sampling dates and investigated plots, a total of 52 nematode species were found in the soil samples (Table 4). The mean number of species varied from 18 to 31, but did not significantly differ between INV and CON plots across all seasons (HSD, p < 0.05). Similarly, INV and CON could not be statistically distinguished in terms of nematode species diversity index (Table 2).

Table 4.
Total abundance of soil nematode species associated with Impatiens parviflora-invaded (INV) and non-invaded control (CON) plots in spring (V), summer (VII) and autumn (IX) 2017 and 2018 (n = 5). c–p: colonizer–persister group.
The majority of the nematode individuals belonged to the species Filenchus vulgaris (10.4%), Aporcelaimellus obtusicaudatus (5.4%), Acrobeloides nanus (4.0%), Plectus acuminatus (3.6%) and the genus Rhabditis (9.2%). The distribution of these and less abundant species/genera in INV and CON plots is given in Table 4. In general, the abundance of F. vulgaris was higher in CON than in INV plots throughout the study, whereas P. acuminatus and Rhabditis were more abundant in INV plots. The abundance of A. obtusicaudatus and A. nanus fluctuated between INV and CON among seasons. Several nematode species were found only under I. parviflora, e.g., Eucephalobus striatus and Protorhabditis xylocola, or only in uninvaded control plots, e.g., Discolaimus major and Psilenchus hilarulus.
RDA revealed significant differences in the nematode community between plots invaded by small balsam (INV), and uninvaded control plots (CON), illustrating distinct proportions of genera in each community (Figure 1 and Figure 2). The CON plots from the 2017 autumn showed some degree of overlap with INV (Figure 2). Throughout all seasons, most nematode taxa tended to exhibit higher abundance in CON plots (Figure 1 and Figure 2). Soil properties such as C, N and C/N ratio were identified as particularly influential factors shaping the distribution of nematode genera between INV and CON plots.
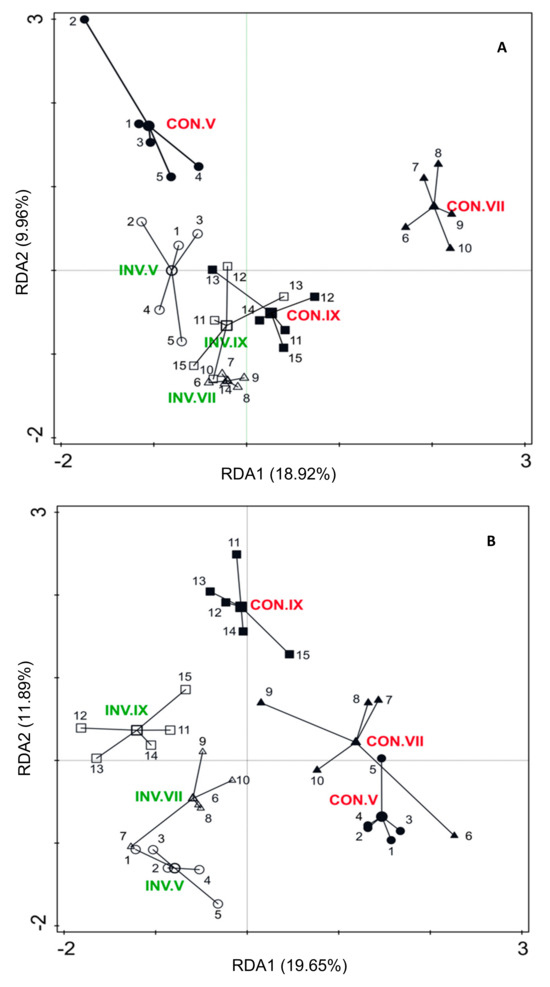
Figure 2.
Ordination of samples and genera (using the first two RDA axes), data ln(y + 1) in Impatiens parviflora-invaded (INV) and control plots (CON) in spring (V), summer (VII) and autumn (IX) 2017 (A) and 2018 (B).
Across all seasons and the investigated INV and CON plots, bacterivores were the most diverse trophic group (14 species), followed by omnivores (11 species), fungivores (8 species), predators (7 species) and obligate and facultative plant parasites (7 and 6 species) (Table 4). I. parviflora invasion significantly increased the relative abundance of bacterivores, while the abundance of plant parasites and root-fungal feeders decreased in INV, particularly during the spring and summer seasons (HSD, p < 0.05). Across all seasons, the number of omnivores, fungivores and predators did not significantly differ between INV and CON plots. Spearman’s correlations identified negative correlations between the number of bacterivores and C and N contents (p < 0.01), and between the number of plant parasites and SM and soil pH (p < 0.01) (Table 3). Root-fungal feeders negatively correlated with soil pH but positively with N content. Furthermore, a favorable correlation between the number of omnivores and C content (p < 0.05) as well as the number of fungivores and SM and N content was recorded (p < 0.05, p < 0.01) (Table 3).
3.2. Functional Diversity and Food Web Diagnostics
MI, MI2–5, ΣMI, PPI, EI, SI and CI values did not differ statistically between INV and CON across all seasons (p > 0.05) (Table 2). F/(F + B) ratio was significantly lower in INV than in CON during both the spring and summer seasons (p > 0.05). Total nematode biomass varied inconsistently across seasons, but did not show significant difference between INV and CON. However, Spearman’s correlations revealed positive interactions between MI, EI, CI, nematode biomass and C and N contents (p > 0.01; p > 0.05) (Table 3). In contrast, CI negatively correlated with SM.
Ordination of nematode species along with the structure index and enrichment index trajectories [22] did not reveal differences between INV and CON in both investigated years (Figure 3). The composition of nematode communities mostly indicated a maturing food web, with low to moderate disturbance in both I. parviflora-invaded and uninvaded forest plots. Decomposition channels of soil food webs in the majority of samples (plots) were balanced between fungal–bacterial mediation (Figure 3). Nonetheless, the relatively low values of CI and F/(F + B) ratio suggesting prevailing bacterial decomposition in INV across all seasons (Table 2) in comparison to CON.
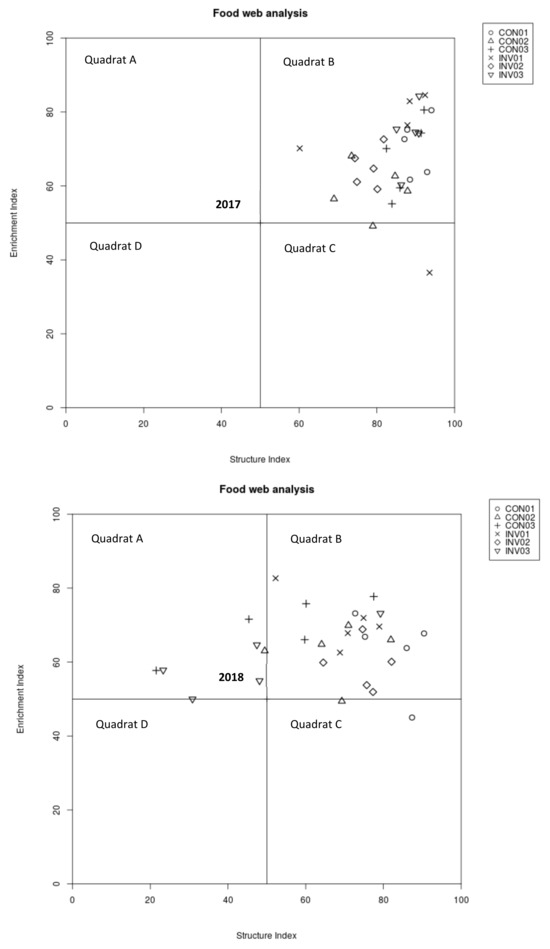
Figure 3.
Nematode food web conditions associated with Impatiens parviflora-invaded (INV) and non-invaded control (CON) plots in 2017 and 2018; spring (01); summer (02); autumn (03); according to the nematode faunal analysis concept by Ferris et al., (2001) [22]. Quadrat A: disturbed food web; Quadrat B: maturing food web; Quadrat C: structured–stable food web; Quadrat D: degraded food web.
4. Discussion
Our study, conducted in beech forest habitat, presented distinct characteristics. It is well established that F. sylvatica exhibits strong competitive abilities against other tree species, resulting in sparse understory vegetation in these habitats [37,38]. This aligns partially with our study, where uninvaded control plots were devoid of ground-floor vegetation across all sampling dates. In contrast, the INV plots displayed an understory dominated by a dense canopy of I. parviflora along with sporadic occurrence of some other herbal species. Interestingly, both INV and CON plots shared similar slope, exposure and tree species composition. This contrasts with findings reported by [16], who suggested that I. parviflora has minimal impact on native species richness in invaded communities, possibly due to its limited ability to form a dense canopy and modest root system. Many invasive plant species, including I. parviflora, are known to be shade-tolerant [4] and thus thrive in closed-canopy forests. It remains unclear why small balsam proliferates in some areas of the Carpathian beech forest selected for our study, but not in others. Extensive research has indicated that small balsam possesses a broad ecological niche, which contributes to its success in colonizing and persisting in various communities, irrespective of soil physical and chemical properties [39]. The soils in our study were strongly acidic or acidic, with no significant differences between INV and CON plots. This finding is consistent with previous studies [11,13], suggesting that I. parviflora thrives in acidic soils and/or that its invasion does not alter soil pH.
Comparing invaded sites with nearby uninvaded sites, most investigations detected increases in soil nutrient stock and/or an increase in resource availability below various invasive plant species, even if the plant species are not nitrogen fixers [40,41,42,43]. The results obtained in our study agree with these studies, but partially contradict the results by [39]. INV plots had significantly higher C and N contents than CON plots across all sampling dates. This phenomenon should be attributed to an enlarged standing crop biomass and the production of a much greater amount of leaf litter, which stimulates the activity of decomposers and may lead to a higher carbon content in the top soil [44]. However, in many cases, changes in nutrient levels are site specific, depending mainly on the initial soil conditions before the invasion process started [42].
Regarding soil fauna, to date, only a few researchers have examined the effect of I. parviflora invasions on soil-inhabiting biota, e.g., yeasts [45] and ants [46]. To our knowledge, no study has distinguished the impact of I. parviflora establishment on soil nematodes anywhere. Thus, the present study is the first to characterize the structure of soil nematode communities in deciduous forest with ground cover colonized by small balsam.
In general, multivariate RDA revealed that the nematode community in plots with I. parviflora differed from that in uninvaded plots, reflecting different proportions of nematode taxa in the two communities. Nevertheless, overall nematode abundance and species diversity, as well as the relative abundance of F, O and P nematodes and nematode biomass did not differ between CON and INV. We hypothesized that colonization by I. parviflora would decrease both nematode abundance and diversity as observed in several other invasive plant species, e.g., Spartina alterniflora [47], H. sosnowskyi [29,48] and F. japonica [30,31]. However, our assumption has not been confirmed. On the other hand, our results are consistent with those from permanent grasslands colonized by the invasive herb Asclepias syriaca [32], as its expansion affected neither nematode species diversity nor nematode abundance. Moreover, A. syriaca did not affect nematode trophic structure, including belowground plant enemies, plant-parasitic nematodes [32], confirming findings by [49] from introduced areas invaded by Ammophila arenaria.
In our deciduous beech forest, plant-parasitic and root-fungal-feeding nematodes belong to the least diverse trophic groups in both INV and CON plots, with their relative abundance similar to that found in natural deciduous forests by [37,50]. Although INV plots had fewer PP and RFF nematodes, especially in spring and summer, compared to CON plots, this decrease was found to be negatively correlated with soil moisture. Because small balsam has a modest root system [16], we assumed that the decrease in PP and RFF abundance was due to changes in soil moisture, rather than the invasion of I. parviflora to forest floor. Our findings did not support the enemy release hypothesis (ERH), which is generally accepted for vertebrates and invertebrates, fungal pathogens and bacterial or viral diseases as main natural plant enemies [51]. The ERH suggests that introduced plants become invasive pests because they benefit from escaping their coevolved enemies [51], contradicting the results by [52], who found I. parviflora under high pest pressure in the introduced range. Interestingly, Solidago gigantea invasion increased overall nematode abundance, mainly due to higher numbers of several plant-parasitic nematode species [53]. This was attributed to high biomass production and the well-developed root system of S. gigantea on which plant parasites can feed. This contradicts our previous findings from H. sosnowskyi [29,48] or F. japonica [30,31] invaded plots, where considerably fewer plant-parasitic nematodes were found than in control plots. This was attributed to the release of chemical compounds (furanocoumarins) by giant hogweed or high tannin concentrations in knotweed tissues that suppress PP nematodes in the soils and thus could facilitate invasive plant expansion. Our current as well as previous results showed that the potential for enemy release varies across invasive species. Enemy release might play a role in some plant invasions, whereas different mechanisms, such as community disturbance, are important in others [54].
Higher plants are quantitatively the most essential producers in terrestrial ecosystems, and several recent studies have clearly shown that individual plant species differ in their effects on various groups of soil organisms they support [55,56,57]. The quality and quantity of plant litter can directly affect the populations and diversity of soil decomposers (bacterivores, fungivores) and their food sources. We assumed that these nematode trophic groups would benefit from plant invasion due to higher supply of food associated with the huge biomass production and improved microclimatic conditions attributed to invasive plant establishment [58,59]. The bacterivores, which feed on soil microbes, were among the most diverse trophic groups in both INV and CON plots in our study. Moreover, I. parviflora invasion overall enhanced their relative abundance, suggesting a prevailed fast bacterial mediated decomposition of organic matter in INV plots. There are only a few reports that show the opposite [31,48]. In contrast, fungivores that feed on fungal biomass were slightly (albeit not significantly) more numerous in CON, suggesting that slow fungal decomposition of organic matter comes mainly from tree litterfall.
The prevalence of bacterivorous nematodes and low and/or decreased abundance of nematodes of higher trophic groups (omnivores and predators) are in general considered as indicators of ecosystem disturbance [19,21,22]. Despite an increase in B nematodes under I. parviflora, invasion by small balsam resulted in a maturing soil food web where numbers of O and P nematodes were similar to those in CON plots across all sampling dates. This finding agrees with previous studies on habitats invaded by H. sosnowskyi [48], A. syriaca [32] or S. gigantea [53], while several reports [31,60] showed the opposite after colonization by F. japonica and Ambrosia trifida, respectively. The functional characteristics (indices) of the beech forest nematode assemblages showed similar patterns in both INV and CON plots, suggesting that enhanced litter input by dense small balsam causes similar responses in the structural and functional parameters of biotic communities. According to the proposal by [19], larger, more diverse nematode assemblage communities indicate healthy soils and are considered desirable. Our findings suggests that the small balsam invasion has no detrimental effect on soil health.
5. Conclusions
It would be interesting to know the composition of the community of organisms that we want to follow before a disturbance and subsequently to evaluate the changes after the disturbance, in our case ecosystem colonization by an invasive plant species. Unfortunately, we cannot predict when and where invasions will take place, thus, we can only compare the community structure of nematodes inhabiting invaded plots with communities in nearby uninvaded plots, assuming both plots had similar nematofauna structure before the invasion. Expected changes in soil nematode communities from I. parviflora invasion were not observed in the present study. Overall, nematode abundance and species diversity, relative abundances of fungivores, omnivores and predators as well as nematode biomass did not differ between invaded and uninvaded plots. Invaded plots had a higher abundance of bacterivores and relatively low values of CI and F(F + B) ratio across all seasons, suggesting that I. parviflora invasion can have a lasting impact on the decomposition pathway. The lower abundance of plant parasitic nematodes under small balsam in some seasons was probably more a consequence of changes in soil moisture than the presence I. parviflora with modest root systems. Moreover, the impact of small balsam on nematode communities did not differ between sampling years and across seasons, indicating a stable and unchanging response to invasive plant growth. Nevertheless, multivariate analysis based on nematode genera distinguished the soil nematode communities in invaded (INV) from those in uninvaded (CON) plots across all seasons, reflecting different proportions of nematode taxa in the two communities. This finding did not correspond with considerably higher carbon (C) and nitrogen (N) contents in invaded plots, whereas soil acidity was similar to that in uninvaded control plots. However, the results presented here are just one case study of I. parviflora invasion into Carpathian beech deciduous forest followed over a relatively short time period. Considering the great fluctuation in environmental variables, such as soil type, physico-chemical characteristics, temperature, elevation or precipitation, parallel and long-term studies in multiple locations with different habitats where invasions take place are needed to corroborate these findings.
Author Contributions
M.R. conceived of the study, led the project and wrote the first draft of the manuscript; M.R. and J.J. collected the material, conducted the study and analyzed the data, A.Č. contributed to data interpretation and commented on and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Slovak scientific agency VEGA, project No. 2/0007/24 “Diversity of soil nematodes and activity of microorganisms of Carpathian forests in relation to climate change”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data in this study are available in presented tables and figures. Raw data are available from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Normand, S.; Svenning, J.C.; Skov, F. National and European perspectives on climate change sensitivity of the habitats directive characteristic plant species. J. Nat. Conserv. 2007, 15, 41–53. [Google Scholar] [CrossRef]
- Lookwood, J.L.; Hoopes, M.P.; Marchetti, M.P. Invasion Ecology; Blackwell: Oxford, UK, 2007. [Google Scholar]
- Von Holle, B.; Delcourt, H.R.; Simberloff, D. The importance of biological inertia in plant community resistance to invasion. J. Veg. Sci. 2003, 14, 425–432. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol. Environ. 2009, 7, 142–149. [Google Scholar] [CrossRef]
- Trepl, L. Über Impatiens parviflora DC. als Agriophyt in Mitteleuropa. Diss. Bot. 1984, 73, 1–400. [Google Scholar]
- Chmura, D.; Sierka, E. Relation between invasive plant and species richness of forest floor vegetation: A study of Impatiens parviflora DC. Pol. J. Ecol. 2006, 54, 417–421. [Google Scholar]
- Jarčuška, B.; Slezák, M.; Hrivnák, R.; Senko, D. Invasibility of alien Impatiens parviflora in temperate forest understories. Flora 2016, 224, 14–22. [Google Scholar] [CrossRef]
- Čuda, J.; Skálová, H.; Janovský, Z.; Pyšek, P. Habitat requirements, short-term population dynamics and coexistence of native and invasive Impatiens species: A field study. Biol. Invasions 2014, 16, 177–190. [Google Scholar] [CrossRef]
- Quinet, M.; Descamps, C.; Coster, Q.; Lutts, S.; Jacquemart, A.L. Tolerance to water stress and shade in the invasive Impatiens parviflora. Int. J. Plant Sci. 2015, 176, 848–858. [Google Scholar] [CrossRef]
- Lanta, V.; Liancourt, P.; Altman, J. Determinants of invasion by single versus multiple plant species in temperate lowland forests. Biol. Invasions 2022, 24, 2513–2528. [Google Scholar] [CrossRef]
- Bobuľská, L.; Macková, D.; Malina, R.; Demková, L. Occurrence and dynamics of Impatiens parviflora depending on various environmental conditions in the protected areas in Slovakia. Eur. J. Ecol. 2016, 2, 87–94. [Google Scholar] [CrossRef]
- Kujawa-Pawlaczyk, J. The spread of Impatiens parviflora DC. in Bialowieza forest. Phytocoen. Semin. Geobot. 1991, 1, 213–222. [Google Scholar]
- Florianová, A.; Münzbergová, Z. Drivers of natural spread of invasive Impatiens parviflora differ between life-cycle stages. Biol. Invasions 2018, 20, 2121–2140. [Google Scholar] [CrossRef]
- Obidziński, T.; Symonides, E. The influence of the groundlayer structure on the invasion of small balsam (Impatiens parviflora DC.) to natural and degraded forest. Acta Soc. Bot. Pol. 2000, 69, 311–318. [Google Scholar] [CrossRef]
- Chmura, D. Biology and Ecology of an Invasion of Impatiens Parviflora DC in Natural and Semi-Natural Habitats, 216; Wydawnictwo Naukowe Akademii Techniczno-Humanistycznej w Bielsku-Białej: Bielsko-Biała, Poland, 2014. [Google Scholar]
- Hejda, M. What is the impact of Impatiens parviflora on diversity and composition of herbal layer communities of temperate forests? PLoS ONE 2012, 7, e395. [Google Scholar] [CrossRef] [PubMed]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Neher, D.A.; Barbercheck, M.E. Diversity and function of soil mesofauna. In Biodiversity in Agroecosystems; Collins, W.W., Qualset, C.O., Eds.; Lewis Publishers: New York, NY, USA, 1998; pp. 27–47. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Wasilewska, L. Soil invertebrates as bioindicators, with special reference to soil-inhabiting nematodes. Russ. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking new ground: Soil communities and exotic plant invasion. Bioscience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Chapin, I.F.S.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Díaz, S. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Roiloa, S.R.; Yu, F.H.; Barreiro, R. Plant invasions: Mechanisms, impacts and management. Flora 2020, 267, 151603. [Google Scholar] [CrossRef]
- Hulme, P.E.; Bremner, E.T. Assessing the impact of Impatiens glandulifera on riparian habitats: Partitioning diversity components following species removal. J. Appl. Ecol. 2006, 43, 43–50. [Google Scholar] [CrossRef]
- Truscott, A.; Palmer, S.C.; Soulsby, C.; Westaway, S.; Hulme, P.E. Consequences of invasion by the alien plant Mimulus guttatus on the species composition and soil properties of riparian plant communities in Scotland. Perspect. Plant Ecol. Evol. Syst. 2008, 10, 231–240. [Google Scholar] [CrossRef]
- Renčo, M.; Kornobis, F.W.; Domaradzki, K.; Jakubska-Busse, A.; Jurová, J.; Homolová, Z. How does an invasive Heracleum sosnowskyi affect soil nematode communities in natural conditions? Nematology 2019, 21, 71–89. [Google Scholar] [CrossRef]
- Čerevková, A.; Ivashchenko, K.; Miklisová, D.; Ananyeva, N.; Renčo, M. Influence of invasion by Sosnowsky’s hogweed on nematode communities and microbial activity in forest and grassland ecosystems. Glob. Ecol. Conserv. 2020, 21, e00851. [Google Scholar] [CrossRef]
- Čerevková, A.; Bobuľská, L.; Miklisová, D.; Renčo, M. A case study of soil food web components affected by Fallopia japonica (Polygonaceae) in three natural habitats in Central Europe. J. Nematol. 2019, 51, e2019-42. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z. Nematode communities indicate the negative impact of Reynoutria japonica invasion on soil fauna in ruderal habitats of Tatra National park in Slovakia. Glob. Ecol. Conserv. 2021, 26, e01470. [Google Scholar] [CrossRef]
- Jurová, J.; Renčo, M.; Gömöryová, E.; Čerevková, A. Effects of the invasive common milkweed (Asclepias syriaca) on nematode communities in natural grasslands. Nematology 2020, 22, 423–438. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.D.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–325. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Yeates, G.W. Modification and qualification of the nematode maturity index. Pedobiologia 1994, 38, 97–101. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Renčo, M.; Čerevková, A.; Gömöryová, E. Soil nematode fauna and microbial characteristics in an early-successional forest ecosystem. Forests 2019, 10, 888. [Google Scholar] [CrossRef]
- Von Oheimb, G.; Friedel, A.; Bertsch, A.; Härdtle, W. The effects of windthrow on plant species richness in a Central European beech forest. Plant Ecol. 2007, 191, 47–65. [Google Scholar] [CrossRef]
- Chmura, D.; Sierka, E.; Orczewska, A. Autecology of Impatiens parviflora DC. in natural forest communities. Roczniki Akademii Rolniczej w Poznaniu. Bot.-Steciana 2007, 11, 17–21. [Google Scholar]
- Vanderhoeven, S.; Dassonville, N.; Meerts, P. Increased topsoil mineral nutrient concentrations under exotic invasive plants in Belgium. Plant Soil 2005, 275, 169–179. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Vanderhoeven, S.; Dassonville, N.; Koutika, L.S.; Meerts, P. Effect of the exotic invasive plant Solidago gigantea on soil phosphorus status. Biol. Fertil. Soils 2006, 42, 481–489. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef]
- Liao, C.; Peng, R.; Luo, Y.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Bo, L. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008, 177, 706–714. [Google Scholar] [CrossRef]
- Steinlein, T. Invasive alien plants and their effects on native microbial soil communities. In Progress in Botany; Lüttge, U., Beyschlag, W., Francis, D., Cushman, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Glushakova, A.M.; Kachalkin, A.V.; Chernov, I.Y. Effect of invasive herb species on the structure of soil yeast complexes in mixed forests exemplified by Impatiens parviflora DC. Microbiology 2015, 84, 717–721. [Google Scholar] [CrossRef]
- Stukalyuk, S.V. Changes in the structure of ant assemblages in broad-leafed forests with domination of Impatiens parviflora Dc. (Balsaminaceae) in herbaceous layer. Russ. J. Biol. Invasions 2016, 7, 383–395. [Google Scholar] [CrossRef]
- Zhang, P.; Neher, D.A.; Li, B.; Wu, J. The impacts of above and belowground plant input on soil microbiota: Invasive Spartina alterniflora versus native Phragmites australis. Ecosystems 2018, 21, 469–481. [Google Scholar] [CrossRef]
- Renčo, M.; Baležentiené, L. An analysis of soil free-living and plant-parasitic nematode communities in three habitats invaded by Heracleum sosnowskyi in central Lithuania. Biol. Invasions 2015, 17, 1025–1039. [Google Scholar] [CrossRef]
- Brinkman, E.P.; van Veen, J.A.; van der Putten, W.H. Endoparasitic nematodes reduce multiplication of ectoparasitic nematodes, but do not prevent growth reduction of Ammophila arenaria (L.) Link (marram grass). Appl. Soil Ecol. 2004, 27, 65–75. [Google Scholar] [CrossRef]
- Háněl, L. Composition and seasonal changes of soil nematode comunity in a Central European oak forest. Acta Soc. Zool. Bohem. 1994, 58, 177–188. [Google Scholar]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2020, 17, 164–170. [Google Scholar] [CrossRef]
- Najberek, K.; Solarz, W.; Chmura, D. Do local enemies attack alien and native Impatiens alike? Acta Soc. Bot. Pol. 2017, 86, 25–31. [Google Scholar] [CrossRef]
- Čerevková, A.; Miklisová, D.; Bobuľská, L.; Renčo, M. Impact of the invasive plant Solidago gigantea on soil nematodes in a semi-natural grassland and a temperate broadleaved mixed forest. J. Helminthol. 2019, 94, e51. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Fountain, M.T.; Barea, J.M.; Christensen, S.; Dekker, S.C.; Duyts, H.; Van der Putten, W.H. Divergent composition but similar function of soil food webs of individual plants: Plant species and community effects. Ecology 2010, 91, 3027–3036. [Google Scholar] [CrossRef]
- Millard, P.; Singh, B.K. Does grassland vegetation drive soil microbial diversity? Nutr. Cycl. Agroecosystems 2010, 88, 147–158. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W.; Williamson, W.; Bonner, K.I. The response of a three trophic level soil food web to the identity and diversity of plant species and functional groups. Oikos 2003, 102, 45–56. [Google Scholar] [CrossRef]
- Aguilera, A.G.; Alpert, P.; Dukes, J.S.; Harrington, R. Impacts of the invasive plant Fallopia japonica (Houtt.) on plant communities and ecosystem processes. Biol. Invasions 2010, 12, 1243–1252. [Google Scholar] [CrossRef]
- Te Beest, M.; Esler, K.J.; Richardson, D.M. Linking functional traits to impacts of invasive plant species: A case study. Plant Ecol. 2015, 216, 293–305. [Google Scholar] [CrossRef]
- Liang, W.J.; Li, F.P.; Li, Q.; Zhang, W.D. Temporal dynamics of soil nematode community structure under invasive Ambrosia trifida and native Chenopodium serotinum. Helminthologia 2007, 44, 29–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).