Abstract
Unprotected areas with endangered species generally face severe human disturbance. Domestic dogs are a special form of human disturbance and are sympatrically distributed with critically endangered Chinese pangolins in remote mountainous regions of Guangdong, China. Conflicts in habitat utilization between domestic dogs and Chinese pangolins have rarely been evaluated, yet these conflicts might result in a decline in population viability in the wild. To address how domestic dogs affect Chinese pangolins (Manis pentadactyla) in spatiotemporal niches, we used camera traps to obtain information on the distribution and activity of Chinese pangolins and GPS collars to track free-ranging domestic dog activity in the Wuqinzhang and Pengzhai forest areas of Guangdong, China. Combined with environmental variables, we used individual and cave locations to predict a potentially suitable habitat for Chinese pangolins with Maxent. We used the minimum convex polygon method (MCP) to obtain the home ranges of the domestic dogs. Then, we calculated the overlap between the potentially suitable habitat for Chinese pangolins and the home ranges of free-ranging domestic dogs. In the temporal niche, we compared the daily activity rhythms between domestic dogs and Chinese pangolins and assessed the influences of domestic dogs on Chinese pangolins through avoidance–attraction ratios (AARs). Our results show that the potentially suitable habitat of the Chinese pangolin comprises only approximately 24% of the Wuqinzhang forest area and 12% of the Pengzhai forest area. The percentages of habitat overlap were approximately 48% and 71% in the Wuqinzhang and Pengzhai forest areas, respectively. There was less overlap in the temporal niche between Chinese pangolins and free-ranging domestic dogs, but their AAR was significant. Our results reveal that the Chinese pangolin is facing severe disturbances from free-ranging domestic dogs in spatial niches in unprotected areas. We suggest that assessments of Chinese pangolins’ survival status should be conducted as soon as possible, especially in unprotected areas. To expand and optimize established nature reserves for the Chinese pangolin, further strengthening of domestic dog management is necessary.
1. Introduction
Domesticated animals originated in the wild, but have been domesticated by humans and are currently pets, livestock, and service animals [1,2]. Due to their migration (both in nature and with humans), domesticated animals are found in all types of ecosystems and habitats and exert large impacts on ecosystems [3]. As the first and most common carnivorous pet, the domestic dog, Canis familiaris, was domesticated by humans approximately 14,000 years ago [4,5]. Dogs can quickly move between human settlements and nearby natural landscapes [6]. Domestic dogs negatively affect wildlife by hunting, spreading diseases, and hybridizing with wild canid animals [7,8,9], reducing the inclusive fitness of local species [10,11]. There are approximately 1 billion domestic dogs in the world, and nearly 75% of them are free-ranging or unowned [1,12]. At least 188 species are threatened by domestic dogs; therefore, domestic dogs are listed as one of the top 100 invasive species [1,13,14]. The number of dogs is sometimes even greater than that of humans, particularly in rural areas [10].
The Chinese pangolin (Manis pentadactyla) is a critically endangered species and is mainly distributed in the southern Yangtze River, Hainan, and Taiwan islands in China and in countries bordering China [15]. They are timid, nocturnal animals that live in caves and are only covered with scales [16,17,18]. Due to its medicinal value in traditional medicine, this species is severely poached and widely traded in Southeast Asia [19,20]. Since the 1960s, the population of Chinese pangolins has declined by about 90%, and the distribution range has decreased by half in eastern China [21,22]. Human disturbance is one of the major contributors to the endangerment of this species. With social and economic development, the construction of roads, houses, and other basic facilities has exacerbated the reduction in and loss of the habitat of the Chinese pangolin, while massive changes in vegetation types have led to a decline in the quality of its habitat. The habitat of Chinese pangolins has decreased and become fragmented, which has made them extremely rare in their original habitat [22,23]. However, assessments of the impacts of free-ranging domestic dogs, a special kind of human disturbance, on wildlife are rare [24,25].
Domestic dogs can be directly or indirectly associated with the Chinese pangolin [26]. Due to domestic dogs’ superior sense of smell and obedience, they are commonly used as hunting tools worldwide and as potential predators of the Chinese pangolin [23,27]. Therefore, Chinese pangolins are constantly attacked by domestic dogs in some provinces of China [28]. According to the Endangered Wildlife Rescue Center rescue records in Pingdong, approximately 20% of rescued Chinese pangolins from 2006 to 2017 were attacked by domestic dogs [29]. With the implementation of conservation policies, the utilization of dogs for hunting has undergone a transformation. Domestic dogs have been employed in scientific research, with researchers successfully training domesticated dogs to locate pangolin caves in their natural habitats and conduct population monitoring [30], potentially exerting pressure on the survival prospects of Chinese pangolins. Otherwise, domestic dogs can affect pangolins’ movements when they pass through human settlements, which may cause them to be detected by other predators. Moreover, domestic dogs, as intermediate hosts, have the potential to transmit viruses and ticks to wild animals [8]. According to rescue records, many Chinese pangolins with ticks attached to their scales have been found [31,32]. Several studies have also shown that Chinese pangolins are infected with CPV [9], which is a virus with a high fatality rate [8]. Even if Chinese pangolins survive treatment, they host the CPV throughout their life [8,9] and spread the virus during mating seasons.
Chinese pangolins are potentially interfered with by free-ranging domestic dogs, especially outside of protected areas. Therefore, to clarify how domestic dogs affect Chinese pangolins, we monitored the activity rhythm of Chinese pangolins and used the Maxent model to predicts potentially suitable habitats for the Chinese pangolin. We also tracked free-ranging domestic dogs using GPS collars to measure the spatial associations and temporal avoidance between Chinese pangolins and free-ranging domestic dogs. Our study reveals the potential impact of this special human disturbance on Chinese pangolins from the perspective of spatiotemporal niches and provides a reference for Chinese pangolin protection in unprotected areas.
2. Materials and Methods
2.1. Study Area and Data Acquisition
Our study was conducted in unprotected areas that are Chinese pangolin hotspots: the Pengzhai Forest (Figure 1A, 101 km2) in Heping County and the Wuqinzhang Forest (Figure 1B, 186 km2) in Huidong County of Guangdong Province, China. We found 349 caves and 428 caves containing Chinese pangolins in the Wuqinzhang forest area (WQZ) and Pengzhai forest area (PZ), respectively.
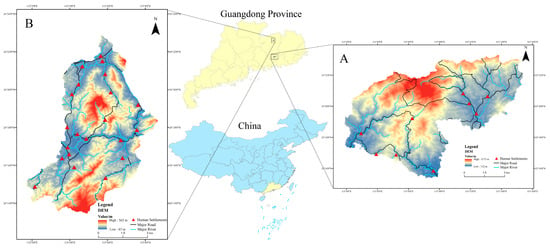
Figure 1.
Study area and distribution of human settlements. (A) Wuqinzhang Forest, (B) Pengzhai Forest.
2.1.1. Occurrences of Chinese Pangolins and Activity Rhythms
We conducted a field investigation of Chinese pangolins through grid and line transect methods from March 2020 to February 2022. We recorded 373 caves and the habitat characteristics (altitude, slope, aspect, vegetation). We installed camera traps in front of the caves to monitor the activity of the Chinese pangolins. We identified which photographs contained Chinese pangolins and extracted their activity rhythms.
2.1.2. GPS Collars Tracking Free-Ranging Domestic Dogs
A total of 8 free-ranging domestic dogs were randomly selected from households in the WQZ and PZs from February 2021 to February 2022. We installed collars on free-ranging domestic dogs with the help of the owners. GPS collars (HQAN40S, 220 g, made by HuanQiuXinShi company in Changsha, China) were worn by the dogs, complying with the requirements of animal telemetry technology. Collars were set to record loci (including latitude, longitude, movement, speed, altitude, etc.) every 1 h due to the rapid movement speed and wide range of activities of domestic dogs. A total of 17,680 valid locations were obtained from the eight domestic dogs.
2.1.3. Environmental Variables
Topography, vegetation, water sources, and human disturbance are the main factors affecting the survival of wildlife. Therefore, according to the life history characteristics of Chinese pangolin, eight environmental factors of four categories were selected to predict the suitable habitat of the Chinese pangolin, including topographic factors, including elevation, slope, and aspect; vegetation factors, including forest type and forest age; human disturbance variables, including distance to roads and human settlement; and distance to water sources (Table 1). Road, human settlement, and river data were obtained from the 1:250,000 basic geographic data of Guangdong Province (2015) from the China National Earth System Science Data Center. We created Euclidean distance layers for roads, human settlements, and rivers in ArcGIS (version 10.8).

Table 1.
Environmental variables used to model the suitable habitat of Chinese pangolins.
2.2. Constructing a Habitat Model for Chinese Pangolins
We used Maxent (3.4.1) to predict a potentially suitable habitat of the Chinese pangolin [33,34]. We rarefied occurrences of Chinese pangolins at a Euclidean distance of 600 m according to Chinese pangolins’ average home range [15,35]. We tested the Spearman correlation coefficients between environmental variables, and no variables were highly correlated (|r| < 0.7). We also set bootstrapping methods for model robustness and used jackknife to assess variable importance. We evaluated the model through the receiver operating characteristic curve (ROC) and test area under the curve (test AUC). The output was set in logistic format (0–1), representing the habitat suitability index (HSI) [36]. The maximum training sensitivity plus specificity threshold (MTSS) was used as the threshold to distinguish potentially suitable or unsuitable habitats; then, potentially suitable habitats were reclassified into high-, medium-, and low-suitability, and the areas were calculated [37,38].
2.3. Spatial Relationship between Chinese Pangolins and Domestic Dogs
We used the 100% minimum convex polygon method (MCP) to calculate the home ranges of free-ranging domestic dogs through the Home Range Tools in ArcGIS (version 10.7). MCP was the first model applied to measure the spatial distribution and habitat use of wildlife, generating home ranges based on activity loci, which can visually describe the entire activity area of the animals [39,40,41,42]. The potentially suitable habitat layer of the Chinese pangolins was overlaid with the home range layer of the domestic dogs. The proportion of the overlapping area was considered the conflict area between the Chinese pangolins and domestic dogs. High-, medium-, and low-conflict areas were considered as high-, medium-, and low-suitability habitats, respectively, for Chinese pangolins.
In addition, considering that the distribution of domestic dogs is highly correlated with settlements, we further evaluated the relationship between the distribution of Chinese pangolins and settlements during the mating and non-mating seasons. We divided the Chinese pangolin activity locations captured by camera traps into mating (May–June, October–December) and non-mating (November–April, July–August) seasons and calculated the Euclidean distance between the Chinese pangolins and human settlements. We used an independent samples t-test to test whether there were significant differences between the mating and non-mating seasons [29,43].
2.4. Temporal Analysis of Chinese Pangolins and Domestic Dogs
We collected videos and photos of Chinese pangolins captured by camera traps and defined photos or videos taken within 30 min as independent detections. We used independent detection of Chinese pangolins and domestic dogs to construct a nonparametric kernel density model with the packages “Overlap” (version 0.3.9) and “Activity” (version 1.3.4) in R (version 4.2.3) [44,45]. We extracted the activity time of the Chinese pangolins via independent detection. We filtered the GPS collar data, excluding records of activity with fewer than 120 or invalid location points, then extracted the activity time of free-ranging domestic dogs. Due to the large amount of data generated by GPS collars, 10% of the total data points were randomly selected as samples for subsequent calculations to facilitate subsequent analysis [46]. We used independent detections to compare intra- and interspecies activity rhythms. First, we compared the activity patterns of Chinese pangolins and domestic dogs during four seasons (spring: February–April; summer: May–September; autumn: October–November; and winter: December–January). Second, we compared the differences in the activity rhythms of Chinese pangolins and domestic dogs in different seasons. Finally, we compared the activity rhythms of Chinese pangolins in the mating and non-mating seasons. Pairwise comparisons of the two species were performed by estimating the coefficients of overlap (∆4) and sample sizes (>75) [47]. Then, we generated 10,000 smoothed bootstraps to estimate the confidence interval (CI) and mean value of ∆4 [44].
We also calculated the avoidance–attraction ratios (AARs) of Chinese pangolins and domestic dogs. The AAR is an index used to calculate the temporal interaction of two species [48,49]. We used a time series of camera traps to test the AAR of the Chinese pangolin after a domestic dog was detected. AAR1 measures the time interval for a Chinese pangolin to be detected after/before a domestic pass (AAR1 = T2/T1), and AAR2 is the time interval between Chinese pangolin detection with/without the influence of a domestic dog (AAR2 = T4/T3, Table 2) [48]. The difference between AAR1 and AAR2 is that AAR1 is affected by prey avoidance and predator attraction, while AAR2 is affected only by prey avoidance. When AAR1 or AAR2 > 1, it implies that the two species have a significant, nonrandom temporal relationship [49].

Table 2.
Percent contribution and permutation importance of environmental variables.
3. Results
3.1. Potentially Suitable Habitats of Chinese Pangolins
The average test AUC of the Maxent models was 0.837 in the WQZ and 0.898 in the PZ, indicating that the prediction results had good reference. In the WQZ, our results demonstrated that the three most important environmental factors were forest type (22%), elevation (16%), and distance to roads (15%). The lowest contributors were distance to a human settlement (6%) and distance from the nearest river (7%) (Table 2). The results of the percent contribution showed that the most influential environmental factors were forest type and elevation, and the least influential factors were still distance to human settlement and rivers. There were slight differences between WQZ and PZ. We used MTSS (0.39) to distinguish suitable habitats from unsuitable habitats. The nature break method was used to further divide suitable habitats into three categories: high-suitability (HSI ≥ 0.56), medium-suitability (0.56 > HSI ≥ 0.47), and low-suitability (0.47 > HSI ≥ 0.39). The areas of high-, medium-, and low-suitability habitats were 9 km2, 16 km2, and 19 km2, respectively, accounting for 5%, 9%, and 10%, respectively, of the WQZ area.
The results of the Maxent model contribution in the PZ showed that the three most important environmental factors were elevation (40%), forest age (21%), and forest type (21%), and the least important factor was slope (0.4%) (Table 2). The results of the percentage contribution showed that the most influential environmental factors were forest type and distance from major rivers. According to the response curves of the environmental factors, elevation was the most important contributing environmental variable, and the Chinese pangolin prefers medium and low elevations. Second, with respect to forest age, the Chinese pangolin prefers relatively pure broad-leaved forests. We used the same methods to distinguish and evaluate habitats, MTSS (0.37). The areas of high-suitability (HSI ≥ 0.57), medium-suitability (0.57 > HSI ≥ 0.46), and low-suitability (0.46 > HSI ≥ 0.37) habitats were 2 km2, 4 km2, and 6 km2, respectively, accounting for 2%, 4%, and 6% of the PZ area.
3.2. Spatial Conflicts between Chinese Pangolins and Free-Ranging Domestic Dogs
The numbers of spatial loci obtained for the four domestic dogs in the WQZ area were 10,390, 3048, 1459, and 2783, respectively, totaling 17,680. The numbers of spatial loci obtained for the domestic dogs in the PZ were 184, 8272, 5006, and 5168, respectively, totaling 18,630.
The ranges of all eight domestic dogs were estimated using a minimum convex polygon (MCP) method with a confidence level of 100%. Specifically, for the four domestic dogs in the WQZ area, the ranges were calculated as follows: 28 km2 (GAF-003), 21 km2 (GAF-004), 27 km2 (GAF-005), and 3 km2 (GAF-006). On the other hand, the ranges for the four domestic dogs in the PZ were determined as follows: 1 km2 (GAF-007), 9 km2 (GAF-009), 12 km2 (GAF-010), and 23 km2 (GAF-012).
We calculated the overlap between the potentially suitable habitats of Chinese pangolins and free-ranging domestic dogs to determine the conflict area (Figure 2). The results showed that the total conflict area in WQZ was 21 km2, accounting for 48% of the potentially suitable habitat of Chinese pangolins; the high-conflict area was 4 km2, accounting for 41% of the high-suitability habitat; the medium-conflict area was 7 km2, accounting for 45% of the medium-suitability habitat; and the low-conflict area was 10 km2, accounting for 50% of the low-suitability habitat (Figure 3A).
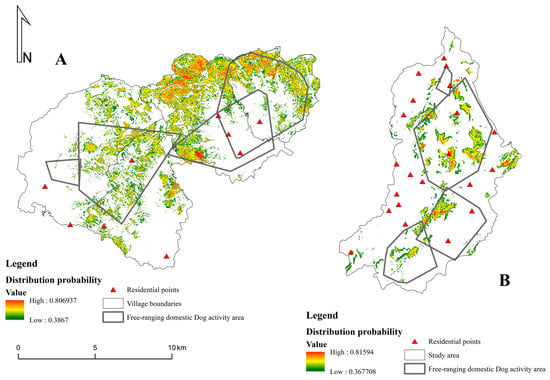
Figure 2.
The distribution probability of Chinese pangolins and the ranges of free-ranging dog home areas (minimum convex polygon), Wuqinzhang forest (A), and Pengzhai forest (B).
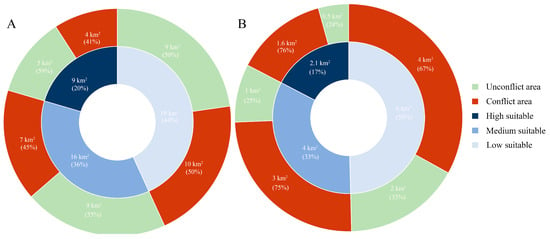
Figure 3.
Conflicting regional distributions of potentially suitable habitats for Chinese pangolins and domestic dog habitats: Wuqinzhang forest area (A), and Pengzhai forest area (B).
The total conflict area in the PZ was 8.6 km2, accounting for 71% of the potentially suitable habitat for Chinese pangolin; the high-conflict area was 1.6 km2, accounting for 76% of the high-suitability habitat; the medium-conflict area was 3 km2, accounting for 75% of the medium-suitability habitat; and the low-conflict area was 4 km2, accounting for 67% of the low-suitability habitat (Figure 3B).
3.3. Temporal Niche Differentiation between Chinese Pangolins and Free-Ranging Domestic Dogs
During monitoring, the camera trap captured a total of 889 Chinese pangolin image videos, and a total of 660 valid detections were recorded. There were significant differences in the activity rhythms of Chinese pangolins and free-ranging domestic dogs. The activity peak of the Chinese pangolins occurred at night, while that of the domestic dogs occurred at dawn and dusk. Specifically, the Chinese pangolins preferred to be active at night, with peaks of activity at 22:00–02:00 and a higher intensity of activity during the day, whereas the domestic dogs preferred to be active at dawn and dusk, with activity peaks at 7:00–10:00 and 18:00–19:00, respectively (Figure 4).
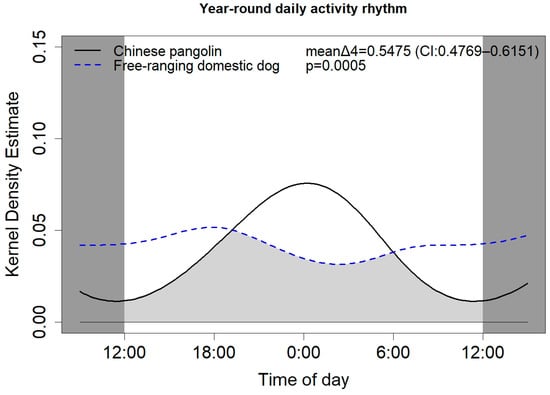
Figure 4.
Overlap of the daily activity rhythm of Chinese pangolins and free-ranging domestic dogs.
We further compared the activity rhythms of free-ranging dogs and Chinese pangolins in different seasons (Figure 5). The results showed that there were significant differences in activity rhythms in all four seasons (spring: p = 0.0014, mean Δ4 = 0.5128, CI: 0.4439–0.5635; summer: p = 0.0004, mean Δ4 = 0.4299, CI: 0.3518–0.4809; autumn: p = 0.0002, mean Δ4 = 0.5066, CI: 0.4055–0.5908; winter: p = 0.0002, mean Δ4 = 0.5410, CI: 0.4575–0.6324). We also compared the activity of Chinese pangolins and free-ranging domestic dogs during the mating and non-mating seasons (Figure 6), and there were significant differences (mating season: p = 0.0001, mean Δ4 = 0.4999, CI: 0.4421–0.5404; non-mating season: p = 0.0019, mean Δ4 = 0.4977, CI: 0.4421–0.5404).
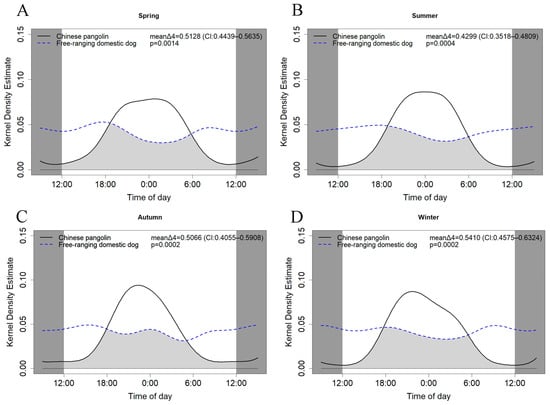
Figure 5.
The daily activity patterns of Chinese pangolins and free-ranging domestic dogs in spring (A), summer (B), autumn (C), and winter (D). Overlaps are represented by the shaded gray area.
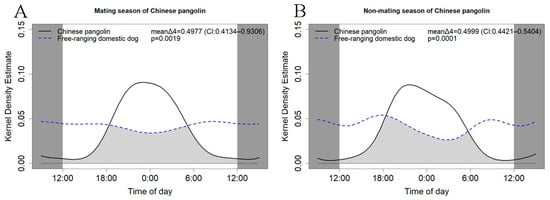
Figure 6.
The daily activity patterns of Chinese pangolin and free-ranging domestic dogs in the mating (A) and non-mating seasons (B) of Chinese pangolin. Overlaps are represented by the shaded gray area.
3.4. Attraction–Avoidance Ratio between Chinese Pangolins and Domestic Dogs
We calculated the attraction–avoidance ratio between Chinese pangolins and domestic dogs from camera trap data. The results indicate that T1 = 6.77, T2 = 13.2, T3 = 11.88, T4 = 14.6, AAR1 = 22.0 > 1, and AAR2 = 1.2 > 1. Both AAR1 > 1 and AAR2 > 1 indicate that free-ranging domestic dogs are attracted by Chinese pangolins and that Chinese pangolins significantly avoid free-ranging domestic dogs.
3.5. Distance to Human Settlement in Different Seasons
We calculated the distance of Chinese pangolins to human settlements in the mating and non-mating seasons. The results showed that the distances traveled during the mating and non-mating seasons were significantly different (p = 0.0014), and Chinese pangolins preferred to stay away from human settlements during the mating season (Figure 7).
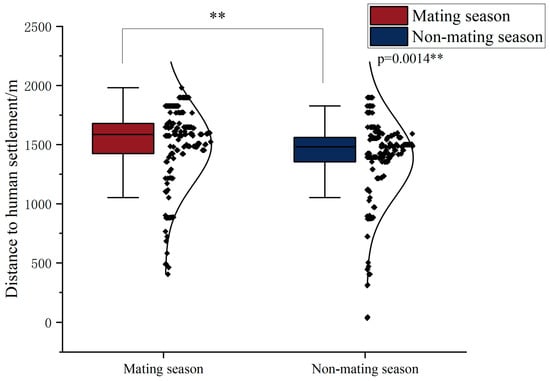
Figure 7.
The distance of Chinese pangolin between human settlements in mating and non-mating seasons. Note: “**” p < 0.01.
4. Discussion
Our research revealed how domestic dogs influence Chinese pangolins in spatiotemporal niches outside of nature reserves. We found that the potential conflict area between Chinese pangolins and free-ranging domestic dogs is severe. The distance of Chinese pangolins to human settlements is greater in the mating season, and Chinese pangolins significantly avoid free-ranging domestic dogs, implying that free-ranging domestic dogs are serious threats to Chinese pangolins outside of protected areas. The daily rhythm of domestic dogs is distinct from that of Chinese pangolins, but the two species have a high overlap in spatial niche, which can threaten the survival of Chinese pangolins outside protected areas.
4.1. Characteristics of Chinese Pangolin Habitats
Habitat plays a crucial role in the survival and perpetuation of wild animals. A suitable habitat provides the necessary material conditions, shelters, and mating grounds for wild animals to thrive [50]. The suitability of a habitat is an important determinant of a species’ normal survival and reproduction, and it is a key factor in assessing the sustainable survival of species.
Our models revealed that elevation (WQZ: 16%; PZ: 40%) and forest type (WQZ: 22%; PZ: 21%) were common and highly contributive environmental factors. This may be because temperature [51], precipitation, vegetation, and prey richness [52,53] change as the elevation gradient increases [54,55,56]. Among these changes, the temperature change is the most significant, and many mountains at higher elevations may experience year-round snow on their summits. The Chinese pangolin has a scaly dorsal covering and poorly insulated armor, with no thick fur to keep warm [15]. The Chinese pangolin is susceptible to cold stress when temperatures are low, so it is widely distributed in warmer areas [57]. Therefore, the Chinese pangolin prefers medium- and low-elevation habitats. Vegetation type, which changes with altitude, is also an important factor influencing the distribution of Chinese pangolins [56]. The forest type may be related to the ants preferred by the Chinese pangolin. The Chinese pangolin prefers relatively pure broad-leaved forests, which, to some extent, reveals the pangolin’s specialization and preference in terms of diet and habitat selection.
Unlike other studies, the contributions of distance from human settlements in this study were only 6.3% (WQZ) and 1.1% (PZ), with less disturbance from human settlements [15]. This may be attributed to the exploitation of agriculture, industry, and the high density of the human population. Consequently, there has been a significant decrease in the habitat area of the Chinese pangolin, leaving fewer patches in which the Chinese pangolin can seek refuge, forcing it to adapt its survival strategy to coexist with human beings [50].
4.2. Spatial Conflicts between Chinese Pangolins and Free-Ranging Domestic Dogs
Currently, many primary predators (tigers, leopards, etc.) have experienced population declines or even extinction [58,59]. As a result, domestic dogs have emerged as top predators. Domestic dogs can affect wild animals through disturbances, viruses, and other factors [1,24,26,49]. Consequently, wild animals’ habitats need to be avoided by domestic dogs.
The ranges of domestic dogs expand with human migration. According to the model’s results, the conflict range is higher in the PZ area (71%) than in the WQZ area (48%). This difference may be attributed to the higher population density in the PZ area compared to the WQZ area, leading to a greater activity range of dogs. Additionally, environmental protection organizations play a role in reconciling conflicts between humans and wildlife. The presence of an environmental protection organization in the WQZ area contributes to a smaller conflict area compared to PZ area.
In contrast, conflict between Chinese pangolins and dogs was found to be significant within all three suitable habitats, with the highest percentage occurring in highly suitable habitats, at 76% within the PZ area. This can be explained by agricultural production dominating the PZ area and free-ranging domestic dogs serving as guard animals for properties located far from towns—overlap with Chinese pangolins’ highly suitable habitats.
Furthermore, our analysis revealed that, during the mating season, Chinese pangolins tend to expand their activity ranges and avoid human settlements for mating opportunities. This indicates that Chinese pangolins choose to stay away from human settlements more frequently during the mating season than the non-mating season as a protective measure against potential disturbance and to increase reproductive success [60,61].
We also calculated whether the site usage of Chinese pangolins changed after the appearance of domestic dogs, with AAR1 and AAR2 both exceeding 1. Those findings indicated that Chinese pangolins were attractive to free-range domestic dogs and that areas where domestic dogs have appeared should be avoided by Chinese pangolins. Both Chinese pangolins and domestic dogs possess keen senses of smell and engage in odor-marking behaviors [15]. While domestic dogs use urine for marking, Chinese pangolins rely on scent-marking glands. Given their well-developed sense of smell and extensive training for scent tracking, domestic dogs can be used to track Chinese pangolins. However, as cave dwellers with diminished visual, but heightened olfactory, senses, Chinese pangolins can detect the scent markers left by domestic dogs and actively avoid those areas where they have appeared.
4.3. Characteristics and Differences in Activity Rhythm between Chinese Pangolins and Free-Ranging Domestic Dogs
Mutual competition among sympatric species shows different activity patterns that are likely to favor the coexistence of the two species [25,62,63,64]. According to the daily activity rhythm (year-round and seasonal), we found that the temporal niche overlap between the two species was low and significantly differentiated through the daily activity rhythms. Domestic dogs had less influence on Chinese pangolins in the terms of temporal niche. The activity peak of the Chinese pangolin is mainly at night; it is a strictly nocturnal animal. Although camera traps have captured Chinese pangolin activity during the daylight, the frequency are extremely rare, and so we assume that this is an emergency measure due to external disturbances. On the other hand, free-ranging domestic dogs are typically diurnal animals, as they have fixed shelters and adequate food supplies, so their peak activity occurs at during dawn and dusk. However, from the GPS collar data, they still showed significant nocturnal activity. We speculate that this is due to the fact that free-ranging domestic dogs in China are mainly used to guard the house, the vast majority of them sleep outdoors in unfenced areas, and their sleep is less efficient.
In terms of seasonal and daily activity rhythms, the overlap between the two species was lowest in summer and highest in winter. This may be due to the fact that our study area was located in Guangdong Province, China, where summer temperatures are high and food resources are abundant. As a result, Chinese pangolins can minimize foraging time and delay their activity peak (2:00–4:00) to avoid nocturnal predators. However, in winter, when temperatures decrease, food becomes scarce, and Chinese pangolins have no fur to combat the cold. Consequently, their activity peak occurs in the warmer first half of the night (18:00–20:00), and they extend their foraging time to obtain sufficient food. On the other hand, free-ranging domestic dogs show little seasonal variation in their activity patterns due to the availability of regular food and shelter. Therefore, the overlap between the two species is highest in winter, when the activity time of Chinese pangolin is advanced.
Due to the earlier activity peak of Chinese pangolins in winter, there is greater overlap between the two species. The temporal niche does not significantly differ between the mating and non-mating seasons. The activity peak during the non-mating season of the Chinese pangolin is earlier than that during the mating season. This could be attributed to the overall colder temperatures during the non-mating season, prompting the Chinese pangolins to be active earlier in order to avoid lower temperatures.
5. Conclusions
By analyzing the spatiotemporal niches of the Chinese pangolin and free-ranging domestic dogs outside nature reserves, we found that free-ranging domestic dogs are great threats to the Chinese pangolin. Although they was less overlap in the temporal niche, there was high overlap in the spatial niche. While most of the activities of free-ranging dogs occur at dawn and dusk, there is also activity at night [65], which may increase the risk of encounters between Chinese pangolins and domestic dogs, increasing the possibility of attack, predation, and disease transmission from free-ranging dogs. Due to the fact that the habitat coverage of Chinese pangolins by Guangdong’ s nature reserves is low, the Chinese pangolin’s habitat is not protected adequately in protected areas [66]. Therefore, we call for an expeditious survey of the wild population of Chinese pangolin to be carried out as soon as possible, especially outside nature reserves, to expand and optimize established nature reserves [66]. At the same time, we need a complete survey on the population of Chinese pangolin and domestic dogs inside and nearby nature reserves, and we request that dogs be banned in reserves and ten kilometers beyond [47]. We should provide regular vaccinations and medical check-ups for dogs, and further limit the range of free-ranging domestic dogs by leashing them at night [47]. Meanwhile, community outreach activities should be stepped up to raise the public’s awareness of conservation efforts and scientific dog breeding.
Author Contributions
Conceptualization, Y.Z., H.G., H.D. and Y.H.; methodology, Y.Z., H.G. and H.D.; software, Y.Z.; validation, H.G. and H.D.; formal analysis, Y.Z. and H.G.; investigation, J.Y. and J.W.; resources, H.G.; data curation, Y.Z. and H.G.; writing—original draft preparation, Y.Z.; writing—review and editing, H.G., H.D., Z.X. and Y.H.; visualization, Y.Z.; supervision, Z.X. and Y.H.; project administration, Z.X. and Y.H.; funding acquisition, Z.X. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Forestry Science and Technology Innovation Project of Guangdong Province (2023KJCX023), Science and Technology Project of Guangzhou, Grant/Award Number: 2023A04J0836.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Guangdong Academy of Forestry (00201056–3/2/2020). With the support of the dog owners.
Data Availability Statement
The data supporting the results of this manuscript are available through direct (and reasonable) request to the corresponding authors.
Acknowledgments
We extend our heartfelt gratitude to dog owners who participate in our research. We greatly appreciate editors and the two anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hughes, J.; Macdonald, D.W. A review of the interactions between free-roaming domestic dogs and wildlife. Biol. Conserv. 2013, 157, 341–351. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Leitão, I.; Santos-Reis, M.; Revilla, E. Human-related factors regulate the spatial ecology of domestic cats in sensitive areas for conservation. PLoS ONE 2011, 6, e25970. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.S.; Dickman, C.R.; Glen, A.S.; Newsome, T.M.; Nimmo, D.G.; Ritchie, E.G.; Vanak, A.T.; Wirsing, A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Conserv. 2017, 210, 56–59. [Google Scholar] [CrossRef]
- Driscoll, C.A.; Macdonald, D.W. Top dogs: Wolf domestication and wealth. J. Biol. 2010, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vilà, C.; Savolainen, P.; Maldonado, J.E.; Amorim, I.R.; Rice, J.E.; Honeycutt, R.L.; Crandall, K.A.; Lundeberg, J.; Wayne, R.K. Multiple and ancient origins of the domestic dog. Science 1997, 276, 1687–1689. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Ríos, G.; Branch, L.C. Altered activity patterns and reduced abundance of native mammals in sites with feral dogs in the high Andes. Biol. Conserv. 2016, 193, 9–16. [Google Scholar] [CrossRef]
- Jin, Y.P.; Zhang, X.K.; Ma, Y.S.; Qiao, Y.C.; Liu, X.B.; Zhao, K.H.; Zhang, C.L.; Lin, D.G.; Fu, X.L.; Xu, X.R.; et al. Canine distemper viral infection threatens the giant panda population in China. Oncotarget 2017, 8, 113910–113919. [Google Scholar] [CrossRef]
- Zhang, L.N.; Wang, K.; An, F.Y.; Zhang, D.L.; Zhang, H.L.; Xu, X.L.; Guo, C.; Yan, H.; Kuang, Y.; Zhang, Z.; et al. Fatal canine parvovirus type 2a and 2c infections in wild Chinese pangolins (Manis pentadactyla) in southern China. Transbound. Emerg. Dis. 2022, 69, 4002–4008. [Google Scholar]
- Wang, S.L.; Tu, Y.C.; Lee, M.S.; Wu, L.H.; Chen, T.Y.; Wu, C.H.; Tsao, E.H.; Chin, S.C.; Li, W.T. Fatal canine parvovirus-2 (CPV-2) infection in a rescued free-ranging Taiwanese pangolin (Manis pentadactyla pentadactyla). Transbound. Emerg. Dis. 2020, 67, 1074–1081. [Google Scholar] [CrossRef]
- Atickem, A.; Bekele, A.; Williams, S.D. Competition between domestic dogs and Ethiopian wolf (Canis simensis) in the Bale Mountains National Park, Ethiopia. Afr. J. Ecol. 2010, 48, 401–407. [Google Scholar] [CrossRef]
- Home, C.; Bhatnagar, Y.V.; Vanak, A.T. Canine Conundrum: Domestic dogs as an invasive species and their impacts on wildlife in India. Anim. Conserv. 2018, 21, 275–282. [Google Scholar] [CrossRef]
- Marshall, H.E.; Gore, M.L.; Ngoprasert, D.; Savini, T. Free-ranging dogs and their owners: Evaluating demographics, husbandry practices and local attitudes towards canine management and dog–wildlife conflict. Integr. Conserv. 2024, 2, 255–270. [Google Scholar] [CrossRef]
- Loss, S.R.; Will, T.; Marra, P.P. The impact of free-ranging domestic cats on wildlife of the United States. Nat. Commun. 2013, 4, 1396. [Google Scholar] [CrossRef]
- Marshall, H.E.; Niti, S.; Dusit, N.; Tommaso, S. The spatial and temporal displacement of native species by domestic dogs. Glob. Ecol. Conserv. 2023, 44, e02504. [Google Scholar] [CrossRef]
- Wu, S.B.; Sun, N.C.M.; Zhang, F.H.; Yu, Y.S.; Ades, G.; Suwal, T.L.; Jiang, Z.G. Chinese pangolin Manis pentadactyla (Linnaeus, 1758). In Pangolins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 49–70. [Google Scholar]
- Wu, S.B.; Liu, N.F.; Ma, G.Z.; Tang, M.; Chen, H.; Xu, Z.R. A Current Situation of Ecology Study on Pangolins. Chin. J. Zool. 2004, 39, 46–52. [Google Scholar]
- Wu, S.B.; Liu, N.F.; Ma, G.Z.; Xu, Z.R.; Chen, H. Habitat selection by Chinese pangolin (Manis pentadactyla) in winter in Dawuling Natural Reserve. Mammalia 2003, 67, 493–502. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Zhao, H.; Zhang, Z.X.; Wang, Z.H.; Wang, H. Allometry of scales in Chinese pangolins (Manis pentadactyla) and Malayan pangolins (Manis javanica) and application in judicial expertise. Zool. Res. 2012, 33, 271–275. [Google Scholar] [CrossRef]
- Challender, D. Asian Pangolins: How behavioural research can contribute to their conservation. In Proceedings of the Workshop on Trade and Conservation of Pangolins Native to South and Southeast Asia, Singapore, 1 July 2008; p. 95. [Google Scholar]
- Challender, D.W.S.; Harrop, S.R.; MacMillan, D.C. Understanding markets to conserve trade-threatened species in CITES. Biol. Conserv. 2015, 187, 249–259. [Google Scholar] [CrossRef]
- Wu, S.B.; MA, G.Z.; Tang, M.; Chen, H.; Liu, N.F. The status and conservation strategy of pangolin resource in China. J. Od Nat. Resour. 2002, 17, 174–180. [Google Scholar]
- Tian, F.; Li, J.; Liu, W.; Liu, Y.; Hu, Y.; Tu, Q.; Li, Y.; Bai, Y.; Shi, M.; Que, T.; et al. Virome in healthy pangolins reveals compatibility with multiple potentially zoonotic viruses. Zool. Res. 2022, 43, 977–988. [Google Scholar] [CrossRef]
- Challender, D.W.; Nash, H.; Waterman, C. Pangolins: Science, Society and Conservation; Academic Press: Cambridge, MA, USA, 2019; p. 71. [Google Scholar]
- Butler, J.; Du Toit, J. Diet of free-ranging domestic dogs (Canis familiaris) in rural Zimbabwe: Implications for wild scavengers on the periphery of wildlife reserves. Anim. Conserv. Forum 2002, 5, 29–37. [Google Scholar] [CrossRef]
- Butler, J.; Du Toit, J.; Bingham, J. Free-ranging domestic dogs (Canis familiaris) as predators and prey in rural Zimbabwe: Threats of competition and disease to large wild carnivores. Biol. Conserv. 2004, 115, 369–378. [Google Scholar] [CrossRef]
- Gompper, M.E. Free-Ranging Dogs and Wildlife Conservation; Oxford University Press: New York, NY, USA, 2014; pp. 73–86. [Google Scholar]
- Ikeya, K. Hunting with dogs among the San in the central Kalahari. Afr. Study Monogr. 1994, 15, 119–134. [Google Scholar]
- Zhang, F.H.; Wang, W.H.; Mahmood, A.; Wu, S.B.; Li, J.Q.; Xu, N. Observations of Chinese pangolins (Manis pentadactyla) in mainland China. Glob. Ecol. Conserv. 2021, 26, e01460. [Google Scholar] [CrossRef]
- Sun, N.C.M.; Arora, B.; Lin, J.S.; Lin, W.C.; Chi, M.J.; Chen, C.C.; Pei, C.J.C. Mortality and morbidity in wild Taiwanese pangolin (Manis pentadactyla pentadactyla). PLoS ONE 2019, 14, e0198230. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Irshad, N.; Hussain, R.; Akrim, F.; Hussain, I.; Anwar, M.; Rais, M.; Nadeem, M.S. Breeding habits of the Indian pangolin (Manis crassicaudata) in Potohar Plateau, Pakistan. Mammalia 2016, 80, 231–234. [Google Scholar] [CrossRef]
- Hassan, M.; Sulaiman, M.H.; Lian, C.J. The prevalence and intensity of Amblyomma javanense infestation on Malayan Pangolins (Manis javanica Desmarest) from Peninsular Malaysia. Acta Trop. 2013, 126, 142–145. [Google Scholar] [CrossRef]
- Khatri-Chhetri, R.; Wang, H.C.; Chen, C.C.; Shih, H.C.; Liao, H.C.; Sun, C.M.; Khatri-Chhetri, N.; Wu, H.Y.; Pei, K.J.C. Surveillance of ticks and associated pathogens in free-ranging Formosan pangolins (Manis pentadactyla pentadactyla). Ticks Tick-Borne Dis. 2016, 7, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wang, M.; Nie, Y.; Swaisgood, R.R.; Wei, W.; Zhou, W.; Zhang, Z.; Wang, G.; Wei, F. Stable seasonal migration patterns in giant pandas. Zool. Res. 2023, 44, 341–348. [Google Scholar] [CrossRef]
- Sun, N.C.M.; Pei, K.J.C.; Lin, J.-S. Attaching tracking devices to pangolins: A comprehensive case study of Chinese pangolin Manis pentadactyla from southeastern Taiwan. Glob. Ecol. Conserv. 2019, 20, e00700. [Google Scholar] [CrossRef]
- Griss, S.; Riemer, S.; Warembourg, C.; Sousa, F.M.; Wera, E.; Berger-Gonzalez, M.; Alvarez, D.; Bulu, P.M.; Hernández, A.L.; Roquel, P. If they could choose: How would dogs spend their days? Activity patterns in four populations of domestic dogs. Appl. Anim. Behav. Sci. 2021, 243, 105449. [Google Scholar] [CrossRef]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Using Maxent modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecol. Inform. 2019, 50, 68–75. [Google Scholar] [CrossRef]
- Abolmaali, S.M.-R.; Tarkesh, M.; Bashari, H. Maxent modeling for predicting suitable habitats and identifying the effects of climate change on a threatened species, Daphne mucronata, in central Iran. Ecol. Inform. 2018, 43, 116–123. [Google Scholar] [CrossRef]
- Seaman, D.E.; Millspaugh, J.J.; Kernohan, B.J.; Brundige, G.C.; Raedeke, K.J.; Gitzen, R.A. Effects of sample size on kernel home range estimates. J. Wildl. Manag. 1999, 739–747. [Google Scholar] [CrossRef]
- Long, J.A.; Nelson, T.A. Time geography and wildlife home range delineation. J. Wildl. Manag. 2012, 76, 407–413. [Google Scholar] [CrossRef]
- Tenorio, F.M.B.; Fernandez, D.A.P.; de Luna, M.C.T.; de Guia, A.P.O.; Balatibat, J.B.; Baril, J.A.; Aurellado, M.E.B. Cat’s Paw: Tracking the Home Range of Domestic Cats in Mount Makiling Forest Reserve. Philipp. J. Sci. 2024, 153, 353–361. [Google Scholar]
- Chen, Y.X.; Yu, Y.; Li, C.; Xiao, Z.S.; Zhou, G.W.; Zhang, Z.J.; Wang, X.W.; Xiang, Z.F.; Chang, J.; Li, M. Population and conservation status of a transboundary group of black snub-nosed monkeys (Rhinopithecus strykeri) between China and Myanmar. Zool. Res. 2022, 43, 523–527. [Google Scholar] [PubMed]
- Sun, N.C.M.; Pei, K.J.C.; Wu, L.Y. Long term monitoring of the reproductive behavior of wild Chinese pangolin (Manis pentadactyla). Sci. Rep. 2021, 11, 18116. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xiao, Z.S.; Zhang, L.; Wang, X.W.; Li, M.; Xiang, Z.F. Activity rhythms of coexisting red serow and Chinese serow at Mt. Gaoligong as identified by camera traps. Animals 2019, 9, 1071. [Google Scholar] [CrossRef]
- You, Z.Y.; Lu, B.G.; Du, B.B.; Liu, W.; Jiang, Y.; Ruan, G.F.; Yang, N. Spatio-Temporal Niche of Sympatric Tufted Deer (Elaphodus cephalophus) and Sambar (Rusa unicolor) Based on Camera Traps in the Gongga Mountain National Nature Reserve, China. Animals 2022, 12, 2694. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Q.; Liu, Y.L.; He, C.W.; Li, T.T.; Li, Q.L.; Ma, C.X.; Wang, D.J.; Li, S. Determining the daily activity pattern of Chinese mountain cat (Felis bieti): A comparative study based on camera-trapping and satellite collar tracking data. Biodivers. Sci. 2022, 30, 22081. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Parsons, A.W.; Bland, C.; Forrester, T.; Baker-Whatton, M.C.; Schuttler, S.G.; McShea, W.J.; Costello, R.; Kays, R. The ecological impact of humans and dogs on wildlife in protected areas in eastern North America. Biol. Conserv. 2016, 203, 75–88. [Google Scholar] [CrossRef]
- Weng, Y.; McShea, W.; Diao, Y.X.; Yang, H.B.; Zhang, X.F.; Gu, B.J.; Bu, H.L.; Wang, F. The incursion of free-ranging dogs into protected areas: A spatio-temporal analysis in a network of giant panda reserves. Biol. Conserv. 2022, 265, 109423. [Google Scholar] [CrossRef]
- Tiller, L.N.; Humle, T.; Amin, R.; Deere, N.J.; Lago, B.O.; Leader-Williams, N.; Sinoni, F.K.; Sitati, N.; Walpole, M.; Smith, R.J. Changing seasonal, temporal and spatial crop-raiding trends over 15 years in a human-elephant conflict hotspot. Biol. Conserv. 2021, 254, 108941. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, J.H.; Oh, K.H.; Lee, B.Y. Vertical distribution of vascular plants in Jungsanri, Mt. Jiri by temperature gradient. Korean J. Environ. Ecol. 2010, 24, 680–707. [Google Scholar]
- Wang, J.J.; Soininen, J.; Zhang, Y.; Wang, B.X.; Yang, X.; Shen, J. Patterns of elevational beta diversity in micro-and macroorganisms. Glob. Ecol. Biogeogr. 2012, 21, 743–750. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 2009, 18, 346–360. [Google Scholar] [CrossRef]
- Underwood, A. Structure of a rocky intertidal community in New South Wales: Patterns of vertical distribution and seasonal changes. J. Exp. Mar. Biol. Ecol. 1981, 51, 57–85. [Google Scholar] [CrossRef]
- Houghton, D.; Stubbings, H. On the vertical distribution of Elminius modestus Darwin. J. Anim. Ecol. 1963, 32, 193–201. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Gao, H.Y.; Dou, H.L.; Wei, S.C.; Sun, S.; Zhang, Y.L.; Hua, Y. Local chronicles reveal the effect of anthropogenic and climatic impacts on local extinctions of Chinese pangolins (Manis pentadactyla) in mainland China. Ecol. Evol. 2022, 12, e9388. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Fallows, C.; Meÿer, M.; Seakamela, S.M.; Orndorff, S.; Kirkman, S.; Kotze, D.; Creel, S. Loss of an apex predator in the wild induces physiological and behavioural changes in prey. Biol. Lett. 2022, 18, 20210476. [Google Scholar] [CrossRef] [PubMed]
- Ordiz, A.; Bischof, R.; Swenson, J.E. Saving large carnivores, but losing the apex predator? Biol. Conserv. 2013, 168, 128–133. [Google Scholar] [CrossRef]
- Kjellander, P.; Hewison, A.J.M.; Liberg, O.; Angibault, J.M.; Bideau, E.; Cargnelutti, B. Experimental evidence for density-dependence of home-range size in roe deer (Capreolus capreolus L.): A comparison of two long-term studies. Oecologia 2004, 139, 478–485. [Google Scholar] [CrossRef]
- Madsen, T. Movements, home range size and habitat use of radio-tracked grass snakes (Natrix natrix) in southern Sweden. Copeia 1984, 3, 707–713. [Google Scholar] [CrossRef]
- Wang, X.; Jia-Li Zhang, J.; Pan, H.; Chen, Y.; Mao, S.; Qi, J.; Shen, Y.; Zhang, M.; Xiang, Z.; Li, M. Unique characteristics of gut microbiota in black snub-nosed monkeys (Rhinopithecus strykeri) reveal an enzymatic mechanism of adaptation to dietary vegetation. Zool. Res. 2023, 44, 357–360. [Google Scholar] [CrossRef]
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.; Collen, B.; Cox, N.; Master, L.L.; O’Connor, S.; et al. A standard lexicon for biodiversity conservation: Unified classifications of threats and actions. Conserv. Biol. 2008, 22, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Pablo-Rodríguez, N.; Aurioles-Gamboa, D.; Montero-Muñoz, J.L. Niche overlap and habitat use at distinct temporal scales among the California sea lions (Zalophus californianus) and Guadalupe fur seals (Arctocephalus philippii townsendi). Mar. Mammal Sci. 2016, 32, 466–489. [Google Scholar] [CrossRef]
- Molloy, S.; Burleigh, A.; Dürr, S.; Ward, M.P. Roaming behaviour of dogs in four remote Aboriginal communities in the Northern Territory, Australia: Preliminary investigations. Aust. Vet. J. 2017, 95, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y.; Dou, H.L.; Wang, K.; Zhang, Y.Q.; Hua, Y. Ensemble SDMs reveal the effect of environmental suitability and nature reserves on conserving Chinese pangolins in Guangdong, China. J. Nat. Conserv. 2024, 79, 126617. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).