Genetic Variation among Aeluropus lagopoides Populations Growing in Different Saline Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. DNA Extraction
2.3. Sequence-Related Amplified Polymorphism Analysis (SRAP)
2.4. Inter-Simple Sequence Repeat (ISSR) Analysis
2.5. Data Scoring and Molecular Analysis
3. Results
3.1. Polymorphism Analysis
3.2. Genetic Diversity
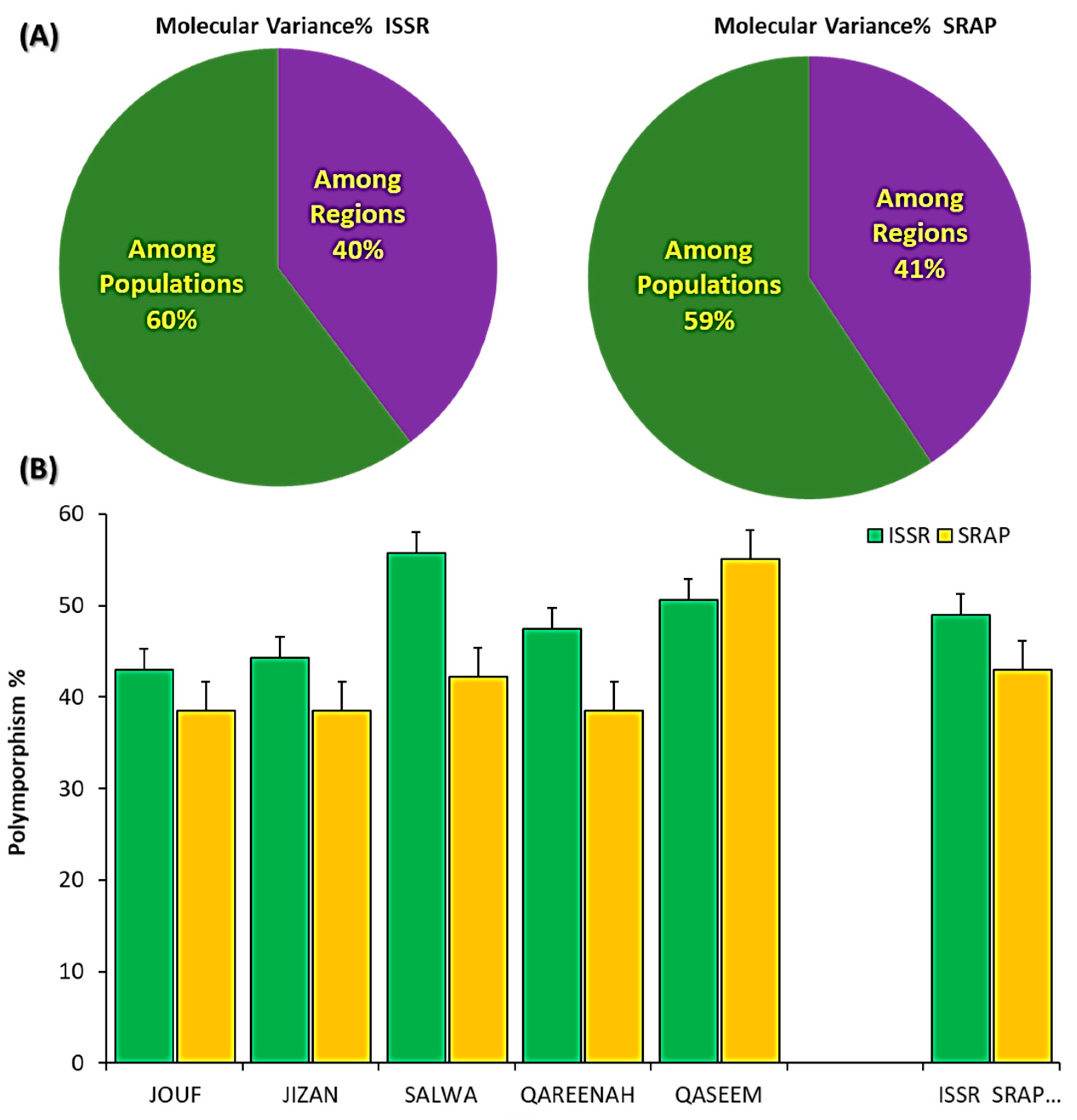
3.3. Molecular Variance and Polymorphism within and among A. lagopoides Population Based on 14 ISSR and 15 SRAP Markers
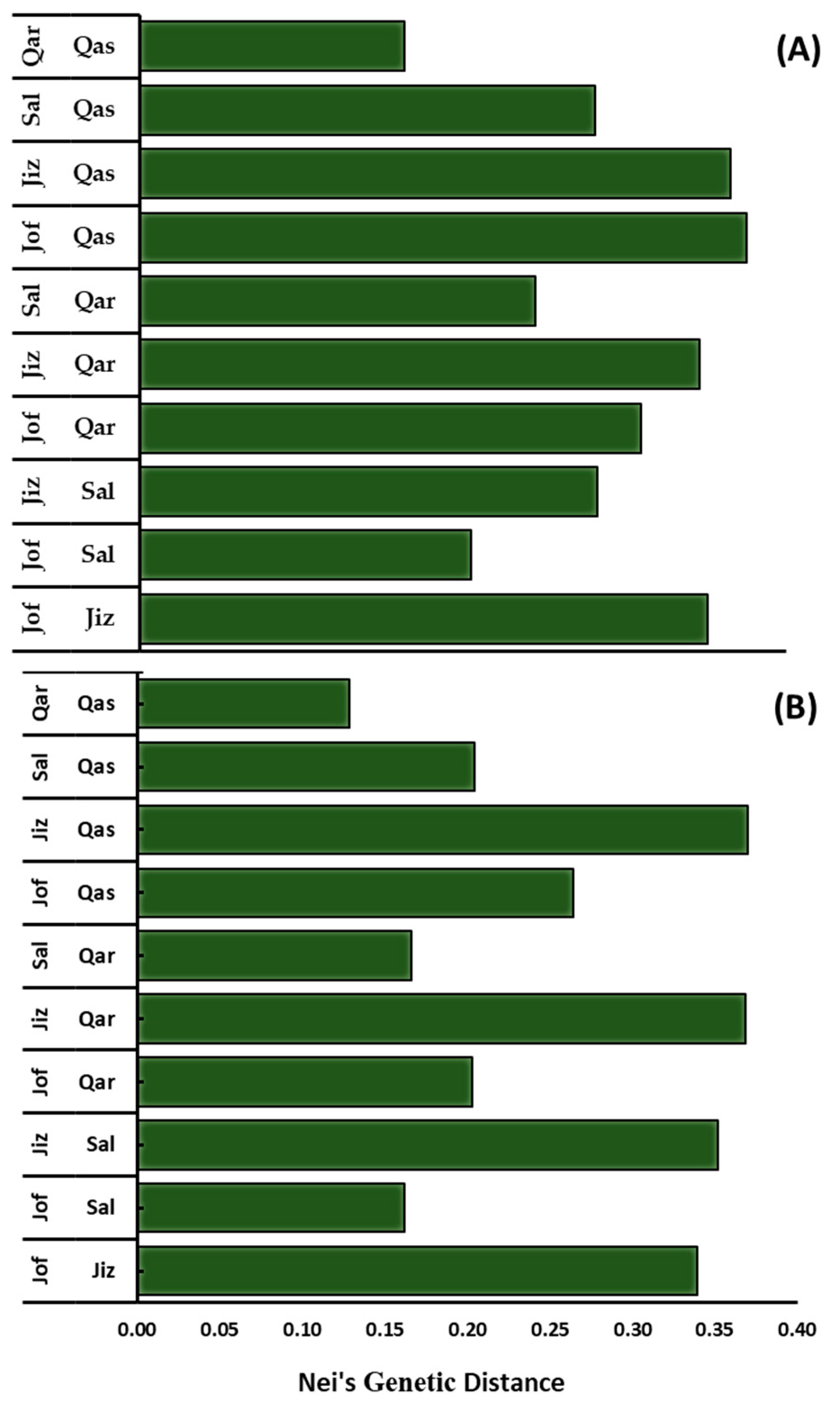
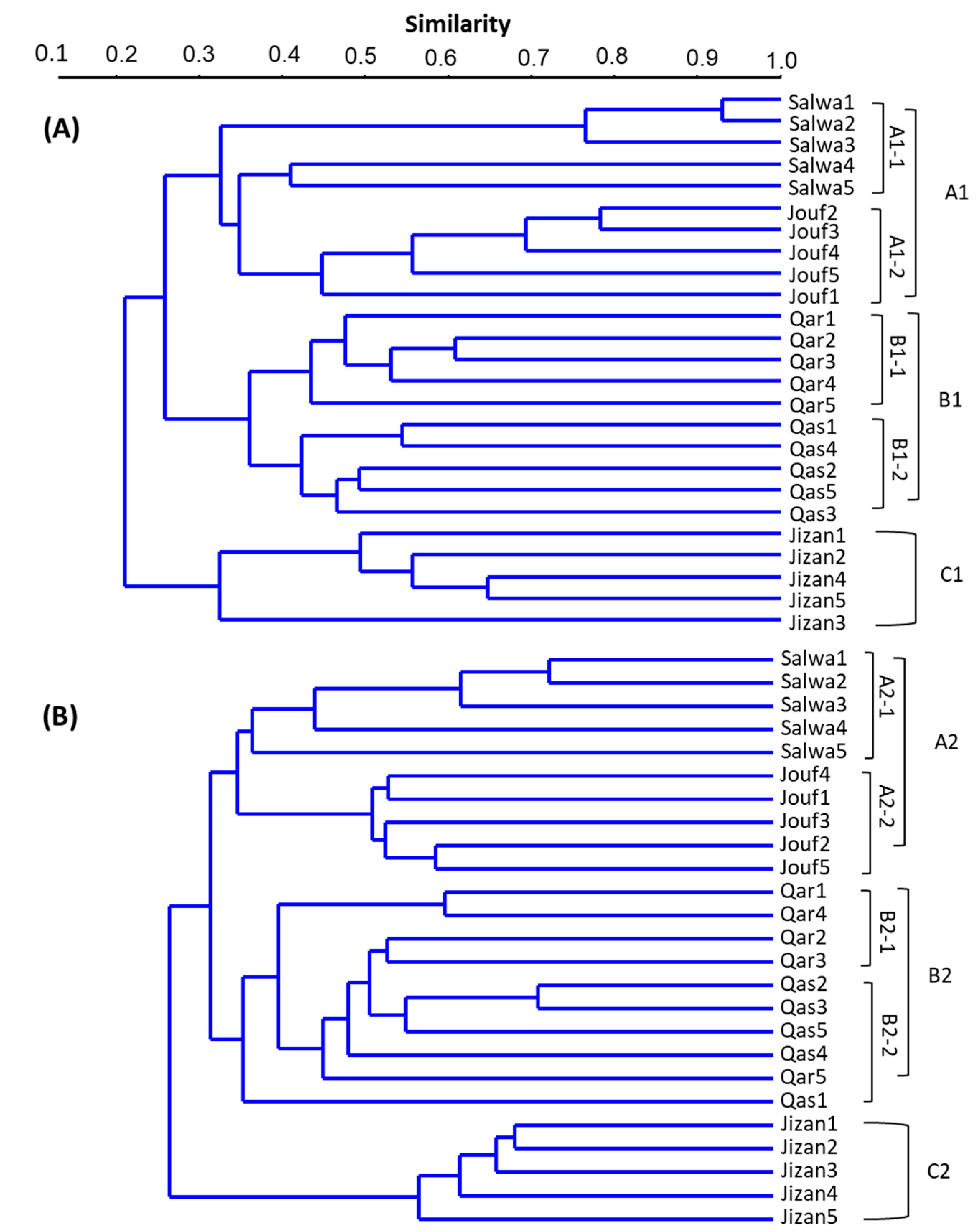
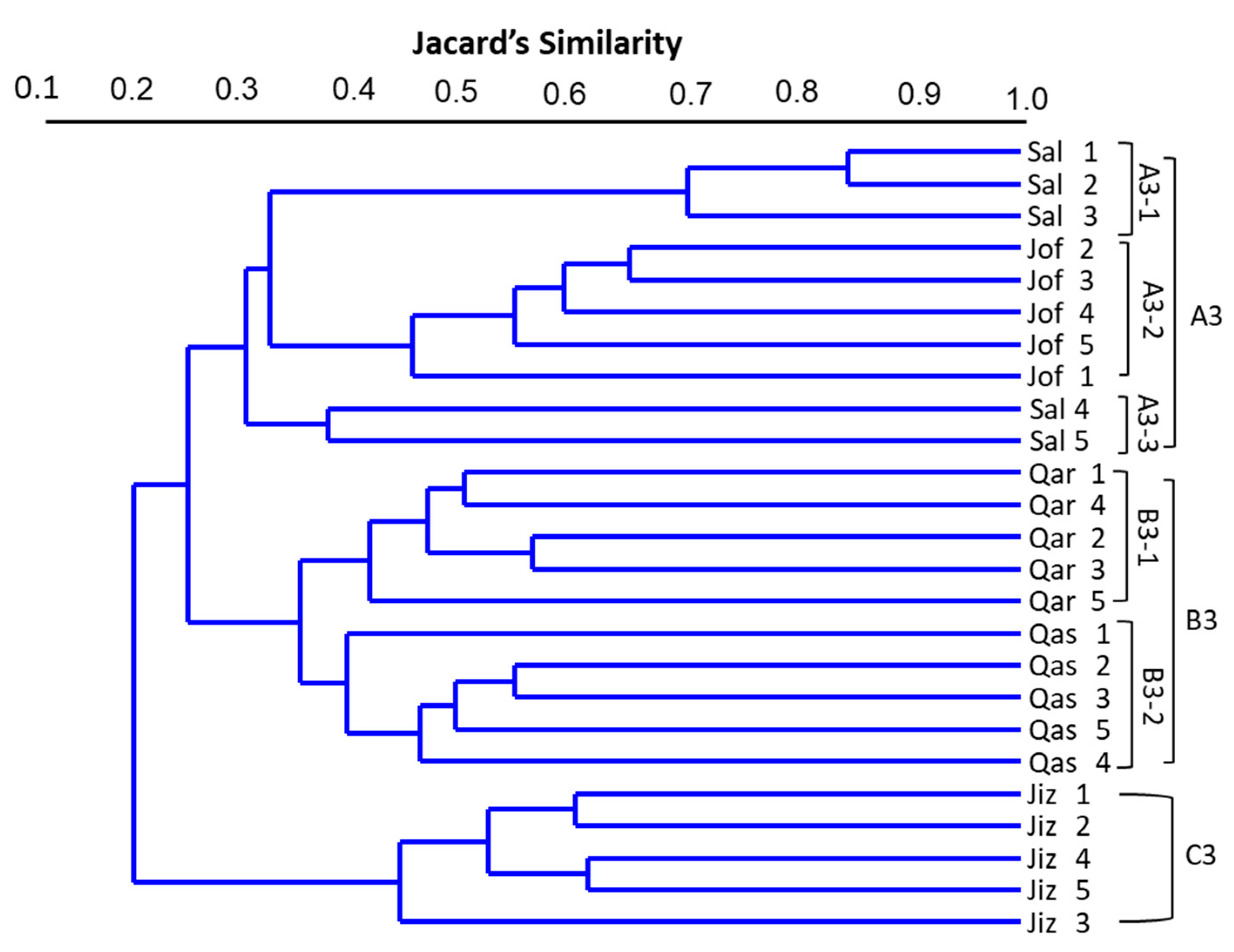
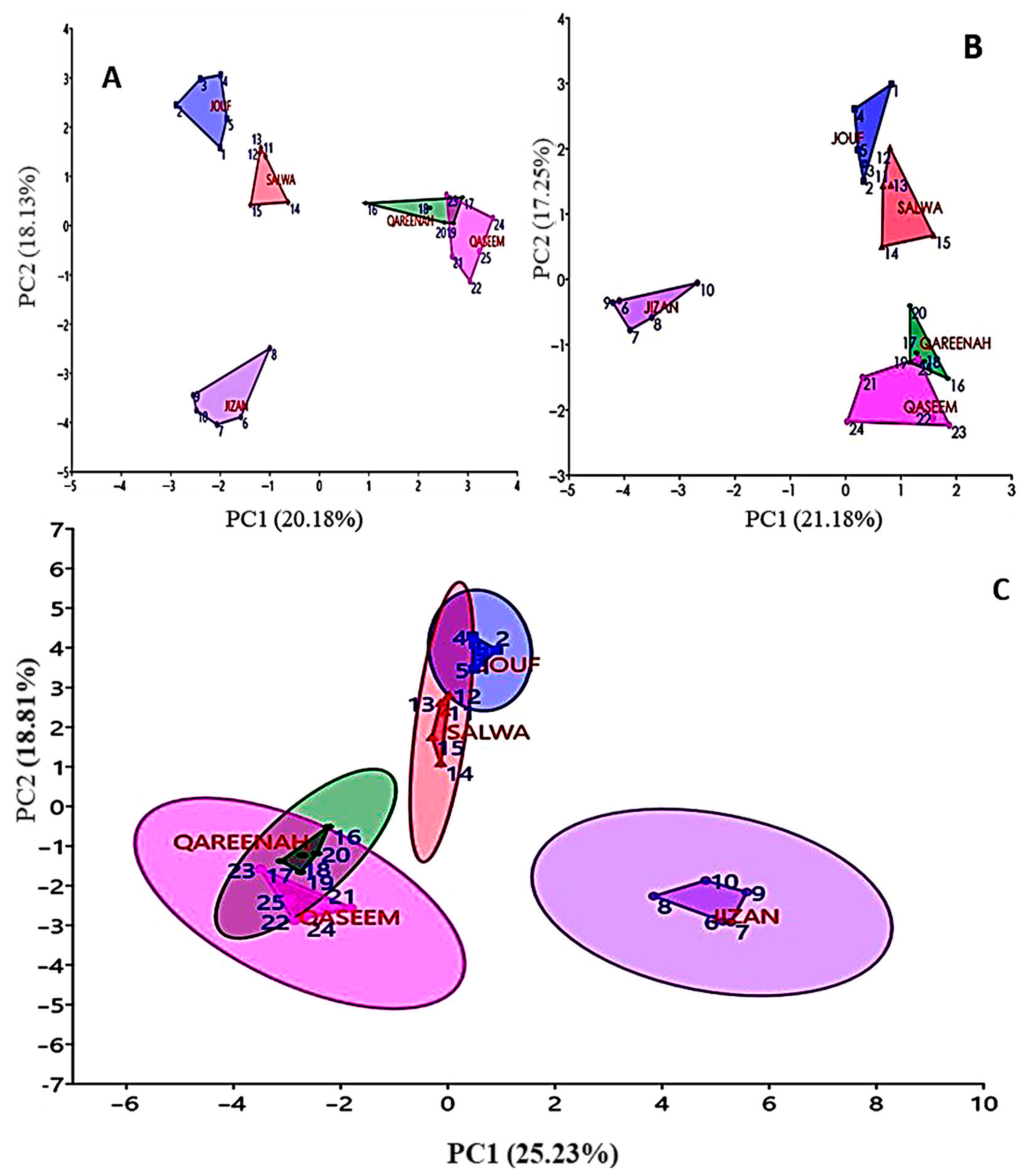
3.4. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudal, R.; Purnell, M. Land resources: Salt affected soils. Reclam. Reveg. Res. 1986, 5, 1–9. [Google Scholar]
- Assaeed, A.M.; Dar, B.A.; Al-Doss, A.A.; Al-Rowaily, S.L.; Malik, J.A.; Abd-ElGawad, A.M. Phenotypic plasticity strategy of Aeluropus lagopoides grass in response to heterogenous saline habitats. Biology 2023, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Phondani, P.C.; Bhatt, A.; Elsarrag, E.; Alhorr, Y.M.; El-Keblawy, A. Criteria and indicator approach of global sustainability assessment system for sustainable landscaping using native plants in Qatar. Ecol. Indic. 2016, 69, 381–389. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Suprasanna, P. Prospects of halophytes in understanding and managing abiotic stress tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Tousif, M.I.; Alamri, A.; Anwar, S.; Alamri, A.; Ahmad, I.; Abdallah, H.H.; Mahomoodally, F.M. A comprehensive phytochemical, biological, toxicological and molecular docking evaluation of Suaeda fruticosa (L.) Forssk.: An edible halophyte medicinal plant. Food Chem. Toxicol. 2021, 154, 112348. [Google Scholar] [CrossRef] [PubMed]
- Cope, T. Flora of Pakistan, No. 143: Poaceae; Pakistan Agricultural Research Council: Islamabad, Pakistan; University of Karachi: Karachi, Pakistan, 1982; p. 678. [Google Scholar]
- Gulzar, S.; Khan, M.A.; Ungar, I.A. Salt tolerance of a coastal salt marsh grass. Commun. Soil Sci. Plant Anal. 2003, 34, 2595–2605. [Google Scholar] [CrossRef]
- Basahi, R.A. Plant diversity of the coastal regions of Gulf of Aqaba, Saudi Arabia. Annu. Res. Rev. Biol. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Dar, B.A.; Assaeed, A.M.; Al-Rowaily, S.L.; Al-Doss, A.A.; Abd-ElGawad, A.M. Vegetation composition of the halophytic grass Aeluropus lagopoides communities within coastal and inland sabkhas of Saudi Arabia. Plants 2022, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Torbatinezhad, N.; Maghsoodlowrad, H.; Gharahbash, A. Nutritive value of Aeluropus littoralis and Aeluropus logopoides in sheep. J. Agric. Sci. Nat. Resour. 2000, 7, 31–46. [Google Scholar]
- Tewari, A. A note on the value of Sporobolus coromandelianus (Trin). Kunth and Aeluropus lagopoides (Linn.) as feed and soil binder. Plant Sci. 1970, 2, 135–136. [Google Scholar]
- Khan, M.A.; Gulzar, S. Light, salinity, and temperature effects on the seed germination of perennial grasses. Am. J. Bot. 2003, 90, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, S.; Malboobi, M.; Razavi, K.; Farrahi-Aschtiani, S. Physiological and molecular responses of Aeluropus lagopoides (Poaceae) to water deficit. Environ. Exp. Bot. 2006, 56, 314–322. [Google Scholar] [CrossRef]
- Vincent, P. Saudi Arabia: An Environmental Overview; CRC Press: London, UK, 2008. [Google Scholar]
- Abd-ElGawad, A.M.; Assaeed, A.M.; Al-Rowaily, S.L.; Dar, B.M.; Malik, J.A. Moisture and salinity drive the vegetation composition of Wadi Hargan, Riyadh, Saudi Arabia. Diversity 2021, 13, 587. [Google Scholar] [CrossRef]
- Gulzar, S.; Khan, M.A. Seed germination of a halophytic grass Aeluropus lagopoides. Ann. Bot. 2001, 87, 319–324. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 2, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Hensen, I.; Oberprieler, C. Effects of population size on genetic diversity and seed production in the rare Dictamnus albus (Rutaceae) in central Germany. Conserv. Genet. 2005, 6, 63–73. [Google Scholar] [CrossRef]
- Brown, A.H.D.; Weir, B.S. Measuring genetic variability in plant populations. In Developments in Plant Genetics and Breeding; Tanksley, S.D., Orton, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 1, pp. 219–239. [Google Scholar]
- Yu, Q.; Liu, Q.; Xiong, Y.; Xiong, Y.; Dong, Z.; Yang, J.; Liu, W.; Ma, X.; Bai, S. Genetic diversity and population divergence of a rare, endemic grass (Elymus breviaristatus) in the southeastern Qinghai-Tibetan plateau. Sustainability 2019, 11, 5863. [Google Scholar] [CrossRef]
- Lousada, J.M.; Lovato, M.B.; Borba, E.L. High genetic divergence and low genetic variability in disjunct populations of the endemic Vellozia compacta (Velloziaceae) occurring in two edaphic environments of Brazilian campos rupestres. Braz. J. Bot. 2013, 36, 45–53. [Google Scholar] [CrossRef]
- Mujeeb-Kazi, A.; Munns, R.; Rasheed, A.; Ogbonnaya, F.C.; Ali, N.; Hollington, P.; Dundas, I.; Saeed, N.; Wang, R.; Rengasamy, P. Breeding strategies for structuring salinity tolerance in wheat. Adv. Agron. 2019, 155, 121–187. [Google Scholar]
- Quilot, B.; Kervella, J.; Génard, M.; Lescourret, F. Analysing the genetic control of peach fruit quality through an ecophysiological model combined with a QTL approach. J. Exp. Bot. 2005, 56, 3083–3092. [Google Scholar] [CrossRef]
- Fu, X.; Ning, G.; Gao, L.; Bao, M. Genetic diversity of Dianthus accessions as assessed using two molecular marker systems (SRAPs and ISSRs) and morphological traits. Sci. Hortic. 2008, 117, 263–270. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Wu, Z.-X.; Lu, J.-J.; Shi, N.-N.; Zhao, Y.; Zhang, Z.-T.; Liu, J.-J. Molecular diversity and relationships among Cymbidium goeringii cultivars based on inter-simple sequence repeat (ISSR) markers. Genetica 2009, 136, 391–399. [Google Scholar] [CrossRef]
- Santhosh, W.; Shobha, D.; Melwyn, G. Assessment of genetic diversity in cashew germplasm using RAPD and ISSR markers. Sci. Hortic. 2009, 120, 411–417. [Google Scholar]
- Massa, A.; Larson, S.; Jensen, K.; Hole, D.J. AFLP variation in Bromus section Ceratochloa germplasm of Patagonia. Crop Sci. 2001, 41, 1609–1616. [Google Scholar] [CrossRef]
- Weising, K.; Freitag, H. Phylogeography of halophytes from European coastal and inland habitats. Zool. Anz. A J. Comp. Zool. 2007, 246, 279–292. [Google Scholar] [CrossRef]
- Lambracht, E.; Westberg, E.; Kadereit, J.W. Phylogeographic evidence for the postglacial colonization of the North and Baltic Sea coasts from inland glacial refugia by Triglochin maritima L. Flora 2007, 202, 79–88. [Google Scholar] [CrossRef]
- Assaeed, A.M.; Al-Faifi, S.A.; Migdadi, H.M.; El-Bana, M.I.; Al Qarawi, A.A.; Khan, M.A. Evaluation of genetic diversity of Panicum turgidum Forssk from Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Shamasbi, F.V.; Nasiri, N.; Shokri, E. Genetic diversity of Persian ecotypes of Indian walnut (Aeluropus littoralis (Gouan) Pari.) by AFLP and ISSR markers. Cytol. Genet. 2018, 52, 222–230. [Google Scholar] [CrossRef]
- Lin, X.C.; Ruan, X.S.; Lou, Y.F.; Guo, X.Q.; Fang, W. Genetic similarity among cultivars of Phyllostachys pubescens. Plant Syst. Evol. 2009, 277, 67–73. [Google Scholar] [CrossRef]
- Uysal, H.; Fu, Y.-B.; Kurt, O.; Peterson, G.W.; Diederichsen, A.; Kusters, P. Genetic diversity of cultivated flax (Linum usitatissimum L.) and its wild progenitor pale flax (Linum bienne Mill.) as revealed by ISSR markers. Genet. Resour. Crop Evol. 2010, 57, 1109–1119. [Google Scholar] [CrossRef]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.; Zietkiewicz, E.; Hofstra, H. Identification of chrysanthemum cultivars and stability of DNA fingerprint patterns. Theor. Appl. Genet. 1995, 91, 439–447. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Li, D.; Sun, J.; Fu, J. Assessing genetic diversity of perennial ryegrass (Lolium perenne L.) from four continents by intersimple sequence repeat (ISSR) markers. Afr. J. Biotechnol. 2011, 10, 19365–19374. [Google Scholar]
- Amin, A.K.; El-Fayoumi, H.H.; Mohamed, N.H.; Tawfik, R.S.; Allam, M.; Karam, M.A. Relationships among some regional species of the genus Lolium L. based on morphological and molecular markers. Int. J. Curr. Res. 2017, 3, 1333–1363. [Google Scholar]
- Tabaripour, R.; Keshavarzi, M. Interspecific Molecular Variation of Lolium L. Based on ISSR, SCoT and ITS. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 1263–1272. [Google Scholar] [CrossRef]
- de Freitas, J.M.B.; Essi, L.; Tedesco, S.B. Genetic diversity and population structure of natural populations of the endangered grass Paspalum rawitscheri (Parodi) Chase ex GH Rua & Valls (Paspaleae, Poaceae). Braz. J. Bot. 2021, 44, 401–409. [Google Scholar]
- Al-Soqeer, A.; Al-Otayk, S.M.; Motawei, M.I. Molecular characterization of new Buffelgrass (Cenchrus ciliaris) genotypes. Plant Omics 2020, 13, 104–107. [Google Scholar] [CrossRef]
- Li, G.; Quiros, C.F. Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: Its application to mapping and gene tagging in Brassica. Theor. Appl. Genet. 2001, 103, 455–461. [Google Scholar] [CrossRef]
- Ai, P.-F.; Zhen, Z.-J.; Jin, Z.-Z. Genetic diversity and relationships within sweet kernel apricot and related Armeniaca species based on sequence-related amplified polymorphism markers. Biochem. Syst. Ecol. 2011, 39, 694–699. [Google Scholar] [CrossRef]
- Budak, H.; Shearman, R.; Parmaksiz, I.; Gaussoin, R.; Riordan, T.; Dweikat, I. Molecular characterization of buffalograss germplasm using sequence-related amplified polymorphism markers. Theor. Appl. Genet. 2004, 108, 328–334. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, X.; Xu, J.; Zheng, Y.; Pu, S.; Duan, Z.; Li, Z.; Liu, G.; Chen, J.; Wang, Z. Phenotypic and molecular marker analysis uncovers the genetic diversity of the grass Stenotaphrum secundatum. BMC Genet. 2020, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, J.; Wang, J.; Wang, X.; Lu, Y.; Ning, Y.; Li, Y. Intra-population genetic diversity of Buchloe dactyloides (Nutt.) Engelm (buffalograss) determined using morphological traits and sequence-related amplified polymorphism markers. 3 Biotech 2019, 9, 97. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, X.-Q.; Lan, Y.; Yang, W.-Y. Evaluation of genetic diversity and relationshipsin orchardgrass (Dactylis glomerata L.) germplasm based on SRAP markers. Can. J. Plant Sci. 2008, 88, 53–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, S.; Liu, J.; Zhao, Y.; Liu, J. Genetic diversity and population structure of Chinese natural bermudagrass [Cynodon dactylon (L.) Pers.] germplasm based on SRAP markers. PLoS ONE 2017, 12, e0177508. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Gilani, S.A.; Kikuchi, A.; Gulzar, S.; Khan, M.A.; Watanabe, K.N. Population diversity of Aeluropus lagopoides: A potential cash crop for saline land. Pak. J. Bot. 2011, 43, 595–605. [Google Scholar]
- Waghmode, A.; Hegde, B. Effect of sodium chloride on pyruvate orthophosphate dikinase of a saline grass Aeluropus lagopoides (Linn.) Trin. ex Thw. Biovigyanam 1984, 10, 209–210. [Google Scholar]
- Doyle, J.J. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Patel, M.P.; Parmar, D.J.; Kalola, A.D.; Kumar, S. Morphological and molecular diversity patterns in castor germplasm accessions. Ind. Crop. Prod. 2017, 97, 316–323. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Société Vaudoise Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Com-Munication; University of Illinois Press: Urbana, IL, USA, 1949; Volume 96. [Google Scholar]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Nguyen, H.T.; Wu, X. Molecular Marker Systems for Genetic Mapping. In The Handbook of Plant Genome Mapping; Meksem, K., Kahl, G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 23–52. [Google Scholar]
- Hamrick, J.L.; Godt, M.W. Effects of life history traits on genetic diversity in plant species. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1996, 351, 1291–1298. [Google Scholar]
- Wang, Z.; Wang, J.-E.; Wang, X.-M.; Gao, H.-W.; Dzyubenko, N.I.; Chapurin, V.F. Assessment of genetic diversity in Galega officinalis L. using ISSR and SRAP markers. Genet. Resour. Crop Evol. 2012, 59, 865–873. [Google Scholar] [CrossRef]
- Nybom, H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 2004, 13, 1143–1155. [Google Scholar] [CrossRef]
- Li, A.; Ge, S. Genetic variation and clonal diversity of Psammochloa villosa (Poaceae) detected by ISSR markers. Ann. Bot. 2001, 87, 585–590. [Google Scholar] [CrossRef]
- Wu, Y.-G.; Guo, Q.-S.; He, J.-C.; Lin, Y.-F.; Luo, L.-J.; Liu, G.-D. Genetic diversity analysis among and within populations of Pogostemon cablin from China with ISSR and SRAP markers. Biochem. Syst. Ecol. 2010, 38, 63–72. [Google Scholar] [CrossRef]
- Peng, X.; Ji, Q.; Fan, S.; Zhang, Y.; Zhang, J. Genetic diversity in populations of the endangered medicinal plant Tetrastigma hemsleyanum revealed by ISSR and SRAP markers: Implications for conservation. Genet. Resour. Crop Evol. 2015, 62, 1069–1078. [Google Scholar] [CrossRef]
- Yan, W.; Li, J.; Zheng, D.; Friedman, C.; Wang, H. Analysis of genetic population structure and diversity in Mallotus oblongifolius using ISSR and SRAP markers. PeerJ 2019, 7, e7173. [Google Scholar] [CrossRef]
- Ma, M.; Wang, T.; Lu, B. Assessment of genetic diversity in Amomum tsao-ko Crevost & Lemarié, an important medicine food homologous crop from Southwest China using SRAP and ISSR markers. Genet. Resour. Crop. Evol. 2021, 68, 2655–2667. [Google Scholar]
- Tang, L.; Xiao, Y.; Li, L.; Guo, Q.; Bian, Y. Analysis of genetic diversity among Chinese Auricularia auricula cultivars using combined ISSR and SRAP markers. Curr. Microbiol. 2010, 61, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, X.; Wang, H.; Wang, J. Genetic diversity and population structure of Salvia miltiorrhiza Bge in China revealed by ISSR and SRAP. Genetica 2010, 138, 241–249. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Z.; Wei, S.; Ouyang, Z.; Wu, K.; Peng, Z. ISSR and SRAP markers in the genetic relationship analysis among Pinellia in China. J. Med. Plants Res. 2012, 6, 3596–3602. [Google Scholar]
- Wu, Z.G.; Li, X.X.; Lin, X.C.; Jiang, W.; Tao, Z.M.; Mantri, N.; Fan, C.Y.; Bao, X.Q. Genetic diversity analysis of yams (Dioscorea spp.) cultivated in China using ISSR and SRAP markers. Genet. Resour. Crop Evol. 2014, 61, 639–650. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Dai, T.-X.; Chang, Y.-T.; Wang, S.-S.; Ou, S.-L.; Chuang, W.-L.; Chuang, C.-Y.; Lin, Y.-H.; Lin, Y.-Y.; Ku, H.-M. Genetic diversity among ‘Ocimum’ species based on ISSR, RAPD and SRAP markers. Aust. J. Crop Sci. 2013, 7, 1463–1471. [Google Scholar]
- Wangsomnuk, P.P.; Khampa, S.; Jogloy, S.; Srivong, T.; Patanothai, A.; Fu, Y.-B. Assessing genetic structure and relatedness of jerusalem artichoke (Helianthus tuberosus L.) germplasm with RAPD, ISSR and SRAP markers. Am. J. Plant Sci. 2011, 2, 753–764. [Google Scholar] [CrossRef][Green Version]
- Loarce, Y.; Gallego, R.; Ferrer, E. A comparative analysis of the genetic relationships between rye cultivars using RFLP and RAPD markers. Euphytica 1996, 88, 107–115. [Google Scholar] [CrossRef]
- Chen, D.; Peng, R.; Li, L.; Sun, N.; Zhong, G.; Cai, Y. Study on genetic diversity of Codonopsis tangshen by SRAP and ISSR markers. Zhongguo Zhong Yao Za Zhi 2009, 34, 255–259. [Google Scholar]
- Lambertini, C.; Gustafsson, M.H.; Frydenberg, J.; Speranza, M.; Brix, H. Genetic diversity patterns in Phragmites australis at the population, regional and continental scales. Aquat. Bot. 2008, 88, 160–170. [Google Scholar] [CrossRef]
- Rahali, N.; Yangui, I.; Boussaid, M.; Messaoud, C. Assessment of genetic diversity and population structure of the endemic Hertia cheirifolia (L.) Kuntze based on ISSR and SRAP molecular markers. Biologia 2022, 77, 3429–3439. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Q.; Niu, Y.; Zhang, Y.; Dong, S.; Zhang, W.; Wang, Z. Diversity analysis and establishment of core collection among Akebia trifoliata (Thunb.) Koidz. in Qinba mountain area of China using ISSR and SRAP markers. Genet. Resour. Crop Evol. 2021, 68, 1085–1102. [Google Scholar] [CrossRef]
- Wang, X.-D.; Li, X.-H.; Zhang, Z.-W.; Wang, K.-J. Genetic diversity and genetic structure of natural populations in an extremely narrowly distributed perennial species Glycine tabacina (Labill.) Benth. on the southeast islands in China. Genet. Resour. Crop Evol. 2019, 66, 989–1008. [Google Scholar] [CrossRef]
| Region | Location | Population | Coordinates | Elevationm a.s.l | EC (dS.m−1) | |
|---|---|---|---|---|---|---|
| N | E | |||||
| Jouf | Inland sabkha in Domat Aljandal | Jouf1 | 29°49.200 | 039°58.393 | 565 | 9.39 ± 1.432 * |
| Jouf2 | 29°49.270 | 039°58.457 | 563 | |||
| Jouf3 | 29°49.850 | 039°58.931 | 558 | |||
| Jouf4 | 29°49.085 | 039°58.149 | 519 | |||
| Jouf5 | 29°49.169 | 039°57.494 | 525 | |||
| Jizan | Coastal sabkha on the Southern Coastal Region | Jizan1 | 16°58.102 | 042°33.849 | 15 | 10.69 ± 3.76 |
| Jizan2 | 16°58.122 | 042°33.707 | 9 | |||
| Jizan3 | 16°58.137 | 042°33.667 | 6 | |||
| Jizan4 | 16°58.144 | 042°34.082 | 4 | |||
| Jizan5 | 16°58.114 | 042°34.016 | 4 | |||
| Salwa | Coastal sabkha as lowland on the coast of the Arabian Gulf | Salwa1 | 24°45.392 | 050°45.225 | −10 | 29.59 ± 1.409 |
| Salwa2 | 25°43.664 | 050°08.274 | −9 | |||
| Salwa3 | 25°43.759 | 050°08.045 | −8 | |||
| Salwa4 | 24°45.071 | 050°45.348 | −11 | |||
| Salwa5 | 25°43.664 | 050°08.274 | −9 | |||
| Qareenah | Inland sabkha in wadi Hargan, Riyadh Region, | Qar1 | 25°03.995 | 046°10.795 | 833 | 12.37 ± 1.026 |
| Qar2 | 25°03.975 | 046°10.802 | 824 | |||
| Qar3 | 25°03.944 | 046°10.824 | 816 | |||
| Qar4 | 25°03.923 | 046°10.852 | 812 | |||
| Qar5 | 25°03.890 | 046°10.897 | 810 | |||
| Qaseem | Inland sabkha of the Al-Aushazia location | Qas1 | 26°03.295 | 44°08.168 | 590 | 24.82 ± 1.024 |
| Qas2 | 26°03.309 | 44°08.253 | 654 | |||
| Qas3 | 26°03.236 | 044°08.220 | 621 | |||
| Qas4 | 26°03.770 | 044°08.272 | 603 | |||
| Qas5 | 26°03.351 | 044°08.144 | 595 | |||
| ISSR Markers | SRAP Markers | |||||
|---|---|---|---|---|---|---|
| Primer Name | Sequences | Annealing Temp. | Primer Name | Forward (5′-3′) | Primer Name | Reverse (5′-3′) |
| FBISSR6 | (GTT)7C | 54 °C | SRAP9F | TGAGTCCAAAACGGTAG | SRAP1R | GACTGCGTACGAATTAAC |
| FBISSR7 | (GTT)7A | 53 °C | SRAP15: | TGAGTCCAAACCGGTTG | SRAP2R | GACTGCGTACGAATTAAT |
| FBISSR8 | (GTT)7T | 53 °C | SRAP7F | TGAGTCCAAACCGGTGA | SRAP3R | GACTGCGTACGAATTACA |
| FBISSR9 | CACACACACACACACACACAG | 57 °C | SRAP22F | TGAGTCCAAACCGGTAG | SRAP4R | GACTGCGTACGAAATACG |
| FBISSR10 | CACACACACACACACACACAC | 57 °C | SRAP21F | TGAGTCCAAACCGGTGC | SRAP7R | GACTGCGTACGAATTCAC |
| FBISSR11 | CACACACACACACACACACAA | 57 °C | SRAP5F | TGAGTCCAAACCGGTCA | SRAP31R | GACTGCGTACGAATTTCA |
| FBISSR12 | CACACACACACACACACACAT | 57 °C | SRAP30F | TGAGTCCAAACCGGAAG | ||
| FBISSR13 | TGTGTGTGTGTGTGTGTGTGG | 57 °C | SRAP19F | TGAGTCCAAACCGGTGC | ||
| FBISSR15 | TGTGTGTGTGTGTGTGTGTGA | 57 °C | SRAP29F | TGAGTCCAAACCGGACC | ||
| FBISSR16 | TGTGTGTGTGTGTGTGTGGTG | 57 °C | ||||
| ISSR15 | GAGAGAGAGAGAGAGAC | 50 °C | ||||
| UBC815 | CTCTCTCTCTCTCTCTG | 50 °C | ||||
| UBC816 | CACACACACACACACAT | 50 °C | ||||
| UBC823 | TCTCTCTCTCTCTCTCC | 50 °C | ||||
| Primer Name | No. of Amplified Bands | No. of Polymorphic Bands | Polymorphism % | Total Bands | DP% | PIC |
|---|---|---|---|---|---|---|
| FBISSR-6 | 7 | 7 | 100 | 20 | 4.49 | 0.15 |
| FBISSR-7 | 7 | 7 | 100 | 21 | 4.49 | 0.15 |
| FBISSR-8 | 8 | 8 | 100 | 76 | 5.13 | 0.40 |
| FBISSR-9 | 20 | 20 | 100 | 142 | 12.82 | 0.35 |
| FBISSR-10 | 3 | 3 | 100 | 15 | 1.92 | 0.30 |
| FBISSR-11 | 13 | 13 | 100 | 95 | 8.33 | 0.34 |
| FBISSR-12 | 10 | 10 | 100 | 79 | 6.41 | 0.34 |
| FBISSR-13 | 9 | 8 | 89 | 97 | 5.77 | 0.39 |
| FBISSR-15 | 12 | 12 | 100 | 151 | 7.69 | 0.47 |
| FBISSR-16 | 11 | 11 | 100 | 150 | 7.05 | 0.40 |
| ISSR 15 | 19 | 19 | 100 | 179 | 12.18 | 0.33 |
| UBC815 | 13 | 13 | 100 | 104 | 8.33 | 0.35 |
| UBC816 | 15 | 15 | 100 | 114 | 9.62 | 0.37 |
| UBC823 | 9 | 8 | 89 | 78 | 5.77 | 0.38 |
| Total | 156 | 156 | 1321 | 100 | 4.73 | |
| Avg/Primer | 11.14 | 11.14 | 94.36 | 7.14 | 0.34 |
| Primer Name (F/R) | Amplified Bands No. | Polymorphic Bands No. | Polymorphism % | Total Bands | DP% | PIC |
|---|---|---|---|---|---|---|
| SRAP9F/SRAP1R | 10 | 10 | 100 | 91 | 9.26 | 0.35 |
| SRAP15F/SRAP2R | 7 | 7 | 100 | 46 | 6.48 | 0.27 |
| SRAP7F/SRAP3R | 7 | 7 | 100 | 65 | 6.48 | 0.40 |
| SRAP7F/SRAP1R | 6 | 6 | 100 | 61 | 5.56 | 0.39 |
| SRAP22F/SRAP2R | 10 | 10 | 100 | 83 | 9.26 | 0.38 |
| SRAP21F/SRAP3R | 8 | 8 | 100 | 81 | 7.41 | 0.38 |
| SRAP5F/SRAP1R | 7 | 6 | 86 | 105 | 6.48 | 0.33 |
| SRAP30F/SRAP3R | 6 | 6 | 100 | 36 | 5.56 | 0.32 |
| SRAP19F/SRAP4R | 4 | 4 | 100 | 6 | 3.70 | 0.15 |
| SRAP22F/SRAP1R | 6 | 6 | 100 | 36 | 5.56 | 0.29 |
| SRAP30F/SRAP1R | 9 | 9 | 100 | 65 | 8.33 | 0.28 |
| SRAP5F/SRAP3R | 6 | 6 | 100 | 59 | 5.56 | 0.30 |
| SRAP9F/SRAP31R | 10 | 10 | 100 | 90 | 9.26 | 0.31 |
| SRAP29F/SRAP7R | 6 | 6 | 100 | 37 | 5.56 | 0.28 |
| SRAP9F/SRAP7R | 6 | 5 | 83 | 18 | 5.56 | 0.20 |
| Total | 108 | 108 | 879 | 100 | 4.63 | |
| Avg/Primer | 7.20 | 7.20 | 100.00 | 58.60 | 6.67 | 0.31 |
| Source | df | SSD | MSD | Est. Var. | Total% | PhiPT | p Value |
|---|---|---|---|---|---|---|---|
| For SRAP | |||||||
| Among Regions | 4 | 202.400 | 50.600 | 7.836 | 41% | ||
| Within Populations | 20 | 228.400 | 11.420 | 11.420 | 59% | ||
| Total | 24 | 430.800 | 19.256 | 100% | 0.397 | 0.001 | |
| For ISSR | |||||||
| Among Regions | 4 | 323.040 | 80.760 | 12.384 | 40% | ||
| Within Populations | 20 | 376.800 | 18.840 | 18.840 | 60% | ||
| Total | 24 | 699.840 | 31.224 | 100% | 0.407 | 0.001 | |
| For (ISSR + SRAP) | |||||||
| Among Regions | 4 | 525.440 | 131.360 | 20.220 | 40% | ||
| Within Populations | 20 | 605.200 | 30.260 | 30.260 | 60% | ||
| Total | 24 | 1130.640 | 50.480 | 100% | 0.401 | 0.001 | |
| Geographical Regions | Na | Ne | I | H | uh | PPB% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISSR | SRAP | ISSR | SRAP | ISSR | SRAP | ISSR | SRAP | ISSR | SRAP | ISSR | SRAP | |
| Jouf | 1.010 ± 0.07 * | 0.881 ± 0.09 | 1.27 ± 0.03 | 1.269 ± 0.03 | 0.24 ± 0.02 | 0.226 ± 0.03 | 0.16 ± 0.02 | 0.154 ± 0.02 | 0.20 ± 0.02 | 0.193 ± 0.02 | 43.04 | 38.53 |
| Jizan | 0.968 ± 0.08 | 0.982 ± 0.09 | 1.31 ± 0.03 | 1.260 ± 0.03 | 0.26 ± 0.02 | 0.223 ± 0.03 | 0.18 ± 0.02 | 0.151 ± 0.02 | 0.22 ± 0.02 | 0.189 ± 0.02 | 44.30 | 38.53 |
| Salwa | 1.209 ± 0.07 | 0.927 ± 0.09 | 1.380 ± 0.03 | 1.261 ± 0.03 | 0.323 ± 0.02 | 0.235 ± 0.03 | 0.220 ± 0.12 | 0.157 ± 0.02 | 0.275 ± 0.02 | 0.196 ± 0.02 | 55.70 | 42.20 |
| Qareenah | 1.051 ± 0.08 | 0.872 ± 0.09 | 1.321 ± 0.03 | 1.277 ± 0.04 | 0.275 ± 0.02 | 0.229 ± 0.03 | 0.186 ± 0.02 | 0.157 ± 0.02 | 0.233 ± 0.02 | 0.196 ± 0.02 | 47.47 | 38.53 |
| Qaseem | 1.082 ± 0.08 | 1.239 ± 0.09 | 1.361 ± 0.03 | 1.379 ± 0.04 | 0.300 ± 0.02 | 0.321 ± 0.03 | 0.206 ± 0.02 | 0.219 ± 0.02 | 0.257 ± 0.02 | 0.273 ± 0.02 | 50.63 | 55.05 |
| Average | 1.065 ± 0.03 | 0.980 ± 0.04 | 1.330 ± 0.01 | 1.289 ± 0.02 | 0.281 ± 0.01 | 0.247 ± 0.01 | 0.191 ± 0.01 | 0.168 ± 0.01 | 0.238 ± 0.01 | 0.210 ± 0.01 | 48.23 ± 2.29 | 42.57 ± 3.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dar, B.A.; Al-Doss, A.A.; Assaeed, A.M.; Javed, M.M.; Ghazy, A.I.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Genetic Variation among Aeluropus lagopoides Populations Growing in Different Saline Regions. Diversity 2024, 16, 59. https://doi.org/10.3390/d16010059
Dar BA, Al-Doss AA, Assaeed AM, Javed MM, Ghazy AI, Al-Rowaily SL, Abd-ElGawad AM. Genetic Variation among Aeluropus lagopoides Populations Growing in Different Saline Regions. Diversity. 2024; 16(1):59. https://doi.org/10.3390/d16010059
Chicago/Turabian StyleDar, Basharat A., Abdullah A. Al-Doss, Abdulaziz M. Assaeed, Muhammad M. Javed, Abdelhalim I. Ghazy, Saud L. Al-Rowaily, and Ahmed M. Abd-ElGawad. 2024. "Genetic Variation among Aeluropus lagopoides Populations Growing in Different Saline Regions" Diversity 16, no. 1: 59. https://doi.org/10.3390/d16010059
APA StyleDar, B. A., Al-Doss, A. A., Assaeed, A. M., Javed, M. M., Ghazy, A. I., Al-Rowaily, S. L., & Abd-ElGawad, A. M. (2024). Genetic Variation among Aeluropus lagopoides Populations Growing in Different Saline Regions. Diversity, 16(1), 59. https://doi.org/10.3390/d16010059











