Abstract
A taxonomic review of the Nepticulidae of Armenia is provided for the first time. Currently, the previously little-known fauna of the pygmy moths of Armenia consists of eight genera and 31 species, including seven new species described and illustrated in the current paper as follows: Stigmella armi Stonis, Dobrynina & Remeikis, sp. nov.; Stigmella garnica Stonis, Dobrynina & Remeikis, sp. nov.; Stigmella inopinoides Dobrynina, sp. nov.; Stigmella magicis Stonis & Dobrynina, sp. nov.; Stigmella ararati Stonis, Dobrynina & Remeikis, sp. nov.; Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.; and Etainia caucasi Remeikis, sp. nov. The most common Nepticulidae species in Armenia, Stigmella muricatella (Klimesch), still remains a taxonomic puzzle. However, the molecular sequences of the Armenian specimens and a specimen of S. muricatella from Greece were found to be similar, though clearly distinct from the widespread S. rolandi van Nieukerken due to numerous hypothetical mutational steps. Mitotype analysis revealed that the Ectoedemia specimens collected in Armenia do not belong to the expected widespread E. spinosella (Joannis) but are more similar to the less-known E. mahalebella (Klimesch). Moreover, some specimens from Armenia identified by us as Simplimorpha promissa (Staudinger) and Ectoedemia (Zimmermannia) longicaudella Klimesch may actually represent possible cryptic taxa, allopatric subspecies or species. However, from a practical point of view, the description of such difficult-to-diagnose taxa is inappropriate. Thus, we have refrained from describing such new taxa based solely on observed molecular differences.
1. Introduction
The task of providing an inventory of biodiversity is a major focus of biological systematics or descriptive taxonomy, and it has the ultimate goal of documenting and realizing the extent of global biological diversity [1,2]. Describing species along with revealing their evolutionary history “is fundamental to understanding the biosphere and the origins of global biodiversity”. Descriptive taxonomy also “opens a treasure of nature-inspired solutions for creating a sustainable future” [3,4]. However, the task of detailing an inventory of biodiversity is colossal [4,5,6,7,8,9] and urgent, particularly in the face of current biological annihilation and the sixth major extinction event [10]. Although hundreds and hundreds of new species are discovered every year [11], the speed of their description is far from sufficient [12], and the majority of the Earth’s organisms, especially smaller ones, still await description [11].
Nepticulidae (commonly called pygmy moths) are a cosmopolitan plant-mining lepidopteran family. Currently, it consists of 1014 species worldwide [13,14], including the seven new species provided from Armenia in the current paper. The family Nepticulidae includes the world’s smallest lepidopterans with a forewing length of less than 1.8 mm or a wingspan of less than 4.0 mm [15]. The larvae of the vast majority of the Nepticulidae species are leaf miners. This family has been extensively characterized in papers or monographic reviews [16,17,18,19,20], with a special reference to Neotropical America recently [14,21].
Armenia is a landlocked country located in the Armenian Highlands in the Southern Caucasus Mountains in Western Asia between the latitudes 38° and 42° N and the meridians 43° and 47° E. The terrain is mostly mountainous, with fast-flowing rivers and a few forests. The land rises to 4090 m above sea level at Mount Aragats, while there is no point below 390 m above sea level. The average elevation of this country’s area is the tenth highest in the world, and it has 85.9% of its terrain as a mountain area [22]. The climate in Armenia is markedly highland continental: the summers are hot, dry, and sunny, while winters are quite cold, with plenty of snow and temperatures ranging between −5 and −10 °C. Armenia contains two major terrestrial ecoregions: the Caucasian mixed forests and the Eastern Anatolian montane steppe [23].
Prior to this study, the taxonomic diversity of the Nepticulidae of Armenia was poorly known. One species, Stigmella armeniana Puplesis 1994, was described on the basis of a few specimens collected in Armenia by Povilas Ivinskis [19], while the primary description of another species, Glaucolepis melanoptera (van Nieukerken & Puplesis, 1991), also included one aberant, non-type specimen from Armenia [19,24]. It should be mentioned that an earlier record of Stigmella inopinata Laštůvka & Laštůvka, 1990, based on specimens collected by Friedrich Kasy in Armenia [25], is considered a case of misidentification in our publication.
The Nepticulidae faunas of neighboring Georgia and Azerbaijan are essentially unknown, with the exception of a few proven species, including those provided in a monograph by Rimantas Puplesis [19]. However, an impressive catalog of the Lepidotera of Iran, a country directly south of Armenia, has been published recently [26]. In this catalog, twenty-nine species of pygmy moths were confirmed for the fauna of Iran, and at least one named species was listed as possible but not yet proven [26].
The goal of the present study was to investigate the world’s smallest lepidopterans of the family Nepticulidae in the Caucasus (Armenia) and, for the first time, to provide a taxonomic review of the Nepticulidae of Armenia, with the naming and describing of newly discovered species, as well as to discuss possible cryptic taxa in the southern Caucasus.
2. Materials and Methods
In 2022, during our targeted fieldwork in Armenia conducted within a framework of the Agreement of Scientific Cooperation between the Scientific Center of Zoology and Hydroecology of the National Academy of Sciences of Armenia and the State Research Institute Nature Research Centre and as part of an informal research project in cooperation with Dr. Mark Kalashian (a co-author of the current publication), we collected about 600 nepticulid specimens (only about 300 were pinned). All these collected specimens, except for the specimens belonging to Glaucolepis melanoptera (van Nieukerken & Puplesis), appeared to be new for the Armenian fauna. Based on the specimens collected in Armenia, we also discovered eight new species, seven of which are described in the current paper. In addition, the type series of the earlier discovered and described Armenian species, Stigmella armeniana Puplesis, was available for our study from the collection of the BRG (the type series will be transferred to the MfN, Berlin, after this study).
During our fieldwork in Armenia (Figure 1), we attracted moths using light from a Philips bulb ML 220–230 V, 160 W, suspended in front of a white screen and powered by the electric mains. Additionally, where electric mains were unavailable, we used old-fashioned fluorescent lanterns powered by D batteries of a dry cell (flashlight batteries) [14] and the currently advertised LepiLED lamp. The latter is lightweight, small, and operated with a 5–13 V voltage DC from powerbank batteries, purposely designed for the sampling of nocturnal insects [27].
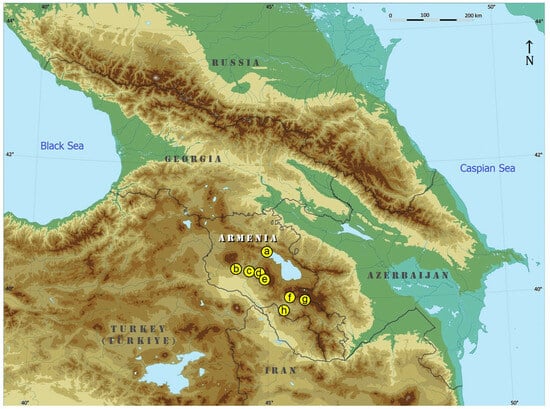
Figure 1.
Map of the region with the chosen locations for our fieldwork in Armenia: (a) Sevan, Tsovagyugh, 2000 m, 40°36′08″ N, 44°57′45″ E; (b) Antarut, 1780–1985 m, 40°22′36″ N, 44°16′03″ E; (c) Victory Park, Yerevan, 1142 m, 40°11′47″ N, 44°31′17″ E; (d) Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E; (e) Garni, 1312–1355 m, 40°07′18″ N, 44°45′17″ E; (f) Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E; (g) Jermuk, 2000 m, 39°50′25″ N, 45°40′07″ E; (h) Noravank, 1468 m, 39°40′57″ N, 45°14′10″ E (Base map courtesy of Dr. Virginijus Gerulaitis, Vytautas Magnus University, Lithuania).
The methods and protocols for the specimen setting, species identification, and description were extensively outlined both earlier [20,28] and recently by Stonis et al. [14]. After the maceration of the abdomen in 10% KOH and subsequent cleaning, male genital capsules were removed from the abdomen and mounted with the ventral side uppermost [14]. The phallus was often removed and mounted alongside the genital armature. Abdominal pelts were not preserved during this study.
Permanent preparations on microscope slides were photographed and studied with a Leica DM2500 microscope and an attached Leica DFC420 digital camera. Adults were measured and studied using a Lomo stereoscopic microscope MBS-10 and photographed using a Leica S6D stereoscopic microscope with a Leica DFC290 digital camera attached.
Collecting Sites. Considering the mosaic of landscapes present at the majority of the collecting sites, there are various habitat types included in some localities characterized below. The habitats of the collecting localities were classified strictly following the EUNIS habitats’ classification [29], adapted to Armenia by Fayvush & Aleksanyan [30], with codes provided in parentheses.
Sevan, Tsovagyugh, Gegharkunik Province, 2000 m, 40°36′08″ N, 44°57′45″ E (Figure 2a,b). The selected habitats near Lake Sevan, within the Sevan National Park, were in the area of a former lakebed following drainage, partly systematic and planted with tree and shrub vegetation, partly penetrated by vegetation from adjacent areas. At the collection site, the following types of habitats were present: highly artificial broadleaf deciduous forestry plantations (G1.C) with the dominance of poplars (Populus spp.) and willows (Salix spp.); mixed riparian floodplain and gallery woodlands (G1.2) tree stands, which were also comprised mainly of poplars and willows, as well as ash trees (Fraxinus spp.), willows (Salix spp.) and maples (Acer spp.); riverine scrub (F9.1) and the sea-buckthorn (Hyppophae rhamnoides) scrub in the Sevan basin (F3.19).

Figure 2.
Collection sites in Armenia: (a,b) Sevan, Tsovagyugh, Gegharkunik Province, near Lake Sevan; habitats were formed on the former lakebed following its drainage, partly systematically planted with tree and shrub vegetation and partly penetrated by vegetation from adjacent areas. (c–f) Antarut, Aragatsotn Province, on the southern slope of Mt Aragats, mainly with Quercus macranthera forests in the canyons and traganth communities with a predominance of Astragalus microcephalus on mountain slopes.
Antarut, Aragatsotn Province, 1780–1985 m, 40°22′36″ N, 44°16′03″ E–40°21′50″ N, 44°16′25″ E (Figure 2c–f). This collecting site is located in central Armenia, on the southern slope of Mt Aragats, mainly in light oak forests (i.e., oak forests with Quercus macranthera, G1.A1D2); tree stands of ash trees (Fraxinus excelsior), maples (Acer spp.) and plum trees (Prunus divaricata) were also present. In the neighboring territory to the collection site, traganth communities with a predominance of Astragalus microcephalus (F7.4I211) were common.
Victory Park, Yerevan, 1142 m, 40°11′47″ N, 44°31′17″ E–40°11′41″ N, 44°31′25″ E (Figure 3a,b). Victory Park (or “Park Pobedy”) is situated somewhat north of Yerevan city center. It was created in the 1930s by planting various decorative trees and shrubs. According to the EUNIS classification, this habitat is classified as a large-scale ornamental garden area (I2.1). The tree stand was formed mainly by maples (Acer spp.), poplars (Populus spp.), plane trees (Platanus orientalis), elms (Ulmus spp.), ash trees (Fraxinus excelsior), and other trees.

Figure 3.
Collection sites in Armenia: (a,b) Victory Park (or “Park Pobedy”), situated somewhat north of Yerevan city center, created in the 1930s by planting various mainly decorative trees and shrubs, including maples, poplars, plane trees, elms, ash trees and other trees; (c–f) Jrvezh Forest Park, in the western area of Yerevan city (belongs to Kotayk Province), created in the mid-1900s by the systematic planting of tree and shrub vegetation, both deciduous and coniferous; the park includes an old artificial pond.
Jrvezh Forest Park, Yerevan, 1515 m, 40°11′07″ N, 44°37′11″ E (Figure 3c–f). This collection site is situated in central Armenia, in the western area of Yerevan city (Jrvezh Forest Park is in Kotayk Province, not Yerevan itself). It was created in the mid-1900s by the systematic planting of tree and shrub vegetation, both deciduous and coniferous. In this territory, a community classified as mixed forestry plantations (G4.F) is present; it includes ash trees (Fraxinus excelsior), maples (Acer spp.), and elms (Ulmus spp.), as well as pines (Pinus spp., mainly P. kochiana and P. sylvestris with small plots of P. pallasiana). In Jrvezh Forest Park, an old artificial pond was created; it belongs to the habitat type C1.3 (i.e., permanent euthrophic lakes, ponds, and pools).
Garni, Kotayk Province (1312–1355 m, 40°07′18″ N, 44°45′17″ E–40°06′41″ N, 44°43′55″ E) and Garni, Ararat Province (1371 m, 40°07′27″ N, 44°45′40″ E) (Figure 4a–f). Both habitats are located in central Armenia, on the sides of a gorge at the confluence of the Rivers Goght and Azat. Along the riversides, mixed riparian floodplain and gallery woodlands (G1.2) occur along old-growth willows (Salix spp.) and poplars (Populus nigra). On the slopes, almond open arid forests (F5.342) are common alongside steppe-like communities in some areas on sand substrates (E1.2H1), as well as bare inland basaltic and ultra-basic cliffs (H3.2H).

Figure 4.
Collection sites in Armenia: (a,d) Garni, Ararat Province; (b,c,e,f) Garni, Kotayk Province; both sites are located on the sides of a gorge at the confluence of the Rivers Goght and Azat; along the riversides, mixed riparian floodplain and gallery woodlands occur with old-growth willows and poplars; on the dry slopes, almond open arid forests are common, and steppe-like communities occur.
Jermuk, Vayots Dzor Province, 2000 m, 39°50′25.8″ N, 45°40′07.6″ E (Figure 5a–c). Jermuk town is situated in the southwestern part of Armenia, on the southern slopes of the Vardenis Range. The collection site is located in the town with remnants of oak forests comprising Quercus macranthera (G1.A1D2); in the tree stands, ash (Fraxinus excelsior), maples (Acer spp.) and plum trees (Prunus divaricata) are present along with artificially planted pines and many other trees and shrubs. The site is located above a gorge with bare inland basaltic and ultra-basic cliffs (H3.2H).

Figure 5.
Collection sites in Armenia: (a–c) Jermuk, Vayots Dzor Province, on the southern slopes of the Vardenis Range, with remnants of oak forests comprising Quercus macranthera along with other artificially planted trees; (d–f) Yeghegis, Vayots Dzor Province, on the southern slopes of the Vardenis Range, with a predominance of hawthorns, plums, nettle trees (Celtis caucasica) and wild roses; (g–i) Noravank, Vayots Dzor Province, on the northern slopes of the Vayots Dzor Range, with thickets of Salix and Tamarix and a predominance of Celtis, Rhamnus cathartica and Rh. pallasii.
Yeghegis, Vayots Dzor Province, 1574 m, 39°52′10″ N, 45°22′12″ E (Figure 5d–f). The Yeghegis river gorge is situated in the southwestern part of Armenia, on the southern slopes of the Vardenis Range. Along the river, mixed riparian floodplain and gallery woodlands (G1.2) are present, comprising old-growth willows (Salix spp.) and a few poplar trees (Populus nigra). Temperate thickets and scrubs (F3.1) are also common on the slopes, with a predominance of hawthorns (Crataegus spp.), plums (Prunus spp.), nettle trees (Celtis caucasica) and wild roses (Rosa spp.), as well as bare inland basaltic and ultra-basic cliffs (H3.2H) on the top.
Noravank, Vayots Dzor Province, 1468 m, 39°40′57″ N, 45°14′10″ E (Figure 5g–i). Noravank (Gnishik River Canyon) is situated in the southwestern part of Armenia, on the northern slopes of the Vayots Dzor (formerly Daralagyaz) Range. Along the River Gnishik that flows through the gorge, thickets of Salix and Tamarix are present (F9.141 and F9.3142, respectively). There are also some plots of traditional vineyards (FB.41) abandoned in the mid-1900s. The slopes of the gorge are covered with vegetation classified into the following habitats: a) nettle tree open arid forests (F5.343) with a predominance of Celtis spp. and buckthorn open arid forests (F5.347) with a predominance of Rhamnus pallasii Fisch. & C.A. Mey. A significant part of the canyon is formed by rocks and scree with vegetation characteristic for such habitats, namely calcareous and the ultra-basic screen of the lower mountain belt of Armenia (H2.6H) and mountain limestone cliffs (H3.2E2).
Molecular analysis. Genomic DNA was obtained from the 96% ethanol-preserved larval stages or adults using the GeneJet Genomic DNA Purification Kit (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) protocol. The primers T3Lep-f (5′-attaaccctcactaaagtcwachaatcataaaratattgg-3′; modified Lep-f1 [31]) and T7Nancy-r (5′-aatacgactcactataggdaraattaraatrtaaacytcwg-3′; modified Nancy [32]) were used to amplify the partial sequences of the mitochondrial DNA cytochrome c oxidase subunit 1 (mtDNA CO1-5′). All PCR mixtures contained 12.5 µL of a 2× DreamTaq Green PCR Master Mix (Thermo Fisher Scientific Baltics, Vilnius, Lithuania), 2.5 µL of 10 pmol/μL of each primer, 1–2 µL of genomic DNA, and deionized water up to a total volume of 25 μL. The PCR thermal regime was as follows: initial denaturation at 95 °C for 5 min; 45 cycles of denaturation at 94 °C for 40 s, annealing at 45 °C for 40 s, and extension at 72 °C for 1 min; the final extension was at 72 °C for 5 min. Each PCR product was electrophoresed on 1.5% agarose gel (Thermo Fisher Scientific Baltics, Vilnius, Lithuania), stained with 10,000× GelRed (Biotium, Fremont, CA, USA) and visualized under UV light; GeneRuler Express DNA Ladder (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) was used for the size measurement of the amplified DNA sequences. PCR products were subsequently purified using the Exonuclease I and FastAP Thermosensitive Alkaline Phosphatase (Thermo Fisher Scientific Baltics, Vilnius, Lithuania) protocol. Automated sequencing in both directions was performed in BaseClear B.V. (Leiden, The Netherlands) using the ABI 3730xl 96-capillary DNA analyzer (Applied Biosystems, Foster City, CA, USA). The obtained DNA sequences were manually aligned using BioEdit 7.2.5 software [33]. All data were deposited in the NCBI GenBank database (https://www.ncbi.nlm.nih.gov/genbank/ accessed on 1 January 2024) under the accession numbers OR793219–OR793227, OR880346, and OR880347. Moreover, for further analysis, sequences were downloaded from the GenBank and the Barcode of Life Data System (BOLD) platform [34,35] (https://www.boldsystems.org accessed on 1 January 2024). The Neighbor-Joining (NJ) trees based on Kimura 2-parameter [36] or Tamura 3-parameter [37] models with a gamma distribution and 1000 or 10,000 bootstrap replications were constructed using the MEGA 7.0 software [38]. Mitotype diversity was obtained using the DnaSP 6 program [39]. ClustalX2 [40] was used for the Fasta format conversion to Nexus. The mitotype networks were generated using the Median Joining Network, Minimum Spanning Network [41], and the TCS Network [42] algorithms, which were implemented in the PopArt 1.7 program (http://popart.otago.ac.nz accessed on 1 January 2024).
Abbreviations for institutions and specimen depository: BRG–Biosystematic Research Group, currently based at the State Research Institute Nature Research Centre, Vilnius, Lithuania; MfN–Museum für Naturkunde, Berlin, formerly known as the Museum der Naturkunde für Humboldt Universität zu Berlin or Museum für Naturkunde/Leibniz-Institut für Evolutions und Biodiversitätsforschung, Berlin, Germany; RMNH–Naturalis Biodiversity Center, Zoological collections, Leiden, The Netherlands; VU–Vrije Universiteit, Amsterdam, The Netherlands.
3. Results
3.1. A Review of the Currently Known Nepticulidae Taxa from Armenia
Based on the results of our collecting and previously known records, the current fauna of the Nepticulidae of Armenia consists of eight genera and 31 species of pygmy moths (Figure 6), including seven new species named and described in this current paper as follows: Stigmella armi Stonis, Dobrynina & Remeikis, sp. nov. (Figure 7g); S. garnica Stonis, Dobrynina & Remeikis, sp. nov. (Figure 7c); S. inopinoides Dobrynina, sp. nov. (Figure 7c); S. magicis Stonis & Dobrynina, sp. nov. (Figure 7a,b); S. ararati Stonis, Dobrynina & Remeikis, sp. nov. (Figure 7e,f); Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov. (Figure 8a,b); and Etainia caucasi Remeikis, sp. nov. (Figure 8c–f).
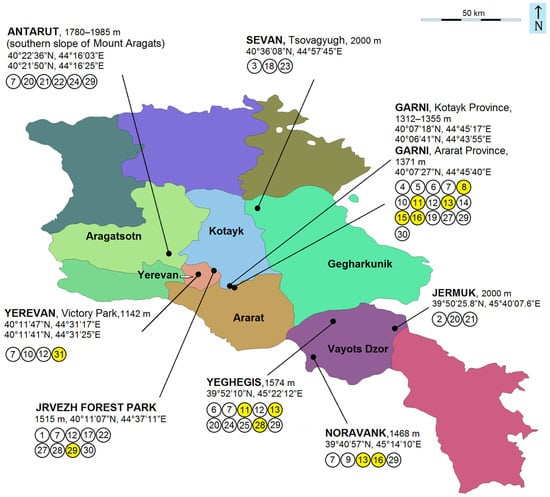
Figure 6.
Nepticulidae species detected in Armenia (the new species are shown in yellow): 1—Simplimorpha promissa (Staudinger); 2—Stigmella confusella (Wood & Walsingham); 3—S. malella (Stainton); 4—S. klimeschi Puplesis; 5—S. armeniana Puplesis; 6—S. kopetdagica Puplesis; 7—S. muricatella (Klimesch); 8—S. armi Stonis, Dobrynina & Remeikis, sp. nov.; 9—S. nivenburgensis (Preissecker); 10—S. aceris (Frey); 11—S. garnica Stonis, Dobrynina & Remeikis, sp. nov.; 12—S. hybnerella (Hübner); 13—S. inopinoides Dobrynina, sp. nov.; 14—S. viscerella (Stainton); 15—S. magicis Stonis & Dobrynina, sp. nov.; 16—S. ararati Stonis, Dobrynina & Remeikis, sp. nov.; 17—S. carpinella (Heinemann); 18—S. lemniscella (Zeller); 19—S. kuznetzovi Puplesis; 20—S. dorsiguttella (Johansson); 21—S. roborella (Johansson); 22—S. basiguttella (Heinemann); 23—Bohemannia pulverosella (Stainton); 24—Ectoedemia longicaudella Klimesch; 25—E. mahalebella (Klimesch); 26—Fomoria septembrella (Stainton); 27—Trifurcula subnitidella (Duponchel); 28—T. vardenisi Stonis, Dobrynina & Remeikis, sp. nov.; 29—Glaucolepis melanoptera (van Nieukerken & Puplesis); 30—G. hamirella (Chrétien); and 31—Etainia caucasi Remeikis, sp. nov.

Figure 7.
Adults of new Nepticulidae species from Armenia; (a,b) Stigmella magicis Stonis & Dobrynina, sp. nov.; (c) S. garnica Stonis, Dobrynina & Remeikis, sp. nov.; (d) S. inopinoides Dobrynina, sp. nov.; (e,f) S. ararati Stonis, Dobrynina & Remeikis, sp. nov.; and (g) S. armi Stonis, Dobrynina & Remeikis, sp. nov.

Figure 8.
Adults of new Nepticulidae species from Armenia; (a,b) Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.; (c,d) Etainia caucasi Remeikis, sp. nov.; (e) androconial patch of E. caucasi on the hindwing’s upper side; (f) androconial patch of E. caucasi on the forewing’s underside (the arrows point to the important diagnostic characters).
- Genus Simplimorpha Scoble, 1983
- 1. Simplimorpha promissa (Staudinger, 1870)
- Nepticula promissa Staudinger, 1870 [43] (pp. 325, 326).
Host plants. Cotinus coggygria Scop. (Anacardiaceae); S. promissa also is known to feed on other species of the same plant family, but this has not yet been confirmed in Armenia: Pistacia atlantica Desf., P. lentiscus L., P. terebinthus L., Rhus coriaria L.
Distribution. S. promissa is known for its distribution in the Mediterranean region and central Europe, from Spain, France, and Italy to Austria, Slovakia, Ukraine, Romania, and Greece. The species also occurs in the Caucasus: Georgia (Abkhaz) [19] and Armenia (this new distribution record was provided in our recent publication [44]). The occurrence of S. promissa in Iran has not yet been confirmed [26].
Material examined. The material examined was 1 ♂, Armenia, Yerevan, Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E, at light, 11 August 2022, leg. J.R. Stonis, genitalia slide no. RA1128 (MfN); 1 ♂, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 21 August 2022, leg. J.R. Stonis, genitalia slide no. RA1110 (MfN). Also, old (vacant) leaf mines on Cotinus coggygria and 9 ♀ adult specimens were collected at light in the Jrvezh Forest Park on 11–21 August 2023, the latter of which were entirely consumed during our DNA studies [44].
Remarks. See Section 3.2.3 for the genetic distinctness of S. promissa from Armenia.
- Genus Stigmella Schrank, 1802
- The lapponica group
- 2. Stigmella confusella (Wood & Walsingham, 1894)
- Nepticula confusella Wood & Walsingham, 1894 [45] (pp. 272, 273).
Host plants. Betula nana L., B. pendula Roth, B. pubescens Ehrh.; in Armenia, B. litwinowii Doluch.
Distribution. Widespread in Europe (except southern and southwestern regions), this species occurs from Ireland and France to northwestern Russia, Ukraine, and Bulgaria. Here, on the basis of documented leaf mines, we provide S. confusella for Armenia, the Caucasus (a new distribution record).
Material examined. Numerous old (vacant) leaf mines were examined on Betula litwinowii, Armenia, Vayots Dzor Province, Jermuk, 2106 m, 39°50′28″ N, 45°40′14″ E, 12 August 2022, leg. J.R. Stonis.
Remarks. For species illustrations, we recommend the following publications: [18] (419, 477, 551), [19] (figures 116–119).
- The former rhamnella group (see Section 3.2.2)
- 3. Stigmella malella (Stainton, 1854)
- Nepticula malella Stainton [46] (p. 304).
Host plants. Malus domestica Borkh., M. sylvestris Mill.
Distribution. This species is widespread in Europe, from Portugal and Ireland to Russia (the southern Ural) and Greece. Recently, this species was confirmed to occur in Iran [26]. Here, on the basis of documented leaf mines, S. malella is provided for Armenia, the Caucasus (a new distribution record). However, this needs to be viewed with caution because no adults have been reared to confirm the species.
Material examined. Armenia, Gegharkunik Province, N of Sevan, 1909 m, 40°37′21″ N, 44°58′21″ E, old (vacant) leaf mines were examined on Malus sp., 9 August 2022, leg. J.R. Stonis.
Remarks. For species illustrations, we recommend the following publications: [18] (423, 482, 556), [19] (figures 225–227).
- 4. Stigmella klimeschi Puplesis, 1988 [47] (pp. 274, 275).
Host plants. Rhamnus spp.
Distribution. Previously, this species was known only in Central Asia (Kazakhstan and Tajikistan) [19]. Here, on the basis of collected adult specimens, we provide S. klimeschi for Armenia, the Caucasus (a new distribution record).
Material examined. The material examined was 1 ♂, Armenia, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 14 August 2022, leg. J.R. Stonis, genitalia slide no. DV059 (MfN); 1 ♂, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 16 August 2022, leg. J.R. Stonis, genitalia slide no. DV051 (MfN).
Remarks. For species illustrations, we recommend the following publications: [19] (figures 241–245), [47].
- 5. Stigmella armeniana Puplesis, 1994 [19] (p. 90).
Host plant. Rhamnus erythroxyloides subsp. sintenisii (Rech.f.) Mabb.
Distribution. This species is known to occur only in Armenia (Ararat Province, E of Garni, Chosrov Reserve) [19].
Material examined. The material examined was 3 ♂ (holotype and paratypes), Armenia, Ararat Province, E of Garni, 30 September 1986, larvae on Rhamnus, ex. l. 22 February 1987, leg. P. Ivinskis, genitalia slide nos AN375 (holotype), AN376 (paratype) (MfN).
Remarks. Here, we provide the first photographic documentation of S. armeniana, a previously little-known species named after Armenia (Figure 9a–f).
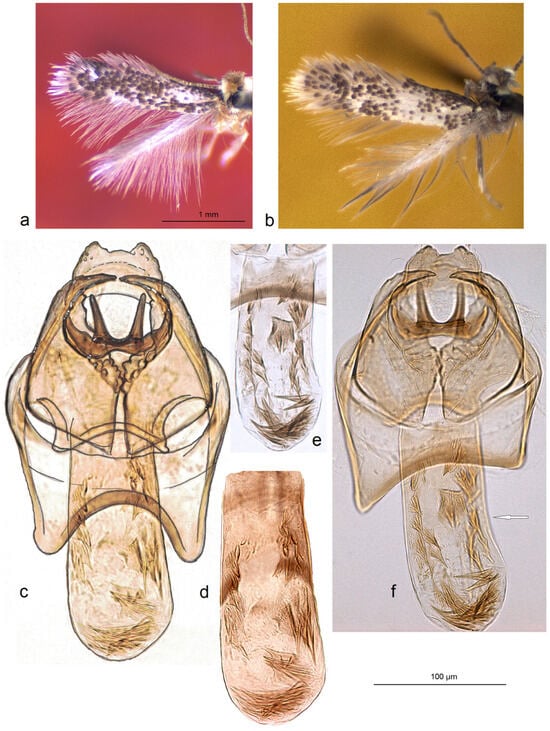
Figure 9.
The first photographic documentation of Stigmella armeniana Puplesis, a previously little-known species named after Armenia [19]: (a) adult, holotype; (b) same, paratype; (c,d) male genitalia, holotype, slide no. AN375; (e,f) the same paratype, slide no. AN376 (MfN) (the arrow points to the most important character).
- 6. Stigmella kopetdagica Puplesis, 1994 [19] (p. 92).
Host plant. Rhamnus coriacea (Regel) Kom.
Distribution. Previously, this species was known only from western Turkmenistan, Central Asia [19]. Here, on the basis of collected adult specimens, we provide S. kopetdagica for Armenia, the Caucasus (a new distribution record).
Material examined. The materials examined was 1 ♂, 1 ♀, Armenia, Kotayk Province, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 23 July 2022, leg. V. Dobrynina, genitalia slide no. DV009♂ (MfN); 1 ♂, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 14 August 2022, leg. J.R. Stonis, genitalia slide no. DV056 (MfN); 1 ♂, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 16 August 2022, leg. J.R. Stonis, genitalia slide no. DV044 (MfN); 1 ♂, Ararat Province, E of Garni,1371 m, 40°07′27″ N, 44°45′40″ E, at light, 30 August 2022, leg. J.R. Stonis, genitalia slide no. DV073 (MfN); 1 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26 July 2022, J.R. Stonis (MfN).
Remarks. For species illustrations, we recommend the following publication: [19] (figures 246–248).
- The former sanguisorbae group (see Section 3.2.2)
- 7. Stigmella muricatella (Klimesch, 1978)
- Nepticula muricatella Klimesch [48] (pp. 266, 267).
Host plants. Rosa spp., incl. R. fedtschenkoana Regel., Sanguisorba minor Scop., S. muricata (Spach) Gremli, Sarcopoterium spinosum (L.) Spach.
Distribution. The species distribution was known in Greece, Turkey (Anatolia), and Tajikistan [19]. However, the adults from Tajikistan differed from the European specimens in the cream background color of the forewing. Here, on the basis of collected adult specimens, we provide S. muricatella for the Caucasus (Armenia) (a new distribution record).
Material examined. The material examined was 4 ♂, 2 ♀, Armenia, Yerevan, Victory Park, 1142 m, 40°11′41″ N, 44°31′25″ E, at light, 29 July–19 August 2022, leg. J.R. Stonis, genitalia slide nos DV125♂, DV127♂, DV128♂ (MfN); 9 ♂, Yerevan, Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E, at light, 11 August –24 August 2022, leg. J.R. Stonis, genitalia slide nos DV112, DV114, DV119 (MfN); 55 ♂, 6 ♀, Kotayk Province, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 23 July–28 August 2022, leg. V. Dobrynina and J.R. Stonis, genitalia slide nos DV002♂*, DV006♂, DV008♂, DV010♂*, DV012♂, DV013♂, DV016♂, DV018♂, DV026♂, DV031♂, DV036♂, DV038♂, DV057♂, DV058♂, DV060♂, DV062♂, DV063♂, DV064♂, DV066♂, DV067♂, DV070♂, DV071♂, DV081♂, DV084♂, DV086♂, DV089♂, DV090♂, DV091♂, DV093♂, DV097♂, DV100♂, DV101♂, DV102♂, VD109♂, RA1134♀ (MfN); 7 ♂, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 30 August 2022, leg. J.R. Stonis, genitalia slide nos DV074♂, DV075♂, DV077♂, DV080♂, DV088♂, DV095♂ (MfN); 1 ♂, Aragatsotn Province, 2 km N of Antarut, 1780 m, at light, 40°21′50″ N, 44°16′25″ E, 2 August 2022, J.R. Stonis; 2 ♂, 4 km N Antarut, 1985 m, 40°22′36″ N, 44°16′03″ E, 12 August 2022, leg. J.R. Stonis, genitalia slide no. RA1121♂ (MfN); 7 ♂, 1 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26 July–15 August 2022, leg. J.R. Stonis and V. Dobrynina, genitalia slide nos DV022♂, DV027♂, DV035♂, RA1108♂, RA1135♀ (MfN); 6 ♂, Noravank, 1468 m, 39°40′57″ N, 45°14′10″ E, at light, 17 August 2022, leg. J.R. Stonis, genitalia slide nos RA1103♂, RA1107♂ (MfN) (* only genitalia preserved, without a pinned specimen).
Remarks. Here, we provide the first photographic documentation of S. muricatella, a previously little-known species described by Josef Klimesch [48] but very common in Armenia (Figure 10a–i).
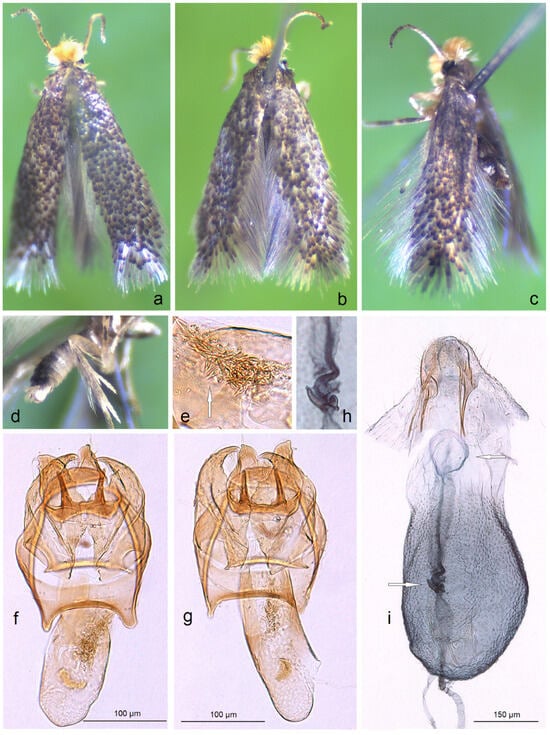
Figure 10.
The first photographic documentation of Stigmella muricatella (Klimesch), previously a little-known Nepticulidae species found to be abundant among the samples of all other hitherto discovered species in Armenia: (a–d) adults; (e,f) male genitalia, slide no. DV060; (g) the same, slide no. DV101 (h,i) female genitalia, slide no. RA1135 (MfN) (the arrows point to the diagnostic characters).
We barcoded three specimens from Armenia; the sequences are available in GenBank under accession IDs: OR793225, OR793226, and OR793227.
- The paliurella group
- 8. Stigmella armi Stonis, Dobrynina & Remeikis, sp. nov.
- https://zoobank.org/NomenclaturalActs/2225ef11-7c42-463b-bd25-360e74b04dab (accessed on 12 December 2023)
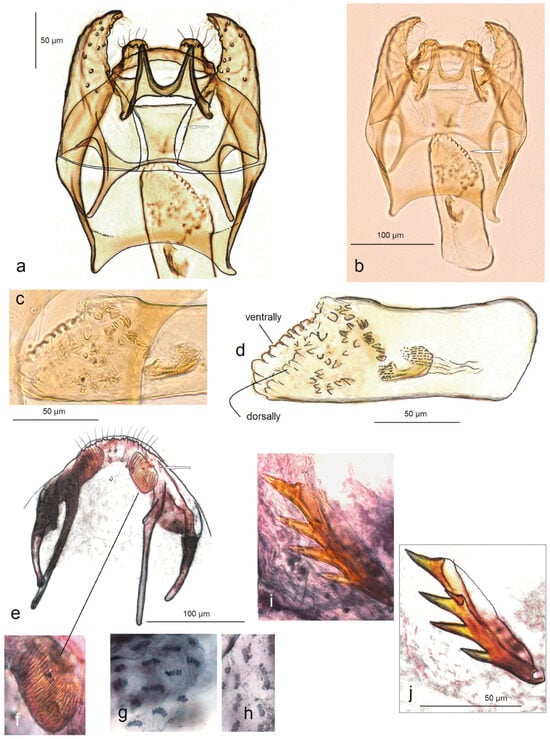 Figure 11. Genitalia of Stigmella armi Stonis, Dobrynina & Remeikis, sp. Nov.: (a–d) holotype, details of male genitalia, slide no. DV122 (MfN); (e–j) paratype, details of female genitalia slide no. DV041 (MfN): I apophyses; (f) ovipositor pad; (g,h) pectinations; and (i,j) vaginal sclerite (the arrow points to the diagnostic character).
Figure 11. Genitalia of Stigmella armi Stonis, Dobrynina & Remeikis, sp. Nov.: (a–d) holotype, details of male genitalia, slide no. DV122 (MfN); (e–j) paratype, details of female genitalia slide no. DV041 (MfN): I apophyses; (f) ovipositor pad; (g,h) pectinations; and (i,j) vaginal sclerite (the arrow points to the diagnostic character).
Diagnosis. Stigmella armi sp. nov. belongs to the paliurella species group. Externally, this new species can be differentiated from other Palearctic members of the group, including the dark-winged Central Asian S. turbatrix Puplesis, by the smoothly scaled, dark brown forewing. In the male genitalia, S. armi is characterized by a unique combination of a large gnathos, trapezoid juxta, well-separated lobes of uncus, and an apically distinctly serrated phallus. In the female genitalia, this new species is also distinctive because of the presence of unique, transversely striated ovipositor pads; furthermore, no other species in this group possesses a vaginal sclerite with long pointed spines.
Description. Male. Forewing length 1.9 mm; wingspan 2.3 mm (n = 1). Similar to the female but damaged (see Remarks). Male genitalia. The genitalia capsule is about 330 µm long and 190 µm wide. The tegumen is band-shaped. The uncus has two well-separated lobes. The gnathos is large, with two well-separated, basally wide caudal processes and short, lobe-like anterior processes. The valva is curved and pointed apically. Juxta is relatively large, trapezoid. The vinculum is relatively wide and long, with short curved lateral lobes. The phallus is 170 µm long, 50 µm wide, distinctly oblique, and serrated apically; the vesica has only some small, indistinctive cornuti.
Female. The forewing length is 2.0 mm; the wingspan is 2.6 mm (n = 1). Head. Palpi cream; frontal brown-black tuft; collar and scape large, cream; antenna is 1/4 shorter the length of forewing with about 19 segments; grey-brown flagellum the on upper side, cream on the underside. Thorax. The tegula, thorax, and forewing are densely covered with dark brown scales with some golden gloss and weak purple iridescence (the latter is more distinct basally and apically); the fringe is brownish grey; the fringe line is absent; the underside of the forewing is brown without spots. The hindwing and its fringe are brownish-grey and, at a certain angle of view, greyish cream. Legs are cream, with brown-grey scales on the upper side. Female genitalia. The ovipositor is short and widely rounded with unique, transversally striated ovipositor pads. Anterior apophyses are distinctly shorter than posterior apophyses. The vaginal sclerite is 85 µm long, strongly chitinized with four 23 µm long, pointed spines and one shorter spine. The corpus bursae with distinctive comb-like pectinations. The ductus spermathecae is unknown (see Remarks).
Bionomics. Adults fly in late July and August. Otherwise, the biology is unknown.
Distribution. This species is known to occur in Garni, at the border of two provinces in Armenia (Ararat and Kotayk), at an elevation of 1300–1400 m.
Etymology. The new species is named after the country where S. armi sp. nov. was discovered. “Arm” is an international abbreviation for Armenia and is commonly used in that country.
Remarks. The male holotype is preserved in a plastic capsule with a broken and missing head. The scaling and color of the thorax, tegulae, and forewing are almost identical to the female paratype. Therefore, the external description of the new species is based on the well-preserved female paratype. Female genitalia were damaged during the preparation of the genitalia mount (slide no. DV041), but the major morphological structures, including the unique ovipositor pads, apophyses, unique vaginal sclerite, and pectinations of bursa, were well-preserved and visible.
Type material. Holotype, ♂, Armenia, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 30 August 2022, leg. J.R. Stonis, genitalia slide no. DV122♂ (MfN). Paratype: 1 ♀, Armenia, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 25 July 2022, leg. V. Dobrynina, genitalia slide DV041♀ (MfN).
- The betulicola group
- 9. Stigmella nivenburgensis (Preissecker, 1942)
- Nepticula nivenburgensis Preissecker [49] (pp. 209–211).
- Stigmella thibaulti Varenne & Nel, 2019 [50] (p. 85), syn. by [51] (p. 303).
Host plants. Salix spp. and Populus nigra L.
Distribution. This species is widespread in Europe (predominantly southern and central), from Spain and Italy to Russia (central and southern European regions) and Greece. It is also known from the western part of Central Asia (Turkmenistan) [19] and East Asia (Japan, China, and Korea) [52]. Here, on the basis of a collected adult specimen, we provide S. nivenburgensis for Armenia, the Caucasus (a new distribution record).
Material examined. The material examined was 1 ♂, Armenia, Vayots Dzor Province, Noravank, 1468 m, 39°40′57″ N, 45°14′10″ E, at light, 17 August 2022, J.R. Stonis, genitalia slide no. RA1104 (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (470, 667, 556), [19].
- The ultima group
- 10. Stigmella aceris (Frey, 1857)
- Nepticula aceris Frey, 1857 [53] (pp. 386, 387).
Host plants. Acer campestre L., A. ginnala Maxim., A. platanoides L., A. tataricum L.
Distribution. This species is widespread in Europe, from Spain and Italy to Norway, Russia (Central European regions), Bulgaria, and Greece. It is also known in the Caucasus (Georgia) [19] and Iran [26]. Here, on the basis of collected adult specimens, we provide S. aceris for Armenia (a new distribution record).
Material examined. Armenia, 1 ♂, Yerevan, Victory Park, 1141 m, 40°11′41″ N, 44°31′12″ E, at light, 1 August 2022, leg. J.R. Stonis, genitalia slide no. DV126 (MfN); 1 ♂, same locality, 19 August 2022, leg. J.R. Stonis, genitalia slide no. RA1109♂ (MfN); 1 ♀, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22 August 2022, leg. J.R. Stonis (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (422, 482, 556), [19] (figures 220–222).
- The irregularis group
- 11. Stigmella garnica Stonis, Dobrynina & Remeikis, sp. nov.
- https://zoobank.org/NomenclaturalActs/3A72E0EF-1C5C-47E2-9677-953E86F4E1A2 (accessed on 12 December 2023)
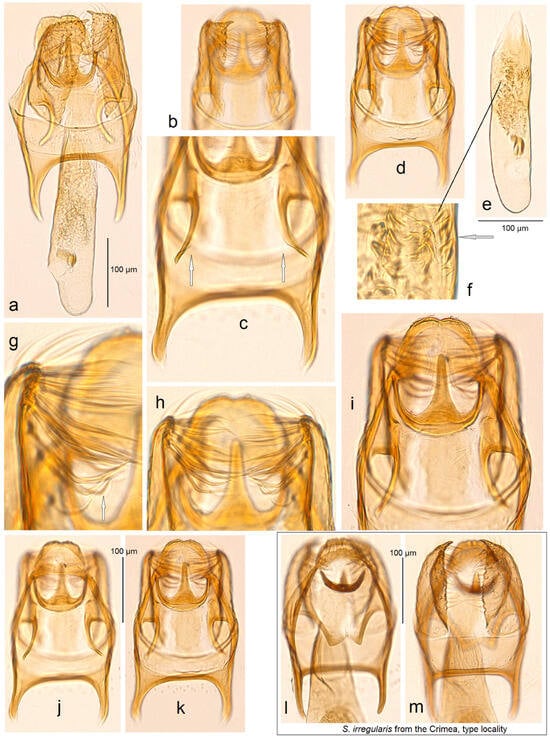 Figure 12. Male genitalia of Stigmella garnica Stonis, Dobrynina & Remeikis, sp. nov. and European S. irregularis Puplesis: (a) S. garnica sp. nov., paratype, slide no. DV055 (MfN); (b–k) same, holotype, genitalia slide no. RA1106 (MfN); and (l,m) S. irregularis Puplesis, a resembling species collected in the Crimea is a locality type with genitalia slide no. RA1129 (MfN) (the arrows point to the most important diagnostic characters).
Figure 12. Male genitalia of Stigmella garnica Stonis, Dobrynina & Remeikis, sp. nov. and European S. irregularis Puplesis: (a) S. garnica sp. nov., paratype, slide no. DV055 (MfN); (b–k) same, holotype, genitalia slide no. RA1106 (MfN); and (l,m) S. irregularis Puplesis, a resembling species collected in the Crimea is a locality type with genitalia slide no. RA1129 (MfN) (the arrows point to the most important diagnostic characters).
Diagnosis. Stigmella garnica sp. nov. belongs to the irregularis species group, formerly comprising only a single named species, the southeast European S. irregularis Puplesis, 1994. Externally, this new species can be differentiated from the resembling S. irregularis by its paler, golden cream forewing. In the male genitalia, the shape of the transtilla and the presence of very long chetae of the valva, and the presence of larger, triangular cornuti distinguish S. garnica sp. nov. from S. irregularis (Figure 12l,m).
Description. Male. The forewing length is 1.6–1.7 mm; the wingspan is 3.7–3.9 mm (n = 2). Head. Palpi cream; frontal tuft yellowish cream but blackish brown centrally; collar and scape glossy whitish cream to golden cream; antenna is slightly shorter than the length of the forewing, with 24 segments; flagellum pale grey to grey, golden glossy. Thorax. Tegula glossy golden cream, with blackish-brown to brown scales distally; thorax glossy golden cream; forewing glossy golden cream, with some blackish-brown and brown scales on the costal margin basally; fringe glossy cream; fringe line absent; forewing underside is brown to pale brown, without spots, or androconia. The hindwing and its fringe are brown to pale brown without androconia. The legs are glossy cream, but the forelegs have blackish-brown scales on the upper side. Male genitalia. The genitalia capsule is 285–325 µm long and 160–195 µm wide. Tegumen is band-shaped. The uncus is large, widely rounded or truncated without clearly differentiated (individualized) lobes. Gnathos has a large caudal process and slender, rounded lateral arms. Valva is relatively wide with a slender, pointed apex and very long chetae. Juxta is absent, but there is a slightly thickened membrane between the valvae ventrally. Transtilla is long, well-separated sublateral processes but without a distinctive transverse bar. The vinculum is short but with very long and slender lateral lobes. The phallus is 115 µm long and 55–60 µm wide; the vesica has numerous tiny, indistinctive cornuti aggregated into an irregular band and some larger, triangular cornuti at the right edge of the band caudally.
Female. Females are similar to the male (but excluded from the type series). Female genitalia. Their ovipositor is short and rounded. Anterior apophyses are shorter than posterior apophyses. The accessory sac is short, wide, and folded. Ductus spermathecae is short, with 1.5 coils and a relatively large vesicle. Corpus bursae is relatively short but wide, covered with comb-like pectinations and with a signum-like band of prominent spine-like pectinations basally.
Bionomics. Adults fly in August. Otherwise, the species biology is unknown.
Distribution. This species is knownin two localities in Armenia: Yeghegis (Vayots Dzor Province) and Garni (at the border of Ararat and Kotayk provinces), at an elevation of 1300–1400 m.
Etymology. The new species is named after Garni, a locality where S. garnica sp. nov. was discovered for the first time.
Type material. Holotype, ♂, Armenia, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 15 August 2022, leg. J.R. Stonis, genitalia slide no. RA1106♂ (MfN). Paratype: 1 ♂, Armenia, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 14 August 2022, leg. J.R. Stonis, genitalia slide no. DV055♂ (MfN); 1 ♀, excluded from the type series, Armenia, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22 August 2022, leg. J.R. Stonis, genitalia slide no. RA1140♀ (MfN).
- The hybnerella group
- 12. Stigmella hybnerella (Hübner, 1796)
- Tinea hybnerella Hübner, 1796 [54] (pl. 34; figure 236).
Host plants. Crataegus spp., incl. C. laevigata (Poir.) DC., C. monogyna Jacq., C. ambigua C. A. Mey. ex A. K. Becker; also known to feed on Amelanchier spp., Cotoneaster spp. and Sorbus aria (L.) Crantz, S. torminalis (L.) Crantz. [18].
Distribution. This species is widespread in Europe and known from the western regions of Asia: the Caucasus (Azerbaijan) and the western part of Central Asia (Turkmenistan: Kopet Dag) [19]. Recently, this species has been proven to occur in Iran [26]. Here, on the basis of collected adult specimens, we provide S. hybnerella for Armenia (a new distribution record).
Material examined. The material examined was 2 ♂, 1 ♀, Armenia, Yerevan, Victory Park 1141 m, 40°11′47″ N, 44°31′17″ E, at light, 19 August 2022, leg. J.R. Stonis (MfN); 3 ♂, Yerevan, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 21 August 2022, leg. J.R. Stonis, genitalia slide no. RA1111 (MfN); 1 ♂, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 14 August 2022, leg. J.R. Stonis, genitalia slide no. DV054; 2 ♂, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 16 August 2022, leg. J.R. Stonis, genitalia slide nos DV043, DV053 (MfN); 2 ♂, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 28 August 2022, leg. J.R. Stonis, genitalia slide nos DV092, DV095 (MfN); 1 ♂, same locality, 22 August 2022, leg. J.R. Stonis (MfN); 2 ♂, 2 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26.vii–15 August 2022, J.R. Stonis, genitalia slide nos DV019♂, DV020♂ (MfN).
Remarks. For the species illustrations, we recommend the following publications: [18] (431, 492, 567), [19] (figures 338–340).
- 13. Stigmella inopinoides Dobrynina, sp. nov.
- https://zoobank.org/NomenclaturalActs/AFC3B5F3-796C-4F77-8A09-1FF0D062346D (accessed on 12 December 2023)
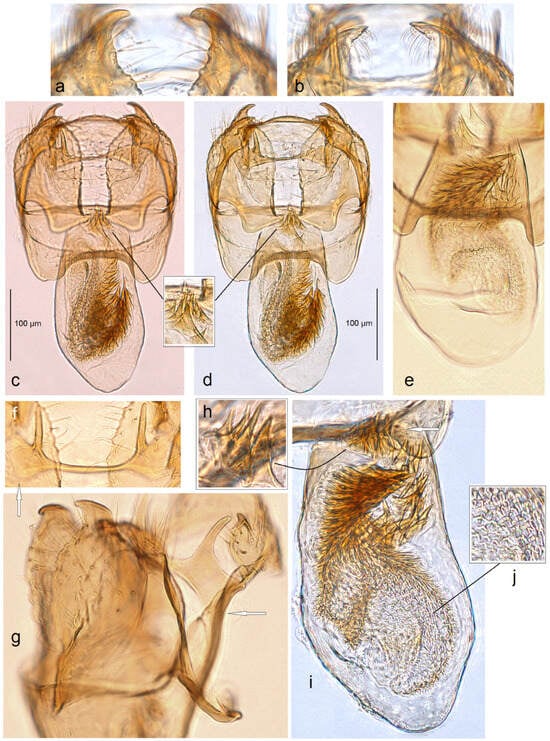 Figure 13. Male genitalia of Stigmella inopinoides Dobrynina, sp. nov.: (a,b) paratype, apical processes of valva, slide no. DV017 (MfN); (c,d) holotype, slide no. DV005 (MfN); (e) paratype, phallus, genitalia slide DV098 (MfN); (f) paratype, gnathos, slide DV037 (MfN); (g) paratype, lateral view of capsule, slide DV037 (MfN); (h–j) and paratype, details of phallus, slide DV017 (MfN) (the arrows point to diagnostic characters).
Figure 13. Male genitalia of Stigmella inopinoides Dobrynina, sp. nov.: (a,b) paratype, apical processes of valva, slide no. DV017 (MfN); (c,d) holotype, slide no. DV005 (MfN); (e) paratype, phallus, genitalia slide DV098 (MfN); (f) paratype, gnathos, slide DV037 (MfN); (g) paratype, lateral view of capsule, slide DV037 (MfN); (h–j) and paratype, details of phallus, slide DV017 (MfN) (the arrows point to diagnostic characters).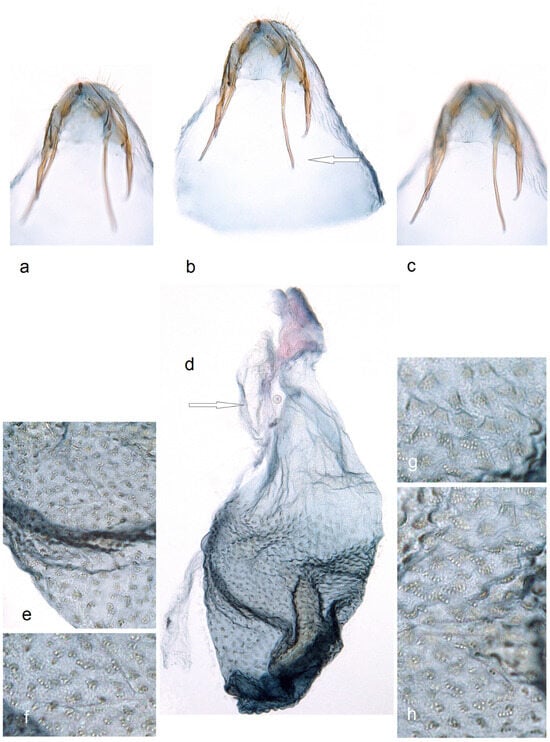 Figure 14. Female genitalia of Stigmella inopinoides Dobrynina, sp. nov., genitalia slide no. RA1132, excluded from the type series: (a–c) apophyses; (d) corpus bursae; and (e–h) pectinations on bursa (MfN) (the arrows point to the most important diagnostic characters).
Figure 14. Female genitalia of Stigmella inopinoides Dobrynina, sp. nov., genitalia slide no. RA1132, excluded from the type series: (a–c) apophyses; (d) corpus bursae; and (e–h) pectinations on bursa (MfN) (the arrows point to the most important diagnostic characters).
Diagnosis. Stigmella inopinoides sp. nov. belongs to the hybnerella species group and closely resembles S. inopinata A. Laštůvka & Z. Laštůvka (1990) [26]. Externally, the new species resembles S. inopinata but tends to be paler, grey, and strongly golden glossy. The male genitalia of S. inopinoides sp. nov. differ from those of S. inopinata in the distinct apical cluster of cornuti in the phallus, as well as the wider, trapezoid anterior excavation of the vinculum and significantly stronger developed apical processes of the valva. In the female genitalia, the new species is characterized by the absence of distal folds of the accessory sac (these folds are distinctive in S. inopinata) and significantly longer posterior apophyses (in S. inopinata, posterior and anterior apophyses are almost equal in length).
Description. Male. The forewing length is 1.9–2.3 mm; the wingspan is 4.3–5.0 mm (n = 23). Head. Palpi and frons grey; frontal tuft orange, medially and usually brown-grey; the collar is comprised white, almost piliform scales; the scape is glossy white; the antenna is slightly shorter than half the length of the forewing, with 29 segments; flagellum brownish grey with a slight golden gloss on the upper side and underside. Thorax. The tegula, thorax, and forewing are smoothly scaled and grey with a strong golden gloss; the fringe is grey with some golden gloss; the fringe line is absent; the forewing underside is dark grey with a slight purple iridescence, without spots or androconia. The hindwing and its fringe are grey to pale grey without androconia. The legs are glossy grey. Male genitalia. The genitalia capsule is 245–250 µm long and 200–225 µm wide. The tegumen is band-shaped. The uncus is wide, laterally, with small, slender lobes (in a permanent genitalia slide, they are often bent ventrally). Gnathos is U-shaped, with a well-separated caudal process and slender transverse bar. The valva is wide with an inwardly slightly curved and pointed apical process and an inwardly slightly curved and the pointed process of the valval inner lobe. The juxta is absent. The transtilla has large, widely rounded, lobe-like sublateral corners but no sublateral processes. The vinculum is short, with short but wide lateral lobes; the anterior excavation is trapezoid. The phallus is 235–265 µm long and 115–125 µm wide; the vesica with a large (usually curved) band of tiny and spine-like cornuti; apart from the band, there is always a distinctive apical cluster of spine-like cornuti.
Female. The forewing length is 2.1 mm; the wingspan is 4.8 mm (n = 1). Externally, they are similar to males (but excluded from the type series). Female genitalia. The ovipositor is short and rounded. Anterior apophyses are short; posterior apophyses are significantly longer. The accessory sac is short and only slightly folded, without distal folds. Ductus spermathecae has 2.5 coils and a relatively slender vesicle. Corpus bursae is wide and covered with distinctive comb-like pectinations in basal 2/3.
Bionomics. Adults fly in July and August. Otherwise, the species biology is unknown.
Distribution. This species is known from the following three localities in Armenia: Garni (Kotayk province), Yeghegis, and Noravank (Vayots Dzor Province), at an elevation of 1300–1550 m. Here, it was assumed that the earlier record of S. inopinata from Armenia [26], based on a few specimens collected by Friedrich Kasy, actually belonged to the new species, S. inopinoides sp. nov. However, these specimens collected in Armenia by Friedrich Kasy were unavailable for our study. It should also be mentioned that the Armenian specimens collected by Kasy were excluded from the type series by the authors during the description of the European S. inopinata [26].
Etymology. The new species is named after the European S. inopinata A. Laštůvka & Z. Laštůvka, a resembling species.
Type material. Holotype, ♂, Armenia, Kotayk Province, Garni, 40°07′18″ N, 44°45′17″ E, 1355 m, 23 July 2022, at light, leg. V. Dobrynina, genitalia slide no. DV005 (MfN); Paratypes (21 ♂): 1 ♂, the same label as holotype, genitalia slide no. DV003 (MfN);18 ♂, Kotayk Province, Garni, 40°07′18″ N, 44°45′17″ E and 40°07′27″ N, 44°45′40″ E, 1355–1371 m, 23 July–30 August 2022, at light, leg. J.R. Stonis, genitalia slide nos DV017, DV034♂, DV042♂, DV046♂*, DV048♂, DV049♂, DV050♂, DV061♂, DV083♂, DV085♂, DV098♂, DV103♂*, DV104♂, DV105♂, DV106♂, DV107♂, DV110♂(MfN); 2 ♂, Vayots Dzor Province, 1 km E Yeghegis, 39°52′10″ N, 45°22′12″ E, 1574 m, 26 July 2022, at light, leg. J.R. Stonis, genitalia slide nos DV028, DV037 (MfN); 1 ♂, Vayots Dzor Province, Noravank, 17 August 2022, 39°40′57″ N, 45°14′10″ E, 1468 m, at light, leg. J.R. Stonis, genitalia slide no. RA1125 (MfN) (* only genitalia preserved, without a pinned specimen).
Excluded from the type series. The following was excluded: 1 ♀, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 25 July 2022, leg. J.R. Stonis, genitalia slide no. RA1132♀ (NRG).
Remarks. Our molecular studies clearly show the uniqueness of this new species (DNA sequences yet unpublished).
- The ulmivora group
- 14. Stigmella viscerella (Stainton, 1853)
- Nepticula viscerella Stainton, 1853 [55] (p. 3958).
Host plants. Ulmus spp.
Distribution. Europe (except northern regions) from Portugal, Great Britain to Russia (central and southern European regions), Bulgaria, and Greece. Here, on the basis of documented leaf mines and a collected adult specimen, we provide S. viscerella for Armenia, the Caucasus (a new distribution record).
Material examined. The material examined was 1 ♂, Armenia, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 14 August 2022, leg. J.R. Stonis, genitalia slide no. DV068 (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (425, 486, 560), [19] (figures 157–160).
- 15. Stigmella magicis Stonis & Dobrynina, sp. nov.
- https://zoobank.org/NomenclaturalActs/CC58B6B5-3365-4383-94AF-B46424A01E4F (accessed on 12 December 2023)
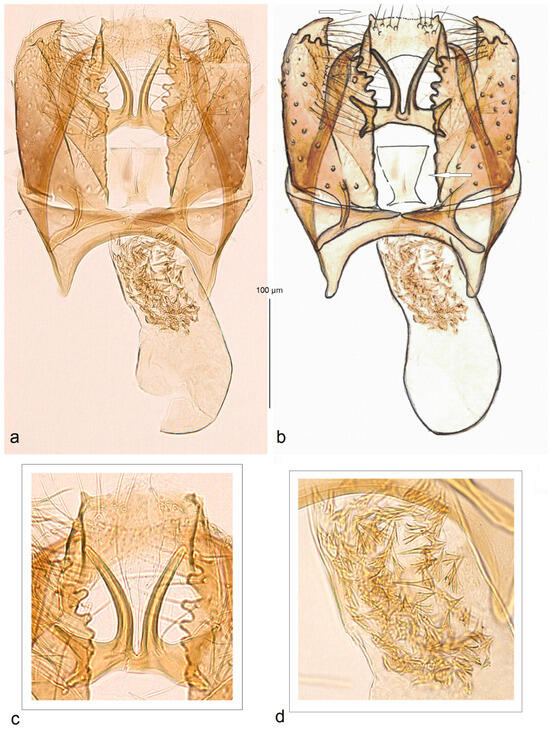 Figure 15. Male genitalia of Stigmella magicis Stonis & Dobrynina, sp. nov., holotype, genitalia slide DV069: (a,b) general view; (c) uncus and gnathos; (d) cornuti (MfN) (the arrow points to the juxta, an important diagnostic character).
Figure 15. Male genitalia of Stigmella magicis Stonis & Dobrynina, sp. nov., holotype, genitalia slide DV069: (a,b) general view; (c) uncus and gnathos; (d) cornuti (MfN) (the arrow points to the juxta, an important diagnostic character).
Diagnosis. Due to some similarities in the male genitalia, including the presence of plate-like juxta, Stigmella magicis sp. nov. was tentatively placed into the ulmivora species group. Externally, the new species can be confused with some other species, including unrelated ones, which also possess a silvery shiny fascia on the forewing. In the male genitalia, S. magicis sp. nov. is characterized by the unique combination of a rectangular uncus with tiny lateral processes, outwardly curved caudal processes and wide lateral arms of the gnathos, and a band of small spine-like cornuti in the phallus.
Description. Male. The forewing length is 1.7 mm; the wingspan is 3.8 mm (n = 1). Head. Palpi cream; the frontal tuft is yellowish cream; the collar and scape are glossy cream; the antenna is distinctly shorter than half the length of the forewing with 22 segments; the flagellum is glossy, pale brown. Thorax. The tegula, thorax, and basal part of the forewing are grey and golden glossy; the forewing has a wide, slivery-shiny postmedian fascia; the fringe is pale grey-brown to pale grey on tornus; the fringe line is absent; the forewing underside pale grey-brown without spots or androconia. The hindwing and its fringe are pale grey without androconia. The legs are brownish cream. Male genitalia. The genitalia capsule is 270 µm long. The tegumen is band-shaped. The uncus is wide and rectangular, with tiny lateral processes. The gnathos has two long and slender, outwardly curved caudal processes and wide lateral arms. The valva is wide, with a short but wide and distally pointed apical process. The juxta is lobe-like, trapezoid, and widens caudally. The transtilla has relatively long and slender sublateral processes; the transverse bar of the transtilla is interrupted in the middle. The vinculum is very short, with medium short, triangular, anteriorly pointed lateral lobes; the anterior excavation is oval-shaped. The phallus is 200 µm long, 70 µm wide medially, and 90 µm wide basally, and the vesica has a short but wide band of various spine-like cornuti.
Bionomics. Adults fly in August. Otherwise, their biology is unknown.
Distribution. This species is known to occur in a single locality in Armenia: Garni (Kotayk Province), at an elevation of 1350 m.
Etymology. The species name is derived from the Latin magicus (magic), in reference to the spectacular male genitalia with curved gnathos.
Type material. Holotype, 1 ♂, Armenia, Kotayk Province, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 16 August 2022, leg. J.R. Stonis, genitalia slide no. DV069 (MfN).
- The salicis group
- 16. Stigmella ararati Stonis, Dobrynina & Remeikis, sp. nov.
- https://zoobank.org/NomenclaturalActs/D8045370-C321-4903-84AD-FE229BC80491 (accessed on 12 December 2023)
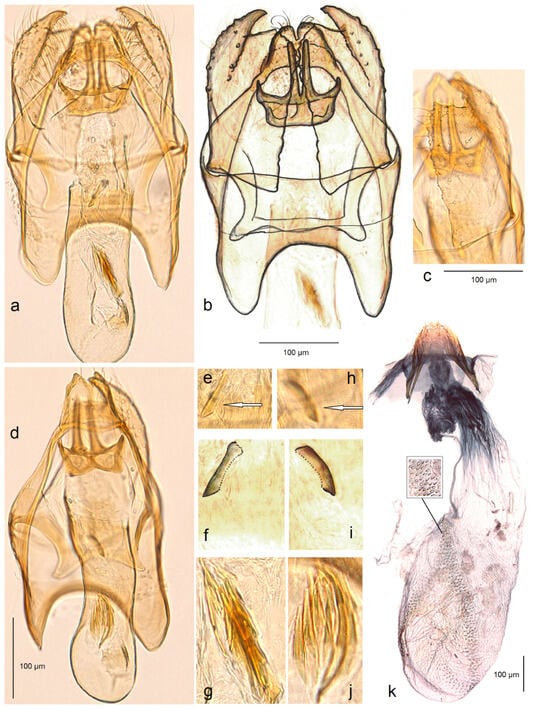 Figure 16. Genitalia of Stigmella ararati Stonis, Dobrynina & Remeikis, sp. nov.: (a,b) general view, holotype, male genitalia, slide no. DV078 (MfN); (c) valva, paratype, male genitalia slide no. DV076 (MfN); (d) general view of male genitalia with left valva missing, slide no. DV076 (MfN); (e–g) apical cornutus and cluster of cornuti, holotype; (h–j) the same, paratype, slide no. DV076 (MfN); and (k) female genitalia, slide no. RA1124 (MfN) (the arrows point to the diagnostic characters).
Figure 16. Genitalia of Stigmella ararati Stonis, Dobrynina & Remeikis, sp. nov.: (a,b) general view, holotype, male genitalia, slide no. DV078 (MfN); (c) valva, paratype, male genitalia slide no. DV076 (MfN); (d) general view of male genitalia with left valva missing, slide no. DV076 (MfN); (e–g) apical cornutus and cluster of cornuti, holotype; (h–j) the same, paratype, slide no. DV076 (MfN); and (k) female genitalia, slide no. RA1124 (MfN) (the arrows point to the diagnostic characters).
Diagnosis. Stigmella ararati sp. nov. belongs to the salicis species group. Externally, the new species is characterized by the glossy cream forewing rather irregularly and sparsely speckled with large blackish brown scales. The male genitalia of S. ararati sp. nov. differ from those of other members of the group, including the Central Asian S. johanssoni Puplesis & Diškus, in having a distinct, large apical cornutus, compact cluster of spine-like cornuti in the phallus and rectangular inner lobe of valva. The female genitalia can be confused with those of some other species of the salicis group.
Description. Male. The forewing length is 2.1–2.4 mm; the wingspan is 4.6–5.3 mm (n = 3). Head. Palpi cream; the frontal tuft comprises cream piliform scales on the frons and contrasting brownish grey piliform scales on the vertex; the collar is cream, comprised of lamellar scales; the scape is cream; antenna is distinctly shorter than half the length of the forewing with 24–25 segments; flagellum is glossy pale brown. Thorax. The tegula is glossy cream, speckled with dark brown scales; the thorax is glossy cream, where brown scales are absent or indistinctive; the forewing is glossy cream, rather irregularly and sparsely speckled with large blackish brown scales, except for an indistinct cream tornal spot abundant on the apex and basally along the costal margin; the fringe is cream to silvery cream; the fringe line is absent; the forewing underside is densely irrorated with small cream or ochre cream scales without spots or androconia. The hindwing and its fringe are ochreous cream, without androconia. The legs are ochreous cream to cream, sometimes covered with pale grey scales on the upper side. Male genitalia. The genitalia capsule is 340–360 µm long and 230 µm wide. The tegumen is band-shaped. The uncus is slender and long, with two triangular lateral lobes. The gnathos has two long, closely set caudal processes and a large central plate. The valva has a large, pointed apical process and a wide, distally angular inner lobe. The juxta is indistinctive and membranous. The transtilla has weakly individualized triangular sublateral processes. The vinculum has large, triangular lateral lobes. The phallus is 290–310 µm long; vesica has a distinct, large apical cornutus and a compact basal cluster of spine-like cornuti.
Female. The forewing length is 2.2 mm; the wingspan is 4.7 mm (n = 1). Externally, they are similar to the male. Female genitalia. The ovipositor is short and rounded. Anterior and posterior apophyses are short and almost equal in length. The accessory sac is small and heavily folded. Ductus spermathecae is very slender, probably without coils (or broken in slide no. RA1124). Corpus bursae is large and oval-shaped, with a band-like signa.
Bionomics. Adults fly in August. Otherwise, the species biology is unknown.
Distribution. This species is known from two localities in Armenia: Garni (Ararat Province) and Noravank (Vayots Dzor Province) at an elevation of 1370–1470 m.
Etymology. The new species is named after Mount Ararat, an iconic mountain of Armenia, viewed in present-day Turkey from the type locality in Garni, Ararat Province.
Type material. Holotype, 1 ♂, Armenia, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22 August 2022, leg. J.R. Stonis, genitalia slide no. DV078♂ (MfN). Paratypes: 2 ♂, same label data as holotype, genitalia slide no DV076♂ (MfN). Excluded from the type series: 1 ♀, Vayots Dzor Province, Noravank, 1468 m, 39°40′57″ N, 45°14′10″ E, at light, 17 August 2022, J.R. Stonis, genitalia slide no. RA1124♀ (MfN).
- The floslactella group
- 17. Stigmella carpinella (Heinemann, 1862)
- Nepticula carpinella Heinemann, 1862 [56] (p. 251).
Host plants. Carpinus betulus L., C. orientalis Mill.
Distribution. This species is widespread in Europe, from Great Britain, France, and Italy to Sweden, Russia (South European regions), and Greece. Recently, this species has been proven to occur in Iran [26]. Here, on the basis of collected adult specimens, we provide S. carpinella from the Caucasus, Armenia (a new distribution record).
Material examined. The material examined was 1 ♂, Armenia, Yerevan, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 13 August 2022, J.R. Stonis (MfN); 1 ♂, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 21 August 2022, J.R. Stonis, genitalia slide no. RA1112 (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (432, 494, 569), [19] (figures 344–346).
- The lemniscella group (= former marginicolella group)
- 18. Stigmella lemniscella (Zeller, 1839)
- Lyonetia lemniscella Zeller, 1839 [57] (p. 215).
Host plants. Ulmus spp.; U. pumila (= U. microphylla) in Armenia.
Distribution. This species is widespread in Europe, from Spain, Italy, and Ireland to Finland, Russia (central and southern European regions), Slovenia, and Bulgaria. Here, on the basis of documented leaf mines, we provide S. lemniscella from the Caucasus, Armenia (a new distribution record).
Material examined. Armenia, Gegharkunik Province, S of Tsovagyugh (north-eastern bank of Lake Sevan), 1909 m, 40°37′21″ N, 44°58′21″ E, numerous documented fresh but vacant leaf mines, 9 August 2022, leg. J.R. Stonis (BRG).
Remarks. For species illustrations, we recommend the following publications: [18] (437, 500, 575), [19] (figures 402–404).
- The hemargyrella group
- 19. Stigmella kuznetzovi Puplesis, 1994 [19] (pp. 152, 153).
- Host plant. Acer turcomanicum Pojark.
Distribution. Previously, the species was known only from the western part of Central Asia (Turkmenistan: Kopet Dag) [19]. Here, on the basis of collected and studied adult specimens, we provide S. kuznetzovi for the Caucasus, Armenia (a new distribution record).
Material examined. The materal examined was 2 ♂, Armenia, Kotayk Province, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 16 August 2022, leg. J.R. Stonis, genitalia slide nos DV045, DV052 (MfN); 1 ♂, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 28 August 2022, leg. J.R. Stonis, genitalia slide no. DV096 (MfN).
Remarks. For species illustrations, we recommend the following publication: [19] (figures 457–460).
- The ruficapitella group
- 20. Stigmella dorsiguttella (Johansson, 1971)
- Nepticula dorsiguttella Johansson, 1971 [58] (p. 251).
Host plants. Quercus spp.; Q. macranthera Fisch & C.A.Mey ex Hohen in Armenia.
Distribution. This species is distributed in Europe from Portugal, Spain and Italy to Sweden, Ukraine (Crimea), Greece, and Turkey. Here, on the basis of collected and studied adult specimens, we provide S. dorsiguttella for the Caucasus, Armenia (a new distribution record).
Material examined. The material examined was 5 ♂, 1 ♀, Armenia, Aragatsotn Province, 2 km N of Antarut, 1780 m, 40°21′50″ N, 44°16′25″ E, at light, 2 August 2022, leg. J.R. Stonis, genitalia slide no. RA1122♂ (MfN); 1 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 15 August 2022, leg. J.R. Stonis, genitalia slide RA1133 (MfN). Also, leaf mines recorded: Armenia, Vayots Dzor Province, Jermuk, 2106 m, 39°50′28″ N, 45°40′14″ E, 12 August 2022, leg. J.R. Stonis (BRG).
Remarks. For species illustrations, we recommend the following publication: [18] (446, 513, 586).
- 21. Stigmella roborella (Johansson, 1971)
- Nepticula roborella Johansson, 1971 [58] (p. 258).
Host plants. Quercus spp.; Q. macranthera Fisch & C.A. Mey ex Hohen in Armenia.
Distribution. This species is widespread in Europe from Spain, Italy, Great Britain to Norway, Finland, Russia (western and central European regions), Greece, and Turkey. S. roborella also occurs in the Caucasus: Georgia and Armenia (the latter is a new distribution record).
Material examined. The material examined was 3 ♂, 1 ♀, Armenia, Yerevan, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 13–23 August 2022, leg. J.R. Stonis, genitalia slide nos DV115♂, RA1114♂ (MfN); 1 ♂, 6 ♀, Aragatsotn Province, 2 km N of Antarut, 1780 m, at light, 40°21′50″ N, 44°16′25″ E, 2 August 2022, leg. J.R. Stonis (MfN); 1 ♂, Aragatsotn Province, 4 km N Antarut, 1985 m, 40°22′36″ N, 44°16′03″ E, 12 August 2022, leg. J.R. Stonis (MfN). Also, leaf mines recorded: Vayots Dzor Province, Jermuk, 2106 m, 39°50′28″ N, 45°40′14″ E, 12 August 2022, leg. J.R. Stonis (BRG).
Remarks. For species illustrations, we recommend the following publications: [18] (448, 515, 588), [19] (figures 477–479).
- 22. Stigmella basiguttella (Heinemann, 1862)
- Nepticula basiguttella Heinemann, 1862 [56] (pp. 258, 259).
Host plants. Castanea sativa Mill. and Quercus spp.; Q. macranthera Fisch & C.A. Mey ex Hohen in Armenia.
Distribution. This species is widespread in Europe from Portugal, Spain, and Italy to Great Britain, Norway, Finland, Russia (central and southern European regions), Bulgaria, and Greece. S. basiguttella also occurs in the Caucasus from western Georgia to southeastern Azerbaijan [19] and in Iran [26]. Here, on the basis of a collected adult specimen, we provide S. basiguttella for Armenia (a new distribution record).
Material examined. 1 ♂, ARMENIA, Aragatsotn Province, 2 km N of Antarut, 1780 m, at light, 40°21′50″ N, 44°16′25″ E, 2 August 2022, leg. J.R. Stonis (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (445, 511, 584), [19] (figures 480–482).
- Genus Bohemannia Stainton, 1859
- The pulverosella group
- 23. Bohemannia pulverosella (Stainton, 1849)
- Trifurcula pulverosella Stainton, 1849 [59] (p. 30).
- Bohemannia piotra Puplesis, 1984 [60] (pp. 149–164).
Host plants. Malus domestica Borkh., M. sylvestris Mill., M. mandshurica (Maxim.) Kom.
Distribution. This species is distributed in Europe from Ireland and Great Britain to Finland, Russia (up to the Ural region), Austria, Hungary, Romania, Croatia, Slovenia and Bulgaria. B. pulverosella also was recorded to occur in continental East Asia (Russia: Primorskiy Kray) [60]. Here, on the basis of examined leaf mines, we provide B. pulverosella for Armenia (a new distribution record).
Material examined. Armenia, Gegharkunik Province, N of Sevan, 1909 m, 40°37′21″ N, 44°58′21″ E, fresh but vacant leaf mines, 9 August 2022, leg. J.R. Stonis.
Remarks. For species illustrations, we recommend the following publication: [18] (453,526,594).
- Genus Ectoedemia Busck, 1907
- Subgenus Zimmermannia Hering, 1940
- 24. Ectoedemia (Zimmermannia) longicaudella Klimesch, 1953 [61] (pp. 193, 194).
Host plants. Quercus petraea (Matt.) Liebl., Q. robur L., Q. macranthera Fisch & C.A. Mey ex Hohen, a native host plant in Armenia, also Castanea sativa Mill. [18,62].
Distribution. This species is distributed predominantly in Europe from Portugal, Spain, and Italy to Belgium, Sweden, Finland, Russia (central European regions), and Greece. The species was also recorded from northeastern Turkey [62] and mentioned from the western Caucasus (Georgia). Here, on the basis of collected adults, we provide E. (Z.) longicaudella for Armenia (a new distribution record).
Material examined. The material examined was 1 ♂, SPAIN, Noguera Teruel, 1600 m, 9–10 August 1989, leg. C. Gielis, genitalia slide no. RA1143 (MfN); 14 ♂, UKRAINE, Crimea, 20 km W Feodosia, Kara-Dag, 2–16 July 1987, leg. R. Puplesis, genitalia slide no. RA1146 (MfN); 1 ♂, UKRAINE, Crimea, 20 km SW Feodosiya, Karadag Reserve, 26 July 2011, at light, leg. J.R. Stonis, A. Navickaitė, G. Varačinskas, genitalia slide no. RA1145 (BRG); 1 ♂, same label (MfN); 1 ♂, ARMENIA, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 15 August 2022, leg. J.R. Stonis, genitalia slide no. RA1105 (MfN); 1 ♂, 3 ♀, Aragatsotn Province, 2 km N of Antarut, 1780 m, at light, 40°21′50″ N, 44°16′25″ E, 2 August 2022, leg. J.R. Stonis, genitalia slide no. RA1144♀ (MfN); 9 ♂, 2 ♀, 4 km N Antarut, 1985 m, 40°22′36″ N, 44°16′03″ E, 12 August 2022, leg. J.R. Stonis, RA1119♂, RA1120♂, RA1123♂ (MfN); 1♂, same label, genitalia slide no. RA1147♂ (BRG).
Remarks. See Section 3.2.3 for the genetic distinctness of E. (Z.) longicaudella from Armenia.
- Subgenus Ectoedemia Busck, 1907
- The rubivora complex
- 25. Ectoedemia mahalebella (Klimesch, 1936)
- Nepticula mahalebella Klimesch, 1936 [63] (pp. 207, 208).
Host plants. Amygdalus nana L., Cerasus avium (L.) Moench, C. fruticosa Pall., C. mahaleb (L.) Mill. and Prunus spp.
Distribution. This species is widespread in Europe, from France and Italy to Slovakia, Ukraine (Crimea), Bulgaria, and Greece. Here, on the basis of collected adults, we provide E. mahalebella from the Caucasus, Armenia (a new distribution record).
Material examined. The material examined was 2 ♂, 2 ♀, Armenia, Kotayk Province, Garni, 1312–1355 m, 40°07′18″ N, 44°45′17″ E–40°06′41″ N, 44°43′55″ E, at light, 14–23 July 2022, leg. J.R. Stonis, genitalia slide nos DV014♂, DV030♂, RA1136♀ (MfN); 1 ♂, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 25 July 2022, leg. V. Dobrynina, genitalia slide no. DV023 (MfN); 2 ♂, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22 August 2022, leg. J.R. Stonis, genitalia slide nos DV079, DV108 (MfN); 1 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26 July 2022, leg. J.R. Stonis (MfN).
- Genus Fomoria Beirne, 1945
- The weaveri group
- 26. Fomoria septembrella (Stainton, 1849)
- Nepticula septembrella Stainton, 1849 [59] (p. 29).
Host plants. Hypericum spp.
Distribution. This species is widespread in Europe, from Portugal, Spain, and Ireland to Norway, Finland, Russia (western and central European regions), Croatia, and Greece. F. septembrella also is known in the Caucasus (Azerbaijan and Georgia) [19], Iran [26], and the western part of Central Asia (Turkmenistan: Kope Dag) [19]. Here, on the basis of the collected adult, we provide F. septembrella for Armenia (a new distribution record).
Material examined. The material examined was 1 ♂, Armenia, Yerevan, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 13 August 2022, leg. J.R. Stonis, genitalia slide RA1130 (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (460, 533, 601), [19] (figures 670–672).
- Genus Trifurcula Zeller, 1848
- The subnitidella group
- 27. Trifurcula subnitidella (Duponchel, 1843)
- Elachista subnitidella Duponchel, 1843 [64] (p. 326, pl. 77, figure 8).
Host plants. Lotus corniculatus L., L. pedunculatus Cav.
Distribution. This species is distributed in Europe from Portugal, Spain, and Italy to Ireland, Sweden, Finland, Russia (up to the Ural region), Bulgaria, and Greece. The species was also recorded in North Africa (Tunisia). Here, on the basis of collected adults, we provide T. subnitidella from the Caucasus, Armenia (a new distribution record).
Material examined. The material examined was 2 ♂, Armenia, Yerevan, Jrvezh Forest Park, 1514 m, 40°11′06″ N, 44°37′11″ E, at light, 11–13 August 2022, leg. J.R. Stonis, genitalia slide no. DV111 (MfN); 1 ♂, Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E, at light, 23 August 2022, leg. J.R. Stonis, genitalia slide no. DV121 (MfN).
Remarks. For species illustrations, we recommend the following publications: [18] (453, 525, 594) and [19] (figures 742–744).
- The cryptella group
- 28. Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.
- https://zoobank.org/NomenclaturalActs/B5B2DC80-167C-4BD2-BE7E-33B4FE7A5608 (accessed on 12 December 2023)
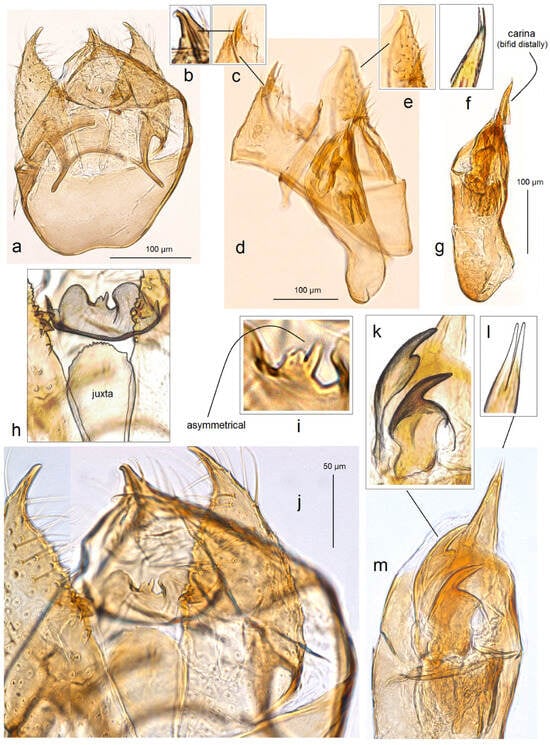 Figure 17. Male genitalia of Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.: (a,b) holotype, ventral view of the capsule with phallus removed, slide no. DV021 (MfN); (c–e) paratype, lateral view of the capsule with phallus removed, slide no. DV118 (MfN); (f,g) phallus, holotype; and (h–m) details of the capsule and phallus of holotype.
Figure 17. Male genitalia of Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.: (a,b) holotype, ventral view of the capsule with phallus removed, slide no. DV021 (MfN); (c–e) paratype, lateral view of the capsule with phallus removed, slide no. DV118 (MfN); (f,g) phallus, holotype; and (h–m) details of the capsule and phallus of holotype.
Diagnosis. Trifurcula vardenisi sp. nov. belongs to the cryptella species group. Externally, the new species can be confused with some other Nepticulidae species possessing an irrorated forewing. The male genitalia of T. vardenisi sp. nov. differ from those of all other members of this group by the unique, asymmetrical gnathos and the specific pair of large apical cornuti of the phallus.
Description. Male. The forewing length is 2.6 mm; the wingspan is 5.7 mm (n = 2). Head. The palpi is cream; the frontal tuft is pale orange to orangish ochre; the collar is distinctive, comprised orangish cream piliform scales; the scape is cream, distally speckled with dark grey scales; the antenna is only slightly longer than half the length of forewing with 31–33 segments; the flagellum is grey on the upper side and underside. Thorax. The tegula, thorax, and forewing is densely irrorated with dark grey-brown or dark grey scales; sometimes, the forewing has a few milky white scales in the apical part; the fringe is greyish white to pale grey; the fringe line is indistinctive or absent; the forewing underside is dark grey-brown, without spots or androconia. The hindwing and its fringe are grey without androconia. The legs are grey and brownish cream distally. Male genitalia. The genitalia capsule is 330–340 µm long and 260 µm wide. The tegumen is not extended into a pseuduncus but is short. The uncus is deeply split longitudinally. The gnathos is asymmetrical, with lateral lobes and a short asymmetrical process medially. The valva is wide, tapering distally, with a slender apical process. The juxta is lobe-like, widened caudally, with serrated caudal margin. The transtilla has slender and relatively long sublateral processes and a slender transverse bar. The vinculum is large, widely rounded, or truncated distally, without lateral lobes. The phallus is 330 µm long, 75–102 µm wide medially, and 95–100 µm wide basally, with long, pointed carina and a pair of large, curved apical cornuti, each possessing a very wide, rounded base.
Female. Unknown.
Bionomics. Adults fly in July and August. Otherwise, this species biology is unknown.
Distribution. This species was discovered in two localities of Armenia: Yeghegis (Vayots Dzor Province) and Jrvezh Forest Park (Yerevan), at an elevation of 1515–1570 m.
Etymology. Trifurcula vardenisi sp. nov. is named after the Vardenis Range (the type locality of this new species, Yeghegis, is situated on the southern slope of this mountain range).
Type material. Holotype, 1 ♂, Armenia, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26 July 2022, leg. J.R. Stonis, genitalia slide no. DV021 (MfN). Paratype: 1 ♂, Yerevan, Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E, at light, 11 August 2022, leg. J.R. Stonis, genitalia slide no. DV118 (MfN).
- Genus Glaucolepis Braun, 1917
- The raikhonae group
- 29. Glaucolepis melanoptera (van Nieukerken & Puplesis, 1991)
- Trifurcula (Glaucolepis) melanoptera van Nieukerken & Puplesis, 1991 [25] (pp. 205–208).
Host plant. Unknown, probably Prunus spp. [25].
Distribution. This species is found in Spain, France, Italy (incl. Sardinia, Sicilia), Ukraine (Crimea), Slovakia, Czech Rep., Austria, Hungary, Croatia, Greece, Armenia, and Turkmenistan [25].
Material examined. The material examined was 5 ♂, Armenia, Yerevan, Jrvezh Forest Park, 1515 m, 40°11′07″ N, 44°37′11″ E, at light, 11–23 August 2022, leg. J.R. Stonis, genitalia slide nos DV113, DV116, DV117 (no pinned specimen, only genitalia), DV120, RA1113 (MfN); 6 ♂, 4 ♀, Kotayk Province, Garni, 1355 m, 40°07′18″ N, 44°45′17″ E, at light, 23 July–16 August 2022, leg. V. Dobrynina and J.R. Stonis, genitalia slide nos DV004♂, DV011♂, DV015♂, DV025, DV047♂, RA1138♀ (MfN); 2 ♂, 1 ♀, Vayots Dzor Province, 1 km E Yeghegis, 1574 m, 39°52′10″ N, 45°22′12″ E, at light, 26.vii–15 August 2022, leg. V. Dobrynina and J.R. Stonis, genitalia slide no. DV029♂ (MfN); 2 ♂, 1 ♀, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22–30 August 2022, leg. J.R. Stonis, genitalia slide nos DV087♂, DV094♂ (MfN); 3 ♂, 1 ♀, Noravank, 1468 m, 39°40′57″ N, 45°14′10″ E, at light, 17 August 2022, leg. J.R. Stonis, genitalia slide no RA1102♂, RA1141♀ (MfN); 2 ♂, Aragatsotn Province, 2 km N of Antarut, 1780 m, at light, 40°21′50″ N, 44°16′25″ E, 2 August 2022, leg. J.R. Stonis (MfN).
Remarks. For species illustrations, we recommend the following publications: [19] (figures 730–734) and [25].
- 30. Glaucolepis hamirella (Chrétien, 1915)
- Nepticula hamirella Chrétien, 1915 [65] (pp. 364, 365).
Host plants. Calamintha spp., Clinopodium calamintha (L.) Stace and Micromeria spp.
Distribution. This species was originally described from Algeria [65] (see Remarks). Here, on the basis of the collected adults, we provide G. hamirella for the Caucasus, Armenia (a new distribution record).
Material examined. The material examined was 2 ♂, Armenia, Kotayk Province, Garni, 1312 m, 40°06′41″ N, 44°43′55″ E, at light, 24 July 2022, leg. V. Dobrynina & J.R. Stonis, genitalia slide nos DV032, DV033 (MfN); 1 ♂, Ararat Province, E of Garni, 1371 m, 40°07′27″ N, 44°45′40″ E, at light, 22 August 2022, leg. J.R. Stonis, genitalia slide no. DV082 (MfN).
Remarks. Recently, the taxonomic status of Glaucolepis saturejae (Parenti), a former synonym of G. hamirella, was restored [52]. However, our Armenian specimens ideally match the North African G. hamirella, not the restored European G. saturejae.
- Genus Etainia Beirne, 1945
- The sericopeza group
- 31. Etainia caucasi Remeikis, sp. nov.
- https://zoobank.org/NomenclaturalActs/F4B3ECA8-0206-4087-A3BE-8453966ADC18 (accessed on 12 December 2023)
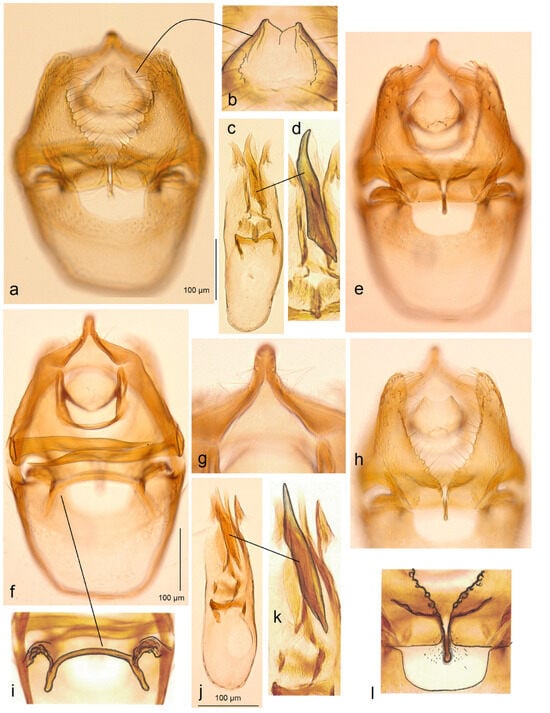 Figure 18. Male genitalia of Etainia caucasi Remeikis, sp. nov.: (a,b) paratype, slide no. RA1115, capsule with phallus removed; (c,d) same, phallus; and (e–l) holotype, slide no. RA1116, details of capsule and phallus (MfN).
Figure 18. Male genitalia of Etainia caucasi Remeikis, sp. nov.: (a,b) paratype, slide no. RA1115, capsule with phallus removed; (c,d) same, phallus; and (e–l) holotype, slide no. RA1116, details of capsule and phallus (MfN).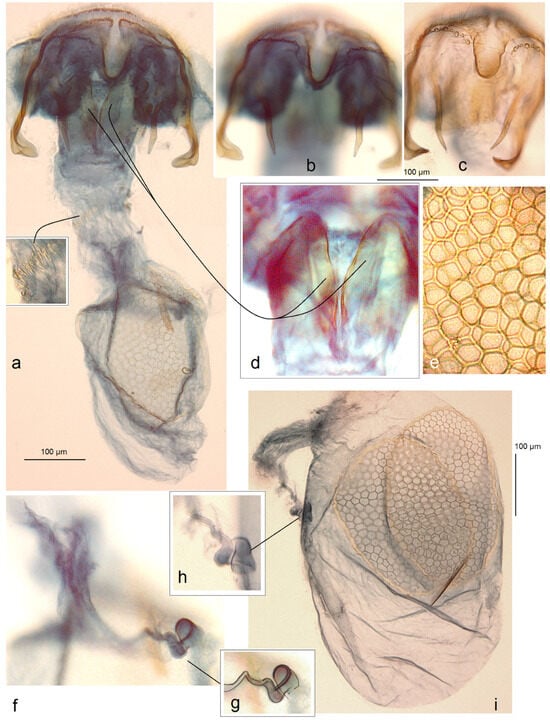 Figure 19. Female genitalia of Etainia caucasi Remeikis, sp. nov., paratypes (MfN): (a) slide no. RA1117, general view; (b) the same, ovipositor and apophyses; (c), the same, slide no. RA1118; (d) slide no. RA1117, lobe-like sclerites; (e) the same, details of signum; (f,g) the same, ductus spermathecae; and (h,i) slide no. RA1118, corpus bursae and ductus spermathecae.
Figure 19. Female genitalia of Etainia caucasi Remeikis, sp. nov., paratypes (MfN): (a) slide no. RA1117, general view; (b) the same, ovipositor and apophyses; (c), the same, slide no. RA1118; (d) slide no. RA1117, lobe-like sclerites; (e) the same, details of signum; (f,g) the same, ductus spermathecae; and (h,i) slide no. RA1118, corpus bursae and ductus spermathecae.
Diagnosis. Etainia caucasi sp. nov. belongs to the sericopeza species group and closely resembles the European E. louisella (Sircom, 1849). However, this new species differs from E. louisella in the caudally truncated or bilobed gnathos possessing a distinctive basal elaboration, as well as in the strongly chitinized, clearly visible basal connection of the valvae ventrally, the longer apical cornutus (or carina) of the phallus, the deep, rectangular posterior excavation of the vinculum in the male genitalia, and the distally strongly bent anterior apophyses, the shape of vaginal sclerites, and details of ovipositor morphology in the female genitalia. Externally, males of E. caucasi sp. nov. are charcterized by a large black androconial patch of the forewing underside, with a few pale-brown scales in the middle surrounded by cream scales and a spot of black androconia on the hindwing’s upper side.
Description. Male. The forewing length is 2.5–2.9 mm; the wingspan is 5.6–6.6 mm (n = 2). Head. The palpi are cream; the frontal tuft is orangish ochre, with some blackish brown piliform scales; the collar is indistinctive, comprised pale ochre-brown or pale ochre piliform scales; the scape is cream to yellow cream; antenna is slightly longer than half the length of forewing with 46 segments; the flagellum is ochre-brown to pale ochre with some dark grey scales. Thorax. The tegula is fuscous, distally yellowish cream; the thorax is densely covered with blackish-grey scales; the forewing is blackish grey-brown, with a distinctive yellowish cream basal spot, wide median fascia, and two postmedian spots on the costal margin and tornus; the fringe is blackish grey and apically pale grey to almost white; the fringe line is absent; the forewing underside is grey or grey-brown, with a large black androconial patch with a few pale brown scales in the middle surrounded by cream scales. The hindwing is grey, with a spot of black androconia basally; the fringe is grey. The legs are brownish cream on the upper side with some brown scales or entirely covered with blackish-grey scales. Male genitalia. The genitalia capsule is 490 µm long and 315–340 µm wide. The uncus is fused with the pseuduncus, which is slender and medium long. Gnathos has a wide, distally truncated, or bifid (or variously split) caudal plate and distinctly elaborated base. The valva is wide, gradually narrowing towards the apex, apically rounded, without apical process; the basal connection between the valvae is strongly chitinized and distinctive; valval basal apodemes are very large. The juxta is absent. Transtilla has relatively long sublateral processes and a long, slender transverse bar. The vinculum is large, anteriorly rounded, or truncated, without lateral lobes, posteriorly (caudally) with a distinctive rectangular excavation. The phallus is 140–160 µm long, distinctive, with well-chitinized lateral carinae and very long, well-developed median carina (or cornutus).
Female. The forewing length is 3.2–3.3 mm; the wingspan is 6.9–7.2 mm (n = 3). Externally, they are similar to males but without androconial spots. Female genitalia. 785 µm long. The ovipositor is very short and widely rounded. Posterior apophyses are slightly longer than anterior apophyses and curved inwardly. The accessory sac is indistinctive or absent. Ductus spermathecae is short but wide, with 1.5 coils and a relatively large vesicle. Corpus bursae is relatively small and oval-shaped with two large signa reticulata, which are almost equal in size (300 and 265 µm long) and occupy most of the 534 µm long bulbous part of the corpus bursae.
Bionomics. Adults fly in July and August. Otherwise, this species biology is unknown.
Distribution. In Armenia, this species is known from a single locality: Yerevan (Victory Park), at an elevation of 1150 m. Here, we also assume that former records of Etainia louisella from Georgia (Lagodekhi Reserve) and the Crimea (Karadag Reserve, Krasnolesye and Mys Martyan) [19] actually belong not to E. louisella but E. caucasi sp. nov; our previous study showed that the males of these specimens possess a truncated or split gnathos; however, the collection series of these peculiar specimens were not re-examined during the current study.
Etymology. The new species is named after the Caucasus, which is the region where the new species occurs.
Type material. Holotype, ♂, ARMENIA, Yerevan, Victory Park, 1141 m, 40°11′41″ N, 44°31′12″ E, at light, 1 August 2022, leg. J.R. Stonis, genitalia slide no. RA1116♂ (MfN). Paratypes: 1 ♀, same label data as holotype, genitalia slide no. RA1117♀ (MfN); 1 ♂, Victory Park, 1142 m, 40°11′41″ N, 44°31′25″ E, at light, 29 July 2022, leg. J.R. Stonis, genitalia slide no. RA1115♂ (MfN); 1 ♀, Victory Park 1141 m, 40°11′47″ N, 44°31′17″ E, at light, 19 August 2022, leg. J.R. Stonis (MfN).
3.2. Molecular Data vs. Morphology
3.2.1. Ectoedemia mahalebella (Klimesch) vs. E. spinosella (Joannis)
Although these two Ectoedemia species can be relatively easily differentiated by the either contorted or sinuous initial gallery of the leaf mine and by the size of the signa in the corpus bursae of the females, they can still be confused if the identification is based solely on the morphology of the male genitalia with adults having rubbed scaling. Considering intraspecific and geographic variability, the male genitalia of these species are indistinguishable. As a result, at the beginning of our research, all collected and subsequently dissected Ectoedemia males with whitish forewing fascia were provisionally identified as E. spinosella (Joannis, 1908). This seemed quite logical as E. spinosella is not only widespread in Europe but is also found abundantly in an area near the Caucasus, specifically on the other side of the Caspian Sea in the Kopet Dag mountains in western Turkmenistan, as well as in Tajikistan [66]. However, a comparison of our new molecular sequences from specimens collected in Armenia with new sequences obtained from specimens of E. mahalebella (Klimesch) collected in Bulgaria and E. spinosella from Croatia and the sequences of both species downloaded from the BOLD database (Figure 20a), clearly confirmed that instead of the expected E. spinosella, E. mahalebella (Figure 20b–d) was detected in Armenia. Moreover, during our mitotype analysis, we found that the sequences of E. spinosella from western Turkmenistan were not identical to the sequences of E. spinosella from Europe but differed in many hypothetical mutational steps (see Figure 20a). It should be mentioned that E. spinosella occurring in western Turkmenistan was initially described as a separate species, E. albiformae Puplesis & Diškus, 2003 [20], but later synonymized with the European E. spinosella [67]. This synonymization has already been recently confirmed without considering molecular data [66]. According to our mitotype network, if E. spinosella from Turkmenistan is not so “typical″ E. spinosella, then it is certainly not E. mahalebella, which is now discovered abundantly in the adjacent Caucasus (Armenia).
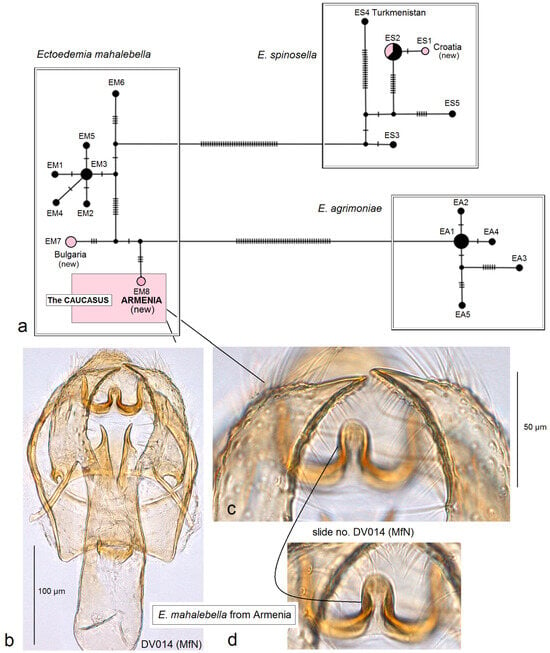
Figure 20.
Ectoedemia mahalebella (Klimesch): (a) the mitotype network of 658 bp long mtDNA CO1-5′ sequences constructed by the Minimum Spanning Network algorithm. It includes six newly analysed (pink color) and twenty-four sequences of E. agrimoniae downloaded from BOLD (black color) (EA1 (LEFIE688-10, LEFIE689-10, LEFIF352-10, LEFIJ1492-12, NEPT153-10 all Finland), EA2 (NEPT022-10 Belgium), EA3 (NEPT077-10 Italy), EA4 (NEPTA051-13 Greece), EA5 (NEPTA052-13 Romania)), E. mahalebella (EM1 (NEPT046-10 France), EM2 (NEPT047-10 France), EM3 (NEPT076-10, NEPT219-10 both Italy; NEPT128-10 France), EM4 (NEPT191-10 Croatia), EM5 (NEPTA1048-15 Switzerland), EM6 (NEPTA146-13 Greece), EM7 (OR793222, OR793223 both Bulgaria, new), EM8 (OR793224 Armenia, new)), and E. spinosella (ES1 (OR793219 Croatia, new), ES2 (OR793220, OR793221 both Croatia, new; NEPT016-10 Netherlands; NEPT070-10, NEPT071-10 both Italy; NEPT111-10 France), ES3 (NEPT140-10 Greece), ES4 (NEPTA1460-15 Turkmenistan), and ES5 (LEASW1368-20 Greece)). The size of each circle is proportional to the mitotype frequency. The unnamed small circles represent mitotypes not sampled in the study but which were predicted to be. Dashes on the lines connecting mitotypes indicate hypothesized mutational steps; (b–d) male genitalia of E. mahalabella from Armenia.
3.2.2. A Study Case of Stigmella muricatella (Klimesch)
During our research, S. muricatella (Klimesch) was found to be incomparably abundant among the samples of all other discovered species in Armenia. The adults of this species were attracted to light traps numerously wherever these light traps were set. However, the most abundant of the Nepticulidae species of Armenia is still a taxonomic puzzle. Knowing that a resembling species, S. rolandi van Nieukerken, is widespread in southern Europe and common in geographically nearby Crimea while also possibly occurring in the Tyan Shan Mts [19], it was expected that our Armenian samples represent S. rolandi,a morphologically similar species. We barcoded two specimens from Armenia (Figure 21c) to compare their sequences with those of S. rolandi and S. muricatella. Hitherto, the only useful barcoded specimen of S. muricatella available for comparison was a female from Greece [68]. The molecular sequences of our Armenian specimens and the female from Greece were not identical, but relatively close, and clearly distinct due to their numerous hypothetical mutational steps from S. rolandi. In a photo of the S. muricatella male genitalia mount of the paratype, which was kindly sent by Erik J. van Nieukerken (Figure 21a,b), it can be seen that the cornuti are very numerous and rather equal sizewise. In comparison, all our Armenian specimens (nearly 60 genitalia slides) possess less and clearly differentiated cornuti: some are very tiny, but others are significantly (or slightly) larger and triangular. Meanwhile, the shapes of the uncus, gnathos, transtilla, and vinculum seem to be variable among our Armenian specimens. Nevertheless, the cornuti of the samples from Armenia are always of the same pattern: they are differentiated sizewise. We also newly checked specimens of the so-called S. muricatella from Tajikistan (BRG/MfN) (documented by Puplesis in 1994 [19]) and found that they possess even less cornuti and a more golden creamy forewing speckled with dark brown scales (the Armenian specimens are rather metallic grey with black scales). Therefore, it can be concluded that S. muricatella is probably a widespread and variable species. Despite the discovered presence of numerous, but not differentiated cornuti in the paratype of S. muricatella, we still treated our Armenian specimens (along with the barcoded female from Greece) as S. muricatella. Otherwise, along with the species “A”, i.e., the “real” S. muricatella by Klimesch, it is necessary to name a separate species “B” from Armenia (including the barcoded specimen from Greece), and one more species “C” on the basis of the specimens from Tajikistan. In this case, S. muricatella and its new “satellites” from Asia can be seen as a bunch of young, closely similar, and hardly differentiated species, like in the known case of the S. oxyacanthella group. From a practical point of view, this is undesirable because of the difficult diagnostics of the species. Therefore, although S. muricatella still remains a puzzle, for now, we followed the first scenario.
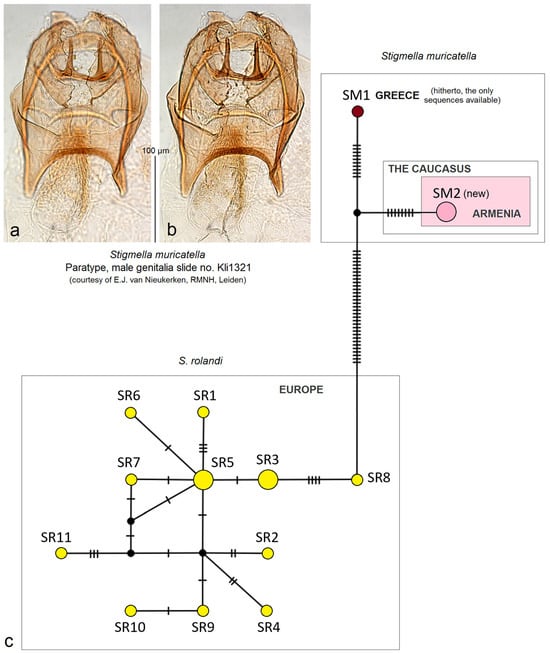
Figure 21.
Stigmella muricatella (Klimesch): (a,b) first photographic documentation of male genitalia, slide no. Kli1321, paratype (courtesy of Erik J.van Nieukerken, RMNH); (c) the mitotype network of 658 bp long mtDNA CO1-5′ sequences constructed using the TCS Network algorithm. It includes three newly analysed (pink color) and sixteen (other colors) sequences of S. muricatella downloaded from BOLD (SM1 (NEPTA594-13 Greece), SM2 (OR793225, OR793226, OR793227 all Armenia, new)) and S. rolandi (SR1 (DEEUR1012-16 Austria), SR2 (DEEUR1024-16 Austria), SR3 (LEATE760-13, LEATH369-14, NEPTA694-13 all Italy), SR4 (LON7167-18 Croatia), SR5 (LON7168-18, NLON450-19 both Croatia; NEPTA692-13 Greece), SR6 (NEPTA1269-15 Croatia), SR7 (NEPTA693-13 Greece), SR8 (NLON443-19 Croatia), SR9 (NLON444-19 Croatia), SR10 (PHLAI086-12 Romania), SR11 (PHLAI091-12 Hungary)) species. For other explanatory notes, see Figure 20.
3.2.3. Designation of the Stigmella rolandi Species Group
The examined Stigmella muricatella belonged to the former S. sanguisorbae species group, an informal group, not a taxonomic unit. In our constructed NJ tree (Figure 22a), the newly obtained molecular sequences of S. muricatella stood “nicely” among the species of the former S. sanguisorbae group as a distinctly separate but sister clade with S. rolandi. However, in this analysis, we also found that despite a very similar morphology, some other species of the former S. sanguisorbae group, S. thuringiaca (Petry) and S. sanguisorbae (Wocke), always took a separate position from the remaining species of the former S. sanguisorbae species group; in all our trees, these species appeared to be a sister clade with the former S. anomalella group. Such a similarity of the analysed molecular sequences between some species of the former sanguisorbae and anomalella groups are confirmed by the similarity of morphological and biological characters. Therefore, it is clear that these three species groups (including the former S. rhamnella group) belong to a common cluster of groups (Figure 22b). Both genital structures of the male and especially differences in the morphology of the female genitalia in the species of this cluster are so minimal, and sometimes overlapping, that it is possible to combine either all three groups into one group, the S. anomalella group (sensu lato), or to merge at least two groups, using the most similar S. sanguisorbae group to the S. rhamnella group, but to leave the S. anomalella group (designated by R. Johansson in 1971 [58]) as a separate but close informal unit. Here, we decided to merge all three groups into one group and, avoiding terminological confusion, named it the S. rolandi group (designated by J.R. Stonis and A. Diškus).
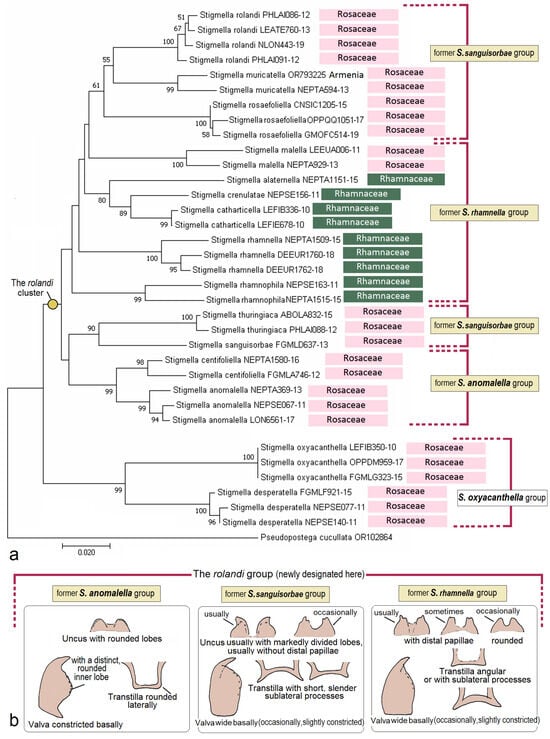
Figure 22.
Stigmella muricatella (Klimesch) and the newly designated S. rolandi group: (a) the NJ tree based on 658 bp long mtDNA CO1-5′ sequences. The analysis involved one new mitotype from Armenia and thirty-three mitotypes downloaded from the BOLD database. The divergence was calculated using the Tamura 3-parameter model and a gamma distribution; (b) pictorial diagnosis of the former species groups of the S. rolandi cluster based on the male genitalia characters.
3.2.4. Cryptic Taxa
During our research in Armenia on the naturally growing host plant Cotinus coggygria [69,70], the leaf-mining Simplimorpha promissa (Staudinger) (Figure 23a–c) was discovered for the first time, and a sample of nine specimens from Jrvezh Forest Park, Yerevan (1515 m) was sequenced. All obtained molecular sequences were characterized by high conservatism among themselves, but they were extraordinarily distant (by approximately 38 hypothetical mutation steps) from those of the studied European specimens [44] (Figure 23d). An examination of the morphology and biology of the specimens collected in Armenia showed no clear diagnostic characters useful for the morphological differentiation of the Armenian sample from the genetically distant European specimens; both the male genitalia and the leaf mines, as well as host plant and seasonality of mining, appeared closely similar. Therefore, having only mitotype differences but the absence of diagnostic morphological and biological characters, we refrained from describing a new possible cryptic taxon (a new subspecies or species) [44].
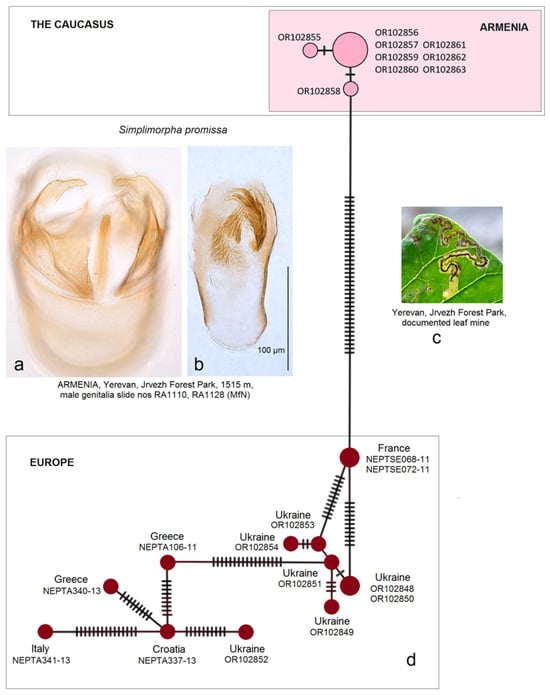
Figure 23.
Simplimorpha promissa (Staudinger): (a,b) male genitalia, Armenia, genitalia slide nos RA1110, RA1128 (MfN); (c) leaf mine; (d) the mitotype network of the 653 bp long mtDNA CO1-5’ sequences, constructed using the Minimum Spanning Network algorithm (after [44]). It includes sixteen newly analyzed sequences and six sequences downloaded from BOLD. For other explanatory notes, see Figure 20.
While studying Ectoedemia (Zimmermannia) longicaudella Klimesch collected in Antarut, Aragatsotn Province, we also found a significant difference between the molecular sequences of the Armenian and European specimens: the Armenian sequences differed from E. (Z.) longicaudella sequences from Bulgaria, Croatia, Italy, Austria, Germany, and the Netherlands by at least 22 hypothetical mutational steps (Figure 24a), and the studied Armenian sample differed even more from another closely related species, E. (Z.) liebwerdella Zimmermann, a type species of the subgenus Zimmermannia Hering (Figure 24a,b). In our NJ tree (Figure 25), the sequences from the Armenian specimens appeared to be clearly separate, but a sister clade to E. (Z.) longicaudella.
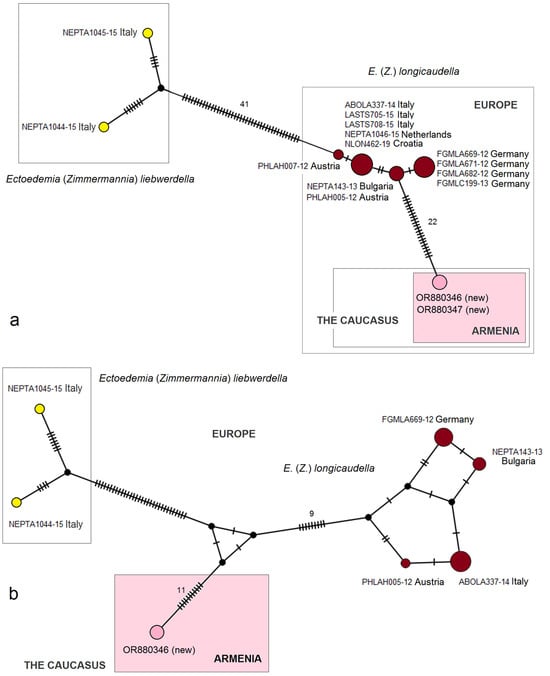
Figure 24.
The mitotype networks of the 658 bp long mtDNA CO1-5′ of Ectoedemia (Zimmermannia) longicaudella Klimesch: (a) constructed using the Median Joining Network algorithm. It includes new sequences from Armenia and sequences downloaded from BOLD; (b) constructed using the Integer NJ Net algorithm. The unnamed small circles represent mitotypes not sampled in the study but which were predicted to be. Dashes on the lines connecting mitotypes indicate hypothesized mutational steps.
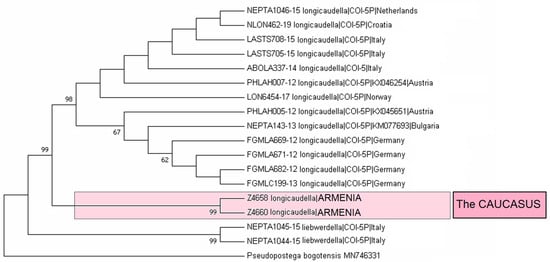
Figure 25.
The Neighbor-Joining tree of the 658 bp long mtDNA CO1-5′ Ectoedemia (Zimmermannia) longicaudella Klimesch sequences. The analysis involved fifteen nucleotide sequences of E. (Z.) longicaudella: two newly analyzed from Armenia and thirteen downloaded from the BOLD database. The Kimura-2 parameter model was used to calculate divergence. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are presented next to the branches (only bootstrap values above 50 are shown). Two sequences of the species E. (Z.) liebwerdella as the morphologically closest species to E. (Z.) longicaudella were used in the analysis. Pseudopostega bogotensis (Opostegidae) was used as an outgroup.
Since the biology of E. (Z.) longicaudella is still poorly known, and our specimens from Armenia were collected even without biological data (caught using light traps), it was not possible to determine any differences in the larval morphology, life cycle, or bark-mine morphology. It did, however, present the task of looking for morphological differences between Armenian and European adult specimens (Figure 26a–k). We found that features of the scaling, including the thorax and forewing pattern, as well as androconial formations of the hindwing and the anal tufts of the abdomen, did not differ. Therefore, we assumed that two separate taxa could not be differentiated on the basis of their external morphology. In the study of the male genitalia, each sclerite was dissected separately, documented, measured, and compared (Figure 27a–m). However, only in the shape of gnathos were differences detected; in the specimens from Armenia, this sclerite possesses lateral arms curved in a slightly different way. Although this examination did not provide the desired results, we also checked some dry genital mounts. These mounts, both of the studied European specimens and our specimens from Armenia, were made at the same time and using the same technique and fixation substitutes. The examination of such dry slides showed some significant differences; in the dry preparations (both in the studied sample of the European E. (Z.) longicaudella and in photographs and drawings of the genitalia provided by other researchers), the gnathos was turned ventrally, while the gnathos did not turn in the dried preparations of the Armenian sample, but remained directed caudally, while the highly elaborate lateral arms of the gnathos were clearly visible (Figure 28e). Moreover, in the dried preparations of the studied Armenian sample, the vinculum appeared to be significantly longer, and inner bulges were visible on the inner side of the valvae (Figure 28d,f). It is interesting that genitalia slide no. 1834 (VU) [71] from a region adjacent to Armenia (Turkey, Anatolia: Kizilchamam) documented by Erik J. van Nieukerken was also identical to our Armenian mounts (see [71] (figure 91). All these features were not observed in mounts of the European specimens (Figure 28a,c). Apparently, although the dissection of separate sclerites did not show obvious differences, the differences may be in the attachment mechanism, elasticity, or other cryptic elements of these sclerites. The female genitalia of the Armenian sample seem to be distinctive enough, as they are characterized by a highly elaborate vaginal sclerite, strongly chitinized ductus spermathecae, and longer and slender anterior apophyses (Figure 29a–d). We assume that the specimens from Armenia, together with the specimen documented by Erik J. van Nieukerken from adjacent Kizilchamam, Turkey, represent a new but cryptic taxon (a new subspecies or species). However, from a practical point of view, the description of one more difficult-to-diagnose taxon among the already rather difficult-to-diagnose species of the subgenus Zimmermannia is inappropriate. Thus, we have been refrained from describing a new taxon.

Figure 26.
Adults of Ectoedemia (Zimmermannia) longicaudella Klimesch: (a,b) lateral view; (c–e) dorsal view; (f) forewing upper side; (g) forewing underside; (h,i) hair-pencil and special scales of the male hindwing; and (j,k) anal tufts.
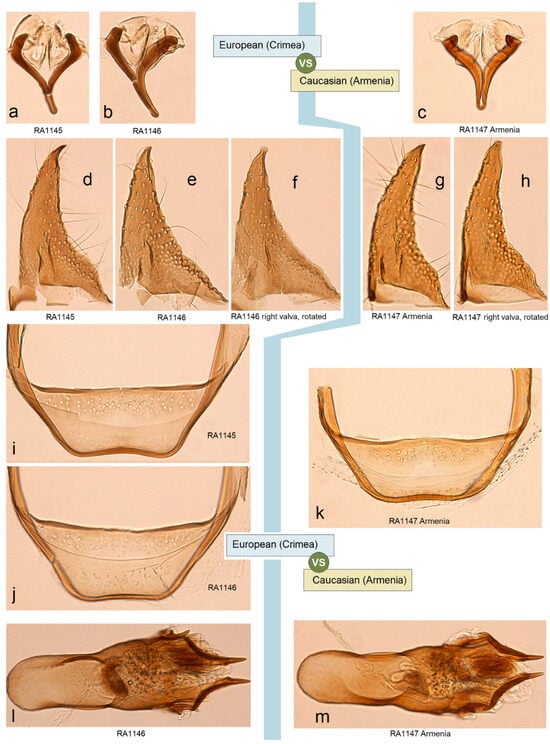
Figure 27.
Dissected sclerites of the male genitalia of Ectoedemia (Zimmermannia) longicaudella Klimesch: (a–c) gnathos; (d–h) valva; (i–k) vinculum; and (l,m) phallus.
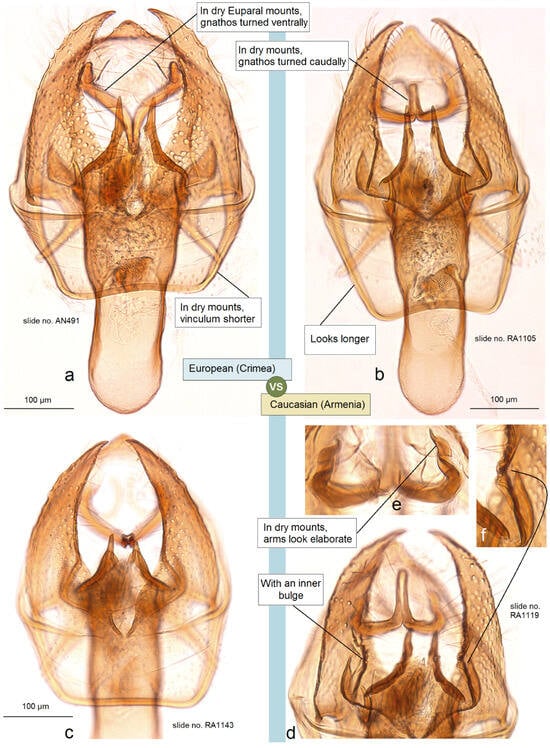
Figure 28.
General view of male genitalia of Ectoedemia (Zimmermannia) longicaudella Klimesch in dry mounts: (a) genitalia slide no. AN491, the Crimea; (b) genitalia slide no. RA1105, Armenia; (c) genitalia slide no. RA1143, the Crimea; and (d–f) genitalia slide no. RA1119, Armenia.
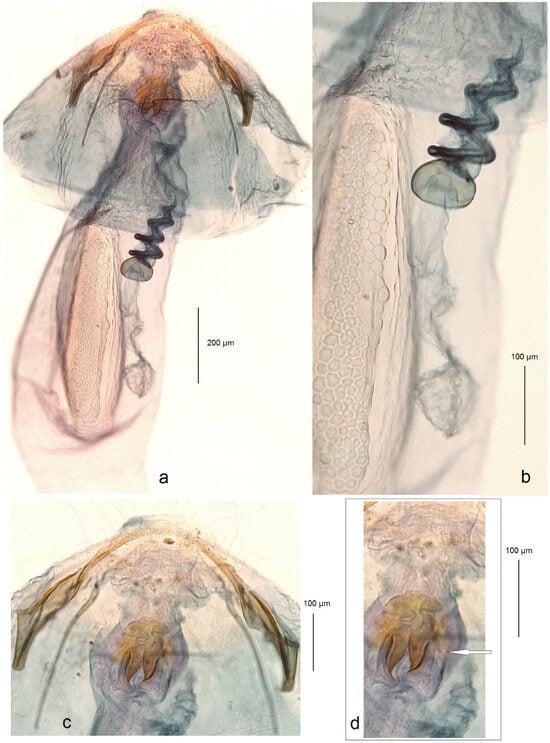
Figure 29.
Female genitalia of Ectoedemia (Zimmermannia) longicaudella Klimesch, slide no. RA1144, Armenia: (a) general view; (b) ductus spermathecae; (c) apophyses and vaginal sclerite; and (d) enlarged vaginal sclerite (MfN).
4. Discussion
In total, the research revealed thirty-one Nepticulidae species in Armenia belonging to eight genera. Only the following two species were known to exist in Armenia previously: Stigmella armeniana Puplesis [19] and Glaucolepis melanoptera (van Nieukerken & Puplesis) [24]. One additional species previously listed for the Armenian fauna (Stigmella inopinata Laštůvka, A. & Laštůvka, Z.) [25,72] was not confirmed and was treated by us as a misidentified record.
Although the currently revealed fauna is characterised by high endemism, a large number of discovered species (9) were hitherto known to be of European distribution (including Euro-Caucasian or Euro-Anatolian species): Stigmella confusella (Wood & Walsingham), S. viscerella (Stainton), S. lemniscella (Zeller), S. dorsiguttella (Johansson), S. roborella (Johansson); Ectoedemia (Zimmermannia) longicaudella Klimesch, E. mahalebella (Klimesch), Trifurcula subnitidella (Duponchel), and Simplimorpha promissa (Staudinger); the latter was first mentioned as a species of the Armenian fauna already in our previous publication [44]. Ten other species identified in the Armenian fauna were previously known to be of Euro-Asian distribution, including at least six species recently confirmed in Iran [26], so their discovery in Armenia is not unexpected: Stigmella muricatella (Klimesch), S. malella (Stainton), S. nivenburgensis (Preissecker), S. aceris (Frey), S. carpinella (Heinemann), S. hybnerella (Hübner), S. basiguttella (Heinemann), Fomoria septembrella (Stainton), Glaucolepis melanoptera (van Nieukerken & Puplesis) and Bohemannia pulverosella (Stainton), the latter being widespread in Europe and also known from Far Eastern Russia.
The smallest group of species discovered during our study were those previously known only from neighboring Central Asia: Stigmella klimeschi Puplesis, S. kopetdagica Puplesis and S. kuznetzovi Puplesis. It should be noted that S. kopetdagica Puplesis, and S. kuznetzovi are known to occur in Asia only in the westernmost part of the region, specifically in the Kopet Dag mountain range (western Turkmenistan), whose biota has clear biogeographical connections with the European biota [19].
Thus, among species of the Armenian fauna that have been clarified at the moment, about 38% of taxa are shared with the fauna of Asia (22% specifically with the fauna of Central Asia), and about 60% with the European fauna (note that these include some species that are associated with both European and Asian faunas).
Rather unexpected was that so many species new to science were discovered during this research: Stigmella armi Stonis, Dobrynina & Remeikis, sp. nov.; S. garnica Stonis, Dobrynina & Remeikis, sp. nov.; S. inopinoides Dobrynina, sp. nov.; S. magicis Stonis & Dobrynina, sp. nov.; S. ararati Stonis, Dobrynina & Remeikis, sp. nov.; Trifurcula vardenisi Stonis, Dobrynina & Remeikis, sp. nov.; Etainia caucasi Remeikis, sp. nov. (moreover, one new Stigmella species was identified but left unnamed and undescribed in the current publication). Thus, the new species account for 25% of the currently known Armenian species (i.e., every fourth species collected in Armenia was new to science). Until other differing data are obtained, all of these species, together with the earlier described S. armeniana Puplesis [19], can be provisionally treated as endemics of the Caucasus (or Armenia) (i.e., 28%, or almost a third of the currently known fauna of Armenia). In addition, some of the species named in this publication are possibly cryptic and are not yet described but endemic taxa. Moreover, at least two species of Stigmella and Acalyptris collected during our research in Armenia were clearly new to science, but they were left undescribed and excluded from this review due to insufficient collection material (we only had female singletons).
Although we only rely on the previous experience of work, we assume that the currently identified species in Armenia may account for only about 40–45% of the species actually occurring in this country, i.e., in total, about 70–80 species of Nepticulidae can be discovered in the future in Armenia. Among those still undiscovered species, we expect a significant portion of species similar to European species but cryptic. We believe that further research is necessary in this fascinating and biogeographically very important region.
Author Contributions
Conceptualization, J.R.S.; research permits, M.Y.K., J.R.S. and V.D.; field collecting and observations, J.R.S., V.D. and M.Y.K.; rearing adults from cocoons, A.D. and J.R.S.; specimen setting and labeling, V.D.; specimen dissections and genitalia slides, V.D., A.R. and A.D.; species identifications, V.D., J.R.S., A.R. and A.D.; measurements, A.R. and J.R.S.; host-plant identifications and habitat characterization, M.Y.K.; molecular analysis, S.O., A.R. and V.D.; methodology, S.O., A.R. and J.R.S.; visualization—adult photography, J.R.S.; genitalia photography, A.R. and J.R.S.; drawings, J.R.S.; SEM photography, J.R.S., V.D. and A.R.; molecular cladograms, S.O., A.R. and J.R.S.; preparation of illustration collages and plates, J.R.S.; sorting and consideration of species groups, A.D. and J.R.S.; writing—original draft, J.R.S., S.O., A.R. and M.Y.K.; reference list, A.D.; writing—review and editing, J.R.S., S.O. and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
J.R.S., V.D., A.R. and S.O. are partially funded by the State Research Institute Nature Research Centre, Vilnius, Lithuania.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The molecular data presented in this study can be found in online repositories (Genbank: https://www.ncbi.nlm.nih.gov/genbank/ accessed on 1 January 2024, and BOLD: https://www.boldsystems.org accessed on 1 January 2024). Open access depositories for the collection material (physical specimens and their genitalia mounts, including of all described new species) are listed in this article.
Acknowledgments
We thank the Scientific Centre of Zoology and Hydroecology of the National Academy of Sciences of Armenia and the Deputy Minister of Environment of Republic of Armenia for the Collecting & Export Permits No. N-2459-146 and No. 2/29.7/1803. We highly appreciate the assistance from our Armenian partners and friends, including those who variously helped us with the fieldwork of 2022: Arman Khachatryan, Artur Barseghyan, Garik Voskanyan (Yerevan) and Hakob Martirosyan (Yeghegis). We are indebted to Erik J. van Nieukerken (RMNH, Leiden) for his helpful discussion and photographs of the paratype of Stigmella muricatella (Figure 21a,b). For the base map of the region (Figure 1), we thank Virginijus Gerulaitis (Vytautas Magnus University, Lithuania). We are indebted to Jos Stratford, Editor of the Nature Research Centre, Vilnius, for help with the English of the text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vane-Wright, R.I. Systematics and global biodiversity strategy. Antenna 1992, 16, 49–56. [Google Scholar]
- Vane-Wright, R.I. Systematics and the Conservation of Biological Diversity. Ann. Mo. Bot. Gard. 1996, 83, 47–57. [Google Scholar] [CrossRef]
- Wheeler, Q.; Bourgoin, T.; Coddington, J.; Gostony, T.; Hamilton, A.; Larimer, R.; Polaszek, A.; Schauff, M.; Solis, M.A. Nomenclatural benchmarking: The role of digital typification and telemicroscopy. ZooKeys 2012, 209, 193–202. [Google Scholar] [CrossRef]
- Wheeler, Q.D.; Knapp, S.; Stevenson, D.W.; Stevenson, J.; Blum, S.D.; Boom, B.M.; Borisy, G.G.; Buizer, J.L.; de Carvalho, M.R.; Cibrian, A.; et al. Mapping the biosphere: Exploring species to understand the origin, organization, and sustainability of biodiversity. Syst. Biodivers. 2012, 10, 1–20. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Accelerating biodiversity descriptions and transforming taxonomic publishing: The first decade of Zootaxa. Zootaxa 2008, 2896, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Describing unexplored biodiversity: Zootaxa in the International Year of Biodiversity. Zootaxa 2011, 2768, 1–4. [Google Scholar] [CrossRef]
- Cutko, A. Biodiversity Inventory of Natural Lands: A How-To Manual for Foresters and Biologists; NatureServe: Arlington, VI, USA, 2009; p. 32. Available online: https://www.natureserve.org/sites/default/files/biodiversityinventorymanual_main.pdf (accessed on 1 January 2024).
- La Salle, J.; Wheeler, Q.; Jackway, P.; Winterton, S.; Hobern, D.; Lovell, D. Accelerating taxonomic discovery through automated character extraction. Zootaxa 2009, 2217, 43–55. [Google Scholar] [CrossRef]
- Pereira, H.; Belnap, J.; Brummitt, N.; Collen, N.; Ding, H.; Gonzales-Espinosa, M.; Gregory, R.; Honrado, J.; Jongman, R.H.G.; Julliard, R.; et al. Global Biodiversity Monitoring. Front. Ecol. Environ. 2010, 8, 459–460. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef]
- Davis, J. Armoured ‘Slug’ among 503 New Species Described by Museum Scientists in 2020. Available online: https://www.nhm.ac.uk/discover/news/2020/december/503-new-species-described-by-museum-scientists-in-2020.html (accessed on 25 October 2023).
- Schmeller, D.S.; Böhm, M.; Arvanitidis, C.; Barber-Meyer, S.; Brummitt, N.; Chandler, M.; Chatzinikolaou, E.; Costello, M.J.; Ding, H.; García-Moreno, J.; et al. Building capacity in biodiversity monitoring at the global scale. Biodivers. Conserv. 2017, 26, 2765–2790. [Google Scholar] [CrossRef]
- Dobrynina, V.; Stonis, J.R.; Diškus, A.; Solis, M.A.; Baryshnikova, S.V.; Young-Min, S. Global Nepticulidae, Opostegidae, and Tischeriidae (Lepidoptera): Temporal dynamics of species descriptions and their authors. Zootaxa 2022, 5099, 450–474. [Google Scholar] [CrossRef] [PubMed]
- Stonis, J.R.; Remeikis, A.; Diškus, A. Neotropical Nepticulidae (A Pictorial Monograph Introducing an Electronic Identification Tool); Nature Research Centre: Vilnius, Lithuania, 2022; p. 363. [Google Scholar]
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Baryshnikova, S.V.; Solis, M.A. What are the smallest moths (Lepidoptera) in the world? Zootaxa 2021, 4942, 269–289. [Google Scholar] [CrossRef] [PubMed]
- van Nieukerken, E.J.; Dop, H. Antennal sensory structures in Nepticulidae (Lepidoptera) and their phylogenetic implications. Z. Zool. Syst. Evolut. Forsch. 1987, 25, 104–126. [Google Scholar] [CrossRef]
- Scoble, M.J. A revised cladistic classification of the Nepticulidae (Lepidoptera) with descriptions of new taxa mainly from South Africa. Transvaal. Mus. Monogr. 1983, 2, 1–105. [Google Scholar]
- Johansson, R.; Nielsen, E.S.; van Nieukerken, E.J.; Gustafsson, B. The Nepticulidae and Opostegidae (Lepidoptera) of north west Europe. Fauna Entomol. Scand. 1990, 23, 1–739. [Google Scholar]
- Puplesis, R. The Nepticulidae of Eastern Europe and Asia: Western, Central and Eastern Parts; Backhuys Publishers: Leiden, The Netherlands, 1994; p. 291, figs. 840. [Google Scholar]
- Puplesis, R.; Diškus, A. The Nepticuloidea & Tischerioidea (Lepidoptera)—A Global Review, with Strategic Regional Revisions; Lututė Publishers: Kaunas, Lithuania, 2003; p. 512. [Google Scholar]
- van Nieukerken, E.J.; Doorenweerd, C.; Nishida, K.; Snyers, C. New taxa, including three new genera show uniqueness of Neotropical Nepticulidae (Lepidoptera). ZooKeys 2016, 628, 1–63. [Google Scholar] [CrossRef] [PubMed]
- National Statistical Service of the Republic of Armenia. Marzes of the Republic of Armenia in Figures. 2002–2006. Available online: https://www.armstat.am/en/?module=publications&mid=6&id=618 (accessed on 18 October 2023).
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- van Nieukerken, E.J.; Puplesis, R. Taxonomy and distribution of the Trifurcula (Glaucolepis) raikhonae group (Lepidoptera: Nepticulidae). Tijdschr. Entomol. 1991, 134, 201–210. Available online: https://biostor.org/reference/49992 (accessed on 28 October 2023).
- Laštuvka, A.; Laštuvka, Z. Stigmella inopinata sp. n. und Parornix mixta (Triberti) in Mitteleuropa (Lepidoptera). Acta Univ. Agric. Fac. Agron. 1990, 38, 197–203. [Google Scholar]
- Rajaei, H.; Aarvik, L.; Arnscheid, W.R.; Baldizzone, G.; Bartsch, D.; Bengtsson, B.Å.; Bidzilya, O.; Buchner, P.; Buchsbaum, U.; Buszko, J.; et al. Catalogue of the Lepidoptera of Iran. Integr. Syst. Stutt. Contrib. Nat. Hist. 2023, 6, 121–459. [Google Scholar] [CrossRef]
- Brehm, G. A new LED lamp for the collection of nocturnal Lepidoptera and a spectral comparison of lighttrapping lamps. Nota Lepidopterol. 2017, 40, 87–108. [Google Scholar] [CrossRef]
- Puplesis, R.; Robinson, G.S. A review of the Central and South American Nepticulidae (Lepidoptera) with special reference to Belize. Bull. Nat. Hist. Mus. Lond. 2000, 69, 3–114. Available online: https://www.biodiversitylibrary.org/page/40885664#page/5/mode/1up (accessed on 1 January 2024).
- Davies, C.E.; Moss, D.; Hill, M.O. EUNIS Habitat Classification Revised 2004; European Environment Agency, European Topic Centre on Nature Protection and Biodiversity: Copenhagen, Denmark, 2004; p. 307. Available online: https://www.researcgate.net/publication/238708061_EUNIS_habitat_classification_revised_2004 (accessed on 1 January 2024).
- Fayvush, G.M.; Aleksanyan, A.S. Habitats of Armenia; NAS RA, Institute of Botany: Yerevan, Armenia, 2016; p. 360, (In Russian, with parts in English). [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Ser. B. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data system. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP v6: DNA Sequence Polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Bandelt, H.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Snell, Q.; Walke, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. In Proceedings of the 16th International Parallel and Distributed Processing Symposium, Ft. Lauderdale, FL, USA, 15–19 April 2002; p. 7. [Google Scholar] [CrossRef]
- Staudinger, O. Beschreibung neuer Lepidopteren des europäischen Faunengebiets. Berl. Entomol. Z. 1870, 14, 273–330. [Google Scholar]
- Orlovskytė, S.; Dobrynina, V.; Stonis, J.R. Unexpected mitotype diversity of Simplimorpha promissa (Lepidoptera: Nepticulidae) in Ukraine and Armenia revealing a possible cryptic taxon. Zootaxa 2023, 5336, 113–124. [Google Scholar] [CrossRef]
- Wood, J.H.; Walsingham, T.G. Nepticula confusella, a new birch-mining species. Entomol. Mon. Mag. 1894, 30, 272–273. [Google Scholar]
- Stainton, H.T. Insecta Britannica. Lepidoptera: Tineina; Lovell Reeve: London, UK, 1854; p. 313. [Google Scholar]
- Puplesis, R. New species of plant mining Lepidoptera (Nepticulidae, Tischeriidae) from Central Asia. Stapfia 1988, 16, 273–290. [Google Scholar]
- Klimesch, J. Beitrag zur Kenntnis der Nepticulidenfauna von Anatolien und der Insel Rhodos (Lepidoptera, Nepticulidae). Tijdschr. Entomol. 1978, 121, 239–278. [Google Scholar]
- Preissecker, F. Zwei neue Nepticula-Arten aus dem Gebiete des heutigen Reichsgaues Wien. Z. Wien. Entomol. Ver. 1942, 27, 208–211. [Google Scholar]
- Varenne, T.; Nel, J. Descriptions de quatre nouvelles espèces de Lépidoptères du sud de la France: Stigmella thibaulti sp. n., Trifurcula lastuvkaorum sp. n., Trifurcula guercheae sp. n., et Elachista bidentate sp. n. (Lepidoptera, Nepticulidae, Elachistidae). Rev. Assoc. Roussil. Entomol. 2019, 28, 85–91. [Google Scholar]
- Huemer, P.; van Nieukerken, E.J. Identity of some recently described Lepidoptera from France—Re-assessed with DNA barcodes and morphology. Zootaxa 2021, 4941, 301–337. [Google Scholar] [CrossRef] [PubMed]
- van Nieukerken, E.J.; Doorenweerd, C.; Hoare, J.R.; Davis, D.R. Revised classification and catalogue of global Nepticulidae and Opostegidae (Lepidoptera, Nepticuloidea). ZooKeys 2016, 628, 65–246. [Google Scholar] [CrossRef] [PubMed]
- Frey, H. Revision der Nepticulen. Linnaea Entomol. 1857, 11, 351–446. [Google Scholar]
- Hübners, J. Sammlung Europäischer Schmetterlinge, 8 Tineae; Selbst-Verlag: Augsburg, Germany, 1796; p. 78, figs. 477; Available online: http://www.biodiversitylibrary.org/item/89072#page/2/mode/2up (accessed on 28 October 2023).
- Stainton, H.T. An introduction to the study of the Nepticulae. Zoologist 1853, 11, 3952–3960. [Google Scholar]
- Heinemann, H. Einige Bemerkungen über die Arten der Gattung Nepticula. Wien. Entomol. Monatsch. 1862, 6, 237–268. [Google Scholar]
- Zeller, P.C. Versuch einer naturgemässen Eintheilung der Schaben. Isis von Oken 1839, 32, 167–224. Available online: https://biostor.org/reference/99970 (accessed on 1 January 2024).
- Johansson, R. Notes on Nepticulidae (Lepidoptera) I. A revision of the Nepticula ruficapitella group. Entomol. Scand. 1971, 2, 241–262. [Google Scholar] [CrossRef]
- Stainton, H.T. An Attempt at a Systematic Catalogue of the British Tineidae & Pterophoridae; John van Voorst: London, UK, 1849; p. 32. [Google Scholar]
- Puplesis, R. Contribution to the classification of the Palearctic Nepticulidae (Lepidoptera). Entomol. Rev. 1984, 3, 149–164. [Google Scholar]
- Klimesch, J. Die europäischen Trifurcula—Und Ectoedemia-Arten (Lep., Nepticulidae). Z. Wien. Entomol. Gesellsch. 1953, 7, 191–196. [Google Scholar]
- van Nieukerken, E.J. A provisional phylogenetic check-list of the western Palaearctic Nepticulidae, with data on hostplants (Lepidoptera). Entomol. Scand. 1986, 17, 1–27. [Google Scholar] [CrossRef]
- Klimesch, J. Ein kleiner Beitrag zur Kenntnis der Nepticuliden-fauna (Lep.) des Vintschgaues (Prov. Bozen). Entomol. Ztg. Hrsg. Entomol. Ver. Stettin. 1936, 97, 194–211. [Google Scholar]
- Duponchel, P.A.J. Nocturnes, Supplément 4. In Histoire Naturelle des Lépidoptères ou Papillons de France; Godart, J.-B., Ed.; Méquignon-Marvis: Paris, France, 1843; pp. 305–512. Available online: http://www.biodiversitylibrary.org/item/39620 (accessed on 1 January 2024).
- Chrétien, P. Contribution a la connaissance des Lépidoptères du Nord de l’Afrique. Ann. Soc. Entomol. Fr. 1915, 84, 289–374. Available online: http://biostor.org/reference/97306 (accessed on 1 January 2024).
- Stonis, J.R.; Remeikis, A.; Diškus, A.; Navickaitė, A. Documenting new and little known leaf-mining Nepticulidae from middle and southwestern areas of the Asian continent. Zootaxa 2020, 4881, 401–452. [Google Scholar] [CrossRef]
- van Nieukerken, E.J.; Laštuvka, A.; Laštuvka, Z. Western Palaearctic Ectoedemia (Zimmermannia) Hering and Ectoedemia Busck s. str. (Lepidoptera, Nepticulidae): Five new species and new data on distribution, hostplants and recognition. ZooKeys 2010, 32, 1–82. [Google Scholar] [CrossRef]
- Doorenweerd, C.; van Nieukerken, E.J.; Hoare, R.J.B. Phylogeny, classification and divergence times of pygmy leaf-mining moths (Lepidoptera: Nepticulidae): The earliest lepidopteran radiation on Angiosperms? Syst. Entomol. 2016, 42, 267–287. [Google Scholar] [CrossRef]
- Takhtajan, A.L. Flora of Armenia. Ericaceae-Elaeagnaceae; Publishing House of the Academy of Sciences of the Armenian SSR: Yerevan, Armenia, 1973; Volume 6, p. 486, (In Russian). Available online: https://ace.aua.am/files/2014/02/Takhtajyan-A.-L.-Flora-of-Armenia.-Volume-6.-Yerevan-Armenian-SSR-Academy-of-Science-Publishing-1973.pdf (accessed on 1 January 2024).
- Shaboyan, N.K.; Moghrovyan, A.V.; Dumanyan, K.H.; Ghukasyan, N.H.; Altunyan, A.A.; Arshakyan, N.I.; Ghazaryan, A.M.; Ulikhanyan, G.R.; Ginosyan, A.L.; Dadayan, A.S.; et al. Phytochemical analysis and antioxidant activity of Cotinus coggygria Scop. from Armenian flora. Pharmacogn. J. 2021, 13, 933–941. [Google Scholar] [CrossRef]
- van Nieukerken, E.J. A taxonomic revision of the Western Palaearctic species of the subgenera Zimmermannia Hering and Ectoedemia Busck s. str. (Lepidoptera, Nepticulidae), with notes on their phylogeny. Tijdschr. Entomol. 1985, 128, 1–98. Available online: https://biostor.org/reference/61076 (accessed on 1 January 2024).
- Laštůvka, A.; Laštůvka, Z. Nepticulidae Mitteleuropas. Ein Illustrierter Begleiter (Lepidoptera); Konvoj Publishers: Brno, Czech Republic, 1997; p. 230. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).