Abstract
Anthropogenic disturbance of natural environments has negative impacts on biodiversity. Amphibians are especially sensitive to deforestation, and there is a high rate of this phenomenon in the Democratic Republic of the Congo. We studied the effects of this modification of forest ecosystems on the diversity of amphibians in the Yoko Forest Reserve. During twenty-four field sampling campaigns organized over a period of twelve months, amphibians were collected from nocturnal surveys supported by three techniques: visual spotting using a headlamp, systematic searching of habitats and acoustic hearing of vocalizations. Twelve sampling sites were chosen, and three habitats were explored at each site. Of these three habitats, one is the undisturbed habitat (primary forest), and two are disturbed habitats (fallow and field). Amphibian abundance, species richness, diversity, evenness and density were compared between these two types of habitat. A total of 4443 amphibians in 10 families, 17 genera and 33 species were recorded throughout the study area. Abundance, species richness and relative density were significantly higher in non-disturbed habitats than in disturbed habitats. On the other hand, primary forest is less diverse than fallow, but not significantly. It is, however, significantly more diverse than the field. The undisturbed habitat is also characterized by lower evenness than the disturbed habitats, with which it shares low similarities. The species Amnirana albolabris, Phrynobatrachus auritus, Leptopelis notatus, Leptopelis millsoni, Xenopus pygmaeus, Hyperolius platyceps, Leptopelis calcaratus, Leptopelis christyi, Leptopelis ocellatus, Arthroleptis tuberosus, Ptychadena perreti, Amietia nutti, Arthroleptis variabilis, Cardioglossa leucomystax, Phrynobatrachus perpalmatus and Chiromantis rufescens were recognized, in order of importance, as primary forest indicators according to the results of this study. All these differences between the two habitat statuses (undisturbed and disturbed) confirm the negative effects of natural habitat alteration on forest amphibians.
1. Introduction
Fragmented landscapes have an impact on amphibians [1], affecting not only species numbers and distribution but also connectivity between populations [2,3,4,5]. The local matrix could be hostile to forest amphibians and limits their dispersal to isolated fragments. This disadvantage is linked to the species’ ecology, which thus makes them vulnerable to natural habitat disturbance [6,7].
Amphibians are key components in ecosystem processes [8,9,10]. Yet habitat modification and loss are the main causes of their extinction [11,12,13,14,15], which is occurring at an accelerating rate in several regions of the world [13,16]. The disturbance of forest habitats currently observed in Central Africa [17] is accompanied by a considerable impact on landscape structure [18]. This could also be the case for forest areas in the Democratic Republic of the Congo (DRC), particularly those in and around Kisangani [19], which are currently threatened by slash-and-burn agriculture and timber exploitation [20,21,22,23,24]. To better understand species–landscape relationships, it is essential to increase data collection across various faunal groups. It is in this context that the present study was carried out on amphibians in the Yoko Forest Reserve (YFRE), one of the forest blocks still more or less intact in the vicinity of Kisangani, despite the anthropic pressure to which the area’s forest ecosystems are subject. Apart from the preliminary survey carried out at four stations in the DRC [25], studies of the anthropogenic effects on amphibians are still in their infancy in and around Kisangani. Thus, the present analysis draws on studies carried out on rodents in the Masako Forest Reserve, located northeast of the city of Kisangani [26,27,28] to understand the effects of natural habitat modification on the fauna in the Kisangani region. It compares amphibian diversity in three habitats: field, fallow and primary forest, the latter serving as a control habitat for understanding the anthropogenic effects on amphibians in the YFRE.
This study verifies the following hypotheses: (i) Given that habitat disturbance affects amphibians in various regions of the world [7,29,30,31,32,33], the anthropogenic activities in the YFRE could have an impact on the distribution of abundances and species richness between the habitats created. (ii) Considering the available results on amphibian diversity in the context of anthropogenic effects [10,25,34,35,36,37,38], abundance, species richness, diversity, evenness and relative density could be significantly higher in the primary forest from its stable ecological conditions than in the other two habitats, and higher in the fallow than in the field. (iii) Based on their supposedly different faunas, primary forest, fallow and field would show low similarities to each other. (iv) As documented at four sites in the DRC [25], of all the families represented in the YFRE, the Hyperoliidae family may contain the highest number of amphibians in the primary forest, while the Ptychadenidae and Dicroglossidae families are expected to provide higher abundances in field and fallow, respectively, based on the habitat conditions and ecological requirements of the species. (v) As anthropogenic effects in the YFRE remain localized, and as demonstrated by previous studies [36,39,40], it is possible that all the species present in the study area are of forest affinity and that none are indicative of disturbed environments despite the affinities that might be found there.
This study aimed to examine the response of amphibians to habitat conditions while assessing their roles as indicators of natural habitat disturbance in the YFRE. Specifically, this research tries to (i) understand the influence of habitat conditions on the distribution of amphibian abundance and species richness, (ii) compare these habitats from their faunas, (iii) determine the rate of similarity between these habitats, (iv) identify the most abundant family in each habitat type and (v) determine which species can be considered as indicators of levels of disturbance in forest habitats to define conservation strategies.
2. Materials and Methods
The YFRE is located near the equator, between 0°15′ and 0°20′ N and 25°14′ and 25°20′ E [41], on the road connecting Kisangani with Ubundu, in the Tshopo Province. It is characterized by an average annual temperature of 25 °C, an average annual rainfall of 1750 mm and by the absence of dry months [42]. Throughout the Kisangani region, rainfall throughout the year is interrupted by two sub-dry seasons. The long rainy season runs from September to December, and the short rainy season covers the months from March to June. On the other hand, the long sub-dry season extends through January and February, and the short sub-dry season covers July and August [43,44]. The hydrographic network is dominated by rivers, the most important of which are the Yoko and Biaro streams. They receive water from numerous small streams before discharging into the Congo River. The Yoko stream, which gave the studied reserve its name, flows from west to east, dividing the study zone into two blocks, north and south. Within each block, six sites were identified (Figure 1).
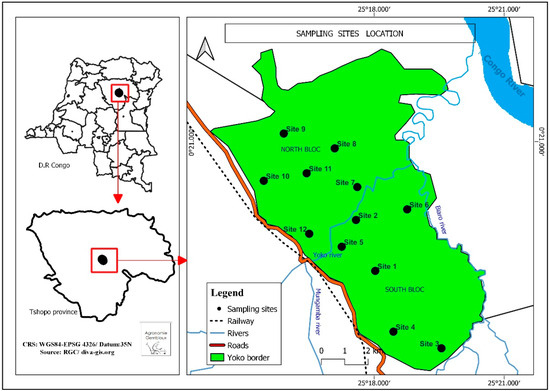
Figure 1.
Location map of the sampling sites in the two blocks of the YFRE, Tshopo Province, DRC (data sources: RGC: https://www.rgc.cd, accessed on 14 October 2023).
According to their ecological characteristics relative to the degree of habitat disturbance as described in the literature [25,29,36,39,45], the exploited habitats in each site were categorized into undisturbed and disturbed. Thus, primary forest corresponds to the undisturbed stage, while field and fallow correspond to the disturbed stages, the former being more disturbed than the latter. Sampling was carried out in these three habitats, which characterize each of the twelve sites. However, the analyses did not focus on habitat categories (undisturbed and disturbed), but rather on the habitats themselves (primary forest, fallow and field).
Primary forest is dominated by Trilepisium madagascariense Dc. (Moraceae), Uapaca guineensis Mull. Arg., Ricinodendron heudelotii (Baillon) Pierre ex Heckel (Euphorbiaceae), Vitex congolensis De Wild. and Th. Dur. (Lamiaceae), Milicia excelsa (Welw.) C.C. Berg., Scorodophloeus zenkeri Harms, Piptadeniastrum africanum (Hooker f.) Brenan (Fabaceae), Chrysophyllum pruniforme Pierre ex Engler (Sapotaceae), Cola griseiflora De Wild., C. acuminata (P. Beauv.) Schott et Endl., Sterculia tragacantha Lindley (Malvaceae), Anonidium mannii (Oliver) Engler and Diels (Annonaceae), Strombosia pustulata Oliver (Strombosiaceae), Panda oleosa Pierre, Microdesmis yafungana J. Léonard (Pandaceae), Turraeanthus africanus (Welw.) Pellegr., Carapa procera DC, Trichilia rubescens Oliver, T. gilgiana Harms (Meliaceae), Heisteria parviflora Smith. (Olacaceae), Aidia micrantha (K. Schum.) F. White (Rubiaceae), Mostuea batesii Baker (Loganniaceae), Rinorea sp, R. oblongifolia (C.H. Wright) Marquand ex Chipp (Violaceae), Campylospermum elongatum (Oliver) Tieghem (Ochnaceae), Afrostyrax lepidophyllum Mildbr (Uaceae), Megaphrynium macrostachyum (Bentham) Milne-Redh, Marantochloa mannii (Bentham) Milne-Redh (Maranthaceae). Fallow is dominated by Manniophyton fulvum Mull. Arg., Uapaca guinneensis Mull. Arg., Tetrorchidium didymostemon (Baillon) Pax et Hoffm., Macaranga monandra Mull. Arg. (Euphorbiaceae), Oncoba welwitschii Oliver, Barteria nigritiana Hooker f. (Flacourtiaceae), Zanthoxylum gilletii (De Wild.) P.G Waterman (Rutaceae), Triumfetta cordifolia Guill., Perr. and A. Rich (Malvaceae), Afromomum sangineum K. Schum. (Zingiberaceae), Pycnanthus angolensis (Welw.) Exell (Myristicaceae), Canarium schweinfurthii Engler (Burseraceae), Terminalia superba Engler and Diels (Combretaceae), Albizia gummifera (J.f. Gmelin) C.A. SM (Fabaceae), Pauridiantha callicarpoides (Hiern) Bremek. (Rubiaceae), Harungana madagascariensis Lam. ex Poiret (Hypericaceae), Ficus exasperata Vahl., F. mucuso Welw. ex Ficalho and Trilepisium madagascariense DC. (Moraceae). The species Oriza sativa L., Zea mays L. (Poaceae), Capsicum annuum L., Solanum melongena L. (Solananceae), Musa sp (Musaceae), Manihot esculenta Crantz (Euphorbiaceae) and Elaeis guineensis Jacq (Arecaceae) are among the most dominant in the field. Orphaned primary forest species such as Julbernardia seretii (De Wild.) Troupin, Pterocarpus soyauxii Taub. (Fabaceae), Pseudospondias microcarpa (A. Rich.) Engler (Anacardiaceae), Ricinodendron heudelotii (Baillon) Pierre ex Heckel (Euphorbiaceae), Schotia biquaertii (De Wild), Petersianthus macrophylla (P. Beauv.) Liben (Lecythidaceae), Khaya anthotheca Dc. (Meliaceae), Nesogordonia kabingaensis (K. Schum.) Capuron (Malvaceae), Margaritaria discoidea (Baillon) and Webster (Phyllanthaceae) are also recorded in the field.
This study covered 4443 amphibians, all belonging to the Anura order and divided into 10 families, 17 genera and 33 species (Table 1).

Table 1.
Amphibian species recorded between December 2020 and November 2021 in three habitats in the YFRE (Democratic Republic of the Congo). N = abundance.
Amphibians were collected according to the method and techniques previously described [46], which deals with the diversity and endemism of amphibians in the YFRE. Two teams, each comprising four researchers, were hired to collect data simultaneously over a period of twelve months, i.e., one hundred and forty-four capture nights. The two teams worked simultaneously in the two blocks, visiting six sites in each block, for six days per mission for each team. Two missions were organized per month, so that each habitat in each site was sampled twice each month by one team during the twelve months of data collection. Thus, each site, through each of its habitats, was visited twenty-four times by four researchers, for a total of twenty-four effective capture nights per site. For the twelve sites, this would be equivalent to two hundred and eighty-eight effective capture nights. As both teams worked simultaneously, this reduced the number of trap nights to one hundred and forty-four for the whole study area, giving a sampling effort of 1152 person-nights.
Amphibian abundances are the numbers of individuals caught per habitat, family or species [47], while species richness corresponds to the number of species present in the habitats exploited [47,48]. Based on the data collected, we expect abundance and species richness to vary between sites across the habitats that characterize them. We also expect the values of these indices to be higher in primary forest than in the other two habitats, and also higher in the fallow than in the field. The Shannon–Wiener diversity index (Equation (1)) was used to assess amphibian diversity in each habitat. This ecological measure quantifies and compares diversity expressed in biologically interpretable units translating the effective number of species [48,49,50].
where H′ is the Shannon index and pi is the proportion of individuals of species i ( equals the number of individuals of species i and N is the total number of individuals collected). Given that ecological conditions are stable in primary forest, we expect this habitat to be characterized by higher diversity than fallow and field. So, to assess how individuals are distributed between species within habitats, we used Pielou’s evenness [51], the value of which is obtained by Relationship (2).
where corresponds to Pielou’s evenness, translates the Shannon–Wiener index or real diversity and Hmax is the maximum diversity. Considering the conditions of variation of the results of this index, we expect to obtain a value close to 1 in the primary forest, in contrast to the values close to 0 expected in the fallow and in the field. Using Relationship (3) developed in [52], we calculated relative densities as the number of individuals per unit area to see whether or not amphibian numbers per hectare are the same in all the habitats characterizing our study area.
where is the relative density (the number of individuals per hectare), is the number of individuals captured in the habitat, is the number of collectors and t is the number of effective capture nights.
Using data from the three habitats with , and elements, respectively, their respective averages , and were calculated between the twelve capture sites for each habitat, the sites being taken as replicates. However, the data are not matched, as only new individuals were considered during capture and marked before release. Thus, the Kruskal–Wallis test was applied to examine whether there were significant differences between habitats about the calculated indices. This test was used because, apart from being independent, the samples did not follow the normal distribution. In fact, before performing the Kruskal–Wallis test, the Shapiro test was applied to check the normality of the data derived from the calculated parameters. All p-values were below 0.01. Whenever the Kruskal–Wallis test indicated significant differences, Dunn’s test was applied to determine which habitats showed significant differences [53]. To examine whether or not amphibian faunas in exploited habitats are similar, we used two indices. The first is Sørensen’s index (4), which is based on presence–absence [50], and the second is the so-called Bray–Curtis dissimilarity (5), which takes into account species abundances, placing much greater emphasis on double presences [50,54].
where is Sørensen’s similarity index, is the number of species common to the habitats compared, and and correspond to the number of species present in only one of the two habitats.
where is the Bray–Curtis dissimilarity (called percentage difference), and W is the sum of the minimum abundances of the different species (this minimum is defined as the abundance in the habitat where the species is rare). and are the sums of the abundances of all species in each of the two habitats considered. The choice of these two indices stems from the need to capture the impact of abundant species when comparing habitats. Since binary data are used to minimize this impact, the Bray–Curtis dissimilarity was used without any data transformation.
Of all the families present in the study area, we expect the Hyperoliidae to score highly in primary forest, compared with the Ptychadenidae and Dicroglossidae, which are expected to be very abundant in the field and fallow, respectively. To do this, we used bar plots showing the occurrences of individuals from each family in each habitat. Despite the degree of habitat disturbance, we expect the species present in the YFRE to be all forest species. Thus, indicator value indices (6) were used to determine this association of species with habitats. These indices are components that can be interpreted as probabilities [55,56] and are potentially unique for each species [57]. The indicator value index for each species in each habitat group is the product of the two components noted and [55,57]. The component is the ratio between the average number of individuals of species in habitat group and the sum of the average numbers of individuals of species in all habitats. This conditional probability is called the specificity or positive predictive value of the species as an indicator of the habitat group. The component corresponds to the frequency of occurrence of species in habitat group . This second conditional probability is called the species’ fidelity or sensitivity as an indicator of the habitat group exploited [50]:
where is the indicator value for species in habitat group , is the measure of specificity and is the measure of fidelity. Thus:
For , is the average number of individuals of species in habitat group , while is the sum of the average numbers of individuals of species in all habitats. is maximal when species is present only in habitat group . For , is the number of sampling sites in habitat where species is present, while is the total number of sampling sites in habitat . is maximal when species is present in all sampling sites of habitat . The indicator value is multiplied by 100 and expressed as a percentage. To complete the analysis of species–habitat association rates, network analysis was carried out [58]. This analysis enables us to understand the various interactions within the YFRE. Field data were processed in Excel 2016. All analyses were performed using R.4.3.1 software (through RStudio 2023.12.1 + 402) [59]. Abundance, species richness, the Shannon–Wiener diversity index and Pielou’s evenness per habitat were calculated using the “diversityresult” function in the “BiodiversityR 2.15-3” package [60]. Amphibian density in habitats was estimated using Equation (3). The means and standard deviations presented in Table 2 were calculated using the “aggregate” function in “Stats”. Boxplots showing variation in abundance and specific richness by habitat, barplots illustrating the occurrence of individuals from different families by habitat and barplots illustrating the relative frequency of each species in each habitat were produced using the “ggplot” function in the “ggplot2 3.4.3” package [61]. The relative frequencies of species by habitat were displayed using the “ggstats 0.7.0” package [62]. For boxplots, the two graphs were put together using the “ggarrance” function from the “ggpubr 0.6.0” package [63]. Dissimilarities between habitats (Sørensen and Bray–Curtis) were calculated using the “vegdist” function in the “Vegan 2.6.4” package [64], a function that always provides dissimilarity [65]. Normality tests were performed using the “Shapiro.test” function, while the Kruskal–Wallis test was performed using the “kruskal.test” function, both functions coming from the “Stats” package, a default package in R. Dunn’s test, meanwhile, was applied using the “dunn_test” function from the “rstatix 0.7.2” package [66]. Indices of indicator values were calculated using the “indval” function in the “labdsv 2.1-0” package [67], retaining only those species with an adjusted p-value less than or equal to 0.05. Bonferoni’s correction method was used to adjust p-values. Network analysis was performed using the “bipartite 2.18” package [68].

Table 2.
Comparison of abundance (), species richness (), Shannon–Wiener diversity (), Pielou’s evenness () and relative density (individuals/ha: ) between habitats. The averages given are those of the parameters calculated for all twelve sites for each habitat. Superscript letters , and symbolize Dunn’s test results. Values bearing the same letters are not statistically different, while differences were obtained between values bearing different letters.
3. Results
Based on the ecological conditions caused by agriculture in the study area, amphibian abundance and specific richness vary between sites across the habitats that characterize them (field, fallow and primary forest). Figure 2 shows these variations, confirming the first hypothesis of this study according to which the anthropogenic activities in the YFRE could have an impact on the distribution of abundances and species richness between habitats.
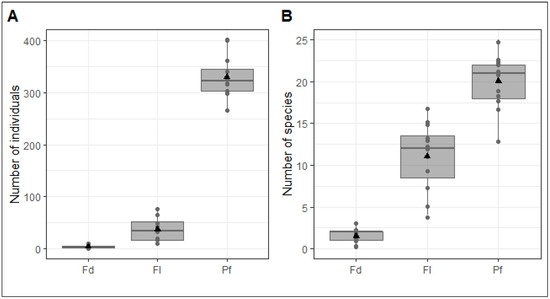
Figure 2.
Distribution of amphibian abundance (A) and species richness (B) at twelve sites, each characterized by three habitats: field (Fd), fallow (Fl) and primary forest (Pf) in the YFRE. The abscissa represents the habitat, the ordinate number of individuals and the number of species, respectively. The points represent the values obtained per site, the triangles the mean values per habitat, and the vertical lines the standard deviations.
A total of 3958 (89.09%), 442 (9.94%) and 43 (0.97%) amphibians were collected in primary forest, fallow and field, respectively (Table 2), for a sampling effort of 1152 person-nights. Species richness was higher in primary forest (S = 32) than in fallow (S = 27) and field (S = 6). The same applies to density. The high diversity found in the fallow does not differ significantly from that obtained in the primary forest. Abundance, species richness and density were significantly higher in the primary forest than in the two disturbed habitats (fallow and field). On the other hand, its diversity was lower than that of the fallow, although significantly higher than that of the field. Primary forest has the lowest evenness of all habitats, and this is significant. These results partially confirm the second hypothesis of this study, according to which abundance, species richness, diversity, evenness and relative density could be significantly higher in the primary forest from its stable ecological conditions than in the other two habitats and higher in the fallow than in the field.
From the point of view of presence–absence occurrences, the primary forest shares only 26% similarity with the field, compared with 88% with the fallow (Table 3). Based on the abundance of amphibians in the habitats (Table 4), this similarity is 18% between primary forest and fallow, giving a dissimilarity of 82%. On the other hand, the similarity rate between primary forest and field is 1%, i.e., 99% dissimilarity. The fall in similarities between primary forest and fallow and between primary forest and field from the Sørensen index to the Bray–Curtis index shows the impact of the high abundance of individuals in the undisturbed habitat (primary forest) compared to the disturbed habitats (fallow and field). This confirms the third hypothesis of this study in terms of the specific composition between primary forest and fallow but invalidates the same hypothesis in terms of amphibian abundances, according to which primary forest, fallow and field would show low similarities to each other.

Table 3.
Habitat similarities (based on Sørensen’s index, ). Primary forest shares a high degree of similarity with fallow. On the other hand, the similarity is low between primary forest and field.

Table 4.
Habitat similarities (based on Bray–Curtis dissimilarity, ). Primary forest shares low similarities with the other two habitats (fallow and field).
The most abundance of amphibians collected in primary forests belongs to the Ranidae family, followed by Arthroleptidae, Phrynobatrachidae, Ptychadenidae and Hyperoliidae (Figure 3). Small numbers were provided by each of the other five families (Rhacophoridae, Pipidae, Bufonidae, Pyxicephalidae and Dicroglossidae). All these families except Pipidae and Pyxicephalidae are represented in the fallow but with almost the same abundance. These results invalidate the fourth hypothesis of this study, according to which, of all the families represented in the YFRE, the Hyperoliidae family may contain the highest number of amphibians in the primary forest, while the Ptychadenidae and Dicroglossidae families are expected to provide higher abundances in field and fallow, respectively. Ptychadenidae, Arthroleptidae and Bufonidae families are present in all habitats; Ranidae, Dicroglossidae, Phrynobatrachidae, Hyperoliidae and Rhacophoridae families live in primary forest and fallow. The Pipidae and Pyxicephalidae families are found only in the primary forest.
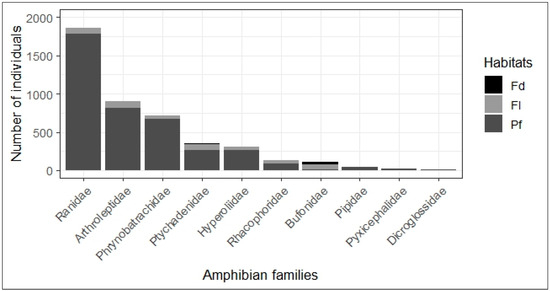
Figure 3.
Amphibian abundance by family in logged habitats. The Ranidae, Arthroleptidae, Phrynobatrachidae, Ptychadenidae and Hyperoliidae families are, in descending order, most abundant in primary forest (Pf). On the other hand, Bufonidae family is most abundant in field (Fd) and fallow (Fl).
Based on the average numbers, frequencies and probabilities of species confirmed by the p-value were adjusted with the Bonferoni correction by multiplying by the number of tests rather than adjusting the significance level, typically 0.05 (Appendix A), Amnirana albolabris, Phrynobatrachus auritus, Leptopelis notatus, Leptopelis millsoni, Xenopus pygmaeus, Hyperolius platyceps, Leptopelis calcaratus, Leptopelis christyi, Leptopelis ocellatus, Arthroleptis tuberosus, Ptychadena perreti, Amietia nutti, Arthroleptis variabilis, Cardioglossa leucomystax, Phrynobatrachus perpalmatus and Chiromantis rufescens are, in order of importance, indicative of primary forest. No species is indicative of fallow or field. This means that the species exploiting these two habitats are forest species that adapt to their disturbance conditions. This trend is confirmed by the results of the network analyses (Figure 4a,b) and helps to confirm the fifth hypothesis of this study, according to which all the species present in the YFRE area are of forest affinity, and none are indicative of disturbed habitats despite the affinities that might be found. Two species, however, show particular behavior relating to an attachment to disturbed habitats. These are Sclerophrys gutturalis and S. pusilla, the former not being present in the undisturbed habitat (primary forest) and the latter being more abundant in the disturbed habitats (fallow and field) than in the undisturbed habitat (primary forest). However, the indicator values obtained in the fallow (Sclerophrys gutturalis: 22.2% with p > 0.05; S. pusilla: 46.1% with p > 0.05) and in the field (Sclerophrys gutturalis: 5.5% with p > 0.05; S. pusilla: 24.0% with p > 0.05) do not qualify these species as indicators of disturbed habitats.
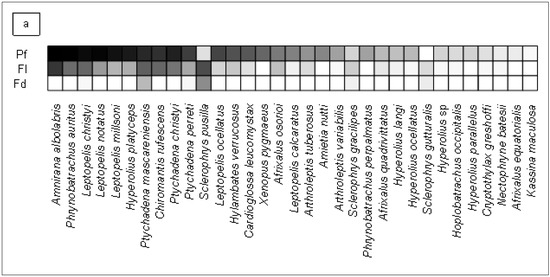
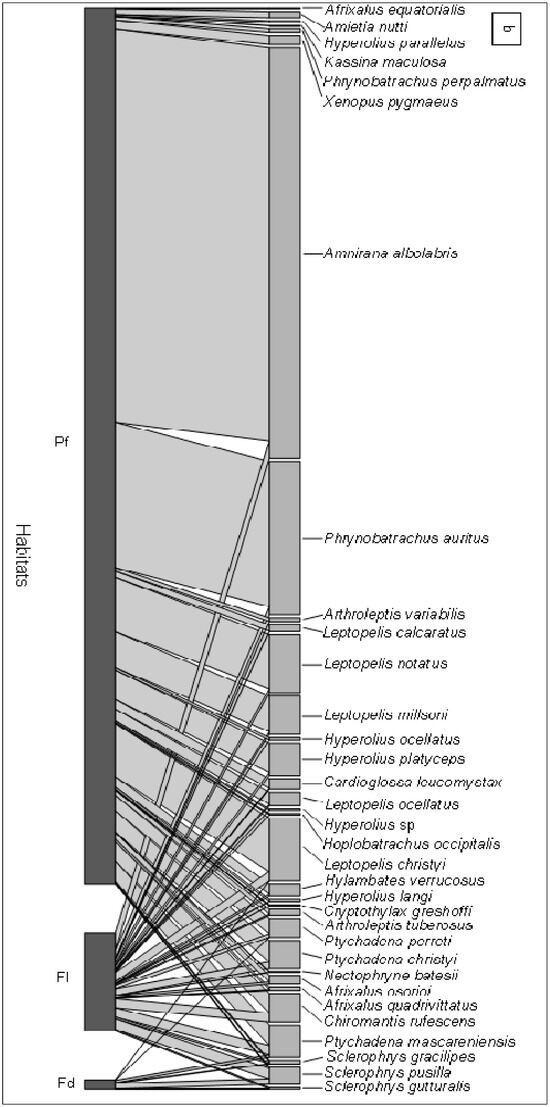
Figure 4.
The network matrix (a) and its bipartite graph (b). The shading of the matrix entries indicates the number of observations. The sequence of species is shown in both network visualizations (line crossings).
4. Discussion
The presence of amphibians in exploited habitats can be seen as the result of the rate of habitat disturbance [31,69,70], which gives each habitat a particular status [25,71]. This modification affects the ecological and microclimatic factors of habitats, as well as the physiological conditions of amphibians. It generates adaptations in the latter, the degree of expression of which is specific to each species [34,36,72,73]. This study showed that the undisturbed habitat (primary forest) is richer in amphibians than the disturbed habitats (fallow and field). This result is confirmed by other studies carried out on amphibians [8,25,36,38]. The high abundance and species richness of amphibians in primary forests are thought to be linked to the high humidity, diversity of ecological niches, availability of food and canopy, all of which have positive effects on individual lifestyles [72,74]. Indeed, the diet of amphibians consists mainly of arthropods [10,75,76], of which coleoptera and diptera are very abundant in the litter of forest environments [77,78]. The primary forest within the YFRE combines all these conditions, making it favorable for amphibians.
Studies carried out have shown that, despite the colonization of disturbed habitats by amphibians, the species richness remains low in these habitats [6,13,25,34,39,69,70]. This low species richness of amphibians in disturbed habitats can be explained by the low biomass [79,80], responsible for the scarcity of prey for many species [81]. Added to this is the high degree of disturbance that characterizes these disturbed habitats [82]. The high diversity of amphibians in the fallow is thought to be the result of the habitat’s high resilience to human activities [83,84,85] or its connectivity with the primary forest. As with rodents, the high diversity that characterizes fallow could be linked to the abundance of food resources that are specific to each of the species present [86]. These factors may be at the root of a better distribution of individuals between species within this habitat. In the context of this study, the high value of the diversity index in the fallow could also be the result of a better partition of species in this habitat than in the primary forest. The high diversity observed in the fallow contrasts with that observed in the field. Indeed, fallow is a vegetation state that tends towards the forest, unlike the field, which is a highly disturbed habitat. This creates the conditions necessary for forest species to survive in the fallow. Anthropogenic activities in the YFRE generally include slash-and-burn agriculture. The field is, therefore, a habitat where the original forest cover has been cut to make way for crops. On the other hand, fallow represents a habitat where agriculture has been stopped and forest dynamics have resumed through the establishment of woody forest species.
The low evenness in the primary forest could be the result of the high abundance in this habitat of the species Amnirana albolabris (n = 1778) and Phrynobatrachus auritus (n = 657), which together account for 61.5% of total species abundance in the primary forest, compared with 26.3% (n = 79 and 37) in the fallow and 0% in the field (n = 0 and 0). Figure 4 illustrates this situation.
One of the negative consequences of habitat disturbance is the modification of their conditions, which become favorable to predators because they favor the growth of their populations. For example, these habitats become very open, which increases the likelihood of amphibians being detected by their predators [30,87,88]. Habitat modification alters ecological processes, affects predator–prey relationships, and reduces amphibian density [30,89]. Beyond the consumption of arthropods and the cannibalism recognized in amphibians, the latter can also serve as prey for many other vertebrates [90]. As the abundance and density of amphibians are closely linked with those of their predators and prey, they maintain themselves in habitats through their trophic roles [10,91,92]. These trophic roles can influence the associations of amphibians with their habitats through the search for prey and shelter from predators.
The high similarity between primary forest and fallow (0.88), in terms of presence–absence occurrences, may be due to the connectivity between the two habitats, imposed by the shade of primary forest woody species. This facilitates amphibian mobility between the two habitats [34,64,74]. Using quantitative data, on the other hand, the Bray–Curtis index indicates a low similarity (0.18) between primary forest and fallow. This difference in results using presence–absence and quantitative data clearly indicates that the two habitats share most species but that the abundances of individuals per species between the two habitats are not at all close. In Masako Forest Reserve, a low similarity between these two habitats was found through rodents [27] using binary data. These differences in similarities between primary forest and fallow in Yoko and Masako Forest Reserves are thought to be a function of the ecological preferences of rodents, which differ profoundly from those of amphibians, and which may be associated with the indices used, but also with the ecological, microclimatic conditions of the habitats in the two Reserves.
According to the results of this study, the Ranidae family is most abundant in primary forests. These observations support previous findings [93] that reported the Ranidae species to be widely distributed in the forests and savannahs of eastern, central and western South Sahara in Africa. In contrast, the preliminary survey carried out previously [25] at four stations in the DRC showed that the Hyperoliidae family is more abundant in primary forests. In the YFRE, the Ranidae family is represented by a single species: Amnirana albolabris. Studies in other environments have shown that this species is largely confined to rainforest woodlands [94], where it inhabits cool, dark places near streams and springs [95]. As previously indicated [96], the high abundance of the species Amnirana albolabris in the primary forest within the YFRE may be justified by its distribution extending across sub-Saharan Africa and its ability to cross geographical barriers and forest refugia. The abundance of species in the Bufonidae family in disturbed habitats is justified by their tolerance of the conditions of these habitats [93,97], which confirms the observations made in the YFRE through three of the four species recorded.
Landscape quality is known to influence species distribution [4,5]. This makes it possible to estimate the importance of each habitat in the landscape [30,98] based on its vegetation, which conditions the presence of amphibians [13,70,99,100,101]. Examples include Leptopelis notatus, Leptopelis calcaratus, Leptopelis millsoni, Leptopelis christyi, Leptopelis ocellatus and Amnirana albolabris, which were frequently recorded on Megaphrynium macrostachyum, Marantochloa mannii, Palisota ambigua and Palisota schweinfurthii, some of the most dominant species of the herbaceous stratum in primary forest. The abundance of these plant species is positively correlated with that of the amphibians that use them [102].
Habitat conditions necessitate adaptations on amphibians [93,103,104] that allow us to distinguish between ubiquitous species, anthropophilous species and forest-dwelling species [105]. Silvicultural or primary forest specialist species are sensitive to the effects of forest change [32,106]. This may be the case for Afrixalus equatorialis ( = 3), Amietia nutti ( = 26), Hyperolius parallelus ( = 7), Kassina maculosa ( = 1), Phrynobatrachus perpalmatus ( = 16) and Xenopus pygmaeus ( = 41), which can be considered as primary forest specialists as they have only been found in this habitat. Only a few species are “anthropophilous” or affiliated with disturbed habitats [31]. These include Sclerophrys kisoloensis and Hoplobatrachus occipitalis [25]. On the other hand, previous studies [39] do not recognize any amphibian species attached to this type of habitat. Similarly, in the YFRE, H. occipitalis was more abundant in the undisturbed habitat (primary forest, = 6) than in the disturbed habitats (fallow and field, = 1 and 0, respectively). According to previous studies [85], generalist species can exploit both undisturbed and disturbed habitats. In addition to the six species found only in primary forest, 21 species were found in both primary forest and fallow, while the Sclerophrys gutturalis was found only in the field and in fallow. Leptopelis christyi, Ptychadena mascareniensis, P. perreti, Sclerophrys gracilipes and Sclerophrys pusilla were found in all habitats. Of the 26 species present concomitantly in primary forest and at least one other habitat, 25 are more abundant in primary forest, with the exception of Sclerophrys pusilla, which is more abundant in fallow and field than in primary forest (Figure 5). As mentioned above, the six species found only in primary forest are likely to be threatened by the loss or transformation of forest habitats. The 21 species found only in primary forest and fallow show adaptation to low levels of disturbance, while the five species found in all habitats show good adaptation to anthropogenic disturbance of forest habitats. The 21 species found in primary forest and fallow can be considered forest generalists. However, as shown in Figure 5, the abundance of species in primary forest indicates that these species are primarily forest dwellers. Only the three species of the Sclerophrys genus show a strong adaptation to the modification of forest habitats. These species could be affiliated with disturbed habitats, particularly Sclerophrys gutturalis, which was absent from the primary forest.
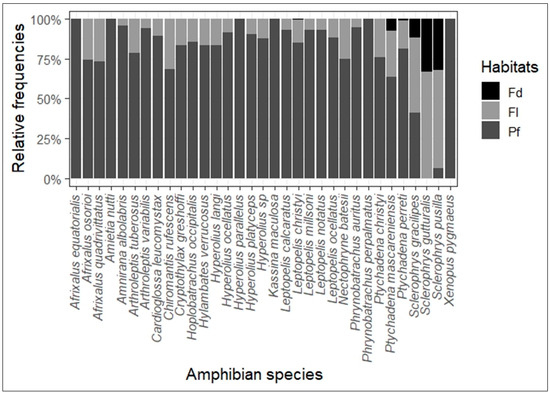
Figure 5.
Relative frequencies of species in habitats within the YFRE. Each barplot represents the proportion of individuals of each species in each habitat.
Based on amphibian adaptations to habitat conditions, some species have developed special diets [78,107]. Others are opportunistic predators [108,109,110] whose abundance in habitats depends on available prey [107,111]. This gives amphibians the power to adapt to ever-changing habitats [110,112]. This is the case for Sclerophrys pusilla and all other species of the Bufonidae family (with the exception of Nectophryne batesii in this study, Figure 5), whose abundance in disturbed habitats is thought to be due to the abundance and diversity of their prey within these habitats [110,113,114]. The same applies to Ptychadena mascareniensis, which is recognized as anthropophilic [105,115]. This is contrary to this study, which shows that this species is more abundant in primary forests than in disturbed habitats. Our observations of Chiromantis rufescens and Hylambates verrucosus in primary forest at YFRE support their previous classification as forest species [97]. Their presence in disturbed habitats could be an adaptation to maintain their populations in the face of current pressure on amphibians. The absence of Sclerophrys gutturalis in the primary forest deserves particular attention. As the species is not exotic in the study area, its local distribution and increase in numbers can be caused by deforestation and agriculture, reflecting changes in the diversity structure. Additional data are needed to ensure that this is not a sampling deficit.
Modification of natural habitats leads to changes in biodiversity at all levels [36]. These changes can be assessed based on the indicator amphibian species for each habitat, the number of which can be linked to the degree of disturbance associated with each species’ ecology and sampling effort. The loss of forest ecosystems hurts terrestrial and aquatic biotopes, altering vegetation and water quality, respectively [7,116,117]. The reduction in forested areas has negative effects on amphibians [118]. These effects can extend to adjacent habitats [119]. The adaptation of species to a wide variety of habitats may be a strategy that enables them to maintain their populations [31,120,121] thanks to the movement of individuals provided by corridors [122].
5. Conclusions
Anthropogenic disturbance of natural environments negatively affects amphibians through their abundance and species richness, which are significantly higher in primary forests than in fallow and fields. The same applies to their density, which decreases as one moves from undisturbed to disturbed habitats. On the other hand, the undisturbed habitat is less diverse than the fallow, although not significantly so, but significantly more diverse than the field. Significantly, of all the harvested habitats, the undisturbed habitat had the lowest evenness, which is a situation that may be mainly associated with the high abundance of the species Amnirana albolabris in this habitat. The differences in abundance, diversity, specific richness and density between primary forest, fallow and field confirm the clear effects of natural habitat modification on amphibians, which are also evident in the low similarities between the two habitat statuses. Based on amphibian adaptations to the ecological conditions of the habitats, the Ranidae family presents a good proportion of amphibians in the undisturbed habitat, where it is followed by Arthroleptidae, Phrynobatrachidae, Ptychadenidae and Hyperoliidae. Also, the presence of Amnirana albolabris, Phrynobatrachus auritus, Leptopelis notatus, Leptopelis millsoni, Xenopus pygmaeus, Hyperolius platyceps, Leptopelis calcaratus, Leptopelis christyi, Leptopelis ocellatus, Arthroleptis tuberosus, Ptychadena perreti, Amietia nutti, Arthroleptis variabilis, Cardioglossa leucomystax, Phrynobatrachus perpalmatus and Chiromantis rufescens) indicates undisturbed habitat. All species are present in the primary forest, with the exception of Sclerophrys gutturalis. However, six species (Afrixalus equatorialis, Amietia nutti, Hyperolius parallelus, Kassina maculosa, Phrynobatrachus perpalmatus and Xenopus pygmaeus) have only been found in primary forest; they have the temperament of primary forest specialists.
The present study has shown the effects of modification of natural habitats on amphibians through the differences observed between undisturbed and disturbed habitats in terms of their abundance, species richness, diversity, evenness and density. The sensitivity of fauna species to habitat modification and the interactions between native vegetation and species calls for the definition of management plans to identify appropriate conservation strategies for sensitive and vulnerable groups such as amphibians. These strategies are based on the landscape approach to biodiversity conservation within fragmented ecosystems. The ecological conditions of Kisangani’s forests are constantly changing, primarily due to shifting cultivation. Integrating this type of agriculture into environmental decision-making at local and national levels will ensure its control while guaranteeing the health of ecosystems, which is justified by the need to maintain biodiversity that is currently under threat. Additional analysis is required to compare this study and previous studies, which may be associated with the local characteristics of the study area. We recommend that the analysis of changes in amphibian diversity, for example, after deforestation, requires repeating the study as it becomes overgrown.
Author Contributions
Conceptualization, L.M., J.B. and L.I.; methodology, L.M., L.I., J.-C.M. and A.M.; validation, L.I. and J.B.; investigation, L.M. and A.M.; supervision, L.I. and J.-C.M.; data curation, L.M. and J.M.; formal analysis, L.M., J.M. and M.D.; writing-original draft preparation, L.M.; writing-review and editing, J.B., L.I., J.-C.M., M.D. and J.M.; visualization, L.M. and J.M.; project administration, J.B. and L.I.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Liège through the “Glocal Health” project. The research received no external funding.
Institutional Review Board Statement
This study was not subject to ethical review and approval as the field data collection methodology used the live capture of animals. Captured animals were marked and released. This allows the population to be re-sampled later without any bias using the same method, which avoids the destruction of the animals and facilitates the maintenance of ecological processes within the habitats.
Data Availability Statement
The data provided are confidential and available upon request from Loving Musubaho and Jan Bogaert.
Acknowledgments
From the Ph.D. grant awarded to Loving Musubaho by the University of Liège through the “Glocal Health” project, all activities related to this research have been financed in their entirety. The authors would like to thank the field teams for their dedication, motivation and loyalty throughout the period of data collection under nocturnal working conditions. They are also grateful to Professors John Katembo of the Institut Supérieur d’Etudes Agronomiques de Bengamisa/Democratic Republic of the Congo, Isaac Shabani of the University of Goma/Democratic Republic of the Congo and Chadrack Kafuti of the University of Gent/Belgium and to Assistant Thom’s Kavali of the University of Kisangani for statistical guidance. Special thanks to Christien Kimbuluma of the Laboratoire d’Ecologie et Aménagement Forestier (LECAFOR), University of Kisangani/Democratic Republic of the Congo for botanical descriptions of the habitats exploited. The managers of the Laboratoire d’Ecologie et Gestion des Ressources Animales (LEGERA) at the University of Kisangani are particularly thanked for the availability of their infrastructure throughout the data collection and processing period.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Species affinities with exploited habitats. Indicator values (IndVal) expressed as percentages and frequencies are confirmed by their adjusted p-values less than or equal to the 0.05 threshold. Aij = measure of specificity and Bij = measure of fidelity.
Table A1.
Species affinities with exploited habitats. Indicator values (IndVal) expressed as percentages and frequencies are confirmed by their adjusted p-values less than or equal to the 0.05 threshold. Aij = measure of specificity and Bij = measure of fidelity.
| Espèce | Habitat | Aij | Bij | IndValij | p-Value | Frequency |
|---|---|---|---|---|---|---|
| Amnirana albolabris | Primary forest | 0.9575 | 1.0000 | 95.7458 | 0.0001 | 23 |
| Phrynobatrachus auritus | Primary forest | 0.9467 | 1.0000 | 94.6686 | 0.0001 | 21 |
| Leptopelis notatus | Primary forest | 0.9316 | 1.0000 | 93.1559 | 0.0001 | 19 |
| Leptopelis millsoni | Primary forest | 0.9310 | 1.0000 | 93.1034 | 0.0001 | 19 |
| Xenopus pygmaeus | Primary forest | 1.0000 | 0.9167 | 91.6667 | 0.0001 | 11 |
| Hyperolius platyceps | Primary forest | 0.9048 | 1.0000 | 90.4762 | 0.0001 | 20 |
| Leptopelis calcaratus | Primary forest | 0.9333 | 0.9167 | 85.5556 | 0.0001 | 13 |
| Leptopelis christyi | Primary forest | 0.8516 | 1.0000 | 85.1590 | 0.0001 | 22 |
| Leptopelis ocellatus | Primary forest | 0.8833 | 0.8333 | 73.6111 | 0.0001 | 14 |
| Arthroleptis tuberosus | Primary forest | 0.7857 | 0.9167 | 72.0238 | 0.0001 | 17 |
| Ptychadena perreti | Primary forest | 0.8140 | 0.8333 | 67.8295 | 0.0007 | 17 |
| Amietia nutti | Primary forest | 1.0000 | 0.6667 | 66.6667 | 0.0002 | 08 |
| Arthroleptis variabilis | Primary forest | 0.9412 | 0.6667 | 62.7451 | 0.0003 | 09 |
| Cardioglossa leucomystax | Primary forest | 0.8958 | 0.6667 | 59.7222 | 0.0003 | 12 |
| Phrynobatrachus perpalmatus | Primary forest | 1.0000 | 0.5833 | 58.3333 | 0.0001 | 07 |
| Chiromantis rufescens | Primary forest | 0.6822 | 0.8333 | 56.8475 | 0.0019 | 16 |
References
- Almeida-Gomes, M.; Vieira, M.V.; Rocha, C.F.D.; Metzger, J.P.; De Coster, G. Patch size matters for amphibians in tropical fragmented landscapes. Biol. Conserv. 2016, 195, 89–96. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; González, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on earth’s ecosystems. Sci. Adv. 2015, 1, e500052. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Archis, J.N.; Akcali, C.; Stuart, B.L.; Kikuchi, D.; Chunco, A.J. Is the future already here? The impact of climate change on the distribution of the eastern coral snake (Micrurus fulvius). PeerJ 2018, 6, e4647. [Google Scholar] [CrossRef] [PubMed]
- Kusza, S.; Nagy, K.; Lanszki, J.; Heltai, M.; Szabo, C.; Czarnomska, S.D. Moderate genetic variability and no genetic structure within the European golden jackal (Canis aureus) population in Hungary. Mamm. Res. 2019, 64, 63–69. [Google Scholar] [CrossRef]
- Almeida-Gomes, M.; Rocha, C.F.D. Habitat loss reduces the diversity of frog reproductive modes in an Atlantic Forest fragmented landscape. Biotropica 2015, 47, 113–118. [Google Scholar] [CrossRef]
- Ribeiro, J.W.; Siqueira, T.; Brejāo, G.L.; Zipkin, E.F. Effects of agricultural and topography on tropical amphibian species and communities. Ecol. Appl. 2018, 28, 1554–1564. [Google Scholar] [CrossRef]
- Koirala, B.K.; Cheda, K.; Penjor, T. Species diversity and spatial distribution of amphibian fauna along the altitudinal gradients in Jigme Dorji Natonal Park, Western Bhutan. J. Threat. Taxa 2019, 11, 14249–14258. [Google Scholar] [CrossRef]
- Gidis, M.; Baskale, E. The herpetofauna of Honaz Mountain National Park (Denizli Province, Turkey) and threatening factors. Amphib. Reptile Conserv. 2020, 14, 147–155.e228. [Google Scholar]
- Vagmaker, N.; Pereira-Ribeiro, J.; Ferreguetti, Á.C.; Boazi, A.; Gama-Matos, R.; Bergallo, H.G.; Rocha, C.F.D. Structure of the leaf litter frog community in an area of Atlantic Forest in Southeastern Brazil. Zoologia 2020, 37, e38877. [Google Scholar] [CrossRef]
- Belasen, A.M.; Bletz, M.C.; Leite, D.S.; Toledo, L.F.; James, T.Y. Long-term habitat fragmentation is associated with reduced MHC IIB diversity and increase infections in amphibian hosts. Front. Ecol. Evol. 2019, 6, 236. [Google Scholar] [CrossRef]
- Betts, M.G.; Wolf, C.; Pfeifer, M.; Banks-Leite, C.; Arroyo-Rodriguez, V.; Ribeiro, D.B.; Barlow, J.; Eigenbrod, F.; Faria, D.; Fletcher, R.J.; et al. Extinction filters mediate the global effects of habitat fragmentation on animals. Science 2019, 366, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Boissinot, A.; Besnard, A.; Lourdais, O. Amphibian diversity in farmlands: Combined influences of breeding-site and landscape attributes in Western France. Agric. Ecosyst. Environ. 2019, 269, 51–61. [Google Scholar] [CrossRef]
- Anjos, A.G.; Costa, R.N.; Brito, D.; Solé, M. Is there an association between the ecological characteristics of anurans from the Brazilian Atlantic Forest and their extinction risk? Ethol. Ecol. Evol. 2020, 32, 336–350. [Google Scholar] [CrossRef]
- Tan, W.C.; Herrel, A.; Rodder, D. A global analysis of habitat fragmentation research in reptiles and amphibians: What have we done so far? Biodivers. Conserv. 2023, 32, 439–468. [Google Scholar] [CrossRef]
- Farasat, H.; Akmali, V.; Sharifi, M. Population genetic structure of the endangered Kaiser’s mountain newt, Neurergus kaiseri (Amphibia: Salamandridae). PLoS ONE 2016, 11, e0149596. [Google Scholar] [CrossRef]
- Brncic, T.M.; Wills, K.J.; Harris, D.J.; Washington, R. Culture or climate? The relative influences of past process on the composition of the lowland Congo rainforest. Proc. R. Soc. 2007, 362, 229–242. [Google Scholar]
- Bogaert, J.; Bamba, I.; Koffi, K.J.; Sibomana, S.; Djibu, K.J.P.; Champluvier, D.; Robbrecht, E.; De Cannière, C.; Visser, M.N. Fragmentation of forest Landscape in Central Africa: Causes, consequences and management. In Patterns and Processes in Forest Landscapes: Multiple and Unstainable Management; Laforteza, R., Chen, J., Sanesi, G., Crow, T.R., Eds.; Springer: New York, NY, USA, 2008; pp. 67–87. [Google Scholar]
- Global Forest Watch. 2024. Available online: https://globalforestwatch.org/dashboards/country/COD/25/7/ (accessed on 10 September 2024).
- Tyukavina, A.; Hansen, M.C.; Potapov, P.; Parker, D.; Okpa, C.; Stehman, S.V.; Kommareddy, I.; Turubanova, S. Congo Basin forest loss dominated by increasing smallholder clearing. Sci. Adv. 2018, 4, eaat2993. [Google Scholar] [CrossRef]
- Kyale, J.K.; Maindo, A.M.N.; Wardell, D.A. Réserve de Biosphère de Yangambi à l’épreuve de la cristallisation des pratiques locales de survie : Une réponse à la faillite de l’État en République Démocratique du Congo. VertigO 2019, 19, 1. [Google Scholar] [CrossRef]
- Kyale, J.K.; Wardell, D.A.; Mikwa, J.F.; Kabuanga, J.M.; Maindo, A.M.N.; Oszwald, J.; Doumenge, C. Dynamique de la déforestation dans la Réserve de biosphère de Yangambi (République démocratique du Congo): Variabilité spatiale et temporelle au cours des 30 dernières années. Bois Forêts Des Trop. 2019, 341, 15–28. [Google Scholar] [CrossRef]
- Masimo, K.J.; Adipalina, G.B.; Ngenda, O.E.; Maestripieri, N.; Saqalli, M.; Rossi, V.; Iyongo, W.M.L. Suivi de l’anthropisation du paysage dans la région forestière de Babagulu, République Démocratique du Congo. VertigO 2020, 20, 2. [Google Scholar] [CrossRef]
- Kipute, D.D.; Mate, J.-P.; Kankeu, R.S.; Ngouhouo-Poufoun, J.; Kahindo, J.-M.; Mampeta, S.; Lelo, U.; Sonwa, D.J.; Joiris, D.V.; Demaze, M.T. Effectiveness of the Yangambi Biosphere Reserve in reducing deforestation in the Democratic of the Congo. Hum. Ecol. 2023, 51, 75–87. [Google Scholar] [CrossRef]
- Musubaho, K.W.L.; Iyongo, W.M.; Ilonga, M.B.; Mapoli, M.J.; Mbumba, M.J.-L.; Neema, S.M.; Tungaluna, G.C.G.; Mukinzi, J.C.I.; Bogaert, J. Méta-analyse exploratoire des effets de perturbations anthropiques sur la diversité des Amphibiens dans les stations de Kasugho, Butembo, Mambasa et Kisangani en République Démocratique du Congo. Tropicultura 2021, 39, 1709. [Google Scholar] [CrossRef]
- Iyongo, W.M.; Visser, M.; Verheyen, E.; Leirs, H.; Iyongo, B.; Ulyel, A.; Bogaert, J. Etude préliminaire des effets de la fragmentation des forêts sur la similarité des habitats et leurs richesses en espèces de Rongeurs (Masako, RD Congo). Ann. ISEA 2009, 4, 177–186. [Google Scholar]
- Iyongo, W.M.L.; Visser, M.; De Cannière, C.; Verheyen, E.; Dudu, A.B.; Ulyel, A.-P.; Bogaert, J. Anthropisation et effets de lisière: Impacts sur la diversité des rongeurs dans la Réserve Forestière de Masako (Kisangani, RD Congo). Trop. Conserv. Sc. 2012, 5, 270–283. [Google Scholar]
- Meniko, T.H.J.P.; Iyongo, W.M.L.; Ulyel, A.-P.J.; Ewango, C.; Dudu, A.B.; Bogaert, J. Diversité des habitats et effets de lisière sur les populations de Rongeurs en zone de contact forêt-jachère à Masako. In Les Forêts de la Tshopo: Écologie, Histoire et Composition; Bogaert, J., Beeckman, H., De Cannière, C., Defourny, P., Ponette, Q., Eds.; Les Presses Universitaires de Liège-Agronomie-Gembloux: Gembloux, Belgium, 2020; pp. 47–56. [Google Scholar]
- Decaëns, T.; Martins, M.B.; Feijoo, A.; Oszwald, J.; Dolédec, S.; Mathieu, J.; de Sartre, X.A.; Bonilla, D.; Brown, G.G.; Criollo, Y.A.C.; et al. Biodiversity loss along a gradient of deforestation in Amazonian agricultural landscapes. Conserv. Biol. 2018, 32, 1380–1391. [Google Scholar] [CrossRef]
- Karraker, N.E.; Fischer, S.; Aowphol, A.; Sheridan, J.; Poo, S. Signals of forest degradation in the demography of common Asian amphibians. PeerJ 2018, 6, e4220. [Google Scholar] [CrossRef]
- Figueiredo, G.T.; Storti, L.F.; Lourenco-De-Moraes, R.; Shibatta, O.A.; Anjos, L. Influence of microhabitat on the richness of anuran species: A case study of different landscapes in the Atlantic Forest of southern Brazil. Ann. Braz. Acad. Sc. 2019, 91, e20171023. [Google Scholar] [CrossRef]
- Sykes, L.; Santini, L.; Etard, A.; Newbold, T. Effects of rarity form on species’ responses to land use. Conserv. Biol. 2020, 34, 688–696. [Google Scholar] [CrossRef]
- Bellotto-Trigo, F.C.; Uezu, A.; Hatfield, J.H.; Morante-Filho, J.C.; dos Anjos, L.; Develey, P.F.; Clegg, T.; Orme, C.D.L.; Banks-Leite, C. Intraspecific variation in sensitivity to habitat fragmentation is influenced by forest cover and distance to the range edge. Biol. Conserv. 2023, 284, 110167. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernandez-Salinas, U.; Martinez-Morales, M.A.; Ramirez-Bautista, A. Amphibian species richness and diversity in a modified tropical environment of central Mexico. Trop. Ecol. 2016, 57, 407–417. [Google Scholar]
- Badillo-Saldaña, L.M.; Ramírez-Bautista, A.; Wilson, L.R. Effects of establishment of grazing areas on diversity of amphibian communities in tropical evergreen forests and mountain cloud forests of the Sierra Madre Oriental. Rev. Mex. Biodivers. 2016, 87, 133–139. [Google Scholar] [CrossRef]
- Decena, S.C.P.; Avorque, C.A.; Decena, I.C.P.; Asis, P.D.; Pacle, B. Impact of habitat alteration on amphibian diversity and species composition in a lowland tropical rainforest in Northeastern Leyte, Philippines. Sci. Rep. 2020, 10, 10547. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, L.; Baccaro, F.B.; Ferreira, E.B.; Sampaio, M.F.; Santos, T.; Justino, R.C.; Angulo, A. The matrix effect: How agricultural matrices shape forest fragment structure and amphibian composition. J. Biogeogr. 2017, 44, 1911–1922. [Google Scholar] [CrossRef]
- Nneji, L.M.; Adeola, A.C.; Okeyonyin, A.; Oladipo, O.C.; Saidu, Y.; Samuel, D.; Usongo, J.Y.; Adedeji, B.E.; Omotoso, O.; Adeyi, A.O.; et al. Diversity and distribution of amphibians and reptiles in Gashaka Gumti National Park, Nigeria. Herpet. Notes 2019, 12, 543–559. [Google Scholar]
- Mindje, M.; Tumushimire, L.; Sinsch, U. Diversity assessment of anurans in the Mugesera wetland (eastern Rwanda): Impact of habitat disturbance and partial recovery. Salamandra 2020, 56, 27–38. [Google Scholar]
- Isingoma, J.; Sande, E.; Kityo, R.; Hughes, D.F. Amphibian communities along a forest degradation gradient in an East African forest reserve. Ecol. Inform. 2023, 75, 102021. [Google Scholar] [CrossRef]
- Boyemba, B.F. Ecologie de Pericopsis elata (Harms) Van Meeuwen (Fabaceae), Arbre de Forêt Tropicale Africaine à Répartition Agrégée. Ph.D. Thesis, Université Libre de Bruxelles, Bruxelles, Belgique, 2011. [Google Scholar]
- Picard, N.; Boyemba, F.; Rossi, V. Reducing the error in biomass estimates strongly depends on model selection. Ann. For. Sci. 2015, 72, 811–823. [Google Scholar] [CrossRef]
- Kahindo, M.J.-M. Potentiel en Produits Forestiers Autres que le bois D’œuvre dans les Formations Forestières de la Région de Kisangani. Cas des Rotins Eremospatha haullevilleana de Wild. et Laccosperma secundiflorum (P.Beauv.) Kuntze de la Réserve Forestière de Yoko (Province Orientale, RD Congo). Ph.D. Thesis, Université de Kisangani, Kisangani, Democratic Republic of the Congo, 2011. [Google Scholar]
- Sabongo, Y. Etude Comparative de la Structure et de la Diversité des Forêts à Gilbertiodendron dewevrei (De Wild.) J. Léonard des Régions de Kisangani et de l’Ituri (RDC). Ph.D. Thesis, Université de Kisangani, Kisangani, Democratic Republic of the Congo, 2015. [Google Scholar]
- Da Silva, F.R.; Rossa-Feres, D.C. Fragmentation gradients differentially affect the species range distributions of four taxonomic groups in semi-deciduous Atlantic forest. Biotropica 2017, 49, 283–292. [Google Scholar] [CrossRef]
- Musubaho, L.; Iyongo, L.; Mukinzi, J.-C.; Mukiranya, A.; Mutahinga, J.; Badjedjea, G.; Lango, L.; Bogaert, J. Diversity and endemism of amphibian fauna in the Yoko Forest Reserve, Democratic Republic of the Congo. Diversity 2024, 16, 457. [Google Scholar] [CrossRef]
- Triplet, P. Dictionnaire Encyclopédique de la Diversité Biologique et de la Conservation de la Nature, 8th ed.; 2022; pp. 1–1315, ISBN 978-2-9552171-6-0. Available online: https://laccreteil.fr/spip.php?article532 (accessed on 5 June 2024).
- Marcon, E. Mesures de la Biodiversité. 2022. Available online: https://github.com/EricMarcon/MesuresBioDiv2/ (accessed on 5 June 2024).
- Jost, L.; DeVries, P.; Walla, T.; Greeney, H.; Chao, A.; Ricotta, C. Partitioning diversity for conservation analyses. Divers. Distrib. 2010, 16, 65–76. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–990. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing Company: Oxford, UK, 2004; pp. 1–256. [Google Scholar]
- Nicolas, V.; Barriere, P.; Colyn, M. Impact of removal pitfall trapping on the community of shrews (Mammalia: Soricidae) in two African tropical forest sites. Mammalia 2003, 67, 133–138. [Google Scholar] [CrossRef]
- Dinno, A. Nonparametric pairwise multiple comparisons in independent groups using Dunn’s test. Stata J. 2015, 15, 292–300. [Google Scholar] [CrossRef]
- Somerfield, C.M. Identification of the Bray-Curtis similarity index: Comment on Yoshioka. Mar. Ecol. Prog. Ser. 2008, 372, 303–306. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Chown, S.L. Scaling up the value of bioindicators. Trend Ecol. Evol. 1998, 13, 46–47. [Google Scholar] [CrossRef]
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Dormann, C.; Frund, J.; Bluthgen, N.; Gruber, B. Indices, graphs and null models: Analysing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 24 January 2024).
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005; pp. 1–196. [Google Scholar]
- Wickham, H. _ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Larmarange, J. –ggstats: Extension to “ggplot2” for Plotting Stats_. R Package Version 0.7.0. 2024. Available online: https://CRAN.R-project.org/package=ggstats (accessed on 25 November 2024).
- Kassambara, A. _ggpubr: ‘ggplot2’ Based Publication Ready Plots_. R Package Version 0.6.6. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 21 July 2024).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 21 July 2024).
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R, 2nd ed.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 1–435. [Google Scholar]
- Kassambara, A. _rstatix: Pipe-Friendly Framework for Basic Statistical Tests_. R Package Version 0.7.2. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 21 July 2024).
- Roberts, D.W. _labdsv: Ordination and Multivariate Analysis for Ecology_. R Package Version 2.1-0. 2023. Available online: https://CRAN.R-project.org/package=labdsv (accessed on 22 July 2024).
- Dormann, C.F.; Fruend, J.; Gruber, B.; Beckett, S.; Devoto, M.; Felix, G.M.F.; Iriondo, J.M.; Opsahl, T.; Pinheiro, R.B.P.; Strauss, R.; et al. _bipartite : Visualising Bipartite Networks and Calculating Some (Ecological) Indices_. R Package Version 2.18, 2022. Available online: https://github.com/biometry/bipartite (accessed on 21 July 2024).
- Bickford, D.; Ng, T.H.; Qie, L.; Kudavidanage, E.P.; Bradshaw, C.J.A. Forest fragment and breeding habitat characteristics explain frog diversity and abundance in Singapore. Biotropica 2010, 42, 119–125. [Google Scholar] [CrossRef]
- Oda, F.H.; Batista, V.G.; Gambale, P.G.; Mise, F.T.; de Souza, F.; Bellay, S.; Ortega, J.C.G.; Takemoto, R.M. Anuran species richness, composition and breeding habitat preferences: A comparison between forest remnants and agricultural landscapes in Southern Brazil. Zool. Stud. 2016, 55, 34. [Google Scholar]
- Vignoli, L.; Pau, F.; Luiselli, L.; Carpaneto, G.M. Co-occurrence patterns of five species of anurans at a pond network in Victoria Lake, Kenya. Afr. J. Ecol. 2010, 48, 275–279. [Google Scholar] [CrossRef]
- Jackson, K.; Blackburn, D.C. A survey of amphibians and reptiles at degraded sites near Pointe-Noire, Kouilou Province, Republic of Congo. Herpetol. Conserv. Biol. 2010, 5, 414–429. [Google Scholar]
- Sinsch, U.; Bocking, H.; Leskovar, C.; Oz, M.; Veith, M. Demography and lifetime growth patterns in viviparous salamanders (genus Lyciasalamandra): Living underground attenuates interspecific variation. Zool. Anz. 2017, 269, 48–56. [Google Scholar] [CrossRef]
- Santos-Barrera, G.; Urbina-Cardona, J.N. The role of the matrix-edge dynamics of amphibian conservation in tropical montane fragmented landscapes. Rev. Mexic. Biodiv. 2011, 82, 679–687. [Google Scholar] [CrossRef]
- Beard, K.H.; Vogt, K.A.; Kulmatiski, A. Top-down effects of a terrestrial frog on forest nutrient dynamics. Oecologia 2002, 133, 583–593. [Google Scholar] [CrossRef]
- Best, M.L.; Welsh, H.H. The trophic role of a forest salamander: Impacts on invertebrates, leaf litter retention and the humification process. Ecosphere 2014, 5, 16. [Google Scholar] [CrossRef]
- Toft, C.A. Feeding ecology of Panamanian litter anurans: Patterns in diet and foraging mode. J. Herpetol. 1981, 15, 139–144. [Google Scholar] [CrossRef]
- Bellakhal, M.; Bellakhal, F.M.; Missaoui, H. Le régime alimentaire de la grenouille verte d’Afrique du Nord, Rana saharica. Rev. Elect. Veterin. 2010, 11, 1–14. [Google Scholar]
- Coleman, J.L.; Barclay, R.M. Prey availability and foraging activity of grassland bats in relation to urbanization. J. Mamm. 2013, 94, 1111–1122. [Google Scholar] [CrossRef]
- Jaganmohan, M.; Vailshery, L.S.; Nagendra, H. Patterns of insect abundance and distribution in urban domestic gardens in Bangalore, India. Diversity 2013, 5, 767–778. [Google Scholar] [CrossRef]
- Hunter, P. The human impact on biological diversity. How species adapt to urban challenges sheds light on evolution and provides clues about conservation. EMBO Rep. 2007, 8, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Frétey, T.; Blanc, C.P. Liste des Amphibiens d’Afrique Centrale: Cameroun, Congo, Gabon, Guinée Equatoriale, République Centrafricaine, République Démocratique du Congo, Sao Tomé et Principe; Association pour le Développement de l’Information Environnementale (ADIE): Libreville, Gabon, 2000; pp. 1–38. [Google Scholar]
- Fournier, A.; Floret, C.; Gnahoua, G.-M. Végétation des jachères et succession post-culturale en Afrique tropicale. In La Jachère en Afrique Tropicale; Floret, C., Pontanier, R., Eds.; John Libbey Eurotext: Paris, France, 2001; pp. 123–168. [Google Scholar]
- Vallod, D.; Wezel, A. Influence des Pratiques Agro-Piscicoles sur la Biodiversité des Étangs de la Dombes (Ain, France) en vue D’une Valorisation de Produits du Terroir. VertigO 2010. Available online: https://journals.openedition.org/vertigo/9980 (accessed on 25 October 2024).
- Tumushimire, L.; Mindje, M.; Sinsch, U.; Dehling, J.M. Anuran diversity of cultivated wetlands in Rwanda: Melting pot of generalists? Salamandra 2020, 56, 99–112. [Google Scholar]
- Mukinzi, I.; Katuala, G.B.; Kennis, J.; Gambalemoke, M.; Kadange, N.; Dudu, A.M.; Colyn, M.; Hutter, R. Preliminary data on the biodiversity of rodents and insectivores (Mammalia) in the periphery of Kisangani (DR Congo). Belg. J. Zool. 2005, 135, 133–140. [Google Scholar]
- Donovan, T.M.; Jones, P.W.; Annand, E.M.; Thompson, F.R. Variation in local-scale edge effects: Mechanisms and landscape context. Ecology 1997, 78, 2064–2075. [Google Scholar] [CrossRef]
- Shapira, I.; Sultan, H.; Shanas, U. Agricultural farming alters predator-prey interactions in nearby natural habitats. Anim. Conserv. 2008, 11, 1–8. [Google Scholar] [CrossRef]
- El Hamoumi, R.; Himm, O. Distribution et état des lieux des peuplements d’Amphibiens dans le complexe de zones humides du bas Loukkos (Larache, Maroc). Bull. L’inst. Sc. 2010, 32, 95–100. [Google Scholar]
- Measey, G.J.; Stevenson, B.C.; Scott, T.; Altwegg, R.; Borchers, D.L. Counting chirps: Acoustic monitoring of cryptic frogs. J. Appl. Ecol. 2017, 54, 894–902. [Google Scholar] [CrossRef]
- Toft, C.A. Resource partitioning in amphibians and reptiles. Copeia 1985, 1, 1–21. [Google Scholar] [CrossRef]
- Toledo, L.F.; Garcia, P.C.A.; Lingnau, R.; Haddah, C.F.B. Description of a new species of Sphaenorhynchus (Anura: Hylidae) from Brazil. Zootaxa 2007, 1658, 57–68. [Google Scholar] [CrossRef]
- Howell, K.M. Field Guide to the Amphibians of the Eastern Arc Mountains and Coastal Forests of Tanzania and Kenya; CPI: Nairobi, Kenya, 2010; pp. 1–316. [Google Scholar]
- Inger, R.F. Amphibia in Exploration Parc National de la Garamba; Fascicule 52: Kinshasa, Democratic Republic of the Congo, 1968; pp. 1–190. [Google Scholar]
- Perret, J.L. Les amphibiens du Cameroun. Zool. Jahr. Abt. Syst. 1966, 8, 289–464. [Google Scholar]
- Jongsma, G.F.M.; Barej, M.F.; Barratt, C.D.; Burger, M.; Conradie, W.; Ernst, R.; Greenbaum, E.; Hirchfeld, M.; Leaché, A.D.; Penner, J.; et al. Diversity and biogeography of frogs in the genus Amnirana (Anura: Ranidae) across sub-Saharan Africa. Mol. Phyl. Evol. 2018, 120, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Laurent, R.F. Amphibiens. Exploration du Parc National des Virunga pour favoriser la recherche scientifique en Afrique. Deuxieme Série 1972, 22, 1–125. [Google Scholar]
- Almeida-Gomes, M.; Prevedello, J.A.; Crouzeilles, R. The use of native vegetation as a proxy for habitat may overestimate habitat availability in fragmented landscapes. Landsc. Ecol. 2016, 31, 711–719. [Google Scholar] [CrossRef]
- Cortés-Gómez, A.M.; Castro-Herrera, F.; Urbina-Cardona, J.N. Small changes in vegetation structure create great changes in amphibian ensembles in the Colombian Pacific rainforest. Trop. Conserv. Sc. 2013, 6, 749–769. [Google Scholar] [CrossRef]
- Carrara, E.; Arroyo-Rodriguez, V.; Vega-Rivera, J.; Schondube, J.E.; Sandra de Freitas, M.; Fahrig, L. Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol. Conserv. 2015, 184, 117–126. [Google Scholar] [CrossRef]
- Guerra, C.; Aràoz, E. Amphibian diversity increases in heterogeneous agricultural landscape. Acta Oecol. 2015, 69, 78–86. [Google Scholar] [CrossRef]
- Amiet, J.L. Aires disjointes et taxons vicariants chez les Anoures du Cameroun: Implications paléoclimatiques. Alytes 1987, 6, 99–115. [Google Scholar]
- Schiøtz, A. The Treefrogs of Eastern Africa; Steenstrupia: Copenhagen, Denmark, 1975; pp. 1–232. [Google Scholar]
- Schiøtz, A. The Treefrogs of Africa; Chimaira: Frankfurt am Main, Germany, 1999; pp. 1–350. [Google Scholar]
- Frétey, T.; Dewynter, M.; Blanc, C.P. Amphibiens d’Afrique Centrale et d’Angola. Clé de Détermination Illustrée des Amphibiens du Gabon et du Mbini; Illustrated Identification key of the Amphibians from Gabon and Mbini; Editions Biotope; Muséum National d’Histoire Naturelle: Paris, France, 2011; pp. 1–232. [Google Scholar]
- Mestre, F. Synergistic effects of climate change and habitat fragmentation on species range shifts and metapopulation persistence. Front. Biogeogr. 2018, 9, e35859. [Google Scholar] [CrossRef]
- Hirai, T.; Matsui, M. Myrmecophagy in a Ranid Frog Rana rugosa: Specialization or week avoidance to ant eating? Zool. Sc. 2000, 17, 459–466. [Google Scholar]
- López, J.A.; Scarabotti, P.A.; Medrano, M.C.; Ghirardi, R. Is the red spotted green frog Hypsiboas punctatus (Anura: Hylidae) selecting its prey? The importance of prey availability. Rev. Biol. Trop. 2009, 57, 847–857. [Google Scholar] [CrossRef]
- Solé, M.; Dias, I.R.; Rodrigues, E.A.S.; Marciano, E., Jr.; Branco, S.M.J.; Cavalcante, K.; Rodder, D. Diet of Leptodactylus ocellatus (Anura: Leptodactylidae) from a cacao plantation in southern Bahia, Brazil. Herpetol. Notes 2009, 2, 9–15. [Google Scholar]
- Santana, D.J.; Ferreira, V.G.; Crestani, G.N.; Neves, M.O. Diet of the Rufous Frog Leptodactylus fuscus (Anura, Leptodactylidae) from two contrasting environments. Herpetozoa 2019, 32, 1–6. [Google Scholar] [CrossRef]
- Hirai, T.; Matsui, M. Feeding habits of the pond frog, Rana nigromaculata, inhabiting rice fields in Kyoto, Japan. Copeia 1999, 4, 940–947. [Google Scholar] [CrossRef]
- López, J.A.; Scarabotti, P.A.; Ghirardi, R. Amphibian trophic ecology in increasingly human-altered wetlands. Herpet. Conserv. Biol. 2015, 10, 819–832. [Google Scholar]
- Carey, C. How physiological methods and concepts can be useful in conservation biology. Integrat. Comparat. Biol. 2005, 45, 4–11. [Google Scholar] [CrossRef]
- Hirai, T.; Matsui, M. Food habits of an endangered Janapese frog, Rana porosa brevipoda. Ecol. Res. 2001, 16, 737–743. [Google Scholar] [CrossRef]
- Rödel, M.-O. Herpetofauna of West Africa 1. Amphibians of the West African Savana; Chimaira: Franckfurt am Main, Germany, 2000; pp. 1–332. [Google Scholar]
- Clément, F.; Ruiz, J.; Rodríguez, M.A.; Blais, D.; Campeau, S. Landscape diversity and forest edge density regulate stream water quality in agricultural catchments. Ecol. Indic. 2017, 72, 627–639. [Google Scholar] [CrossRef]
- Matos, F.A.R.; Magnago, L.F.S.; Gastauer, M.; Carreiras, J.M.B.; Simonelli, M.; Meira-Neto, J.A.A.; Edwards, D.P. Effects of landscape configuration and composition on phylogenetic diversity of trees in a highly fragmented tropical forest. J. Ecol. 2017, 105, 265–276. [Google Scholar] [CrossRef]
- Van der Hoek, Y.; Tuyisingize, D.; Eckardt, W.; Garriga, N. Spatial variation in anuran richness, diversity and abundance across montane wetland habitat in Volcanoes National Park, Rwanda. Ecol. Evol. 2019, 9, 4220–4230. [Google Scholar] [CrossRef]
- Quesnelle, P.E.; Lindsay, K.E.; Fahring, L. Relative effects of landscape-scale wetland amount and landscape matrix quality on wetland vertebrates: A meta-analysis. Ecol. Appl. 2015, 25, 812–825. [Google Scholar] [CrossRef]
- Decout, S.; Manel, S.; Miaud, C.; Luque, S. Integrative approach for landscape-based graph connectivity analysis: A case study with the common frog (Rana temporaria) in human-dominated landscapes. Landsc. Ecol. 2012, 27, 267–279. [Google Scholar] [CrossRef]
- Holzer, K.A.; Bayers, R.P.; Nguyen, T.T.; Lawler, S.P. Habitat value of cities and rice paddies for amphibians in rapidly urbanizing Vietnam. J. Urban Ecol. 2017, 1–12. [Google Scholar] [CrossRef]
- Cox, K.; Maes, J.; van Calster, H.; Mergeay, J. Effect of the landscape matrix on gene flow in a coastal amphibian metapopulation. Conserv. Genet. 2017, 18, 1359–1375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).